Abstract
As an adverse immune phenomenon, graft-versus-host disease often occurs after allogeneic hematopoietic stem cell transplantation. The incidence of acute and chronic graft-versus-host disease is about 40–60% and the mortality rate can reach 15%, which is a potentially fatal disease. There are rare GvHD cases involving the central nervous system. We reported a rare case of diffuse white matter changes after haploid bone marrow transplantation, summarizing its clinical manifestations and diagnosis and treatment in conjunction with the literature.
Keywords: graft-versus-host disease, central nervous system, diffuse white matter lesions, immunosuppression, bone marrow transplantation
Introduction
A 22-year-old woman suffered headaches, vomiting, progressive unconsciousness, left hemiplegia and dysarthria. She was diagnosed as leukemia and received an allogeneic hematopoietic cell transplantation (HCT). Neurological examination showed drowsiness, dysphoria, dysphasia, gaze to the right, left hemiplegia, positive left Babinski sign, and positive Kernig sign. Brain magnetic resonance imaging (MRI) showed diffuse white matter and corpus abnormal signal. Intracranial pressure was more than 400 mmH2O. Cerebrospinal fluid (CSF) test revealed normal protein and white blood cell, mildly increased glucose, and elevated immunoglobulin G.
Case Presentation
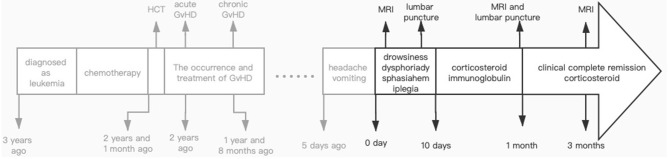
A 22-year-old woman was accepted to our department for the main complaint of paroxysmal occipital headache and vomiting, following progressive unconsciousness, left hemiplegia and dysarthria for 5 days. For medical history, she was diagnosed as acute myeloid leukemia (AML) M5 3 years ago and received induction remission treatment 1 week after the diagnosis. After that, she underwent intermittent chemotherapy for 10 months (until 2 years and 1 month before admitted) to achieve complete remission and then received a haploid hematopoietic stem cell transplant with father donor. Liver acute GVHD occurred in the 4th week after transplantation (2 years before admitted), and lung injury manifested as bronchiolitis obliterans in the 5th month (1 year and 8 months before admitted). Chronic GvHD involving the gastrointestinal tract and skin appeared at 1 year and 6 months before admitted. In the following 6 months, the symptoms of GVHD were relieved after treatment with corticosteroids and cyclosporine as anti-rejection drugs. Regular examination of bone marrow puncture showed a state of remission. Anorexia symptoms appeared when steroids gradually decreased, and central nervous system symptoms appeared 1 month later. Neurological examination showed drowsiness, dysphoria, dysphasia, gaze to the right, left hemiplegia, positive left Babinski sign, and positive Kernig sign.
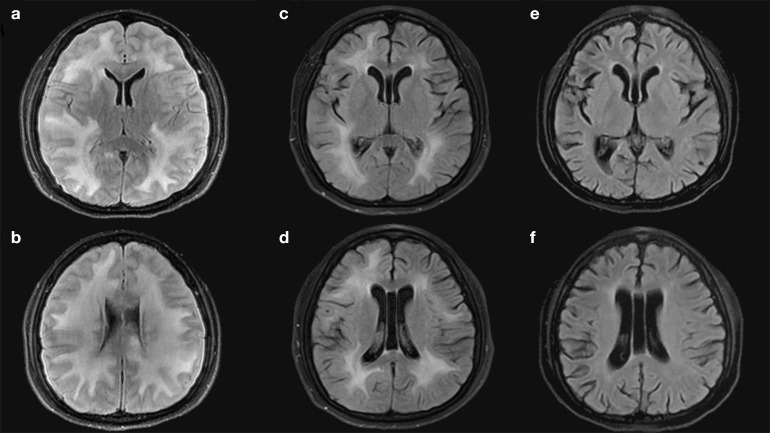
Brain magnetic resonance imaging (MRI) showed diffuse white matter and corpus abnormal signal with slightly lower T1WI signal, slightly higher T2WI and FLAIR signal, bilateral lateral ventricles and partial sulcus were narrowed, and cerebral gyrus was swelling (Figures 1a,b). The dark-fluid presents a roughly symmetrical high signal, the ADC signal is slightly higher, and DWI has no dispersion limitation. Laboratory examination including rheumatoid factor, antinuclear antibody, antineutrophil cytoplasmic antibody, single and double strand DNA revealed almost normal exclude mild hyponatremia (Na+ 131.2 mmol/L, normal range 137–147 mmol/L). Serum cryptococcal antigen was not detected and the panel PCR assay (including IgM Antibody assay for AdV, HSV, RuV, RSV, toxoplasma, Legionella, and PCR for EBV, CMV, VZV, HHV) revealed negative. Intracranial pressure was more than 400 mmH2O. Cerebrospinal fluid (CSF) test revealed normal protein (0.40 g/L) and white blood cell (WBC, 4 × 106 /L), mildly increased glucose (4.6 mmol/L, normal range 2.3~4.1 mmol/L), and elevated immunoglobulin G (IgG, 61.3 mg/L, normal range 0–34.0 mg/L). No malignant phenotype was found in flow cytometry. Autoimmune disease markers of both serum and CSF were also negative, which including NMDA-R-Ab, CASPR2-Ab, AMPA1-R-Ab, AMPA2-R-Ab, LGl1-Ab, GABAB-R-Ab, and GAD 65-Ab. Besides, serum toxicological screening was also negative. The patient was diagnosed with white matter lesions, and we speculate that it is more likely to be caused by central nervous system graft-versus-host disease.
Figure 1.
(a–f) MRI done in different periods.
In addition to the ongoing systemic immunosuppression, the patient received intravenous corticosteroid (methylprednisolone 80 mg/day) for 14 days, followed intravenous immunoglobulin (0.4 g per kilogram of body weight) for 5 days. Consciousness and hemiplegia improved significantly within 2 days. After 3 weeks of treatment, the paralysis symptom was completely recovered, and anorexia and vomiting were relieved. Brain magnetic resonance imaging (MRI) appearances were slightly improved, edema lessened than before (Figures 1c,d). Reexamination of lumbar puncture showed that the cranial pressure was lower (250 mmH2O). Cerebrospinal fluid test showed decrease IgG (44.4 mg/L). Intravenous hormone dosage decreased to methylprednisolone 40 mg/day and sequentially reduced. A follow-up brain MRI, at 3 months from the start of methylprednisolone treatment (Figure 2), showed disappearance of lesions in the brain white matter (Figures 1e,f).
Figure 2.
Historical and current information from this episode of care organized as timeline.
Short Review of GvHD
As a rather common treatment of blood system diseases nowadays, allogeneic HCT still has its limitation. Its main obstacle is graft-versus-host disease (GvHD), an immune-mediated disease that could affect many tissues and organs (1). Among patients receiving HCT, the incidence of GVHD is as high as 40 to 60% (2, 3). In this potentially fatal disease, the mortality rate may be close to 15% (2). Studies also confirmed the importance of donor selection. The prevalence of acute GVHD and chronic GVHD and treatment-related mortality increased significantly in recipients of HLA mismatched donors (4). Although the neurological complications of acute and chronic graft-versus-host disease are rare, they can significantly affect the quality of life and have a high mortality rate (5).
The diagnostic criteria for acute CNS-GvHD disease have not been clearly proposed in the literature. Openshaw described six diagnostic criteria for chronic CNS-GVHD in 2009, including: 1. Occurrence with chronic GVHD affecting other organs; 2. Neurological signs of CNS involvement without other explanation; 3. Corresponding brain MRI abnormality; 4. Abnormal CSF studies (pleocytosis, elevated protein or immunoglobulin G, oligoclonal bands); 5. Pathological brain biopsy or post-mortem examination; 6. Response to immunosuppressive therapy (6). In 2010, a report from the Consensus Conference on Clinical Practice in chronic CNS-GvHD suggested that for clinical purposes, chronic CNS-GVHD can be diagnosed by meeting two of the first two criteria and at least two of the four remaining criteria (5).
Relevant articles were selected by searching the keyword “central nervous system GvHD” for relevant case reports via pubmed. Then we reviewed references of all selected articles to research additional articles. We selected 29 articles published between 1990 and December 2019, including 46 cases (Table 1) (7–35), 22 with histological analysis (brain biopsy, spine biopsy, or autopsy) (7, 8, 10, 13, 17–19, 22, 25, 27, 29, 30, 32–34). Patients who had received an allogeneic HCT for hematological disease were selected if they had CNS abnormalities and other diagnosis (autoimmune disease, infectious diseases, hematologic malignancies relapse, or post transplantation lymphoproliferative disorders) were excluded. In these cases, the male: female ratio was 1.42, which shows that males account for more. The median age of onset was 41 years old (range 9~68 years old). Primary diseases mainly include hematological malignancies and a few other diseases that require stem cell transplantation (Supplementary Chart 1). Sixteen patients received bone marrow from matched-related donor, 11 patients received from matched-unrelated donor, four patients received from mismatch-unrelated donor, two patients received cord blood, one patient received one-allele mismatched related donor, and one patient received haplo-identical T depleted cells. A total of 25 cases reported the history of acute GvHD. In addition, 29 cases reported the onset of chronic GvHD before or during the onset of neurological symptoms. In these cases, median neurology symptoms onset was 390 days after HCT (7-7300). In order to prevent or treat immune rejection, immunosuppression therapy is widely used in patients undergoing bone marrow transplantation. It is also considered to be one of the trigger factors of graft-versus-host disease (36). In these cases, 40 patients received immunosuppressive therapy to prevent GvHD, and 16 of them stopped immunosuppressive therapy before the onset of the disease. The main clinical manifestations vary, we made statistics on the patients' neurological symptoms (Supplementary Chart 2). Their clinical characteristics are heterogeneous: 11 of these showed a stroke-like episodes or lacunar syndrome, 13 patients had ADEM or multiple sclerosis-like presentation, 17 patients had symptoms of encephalopathy or encephalitis, and the clinical manifestations of the remaining four patients were not typical. Twelve patients underwent electroencephalograms (EEG). Ten people's EEG showed diffuse slow wave activity, and two of those ten detected seizures. The other two EEG results showed that one was conformed encephalitis, and the other has no obvious abnormality. A total of 40 patients were tested for cerebrospinal fluid. Eleven of them (27.5%) had no abnormalities, and elevated protein (n = 23, 57.5%) was the most common abnormality (Table 2). Except for the first patient reported in 1990, the remaining 45 people all underwent brain MRI, only three people indicated normal. Abnormal imaging manifestations are mainly divided into three categories, hemorrhage or ischemia, white matter lesions, and others (Supplementary Table 1). The features of brain biopsy or autopsy data included: Perivascular inflammation (n = 16, 72.8%), vasculitis (n = 4, 18.2%), gliosis, microglia proliferation or activation (n = 8, 36.4%), infiltration of CD3+ / CD4+ T cells (n = 1, 4.5%), infiltration of CD3+ /CD8+ T cells (n = 6, 27.3%), parenchyma lymphocytic infiltration (n = 4, 18.2%), demyelination (n = 7, 31.8%), granulomatous infiltration (n = 3, 13.6%). Among the available information, 41 cases reported treatment methods and 40 of them received immunosuppressive treatment (Supplementary Table 2). We have ascertained the treatment effects and prognosis of all cases, three of them are not available. Most patients have achieved complete response (n = 15) or partial response (n = 17) in clinical and/or imaging studies after treatment, while other patients showed stable (n = 3), progress (n = 6), or transient remission (n = 2), respectively. Since only part of cases have been followed up, and the follow-up time ranges from 3 months to 6 years, it is difficult for us to conduct an effective prognosis assessment. At the end of the follow-up, six patients died and one patient survived (Supplementary Table 3).
Table 1.
Detailed information of the cases.
| Time of report | Age | Sex | Initial disease | GvHD history | Clinical characteristics, imaging, and biological abnormalities | Histology | Immunosuppressive therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1990 (7) |
32 | m | CML | Chronic GvHD | Clinic: dysphagia, dysarthria; CSF: normal | Perivascular infiltrates of CNS | NA | Deceased |
| 1993 (8) |
9 | f | Posthepatitis aplastic anemia | Chronic GvHD | Onset: 8 mo after HSCT; Clinic: seizure, spasticity, disturbance of consciousness; CSF: pleocytosis, elevated protein; MRI: cortical atrophy, ventricular dilatation | Diffuse infiltration of white matter with CD3 lymphocytes, Inflammatory cell perivascular infiltration, Focal CD68+ microglia proliferation (HLA-DR+) | NA | Deceased 13 mo after neurological symptoms of liver and kidney failure |
| 1993 (8) |
13 | m | ALL | Chronic GvHD | Onset: 100 d after HSCT; Clinic: cognitive deficits; MRI: white matter lesions in cerebellum | Diffuse CD3 lymphocytes infiltration and gliosis, inflammatory cells were mostly confined to perivascular spaces | NA | Deceased 5 mo after neurological symptoms of interstitial pneumonia |
| 1996 (9) |
14 | f | Lymphoblastic lymphoma B | Acute GvHD | Onset: 71 d after HSCT; Clinic: disorientation and myoclonus; CSF: elevated protein (0.9 g/L), elevated CSF Ig; MRI: multiple foci of hyperintense signal on T2-weighted images within the brain stem and deep white matter; EEG: diffuse slowing | No | MP 1g/d | Clinical and MRI PR Deceased 123 d after neurological symptoms of severe sepsis |
| 1999 (10) |
43 | m | CML | Acute and chronic GvHD | Onset: 18 mo after HSCT Clinic: acute vertigo, hemiparesis, aphasia, cortical blindness; MRI: multiple hematoma; CSF: elevated protein Angiography: normal | T cells, B cells, and monohistiocytes vessel wall and perivascular infiltration, sporadic demyelination and micro glia reaction | Corticosteroids (1.5mg/kg) and cyclophosphamide (bolus) | CR Deceased 5 mo after neurological symptoms of pneumonia |
| 1999 (10) |
32 | f | AML | Acute and chronic GvHD | Onset: 28 mo after HSCT; Clinic: aphasia, hemiparesis and seizure; MRI: ischemic areas, white matter lesion; CSF: normal | No | Corticosteroids (1.5mg/kg) and cyclophosphamide (bolus) | Progression with cerebral infarctions Deceased of tentorial herniation |
| 1999 (10) |
19 | m | ALL | Chronic GvHD | Onset: 31 mo after HSCT; Clinic: sub-acute confusion, spastic right hemiparesis Perfusion; MRI: leukoencephalopathy; CSF: normal; Angiography: normal | No | Corticosteroids (1.5mg/kg) | CR |
| 1999 (10) |
32 | m | CLL | Acute and chronic GvHD | Onset: 5 y after HSCT; Clinic: aphasia, apraxia, dementia, tetra paresis; MRI: leukoencephalopathy, hemorrhage; CSF: normal Perfusion; Angiography: normal | No | Corticosteroids (1.5mg/kg) and cyclophosphamide (bolus) | Clinical and MRI stability |
| 1999 (10) |
53 | m | CLL | Acute and chronic GvHD | Onset: 30 mo after HSCT; Clinic: acute aphasia and cognitive deficit; MRI: periventricular white matter lesion, frontoparietal ischemia; CSF: pleocytosis; Angiography: MCA branch occlusion | No | NA | Progression |
| 2000 (11) |
22 | f | ALL | Acute GvHD | Onset: 9 mo after HSCT; Clinic: hemiparesis MRI: white matter lesions in frontal lobe Angiography: multiple stenosis and occlusions in the peripheral branches of the anterior and middle cerebral arteries | No | Corticosteroids | Transient improvement |
| 2001 (12) |
25 | m | T-cells lymphoma | Chronic GvHD | Onset: 380 d after HSCT Clinic: cerebellar and pyramidal syndromes and peripheral neuropathy CSF: oligoclonal bands, pleocytosis MRI: multiple | No | MP 1g/d and plasmapheresis | Clinical PR and MRI stability |
| demyelinating-like, asymmetric, hyperintense areas on T2-weighted images at the brainstem, cerebellar and corona radiata white matter. | ||||||||
| 2002 (13) |
18 | f | AML | Acute GvHD | Onset: 2 mo after HSCT Clinic: seizure, spastic, cognitive dysfunction MRI: diffuse atrophy EEG: bilateral slowing and voltage suppression over the left hemisphere CSF: elevated protein and pleocytosis | Cerebral and meningeal angiitis, granulomatous infiltration, lymphocytic infiltration with microglial proliferation | Corticosteroids | PR |
| 2003 (14) |
51 | f | AML | Acute and chronic GvHD | Onset: 10 mo after HSCT Clinic: diplopia, dysarthria, and gait disturbance MRI: multifocal abnormal high signal intensity mainly in the white matter of both cerebral hemispheres as well as in the cerebellum and brainstem CSF: normal | No | NA | Clinical CR and MR PR Alive 15 mo after |
| 2005 (15) |
55 | f | NHL | Chronic GvHD | Onset: 23 mo after HSCT Clinic: acute cerebellar syndrome CT: acute intra_x005f parenchymal hemorrhage in left cerebellum MRI: white matter lesions peri-ventricular Angiography: aneurysm of the left posterior inferior cerebellar artery and dilated branches of cerebral arteries | No | Tacrolimus | PR |
| 2006 (16) |
48 | m | NHL | Acute GvHD | Onset: 14 mo after HSCT Clinic: headaches, personality change, cognitive dysfunction MRI: diffuse deep white matter changes with periventricular predominance CSF: pleocytosis, elevated protein EEG: slow wave activity consistent with a diffuse encephalopathy | No | MP 2 mg/kg | CR |
| 2007 (17) |
58 | f | ALL | Acute and chronic GvHD | Onset: 178 d after HSCT Clinic: encephalopathy and seizure CSF: elevated protein (0.67 g/L) MRI: patchy areas of white matter lesions | Leptomeningeal perivascular infiltration of CD3+/CD8+ T cells | Corticosteroids | Transient improvement Deceased 123 d after neurological symptoms of multiorgan failure |
| 2007 (17) |
45 | f | T-cells lymphoma | Acute and chronic GvHD | Onset: 18 mo after HSCT; Clinic: right hemiparesis and seizure; CSF: normal; MRI: white matter lesions in fronto-parietal lobe | Perivascular inflammation mainly composed of CD3+/CD4+ T cells of the donor (FISH XY) | MP 1g/d(5d) | Clinical then MRI CR Alive 8y after HSCT |
| 2007 (18) |
41 | m | Follicular lymphoma | Chronic GvHD | Onset: 3 year after; HSCT Clinic: progressive left hemiparesis; CSF: normal; MRI: large mass of right parietal lobe | Focal infiltration of lympho_x005f histiocytic inflammatory cell and noncaseating granuloma with perivascular predominance IHC: CD3+ T cells | Corticosteroids and ciclosporin | Clinical and MRI PR |
| 2009 (19) |
56 | m | NHL | Chronic GvHD | Clinic: dizziness, tinnitus, vertigo, proximal weakness; MRI: large lesion of the corpus callosum | Perivascular inflammation and scattered CD3+/CD8+ T cells associated with microglia activation and macrophages in brain parenchyma | Corticosteroids and MMF | Progression Deceased 2 mo later |
| 2009 (20) |
32 | f | MDS | Chronic GvHD | Onset: 7 mo after HSCT; Clinic: bilateral papillar edema with almost blindness, weakness of lower limbs, urinary retention; CSF: normal; MRI: multiple white matter lesions mainly in internal capsule, thalamus and thorax spine, evocative of multiple sclerosis | No | Corticosteroids (bolus then 0.5 mg/kg) and ciclosporin | Clinical and MRI PR Alive 2y after HSCT |
| 2009 (21) |
40 | f | Follicular lymphoma | Acute GvHD | Onset: 10 d after HSCT; Clinic: encephalitis and seizure; CSF: elevated protein (6.75 g/L), pleocytosis (96.8% of donor cells); MRI: normal | No | Corticosteroids (3 bolus) followed by etoposide (50 mg/m2) because of HLH evidence | Progression Deceased 32 d after HSCT |
| 2010 (22) |
35 | m | CML | Acute and chronic GvHD | Onset: 4 y after HSCT; Clinic: seizure; MRI: cortical/sub cortical acute ischemic lesions in peri-insular region, left frontal and parietal lobe; EEG: temporal slowing without epileptic discharges | Micro-angiopathy | Corticosteroids (3 bolus then 1mg/kg) and cyclophosphamide (bolus) Then methotrexate (10mg/week) | CR |
| 2010 (22) |
28 | f | AML | Acute and chronic GvHD | Onset: 2 y after HSCT; Clinic: progressive depression, cognitive deficits, cortical blindness, seizure, ataxia, tetraparesis; CSF: elevated protein, oligo-clonal bands; MRI: leukoencephalopathy then internal brain atrophy; EEG: generalized slowing and epileptic discharges | Perivascular infiltration of donor CD3+ CD8+ lympho-monuclear cells (FISH XY), loss of myelin and axon preservation | Corticosteroids (5 bolus then 1mg/kg) and cyclophosphamide (1 bolus then 100mg/j) | CR |
| 2010 (22) |
20 | m | SCID | Chronic GvHD | Onset: 20 y after HSCT; Clinic: hemiparesis, ataxia, cortical blindness and deafness; CSF: elevated protein, pleocytosis; MRI: multiple focal ischemic lesions and hemorrhage | Perivascular infiltration of CD3+ CD8+ lympho-monuclear cells, loss of myelin and axon preservation | Corticosteroids and cyclophosphamide (4 bolus) | PR Deceased 1 y after neurological symptoms of severe sepsis |
| 2010 (22) |
33 | m | CLL | Acute and chronic GvHD | Onset: 2 y after HSCT; Clinic: ataxia, cortical blindness, spastic tetraparesis, acute pseudo-bulbar syndrome; CSF: elevated protein, pleocytosis, intrathecal IgG synthesis; MRI: frontally accentuated brain atrophy | Cerebral and meningeal angiitis | Corticosteroids (3 bolus then 1.5mg/kg) and cyclophosphamide (1 bolus) | Small improvement Deceased 4 y after neurological symptoms of acute aspiration |
| 2010 (23) |
57 | m | CMML | No | Onset: 4 w after HSCT Clinic: recurrent myelitis with mild paraparesis, urinary difficulty; CSF: elevated protein; MRI: multiple white matter lesion of spinal cord without cerebral anomalies | No | Corticosteroids (5 bolus then 1mg/kg) then cyclophosphamide (7 bolus) | CR but relapse of myelitis 1,5 y later Treatment by cyclophosphamide with PR |
| 2010 (23) |
65 | m | AML | No | Onset: 3 y after HSCT Clinic: recurrent myelitis with mild paraparesis and lower limb hypoesthesia; CSF: elevated protein and oligoclonal bands; MRI: multiple white matter lesion of spinal cord, T2-hyperintense paraventricular left hemispheric lesion | No | Corticosteroids (5 bolus) | CR but relapse of myelitis 1 mo after Treatment by corticosteroids with CR |
| 2012 (24) |
54 | m | AML | No | Onset: 390 d after HSCT; Clinic: progressive ascending weakness, areflexia and cranial neuropathy; CSF: elevated protein; MRI: multiple | Loss of myelin and axon preservation | Corticosteroids and IV Ig (5 courses) | PR Deceased 5 y later |
| sub-cortical lesions, one with a relatively open ring sign | ||||||||
| 2012 (24) |
59 | m | AML | No | Onset: 240 d after HSCT; Clinic: progressive ascending weakness, areflexia and cranial neuropathy; CSF: elevated protein, oligoclonal bands; MRI: pontine white matter lesion | No | IV Ig (5 courses) | PR Alive 9 y later |
| 2012 (24) |
29 | m | AML | No | Onset: 63 d after HSCT; Clinic: progressive ascending weakness, areflexia and cranial neuropathy; CSF: elevated protein, oligoclonal bands; MRI: multiple white matter lesions of cervical spine and few in brain | Biopsy of spinal cord Loss of myelin and axon preservation | Corticosteroids and IV Ig (5 courses) | PR Deceased 2 y later of severe chronic GvHD |
| 2014 (25) |
63 | m | CLL | Acute GvHD | Onset: 92 d after HSCT; Clinic: cognitive impairment, tremor; CSF: elevated protein; MRI: multifocal subcortical and juxtacortical white matter lesions | No | Prednisolone 0.5 mg/kg/d | CR Alive 1 y after |
| 2015 (26) |
7 | m | Idiopathic aplastic anemia | Chronic GvHD | Onset: 15 mo after HSCT; Clinic: depression and seizure; CSF: normal; MRI: bilateral uncus lesions; EEG: slowing background and one epileptic focus VGKC and ILGI1 antibodies positives | No | Corticosteroids (5 boluses then 1mg/kg) and IV Ig (5 courses) | Clinical PR |
| 2017 (27) |
33 | m | Fanconi disease | Acute and chronic GvHD | Onset: 308 d after HSCT; Clinic: myelitis with motor and sensitive neuronopathy, memory disorders; MRI: Pan-myelitis and posterior lepto-meningitis; EMG: Motor and sensitive neuronopathy and muscular junction damage; CSF: Pleocytosis with majority of T cells, elevated protein, oligoclonal bands | Lymphohistiocytic vasculitis without necrosis, perivascular infiltration with CD3+ CD8+ T cells | Corticosteroids (10 bolus then 1mg/kg), IV Ig (3 courses), plasmapheresis (10 courses), MMF | Clinical stability Alive 37 m after CNS symptoms |
| 2017 (27) |
62 | m | MPN | Acute GvHD | Onset: 152 d after HSCT; Clinic: confusion, coma; MRI: Normal; EEG: diffuse brain suffering and frontal peak-waves discharges; CSF: Pleocytosis and elevated protein | Diffuse lymphocyte T infiltrate with small perivascular predominance and diffuse gliosis | Corticosteroids (1mg/kg) | Progression Deceased 17 d after CNS symptoms |
| 2017 (27) |
68 | f | MPN | Acute GvHD | Onset: 9 d after HSCT; Clinic: encephalitis; MRI: Hyper T2 focal lesion of the left hemisphere; EEG: Encephalitis; CSF: Pleocytosis | No | Corticosteroids (2mg/kg) | Progression Deceased 5 d after CNS symptoms |
| 2017 (27) |
29 | f | Fanconi disease | Chronic GvHD | Onset: 378 d after HSCT; Clinic: cerebellar syndrome, cranial nerves deficits and atypical poly-radiculonevritis; MRI: normal; CSF: elevated protein | No | Corticosteroids (3 bolus then 1mg/kg), plasmapheresis (5 courses), IV Ig (6 courses) | PR Deceased 30 mo after CNS symptoms |
| 2017 (27) |
50 | m | MPN | Chronic GvHD | Onset: 2090 d after HSCT; Clinic: transient and focal deficits (right hemiparesis and paresthesia); MRI: T2 hyper-signals compatible with multiple sclerosis; CSF: normal | No | Ciclosporin A (6mg/kg) | CR Alive 8, 3 mo after CNS symptoms |
| 2017 (27) |
16 | f | AML | Acute GvHD | Onset: 255 d after HSCT; Clinic: encephalitis, extra pyramidal syndrome; MRI: peri-ventricular and posterior leuko-encephalopathy associated with hemispheric cerebellar lesions with contrast enhancement; EEG: Global and diffuse slowing | No | Corticosteroid (1mg/kg) | PR Deceased 4, 2 mo after CNS symptoms |
| 2017 (27) |
36 | m | CML | Acute and chronic GvHD | Onset: 199 d after HSCT; Clinic: cerebellar and vestibular syndromes, left hemiparesis and hypoesthesia, and cranial nerves deficits; MRI: lacunar infarct, cerebral vasculopathy associated with focal lesion Angiography: normal; CSF: Elevated protein with IgG polyclonal | No | Corticosteroids (1mg/kg) | PR then secondary aggravation Deceased 8 mo after CNS symptoms |
| 2017 (28) |
66 | m | AML | Acute GvHD | Onset: 10 mo after HSCT; Clinic: drowsiness, apathy, diffuse cognitive impairment, frontal lobe involvement; MRI: hyperintensities of the centrum ovule and the lateral ventricles without gadolinium enhancement; CSF: normal; EEG: unremarkable | No | Corticosteroids (1mg/kg),fingolimod (0.5 mg/d) | CR but relapse of myelitis 7 mo later Alive 2, 3 y after CNS symptoms |
| 2017 (29) |
46 | f | AML | Acute and chronic GvHD | Onset: 19 mo after HSCT; Clinic: headaches, tremor, memory loss; MRI: areas of confluent and asymmetrical hyperintensity on T2WI and FLAIR in the bilateral cerebral white matter; CSF: pleocytosis, protein elevation, and low glucose Gadolinium-enhanced; MRI: multiple punctate and curvilinear lesions | Perivascular infiltration of CD3+ CD8+ lympho-myonuclear cells, non-caseating granulomas, loss of myelin and axon preservation | Corticosteroids and tacrolimus | CR |
| 2018 (30) |
60 | m | AML | Acute GvHD | Onset: 7 d after HSCT; Clinic: impaired consciousness, psychomotor agitation; CSF: elevated protein; MRI: multiple hyperintense lesions on T2 and fluid-attenuated inversion recovery of the deep white matter in frontal as well on the splenium of the corpus callous | Autopsy after CR showed no lymphocytic infiltration in the central nervous system. | Intrathecal methylprednisolone 40mg/w | CR Deceased 5 mo after neurological symptoms of invasive bronchopulmonary aspergillosis |
| 2018 (31) |
58 | m | ALL | Chronic GvHD | Onset: 1 y after HSCT; Clinic: bradypsychia, cognitive impairment, dyspraxia, ataxia, a pyramidal syndrome, autonomic dysfunction; MRI: infra- and supratentorial leukoencephalopathy, stable in comparison with 1 year before; EEG: diffuse moderate slowing of the dominant rhythm without paroxysmal activity; CSF: elevated protein, oligoclonal bands, elevated IgG; Serum analyses: Caspr2+; PET-CT: diffuse cortical and subcortical hypometabolism | No | MP 1g/d(5d), Cy 500 mg/m2/3w, rituximab 375 mg/m2/w(4 administrations) | CR Alive 13 mo after CNS symptoms |
| 2018 (32) |
59 | f | CLL | Acute and chronic GvHD | Onset: 33 mo after HSCT; Clinic: right upper extremity weakness, dysarthria; MRI: left frontal cortex infarction; MRA: severe stenosis of the left MCA; CSF: mild pleocytosis | Inflammatory cell infiltrations of perivascular areas | Surgical revascularization | Clinical and MR stability Alive 6 mo after CNS symptoms |
| 2019 (33) |
37 | f | MDS | Chronic GvHD | Onset: 2 y after HSCT; Clinic: generalized tonic-clonic type seizures, headache, blurred vision, symmetrical lower leg weakness, increased tendon reflex, hypoesthesia; MRI: multifocal ring enhancement lesions consistent with demyelinating features; multifocal nodular leptomeningeal enhancement and nodular intramedullary enhancing lesions along the spinal cord; CSF: elevated IgG | A loss of myelin fibers, perivascular T-cell infiltration (CD3+), macrophage infiltration associated with reactive gliosis | MP 1g/d(7d), plasmapheresis | CR Alive 1 y after CNS symptoms |
| 2019 (34) |
68 | m | MDS | No | Onset: 742 d after HSCT; Clinic: right-sided pyramidal distribution of weakness and a stimulus sensitive, myoclonus, increased tendon reflexes; MRI: multiple scattered T2 FLAIR hyperintense, cortical and subcortical lesions in both cerebral hemispheres; CSF: elevated protein EEG: diffuse slow wave activity | Perivascular T-cell infiltration (CD3+), microglial proliferation | MP 1g/d(5d), plasmapheresis | PR then secondary aggravation Deceased 195 d after CNS symptoms |
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CNS, central nervous system; CR, complete response; CSF, cerebrospinal fluid; EEG, electroencephalogram; GvHD, graft versus host disease; HLH, hemophagocytic lymphohistiocytosis; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; MRI, magnetic resonance imaging; NA, not available; PR, partial response.
Table 2.
Cerebrospinal fluid test results.
| Cerebrospinal fluid test results |
Number percentage (n = 40) |
|---|---|
| Protein elevates | 23 57.5% |
| Pleocytosis | 13 32.5% |
| Positive oligoclonal bands | 7 17.5 |
| IgG increase | 5 12.5% |
| Low glucose | 1 2.5% |
| Normal | 11 27.5% |
| Not available | 5 12.5% |
The histological changes of lymphocytic vasculitis are common in skin GvHD, and the specific manifestation is the infiltration of lymph plasma cells around blood vessels and appendages (36). We found that lymphocytic vasculitis has a prominent position in the existing CNS-GvHD histological data. Interestingly, the imaging manifestations of primary and secondary central nervous system vasculitis found in the past are mostly multiple cerebral infarctions, multiple white matter lesions and multiple cerebral hemorrhages, which are also consistent with many current imaging manifestations of CNS-GvHD (37, 38). At present, there is insufficient evidence for whether there is a connection between CNS-GvHD and central nervous system vasculitis, but we will introduce the results of animal model studies in the following section.
Based on the treatment of the above cases, steroid-based immunosuppressive therapy is currently the main first-line therapy for acute and chronic GvHD. These therapies are limited by drug toxicity, non-specific immunosuppression, and long-term treatment requirements. The development of new therapies to improve efficacy and reduce steroid complications is still urgent.
An in-depth understanding of the regulatory cells and molecular pathways involved in the immune response creates opportunities for us to use these cell subsets to prevent GvHD. At present, a variety of cell subsets with immunomodulatory effects, including Treg cells and mesenchymal stem cells (MSC), are being regarded as promising new treatment approaches for GvHD (39). A meta-analysis showed that compared with conventional treatment, MSC infusion showed a significant improvement in the complete remission of chronic GvHD and overall survival, but there was no substantial improvement in the incidence and rate of acute GvHD (40). And, it shows a positive effect for patients who already have acute GVHD (40).
Possible Mechanism of GvHD
Reviewing these cases, we found that the most common clinical manifestations of patients with CNS graft-versus-host disease were cognitive dysfunction, cranial neuropathy, and seizure. The histology of these cases showed that high frequency of infiltration around the blood vessels (7, 8, 10, 17–19, 22, 27, 29, 32–34), followed by activation or proliferation of microglia, demyelination, infiltration of CD3+/CD8+ T cells (18, 19, 22, 27, 34). In two of them, perivascular infiltrating T cells were confirmed to be donor sources. Animal studies have explored the possible pathological mechanisms.
MHC Molecular Expression in CNS
MHC class I molecules on target cell provide specific fragment of foreign for cytotoxic T cells, while MHC class II molecules located on antigen rendering cells are involved in activating helper T cells (41, 42). T lymphocytes and the cellular expression of major histocompatibility complex gene products is practically undetectable in the central nervous system of healthy animals (43). In a parental lymphocyte induced GvHD F1 hybrid rat model, immunohistochemical examination of the central nervous system showed that host class I and II MHC-encoded cell surface molecules were widely expressed in parenchyma and blood vessels. Moreover, scattered T lymphocytes were occasionally found in the central nervous system of these animals (43). Another GvHD F1 hybrid rat model induced by parental splenocytes confirmed that almost all microglia in the rat brain expressed MHC class II molecules 10 days after injection (44).
Microglia Activation
Parenchymal lymphocyte inflammation, microglia activation and mild cerebrovascular inflammation-like changes were observed in allogeneic transplanted animals (45). A recent study provided a new possibility to explain the role of microglia activation in CNS-GvHD (46). It pointed out that the expression of MHC class II molecules and CD80 and the productions of TNF were increased on the microglia of mice with GVHD (46). However, in glial cells induced GvHD mice with TNF genetic deletion in, cortical and meningeal infiltration of CD3+ T cells was significantly reduced (46). RNA-seq analysis of microglia cells in GvHD models found enhanced proinflammatory MAPK/NF-κB/TAK1 signal in microglia cells and activated various signal cascades in immune cells (46). MHC-unmatched bone marrow or splenocytes induced BALA/c mouse GvHD model revealed significant increased levels of IFN-γ, TNF-α, and IL-6 mRNA in the brain tissue (47). Therapeutic TAK1 inhibition can reduce microglial inflammation during CNS-GvHD (46).
T Lymphocyte Infiltration
To establish a mouse model of acute GvHD, allogeneic T cells removed bone marrow was used in allogeneic HCT, while syngeneic were used as the control group (48). It was observed that in the GvHD model, CD3+ and CD4+ T cells were always found in the cortex, hippocampus, midbrain (thalamus, hypothalamus, basal ganglia), cerebellum, medulla oblongata, choroid plexus (48). CD3+ T lymphocytes were also detected in meninges, ependyma, spinal cord neuropil, and sciatic nerve (48). However, absolute numbers showed that the parenchymal infiltration of all these parts is equal, which is equivalent to infection, ischemia and the model of spontaneous inflammatory disease is consistent (48). A study of GvHD rhesus macaques models proved that brain is the target of GvHD in the rhesus macaques transplant model, and CNS GvHD is mainly mediated by CD8+ T cells expressing integrins (49). Additionally, CD163+ cells (macrophages/monocytes) have also been observed centrally infiltrated in GvHD, suggesting that infiltration of antigen-presenting cells may also be a pathological feature of CNS GvHD (49). They observed two GvHD prevention programs (tacrolimus/methotrexate or rapamycin monotherapy), both of which significantly reduced GvHD-related CD8+ infiltration, but failed to completely inhibit the proliferation of CD8+ T lymphocytes (49). In the allogeneic HCT mice model, the pathological manifestations of chronic CNS-GvHD was described as diffuse infiltration of the parenchyma and pia mater of CD45+ cells, perivascular inflammation, and microglia activation (45).
Increased Inflammatory Cytokines
A study published in 2013 also pointed out that the recipients of allogeneic HCT had significantly lower brain weight than the syngeneic HCT (syngeneic hematopoietic stem cell transplantation) control group, and significant neuronal and glial cell apoptosis (48). This reminds us of the imaging features of brain atrophy in some patients in clinical case reports. After evaluation, it was confirmed that the spatial learning and memory abilities of these GvHD model mice were significantly impaired (48). In experiments with rhesus macaques as experimental subjects, the animals showed obvious significant lassitude, and acute behavioral depression (49). In animal models, IL-6 is related to mood and cognitive dysfunction, and IL-6 receptors are expressed on microglia and astrocytes in the brain (50, 51). For the performance of abnormal animal behavior, researchers had conducted a more in-depth study with host interleukin 6 production as the entry point. The forced swimming test was used to evaluate animal behavior. It was observed that the struggle behavior of GVHD animals in the test increased significantly, which is a dysfunctional response (47). Mice given anti-IL-6R antibodies significantly reduced this effect (47). Blocking the IL-6 pathway can significantly reduce the accumulation of donor T cells, the expression of inflammatory cytokines and the proliferation of host microglia, but it cannot reverse all metabolic abnormalities during GvHD (47). This study explains why drugs that inhibit the peripheral IL-6 signaling pathway, such as tocilizumab, may not completely eliminate the neuroinflammation that occurs in GVHD patients (47).
Other Findings
Another study used parental strain's lymph node cells injecting into genetically tolerated F1 hybrid newborn rats to induce GvHD showed that the total cytoplasmic RNA of the cerebellum and the biological activity of each unit of RNA in the experimental group was found decreased (52). The early gene product c-Fos is a reliable sign of neuron activation, and peripheral messengers generated during graft versus host reaction can directly or indirectly trigger the initial activation of neurons in a specific region of the brain (53). The researchers observed a strong c-Fos immune response in the piriform cortex and several other olfactory-related areas (IGr, TT, AO, Ent), occipital visual cortex, and prefrontal cortex (53). The relationship between these changes and the clinical manifestations of GvHD patients had not been confirmed.
Case Discussion
Compared with the previous cases, our case is a particular one. The patient's clinical characteristics met the diagnostic criteria for chronic CNS-GvHD, but the up to 3 years delayed diffuse white matter lesions shown in MRI were not common in previous cases. This particular imaging change is different from encephalitis and does not have the characteristic of bilateral symmetrical diffuse lesions, which is closer to the change of poisoning or metabolic encephalopathy. Significantly high intracranial pressure and swelling of brain have not been reported in previous cases. In addition, the patient also exhibited chronic GvHD of the skin that coincided with symptoms of the central nervous system, suggesting the CNS manifestations might be explained by the same reason. After starting empirical immunosuppressive therapy, MRI lesions and clinical symptoms of both CNS and skin were completely relieved, which also confirmed that GvHD might be etiologic factors of both skin and CNS damage. Unfortunately, the patient's brain tissue biopsy could not be performed, while according to previous case reports and basic research, we speculate that the cause of the patient may be related to the perivascular inflammation caused by GvHD and the destruction of the blood-brain barrier. If chronic GvHD causes a large number of inflammatory factors and inflammatory cells released into the blood, it may spread into the brain tissue through the destroyed blood-brain barrier, causing white matter degeneration and brain swelling, which in turn leads to the occurrence of high intracranial pressure. With the start of immunosuppressive therapy, the patient's symptoms improved rapidly, and the cerebrospinal fluid pressure dropped to normal level.
The patient's nervous system has been relieved in imaging and symptoms. The treatment produced a very rapid treatment response and was well tolerated by the patient. With subsequent treatment, there should be an optimistic prognosis in terms of CNS-GvHD. However, she still faces the risk of disease recurrence, complications, infection and drug side effects in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
ML performed case information collection, literature review, literature information statistics, and drafted the manuscript. YZhan contributed to case information collection and literature information statistics. YG, ZZ, and HDon contributed to literature review and manuscript preparation. YZhao and HDen performed manuscript review and final version approval. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.621392/full#supplementary-material
References
- 1.Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatol Clin. (2019) 37:569–82. 10.1016/j.det.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 2.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. (2012) 119 296–307. 10.1182/blood-2011-06-364265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson K, Horowitz MM, Gale RP, van Bekkum DW, Gluckman E, Good RA, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. (1990) 75:2459–64. 10.1182/blood.V75.12.2459.bloodjournal75122459 [DOI] [PubMed] [Google Scholar]
- 4.Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. (2019) 134:924–34. 10.1182/blood.2019001212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grauer O, Wolff D, Bertz H, Greinix H, Kuhl JS, Lawitschka A, et al. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain. (2010) 133:2852–65. 10.1093/brain/awq245 [DOI] [PubMed] [Google Scholar]
- 6.Openshaw H. Neurological manifestations of chronic graft versus host disease. In: Vogelsang GB, Pavletic SZ. editors. Chronic Graft versus Host Disease. New York, NY: Cambridge University Press; (2009). p. 243–51. 10.1017/CBO9780511576751.024 [DOI] [Google Scholar]
- 7.Marosi C, Budka H, Grimm G, Zeitlhofer J, Sluga E, Brunner C, et al. Fatal encephalitis in a patient with chronic graft-versus-host disease. Bone Marrow Transplant. (1990) 6:53–7. [PubMed] [Google Scholar]
- 8.Iwasaki Y, Sako K, Ohara Y, Miyazawa M, Minegishi M, Tsuchiya S, et al. Subacute panencephalitis associated with chronic graft-versus-host disease. Acta Neuropathol. (1993) 85:566–72. 10.1007/BF00230498 [DOI] [PubMed] [Google Scholar]
- 9.Provenzale JM, Graham ML. Reversible leukoencephalopathy associated with graft-versus-host disease: MR findings. AJNR Am J Neuroradiol. (1996) 17:1290–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Padovan CS, Bise K, Hahn J, Sostak P, Holler E, Kolb HJ, et al. Angiitis of the central nervous system after allogeneic bone marrow transplantation? Stroke. (1999) 30:1651–6. 10.1161/01.STR.30.8.1651 [DOI] [PubMed] [Google Scholar]
- 11.Takatsuka H, Okamoto T, Yamada S, Fujimori Y, Tamura S, Wada H, et al. New imaging findings in a patient with central nervous system dysfunction after bone marrow transplantation. Acta Haematol. (2000) 103:203–5. 10.1159/000041050 [DOI] [PubMed] [Google Scholar]
- 12.Solaro C, Murialdo A, Giunti D, Mancardi G, Uccelli A. Central and peripheral nervous system complications following allogeneic bone marrow transplantation. Eur J Neurol. (2001) 8:77–80. 10.1046/j.1468-1331.2001.00160.x [DOI] [PubMed] [Google Scholar]
- 13.Ma M, Barnes G, Pulliam J, Jezek D, Baumann RJ, Berger JR. CNS angiitis in graft vs host disease. Neurology. (2002) 59:1994–7. 10.1212/01.WNL.0000038948.09158.A7 [DOI] [PubMed] [Google Scholar]
- 14.Tomonari A, Tojo D, Adachi T, Iseki J, Ooi N, Shirafuji K, et al. Acute disseminated encephalomyelitis (ADEM) after allogeneic bone marrow transplantation for acute myeloid leukemia. Ann Hematol. (2003) 82:37–40. 10.1007/s00277-002-0573-1 [DOI] [PubMed] [Google Scholar]
- 15.Campbell JN, Morris PP. Cerebral vasculitis in graft-versus-host disease: a case report. AJNR Am J Neuroradiol. (2005) 26:654–6. Available online at: http://www.ajnr.org/content/26/3/654 [PMC free article] [PubMed] [Google Scholar]
- 16.Shortt J, Hutton E, Faragher M, Spencer A. Central nervous system graft-versus-host disease post allogeneic stem cell transplant. Br J Haematol. (2006) 132:245–7. 10.1111/j.1365-2141.2005.05864.x [DOI] [PubMed] [Google Scholar]
- 17.Kew AK, Macaulay R, Burrell S, Rubin S, Dow G, Couban S. Central nervous system graft-versus-host disease presenting with granulomatous encephalitis. Bone Marrow Transplant. (2007) 40:183–4. 10.1038/sj.bmt.1705709 [DOI] [PubMed] [Google Scholar]
- 18.Kamble RT, Chang CC, Sanchez S, Carrum G. Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant. (2007) 39:49–52. 10.1038/sj.bmt.1705540 [DOI] [PubMed] [Google Scholar]
- 19.Saad AG, Alyea EP, 3rd, Wen PY, Degirolami U, Kesari S. Graft-versus-host disease of the CNS after allogeneic bone marrow transplantation. J Clin Oncol. (2009) 27:e147–9. 10.1200/JCO.2009.21.7919 [DOI] [PubMed] [Google Scholar]
- 20.Matsuo Y, Kamezaki K, Takeishi S, Takenaka K, Eto T, Nonami A, et al. Encephalomyelitis mimicking multiple sclerosis associated with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Intern Med. (2009) 48:1453–6. 10.2169/internalmedicine.48.2003 [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Uchida N, Ishiwata K, Araoka H, Takagi S, Tsuji M, et al. Possible graft-versus-host disease involving the central nervous system soon after cord blood transplantation. Am J Hematol. (2009) 84:764–6. 10.1002/ajh.21518 [DOI] [PubMed] [Google Scholar]
- 22.Sostak P, Padovan CS, Eigenbrod S, Roeber S, Segerer S, Schankin C, et al. Cerebral angiitis in four patients with chronic GVHD. Bone Marrow Transplant. (2010) 45:1181–8. 10.1038/bmt.2009.323 [DOI] [PubMed] [Google Scholar]
- 23.Voss M, Bischof F. Recurrent myelitis after allogeneic stem cell transplantation. Report of two cases. BMC Neurol. (2010) 10:76. 10.1186/1471-2377-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redouan E, Emmanuel P, Michelle P, Bernard C, Josiane C, Cedric D. Evaluation of antioxidant capacity of ulvan-like polymer obtained by regioselective oxidation of gellan exopolysaccharide. Food Chem. (2011) 127:976–83. 10.1016/j.foodchem.2011.01.067 [DOI] [PubMed] [Google Scholar]
- 25.Delios AM, Rosenblum M, Jakubowski AA, DeAngelis LM. Central and peripheral nervous system immune mediated demyelinating disease after allogeneic hemopoietic stem cell transplantation for hematologic disease. J Neurooncol. (2012) 110:251–6. 10.1007/s11060-012-0962-9 [DOI] [PubMed] [Google Scholar]
- 26.Harvey CM, Gottipati R, Schwarz S, Auer D, O'Donoghue M, Russell NH, et al. Acute disseminated encephalomyelitis following allo-SCT: central nervous system manifestation of GVHD. Bone Marrow Transplant. (2014) 49:854–6. 10.1038/bmt.2014.29 [DOI] [PubMed] [Google Scholar]
- 27.Ruggiu M, Cuccuini W, Mokhtari K, Meignin V, Peffault de Latour R, Robin M, et al. Case report: Central nervous system involvement of human graft versus host disease: report of 7 cases and a review of literature. Medicine (Baltimore). (2017) 96:e8303. 10.1097/MD.0000000000008303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauthier J, Vermersch P, Chauvet P, Varlet P, Coiteux V, Magro L, et al. Successful treatment with fingolimod of graft-versus-host disease of the central nervous system. Blood Adv. (2018) 2:10–3. 10.1182/bloodadvances.2017011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terada M, Nakamagoe K, Obara N, Ogawa S, Sakamoto N, Sato T, et al. Chronic graft-versus-host disease presenting with multiple punctate intracranial lesions on contrast-enhanced magnetic resonance imaging. Intern Med. (2017) 56:363–8. 10.2169/internalmedicine.56.7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polchlopek Blasiak K, Simonetta F, Vargas MI, Chalandon Y. Central nervous system graft-versus-host disease (CNS-GvHD) after allogeneic haematopoietic stem cell transplantation. BMJ Case Rep. (2018) 2018:1–4. 10.1136/bcr-2017-221840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirotte M, Forte F, Lutteri L, Willems E, Duran U, Belle L, et al. Neuronal surface antibody-mediated encephalopathy as manifestation of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. J Neuroimmunol. (2018) 323:115–8. 10.1016/j.jneuroim.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 32.Nakayama Y, Kamio Y, Kato N, Murayama Y. Extracranial-intracranial bypass for cerebral vasculitis after graft-versus-host disease: case report and review of the literature. World Neurosurg. (2019) 123:193–6. 10.1016/j.wneu.2018.11.256 [DOI] [PubMed] [Google Scholar]
- 33.Min GJ, Park S, Park SS, Yoon JH, Lee SE, Cho BS, et al. A case of central nervous system graft-versus-host disease following allogeneic stem cell transplantation. Int J Hematol. (2019) 110:635–9. 10.1007/s12185-019-02702-1 [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Billick M, Monsour D, Liu J. Central nervous system graft-versus-host disease in a 68-year-old man presenting with myoclonus. CMAJ. (2019) 191:E1078–81. 10.1503/cmaj.190216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathore GS, Leung KS, Muscal E. Autoimmune encephalitis following bone marrow transplantation. Pediatr Neurol. (2015) 53:253–6. 10.1016/j.pediatrneurol.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 36.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. (2009) 373:1550–61. 10.1016/S0140-6736(09)60237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. (2009) 66:704–9. 10.1001/archneurol.2009.76 [DOI] [PubMed] [Google Scholar]
- 38.Hajj-Ali RA, Calabrese LH. Central nervous system vasculitis. Curr Opin Rheumatol. (2009) 21:10–8. 10.1097/BOR.0b013e32831cf5e6 [DOI] [PubMed] [Google Scholar]
- 39.Blazar BR, MacDonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. (2018) 131:2651–60. 10.1182/blood-2017-11-785865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Chen S, Yang P, Cao H, Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. (2019) 10:182. 10.1186/s13287-019-1287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. (1979) 27:51–177. 10.1016/S0065-2776(08)60262-X [DOI] [PubMed] [Google Scholar]
- 42.Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol. (1984) 2:395–428. 10.1146/annurev.iy.02.040184.002143 [DOI] [PubMed] [Google Scholar]
- 43.Hickey WF, Kimura H. Graft-vs.-host disease elicits expression of class I and class II histocompatibility antigens and the presence of scattered T lymphocytes in rat central nervous system. Proc Natl Acad Sci U S A. (1987) 84:2082–6. 10.1073/pnas.84.7.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedgwick JD, Ford AL, Foulcher E, Airriess R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol. (1998) 160:5320–30. [PubMed] [Google Scholar]
- 45.Padovan CS, Gerbitz A, Sostak P, Holler E, Ferrara JL, Bise K, et al. Cerebral involvement in graft-versus-host disease after murine bone marrow transplantation. Neurology. (2001) 56:1106–8. 10.1212/WNL.56.8.1106 [DOI] [PubMed] [Google Scholar]
- 46.Mathew NR, Vinnakota JM, Apostolova P, Erny D, Hamarsheh S, Andrieux G, et al. Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia. J Clin Invest. (2020) 130:1315–29. 10.1172/JCI130272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belle L, Zhou V, Stuhr KL, Beatka M, Siebers EM, Knight JM, et al. Host interleukin 6 production regulates inflammation but not tryptophan metabolism in the brain during murine GVHD. JCI Insight. (2017) 2:e93726. 10.1172/jci.insight.93726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartrampf S, Dudakov JA, Johnson LK, Smith OM, Tsai J, Singer NV, et al. The central nervous system is a target of acute graft versus host disease in mice. Blood. (2013) 121:1906–10. 10.1182/blood-2012-09-456590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaliyaperumal S, Watkins B, Sharma P, Furlan S, Ramakrishnan S, Giver C, et al. CD8-predominant T-cell CNS infiltration accompanies GVHD in primates and is improved with immunoprophylaxis. Blood. (2014) 123:1967–9. 10.1182/blood-2014-01-547612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bay-Richter C, Janelidze S, Sauro A, Bucala R, Lipton J, Deierborg T, et al. Behavioural and neurobiological consequences of macrophage migration inhibitory factor gene deletion in mice. J Neuroinflammation. (2015) 12:163. 10.1186/s12974-015-0387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. (2012) 8:1254–66. 10.7150/ijbs.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffin WS, Head JR, Pardue S, Morrison MR. Changes in RNA synthesis and messenger RNA content in the cerebellum of rats with graft versus host disease. J Neurochem. (1980) 35:880–8. 10.1111/j.1471-4159.1980.tb07086.x [DOI] [PubMed] [Google Scholar]
- 53.Furukawa H, Yamashita A, del Rey A, Besedovsky H. c-Fos expression in the rat cerebral cortex during systemic GvH reaction. Neuroimmunomodulation. (2004) 11:425–33. 10.1159/000080154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.