Abstract
Rotator cuff (RC) muscle fatty infiltration (FI) is an important factor that determines the clinical outcome of patients with RC repair. There is no effective treatment for RC muscle FI at this time. The goal of this study is to define the role Trichostatin A (TSA), a histone deacetylase (HDAC) inhibitor in regulating muscle fibro/adipogenic progenitors (FAPs) adipogenesis and treating muscle fatty degeneration after massive RC tears in a mouse model. We hypothesize that TSA reduces muscle FI after massive RC tears. HDAC activity was measured in FAPs in RC muscle after tendon/nerve transection or sham surgery. FAPs were treated with TSA for 2 weeks and FAP adipogenesis was evaluated with perilipin and Oil Red O staining, as well as reverse transcript-polymerase chain reaction for adipogenesis-related genes. About 0.5 mg/kg TSA or dimethyl sulfoxide was administered to C57B/L6 mice with massive rotator cuff tears through daily intraperitoneal injection for 6 weeks. Supraspinatus muscles were harvested for biochemical and histology analysis. We found that FAPs showed significantly higher HDAC activity after RC tendon/nerve transection. TSA treatment significantly reduced HDAC activity and inhibited adipogenesis of FAPs. TSA also abolished the role of bone morphogenetic protein-7 in inducing FAP adipogenesis and promoted FAP brown/beige adipose tissue (BAT) differentiation. TSA injection significantly increased histone H3 acetylation and reduced FI of rotator cuff muscles after massive tendon tears. Results from this study showed that TSA can regulate FAP adipogenesis and promote FAP BAT differentiation epigenetically. HDAC inhibition may be a new treatment strategy to reduce muscle FI after RC tears and repair.
Keywords: adipogenesis, fibro-adipogenic progenitor, HDAC inhibitor, muscle fatty infiltration, rotator cuff tears
1 |. INTRODUCTION
Muscle fatty infiltration (FI), characterized by the heterogeneous adipose tissue presence within a muscle, is one of the major pathologic changes of rotator cuff (RC) muscles after acute or chronic tendon tears. The degree of muscle atrophy and FI are directly correlated with high re-tear rates and poor clinical outcomes with large or massive RC tears.1 Smaller tear also have been found to have atrophy and FI, and thus the clinical problem is likely more common than previously believed.2 The mechanism of RC muscle FI is not fully understood and, therefore, there is no effective treatment for this disorder. Recent work has revealed that muscle residential interstitial progenitor cells, fibro/adipogenic progenitors (FAPs), are the major cellular source of adipocytes in muscle FI.2,3 Our work further demonstrated that FAPs are the cellular origin of adipocytes in RC muscle FI in a mouse model.4
FAPs are known to differentiate into mature white adipocytes (WAT) in vitro and in vivo.3,4 A recent study showed that FAPs can also differentiate into brown fat-like adipocytes as evidenced by expression of brown fat adipose tissue (BAT) hallmark of uncoupling protein-1 (UCP-1).5 Beige fat is an interchangeable status between WAT and BAT. Inducing beige fat activity, also known as “browning of white fat” is considered as a promising strategy in curing obesity and FI.6,7 Transforming growth factor beta (TGFβ) and bone morphogenetic protein-7 (BMP-7) have been found to play important roles in stem cell adipogenesis.8 In our previous studies, we observed significantly increased TGFβ and BMP-7 during the development of muscle fatty infiltrated after RC tears.9,10 Inhibition TGFβ and BMP pathways decrease RC muscle FI after massive tendon tears.11 Thus, the TGFβ pathways appear to have an important role in FAP adipogenic differentiation, although the mechanisms are not clearly defined.
Epigenetic regulation is an important mechanism of controlling gene expression in eukaryotic cells. Common epigenetic modifications of genes include histone acetylation and deacetylation, histone methylation, DNA methylation, and noncoding messenger RNAs. Epigenetic control of gene expression has recently been identified as a mechanism involved in the regulation of muscle mass, function, and phenotype in health and disease.12,13 Histone deacetylases (HDACs) are a class of enzymes that remove acetyl groups from histones, causing tighter DNA wrapping on histone. Working with histone acetylases, which add acetyl groups to histones, HDAC controls many gene expression by regulating DNA wrapping around histones through histone acetylation and deacetylation, and are thought to be a central mechanism in regulating oncogenic transformations.14–16 HDAC activity is important for muscle stem cell differentiation.17 HDAC inhibitors have been found to be effective in treating muscle atrophy and degeneration in animal studies,18,19 at least partially through FAPs.20
In this study, we examined HDAC activity of FAPs from RC muscle in a mouse massive RC tear model. We further tested the role of Trichostatin A (TSA), an HDAC inhibitor in regulating FAPs adipogenesis. Lastly, we tested the role of TSA in treating RC muscle FI after massive RC tears in a mouse model. We hypothesize that HDAC plays a critical role in regulating FAP adipogenesis and TSA inhibits FAP adipogenesis thus can be used to treat RC muscle atrophy and FI.
2 |. METHODS
2.1 |. Animal procedures
Twenty adult female wild-type C57B/L6 mice (Jackson laboratory Corp.; Stock # 000664) and eight PDGFRα-GFP FAP reporter mice (Jackson laboratory Corp.; Stock # 007669) underwent unilateral massive RC tear surgery at 3 months old with complete supraspinatus (SS) and infraspinatus (IS) tendon transection plus suprascapular denervation transection (TT + DN) as described previously.21 A sham surgery, in which the tendons and nerve were exposed but not transected, was performed on the contralateral side. This sham surgery procedure has been proven with no effect on RC muscle atrophy or FI.22 Four C57B/L6 mice were killed at 2 weeks after surgery and SS muscles from both RC tear and sham sides were harvested for FAPs. Two weeks after injury was chosen because our previous studies have determined that it is a key time point determining FAP differentiation.4,9 The rest of the animals were randomly divided into TSA and the vehicle control treatment group (8 of C57B/L6 and 4 of PDGFRα reporter mice in each group). In the TSA treatment group, 0.5 mg/kg TSA dissolved in 0.1 ml 1% dimethyl sulfoxide (DMSO) was administered to mice from the same day of surgery through intraperitoneal injection (5 days per week). The same volume of 1% DMSO was administered to the mice in the vehicle control group. No side effects of DMSO at this dose and treatment regimen were observed in our previous studies.8,9 Mice were killed at 6 weeks after surgery when FI became obvious. In each group, four of C57B/L6 and four of PDGFRα reporter mice were used for histologic analysis, and four of C57B/L6 mice were used for histone acetylation assay. The SFVAMC Institutional Animal Care and Use Committee approved all procedures and handling of the animals. A schematic flowchart of the experiment design is included is Figure 1.
FIGURE 1.
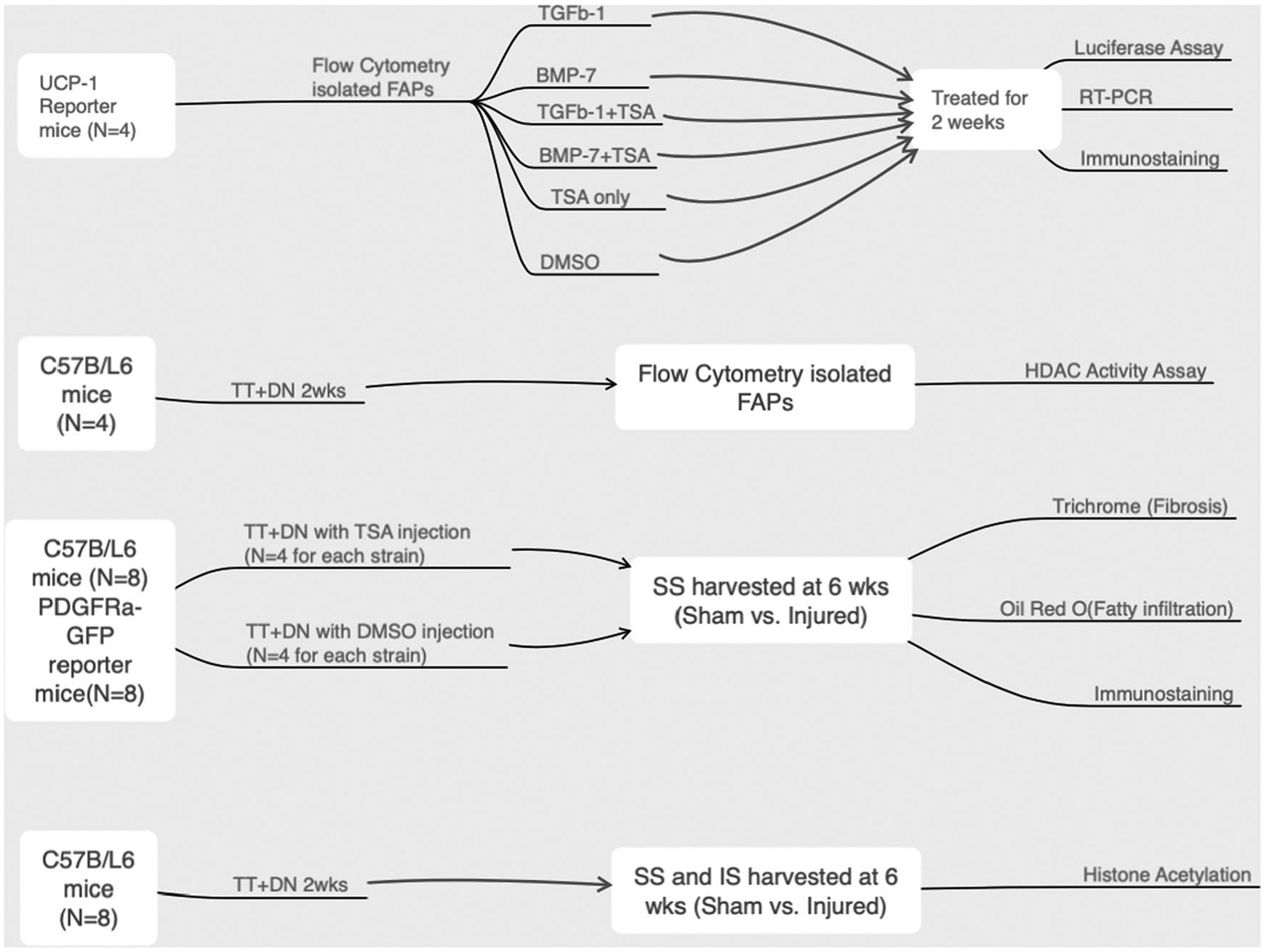
The schematic flow of the experiment design. BMP-7, bone morphogenetic protein-7; DMSO, dimethyl sulfoxide; DN, denervation transection; FAPs, fibro/adipogenic progenitors; HDAC, histone deacetylase; IS, infraspinatus; RT-PCR, real-time polymerase chain reaction; SS, supraspinatus; TGFβ, transforming growth factor beta; TSA, Trichostatin A; TT, tendon transection; UCP-1, uncoupling protein-1
2.2 |. Muscle harvesting and histology
Bilateral SS muscles were harvested and the remaining tendon and scar tissue were removed at the muscle/tendon junction after mice were killed. The muscles were then snap frozen in liquid nitrogen-cooled isopentane and sectioned at a thickness of 10 μm with a cryostat. Oil-red-O (Sigma-Aldrich) staining was conducted to evaluate the FI as described previously.16 For immunostaining, after fixing in 4% paraformaldehyde and rinsing in phosphate-buffered solution (PBS), sections were incubated in blocking solution (0.3% Triton X-100, 5% bovine serum albumin in PBS) for 1 h, and then incubated with the primary antibodies (anti-Laminin, L9393, Sigma-Aldrich, 1:500 diluted; anti-UCP-1, sc-6529, Santa Cruz Biotechnology, 1:50 diluted) at 4°C for more than 15 h. After PBS rinses, the sections were treated with secondary antibodies (1:250 diluted) for 2 h. For PDGFRα-GFP reporter mice, secondary antibodies were Alexa Fluor 594- conjugated antirabbit IgG (ab150076) and Alexa Fluor 647-conjugated anti-goat IgG (ab150131). For C57BL/6J mice, secondary antibodies were Alexa Fluor 488-conjugated antirabbit IgG (ab150077) and Alexa Fluor 594-conjugated anti-goat IgG (ab150140). After a PBS rinse, we mounted slides using VectaShield with 4′,6-diamidino-2-phenylindole (DAPI) and a coverslip. Histologic pictures were taken with the Zeiss digital camera on an Axio Imager 2 microscope (Zeiss). The fat area fraction was evaluated by dividing the Oil-Red-O stained area by the entire sample.16
2.3 |. Muscle digestion and FAPs isolation
FAPs were isolated from SS muscles in C57B/L6 wild-type mice at 2 weeks after TT + DN surgery (N = 4). To evaluate FAP BAT differentiation, additional FAPs were isolated from SS muscles in healthy UCP-1 reporter mice (Ucp1-luc2-tdTomato)1Kajim/J, a kind gift from Dr. Shingo Kajimura at UCSF; N = 4). In brief, SS muscles from surgical and sham sides were minced into 1 mm small pieces with sterile scissors in cell culture hood. The mixture was incubated with 0.2% Collagenase II for 90 min at 37°C sterile water bath. Forty-milliliter washing buffer (F/10, 10% horse serum, 1× HEPES) was added into the mixture and centrifuged at 1500 rpm for 5 min at room temperature. The supernatant was then transferred to a new 50 ml centrifuge tube. The remnant was rinsed with washing buffer and span down at 1500 rpm for 5 min. The supernatant was collected and combined with that from the last round. D2 solution (0.06% Collagenase II, 0.15% dispase with washing buffer) was added and incubated for 30 min at 37°C. The solutions were then passed through a 70 μm cell strainer (VWR International) followed with a 40-μm cell strainer (VWR International). The filtered cells were washed with 40 ml FACS buffer (2.5% FBS, 20 mM EDTA, and 1×PBS) and spun down at 1500 rpm for 5 min. The supernatant was discarded, and the cell pellets were re-suspended with 500 μl of FACS buffer. The cells were incubated with anti-CD31-fluorescein isothiocyanate (FITC; BD bioscience, Clone 390), anti-CD45-FITC (BD bioscience, Clone WM59), anti-integrin α7-APC (R&D Systems, Clone #334908), and PE-Cy7-Sca1 (BD bioscience, Clone. E13–161.7) for 30 min before being sorted with FACSAria™ II (BD bioscience). FAPs were collected as the CD31-/CD45-/ITGA7-/Sca1+ cell population.4
2.4 |. Cell culture
Twenty-four well cell plates were coated with 1% Matrigel in Dulbecco’s modified Eagle’s medium for 1 h at room temperature before cell seeding. After sorting, FAPs were seeded into Matrigel precoated 24 well plates at a density of 5000 cells per well. Cells were cultured for 1 week with standard cell culture medium (SM; Ham′s F-10, 10% FBS, 10 ng/ml basic fibroblast growth factor [bFGF], and 1% antibiotic–antimycotic solution, Thermo Fisher Scientific). For adipogenic differentiation, the cells were cultured with adipogenic differentiation medium (100 μM IBMX, 2.5 μM DEX, 100 mM indomethacin, and 10 μg/ml insulin) for 2 weeks.
2.5 |. In vitro drug treatment
To test the role of HDAC in FAP differentiation, HDAC inhibitor TSA dissolved in 0.01% DMSO was added to standard culture medium for FAPs. To test the role of TGFβ and BMP signaling in FAP adipogenesis, 5 ng/ml of TGFβ−1 (R&D Systems) or 100 ng/ml of BMP-7 (R&D Systems) were added into SM (Ham′s F-10, 10% FBS, 10 ng/ml bFGF, and 1% antibiotic–antimycotic) cultured FAPs. The medium was changed every other day with fresh TSA, TGFβ−1, or BMP-7. Cells were fixed at 14 days after treatment followed by RNA extraction or immunostaining.
2.6 |. Real-time qPCR
Total RNA was isolated using TRIzol reagent (Invitrogen Inc.) according to the manufacturer’s instructions. One microgram of total RNA was used to generate complementary DNA (cDNA) using Maxima First Strand cDNA Synthesis Kit (Roche Applied Bioscience) for each sample real-time quantitative polymerase chain reaction (RT-qPCR).23 Six technical repeats were included in each group. The amount of RNA was used in this experiment was 1000 ng. The sequence of primers used in this experiment is listed in Table 1.
TABLE 1.
Primers used in the experiment
| Gene | Forward | Reverse |
|---|---|---|
| s26 | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
| PPARg | GCATGGTGCCTTCGCTGA | TGGCATCTCTGTGTCAACCATG |
| Adiponectin | CCCAAGGGAACTTGTGCAGGTTGGATG | GTTGGTATCATGGTAGAGAAGAAAGCC |
| ACTA2 | CCGACCGAATGCAGAAGGA | ACAGAGTATTTGCGCTCCGAA |
| Col-I | CAGCCGCTTCACCTACAGC | TTTTGTATTCAATCACTGTCTTGCC |
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGAAGGGC |
| PRDM16 | 5’-CCCACCAGACTTCGAGCTAC | ATCCGTCAGCATCTCCCATC |
| Hdac1 | TGGGGCTGGCAAAGGCAAGT | GACCACTGCACTAGGCTGGAACA |
| Hdac2 | CGTACAGTCAAGGAGGCGGCAA | TGAGGCTTCATGGGATGACCCTGG |
| Hdac3 | ACGTGCATCGTGCTCCAGTGT | AGTGTAGCCACCACCTCCCAGT |
| Hdac4 | AGCTCTGGCAACGTCAGCACT | AAGTGGGGCGACTGAGCCTTCT |
| Hdac5 | ACGTAAATGTGGCGTGGACAGGA | TTCAACAGCATCAAACCCAGCGGA |
| Hdac6 | TGGGGCTTCAAGGGCTGGATCT | TGCTCTCTGATGGCATGGAGCC |
| Hdac7 | GCTCAGCATGTGCATGTGGAACAC | TGAGAGCCTGGTGTGTCTGGCT |
| Hdac8 | ATGACACCAGTGGTCGGCAA | ACGAGCCGTGTTGGCAAGGTT |
| Hdac9 | TGCACCTTTGCCTCAGAGCACG | TGGCTGCCTGGTTGCTTCAGT |
| Hdac10 | TAGCAGCCAAACATGCCAAGCAGA | ATGCTCATAGCGGTGCCAAGAGAAA |
| Hdac11 | GCTGGGAAATGGGGCAAGGTGA | AGCTCGTTGAGATAGCGCCTCGT |
| p300 | CGCTTTGTCTACACCTGCAA | TGCTGGTTGTTGCTCTCATC |
| sirt1 | CGGCTACCGAGGTCCATATAC | CAGCTCAGGTGGAGGAATTGT |
| sirt2 | GAGCCGGACCGATTCAGAC | AGACGCTCCTTTTGGGAACC |
| sirt3 | GGATTCGGATGGCGCTTGA | CACCTGTAACACTCCCGGAC |
| sirt4 | GAGCATTCTTACTAGGGATTCCA | AACGGCTAAACAGTCGGGTT |
| sirt5 | GCCACCGACAGATTCAGGTT | CCACAGGGCGGTTAAGAAGT |
| sirt6 | CCAAATCGTCAGGTCAGGGA | CAGAGTGGGGTACAGGGATG |
| sirt7 | CTAAGCGAAGCGGAGCCTAC | GTGGAGCCCATCACAGTTCT |
2.7 |. Immunostaining
To evaluate FAPs adipogenesis, FAPs were stained for goat anti-mouse perilipin (Cat#ab61682, 1:1000, Abcam) a mature adipocyte marker, preconjugated mouse anti-mouse Cy 3 alpha-smooth muscle actin (αSMA; clone 1A4, 1:500, Sigma-Aldrich) as a fibrosis marker, rabbit anti-mouse UCP-1 (Cat#ab10983, brown fat marker, 1:100, Abcam), and DAPI (1:10,000, Sigma-Aldrich) at all time points for overnight. The FAPs were then stained with Donkey anti-goat FITC (Cat#ab150129, 1:2000, Abcam) and Donkey anti Rabbit Cy5 (Cat#ab150075, 1:2000, Abcam). Three replicates per condition were included. The adipogenesis index was determined by dividing the number of cells expressing perilipin by the total number of nuclei in each image.
2.8 |. HDAC activity assay
HDAC activity in FAPs was measured by HDAC activity assay kit (Epigentek Inc., # P-4002–96). FAPs were collected with FACS and then lyzed with nuclear extract lysis buffer. The same amount of nuclear proteins from FAPs were was added into 96 well plates for HADC enzyme activity measurement by a Bio-rad plate reader (Bio-Rad Inc.) with 560 nm wavelength according to manufacture′s instruction.
2.9 |. Histone acetylation assay
Histone acetylation assay was performed on the mice treated with TSA and vehicle group with EpiQuik Global Histone H3 Acetylation Assay Kit (EpiGentek). SS and IS muscles from the surgery side were homogenized with a Dounce homogenizer. The homogenized mixture was then transferred to a 15-ml conical tube and 100% TCA solution was used to precipitate Histone. The samples were read absorbance on a plate reader at 450 nm. The fold change of histone acetylation in SS muscle the TSA-treated group to the vehicle-treated group was calculated.
2.10 |. Luciferase assay
UCP-1 reporter gene expression level in FAPs collected from UCP-1 reporter mice was measured with UCP-1 promoter-driven Luc2 luciferase activity using a plate reader (Bio-Rad Inc.) under 560 nm wavelength after adding the substrate from Firefly Luciferase Kit (Promega, #G7940). The reading was normalized with blank and negative control (WT FAPs with substrate).
2.11 |. Statistical analysis
The number of animals required for this aim was determined based upon power analysis using our best approximation of true effect and anticipated sample variability using the assumption: α = .05, β = .80, α1 = 1.5α2 with the outcome of a 20% difference. With these calculations, we concluded that a minimum number of four animals were required in each group. All data were presented in the form of mean ± standard deviation. Statistical analyses were performed using two-tailed Student′s t-test with significance at p < .05.
3 |. RESULTS
3.1 |. RC tears cause an increase of HDAC activity in FAPs
Animals tolerated the surgery and treatment well. No adverse effect was observed from either RC surgery itself or TSA treatment. After 2 weeks of injury, HDAC activity in FAPs from SS muscle in the surgery side (67.47 ± 3.18 pmol) was significantly higher compared with the sham side (21.43 ± 1.58 pmol; p < .01, N = 4, Figure 2A). We then examined gene expression levels of HDAC1–11, Sir1–4, and p300 histone acetyltransferase (opposite role of HDAC) in FAPs. Among those genes, we found HDAC1, 3, 4, 5, p300, and sir1 expression levels were significantly increased in FAPs from the surgical side compared with those from the sham side (p < .05, Figure 2B).
FIGURE 2.
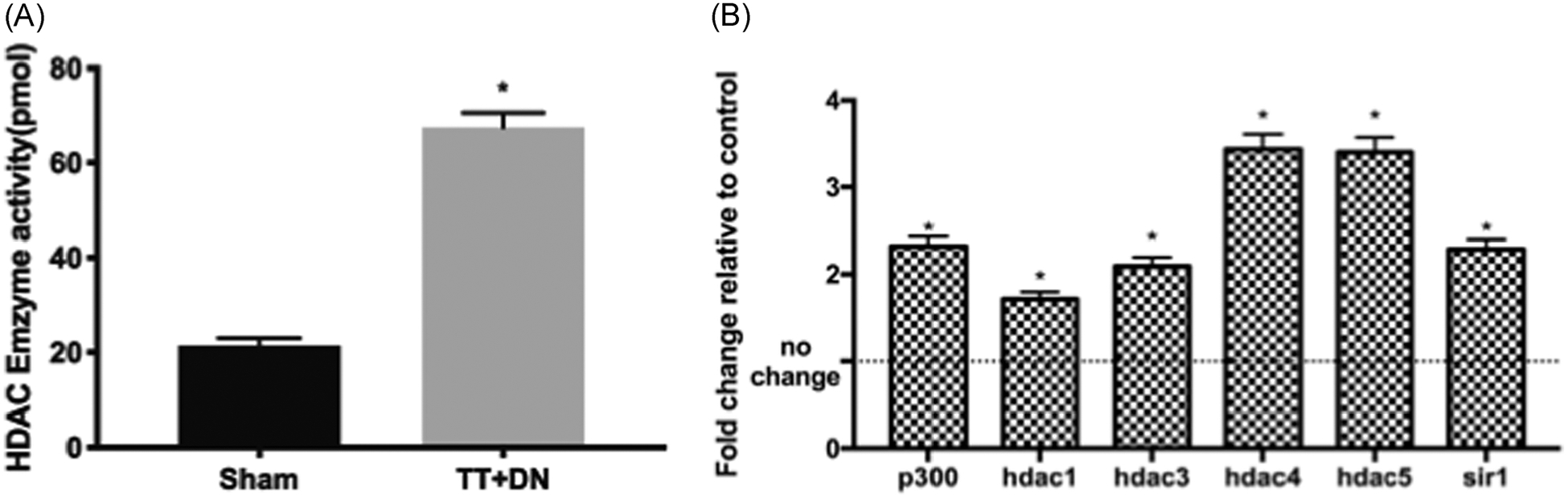
(A) HDAC enzyme activity of FAPs increased after 2 weeks of TT + DN injury. (B) HDAC1, 3, 4, 5, p300, and sir1 messenger RNA expression significantly increased in FAPs from the surgical side compared with the sham side (*p < .05). DN, denervation transection; FAPs, fibro/adipogenic progenitors; HDAC, histone deacetylase; TT, tendon transection
3.2 |. TSA decreased the adipogenesis of FAPs
Two weeks of TSA treatment significantly decreased the perilipin A expression in FAPs (Figure 3A–C). In DMSO-treated control group, 95.38% of FAPs express perilipin A, a hallmark for adipocytes. However, only 70.3% of FAPs expressed perilipin A in the TSA-treated group (p < .05).
FIGURE 3.

(A) Typical immunofluorescence staining of FAPs treated the DMSO and TSA for 2 weeks (red-UCP-1, green-perilipin A, and blue-DAPI). Typical FAPs expressed UCP-1 were indicated with arrows. (B) Quantification of perilipin A(+) FAPs in TSA and DMSO-treated groups. (C) quantification of αSMA(+) FAPs in TSA and DMSO-treated groups. (D) RT-PCR showed that TSA treatment could increase the UCP1, PRDM16, Collagen I, and ACTA2 gene expression but decreased the adiponectin and PPARγ gene expression in FAPs. (E) Luciferase assay of UCP-1 driven luciferase reporter gene activity of UCP-1 reporter FAPs was significantly higher in the TSA-treated group compared with the DMSO-treated group (*p < .05). DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; FAPs, fibro/adipogenic progenitors; RT-PCR, real-time polymerase chain reaction; TSA, Trichostatin A; UCP-1, uncoupling protein-1; αSMA, alpha-smooth muscle actin
Moreover, TSA significantly decreased adiponectin and PPARγ gene expression in FAPs compared with DMSO (Figure 3D).
3.3 |. TSA-induced FAP BAT differentiation
Two weeks of TSA treatment significantly increased the activity of UCP-1 promoter-driven luciferase reporter gene 5.3 ± 0.44 fold in UCP-1 reporter FAPs compared with that in the control group (Figure 3E). The TSA treatment increased the gene expression level of two BAT markers of UCP1 and PRDM16 gene expression by 16.21 ± 5.32 and 14.29 ± 3.83 fold, respectively in FAPs compared those in the control group (Figure 3D).
3.4 |. TSA abolished the role of BMP-7 in inducing FAP adipogenesis
TGF β1 significantly inhibited FAP adipogenesis and promoted FAP fibrogenesis as evidenced by decreased perilipin A expression and increased αSMA in FAPs treated with TGF β1. TSA did not change the percentage of perilipin A(+) or αSMA(+) FAPs under TGF β1 treatment though RT-PCR results showed that TSA decreased gene expression of PPARγ and Collagen I in FAPs (Figure 4). BMP-7 significantly increased FAP adipogenesis and inhibited FAP fibrogenesis as evidenced by increased perilipin A expression and decreased αSMA in FAP treated with BMP-7. However, the role of BMP-7 was abolished in the presence of TSA. FAPs treated with the combination of TSA and BMP-7 had significantly reduced perilipin A and increased αSMA expression compared with those treated BMP-7 alone. RT-PCR showed that TSA significantly decreased adiponectin and αSMA gene expression but increased Collagen I and ACTA2 (αSMA) gene expression in BMP-7 treated FAPs. TSA also significantly increased gene expression of UCP1in FAPs treated with BMP-7 (Figure 5).
FIGURE 4.

(A) Typical immunostaining image of FAPs under treatment TGFβ−1 and/or TSA. (B) Quantification of the percentage of perilipin A (+) FAPs in all treatment groups. Typical FAPs expressed UCP-1 were indicated with arrows in the TSA treatment group compared with FAPs without expression of UCP-1 in the DMSO group. (C) Quantification of the percentage of αSMA positive FAPs in all treatment groups. (D) RT-PCR data showed TSA significantly decreased PPARγ and Collagen I gene expression in FAPs in presence of TGFβ−1 (*p < .05). DMSO, dimethyl sulfoxide; FAPs, fibro/adipogenic progenitors; RT-PCR, real-time polymerase chain reaction; TGFβ−1, transforming growth factorβ−1;TSA, Trichostatin A; UCP-1, uncoupling protein-1
FIGURE 5.

(A) Typical immunostaining image of FAPs under treatment BMP-7 and/or TSA. (B) Quantification of the percentage of perilipin A (+) FAPs in all treatment groups. Typical FAPs expressed UCP-1 were indicated with arrows in the TSA treatment group compared with FAPs without expression of UCP-1 in the DMSO group. (C) Quantification of the percentage of αSMA positive FAPs in all treatment groups. (D) RT-PCR data showed TSA significantly decreased adiponection and PPARγ gene expression, but increased UCP-1, collagen 1, and ACTA2 gene expression in FAPs in presence of BMP-7 (*p < .05). BMP-7, bone morphogenetic protein-7; DMSO, dimethyl sulfoxide; FAPs, fibro/adipogenic progenitors; RT-PCR, real-time polymerase chain reaction; TSA, Trichostatin A; UCP-1, uncoupling protein-1
3.5 |. TSA decreases muscle FI and fibrosis after massive RC tears in vivo
Oil Red O and Masson’s trichrome staining showed that TSA treatment significantly decreased FI and fibrosis in SS after massive tendon/nerve transection (Figure 6). TSA treatment also induced FAP BAT differentiation in SS after RC tears as evidence with UCP-1 expression (Figure 7A). TSA significantly increased histone acetylation level in SS muscle compared with DMSO-treated control (Figure 7B).
FIGURE 6.

Trichostatin A (TSA) treated TT + DN decreases fatty infiltration and fibrosis. (A) Typical image of Oil Red O. Typical lipid droplets were indicated with arrows. (B) Quantification of a fatty index. TSA treatment decreases the percentage of fat. Red-lipid. (C) Typical image of trichome staining. Blue-collagen, red-muscle, and purple-nucleus. Fibrosis was indicated with arrows. DN, denervation transection; TT, tendon transection
FIGURE 7.

(A) TSA treatment-induced FAP BAT differentiation as evidenced by UCP-1(+) PDGFRα (+) BAT-FAPs (arrows) in SS muscle after TT + DN. (B) TSA increased HDAC activity in both supraspinatus (SS) and infraspinatus (IS) muscles after TT + DN (*p < .05). BAT, brown fat adipose tissue; DN, denervation transection; FAPs, fibro/adipogenic progenitors; TSA, Trichostatin A; TT, tendon transection; UCP-1, uncoupling protein-1
4 |. DISCUSSION
There are currently 18 known human HDACs, which are grouped into four classes. Classes I, II, and IV HDACs are zinc-dependent proteins, while Class III HDACs require NAD+.24–26 HDACs have been found to play a role in muscle development, as well as muscle wasting and degeneration wasting.26 Inhibiting HDAC with TSA stimulates myoblast recruitment and fusion with existing myotubes. In vitro, HDAC inhibition with sodium butyrate promoted satellite cell fusion into existing myotubes. TSA treatment of damaged muscles had increased the expression of markers of myogenesis, supporting a beneficial effect of TSA on muscle regeneration.17 HDAC may also regulate muscle development by controlling the promoter accessibility of key myogenic genes by changing chromatin configuration. For example, HDAC1 is found to be present on Myog, Myh10, and Ckm promoters in myoblasts,27–29 and HDAC2 has been detected on the Ckm and Des (desmin) loci.30 The mechanism of HDACs and HDAC inhibitors on muscle growth may go beyond their canonical role of modification of histone. For example, the activation of several transcription factors, including signal transducer and activator of transcription 1, nuclear factor-κB, p53, and FOXO can be affected by acetylation of their specific lysine residues. Thus, HDACs may indirectly or directly regulate the activation of those transcription factors and effect on their target gene expression. Future work is needed to define the detailed mechanism of HADCs and HDAC inhibitor on FAP differentiation and RC muscle FI.
FAPs are a group of newly identified muscle residential progenitors. FAPs have gained increasing interest due to their important role in muscle fibrosis and fatty degeneration. Recent studies demonstrated the existence of FAPs in human skeletal muscle and their role in muscle pathologies.31–33 Multiple studies have shown that FAPs are responsible for increased intramuscular fibrotic and adipose tissue after direct and indirect muscle injuries.34,35 However, information regarding the role of HDAC and HDAC inhibitors on FAP differentiation is limited. In a previous study, Mozzetta et al.20 reported that the HDAC inhibitor TSA inhibited FAP adipogenic potential while enhancing their ability to promote differentiation of adjacent satellite cells. By contrast, FAPs from old mdx mice were resistant to HDACi-mediated inhibition of adipogenesis and constitutively repressed satellite cell-mediated formation of myotubes.20 Consistent with their finding, we found TSA inhibited FAP adipogenesis and reduced RC muscle FI in young C57B/L6 mice. Furthermore, we found that TSA stimulates FAP brown/beige adipose tissue (BAT) differentiation. RC tear is a known aging-related disorder. Employing only young mice is a limitation of this current study. Future studies are warrantied with aged animals to understand the role of aging of epigenetic regulation of FAP adipogenesis after RC tears.
FAPs can differentiate into WAT-like mature adipocytes in vitro and in vivo during muscle fatty degeneration.2,3 Recently, Gorski et al.5 reported that FAPs can differentiate into brown/beige-like adipocytes (BAT) that express UCP-1. In our recent study, we discovered that FAPs from muscles with full-thickness tendon tears patients could be induced to beige fat.36 A unique characteristic of beige fat is that it can return to brown-like phenotype in sprite of its white fat-like appearance under certain “browning” stimulation conditions. White fat “browning” reagents, such as Rosiglitazone, a PPARγ agonist, have been shown to be able to induce white fat-like beige fat returning to a brown fat-like phenotype.37 In our previous study, we have discovered “browning” of white fat in RC muscle with the treatment of Beta-3 adrenergic receptor agonist.38 Transplantation of beige FAPs also significantly reduced RC muscle atrophy/FI and improved shoulder function after tendon injury and repair.39,40 In this study, we found TSA can induce FAP BAT differentiation as evidenced as significantly increased UCP-1 expression in FAP after TSA treatment. Thus, TSA treatment reduces RC muscle FI may be related to “white fat browning.”
Many signaling pathways have been identified to modulate RC muscle FI. For example, Fatty acid-binding protein 4 has been reported to regulate FI after RC tear by hypoxia-inducible factor 1 in mice.41 PDGFRα has also been identified as an important pathway controlling RC muscle FI by modulating FAP differentiation.42 The underlining mechanism of TSA in regulating FAP differentiation and RC muscle FI are not fully understood. Future works are warrantied to study the relationship of HDAC inhibition and those pathways in RC muscle FI. The systematic effect of TSA on innate immune system regulation, DNA synthesis, myogenesis as well as bone marrow mesenchymal stem cell proliferation and differentiation also need be to study in future works.
TGFβ and BMP are important pathways in regulating FAP differentiation. Canonical TGFβ and BMP pathway share the common downstream Smads factors, although TGFβ prefers Smad2/3 and BMP prefers Samd1/5/8.43 Our previous studies have shown two important TGFβ and BMP family members—TGFβ−19 and BMP-710 are overexpressed in muscle after RC injury. In this study, we showed that TGFβ−1 suppresses FAP adipogenesis while BMP-7 promotes FAP adipogenesis. In this study, we found that TSA did not interfere with the inhibitory role TGFβ−1 on FAP adipogenesis, but it significantly inhibited the role of BMP-7 in promoting FAP adipogenesis. Future work is warranted to define the exact mechanism of how TSA modifies FAP response to the TGFβ/BMP pathway.
HDAC inhibitors (HDACi) are mostly studied as anticancer agents, but there is a growing body of evidence suggesting these enzymes play an important role in other diseases such as neurological disorders, inflammatory processes, and viral infections.44 Though TSA has not received the approval from Food and Drug Administration (FDA) for clinical use, some other HDAC inhibitors have been approved by FDA.45–48 Phase III clinical trials of HDAC inhibitor of Givinostat (Italfarmaco) in treating Duchenne and Becker muscle dystrophy are currently ongoing (https://clinicaltrials.gov). If successful, the first HDAC inhibitor for muscle disease may be available on market in the next few years. With more and more HDAC inhibitors available on a marker, future clinical trials with HDAC inhibitors in treating RC muscle fatty degeneration may become feasible in near future.
In summary, we found significantly increased HDAC activity in FAPs in RC muscle after tendon tears. HDAC inhibitor TSA inhibits FAP adipogenesis, stimulates FAP BAT differentiation, and significantly reduced RC muscle FI in this study. This data suggest that modification of FAP differentiation epigenetically with HADC inhibitor may be a new direction for treating RC muscle fatty degeneration.
ACKNOWLEDGMENTS
This study was supported by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant (1I01BX002680, PI: Kim), NIH/NIAMS Research Grant (1R01AR072669-01A1, PI: Feeley), and Orthopedic Research and Education Foundation Career Development and Stem Cell Research Grants (PI: Feeley). Dr. Zili Wang was supported by the China Scholarship Council in the present study. We thank Dr. Shingo Kajimura (UCSF) for his kind gifts of UCP-1 reporter and UCP-1 knockout mice)
Funding information
Orthopedic Research and Education Foundation; NIH/NIAMS, Grant/Award Number: 1R01AR072669-01; VA BLRD Merit Review grant, Grant/Award Number: 1I01BX002680; China Scholarship Council
REFERENCES
- 1.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007; 35(5):719–728. [DOI] [PubMed] [Google Scholar]
- 2.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–152. [DOI] [PubMed] [Google Scholar]
- 3.Joe AWB, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Ning AY, Chen Chang N, et al. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorski T, Mathes S, Krutzfeldt J. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle. 2018;9(2):384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer S, Harms M, Boucher J. The colorful versatility of adipocytes: white-to-brown transdifferentiation and its therapeutic potential in man [published online ahead of print July 03, 2020]. FEBS J. 2020. 10.1111/febs.15470 [DOI] [PubMed] [Google Scholar]
- 7.Alipoor E, Hosseinzadeh-Attar MJ, Rezaei M, Jazayeri S, Chapman M. White adipose tissue browning in critical illness: a review of the evidence, mechanisms and future perspectives [published online ahead of print July 01, 2020]. Obes Rev. 2020. 10.1111/obr.13085 [DOI] [PubMed] [Google Scholar]
- 8.Zoelen EJ, Duarte I, Hendriks JM, Woning SP. TGFbeta-induced switch from adipogenic to osteogenic differentiation of human mesenchymal stem cells: identification of drug targets for prevention of fat cell differentiation. Stem Cell Res Ther. 2016;7(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Joshi SK, Ravishankar B, Laron D, Kim HT, Feeley BT. Upregulation of transforming growth factor-beta signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg. 2014;23(11):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Joshi S, Ravishankar B, Laron D, Kim HT, Feeley BT. Bone morphogenetic protein signaling in rotator cuff muscle atrophy and fatty infiltration. Muscles Ligaments Tendons J. 2015;5(2):113–119. [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MR, Liu X, Lee L, et al. TGF-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One. 2016;11(5):e0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreiro E, Tajbakhsh S. Epigenetic regulation of muscle development. J Muscle Res Cell Motil. 2017;38(1):31–35. [DOI] [PubMed] [Google Scholar]
- 13.Howlett KF, McGee SL. Epigenetic regulation of skeletal muscle metabolism. Clin Sci. 2016;130(13):1051–1063. [DOI] [PubMed] [Google Scholar]
- 14.López-Rodas G, Brosch G, Georgieva EI, Sendra R, Franco L, Loidl P. Histone deacetylase: a key enzyme for the binding of regulatory proteins to chromatin. FEBS Lett. 1993;317(3):175–180. [DOI] [PubMed] [Google Scholar]
- 15.Wolffe AP. Histone deacetylase: a regulator of transcription. Science. 1996;272(5260):371–372. [DOI] [PubMed] [Google Scholar]
- 16.Wade PA, Wolffe AP. Histone acetyltransferases in control. Curr Biol. 1997;7(2):R82–R84. [DOI] [PubMed] [Google Scholar]
- 17.Sincennes MC, Brun CE, Rudnicki MA. Concise review: epigenetic regulation of myogenesis in health and disease. Stem Cells Transl Med. 2016;5(3):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohseni J, Zabidi-Hussin ZA, Sasongko TH. Histone deacetylase inhibitors as potential treatment for spinal muscular atrophy. Genet Mol Biol. 2013;36(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iezzi S, Di Padova M, Serra C, et al. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell. 2004;6(5):673–684. [DOI] [PubMed] [Google Scholar]
- 20.Mozzetta C, Consalvi S, Saccone V, et al. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5(4):626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012;94(7):e41. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Joshi SK, Samagh SP, et al. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res. 2012;30(9):1440–1446. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Lee C, Laron D, et al. Role of pulsed electromagnetic fields (PEMF) on tenocytes and myoblasts-potential application for treating rotator cuff tears. J Orthop Res. 2017;35(5):956–964. [DOI] [PubMed] [Google Scholar]
- 24.Penna F, Costelli P. New developments in investigational HDAC inhibitors for the potential multimodal treatment of cachexia. Expert Opin Investig Drugs. 2019;28(2):179–189. [DOI] [PubMed] [Google Scholar]
- 25.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. [DOI] [PubMed] [Google Scholar]
- 26.Walsh ME, Van H. Remmen, Emerging roles for histone deacetylases in age-related muscle atrophy. Nutr Healthy Aging. 2016;4(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caretti G. The polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18(21):2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mal A, Harter ML. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc Natl Acad Sci USA. 2003;100(4):1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkawa Y, Yoshimura S, Higashi C, et al. Myogenin and the SWI/SNF ATPase Brg1 maintain myogenic gene expression at different stages of skeletal myogenesis. J Biol Chem. 2007;282(9):6564–6570. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa Y, Marfella CG, Imbalzano AN. Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brg1. EMBO J. 2006;25(3):490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uezumi A, Nakatani M, Ikemoto-Uezumi M, et al. Cell-surface protein profiling identifies distinctive markers of progenitor cells in human skeletal muscle. Stem Cell Reports. 2016;7(2):263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uezumi A, Kasai T, Tsuchida K. Identification, isolation, and characterization of mesenchymal progenitors in mouse and human skeletal muscle. Methods Mol Biol. 2016;1460:241–253. [DOI] [PubMed] [Google Scholar]
- 33.Uezumi A, Fukada S, Yamamoto N, et al. Identification and characterization of PDGFRalpha+mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sciorati C, Clementi E, Manfredi AA, Rovere-Querini P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: cellular and molecular players. Cell Mol Life Sci. 2015;72(11):2135–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiore D, Judson RN, Low M, et al. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 2016;17(1):161–169. [DOI] [PubMed] [Google Scholar]
- 36.Feeley BT, Liu M, Ma CB, et al. Human rotator cuff tears have an endogenous, inducible stem cell source capable of improving muscle quality and function after rotator cuff repair. Am J Sports Med. 2020; 48(11):2660–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asano H, Kanamori Y, Higurashi S, et al. Induction of beige-like adipocytes in 3T3-L1 cells. J Vet Med Sci. 2014;76(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Liu X, Liu M, et al. Beta-3 adrenergic receptor agonist treats rotator cuff fatty infiltration by activating brown/beige fat in mice [published online ahead of print June 27, 2020]. J Shoulder Elbow Surg. 2020. 10.1016/j.jse.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee C, Liu M, Agha O, Kim HT, Feeley BT, Liu X. Beige FAPs transplantation improves muscle quality and shoulder function after massive rotator cuff tears. J Orthop Res. 2020;38(5):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C, Liu M, Agha O, Kim HT, Liu X, Feeley BT. Beige fibro- adipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears. J Shoulder Elbow Surg. 2020;29(4):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YS, Kim JY, Oh KS, Chung SW. Fatty acid-binding protein 4 regulates fatty infiltration after rotator cuff tear by hypoxia-inducible factor 1 in mice. J Cachexia Sarcopenia Muscle. 2017;8(5): 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirasawa H, Matsumura N, Shimoda M, et al. Inhibition of PDGFR signaling prevents muscular fatty infiltration after rotator cuff tear in mice. Sci Rep. 2017;7:41552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7(12):1191–1204. [DOI] [PubMed] [Google Scholar]
- 44.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140(6):935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. J Antibiot. 2011;64(8):525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garnock-Jones KP. Panobinostat: first global approval. Drugs. 2015; 75(6):695–704. [DOI] [PubMed] [Google Scholar]


