Abstract
Bound by lineage-determining transcription factors and signaling effectors, enhancers play essential roles in controlling spatiotemporal gene expression profiles during development, homeostasis and disease. Recent synergistic advances in functional genomic technologies, combined with the developmental biology toolbox, have resulted in unprecedented genome-wide annotation of heart enhancers and their target genes. Starting with early studies of vertebrate heart enhancers and ending with state-of-the-art genome-wide enhancer discovery and testing, we will review how studying heart enhancers in metazoan species has helped inform our understanding of cardiac development and disease.
Keywords: gene regulation, cardiac gene expression, transcription factor (TF), epigenomics and epigenetics, comparative genomics, enhancer
Introduction
The heart is a vital organ whose primary role is to pump blood through the circulatory system to reach different organs. Heart-like structures are ancient and observed across diverse metazoans, including arthropods (such as Drosophila), mollusks (such as octopus) and chordates. Heart structures vary widely across metazoans ranging from a single-layered tubular heart in arthropods and tunicates (including Ciona), three separate hearts in some cephalopods (including octopus), a two-chambered heart in jawed fish, a three-chambered heart in amphibians, to a four-chambered heart in other tetrapods (reviewed in Stephenson et al., 2017; Poelmann and Gittenberger-de Groot, 2019). This lineage-specific tuning of cardiac structures is accompanied by changes in the whole circulatory system and highly adapted to the specific physiological needs of different animals. Despite these differences in heart structure, which are mostly related to later-stage heart morphogenesis, many cellular events and molecular regulators involved in early heart development are broadly shared across metazoan species.
A core set of cardiac transcription factors (TFs), including NK2 (Drosophila homolog: Tinman), MEF2 (Drosophila homolog: Mef2), GATA (Drosophila homolog: Pannier), TBX (Drosophila homolog: Nmr1/2, Doc1/2/3, etc.), and HAND (Drosophila homolog: Hand) families, interact with enhancers to control cardiac gene expression and cell fates in Drosophila, fish, and tetrapods (reviewed in Olson, 2006; Tolkin and Christiaen, 2012; Waardenberg et al., 2014). Though specific usage of paralogs and dosage sensitivities may vary between different species, these core TFs form the “cardiac regulatory kernel” (Tolkin and Christiaen, 2012; Waardenberg et al., 2014) in metazoans by closely interacting with each other and extracellular signaling cues. The requirement of extracellular signaling pathways in cardiogenesis also shows a high degree of conservation. The core signaling pathways, such as WNT, FGF, NOTCH, and BMP, play essential cardiogenic roles in both Drosophila and vertebrates (reviewed in Noseda et al., 2011).
Early vertebrate heart development involves a conserved sequence of cellular events that are seen in most, if not all, classes of vertebrate species (reviewed in Miquerol and Kelly, 2013). These events include: the emergence of specified cardiac progenitors within the anterior lateral plate mesoderm; migration of the cardiac progenitors to the midline to form the linear heart tube; rightward looping and elongation of the primitive heart tube; ballooning of the atrial and ventricular chambers out from the looped tube; and cardiac cushion and valve formation at the atrioventricular canal and outflow tract. This conserved set of events involve the complex interplay of multiple cardiac cell types, including the first heart field progenitors (FHF) that give rise to the linear heart tube and second heart field progenitors (SHF) that provide later addition to both poles of the heart tube (Kelly, 2012). Although cardiomyocytes make up a significant portion of mature hearts, other cell types, such as endocardial cells, smooth muscle cells, and cardiac fibroblasts, are also involved in cardiac development and physiological function (Hu et al., 2018; Honkoop et al., 2019; Tucker et al., 2020).
Understanding the interplay between multiple cardiac TFs and signaling pathways, within and between the cell types involved in cardiogenesis, requires a detailed knowledge of the cis-regulatory elements (CREs) that comprise heart enhancers. The regulatory logic encoded within CREs is readily understood by the embryo and is sufficient to organize multiple cardiac TFs and signaling pathways that ultimately result in a fully formed and functioning heart. In contrast, it has taken decades of experimental advances and insights to develop systems and technologies where cardiac CREs can be discovered and tested.
In this review, we discuss the genetic control of heart development and disease from an enhancer-centric perspective. From early gene-centric enhancer dissection in the 1990s to genome-wide characterization of heart enhancers in development and disease today, the discovery of heart enhancers has substantially shaped our understanding of the principles in cardiac gene regulation. We begin with a brief overview of developmental enhancers followed by a discussion of regulatory principles gained from pre-genomics enhancer studies. We then discuss how rapid advances in genome-wide approaches have transformed our knowledge regarding the locations, interactions, temporal dynamics and functions of heart enhancers. Our review will incorporate evolutionary characteristics of heart enhancers and discuss how new methods for dissecting heart enhancer functions promises to improve our understanding of heart development and cardiovascular diseases.
Enhancer Structure and Function in Development: A Primer
Enhancers are traditionally defined as short non-coding DNA sequences with the ability to drive gene expression regardless of the genomic distance, position, and orientation relative to the cognate genes [i.e., (Blackwood and Kadonaga, 1998) recently reviewed by Field and Adelman, 2020]. Enhancers can influence gene expression over short (hundreds of base pairs, bp) or large (megabases) genomic distances. These distal enhancers form long-range chromatin interactions with their target genes, such as the well-studied ZRS enhancer that is 1 Mb away from its target Shh (Lettice et al., 2003). This flexibility allows a single gene to be regulated by multiple enhancers with different spatiotemporal activities, as well as a single enhancer to contribute to the regulation of multiple genes, which was shown in recent genome-wide enhancer interaction maps (Montefiori et al., 2018; Jung et al., 2019). Together this many-to-many relationship sets up a complex regulatory network to achieve the highly diverse tissue-specific expression patterns evident in development.
Spatial-temporal developmental gene expression is achieved through the combinatorial recruitment of a discrete set of TFs to enhancers (for a recent review of how TFs recognize CREs see Zeitlinger, 2020). TFs interact with enhancers through short degenerate DNA sequence motifs. Recent work investigating the regulatory logic of a typical developmental enhancer supports an overarching principle that specific developmental gene expression relies on sub-maximal TF recognition motifs (Farley et al., 2015). Layered on top of TF motif affinity is the motif syntax within an enhancer, where the spacing, orientation, and order of the motifs themselves can impact the ability of the enhancer to drive developmental gene expression (Farley et al., 2016). It is also important to recognize that developmental genes are commonly regulated by additional redundant enhancers and ascertaining the contributions of individual enhancers remains an outstanding challenge for the majority of developmentally expressed genes (Cannavò et al., 2016; Osterwalder et al., 2018).
Some lineage-determining TFs can bind to compact chromatin regions that are largely inaccessible to other factors. These pioneer factors recruit chromatin-remodeling complexes that promote nucleosome eviction, facilitating the subsequent binding of other collaborating TFs and signal effectors (McPherson et al., 1993; Cirillo et al., 2002; reviewed in Zaret, 2020). To impact gene expression, TFs recruit transcriptional cofactors to enhancers. Cofactors can in turn modify chromatin states by catalyzing post-translational histone modifications (e.g., P300/CBP, MLL3/4), initiate chromatin remodeling (e.g., BRG1), bridge the gap between promoters and enhancer-bound transcription machinery (e.g., Mediator), or affect the affinity of TF binding at enhancers (Malik and Roeder, 2010; Siggers et al., 2011; Slattery et al., 2011; Krasnov et al., 2016). Despite these advances (and many others), much remains to be learned about the mechanisms underlying the recruitment of pioneer factors to a small subset of genomic sites and the molecular events that follow.
Enhancer activation in development is accompanied by progressive changes at the chromatin level, which in turn can be used to annotate enhancer states. Repressed enhancers are located in nucleosome dense regions. Certain repressed regions are characterized by the post-translational histone modification H3K27me3 which is deposited by the Polycomb repressive complex 2 (PRC2). The binding of pioneer factors and chromatin-remodeling complexes may switch enhancers to a poised state, in which enhancers share many features with those in an active state. Poised enhancers show features of low nucleosome occupancy, limited TF binding, and post-translational histone modifications H3K4me1 and H3K4me2 without the presence of H3K27ac, a histone mark of active developmental enhancers (Creyghton et al., 2010; Rada-Iglesias et al., 2011; Zentner et al., 2011). These poised developmental enhancers may even retain the repressive mark H3K27me3 (Rada-Iglesias et al., 2011; Zentner et al., 2011). Upon full activation, transcription co-factor P300 and RNA polymerase II are recruited to enhancers, leading to bi-directional transcription of enhancer RNAs and active enhancer regions marked with H3K27ac (reviewed by Calo and Wysocka, 2013; Heinz et al., 2015).
Enhancer activities are influenced by both local chromatin interactions and higher-order chromatin architectures. Eukaryotic genomes are compartmentalized into large self-interacting chromatin domains, termed topologically associated domains (TADs) (Dixon et al., 2012; Rao et al., 2014). TADs largely constrain the chromatin span that enhancers search through and define the regulatory domains within which enhancer-promoter interactions most frequently occur (Long et al., 2016). For example, promoter capture Hi-C experiments have revealed that 60–80% of the detected promoter interactions occur within TADs (Javierre et al., 2016; Choy et al., 2018; Montefiori et al., 2018). Early studies have noticed that TAD boundaries are shared between different cell types and conserved between species (Dixon et al., 2012; Vietri Rudan et al., 2015), however, these two concepts have been revised more recently. An increasing number of studies reported dynamic loss and gain of TADs and changes of TAD sizes during differentiation (Bonev et al., 2017; Bertero et al., 2019; Zhang et al., 2019). While evolutionarily conserved TADs correspond to regions of conserved synteny harboring important developmental genes and enhancers (Harmston et al., 2017), new analyses have questioned the extent to which TAD boundaries themselves correspond to evolutionary breakpoints (Eres et al., 2019; Eres and Gilad, 2020; Torosin et al., 2020). The importance of understanding how TADs relate to gene regulation is underscored by the increasing number of experiments showing that the disruption of TAD boundaries and sub-TAD domains can rewire enhancer-promoter interactions and fundamentally change the regulatory environment (Guo et al., 2015; Lupiáñez et al., 2015; Flavahan et al., 2016; Franke et al., 2016; Liang et al., 2020).
In sum, the precise and robust transcriptional regulation that occurs during development is achieved by the complex interplay between enhancers, TFs, co-factors, and epigenetic modifications, which together are organized under higher orders of chromatin architectures.
Heart Enhancers: Fundamental Insights, One Cre at a Time
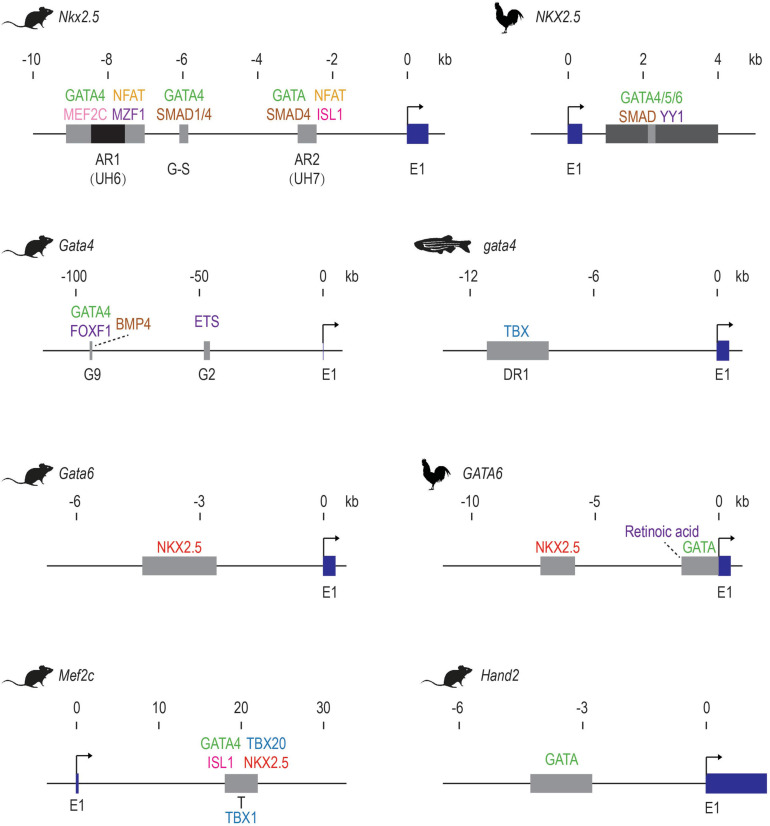
Studies of heart enhancers initiated from targeted searches around cardiac genes. Putative enhancer regions were screened by “promoter bashing,” in which regulatory regions near the TSS are narrowed down via a series of deletions/mutations to produce overlapping DNA segments that are tested in reporter assays (Table 1). One of the best-studied examples is the mouse Nkx2.5 locus. LacZ reporter assays identified enhancer elements that specifically drove Nkx2.5 expression in different chambers of the hearts, as well as in thyroid, pharynx, and stomach within a 14 kb window around the TSS, revealing previously unappreciated complex enhancer modules underlying the control of cardiac TFs. Similar complexities were seen at genes encoding other cardiac TFs, such as Hand2 (heart and pharyngeal specific enhancers) (McFadden et al., 2000; Charité et al., 2001; Iklé et al., 2012), Mef2c (anterior heart field and somite specific enhancers) (Wang et al., 2001; Dodou et al., 2004), and Gata4 (lateral mesoderm, endocardium, and endoderm specific) (Rojas et al., 2005, 2009; Schachterle et al., 2012). Although limited in number and biased toward proximal gene promoter regions, these studies (and many others) have revealed fundamental principles and mechanisms underlying cardiac gene regulation.
TABLE 1.
Functionally characterized enhancer regions near cardiac genes.
| Target genes | Enhancer length | Genomic position | Expression domain | Upstream regulators or function | References |
| Mouse Nkx2.5 | 14 kb | 5′ flanking sequence of TSS | cardiac crescent, ventricles, outflow tract, pharynx, thyroid, stomach | NKX2.5 (negatively regulate this enhancer) | Tanaka et al., 1999 |
| Mouse Nkx2.5 | 4, 3.3 kb | 5′ flanking sequence of TSS | outflow tract, basal portion of the right ventricle, pharynx, thyroid | Tanaka et al., 1999 | |
| Mouse Nkx2.5 | 6 kb | 3′ flanking sequence of TSS | right ventricle | Tanaka et al., 1999 | |
| Mouse Nkx2.5 | 8 kb | [−14, −6 kb] of TSS | medial wall and inner trabeculae of ventricles | Tanaka et al., 1999 | |
| Mouse Nkx2.5 | 2.1 kb, two separate fragments (513, 686 bp) (AR1) | [−9.4, −7.3 kb] of TSS | endogenous cardiac expression of Nkx2.5 | GATA4, MEF2C, NFAT, MZF1 | Lien et al., 1999; Chen and Cao, 2009; Clark et al., 2013; Doppler et al., 2014 |
| Mouse Nkx2.5 | 505 bp (AR2) | [−3, −2.5 kb] of TSS | anterior cardiac crescent, right ventricle, outflow tract, developing spleen, pharyngeal pouches | GATA, SMAD4, NFAT, ISL1 | Searcy et al., 1998; Liberatore et al., 2002; Lien et al., 2002; Takeuchi et al., 2005; Chen and Cao, 2009; Quinodoz et al., 2018 |
| Mouse Nkx2.5 | 2, 1.5 kb | [−10.7, −3.5 kb] of TSS | early heart tube, outflow tract, right ventricle | GATA | Reecy et al., 1999 |
| Mouse Nkx2.5 | 237 bp (G-S) | [−6.2, −5.79 kb] of TSS | cardiac crescent, heart, forebrain | GATA4, SMAD1/4 | Brown et al., 2004 |
| Mouse Nkx2.5 | 10 kb (FL) | 5′ flanking sequence of TSS | test in cell lines (10T1/2, P19) | GATA4, SMAD1/4, TBX20 | Brown et al., 2004; Takeuchi et al., 2005 |
| Mouse Nkx2.5 | 2.6 kb (UH5) | [−16, −14 kb] of TSS (estimated) | heart tube, both atria, left ventricle, foregut | Chi et al., 2005 | |
| Mouse Nkx2.5 | 7.3 kb (UH6) | [14, −6 kb] of TSS (estimated) | right ventricle, interventricular septum, atrial ventricular canal | Chi et al., 2005 | |
| Chicken Nkx2.5 | 3 kb, 200 bp | [+976 bp, +3.97 kb], [+2.1, +2.3 kb] of TSS | anterior cardiac cresent, outflow tract, right ventricle, pharyngeal arches (test in mouse) | GATA4/5/6, SMAD, YY1 | Lee et al., 2004 |
| Mouse Gata4 | 4.4 kb (G2) | [−45.3, −40.9 kb] of TSS | lateral mesoderm | FOXF1, GATA4, BMP4 | Rojas et al., 2005 |
| Mouse Gata4 | 1.9 kb (G9) | 93 kb upstream of TSS | cardiac crescent, linear heart tube, endocardium | EST factors (ETS1, ERG) | Schachterle et al., 2012 |
| Zebrafish gata4 | 14.8, 12 kb | 5′ flanking sequence of TSS | lateral plate mesoderm, both atrium and ventricle | Heicklen-Klein and Evans, 2004 | |
| Zebrafish gata4 | 7.8, 5.5 kb | 5′ flanking sequence of TSS | ventricle and the bulboventricular valve | Heicklen-Klein and Evans, 2004 | |
| Zebrafish gata4 | 3 kb (DR1), 1.3 kb (DR1A) | [−11, −8 kb] of TSS | lateral plate mesoderm, both atrium and ventricle | TBX | Heicklen-Klein and Evans, 2004 |
| Chicken GATA5 | 500 bp | [−5, −4.5 kb] of TSS | cardiac crescent, septum trans-versum and epicardium, ventricle, AV canal (test in mice) | MacNeill et al., 2000 | |
| Mouse Gata6 | 6.8, 1.8 kb | [−4.3, +2.5 kb], [−4.3, −2.5 kb] of TSS | cardiac cresent, high expression in outflow tract | NKX2.5 | Molkentin et al., 2000 |
| Chicken GATA6 | 1.4 kb | 6.2 kb upstream of TSS | cardiac crescent, high expression in the outflow tract (test in mouse) | NKX2.5 | Davis et al., 2000 |
| Chicken GATA6 | 10 kb | [−9.2, +0.8 kb] of TSS | cardiac specific (test in mice) | He and Burch, 1997 | |
| Chicken GATA6 | 2.3, 1.5 kb | [−1.5, +0.8 kb], [−1.5 kb, 0] of TSS | posterior region of the heart field, atrioventricular conduction system (test in mice) | Retinoic acid | He and Burch, 1997; Davis et al., 2001 |
| Chicken GATA6 | 317, 187, 102, 47 bp | [−1.4, −1.1 kb] of TSS | atrioventricular conduction system (test in mice) | GATA | Adamo et al., 2004 |
| Mouse Hand2 | 1.5 kb | [−4.2, −2.7 kb] of TSS | cardiac crescent, right ventricle, outflow tract | GATA | McFadden et al., 2000 |
| Mouse Mef2c | 6, 3.9 kb, 449 bp | [+16.3, +22.5 kb] of TSS | anterior (second) heart field | GATA4, ISL1, NKX2.5, TBX20, TBX1 (negative regulator) | Dodou et al., 2004; Takeuchi et al., 2005; Caputo et al., 2015; Pane et al., 2018 |
| Mouse Hey2 | 2.5, 1.6 kb, 649 bp | 211 kb upstream of TSS | cardiac crescent, ventricle and outflow tract | TBX20, GATA4 | Ihara et al., 2020 |
| Zebrafish hey2 | 626 bp (aCNE21) | 24 kb upstream of TSS | distal linear heart tube, ventricle, outflow tract | Gibb et al., 2018; Yuan et al., 2018 | |
| Mouse Tbx1 | 200 bp (require another non-cardiac element) | [−12.8, −12.6 kb] of TSS | second heart field, right ventricle, outflow tract, pulmonary trunk, and pulmonary valves | FOX (likely FOXC1 or FOXC2) | Maeda et al., 2006 |
| Human TBX5 | 368 bp (enhancer 2) | 380 kb downstream of TSS | both ventricles and atria | Harbor a CHD-associated variant | Smemo et al., 2012 |
| Human TBX5 | 3.5 kb (enhancer 9) | 140 kb downstream of TSS | ventricles, interventricular septum, atrioventricular canal | Smemo et al., 2012 | |
| Human TBX5 | 5 kb (enhancer 16) | 9 kb upstream | ventricles, interventricular septum, atrioventricular canal, and weakly in atria | Smemo et al., 2012 | |
| Mouse Isl1 | 2.9 kb | 120 kb downstream | embryonic and adult sinoatrial node (SAN) | SAN hypoplasia and sinus arrhythmia in enhancer knockout, contain SNPs associated with heart rate | Galang et al., 2020 |
| Mouse Fgf8 | 900 bp | [−5.4, −4.5 kb] of TSS | outflow tract, pharyngeal arches | TBX1 | Hu et al., 2004 |
| Mouse Fgf10 | 1.7 kb | [+44, +46 kb] of TSS | anterior second heart field, pharyngeal mesoderm | TBX1, NKX2.5 (negative), ISL1 | Watanabe et al., 2012 |
| Mouse Srf | 1 kb, 541 bp | 3′ UTR sequence | cardiac crescent, heart tube, tail | TBX2 TBX5, TIP60 | Barron et al., 2005 |
Establishing Molecular Cascades Regulating Heart Development
Enhancers represent information hubs that integrate multiple upstream regulatory inputs such as lineage-determining master TFs and signaling effectors. Dissecting the transcription factors that bind to enhancers unveils these direct upstream regulators (Figure 1 and Table 1). By combining motif mutagenesis, gel shift, and transgenic assays, Nkx2.5 enhancer studies revealed that GATA4 and SMAD-mediated BMP signaling directly activated Nkx2.5 expression through multiple enhancer regions (Searcy et al., 1998; Lien et al., 1999, 2002; Liberatore et al., 2002; Brown et al., 2004) (Figure 1). Dissections of Nkx2.5 enhancers in the following years added ISL1, TBX20, MEF2C, and NFAT into direct upstream regulators that collectively drove Nkx2.5 expression in cardiac cells (Takeuchi et al., 2005; Chen and Cao, 2009; Clark et al., 2013). Furthermore, mining known heart enhancers can also lead to discoveries of novel cardiac regulators. For example, MZF1, previously known as a hematopoietic TF, was found to bind to an Nkx2.5 enhancer from in silico motif analysis and validated in embryonic stem cell (ESC) differentiation. Overexpression of MZF1 at different stages of cardiac differentiation revealed its novel, stage-dependent roles in cardiogenesis (Doppler et al., 2014).
FIGURE 1.
Early examples of validated of cardiac TF-enhancer interactions. The first exons of the cardiac genes are shown in dark blue. Enhancer elements are shown as gray boxes. The AR1 enhancer of mouse Nkx2.5 contains a repressive element in the middle, which is shown in black. Direct activators are listed above the enhancer elements while repressors are shown below. Upstream factors without direct binding evidence are indicated with dotted lines. E1: exon 1. These schematics are generated based on data from these publications: mouse Nkx2.5 (Searcy et al., 1998; Lien et al., 1999, 2002; Liberatore et al., 2002; Brown et al., 2004; Chi et al., 2005; Takeuchi et al., 2005; Chen and Cao, 2009; Clark et al., 2013; Doppler et al., 2014; Quinodoz et al., 2018); Chicken NKX2.5 (Lee et al., 2004); Mouse Gata4 (Rojas et al., 2005; Schachterle et al., 2012); zebrafish gata4 (Heicklen-Klein and Evans, 2004); mouse Gata6 (Molkentin et al., 2000); Chicken GATA6 (He and Burch, 1997; Davis et al., 2001; Adamo et al., 2004); mouse Mef2c (Dodou et al., 2004; Takeuchi et al., 2005; Pane et al., 2018); mouse Hand2 (McFadden et al., 2000).
Through similar enhancer dissection, the upstream signals of many other cardiac TFs have been identified (Table 1 and Figure 1). For example, the lateral mesoderm expression of mouse Gata4 relies on transcriptional inputs from FOXF1, BMP4, and its autoregulation (Rojas et al., 2005), while its expression in endocardia requires binding of ETS factors such as ETS1 and ERG (Schachterle et al., 2012). The anterior heart field (AHF) expression of Mef2c is positively regulated by GATA4, ISL1, and TBX20 and repressed by TBX1 through an intronic enhancer (Dodou et al., 2004; Takeuchi et al., 2005; Pane et al., 2018). Ventricular expression of Hey2 is dependent on TBX20 and GATA factor binding, but not NK-2 proteins. Summarizing the existing examples, it is clear that GATA factors, which regulate the expression of many other cardiac TFs (NKX2.5, HAND2, HEY2, MEF2C, etc.), sit among the top of the cardiac molecular cascade. Importantly, sustained cardiac expression of GATA itself requires the transcriptional inputs of other cardiac genes such as NKX2.5 and TBX factors, likely establishing a reciprocal feedback loop to maintain the robustness of the cardiac regulatory network.
Cardiac TF Crosstalk
Enhancer activation requires the cooperative binding of multiple TFs, therefore studying heart enhancers reveals cooperation and competition between these upstream factors. By co-expressing different combinations of factors together with a specific enhancer, the synergistic effect of factors in activating the enhancer can be revealed by quantitative measures like luciferase assays. Using this type of approach, GATA4 and SMAD1/4 were found to work as mutual co-activators in activating Nkx2.5 expression through a distal enhancer (commonly referred to as the G-S enhancer) (Brown et al., 2004). At another Nkx2.5 enhancer (AR1), GATA binding is indispensable for the transcriptional activation mediated by NFAT, likely through cooperative binding (Chen and Cao, 2009). Besides cooperativity, competitive binding between different TFs at heart enhancers can also play an important role in cardiac lineage specification. For example, the two homeodomain TFs, NKX2.5 and ISL1 compete for the same binding sites within an anterior second heart field enhancer of Fgf10, reflecting the antagonism between NKX2.5 and ISL1 during the differentiation from SHF progenitors to cardiomyocytes (Watanabe et al., 2012).
Putting Enhancers to Work
Besides providing direct evidence for building cardiac transcriptional networks, validated cardiac enhancers also frequently serve as genetic tools to label a specific cardiac population of interest for developmental studies. Transgenic mice in which Cre recombinase expression is driven by the Mef2c AHF enhancer have been used to determine anterior heart field derived structures and conditionally knock-out many developmental genes (Mef2c, Tbx1, β-catenin, Ezh2) to reveal their specific roles in anterior heart field development and congenital heart disease (Verzi et al., 2005; Delgado-Olguín et al., 2012; Barnes et al., 2016; Racedo et al., 2017). A GFP line driven by the Nkx2.5 AR1 enhancer was used to discover an immature cardiomyoblast population in neonatal mice that was required for normal heart development (Serpooshan et al., 2017). Recently, this Nkx2.5 enhancer was found to be reactivated after myocardial infarction in the adult heart, suggesting the role of this enhancer in responses to heart injuries (Deutsch et al., 2018). A mouse Smarcd3 enhancer was found to label early cardiac progenitor cells before the expression of known cardiac markers (Nkx2.5, Isl1, Tbx5) in mice, indicating an early molecular distinction between cardiac progenitors and neighboring cells (Devine et al., 2014). This enhancer was later shown to function similarly in zebrafish and helped identify ∼160 putative cardiac enhancers conserved between zebrafish and mammals (Yuan et al., 2018). One of these deeply conserved heart enhancers recapitulated the cardiac expression of the nearby gene hey2 thus was subsequently used in dissecting how hey2 restricted cardiac progenitor proliferation (Gibb et al., 2018).
In sum, deeply dissecting cardiac enhancers reveals both molecular tools for visualizing, isolating, and manipulating cardiac populations as well as cis- and trans-regulatory mechanisms that control cardiac gene expression.
Unmasking Heart Enhancers With Comparative and Functional Genomics
Enhancer Hunting: Tools of the Trade
Comparative genomics has long been used to identify putative enhancer regions (Tagle et al., 1988; Aparicio et al., 1995). Such comparative approaches are based on the assumption that functionally relevant enhancer sequences will be under negative selection and will thus show higher sequence constraints than non-functional regions. This assumption is supported by the genome-wide identification of conserved non-coding elements (CNEs) and the following discoveries that many CNEs work as developmental enhancers (Nobrega et al., 2003; Bejerano et al., 2004; Johnson et al., 2004; de la Calle-Mustienes et al., 2005; Shin et al., 2005; Woolfe et al., 2005; Pennacchio et al., 2006). Substantial work using a variety of approaches including transitive alignment (Hiller et al., 2013; Braasch et al., 2016), ancestral reconstruction (Hiller et al., 2013), and conserved microsynteny (Irimia et al., 2012; Clément et al., 2020; Wong et al., 2020) have further enhanced our ability to detect more distantly related conserved non-coding elements.
Although CNEs are enriched for developmental enhancers, the vast majority of enhancers appear to evolve more rapidly, with many being lineage- or species-specific. This feature has been demonstrated in many different tissues or cell types and in both vertebrates and invertebrates (Odom et al., 2007; Kunarso et al., 2010; Schmidt et al., 2010b; Mikkelsen et al., 2010; Cotney et al., 2013; Paris et al., 2013; Arnold et al., 2014; Villar et al., 2015). Although enhancers in different tissues or at different developmental stages may be under varied selection pressures (Blow et al., 2010; Nord et al., 2013; Visel et al., 2013), rapid evolution is an overall feature of enhancer sequences, which suggests that many enhancers would be missed in detection approaches based on sequence conservation alone.
Over the past 15 years, large scale genomic assays have enabled enhancer discoveries at an unprecedented scale (Table 2). In particular, chromatin immunoprecipitation with high-throughput sequencing (ChIP-seq) can locate enhancers by profiling the co-occupancy of lineage-specific TFs, binding of co-factors, or post-transcriptional modifications that marks active enhancers (reviewed in Buecker and Wysocka, 2012). As ChIP-seq requires large numbers of input cells, which is often difficult to obtain from early embryonic tissues, many low input ChIP methods (O’Neill et al., 2006; Brind’Amour et al., 2015) and alternative strategies, such as enzyme-tethering based approaches have been established (e.g., CUT&RUN, CUT&Tag, and CUTAC) (Skene and Henikoff, 2017; Kaya-Okur et al., 2019; Meers et al., 2019; Henikoff et al., 2020).
TABLE 2.
Genomic approaches for enhancer mapping.
| Method category | Method strategy | Description | References |
| ChIP-seq (detect DNA-binding factor occupancy and histone modification profiles) | Co-factors (EP300, Mediator) | Assays enhancers mediated by specific co-factors; TFs need not be specified in advance. | Blow et al., 2010; He et al., 2011; May et al., 2012 |
| Co-occupancy of multiple TFs | Reveals specific trans factors but requires specific antibodies for each factor and often each species. Typically requires large numbers of nuclei. | He et al., 2011, 2014; Luna-Zurita et al., 2016; Akerberg et al., 2019 | |
| Active histone marks (H3K27ac, H3K4me1) | Robust antibodies that work across metazoans; reveals enhancer states;requires less input than for TFs. | Wamstad et al., 2012; Nord et al., 2013; He et al., 2014 | |
| Enzyme tethering ChIP alternative (use factor-mediated in-situ genome fragmentation to profile epigenome) | CUT&RUN (pA-MNase fusion protein) | Unfixed in-situ procedure, requires lower cell numbers (∼100 for histone modification) and less sequencing reads | Skene and Henikoff, 2017; Meers et al., 2019 |
| CUT&Tag (pA-Tn5) | Similar to CUT&RUN with a simpler barcoding step; streamlined workflow in a single tube; works on low cell numbers or even single cells | Kaya-Okur et al., 2019; Henikoff et al., 2020 | |
| CUTAC (pA-Tn5, low salt) | Similar to CUT&Tag with a small modification that detects accessible chromatin in parallel with adjacent histone modifications | Henikoff et al., 2020 | |
| Accessible chromatin profiling (detect nucleosome-depleted regions that are enriched for enhancers) | DNase-seq | High quality TF footprintscan be generated. | Thurman et al., 2012; Vierstra et al., 2014, 2020 |
| ATAC-seq | Simple and robust method that requires low cell numbers, widely applied; can be used on frozen sections; produces a comprehensive list of where CREs may be located. | Buenrostro et al., 2013; Corces et al., 2017 | |
| Nascent RNA sequencing run-on assays (depict the real-time activity of RNA polymerases and detect eRNAs) | GRO-seq | Detect actively transcribed eRNAs which is a hallmark of active enhancers | Core et al., 2008 |
| PRO-seq | Refined version of GRO-seq that uses biotinylated nucleotide to reach nucleotide-resolution, low background, and large dynamic ranges | Kwak et al., 2013; Core et al., 2014 | |
| ChRO-seq | Similar to PRO-seq but use chromatin as starting materials; can be applied to solid tissues and samples with degraded RNAs | Chu et al., 2018 | |
| Chromosome conformation capture (use proximity ligation and detect enhancer-promoter interaction) | Hi-C | Maps genome-wide chromatin contacts (‘all-to-all’); requires substantial sequencing to reveal local enhancer-promoter interactions | Lieberman-Aiden et al., 2009 |
| Promoter capture Hi-C | Maps promoter-centric chromatin interactions; requires less reads for detecting promoter-enhancer interactions | Mifsud et al., 2015; Schoenfelder et al., 2015 | |
| ChIA-PET | Detect chromatin interactions mediated by a specific DNA-binding factor; can enrich rare factor-specific chromatin interactions | Fullwood et al., 2009; Grubert et al., 2020 | |
| HiChIP& PLAC-seq (Use in-situ Hi-C followed by ChIP) | Detects factor-centric chromatin interaction similar to ChIA-PET but require 10-fold to 100-fold fewer cells, also more robust and less time-consuming | Fang et al., 2016; Mumbach et al., 2016 | |
| 4C | Identifies all genomic regions that interacts a reference locus (‘one-to-all’); can be used for studying specific enhancers | Simonis et al., 2006 |
Chromatin accessibility profiling provides a comprehensive view of the candidate regions most likely to harbor CREs, making them arguably the most widely used assay to identify putative enhancers (Thurman et al., 2012; Buenrostro et al., 2013; Vierstra et al., 2014, 2020; Corces et al., 2017). Since active enhancers are transcribed bidirectionally to produce eRNA, nascent RNA sequencing technologies, specifically the run-on assays (GRO-seq, PRO-seq, ChRO-seq, etc.), can be used as a direct readout of enhancer activity. Furthermore, when coupled with chromatin accessibility assays (i.e., ATAC-seq), run-on assays can distinguish active enhancers (producing bi-directional RNAs) from other CREs such as CTCF bound insulators (reviewed in Wissink et al., 2019).
After discovering a distal putative enhancer, one of the most pressing questions is to discover what gene or genes it associates within a cell type and condition of interest. To address this, chromosome conformation capture (3C) based assays (including 4C, 5C, HiChIP, promoter capture Hi-C, and Hi-C) are commonly used to characterize enhancer-promoter interactions (Denker and De Laat, 2016; Fang et al., 2016; Mumbach et al., 2016). Capture Hi-C approaches, such as promoter-capture Hi-C and HiCap (Mifsud et al., 2015; Sahlén et al., 2015; Schoenfelder et al., 2015), are increasingly being used to reveal promoter-centric chromatin interactions at high resolution. Capture-based methods that target putative enhancer regions, such as those discovered by DNAse-seq (Sönmezer et al., 2020), could also be used for ‘enhancer-capture’ Hi-C. Naturally, the choice of 3C-based methods depends on the research question and practical considerations such as the quantity of sample material, genome size, capture probe availability, and sequencing costs.
These widely used genome-scale assays, each with their own strengths (Table 2), continue to reveal new insights into enhancer location, activity and function. The increasing number of high-quality datasets are also creating new opportunities and challenges for integrative data analysis that will further expand our understanding of metazoan heart development and human disease.
Heart Enhancers: From Genome-Wide Mapping to Metazoan Regulatory Logic
The development of ChIP-chip, ChIP-seq, and other genomic techniques has enabled genome-wide enhancer discoveries and analysis of distinct cardiac samples obtained from diverse model systems (Table 3). Pioneering studies in Drosophila using ChIP-chip against master regulators (Twi, Tin, Mef2, Bag, Bin, Doc, and Pnr) and signaling effectors (dTCF and pMad) required for the specification of cardiac mesoderm revealed fundamental principles of combinatorial TF binding dynamics and TF-signaling interactions at cardiac enhancers (Zinzen et al., 2009; Junion et al., 2012). These Drosophila cardiac TF mapping studies, together with a comparative analysis of Twi, Tin, Mef2, Bin, and Bap in two distant Drosophila species, underscore the conserved presence of combinatorial TF binding, even when the underlying DNA sequence has changed (Khoueiry et al., 2017). The Junion et al. (2012) study led to a “transcription factor collective” model of TF binding where TFs use both protein-DNA and protein–protein interactions to regulate gene expression (reviewed by Spitz and Furlong, 2012), which was later supported by the comparative Khoueiry et al. (2017) study.
TABLE 3.
Genome-wide metazoan heart enhancer profiling datasets generated using chromatin immunoprecipitation of post translational histone modifications, transcription factors, and cofactors.
| Method | Species | Factor | Sample | Condition | Stage | References |
| BiTS-ChIP-Seq | Drosophila | H3, H3K4me3, H3K4me1, H3K27ac, H3K27me3, H3K36me3, H3K79me3 | Mesoderm | WT | stages 10–11 (6–8 h AEL, cardiac mesoderm specified) | Bonn et al., 2012 |
| ChIP-seq | Zebrafish | H3.3 | myl7:GFP+ cardiomyocytes | Uninjured, 14 days post ablation, 7 days post Nrg1 treatment | Adult | Goldman et al., 2017 |
| ChIP-seq | Zebrafish | H3K27ac | myl7:GFP+ cardiomyocytes | Uninjured, 14 days post ablation | Adult | |
| ChIP-seq | Mouse | H3K27ac, H3K4me1, H3K4me3, H3K27me3 | ESCs, ESC-differentiated cells | WT | ESCs, mesoderm, cardiac precursors, cardiomyocytes | Wamstad et al., 2012 |
| ChIP-seq | Mouse | H3K27ac | Hearts | WT | E11.5, E14.5, E17.5, P0, P7, P21, P56 | Nord et al., 2013 |
| ChIP-seq | Mouse | H3K4me1, H3K27me3, H3K4me3 | Ventricle | WT | E12.5 and adult | He et al., 2014 |
| ChIP-seq | Mouse | H3K27ac | Ventricle | WT, GATA4 KO | E12.5 (WT, GATA4 KO), adult (normal) | |
| ChIP-seq | Mouse | H3K27ac | Heart | WT | E12.5 | Zhou et al., 2017 |
| ChIP-seq | Mouse | H3K27ac | iCLM (induced cardiac-like myocytes) reprogrammed from MEF | Transfected with GMT, GHMT, AGHMT or mock control | Day 2 and 7 in reprogramming | Hashimoto et al., 2019 |
| ChIP-seq | Mouse | H3K27ac | iCLM (induced cardiac-like myocytes) reprogrammed from MEF | Transfected with single factors | Day 2 in reprogramming | |
| ChIP-seq | Mouse | H3K27ac | Ventricle, atrium | WT | P4 | |
| ChIP-seq | Human | H3K4me3, H3K27me3, H3K36me3 | ESCs, ESC-differentiated cells | WT | pluripotent cells, mesodermal progenitors, specified tripotential cardiovascular progenitors, committed cardiovascular cells, definitive cardiovascular cells | Paige et al., 2012 |
| ChIP-seq | Human | H3K4me3, H3K36me3, H3K27ac, H3K27me3 | iPSC-differentiated cells | WT, GATA4_G296S | iPSC-derived cardiomyocytes | Ang et al., 2016 |
| ChIP-seq | Human | H3K27ac, H3K9ac, H3K4me3, H3K4me1, H3K36me3 | Left ventricle | Healthy donor and patients with heart failure | fetal, infant, adult (non-failing and failing heart) | Gilsbach et al., 2018 |
| ChIP-seq | Human | H3K27ac | Left ventricle | healthy donors and patients with dilated cardiomyopathy | Adult | Spurrell et al., 2019 |
| ChIP-seq | Human | H3K27ac | ESCs, ESC-differentiated cells | WT | ESCs, mesodermal cells, cardiac mesodermal cells, cardiac progenitors, primitive cardiomyocytes, and ventricular cardiomyocytes | Zhang et al., 2019 |
| ChIP-seq | Human | H3K4me1, H3K4me2, H3K4me3, H3K27ac, H3K27me3, H3K9me3, H3K36me3 | Heart | Healthy donor | CS13, CS14, CS16, CS17, CS18, CS19, CS20, CS21, CS23 (Carnegie stage, corresponding to PCW 4–8) | Vanoudenhove et al., 2020 |
| ChIP-chip | Drosophila | Twist, Tinman (Nkx2.5) | Whole embryo | WT | Stage 5–7, stage 8–9 (dorsal mesoderm specified), stage 10–11 (cardiac mesoderm specified) | Zinzen et al., 2009 |
| ChIP-chip | Drosophila | Mef2 | Whole embryo | WT | Stage 5–7, stage 8–9, stage 10–11 stage 12–13, stage 13–15 | |
| ChIP-chip | Drosophila | Bagpipe | Whole embryo | WT | Stage 10–11 | |
| ChIP-chip | Drosophila | Biniou | Whole embryo | WT | Stage 10–11, stage 12–13, stage 13–15 | |
| ChIP-chip | Drosophila | Dorsocross, Pannier, dTCF, and pMad | Whole embryo | WT | Stage 8–9, stage 10–11 | Junion et al., 2012 |
| BiTS-ChIP-seq | Drosophila | Mef2, Rpb3-Pol II | Mesoderm | WT | stages 10–11 | Bonn et al., 2012 |
| ChIP-seq | Drosophila | Mef2 | Whole embryo | WT | ||
| ChIP-seq | Drosophila melanogaster and Drosophila virilis | Twist | Whole embryo | WT | Stage 5–7, stage 8–9, stage 10–11 | Khoueiry et al., 2017 |
| Tinman | Stage 8–9, stage 10–11 | |||||
| Mef2 | Stage 5–7, stage 8–9, stage 10–11 stage 12–13, stage 13–15 | |||||
| Bagpipe | Stage 10–11 | |||||
| Biniou | Stage 10–11, stage 12–13, stage 13–15 | |||||
| ChIP-seq | Mouse | P300 | Heart | WT | E11.5 | Blow et al., 2010 |
| ChIP-seq | Mouse | P300 | Heart | WT | P2 | May et al., 2012 |
| ChIP-seq | Mouse | GATA4 (flag or biotin-tagged) | Ventricle | WT | E12.5 | He et al., 2014 |
| ChIP-seq | Mouse | GATA4 (flag or biotin epitope-tagged) | Ventricle | Normal, banding (surgically placed ligature around the aorta), sham | Adult | |
| ChIP-seq | Mouse | GATA4, TBX3, NKX2.5, P300 | Heart | WT | Adult | van den Boogaard et al., 2012 |
| ChIP-seq | Mouse | HAND2 (flag-tagged) | Limb bud, hearts, branchial arches | WT | E10.5 | Osterwalder et al., 2014 |
| ChIP-seq | Mouse | NKX2.5 | Heart | WT | E11.5 | Dupays et al., 2015 |
| ChIP-exo | Mouse | GATA4, NKX2.5, and TBX5 | ESCs, ESC-differentiated cells | WT, NKX2.5 KO, TBX5 KO, double KO | cardiac precursors and cardiomyocytes | Luna-Zurita et al., 2016 |
| ChIP-seq | Mouse | P300 (biotin-tagged) | Heart | WT | E12.5, Adult | Zhou et al., 2017 |
| ChIP-seq | Mouse | P300 (biotin-tagged) | Endocardial and endothelial cells in the heart | WT | Adult | |
| ChIP-seq | Mouse | CTCF | Left ventricle (isolated cardiomyocytes) | WT, CTCF KO | Adult | Rosa-Garrido et al., 2017 |
| ChIP-seq | Mouse | HAND2 (flag-tagged) | Heart | WT | E10.5 | Laurent et al., 2017 |
| ChIP-seq | Mouse | TBX20 (GFP-tagged) | Heart | WT | E11.5 | Boogerd et al., 2017 |
| ChIP-seq | Mouse | GATA4, HAND2 (3XTy1 tag), MEF2C (3XTy1 tag), TBX5 | iCLM (induced cardiac-like myocytes) reprogrammed from MEF | Transfected with GHMT, AGHMT or single factors | Day 2 in reprogramming | Hashimoto et al., 2019 |
| ChIP-seq | Mouse | GATA4, MEF2C (3XTy1 tag), TBX5 | iCLM (induced cardiac-like myocytes) reprogrammed from MEF | Transfected with GMT | Day 2 in reprogramming | |
| ChIP-seq | Mouse | GATA4, TBX5 | Ventricle | WT | P4 | |
| ChIP-seq | Mouse | MEF2A, MEF2C, NKX2.5, SRF, TBX5, TEAD1 (biotin -tagged) | Heart | WT | E12.5 | Akerberg et al., 2019 |
| ChIP-seq | Mouse | MEF2A, NKX2.5, SRF, TBX5, TEAD1 (biotin-tagged) | Heart | WT | Adult (P42) | |
| ChIP-seq | Human | NKX2.5, GATA4, TBX5, SRF, MEF2A, P300 (all TFs biotin-tagged) | HL1 cardiomyocyte cell line | WT | cell line | He et al., 2011 |
| ChIP-seq | Human | P300 | Heart | WT | Fetal (gestational week 16), adult | May et al., 2012 |
| ChIP-seq | Human | GATA4, TBX5, MED1 | iPSC-differentiated cells | WT, GATA4_G296S | iPS-derived cardiomyocytes | Ang et al., 2016 |
| ChIP-seq | Human | HEY2, NR2F2, and TBX5 | iPSC-differentiated cells | WT | cardiomyocytes | Churko et al., 2018 |
| ChIP-seq | Human | CTCF | ESCs, ESC-differentiated cells | WT | ESCs, mesodermal cells, cardiac mesodermal cells, cardiac progenitors, primitive cardiomyocytes, and ventricular cardiomyocytes | Zhang et al., 2019 |
Data from consortiums (ENCODE, FANTOM, and Roadmap Epigenomics Projects) are not listed. The table separates post translational histone modifications from TF/cofactor data. For each data type, the experiments are sorted by species first and then by publication date.
To demarcate the location of putative enhancers active in embryonic and adult hearts, pioneering mammalian studies performed ChIP-seq for the histone acetyltransferase EP300 and the active post-translational histone modification H3K27ac (Blow et al., 2010; May et al., 2012). To overcome the challenge of having to obtain specific antibodies for each TF of interest, many ChIP-seq studies have used tagging methods to biotinylate DNA binding proteins including EP300 and cardiac TFs to define heart enhancers in Drosophila (Bonn et al., 2012), mouse embryos (He et al., 2014; Zhou et al., 2017; Akerberg et al., 2019) and human cardiomyocyte cell lines (He et al., 2011). These biotin tagging-based approaches achieve more sensitive and reliable identifications of heart enhancers and enable enhancers discoveries in specific cardiac cell types (Zhou et al., 2017).
Like in Drosophila, the combinatorial binding of cardiac TFs defines mammalian heart enhancers (He et al., 2011; Akerberg et al., 2019). However, it is still unclear whether the mammalian cardiac enhancers discovered by these and other studies fit the “TF collective model” proposed for Drosophila; the “billboard model (Arnosti and Kulkarni, 2005),” in which specific sets of TFs are recruited to enhancers with flexible motif grammar; or a mixture of models (Long et al., 2016). For example, the importance of heterotypic interactions between mouse TBX5 and NKX2-5 was demonstrated using co-crystal structure together with DNA, as well as ChIP-exo experiments (Luna-Zurita et al., 2016). Intriguingly, the genetic loss of either Tbx5 or Nkx2-5 led to ectopic interactions of the other remaining TF. Unlike the more flexible “TF collective” or “billboard” models, TBX5 and NKX2-5 co-occupancy highlighted in this study featured preferred motif arrangements. Most recently, a novel single molecule footprinting (SMF) method was used to ascertain TF co-occupancy in mouse embryonic stem cells (Sönmezer et al., 2020). In this study, simultaneous TF binding did not depend on the identity of the TFs involved, and the co-occupancy of TFs on chromatin lacked of strict motif organization, which the authors proposed agreed with the “billboard model” (Sönmezer et al., 2020). Indeed, comparative approaches using this SMF method to study enhancer logic during metazoan cardiac development will be insightful for both learning general principles governing enhancer regulation as well as the biologically important exceptions that define key physiological processes.
To study cardiac enhancer dynamics across multiple stages of in vitro cardiac differentiation or in vivo development, several studies from individual labs as well as consortiums, have utilized robust genome-wide assays that do not rely on mapping specific transcription factors, namely ChIP-seq for histone modifications (Paige et al., 2012; Wamstad et al., 2012; Nord et al., 2013; Vanoudenhove et al., 2020), and DNase-seq and ATAC-seq for chromatin accessibility (Bertero et al., 2019; Gorkin et al., 2020; Meuleman et al., 2020). These studies revealed highly dynamic chromatin states accompanying cardiac differentiation and development. Specifically, ATAC-seq is widely used on precious in vivo cardiac samples to identify genomic regions that are enriched for TF binding and functional enhancer elements (Jia et al., 2018; Yuan et al., 2018; Pawlak et al., 2019; Racioppi et al., 2019). Recently, accessible chromatin profiling has also enabled the discovery of enhancers specific to cardiac subpopulations, such as pacemaker cells (Galang et al., 2020; van Eif et al., 2020) and endocardial populations (Boogerd et al., 2017).
Functional insights into cardiac enhancer regions continue to be made by studying TF occupancy and chromatin states upon the perturbation of cardiac TFs or signaling pathways in multiple organisms (e.g., Gata4, gata5, Nkx2.5, Tbx5/tbx5, Tbx20, Hand2/hand2, Isl1, Foxf, Fgfr, Mek, and Ras) (He et al., 2014; Luna-Zurita et al., 2016; Boogerd et al., 2017; Jia et al., 2018; Pawlak et al., 2019; Racioppi et al., 2019), as well as in a human congenital heart disease (CHD) model (cardiomyocytes with a disease-associated missense mutation of GATA4) (Ang et al., 2016). These studies reveal the master regulatory roles of cardiac TFs at the chromatin level. For example, GATA4 is essential for establishing open chromatin, promoting active epigenetic modification (H3K27ac) and recruiting TBX5 to the proper cardiac enhancers (He et al., 2014; Ang et al., 2016). On the other hand, TBX5 and NKX2.5 are important for preventing ectopic binding of GATA4 during cardiac differentiation, highlighting the importance of interdependent co-occupancy of these cardiac TFs in precisely controlling cardiac gene expression (Luna-Zurita et al., 2016). This interdependent co-occupancy is also essential in cardiac reprogramming, as only co-expression of cardiac factor cocktails (GATA4, HAND2, TBX5, MEF2C, etc.), but not single-TF overexpression, can leads to robust cardiac TF occupancy to reprogramming enhancers (Hashimoto et al., 2019).
Heart Enhancers in Space: Chromatin Interactions and Architectures
Heart enhancer activity not only requires proper TF binding, but is under the control of local chromatin interactions and higher-order chromatin architectures. Several groups have conducted promoter capture Hi-C in ESC/iPSC-derived cardiomyocytes or adult hearts to map enhancer-promoter interactions (Choy et al., 2018; Montefiori et al., 2018; Jung et al., 2019). These promoter capture Hi-C studies identified potential target genes for a substantial fraction of candidate heart enhancers. Interestingly, on average 25–35 distal interacting regions per gene and 40–60% of distal regions interacting with more than one gene. Hi-C has also been recently used to profile high-order chromatin architectures such as TADs and compartments across closely sampled time points during the differentiation from stem cells to cardiomyocytes (Bertero et al., 2019; Zhang et al., 2019). These studies showed extensive rearrangement of chromatin architectures during cardiac cell differentiation, with 19% genome switching compartments and 20–40% of TADs being stage-specific. Integrated analyses based on these datasets also revealed important regulatory mechanisms and unknown regulators in heart development. For example, Bertero et al. (2019) detected spatial coalescence of multiple cardiac genes from different chromosomes. This coalescence formed a trans-interacting chromatin domain that recruited the muscle-specific splicing factor RBM20 for efficient pre-mRNA splicing (Bertero et al., 2019).
The importance of chromatin interactions and architecture in heart development and function is also revealed by the essentiality of genome organizing factors such as CTCF and the cohesin complex. CTCF knock-out in cardiac progenitor cells leads to severe defects in cardiac cell maturation due to the disruption of enhancer-promoter interaction and subsequent misregulation of cardiac genes (Gomez-Velazquez et al., 2017). In the adult heart, CTCF depletion is sufficient to induce pathological consequences that are very similar to heart failure (Rosa-Garrido et al., 2017). Knock-out of Stag2 (which encodes a cohesin subunit) in embryonic mice leads to lethality by E10.5 due to severe morphogenesis defects in SHF-derived structures (right ventricle, outflow tract and septation), however, loss of Stag2 in adults only moderately reduces their fitness, indicating a strong developmental role of Stag2 (De Koninck et al., 2020). Perturbation of the cohesin loading factor NIPBL in both mouse and zebrafish results in multi-organ defects (including heart abnormalities) reminiscent to the Cornelia de Lange Syndrome, a congenital disease linked to NIPBL mutation (Kawauchi et al., 2009; Muto et al., 2011; Santos et al., 2016). The different phenotypes observed upon the loss of cohesin complex members, cohesin associated loading proteins, and CTCF indicate that in addition to their roles in sister chromatid cohesion and chromatin organization (Merkenschlager and Nora, 2016; Hanssen et al., 2017; Pugacheva et al., 2020), there are likely more subtle and CTCF-independent roles (i.e., Schmidt et al., 2010a) for these proteins in cardiac gene regulation.
Enhancing Enhancers With Enhancer-Associated RNAs
Upon activation, many enhancers are transcribed into non-coding RNAs, which are broadly referred to as enhancer RNAs (eRNAs). The expression of eRNAs is well correlated with their putative target gene expression (Kim et al., 2010; Kaikkonen et al., 2013; Li et al., 2013; Andersson et al., 2014; Arner et al., 2015). eRNAs may not only serve as hallmarks of enhancer activation, but also exert important functions in driving target gene expression by promoting chromatin accessibility (Mousavi et al., 2013), mediating enhancer-promoter interaction (Lai et al., 2013; Li et al., 2013; Hsieh et al., 2014), regulating chromatin remodeling (Kaikkonen et al., 2013), and facilitating PolII pause-release at promoters (Schaukowitch et al., 2014; Shii et al., 2017). However, for the vast majority of eRNAs, it remains unclear whether they are simply by-products of enhancer transcription or whether they possess functional roles based on the transcriptional process itself, or through additional molecular interactions in cis or in trans (reviewed in Li et al., 2016; Arnold et al., 2020).
Though early discoveries described eRNAs as short, non-polyadenylated, bidirectionally transcribed RNAs (Kim et al., 2010; Andersson et al., 2014), a diverse group of molecules with other structures (long, polyadenylated, or unidirectionally transcribed) have been attributed to eRNAs (Koch et al., 2011; Kaikkonen et al., 2013; Li et al., 2013; Alvarez-Dominguez et al., 2017). The structure and functional similarities between some eRNAs and cis-acting long non-coding RNAs (lncRNAs) have raised an emerging concept that they represent overlapping categories of regulatory non-coding RNAs (ncRNAs) (Espinosa, 2016; Paralkar et al., 2016; Arnold et al., 2020; Gil and Ulitsky, 2020).
The roles of eRNAs and lncRNAs in the contexts of heart development have been explored by many studies (Grote et al., 2013; Klattenhoff et al., 2013; Ounzain et al., 2014, 2015; Anderson et al., 2016; Alexanian et al., 2017; Yang et al., 2017; Turton et al., 2019; Nicole Ritter et al., 2019). Given the challenge in categorizing these ncRNAs, we consider all ncRNAs that are associated with heart enhancers and discuss the different ways through which they may regulate heart development using two examples: (1) The ncRNA transcript itself is involved in target gene regulation. For example, using anti-sense mediated RNA knockdown, Yang et al. (2017) showed that the expression of Ryr2, a TBX5 target that is critical for maintaining cardiac rhythm, depends on a novel TBX5-dependent eRNA, RACER; and (2) Instead of the ncRNA molecule, it is the transcriptional activity of the ncRNA locus that appears to be important for controlling the target genes. Two such ncRNAs come from the Hand2 locus, upperhand (Uph) (Anderson et al., 2016) and handsdown (Hdn) (Nicole Ritter et al., 2019). Particularly, the Hdn locus interacts with the Hand2 promoter and putative cardiac enhancers, suggesting it may regulate Hand2 expression via direct chromatin interaction, reminiscent of CREs (Nicole Ritter et al., 2019). The transcription of Uph over a cardiac enhancer upstream of Hand2 allows the binding of GATA4 and deposition of H3K27ac to this enhancer (Anderson et al., 2016). Together, these examples showcase a few models of the complex interactions between enhancers and the ncRNAs associated with them.
The field of enhancer-associated ncRNAs in heart development has many unanswered questions. Future studies that use chromatin run-on assays (GRO-seq, PRO-seq) or generate deeply sequenced RNA-seq datasets coupled with enhancer annotations should help to understand the dynamic changes of eRNAs in development. Functional experiments such as those use RNA targeting Cas protein (Cas13) (Abudayyeh et al., 2017), shRNA (Lambeth and Smith, 2013), or antisense oligonucleotide-mediated knockdown (Dias and Stein, 2002) will also be essential for teasing out the roles of enhancer-associated ncRNAs in gene-regulation independent of the enhancer elements themselves.
Heart Enhancers: Keeping Track of Time
As the activity of enhancers are not only tissue-specific but also stage-specific, it is important to obtain high-resolution temporal profiles of heart enhancers to truly understand their function. This is specifically highlighted by the in vitro cardiac differentiation study from Wamstad et al. (2012), which showed that enhancers active in ESC, mesoderm progenitors, cardiac progenitors, and cardiomyocytes were largely non-overlapping (Wamstad et al., 2012). Consistently, Luna-Zurita et al. (2016) discovered thousands of GATA4, NKX2.5, and TBX5 binding sites were specific to either cardiac progenitor cells or cardiomyocytes. Similar results have also been reported for in vivo development, for example, 80% of the GATA4 binding sites in fetal heart are not occupied by GATA4 in adult heart (He et al., 2014).
Since the heart is the first organ formed in embryogenesis, the embryonic stage that is required to capture the initial phase of cardiogenesis is especially early in development, and is likely during early gastrulation (Scott, 2012; Devine et al., 2014; Lescroart et al., 2014). Though heart enhancers have been extensively characterized across many developmental stages in various species [such as Nord et al. (2013) and Vanoudenhove et al. (2020) and many others in Tables 3–5], there is a paucity of datasets that characterize enhancers active at the initial stage of vertebrate heart development, such as the transition from mesoderm progenitors to cardiac lineages. The majority of in vivo studies in vertebrates used relatively mature cardiac samples, including embryonic hearts with defined chamber structures (e.g., E10.5 and onward in mice) or postnatal heart tissues (Tables 3–5). As these stages are later than when cardiac lineage commitment occurs, these studies may not capture the enhancers that specifically drive early cardiogenesis.
TABLE 5.
Chromatin accessibility datasets used for annotating heart enhancers.
| Methods | Species | Samples | Condition | Stage | References |
| ATAC-seq | Ciona | B7.5 lineage | WT | 6 hpf (native mesoderm),18 hpf (committed heart and pharyngeal muscle precursors) | Racioppi et al., 2019 |
| ATAC-seq | Ciona | B7.5 lineage | WT, Fgfr dominant-negative, Mek constitutively active, Foxf-CRISPR, M-Ras constitutively active | 10 hpf (multipotent cardiopharyngeal progenitors) | |
| ATAC-seq | Zebrafish | myl7:GFP+ cardiomyocytes | WT, gata5 -/-, hand2 -/-, tbx5 -/- mutants | 72 hpf | Pawlak et al., 2019 |
| ATAC-seq | Mouse | Heart | WT | E12.5 | Zhou et al., 2017 |
| ATAC-seq | Mouse | Heart | WT | P1, P14, P56 | Quaife-Ryan et al., 2017 |
| ATAC-seq | Mouse | endocardial cells | WT and TBX20 KO | E12.5 | Boogerd et al., 2017 |
| ATAC-seq | Mouse | Nkx2-5+ cardiac progenitor cells | WT | E7.5, E8.5, E9.5 | Jia et al., 2018 |
| ATAC-seq | Mouse | Isl1+ cardiac progenitor cells | WT and Isl1 KO | E8.5, E9.5 | |
| ATAC-seq | Mouse | Isl1+/CD31+, Isl1+/CD31- cardiac progenitor cells | WT | E8.5, E9.5 | |
| ATAC-seq | Mouse | Isl1+ cardiac progenitor cells | Nkx2.5 overexpression in Isl1+ cells | E9.5, E12.5 | |
| Single-cell ATAC-seq | Mouse | Isl1+ cardiac progenitor cells | WT | E8.5, E9.5 | |
| Omni-ATAC-seq | Mouse | Heart | WT | Adult | Liu et al., 2019 |
| ATAC-seq | Mouse | Ventricle cardiomyocytes | WT | E12.5 | Akerberg et al., 2019 |
| ATAC-seq | Mouse | Cardiac pacemaker cells (PCs), right atrial cardiomyocytes (RACMs) | WT | Neonatal (P0-P2) | Galang et al., 2020 |
| Single-cell ATAC-seq | Mouse | Ventricle | myocardial infarction (MI) or sham surgeries | P1, P8 (3days post surgeries for both) | Wang et al., 2020 |
| ATAC-seq | Human | ESCs, ESC-differentiated cells | WT | ESCs, mid primitive streak, lateral mesoderm, cardiac mesoderm | Loh et al., 2016 |
| ATAC-seq | Human | iPSC-differentiated cells | WT, GATA4_G296S | iPS-derived cardiac progenitor cells | Ang et al., 2016 |
| ATAC-seq | Human | ESCs, ESC-differentiated cells, iPSCs, iPSC-differentiated cells | WT | ESCs and iPSCs, mesoderm, cardiac mesoderm, cardiomyocyte | Liu et al., 2017 |
| ATAC-seq | Human | ESCs, ESC-differentiated cells, iPSCs, iPSC-differentiated cells | Control and INN (isotretinoin) treatment | ESCs and iPSCs, mesoderm, cardiac mesoderm | Liu et al., 2018 |
| ATAC-seq | Human | ESCs, ESC-differentiated cells | WT | ESCs, mesoderm, cardiac progenitors, cardiomyocytes | Bertero et al., 2019 |
| ATAC-seq | Human | ESC-differentiated sinoatrial node-like pacemaker cells (SANLPC), ventricle-like cardiomyocytes (VLCM), | WT | ESC-differentiated cardiomyocytes | van Eif et al., 2020 |
Data from large consortiums (ENCODE, FANTOM, and Roadmap Epigenomics Projects) are not listed. Datasets are sorted by species first and then by publication dates.
TABLE 4.
Chromatin interaction datasets used for annotating heart enhancers.
| Methods | Species | Sample | Condition | Stage | References |
| Hi-C | Mouse | Left ventricle (isolated cardiomyocytes) | Control, Transverse Aortic Constriction, CTCF KO | Adult | Rosa-Garrido et al., 2017 |
| Hi-C | Human | ESCs, ESC-differentiated cells | WT | ESC-derived mesendoderm cells | Dixon et al., 2015 |
| Hi-C | Human | Left ventricle | WT | Adult | Leung et al., 2015 |
| Hi-C | Human | Right ventricle | WT | Adult | Schmitt et al., 2016 |
| PCHi-C | Human | iPSCs, iPSC-differentiated cells | WT | iPSC, iPSC-derived cardiomyocytes | Montefiori et al., 2018 |
| PCHi-C | Human | ESCs, ESC-differentiated cells | WT | ESC-derived cardiomyocytes | Choy et al., 2018 |
| PCHi-C | Human | Left ventricle | WT | adult | Jung et al., 2019 |
| Hi-C | Human | ESCs, ESC-differentiated cells, iPSCs, iPSC-differentiated cells | WT | ESCs, iPSCs, mesoderm, cardiac progenitors, cardiomyocytes, fetal heart | Bertero et al., 2019 |
| Hi-C | Human | ESCs, ESC-differentiated cells | WT | ESCs, mesodermal cells, cardiac mesodermal cells, cardiac progenitors, primitive cardiomyocytes, and ventricular cardiomyocytes | Zhang et al., 2019 |
Data from large consortiums (ENCODE, FANTOM, and Roadmap Epigenomics Projects) are not listed. Datasets are sorted by species first and then by publication dates.
A few recent in vivo studies confirm the observations made from in vitro differentiation that enhancer-associated chromatin states are highly dynamic, especially during early cardiac lineage specification. A recent study that profiled mouse Nkx2.5+ cardiac progenitor cells revealed major changes in chromatin accessibility between E7.5 and E8.5 but only minor differences between E8.5 and E9.5 (Jia et al., 2018). This suggests that early lineage fate transitions may be accompanied by major changes of chromatin states, which become more stabilized in committed cell types. Similar trends are observed in cardiopharyngeal lineage specification in the tunicate Ciona, in which most significant chromatin changes occur between the transition from mesoderm progenitors to cardiopharyngeal progenitors compared to later stages (Racioppi et al., 2019). These examples reveal intriguing dynamics of the enhancers involved in early cardiac lineage decisions, however, much remains to be explored. Filling this knowledge gap, especially in the context of developing embryos, can bring valuable insights into key cellular events in early cardiogenesis.
Evolutionary Mysteries of Heart Enhancers
Intriguing results have emerged from evolutionary studies of heart enhancers. Although the TFs controlling heart enhancers are highly conserved, validated heart enhancers show weak DNA constraint compared to brain enhancers identified at the same developmental stage (E11.5) (Blow et al., 2010). For instance, only 6% of the candidate heart enhancers were deemed to possess high DNA constraint (phastCon score > 600) compared to 44% of forebrain, 39% of midbrain, and 30% of limb enhancers. This could be in part due to the fact that molecularly, the brain seems to be a more conserved organ in terms of the low proportion of positively select genes, old phylogenetic ages of the transcriptomes, and the low percentage of genes showing trajectory changes between different species (Cardoso-Moreira et al., 2019).
It remains an open and intriguing question how heart enhancers that lack evolutionary conservation work together with many conserved cardiac TFs to orchestrate the development of the heart. Several reasons may contribute to this phenomenon. First, it has been demonstrated by many studies that enhancers are rapidly evolving with pervasive turnovers of TF binding sites (TFBSs) (Kunarso et al., 2010; Mikkelsen et al., 2010; Schmidt et al., 2010b; Cotney et al., 2013; Paris et al., 2013; Arnold et al., 2014; Ballester et al., 2014; Villar et al., 2015; Khoueiry et al., 2017). The rapid changes in the sequence, orientation, spacing and numbers of TFBSs within enhancers may not necessarily alter the functional roles of enhancers but do make it hard to detect enhancer sequence homology via genomic sequence alignment. As a consequence, some functionally conserved enhancers will not share detectable sequence homology. A recent and striking example is a sponge Islet enhancer, which drives expression that overlaps endogenous islet gene (isl2a) expression in zebrafish, despite the absence of homologous sequence in the vertebrate genomes. Nevertheless, enhancers with similar TFBS compositions can be found in human and mouse ISLET/Islet regions and their activities resemble that of the sponge enhancer in zebrafish (Wong et al., 2020). A similar strategy based on motif composition also identified conserved brain enhancers between chordates and hemichordates, which would not have been detected by sequence alignment alone (Yao et al., 2016). These two examples and many others i.e. (Fisher et al., 2006; Hare et al., 2008; Friedli et al., 2010; Chatterjee et al., 2011) indicate that a grammar more flexible than strict sequence conservation is used in some enhancers to produce conserved transcriptional “output.” Overall, the discordance between sequence and functional conservation may account for a significant portion of the weakly conserved heart enhancers.
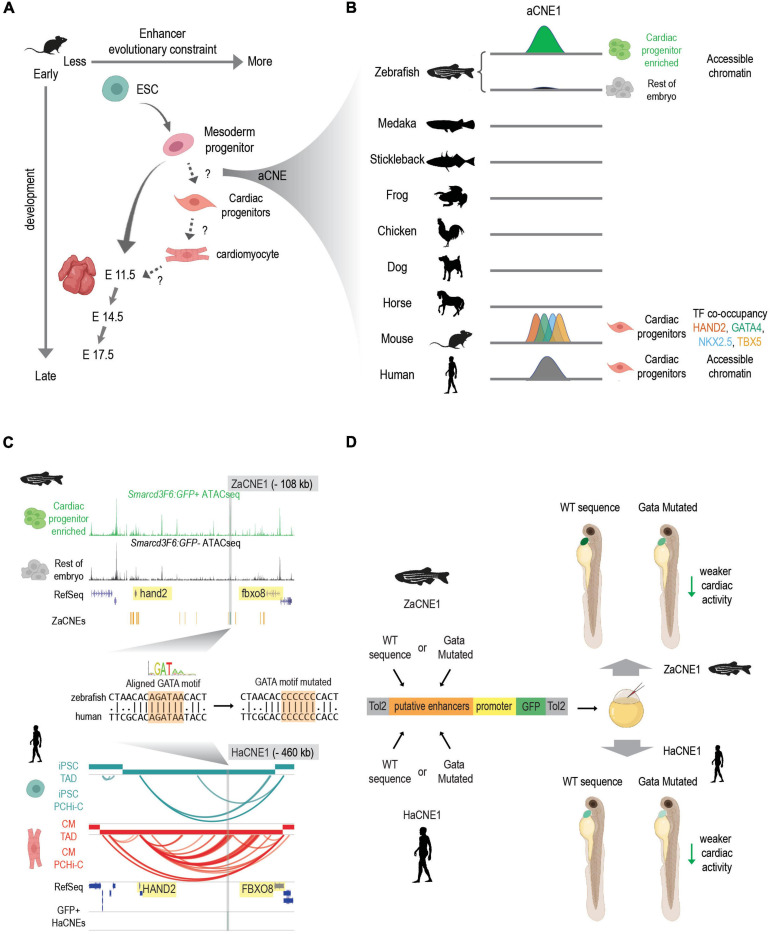
Second, an increasing number of studies indicate that the conservation of enhancers active in early embryonic development follows an hour-glass like pattern (Bogdanovic et al., 2012; Bogdanović et al., 2016; Liu et al., 2020) similar to that of transcriptomes (Irie and Sehara-Fujisawa, 2007; Domazet-Lošo and Tautz, 2010; Kalinka et al., 2010; Irie and Kuratani, 2011; Yanai et al., 2011). However, much less is known about “phylotypic enhancers” that presumably are established prior to organogenesis to set up conserved vertebrate gene expression patterns. A temporal study of developmental enhancers compared the H3K27ac (a mark of active enhancers) profiles across the development of three mouse tissues (heart, brain, and liver) from ESC to adults (Nord et al., 2013). They showed that both sequence constraints (PhastCon scores) and evolutionary ages of candidate active enhancers peak at different developmental stages in different tissues. Though enhancers active in the brain show the highest conservation at E11.5, heart enhancers active at mouse E11.5 are less conserved compared to those active during earlier cardiac lineage specification (Figure 2A). This suggests that although enhancer turnover is a typical property of heart enhancers, deeply conserved CREs are more likely to be active in early cardiogenesis or even prior to cardiac lineage commitment.
FIGURE 2.
Discovering conserved heart enhancers during early heart development: a case study. (A) Enhancers that are active at different stages of heart development show different evolutionary constraints. In mouse, enhancers that are active in mesoderm progenitors show higher sequence conservation than enhancers active in ESC and E11.5 embryonic hearts. But conservation levels of enhancers that active during the transition of mesoderm progenitors to cardiac progenitors and cardiac progenitors to cardiomyocytes remain less characterized. aCNEs, the accessible chromatin shared between zebrafish and human (or zebrafish and mouse) were identified within the mesoderm to cardiac progenitor transition (Yuan et al., 2018). Schematics generated based on Figure 5 (Nord et al., 2013). (B) Schematics showing sequence homology and shared enhancer signatures for aCNE1 locus across multiple species. aCNE1 was first discovered as an accessible chromatin region specific for an early cardiac progenitor-enriched population in zebrafish. Gray lines indicate the existence of orthologous sequences to aCNE1 in the given species (based on CNEs identified in Hiller et al., 2013). In mouse, aCNE1 regions are co-occupied by multiple cardiac TFs in cardiac cells (based on data from Luna-Zurita et al., 2016; Laurent et al., 2017). Human aCNE1 region shows chromatin accessibility in cardiac progenitor cells (based on data from Paige et al., 2012). The stickleback and the frog icons were created by Milton Tan and Soledad Miranda-Rottmann, respectively, and shared through (http://phylopic.org/) under the following license (https://creativecommons.org/licenses/by/3.0/). (C) Genome browser view of aCNE1 in zebrafish (ZaCNE1) and human (HaCNE1) genome. aCNE1 is located 108 kb upstream of hand2 in the zebrafish genome and 406 kb upstream of HAND2 in the human genome. Yellow boxes highlight the genes flanking aCNE1, indicating the conserved synteny that aCNE1 resides in. ATAC-seq data from Yuan et al. (2018) is plotted for ZaCNE1 and promoter capture Hi-C data from Montefiori et al. (2018) is plotted for HaCNE1. Note that aCNE1 display conserved cardiac-specific activity in both zebrafish (accessibility) and human (interacting with cardiac gene HAND2). ZaCNE1 and HaCNE1 shares an aligned GATA motif, the mutation of which can be used to determine if the activity of aCNE1 depends on this GATA motif. (D) Functional enhancer assays of WT and GATA motif mutated zebrafish and human aCNE1 sequence in zebrafish embryos. Candidate sequences are cloned into an enhancer vector to drive GFP expression. The whole cassette will be chromatinized after injecting into zebrafish embryos. For both ZaCNE1 and HaCNE1, GATA motif mutation leads to decreased enhancer activity compared to the respective WT sequences. This example illustrates that human and zebrafish aCNE1 share conserved activity and regulation despite less than 60% sequence identity. Schematics generated based on data from Yuan et al. (2018). Parts of this figure were created with BioRender.com.
To explore the existence of pre-cardiac enhancers that could contribute to the initiation of cardiac gene regulatory networks, we recently characterized the open chromatin landscape of a cardiac-enriched population in zebrafish embryos before the expression of the canonical cardiac marker nkx2.5 (Yuan et al., 2018). This approach allowed us to detect cardiac CREs that were primed early in development prior to cardiac lineage commitment. We present this work in Figure 2 as a general example of how comparative genomic resources in combination with epigenomic profiling in two or more species can give insight into functionally conserved developmental enhancers. To determine to what extent deeply conserved CREs were involved in early heart development we exploited conserved non-coding element (CNE) datasets established using both direct alignment and indirect approaches (Hiller et al., 2013; Braasch et al., 2016) and found more than 160 human-zebrafish conserved candidate heart enhancers (referred to as aCNEs). Though most of these aCNEs remain to be tested in vivo, the majority of the aCNEs tested (15/18) drive robust cardiac expression in zebrafish. This example illustrates a comparative strategy for discovering early heart enhancers underscores that at least some of the regulatory logic driving vertebrate heart development can be found in orthologous sequences shared between humans and fish.
In sum, despite the overall rapid evolution of heart enhancers, a small fraction of deeply conserved heart enhancers likely contributes to the regulation of early cardiogenesis. The lack of overt sequence conservation in heart enhancers may be partially due to the rapid turnover of TFBSs. On the other hand, variants in heart enhancers that alter gene expression are likely to contribute to morphological differences of cardiac structures between species.
Heart Enhancers: One Cell at a Time
Currently, most of the data for annotating heart enhancers was generated at the bulk population level (Tables 3–5); however, both in vitro differentiated cardiac cells and animal hearts contain heterogeneous populations (reviewed in Paik et al., 2020). This was largely due to the challenges in isolating closely related developmental lineages and collecting enough material from early embryos for enhancer profiling. But as enhancer activity is highly context-specific, the existing data bias likely limits the discoveries of enhancers that are active only in specific subpopulations (e.g., SHF progenitors, endocardial cells, cardiac smooth muscle cells, etc.) or at certain stages.
Rapid advances in single-cell genomics techniques have brought unprecedented opportunities to circumvent the difficulties in cell type isolation. Specifically, single-cell ATAC-seq (scATAC-seq) has become more and more commonly used in delineating cell-type-specific CREs within diverse cellular populations (Buenrostro et al., 2015; Cusanovich et al., 2015). scATAC-seq of Isl1+ cells from E8.5 and E9.5 mouse embryonic hearts revealed the TF regulators involved in the different stages of two distinct developmental trajectories, the cardiomyocyte and endothelial trajectories (Jia et al., 2018). More recently, scATAC-seq of neonatal hearts post-injury uncovered previously uncharacterized TFs that potentially regulate specific cell types in mammalian heart regeneration and decoded the cis and trans regulators underlying regenerative and non-regenerative injury responses (Wang et al., 2020). Moreover, large single-cell atlases of chromatin accessibility have been generated for 13 adult mouse organs (∼100,000 nuclei) and 15 fetal human tissues (∼800,000 nuclei), illustrating the regulatory programs that define the cell repertoire for many mammalian organs including the heart (Cusanovich et al., 2018a; Domcke et al., 2020). Embryonic single-cell accessible chromatin landscapes have been profiled for E8.25 mouse embryos (∼19,000 nuclei) and Drosophila embryos (∼20,000 nuclei) spanning early blastoderm to terminally differentiated lineages (Cusanovich et al., 2018b; Pijuan-Sala et al., 2020). As all the above studies provide a variety of processed data and interactive web sessions for convenient exploration of the chromatin accessibility of one’s favorite genes or loci, they can be very useful resources for exploring cell type-specific cardiac enhancers.
Furthermore, with single-cell multimodal omics being selected as the Methods of the Year 2019 (Nature Methods, 2020), techniques for simultaneous measuring multiple modalities in the same single cells are blooming rapidly. Related to epigenomics, it has become possible to simultaneous profile accessible chromatin and transcriptome (Cao et al., 2018; Chen et al., 2019; Li et al., 2019; Moudgil et al., 2020), methylome and transcriptome (Angermueller et al., 2016), methylome and chromatin conformation (Li et al., 2019), or even three modalities altogether (Pott, 2017; Clark et al., 2018) within the same cells. The combinatorial use of single-cell epigenomics techniques on cardiac samples will potentially provide a holistic view of enhancer activities in all subtypes of cardiac cells across all stages in heart development. The multi-omics measurements not only enable a more comprehensive and accurate delineation of the state of the single cells but also provide unique opportunities in identifying the potential causal factors across multiple regulatory layers, by correlating changes from genetic, epigenetic, or chromatin conformation levels to the gene expression differences. Although technology and analytic challenges still lie ahead, the application of single-cell epigenomics, especially the multi-omics approaches, into heart development, will likely transform the way that we study and understand heart enhancers and cardiac gene regulatory networks.
Computing Heart Enhancers
With the rapid accumulation of hundreds of epigenomic and transcriptomic datasets from cardiac tissues, efforts have been made toward compiling them and extract sequence features from known cardiac enhancers to predict unknown ones. Dickel et al. (2016) conducted an integrative analysis of over 35 genome-wide H3K27ac or P300 profiles from mouse or human heart samples to compile a compendium of more than 80,000 heart enhancers, which serves as one of the most comprehensive putative heart enhancer lists available to date. The abundance of genomics datasets and the growing number of in vivo validated heart enhancers also provide ample input for building computational models for novel heart enhancer prediction. One kind of model is purely based on the sequence features of the gold standard heart enhancers experimentally validated in vivo. For example, Narlikar et al. (2010) combined motif discovery, Markov sequence feature characterization, and linear regression to build a heart enhancer classifier from ∼70 validated heart enhancers. They used this classifier to discover more than 40,000 putative heart enhancers within the conserved CNEs in the human genome, with an in vivo validation rate > 60% (Narlikar et al., 2010). By comparing validated cardiac and non-cardiac enhancer sequences from Drosophila, Jin et al. (2013) identified a novel motif as a classifier for heart enhancer prediction. They further showed that this motif was essential for driving cardiac activity in 3/8 enhancers tested. One widely used sequence-based machine learning method, gapped k-mer support-vector-machine (gkm-SVM) (Ghandi et al., 2014), has been applied to learn the sequence features from previously identified open chromatin regions. It predicted an addition of 80,000 putative cardiac CREs and the cognate TFs that bind to them (Lee et al., 2018).
Several studies have explored how including different genomics features in training models could affect their performance in enhancer prediction. A study in Drosophila added ChIP signals on top of sequence motifs into their classifiers and found this combined strategy significantly boosted the prediction accuracy of cell-type-specific cardiac enhancers than motif sequence alone (Ahmad et al., 2014). By further including ChIP data for a larger set of cardiac TFs and histone modifications, their updated model was able to distinguish enhancers active in distinct subpopulations of cardiac cells and pericardial cells in Drosophila embryos (Busser et al., 2015). Similarly, Akerberg et al. (2019) took advantage of the variety of ChIP-seq data that they generated for mouse hearts and compared the performance of different chromatin features (open chromatin, H3K27ac histone modification, cardiac TF occupancy) alone or combined in predicting heart enhancers. They found open chromatin had high sensitivity while TF binding profiles yielded high precision in enhancer prediction. Ultimately, the number of co-bound cardiac TFs turned out to be the most important classifier in heart enhancer prediction compared to signal intensities (Akerberg et al., 2019). With the rapid evolvement of the machine learning field, computational classification and predictions will become an important component that is complementary to experimental data in heart enhancer characterization. The two strategies will benefit from the advancement of each other and together expand our understanding of enhancer biology.
Heart Enhancers in Cardiovascular Disease
Heart diseases are a leading cause of death worldwide (Mozaffarian et al., 2015). As the most prevalent human birth defects, congenital heart disease (CHD) affects roughly 0.8% of newborns (Fahed et al., 2013). Though disruption of a set of developmental and structural genes have been recognized as the causes of a portion of CHD, the genetic factors underlying a large number of cases remain ambiguous (Fahed et al., 2013; Barnett and Postma, 2015; Postma et al., 2015; Richter et al., 2020). Genome-wide association studies (GWAS) have been carried out to identify the underlying genetic causes of a wide range of cardiovascular phenotypes and diseases, including CHD, cardiac arrest, coronary artery disease (CAD), cardiac arrhythmia, cardiomyopathy, and myocardial infarction (Arking et al., 2014; Nikpay et al., 2015; Eppinga et al., 2016; Nelson et al., 2017). Currently, thousands of variants have been implicated in heart-related disease risks (NHGRI GWAS catalog1).
Whole-genome sequencing (WGS) is becoming the method of choice for discovering de novo variants in CHD. Supporting the use of WGS for discovering molecular mechanisms underlying CHD, a recent study illustrated that the potential contribution from disruptive non-coding variants was at least as high as that from coding-variants (Richter et al., 2020). However, several factors complicate the functional annotation of disease-associated non-coding variants (Zhang and Lupski, 2015). In the case of common genetic variation associated with CHD-related phenotypes uncovered by GWAS, the tagged SNPs used will be in linkage disequilibrium (LD) with other SNPs that may represent the true causal variant. Even if a likely pathogenic non-coding mutation or copy number variation is nominated, one must then ascertain when and where this change impacts development and disease. In the following section, we briefly review insights into heart enhancer function revealed by human genetic studies.
Connecting Non-coding Variants to Cardiovascular Diseases
Only a handful of non-coding variants linked to cardiovascular diseases have been functionally dissected (Table 6). Compared to studying the function of a protein coding gene mutation, the functional characterization of non-coding disease associated variants is challenging. An early example of this was done for a genetic variant on human chromosome 9p21 harboring multiple SNPs associated with myocardial infarction and CAD (reviewed by Samani and Schunkert, 2008). A large 70 kb deletion of the whole orthologous sequence in the mouse genome severely reduced the expression of the nearby cardiac genes (Cdkn2a/b) and affected aortic smooth muscle cell proliferation and senescence. Allele-specific analysis of Cdkn2b transcripts in the heterozygous mice revealed a lack of cis-acting enhancers as the main mechanism underlying Cdkn2b downregulation, suggesting this genetic susceptibility interval contains enhancers that could be affected by the discovered sequence polymorphisms (Visel et al., 2010). However, disruption of cis-regulatory elements is not the only mechanism that contributes to diseases risk. Other studies revealed that expression of the long non-coding RNA (lncRNA) ANRII, which resides in chromosome 9q21, was affected by several SNPs within this region, and ANRII, in turn, could regulate other genes involved in vascular cell proliferation, adhesion, apoptosis, and remodeling (Holdt et al., 2010; Congrains et al., 2012a, b).
TABLE 6.
Functionally characterized non-coding SNPs implicated in cardiovascular disease.
| SNP | SNP position | Gene(s) | Disease | Evidence | References |
| SNPs within a 58 kb interval, include cis-regulatory elements | chr 9p21 | Cdkn2a/b | coronary artery disease | Deletion of the mouse orthologous interval severely impairs Cdkn2a/b expression nearby through a cis-acting mechanism. | Visel et al., 2010 |
| chr12:114704515: G>T, overlaps a TBX5 enhancer | 90 kb downstream of TBX5 | TBX5 | Septal defects | The risk allele ablates the cardiac enhancer activity | Smemo et al., 2012 |
| rs118026695:A>G and g.4574C>deletion | NKX2.5 promoter | NKX2.5 | ventricular septal defect | Risk alleles significantly upregulate the promoter activity | Pang et al., 2012 |
| g.17483564C>T and g.17483576C>G | NKX2.5 enhancer, 10 kb upstream | NKX2.5 | ventricular septal defect | Conserved with mouse AR1 Nkx2.5 enhancer, risk alleles significantly decrease the enhancer activity | Huang et al., 2013 |
| rs12190287:C>G rs12524865:C>A overlap enhancers | 3′ UTR of TCF21 | TCF21 | coronary heart disease | The protective alleles disrupts AP-1 binding and enhancer-associated histone modification, leading to TCF21 expression changes. | Miller et al., 2013 |
| rs12190287:C>G, overlaps a miRNA binding site | 3′ UTR of TCF21 | TCF21 | coronary heart disease | The protective allele (G) changes TCF21 transcript structure and disrupts miR-224 binding and post-transcriptional repression mediated by this miRNA. TGF-b and PDGF-bb signaling act upstream of miR-224 mediated allele-specific expression. | Miller et al., 2014 |
| rs6801957:G>A, overlaps an enhancer | Intron of SCN10A | SCN5A | cardiac rhythm disorder | The enhancer interacts with the SCN5A promoter. The minor allele disrupts a Tbox binding site and impairs the enhancer activity in the cardiac conduction system. | van den Boogaard et al., 2012, 2014 |
| rs7539120:A>T | An upstream enhancer of NOS1AP | NOS1AP | QT interval variations | The risk allele leads to increased enhancer activity. Overexpression of NOS1AP result in altered electrophysiology in cardiomyocytes | Kapoor et al., 2014 |
| rs4897612:G>T | −137 in VNN1 promoter | VNN1 | HDL cholesterol levels | eQTL of VNN1, allele-specific transcriptional activity, chromatin accessibility, binding of nuclear protein including SP-1, | Kaskow et al., 2014 |
| rs2050153:G>A | −587 in VNN1 promoter | VNN1 | HDL cholesterol levels | eQTL of VNN1, allele-specific chromatin accessibility, methylation and chromatin condensation | |
| rs138912749:T>C overlaps a miRNA binding site | 3′ UTR of SHOX2 | SHOX2 | atrial fibrillation | The minor allele creates a functional binding site for miR-92b-5p, which leads to reduced expression of SHOX2. | Hoffmann et al., 2016 |
| rs6489956:C>T overlaps two miRNA binding sites | 3′ UTR of TBX5 | TBX5 | CHD susceptibility | The minor allele shows increased binding to miR-9/30a, which leads to reduced expression of TBX5 | Wang et al., 2017 |
| rs7373779, rs41312411, rs11710077, rs13097780, rs6801957 | SCN5A-SCN10A GWAS locus | SCN5A | QT interval variations | Allele-specific enhancer activity and nuclear factor binding. (More putative variants were identified other than these five representative ones) | Kapoor et al., 2019 |
Another well-studied example is rs12190287, a CAD-associated variant located within the 3′ UTR of the TCF21 gene. Two continuous studies together revealed a dual mechanism of this SNP in modulating TCF21 expression at both transcriptional and post-transcriptional levels (Miller et al., 2013, 2014). Overlapping a TCF21 enhancer, this variant causes dysregulation of TCF21 through allele-specific histone modifications (H3K4me1, H3K27ac, H3K27me1) and AP-1 factor (c-Jun, JunD, ATF3) binding. These allele-specific chromatin effects are further augmented upon PDGFR-β stimulation, which indicates that the vascular growth factor signaling also acts differently on this variant (Miller et al., 2013). Moreover, the same minor allele disrupts a miR-224 binding site within the 3′ UTR of TCF21, therefore, prevents the post-transcriptional repression of TCF21 mediated by this miRNA (Miller et al., 2014).
The ion channel genes SCN5A/SCN10A locus is another hotspot heavily loaded with variants linked to cardiac arrhythmia and conduction system disorders (Veerman et al., 2015). One cardiac arrhythmia-associated SNP rs6801957 is located within the intron of SCN10A but is encompassed by a human-mouse conserved enhancer that interacts with the nearby gene SCN5A (van den Boogaard et al., 2014). This variant, but not other variants in LD disrupts the binding of TBX3/TBX5 in vitro and reduces the activity of this enhancer in the cardiac conduct system (van den Boogaard et al., 2012). Overall, these variant-oriented studies revealed the molecular mechanisms through which single nucleotide substitutions could alter enhancer activity and lead to pathological gene expression.
Discovering Disruptive Non-coding Variants Near Cardiac Genes
The CREs controlling the expression of TFs (i.e., the regulators of the regulators) are prime candidate regions for discovering damaging mutations that lead to gene dosage-related phenotypes (van der Lee et al., 2020). Indeed, hypothesis driven dissection of enhancers near cardiac genes have revealed several examples of disease causing non-coding mutations that control haploinsufficient cardiac genes TBX5, NKX2.5, and SHOX2 (reviewed in Chung and Rajakumar, 2016; Steimle and Moskowitz, 2017; Li et al., 2018).
It had been known for over a decade that heterozygous mutations within TBX5 lead to Holt-Oram syndrome in humans (Basson et al., 1997, 1999) when Smemo et al. (2012) went searching for disease-causing enhancer mutations around the TBX5 gene in families with septal defects, the predominant cardiac defect of Holt-Oram syndrome. This study, which involved scanning more than 700 kb for conserved non-coding sequences revealed three enhancer elements which together recapitulated the endogenous TBX5 heart expression in developing mouse embryos. Targeted sequencing revealed homozygous mutations in one of the enhancer elements in individuals with, but not in family members without, the disease. Another targeted sequencing of the NKX2.5 locus in ventricular septal defect patients revealed novel variants within the NKX2.5 promoter and a known distal enhancer (AR1). These novel variants significantly altered the transcriptional activity of the Nkx2.5 promoter and AR1 enhancer in luciferase assays (Pang et al., 2012; Huang et al., 2013). These tour de force experiments illustrate the lengths one must go to implicate regulatory mutations as a disease causing mechanism, and demonstrates how understanding the molecular mechanisms underlying human disease can reveal fundamental biological insights in cardiac enhancer elements.
In addition to enhancers and promoters, non-coding regulatory variation can impact miRNA binding sites, lncRNAs, or even several of these functional elements at the same time. In principle this could occur by disrupting or creating TF/miRNA binding sites, changing chromatin states, mediating different responses to extracellular signaling, or affecting lncRNA expression which in turn can affect gene regulation in trans (Table 6). For example a variant associated with increased CHD susceptibility was identified within the 3′ UTR of TBX5. This variant was shown to increase the binding of two miRNAs with the minor allele leading to a significant reduction in the expression of TBX5 through transcriptional and translational regulation (Wang et al., 2017). NKX2.5 mutations have also been implicated in diverse types of CHD, including ventricular septal defects (reviewed in Chung and Rajakumar, 2016). Similarly, target sequencing of the SHOX2 region in atrial fibrillation (AF) patients identified an AF-associated SNP within the 3′ UTR. The 3′ UTR allele created a binding site for an mRNA miR-92b-5p, which significantly reduced the SHOX2 3′UTR reporter activity in a luciferase assay (Hoffmann et al., 2016).
While there are relatively few hard-won examples of non-coding mutations that explain the molecular mechanism behind CHD, it is clear that a comprehensive annotation of heart enhancer location and function will accelerate molecular-based diagnoses and our understand of heart gene regulation.
Interpreting Non-coding Variants With Genome-Wide Enhancer Annotation
With the burst of cardiac epigenomic datasets in the past decade, the interpretation of heart disease-associated variants has developed from susceptible locus-centric to a genome-wide manner. Continuous efforts have been made to first establish a comprehensive enhancer annotation and then use for the fine-mapping non-coding variants (Dickel et al., 2016; Choy et al., 2018; Montefiori et al., 2018). For example, the heart enhancer list that they curated from ChIP-seq datasets, Dickel et al. (2016) found more than 2000 enhancer-overlapping variants that were associated with heart phenotypes. When deleting two of the variant-containing enhancers that were upstream of cardiac structure genes (Myl7 and Myl2), they showed that both enhancers are required for normal cardiac gene expression, cardiomyocyte morphology, and heart functions. On top of enhancer identification, chromatin conformation capture assays are especially helpful for linking cardiac GWAS SNPs to their targeted genes. The promoter capture Hi-C datasets generated in differentiated cardiomyocytes arguably pinpoint the true target genes of many GWAS and LD SNPs, some of which were different from the target genes proposed based on proximity (Choy et al., 2018; Montefiori et al., 2018). Remarkably, Montefiori et al. (2018) reported that 90% of the SNP-gene interactions skipped at least one gene promoter, arguing against the intuitive approach of assigning SNPs to their neighboring genes when interpreting possible causal mechanisms. In line with the cell-type-specificity of enhancer activities, the interaction networks identified using cardiomyocyte promoter capture Hi-C data turned out to be most informative to interpret cardiac arrhythmia phenotypes (which directly results from cardiomyocyte dysfunction) as compared to CHD, CAD, heart failure, and myocardial infarction (all of which involved cellular systems other than cardiomyocytes) (Choy et al., 2018; Montefiori et al., 2018). This indicates that generating chromatin maps for other cardiac cell types or at other differentiation stages could more effectively facilitate the mechanistic dissection of other types of cardiovascular diseases.
With the promising future of functional genomics in non-coding variants dissection, generation and curation of transcriptome and epigenome datasets have been tailed toward studying a specific type of heart disease to achieve higher precision. For example, to understand causal variants for atrial fibrillation (AF), RNA-seq data and ATAC-seq specifically from the left atria were generated to identify potential CREs and target genes that were likely to be affected by the genetic variants within 104 AF-associated loci (van Ouwerkerk et al., 2019). Following this study, a functional enhancer screening of these AF-associated loci using STARR-seq found 24/55 the variant-containing enhancers with allele-specific activities, demonstrating the robustness of this approach. Deletion of the orthologous region of one such enhancer near Hcn4 in the mouse genome caused a loss of Hcn4 expression and cardiac defects (van Ouwerkerk et al., 2020).
In addition to our growing understanding of the regulatory logic underlying developmental gene expression, it is also important to acknowledge the contribution of pro-inflammatory processes on heart enhancer usage and gene expression. For instance, the rapid pro-inflammatory gene expression by the NF-κB transcription factor complex, which across cell types utilizes clusters of strong enhancers (also known as “super enhancers”) to rapidly deploy pro-inflammatory gene expression (Brown et al., 2014; Schmidt et al., 2015). This mode of gene regulation can recruit transcriptional machinery from cell-lineage genes in a process known as cofactor squelching (Schmidt et al., 2015, 2016). Indeed a detailed knowledge of acute and chronic inflammatory enhancer biology during heart development and disease is essential and integrating this information with emerging compendiums of heart epigenomic data (such as Vanoudenhove et al., 2020) will be valuable.
Integrating enhancer information into the functional annotation of non-coding variants is no doubt a powerful approach; however, it should be noted that disrupting enhancer activities is not the only mechanism underlying the pathological consequences of non-coding variants. Even with extensive efforts in curating heart enhancers, nearly 90% of the heart disease-associated LD SNPs did not overlap any heart enhancers in the compendium (Dickel et al., 2016) and more than 80% of them could not be linked to gene promoters based on cardiomyocytes promoter capture Hi-C data (Montefiori et al., 2018). Apart from other possible technical reasons, this small overlap suggests regulatory mechanisms other than altering heart enhancers could account for a substantial portion of non-coding variants-mediated disease risk. In fact, unbiased examination of 98 amplicons (250–600 bp) containing 106 SNPs linked to QT interval phenotypes at the SCN5A locus found that 35% of the reference allele-containing amplicons showed enhancer activity while another 44% worked as silencers in luciferase assays (Kapoor et al., 2019), suggesting disease-associated SNPs likely fall into not only enhancers but also silencers. Besides CREs, functional non-coding variants have also been mapped to miRNA-binding sites and lncRNAs (Table 6). A recent CHD genomic analysis has demonstrated significant enrichment of RNA-binding-protein regulatory sites in de novo variants identified in CHD patients, indicating contribution from disrupted post-transcriptional regulation to CHD (Richter et al., 2020). Moreover, it has been shown that the same minor allele of a variant could regulate the target gene expression through both transcriptional and post-transcriptional mechanisms and, even more strikingly, in an opposite manner, highlighting the complexity of sequence polymorphisms in affecting gene expression (Miller et al., 2013, 2014). Therefore, a comprehensive annotation of different types of cardiac CREs that are not limited to enhancers, together with a good non-coding RNA annotation, will be necessary for truly understanding the mechanisms of the heart disease from the non-coding variant perspective. Additionally, it is likely that several coding and/or non-coding variants collectively explain a complex cardiovascular phenotype. Thus while it is important to dissect disease phenotype associated variants individually, more complex studies looking at genetic interactions and addictive effects may well be required.
Emerging Techniques for the Functional Dissection of Heart Enhancers
So far, numerous putative heart enhancers have been identified in different conditions and cell types from several model organisms. However, compared to enhancer mapping, the throughput of current approaches for enhancer functional dissection, especially in vivo, remains a major bottleneck. Traditionally, each candidate enhancer is accessed individually via being placed upstream of a reporter gene and introduced into cells or in vivo organisms. Collective efforts using this approach have led to the establishment of central resources of validated enhancers, such as the Vista Enhancer Browser2 (Visel et al., 2007). To measure enhancer activity in a more high throughput manner, several methods have been developed through the years, such as massively parallel reporter assays (MPRA) (Melnikov et al., 2012; Patwardhan et al., 2012; Sharon et al., 2012), and self-transcribing active regulatory region sequencing (STARR-seq) (Arnold et al., 2013). However, most of these approaches are typically carried out in vitro or in the absence of chromatin contexts, raising the question of how faithfully their results reflect the native activities of the candidate regions. Recently, the development of more robust and scalable in vivo enhancer assays, such as the site-directed enhancer-reporter assay (enSERT), has allowed systematic assessment of more than 100 variants in an essential limb enhancer (Kvon et al., 2020). For invertebrates like Drosophila, unbiased, automated enhancer mutational scanning has been established using robotic systems, which permits multi-stage quantitative measurement of enhancer activities in development (Fuqua et al., 2020). Developing similar systems for vertebrates will greatly improve our capacity in assessing vertebrate enhancer functions and advance our understanding of how regulatory information is encoded in developmental enhancers.
Compared to all enhancer reporter assays, which introduces an atypical distance between candidate enhancers and the reporter genes, a complementary perhaps preferred way to understand enhancer functions is to dissect their activity and function in their endogenous loci. The ever-growing CRISPR-Cas9 toolbox provides many options for in situ enhancer dissection (reviewed in Klein et al., 2018; Pickar-Oliver and Gersbach, 2019; Xu and Qi, 2019). Individual enhancer deletions or substitutions have been routinely used to characterize enhancer functions in specific developmental processes (Dickel et al., 2016, 2018; Kvon et al., 2016; Osterwalder et al., 2018; van Eif et al., 2020; van Ouwerkerk et al., 2020). To increase the throughput, a variety of CRISPR-based enhancer screens have been developed for in vitro systems, such as the saturated tilling arrays that can unbiased assess certain genomic loci for functional enhancers (Korkmaz et al., 2016; Diao et al., 2017; Gasperini et al., 2017) and epigenetic screens against candidate enhancers using deactivated Cas9 (dCas9) coupled with transcriptional activators or repressors (Klann et al., 2017; Simeonov et al., 2017; Fulco et al., 2019; Gasperini et al., 2019). Specifically, by using single-cell RNA-seq as readouts, CRISPR-mediated epigenetic screens have been successfully applied to perturb thousands of candidate enhancers in cell lines to determine their functional importance and target genes (Fulco et al., 2019; Gasperini et al., 2019). Though achieving the same throughput in vivo may still be challenging, increasing efforts have been made toward applying these powerful systems in animals. Very recently, a single-cell-based in vivo CRISPR/Cas9 screen (Perturb-seq) has been successfully used to screen 35 genes in the mouse developing neuronal cortex in utero (Jin et al., 2020). Though not large-scale yet, this study offers a very encouraging framework to achieve systematic assessment of genes or CREs in vivo. Moreover, dCas9-mediated epigenetic perturbation, which is likely more suitable for enhancer screens, has been continuously optimized over the years and showed a promising future of targeting enhancers in a more scalable manner in developing animals (Morita et al., 2016; Zhou et al., 2018; Li et al., 2020).
Discussion, Concluding Remarks, and Future Perspectives
The past decade has witnessed an exponential growth of the numbers of putative heart enhancer regions identified, largely owing to rapid advances in epigenomic profiling approaches. These techniques are still growing at an ever-increasing speed and will undoubtedly continue to revolutionize the way that researchers annotate and interpret enhancer activities. Single-cell epigenomic techniques, especially the multi-omics approaches, will likely become one of the main driving forces in expanding the horizon of cardiac enhancers and regulatory networks in the next decade. However, it should be noted that many analytical challenges are inherently associated with single-cell epigenomic datasets that currently remain sparse and noisy (reviewed in Schwartzman and Tanay, 2015; Verma and Kumar, 2019). Robust computational and statistical models are needed to extract biological information from other irrelevant signals (e.g., technical noises, batch effect) and for integrating the multimodal data of different characteristics, dimensionalities, and coverages to model them in a single space. Methods addressing these challenges are rapidly emerging (reviewed in Forcato et al., 2020; Hao et al., 2020) but still in the early stages in terms of accommodating all different data types and features. Both technical improvements of assay sensitivity and the development of analytic methods are essential for successfully applying these single-cell genomics techniques to understanding enhancer biology.
In vivo functional characterization of enhancers, especially developmental enhancers, is still one of the biggest challenges lying ahead. As developmental genes are usually regulated by multiple enhancers with overlapping activities, it is reasonable to assume that most enhancers may have redundant functions in normal development (Frankel et al., 2010; Perry et al., 2010; Cannavò et al., 2016; Dickel et al., 2018; Osterwalder et al., 2018). While these redundant enhancers may be seemingly dispensable in normal conditions, they could be required in stressed environments or sensitized genetic backgrounds (e.g., such as heterozygous deletion of developmental TFs) (Frankel et al., 2010; Perry et al., 2010; Osterwalder et al., 2018). It therefore becomes a very complicated task to determine the specific contexts in which a given developmental enhancer is required.
On the other hand, we are in an era with unprecedented opportunities to overcome these challenges. The combined use of CRISPR technologies and single-cell genomics is likely to make a substantial contribution to functional enhancer dissections in the near future. With the concurrent advancement of these two technologies, it probably will not be too far until we can conduct mid- to large-scale in vivo enhancer screening. Moreover, coupling CRISPR with other single-cell epigenomic assays (e.g., single-cell accessibility chromatin) to target TFs or chromatin modifiers (Rubin et al., 2019; Sanjana et al., 2020), can provide information complementary to enhancer screens and together build toward a comprehensive regulatory network.
From traditional approaches to the newest genomic assays, the rich history of heart enhancer studies has not only left us with a wealth of knowledge about the genomic locations, functional roles, evolutionary conservation, and disease implications of heart enhancers but also opened up many challenges and unanswered questions. What are the best experimental designs and analytic strategies of single-cell epigenomic assays? How can we increase the scalability of functional enhancer assays and efficiently adopt them into in vivo contexts? Could we develop more robust and transferable computational methods that can not only predict heart enhancers but also determine their chamber-, cell-type or developmental-stage specific activities and how the activity of enhancers can be affected by non-coding variants? We may not be sure when these questions will be fully answered, but we can confidently anticipate that efforts made in tackling these challenges will push our understanding of heart enhancers and cardiac regulatory network to an unprecedented level.
Author Contributions
XY researched, conceived the structure, created the figures and tables, and led the writing of the review. MW and IS developed the ideas and provided text for the review. All the authors read and edited the review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to the authors whose work could not be covered or thoroughly discussed in this review due to space limitations. We would like to thank Mengyi Song, Huayun Hou, and Anna Prentice for reading the manuscript and providing feedback.
Funding. This work was supported in part by The Hospital for Sick Children Restracomp Studentship and Connaught International Scholarship to XY and CIHR (FRN 156318 to MW and IS). MW was supported by the Canada Research Chairs Program and an Early Researcher Award from the Ontario Ministry of Research and Innovation.
References
- Abudayyeh O. O., Gootenberg J. S., Essletzbichler P., Han S., Joung J., Belanto J. J., et al. (2017). RNA targeting with CRISPR-Cas13. Nature 550 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo R. F., Guay C. L., Edwards A. V., Wessels A., Burch J. B. E. (2004). GATA-6 gene enhancer contains nested regulatory modules for primary myocardium and the embedded nascent atrioventricular conduction system. Anat. Rec. 280A 1062–1071. 10.1002/ar.a.20105 [DOI] [PubMed] [Google Scholar]
- Ahmad S. M., Busser B. W., Huang D., Cozart E. J., Michaud S., Zhu X., et al. (2014). Machine learning classification of cell-specific cardiac enhancers uncovers developmental subnetworks regulating progenitor cell division and cell fate specification. Dev. 141 878–888. 10.1242/dev.101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerberg B. N., Gu F., VanDusen N. J., Zhang X., Dong R., Li K., et al. (2019). A reference map of murine cardiac transcription factor chromatin occupancy identifies dynamic and conserved enhancers. Nat. Commun. 10 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanian M., Maric D., Jenkinson S. P., Mina M., Friedman C. E., Ting C. C., et al. (2017). A transcribed enhancer dictates mesendoderm specification in pluripotency. Nat. Commun. 8 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez J. R., Knoll M., Gromatzky A. A., Lodish H. F. (2017). The super-enhancer-derived alncRNA-EC7/bloodlinc potentiates red blood cell development in trans. Cell Rep. 19 2503–2514. 10.1016/j.celrep.2017.05.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. M., Anderson D. M., McAnally J. R., Shelton J. M., Bassel-Duby R., Olson E. N. (2016). Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 2 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R., Gebhard C., Miguel-Escalada I., Hoof I., Bornholdt J., Boyd M., et al. (2014). An atlas of active enhancers across human cell types and tissues. Nature 507 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang Y.-S., Rivas R. N., Ribeiro A. J. S., Srivas R., Rivera J., Stone N. R., et al. (2016). Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell 167 1734.e22–1749.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermueller C., Clark S. J., Lee H. J., Macaulay I. C., Teng M. J., Hu T. X., et al. (2016). Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat. Methods 13 229–232. 10.1038/nmeth.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio S., Morrison A., Gould A., Gilthorpe J., Chaudhuri C., Rigby P., et al. (1995). Detecting conserved regulatory elements with the model genome of the Japanese puffer fish. Fugu rubripes. Proc. Natl. Acad. Sci. U.S.A. 92 1684–1688. 10.1073/pnas.92.5.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking D. E., Pulit S. L., Crotti L., van der Harst P., Munroe P. B., Koopmann T. T., et al. (2014). Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat. Genet. 46 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner E., Daub C. O., Vitting-Seerup K., Andersson R., Lilje B., Drabløs F., et al. (2015). Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 347 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C. D., Gerlach D., Spies D., Matts J. A., Sytnikova Y. A., Pagani M., et al. (2014). Quantitative genome-wide enhancer activity maps for five Drosophila species show functional enhancer conservation and turnover during cis-regulatory evolution. Nat. Genet. 46 685–692. 10.1038/ng.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C. D., Gerlach D., Stelzer C., Boryn L. M., Rath M., Stark A. (2013). Genome-wide quantitative enhancer activity maps identified by STARR-seq. Science 339 1074–1077. 10.1126/science.1232542 [DOI] [PubMed] [Google Scholar]
- Arnold P. R., Wells A. D., Li X. C. (2020). Diversity and emerging roles of enhancer RNA in regulation of gene expression and cell fate. Front. Cell Dev. Biol. 7:377. 10.3389/fcell.2019.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N., Kulkarni M. M. (2005). Transcriptional enhancers: intelligent enhanceosomes or flexible billboards? J. Cell. Biochem. 94 890–898. 10.1002/jcb.20352 [DOI] [PubMed] [Google Scholar]
- Ballester B., Medina-Rivera A., Schmidt D., Gonzàlez-Porta M., Carlucci M., Chen X., et al. (2014). Multi-species, multi-transcription factor binding highlights conserved control of tissue-specific biological pathways. eLife 3 1–29. 10.1515/bc.2003.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes R. M., Harris I. S., Jaehnig E. J., Sauls K., Sinha T., Rojas A., et al. (2016). MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1. Development 143 774–779. 10.1242/dev.126383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett P., Postma A. V. (2015). Genetics of congenital heart disease: beyond half-measures. Trends Cardiovasc. Med. 25 302–304. 10.1016/j.tcm.2014.11.012 [DOI] [PubMed] [Google Scholar]
- Barron M. E., Belaguli N. S., Shu X. Z., Trinh M., Iyer D., Merlo X., et al. (2005). Serum response factor, an enriched cardiac mesoderm obligatory factor, is a downstream gene target for Tbx genes. J. Biol. Chem. 280 11816–11828. 10.1074/jbc.m412408200 [DOI] [PubMed] [Google Scholar]
- Basson C. T., Bachinsky D. R., Lin R. C., Levi T., Elkins J. A., Soults J., et al. (1997). Mutations in human cause limb and cardiac malformation in Holt-Oram syndrome. Nat. Genet. 15 30–35. 10.1038/ng0197-30 [DOI] [PubMed] [Google Scholar]
- Basson C. T., Huang T., Lin R. C., Bachinsky D. R., Weremowicz S., Vaglio A., et al. (1999). Different TBX5 interactions in heart and limb defined by Holt-Oram syndrome mutations. Proc. Natl. Acad. Sci. U.S.A. 96 2919–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejerano G., Pheasant M., Makunin I., Stephen S., Kent W. J., Mattick J. S., et al. (2004). Ultraconserved elements in the human genome. Science 304 1321–1325. 10.1126/science.1098119 [DOI] [PubMed] [Google Scholar]
- Bertero A., Fields P. A., Ramani V., Bonora G., Yardimci G. G., Reinecke H., et al. (2019). Dynamics of genome reorganization during human cardiogenesis reveal an RBM20-dependent splicing factory. Nat. Commun. 10 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood E. M., Kadonaga J. T. (1998). Going the distance: a current view of enhancer action. Science 281 60–63. 10.1126/science.281.5373.60 [DOI] [PubMed] [Google Scholar]
- Blow M. J., McCulley D. J., Li Z., Zhang T., Akiyama J. A., Holt A., et al. (2010). ChIP-Seq identification of weakly conserved heart enhancers. Nat. Genet. 42 806–810. 10.1038/ng.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic O., Fernandez-Miñán A., Tena J. J., de la Calle-Mustienes E., Hidalgo C., van Kruysbergen I., et al. (2012). Dynamics of enhancer chromatin signatures mark the transition from pluripotency to cell specification during embryogenesis. Genome Res. 22 2043–2053. 10.1101/gr.134833.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanović O., Smits A. H., de la Calle Mustienes E., Tena J. J., Ford E., Williams R., et al. (2016). Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 48 417–426. 10.1038/ng.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev B., Mendelson Cohen N., Szabo Q., Fritsch L., Papadopoulos G. L., Lubling Y., et al. (2017). Multiscale 3D genome rewiring during mouse neural development. Cell 171 557.e24–572.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn S., Zinzen R. P., Girardot C., Gustafson E. H., Perez-Gonzalez A., Delhomme N., et al. (2012). Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat. Genet. 44 148–156. 10.1038/ng.1064 [DOI] [PubMed] [Google Scholar]
- Boogerd C. J., Aneas I., Sakabe N., Dirschinger R. J., Cheng Q. J., Zhou B., et al. (2017). Probing chromatin landscape reveals roles of endocardial TBX20 in septation. J. Clin. Invest. 126 3023–3035. 10.1172/jci85350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I., Gehrke A. R., Smith J. J., Kawasaki K., Manousaki T., Pasquier J., et al. (2016). The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 48 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brind’Amour J., Liu S., Hudson M., Chen C., Karimi M. M., Lorincz M. C. (2015). An ultra-low-input native ChIP-seq protocol for genome-wide profiling of rare cell populations. Nat. Commun. 6 1–8. [DOI] [PubMed] [Google Scholar]
- Brown C. O., Chi X., Garcia-Gras E., Shirai M., Feng X. H., Schwartz R. J. (2004). The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J. Biol. Chem. 279 10659–10669. 10.1074/jbc.m301648200 [DOI] [PubMed] [Google Scholar]
- Brown J. D., Lin C. Y., Duan Q., Griffin G., Federation A. J., Paranal R. M., et al. (2014). Nf-kb directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 56 219–231. 10.1016/j.molcel.2014.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buecker C., Wysocka J. (2012). Enhancers as information integration hubs in development: lessons from genomics. Trends Genet. 28 276–284. 10.1016/j.tig.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro J. D., Giresi P. G., Zaba L. C., Chang H. Y., Greenleaf W. J. (2013). Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10 1213–1218. 10.1038/nmeth.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro J. D., Wu B., Litzenburger U. M., Ruff D., Gonzales M. L., Snyder M. P., et al. (2015). Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523 486–490. 10.1038/nature14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busser B. W., Haimovich J., Huang D., Ovcharenko I., Michelson A. M. (2015). Enhancer modeling uncovers transcriptional signatures of individual cardiac cell states in Drosophila. Nucleic Acids Res. 43 1726–1739. 10.1093/nar/gkv011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E., Wysocka J. (2013). Modification of enhancer chromatin: what, how, and why? Mol. Cell 49 825–837. 10.1016/j.molcel.2013.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavò E., Khoueiry P., Garfield D. A., Geeleher P., Zichner T., Gustafson E. H., et al. (2016). Shadow enhancers are pervasive features of developmental regulatory networks. Curr. Biol. 26 38–51. 10.1016/j.cub.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Cusanovich D. A., Ramani V., Aghamirzaie D., Pliner H. A., Hill A. J., et al. (2018). Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361 1380–1385. 10.1126/science.aau0730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo L., Witzel H. R., Kolovos P., Cheedipudi S., Looso M., Mylona A., et al. (2015). The Isl1/Ldb1 complex orchestrates genome-wide chromatin organization to instruct differentiation of multipotent cardiac progenitors. Cell Stem Cell 17 287–299. 10.1016/j.stem.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Moreira M., Halbert J., Valloton D., Velten B., Chen C., Shao Y., et al. (2019). Gene expression across mammalian organ development. Nature 571 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charité J., McFadden D. G., Merlo G., Levi G., Clouthier D. E., Yanagisawa M., et al. (2001). Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev. 15 3039–3049. 10.1101/gad.931701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Bourque G., Lufkin T. (2011). Conserved and non-conserved enhancers direct tissue specific transcription in ancient germ layer specific developmental control genes. BMC Dev. Biol. 11:63. 10.1186/1471-213X-11-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lake B. B., Zhang K. (2019). High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat. Biotechnol. 37 1452–1457. 10.1038/s41587-019-0290-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Cao X. (2009). NFAT directly regulates Nkx2-5 transcription during cardiac cell differentiation. Biol. Cell 101 335–350. 10.1042/bc20080108 [DOI] [PubMed] [Google Scholar]
- Chi X., Chatterjee P. K., Wilson W., Zhang S. X., DeMayo F. J., Schwartz R. J. (2005). Complex cardiac Nkx2-5 gene expression activated by noggin-sensitive enhancers followed by chamber-specific modules. Proc. Natl. Acad. Sci. U.S.A. 102 13490–13495. 10.1073/pnas.0504295102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy M.-K., Javierre B. M., Williams S. G., Baross S. L., Liu Y., Wingett S. W., et al. (2018). Promoter interactome of human embryonic stem cell-derived cardiomyocytes connects GWAS regions to cardiac gene networks. Nat. Commun. 9:2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T., Rice E. J., Booth G. T., Salamanca H. H., Wang Z., Core L. J., et al. (2018). Chromatin run-on and sequencing maps the transcriptional regulatory landscape of glioblastoma multiforme. Nat. Genet. 50 1553–1564. 10.1038/s41588-018-0244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I.-M., Rajakumar G. (2016). Genetics of congenital heart defects: the NKX2-5 Gene, a Key player. Genes 7: 6. 10.3390/genes7020006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churko J. M., Garg P., Treutlein B., Venkatasubramanian M., Wu H., Lee J., et al. (2018). Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat. Commun. 9:4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo L. A., Lin F. R., Cuesta I., Friedman D., Jarnik M., Zaret K. S. (2002). Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9 279–289. 10.1016/s1097-2765(02)00459-8 [DOI] [PubMed] [Google Scholar]
- Clark C. D., Zhang B., Lee B., Evans S. I., Lassar A. B., Lee K. H. (2013). Evolutionary conservation of Nkx2.5 autoregulation in the second heart field. Dev. Biol. 374 198–209. 10.1016/j.ydbio.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. J., Argelaguet R., Kapourani C. A., Stubbs T. M., Lee H. J., Alda-Catalinas C., et al. (2018). ScNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells e. Nat. Commun. 9 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément Y., Torbey P., Gilardi-Hebenstreit P., Crollius H. R. (2020). Enhancer-gene maps in the human and zebrafish genomes using evolutionary linkage conservation. Nucleic Acids Res. 48 2357–2371. 10.1093/nar/gkz1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congrains A., Kamide K., Katsuya T., Yasuda O., Oguro R., Yamamoto K., et al. (2012a). CVD-associated non-coding RNA, ANRIL, modulates expression of atherogenic pathways in VSMC. Biochem. Biophys. Res. Commun. 419 612–616. 10.1016/j.bbrc.2012.02.050 [DOI] [PubMed] [Google Scholar]
- Congrains A., Kamide K., Oguro R., Yasuda O., Miyata K., Yamamoto E., et al. (2012b). Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis 220 449–455. 10.1016/j.atherosclerosis.2011.11.017 [DOI] [PubMed] [Google Scholar]
- Corces M. R., Trevino A. E., Hamilton E. G., Greenside P. G., Sinnott-Armstrong N. A., Vesuna S., et al. (2017). An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14 959–962. 10.1038/nmeth.4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core L. J., Martins A. L., Danko C. G., Waters C. T., Siepel A., Lis J. T. (2014). Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46 1311–1320. 10.1038/ng.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core L. J., Waterfall J. J., Lis J. T. (2008). Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322 1845–1848. 10.1126/science.1162228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotney J., Leng J., Yin J., Reilly S. K., Demare L. E., Emera D., et al. (2013). The evolution of lineage-specific regulatory activities in the human embryonic limb. Cell 154 185–196. 10.1016/j.cell.2013.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton M. P., Cheng A. W., Welstead G. G., Kooistra T., Carey B. W., Steine E. J., et al. (2010). Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. U.S A. 107 21931–21936. 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich D. A., Daza R., Adey A., Pliner H. A., Christiansen L., Gunderson K. L., et al. (2015). Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348 910–914. 10.1126/science.aab1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich D. A., Hill A. J., Aghamirzaie D., Daza R. M., Pliner H. A., Berletch J. B., et al. (2018a). A single-cell atlas of in vivo mammalian chromatin accessibility. Cell 174 1309.e18–1324.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich D. A., Reddington J. P., Garfield D. A., Daza R. M., Aghamirzaie D., Marco-Ferreres R., et al. (2018b). The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature 555 538–542. 10.1038/nature25981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. L., Edwards A. V., Juraszek A. L., Phelps A., Wessels A., Burch J. B. E. (2001). A GATA-6 gene heart-region-specific enhancer provides a novel means to mark and probe a discrete component of the mouse cardiac conduction system. Mech. Dev. 108 105–119. 10.1016/s0925-4773(01)00500-7 [DOI] [PubMed] [Google Scholar]
- Davis D. L., Wessels A., Burch J. B. E. (2000). An Nkx-dependent enhancer regulates cGATA-6 gene expression during early stages of heart development. Dev. Biol. 217 310–322. 10.1006/dbio.1999.9561 [DOI] [PubMed] [Google Scholar]
- De Koninck M., Lapi E., Badía-Careaga C., Cossío I., Giménez-Llorente D., Rodríguez-Corsino M., et al. (2020). Essential roles of cohesin STAG2 in Mouse embryonic development and adult tissue homeostasis. Cell Rep. 32:108014. 10.1016/j.celrep.2020.108014 [DOI] [PubMed] [Google Scholar]
- de la Calle-Mustienes E., Feijóo C. G., Manzanares M., Tena J. J., Rodríguez-Seguel E., Letizia A., et al. (2005). A functional survey of the enhancer activity of conserved non-coding sequences from vertebrate Iroquois cluster gene deserts. Genome Res. 15 1061–1072. 10.1101/gr.4004805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Olguín P., Huang Y., Li X., Christodoulou D., Seidman C. E., Seidman J. G., et al. (2012). Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat. Genet. 44 343–347. 10.1038/ng.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A., De Laat W. (2016). The second decade of 3C technologies: detailed insights into nuclear organization. Genes Dev. 30 1357–1382. 10.1101/gad.281964.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch M. A., Doppler S. A., Li X., Lahm H., Santamaria G., Cuda G., et al. (2018). Reactivation of the Nkx2.5 cardiac enhancer after myocardial infarction does not presage myogenesis. Cardiovasc. Res. 114 1098–1114. 10.1093/cvr/cvy069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine W. P., Wythe J. D., George M., Koshiba-Takeuchi K., Bruneau B. G. (2014). Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 3:e03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y., Fang R., Li B., Meng Z., Yu J., Qiu Y., et al. (2017). A tiling-deletion-based genetic screen for cis-regulatory element identification in mammalian cells. Nat. Methods 14 629–635. 10.1038/nmeth.4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias N., Stein C. A. (2002). Antisense oligonucleotides: basic concepts and mechanisms. Mol. Cancer Ther. 1 347–355. [PubMed] [Google Scholar]
- Dickel D. E., Barozzi I., Zhu Y., Fukuda-Yuzawa Y., Osterwalder M., Mannion B. J., et al. (2016). Genome-wide compendium and functional assessment of in vivo heart enhancers. Nat. Commun. 7:12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickel D. E., Ypsilanti A. R., Rubenstein J. L. R., Pennacchio L. A., Correspondence A. V., Gov D. (2018). Ultraconserved enhancers are required for normal development. Cell 172 491–499. 10.1016/j.cell.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. R., Jung I., Selvaraj S., Shen Y., Antosiewicz-Bourget J. E., Lee A. Y., et al. (2015). Chromatin architecture reorganization during stem cell differentiation. Nature 518 331–336. 10.1038/nature14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., et al. (2012). Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485 376–380. 10.1038/nature11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou E., Verzi M. P., Anderson J. P., Xu S.-M., Black B. L. (2004). Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131 3931–3942. 10.1242/dev.01256 [DOI] [PubMed] [Google Scholar]
- Domazet-Lošo T., Tautz D. (2010). A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns. Nature 468 815–819. 10.1038/nature09632 [DOI] [PubMed] [Google Scholar]
- Domcke S., Hill A. J., Daza R. M., Cao J., O’Day D. R., Pliner H. A., et al. (2020). A human cell atlas of fetal chromatin accessibility. Science 370:eaba7612. 10.1126/science.aba7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler S. A., Werner A., Barz M., Lahm H., Deutsch M. A., Dreßen M., et al. (2014). Myeloid zinc finger 1 (Mzf1) differentially modulates murine cardiogenesis by interacting with an Nkx2.5 cardiac enhancer. PLoS One 9:e113775. 10.1371/journal.pone.0113775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupays L., Shang C., Wilson R., Kotecha S., Wood S., Towers N., et al. (2015). Sequential binding of MEIS1 and NKX2-5 on the Popdc2 gene: a mechanism for spatiotemporal regulation of enhancers during cardiogenesis. Cell Rep. 13 183–195. 10.1016/j.celrep.2015.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinga R. N., Hagemeijer Y., Burgess S., Hinds D. A., Stefansson K., Gudbjartsson D. F., et al. (2016). Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat. Genet. 48 1557–1563. 10.1038/ng.3708 [DOI] [PubMed] [Google Scholar]
- Eres I. E., Gilad Y. (2020). A TAD Skeptic: is 3D genome topology conserved? Trends Genet. S0168-9525, 30298–30305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eres I. E., Luo K., Hsiao C. J., Blake L. E., Gilad Y. (2019). Reorganization of 3D genome structure may contribute to gene regulatory evolution in primates. PLoS Genet. 15:e1008278. 10.1371/journal.pgen.1008278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa J. M. (2016). Revisiting lncRNAs: how do you know yours is not an eRNA? Mol. Cell 62 1–2. 10.1016/j.molcel.2016.03.022 [DOI] [PubMed] [Google Scholar]
- Fahed A. C., Gelb B. D., Seidman J. G., Seidman C. E. (2013). Genetics of congenital heart disease: the glass half empty. Circ. Res. 112 707–720. 10.1161/circresaha.112.300853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Yu M., Li G., Chee S., Liu T., Schmitt A. D., et al. (2016). Mapping of long-range chromatin interactions by proximity ligation-assisted ChIP-seq. Cell Res. 26 1345–1348. 10.1038/cr.2016.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley E. K., Olson K. M., Zhang W., Brandt A. J., Rokhsar D. S., Levine M. S. (2015). Suboptimization of developmental enhancers. Science 350 325–328. 10.1126/science.aac6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley E. K., Olson K. M., Zhang W., Rokhsar D. S., Levine M. S. (2016). Syntax compensates for poor binding sites to encode tissue specificity of developmental enhancers. Proc. Natl. Acad. Sci. U.S.A. 113 6508–6513. 10.1073/pnas.1605085113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A., Adelman K. (2020). Evaluating enhancer function and transcription. Annu. Rev. Biochem. 89 213–234. 10.1146/annurev-biochem-011420-095916 [DOI] [PubMed] [Google Scholar]
- Fisher S., Grice E. A., Vinton R. M., Bessling S. L., McCallion A. S. (2006). Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science 312 276–279. 10.1126/science.1124070 [DOI] [PubMed] [Google Scholar]
- Flavahan W. A., Drier Y., Liau B. B., Gillespie S. M., Venteicher A. S., Stemmer-Rachamimov A. O., et al. (2016). Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529 110–114. 10.1038/nature16490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcato M., Romano O., Bicciato S. (2020). Computational methods for the integrative analysis of single-cell data. Brief. Bioinform. 2020 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke M., Ibrahim D. M., Andrey G., Schwarzer W., Heinrich V., Schöpflin R., et al. (2016). Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature 538 265–269. 10.1038/nature19800 [DOI] [PubMed] [Google Scholar]
- Frankel N., Davis G. K., Vargas D., Wang S., Payre F., Stern D. L. (2010). Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466 490–493. 10.1038/nature09158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedli M., Barde I., Arcangeli M., Verp S., Quazzola A., Zakany J., et al. (2010). A systematic enhancer screen using lentivector transgenesis identifies conserved and non-conserved functional elements at the Olig1 and Olig2 locus. PLoS One 5:e15741. 10.1371/journal.pone.0015741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco C. P., Nasser J., Jones T. R., Munson G., Bergman D. T., Subramanian V., et al. (2019). Activity-by-contact model of enhancer–promoter regulation from thousands of CRISPR perturbations. Nat. Genet. 51 1664–1669. 10.1038/s41588-019-0538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood M. J., Liu M. H., Pan Y. F., Liu J., Xu H., Mohamed Y. B., et al. (2009). An oestrogen-receptor-α-bound human chromatin interactome. Nature 462 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua T., Jordan J., Elize van Breugel M., Halavatyi A., Tischer C., Polidoro P., et al. (2020). Dense and pleiotropic regulatory information in a developmental enhancer. Nature 587 235–239. 10.1038/s41586-020-2816-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galang G., Mandla R., Ruan H., Jung C., Sinha T., Stone N. R., et al. (2020). ATAC-seq reveals an Isl1 enhancer that regulates sinoatrial node development and function. Circ. Res. 127 1502–1518. 10.1161/circresaha.120.317145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini M., Findlay G. M., McKenna A., Milbank J. H., Lee C., Zhang M. D., et al. (2017). CRISPR/Cas9-mediated scanning for regulatory elements required for HPRT1 expression via thousands of large, programmed genomic deletions. Am. J. Hum. Genet. 101 192–205. 10.1016/j.ajhg.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini M., Hill A. J., McFaline-Figueroa J. L., Martin B., Kim S., Zhang M. D., et al. (2019). A genome-wide framework for mapping gene regulation via cellular genetic screens. Cell 176 377.e19–390.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi M., Lee D., Mohammad-Noori M., Beer M. A. (2014). Enhanced regulatory sequence prediction using gapped k-mer features. PLoS Comput. Biol. 10:e1003711. 10.1371/journal.pcbi.1003711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb N., Lazic S., Yuan X., Deshwar A. R., Leslie M., Wilson M. D., et al. (2018). Hey2 regulates the size of the cardiac progenitor pool during vertebrate heart development. Development 145:dev167510. 10.1242/dev.167510 [DOI] [PubMed] [Google Scholar]
- Gil N., Ulitsky I. (2020). Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 21 102–117. 10.1038/s41576-019-0184-5 [DOI] [PubMed] [Google Scholar]
- Gilsbach R., Schwaderer M., Preissl S., Grüning B. A., Kranzhöfer D., Schneider P., et al. (2018). Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat. Commun. 9:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. A., Kuzu G., Lee N., Karasik J., Gemberling M., Foglia M. J., et al. (2017). Resolving heart regeneration by replacement histone profiling. Dev. Cell 40 392.e6–404.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Velazquez M., Badia-Careaga C., Lechuga-Vieco A. V., Nieto-Arellano R., Tena J. J., Rollan I., et al. (2017). CTCF counter-regulates cardiomyocyte development and maturation programs in the embryonic heart. PLoS Genet. 13:e1006985. 10.1371/journal.pgen.1006985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorkin D. U., Barozzi I., Zhao Y., Zhang Y., Huang H., Lee A. Y., et al. (2020). An atlas of dynamic chromatin landscapes in mouse fetal development. Nature 583 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote P., Wittler L., Hendrix D., Koch F., Währisch S., Beisaw A., et al. (2013). The tissue-specific lncRNA fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 24 206–214. 10.1016/j.devcel.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubert F., Srivas R., Spacek D. V., Kasowski M., Ruiz-Velasco M., Sinnott-Armstrong N., et al. (2020). Landscape of cohesin-mediated chromatin loops in the human genome. Nature 583 737–743. 10.1038/s41586-020-2151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Xu Q., Canzio D., Shou J., Li J., Gorkin D. U., et al. (2015). CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell 162 900–910. 10.1016/j.cell.2015.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen L. L. P., Kassouf M. T., Oudelaar A. M., Biggs D., Preece C., Downes D. J., et al. (2017). Tissue-specific CTCF-cohesin-mediated chromatin architecture delimits enhancer interactions and function in vivo. Nat. Cell Biol. 19 952–961. 10.1038/ncb3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Hao S., Andersen-Nissen E., Mauck W. M., III, Zheng S., Butler A., et al. (2020). Integrated analysis of multimodal single-cell data. bioRxiv [Preprint]. 10.1101/2020.10.12.335331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare E. E., Peterson B. K., Iyer V. N., Meier R., Eisen M. B. (2008). Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genet. 4:e1000106. 10.1371/journal.pgen.1000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmston N., Ing-Simmons E., Tan G., Perry M., Merkenschlager M., Lenhard B. (2017). Topologically associating domains are ancient features that coincide with Metazoan clusters of extreme noncoding conservation. Nat. Commun. 8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Wang Z., Garry G. A., Malladi V. S., Botten G. A., Ye W., et al. (2019). Cardiac reprogramming factors synergistically activate genome-wide cardiogenic stage-specific enhancers. Cell Stem Cell 25 69.e5–86.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A., Gu F., Hu Y., Ma Q., Ye L. Y., Akiyama J. A., et al. (2014). Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 5:4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A., Kong S. W., Ma Q., Pu W. T. (2011). Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc. Natl. Acad. Sci. U.S.A. 108 5632–5637. 10.1073/pnas.1016959108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. Z., Burch J. B. E. (1997). The chicken GATA-6 locus contains multiple control regions that confer distinct patterns of heart region-specific expression in transgenic mouse embryos. J. Biol. Chem. 272 28550–28556. 10.1074/jbc.272.45.28550 [DOI] [PubMed] [Google Scholar]
- Heicklen-Klein A., Evans T. (2004). T-box binding sites are required for activity of a cardiac GATA-4 enhancer. Dev. Biol. 267 490–504. 10.1016/j.ydbio.2003.09.042 [DOI] [PubMed] [Google Scholar]
- Heinz S., Romanoski C. E., Benner C., Glass C. K. (2015). The selection and function of cell type-specific enhancers. Nat. Rev. Mol. Cell Biol. 16 144–154. 10.1038/nrm3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J., Kaya-Okur H. S., Ahmad K. (2020). Efficient chromatin accessibility mapping in situ by nucleosome-tethered tagmentation. eLife 9:e63274. 10.7554/eLife.63274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller M., Agarwal S., Notwell J. H., Parikh R., Guturu H., Wenger A. M., et al. (2013). Computational methods to detect conserved non-genic elements in phylogenetically isolated genomes: application to zebrafish. Nucleic Acids Res. 41:e151. 10.1093/nar/gkt557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Clauss S., Berger I. M., Weiß B., Montalbano A., Röth R., et al. (2016). Coding and non-coding variants in the SHOX2 gene in patients with early-onset atrial fibrillation. Basic Res. Cardiol. 111 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt L. M., Beutner F., Scholz M., Gielen S., Gäbel G., Bergert H., et al. (2010). ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler. Thromb. Vasc. Biol. 30 620–627. [DOI] [PubMed] [Google Scholar]
- Honkoop H., de Bakker D. E. M., Aharonov A., Kruse F., Shakked A., Nguyen P. D., et al. (2019). Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. eLife 8 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. L., Fei T., Chen Y., Li T., Gao Y., Wang X., et al. (2014). Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc. Natl. Acad. Sci. U.S.A. 111 7319–7324. 10.1073/pnas.1324151111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Liu J., Zhao J., Wilkins B. J., Lupino K., Wu H., et al. (2018). Single-nucleus transcriptomic survey of cell diversity and functional maturation in postnatal mammalian hearts. Genes Dev. 32 1344–1357. 10.1101/gad.316802.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T., Yamagishi H., Maeda J., McAnally J., Yamagishi C., Srivastava D. (2004). Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development 131 5491–5502. 10.1242/dev.01399 [DOI] [PubMed] [Google Scholar]
- Huang W., Meng H., Qiao Y., Pang S., Chen D., Yan B. (2013). Two novel and functional DNA sequence variants within an upstream enhancer of the human NKX2-5 gene in ventricular septal defects. Gene 524 152–155. 10.1016/j.gene.2013.04.043 [DOI] [PubMed] [Google Scholar]
- Ihara D., Watanabe Y., Seya D., Arai Y., Isomoto Y., Nakano A., et al. (2020). Expression of Hey2 transcription factor in the early embryonic ventricles is controlled through a distal enhancer by Tbx20 and Gata transcription factors. Dev. Biol. 461 124–131. 10.1016/j.ydbio.2020.02.001 [DOI] [PubMed] [Google Scholar]
- Iklé J. M., Artinger K. B., Clouthier D. E. (2012). Identification and characterization of the zebrafish pharyngeal arch-specific enhancer for the basic helix-loop-helix transcription factor Hand2. Dev. Biol. 368 118–126. 10.1016/j.ydbio.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N., Kuratani S. (2011). Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat. Commun. 2:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N., Sehara-Fujisawa A. (2007). The vertebrate phylotypic stage and an early bilaterian-related stage in mouse embryogenesis defined by genomic information. BMC Biol. 5:1. 10.1186/1741-7007-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia M., Tena J. J., Alexis M. S., Fernandez-Miñan A., Maeso I., Bogdanovic O., et al. (2012). Extensive conservation of ancient microsynteny across metazoans due to cis-regulatory constraints. Genome Res 22 2356–2367. 10.1101/gr.139725.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javierre B. M., Burren O. S., Wilder S. P., Kreuzhuber R., Hill S. M., Sewitz S., et al. (2016). Lineage-specific genome architecture links enhancers and non-coding disease variants to target gene promoters. Cell 167 1369.e19–1384.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Preussner J., Chen X., Guenther S., Yuan X., Yekelchyk M., et al. (2018). Single cell RNA-seq and ATAC-seq analysis of cardiac progenitor cell transition states and lineage settlement. Nat. Commun. 9:4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Stojnic R., Adryan B., Ozdemir A., Stathopoulos A., Frasch M. (2013). Genome-wide screens for in vivo tinman binding sites identify cardiac enhancers with diverse functional architectures. PLoS Genet. 9:e1003195. 10.1371/journal.pgen.1003195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Simmons S. K., Guo A., Shetty A. S., Ko M., Nguyen L., et al. (2020). In vivo Perturb-Seq reveals neuronal and glial abnormalities associated with autism risk genes. Science 370:eaaz6063. 10.1126/science.aaz6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. S., Davidson B., Brown C. D., Smith W. C., Sidow A. (2004). Noncoding regulatory sequences of Ciona exhibit strong correspondence between evolutionary constraint and functional importance. Genome Res. 14 2448–2456. 10.1101/gr.2964504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung I., Schmitt A., Diao Y., Lee A. J., Liu T., Yang D., et al. (2019). A compendium of promoter-centered long-range chromatin interactions in the human genome. Nat. Genet. 51 1442–1449. 10.1038/s41588-019-0494-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junion G., Spivakov M., Girardot C., Braun M., Gustafson E. H., Birney E., et al. (2012). A transcription factor collective defines cardiac cell fate and reflects lineage history. Cell 148 473–486. 10.1016/j.cell.2012.01.030 [DOI] [PubMed] [Google Scholar]
- Kaikkonen M. U., Spann N. J., Heinz S., Romanoski C. E., Allison K. A., Stender J. D., et al. (2013). Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell 51 310–325. 10.1016/j.molcel.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinka A. T., Varga K. M., Gerrard D. T., Preibisch S., Corcoran D. L., Jarrells J., et al. (2010). Gene expression divergence recapitulates the developmental hourglass model. Nature 468 811–816. 10.1038/nature09634 [DOI] [PubMed] [Google Scholar]
- Kapoor A., Lee D., Zhu L., Soliman E. Z., Grove M. L., Boerwinkle E., et al. (2019). Multiple SCN5A variant enhancers modulate its cardiac gene expression and the QT interval. Proc. Natl. Acad. Sci. U.S.A. 166 10636–10645. 10.1073/pnas.1808734116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Sekar R. B., Hansen N. F., Fox-Talbot K., Morley M., Pihur V., et al. (2014). An enhancer polymorphism at the cardiomyocyte intercalated disc protein NOS1AP locus is a major regulator of the QT interval. Am. J. Hum. Genet. 94 854–869. 10.1016/j.ajhg.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskow B. J., Diepeveen L. A., Michael Proffitt J., Rea A. J., Ulgiati D., Blangero J., et al. (2014). Molecular prioritization strategies to identify functional genetic variants in the cardiovascular disease-associated expression QTL Vanin-1. Eur. J. Hum. Genet. 22 688–695. 10.1038/ejhg.2013.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S., Calof A. L., Santos R., Lopez-Burks M. E., Young C. M., Hoang M. P., et al. (2009). Multiple organ system defects and transcriptional dysregulation in the Nipbl+/- Mouse, a model of cornelia de lange syndrome. PLoS Genet. 5:e1000650. 10.1371/journal.pgen.1000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya-Okur H. S., Wu S. J., Codomo C. A., Pledger E. S., Bryson T. D., Henikoff J. G., et al. (2019). CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10:1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. G. (2012). The Second Heart Field. Amsterdam: Elsevier Inc. [Google Scholar]
- Khoueiry P., Girardot C., Ciglar L., Peng P. C., Hilary Gustafson E., Sinha S., et al. (2017). Uncoupling evolutionary changes in DNA sequence, transcription factor occupancy and enhancer activity. eLife 6 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-K., Hemberg M., Gray J. M., Costa A. M., Bear D. M., Wu J., et al. (2010). Widespread transcription at neuronal activity-regulated enhancers. Nature 465 182–187. 10.1038/nature09033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann T. S., Black J. B., Chellappan M., Safi A., Song L., Hilton I. B., et al. (2017). CRISPR-Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nat. Biotechnol. 35 561–568. 10.1038/nbt.3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattenhoff C. A., Scheuermann J. C., Surface L. E., Bradley R. K., Fields P. A., Steinhauser M. L., et al. (2013). Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152 570–583. 10.1016/j.cell.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. C., Chen W., Gasperini M., Shendure J. (2018). Identifying novel enhancer elements with CRISPR-Based screens. ACS Chem. Biol. 13 326–332. 10.1021/acschembio.7b00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Fenouil R., Gut M., Cauchy P., Albert T. K., Zacarias-Cabeza J., et al. (2011). Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat. Struct. Mol. Biol. 18 956–963. 10.1038/nsmb.2085 [DOI] [PubMed] [Google Scholar]
- Korkmaz G., Lopes R., Ugalde A. P., Nevedomskaya E., Han R., Myacheva K., et al. (2016). Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9. Nat. Biotechnol. 34 192–198. 10.1038/nbt.3450 [DOI] [PubMed] [Google Scholar]
- Krasnov A. N., Mazina M. Y., Nikolenko J. V., Vorobyeva N. E. (2016). On the way of revealing coactivator complexes cross-talk during transcriptional activation. Cell Biosci. 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunarso G., Chia N.-Y., Jeyakani J., Hwang C., Lu X., Chan Y.-S., et al. (2010). Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat. Genet. 42 631–634. 10.1038/ng.600 [DOI] [PubMed] [Google Scholar]
- Kvon E. Z., Kamneva O. K., Melo U. S., Barozzi I., Osterwalder M., Mannion B. J., et al. (2016). Progressive loss of function in a limb enhancer during snake evolution. Cell 167 633.e11–642.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvon E. Z., Zhu Y., Kelman G., Visel A., Dickel D. E., Pennacchio L. A. (2020). Comprehensive in vivo interrogation reveals phenotypic impact of human enhancer variants. Cell 180 1262.e15–1271.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H., Fuda N. J., Core L. J., Lis J. T. (2013). Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science 339 950–953. 10.1126/science.1229386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F., Orom U. A., Cesaroni M., Beringer M., Taatjes D. J., Blobel G. A., et al. (2013). Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 494 497–501. 10.1038/nature11884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth L. S., Smith C. A. (2013). Short hairpin RNA-mediated gene silencing. Methods Mol. Biol. 942 205–232. 10.1007/978-1-62703-119-6_12 [DOI] [PubMed] [Google Scholar]
- Laurent F., Girdziusaite A., Gamart J., Barozzi I., Osterwalder M., Akiyama J. A., et al. (2017). HAND2 target gene regulatory networks control atrioventricular canal and cardiac valve development. Cell Rep. 19 1602–1613. 10.1016/j.celrep.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Kapoor A., Safi A., Song L., Halushka M. K., Crawford G. E., et al. (2018). Human cardiac cis-regulatory elements, their cognate transcription factors, and regulatory DNA sequence variants. Genome Res. 28 1577–1588. 10.1101/gr.234633.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Evans S., Ruan T. Y., Lassar A. B. (2004). SMAD-mediated modulation of YY1 activity regulates the BMP response and cardiac-specific expression of a GATA4/5/6-dependent chick Nkx2.5 enhancer. Development 131 4709–4723. 10.1242/dev.01344 [DOI] [PubMed] [Google Scholar]
- Lescroart F., Chabab S., Lin X., Rulands S., Paulissen C., Rodolosse A., et al. (2014). Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 16 829–840. 10.1038/ncb3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettice L. A., Heaney S. J. H., Purdie L. A., Li L., de Beer P., Oostra B. A., et al. (2003). A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12 1725–1735. 10.1093/hmg/ddg180 [DOI] [PubMed] [Google Scholar]
- Leung D., Jung I., Rajagopal N., Schmitt A., Selvaraj S., Lee A. Y., et al. (2015). Integrative analysis of haplotype-resolved epigenomes across human tissues. Nature 518 350–354. 10.1038/nature14217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Liu Y., Zhang Y., Kubo N., Yu M., Fang R., et al. (2019). Joint profiling of DNA methylation and chromatin architecture in single cells. Nat. Methods 16 991–993. 10.1038/s41592-019-0502-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Liu Y., Cao H., Zhang Y., Gu Z., Liu X., et al. (2020). Interrogation of enhancer function by enhancer-targeting CRISPR epigenetic editing. Nat. Commun. 11 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Wang Z. S., Wang X. H., Xu Y. J., Qiao Q., Li X. M., et al. (2018). A SHOX2 loss-of-function mutation underlying familial atrial fibrillation. Int. J. Med. Sci. 15 1564–1572. 10.7150/ijms.27424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Notani D., Ma Q., Tanasa B., Nunez E., Chen A. Y., et al. (2013). Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498 516–520. 10.1038/nature12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Notani D., Rosenfeld M. G. (2016). Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat. Rev. Genet. 17 207–223. 10.1038/nrg.2016.4 [DOI] [PubMed] [Google Scholar]
- Liang M., Soomro A. U., Tasneem S., Abatti L. E., Alizada A., Yuan X., et al. (2020). Enhancer-gene rewiring in the pathogenesis of quebec platelet disorder. Blood 136 2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore C. M., Searcy-Schrick R. D., Vincent E. B., Yutzey K. E. (2002). Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev. Biol. 244 243–256. 10.1006/dbio.2002.0604 [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E., Van Berkum N. L., Williams L., Imakaev M., Ragoczy T., Telling A., et al. (2009). Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326 289–293. 10.1126/science.1181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien C. L., McAnally J., Richardson J. A., Olson E. N. (2002). Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev. Biol. 244 257–266. 10.1006/dbio.2002.0603 [DOI] [PubMed] [Google Scholar]
- Lien C. L., Wu C., Mercer B., Webb R., Richardson J. A., Olson E. N. (1999). Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 126 75–84. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang M., Wei X., Wu L., Xu J., Dai X., et al. (2019). An ATAC-seq atlas of chromatin accessibility in mouse tissues. Sci. Data 6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Viales R. R., Khoueiry P., Reddington J. P., Girardot C., Furlong E. E. M., et al. (2020). The hourglass model of evolutionary conservation during embryogenesis extends to developmental enhancers with signatures of positive selection. bioRxiv [Preprint]. 10.1101/2020.11.02.364505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Jiang C., Xu J., Zhao M. T., Van Bortle K., Cheng X., et al. (2017). Genome-wide temporal profiling of transcriptome and open chromatin of early cardiomyocyte differentiation derived from hiPSCs and hESCs. Circ. Res. 121 376–391. 10.1161/circresaha.116.310456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Van Bortle K., Zhang Y., Zhao M. T., Zhang J. Z., Geller B. S., et al. (2018). Disruption of mesoderm formation during cardiac differentiation due to developmental exposure to 13-cis-retinoic acid. Sci. Rep. 8 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K. M., Chen A., Koh P. W., Deng T. Z., Sinha R., Tsai J. M., et al. (2016). Mapping the pairwise choices leading from pluripotency to human bone. Heart, and other mesoderm cell types. Cell 166 451–467. 10.1016/j.cell.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H. K., Prescott S. L., Wysocka J. (2016). Ever-changing landscapes: transcriptional enhancers in development and evolution. Cell 167 1170–1187. 10.1016/j.cell.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Zurita L., Stirnimann C. U., Glatt S., Kaynak B. L., Thomas S., Baudin F., et al. (2016). Complex interdependence regulates heterotypic transcription factor distribution and coordinates cardiogenesis. Cell 164 999–1014. 10.1016/j.cell.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupiáñez D. G., Kraft K., Heinrich V., Krawitz P., Brancati F., Klopocki E., et al. (2015). Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161 1012–1025. 10.1016/j.cell.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeill C., French R., Evans T., Wessels A., Burch J. B. E. (2000). Modular regulation of cGATA-5 gene expression in the developing heart and gut. Dev. Biol. 217 62–76. 10.1006/dbio.1999.9539 [DOI] [PubMed] [Google Scholar]
- Maeda J., Yamagishi H., McAnally J., Yamagishi C., Srivastava D. (2006). Tbx1 is regulated by forkhead proteins in the secondary heart field. Dev. Dyn. 235 701–710. 10.1002/dvdy.20686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S., Roeder R. G. (2010). The metazoan mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11 761–772. 10.1038/nrg2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May D., Blow M. J., Kaplan T., McCulley D. J., Jensen B. C., Akiyama J. A., et al. (2012). Large-scale discovery of enhancers from human heart tissue. Nat. Genet. 44 89–93. 10.1038/ng.1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. G., Charité J., Richardson J. A., Srivastava D., Firulli A. B., Olson E. N. (2000). A GATA-dependent right ventricular enhancer controls dHAND transcription in the developing heart. Development 127 5331–5341. [DOI] [PubMed] [Google Scholar]
- McPherson C. E., Shim E. Y., Friedman D. S., Zaret K. S. (1993). An active tissue-specific enhancer and bound transcription factors existing in a precisely positioned nucleosomal array. Cell 75 387–398. 10.1016/0092-8674(93)80079-t [DOI] [PubMed] [Google Scholar]
- Meers M. P., Bryson T. D., Henikoff J. G., Henikoff S. (2019). Improved CUT&RUN chromatin profiling tools. eLife 8:e46314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikov A., Murugan A., Zhang X., Tesileanu T., Wang L., Rogov P., et al. (2012). Systematic dissection and optimization of inducible enhancers in human cells using a massively parallel reporter assay. Nat. Biotechnol. 30 271–277. 10.1038/nbt.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., Nora E. P. (2016). CTCF and cohesin in genome folding and transcriptional gene regulation. Annu. Rev. Genomics Hum. Genet. 17 17–43. 10.1146/annurev-genom-083115-022339 [DOI] [PubMed] [Google Scholar]
- Meuleman W., Muratov A., Rynes E., Halow J., Lee K., Bates D., et al. (2020). Index and biological spectrum of human DNase I hypersensitive sites. Nature 584 244–251. 10.1038/s41586-020-2559-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud B., Tavares-Cadete F., Young A. N., Sugar R., Schoenfelder S., Ferreira L., et al. (2015). Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat. Genet. 47 598–606. 10.1038/ng.3286 [DOI] [PubMed] [Google Scholar]
- Mikkelsen T. S., Xu Z., Zhang X., Wang L., Gimble J. M., Lander E. S., et al. (2010). Comparative epigenomic analysis of murine and human adipogenesis. Cell 143 156–169. 10.1016/j.cell.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Anderson D. R., Kundu R. K., Raiesdana A., Nürnberg S. T., Diaz R., et al. (2013). Disease-related growth factor and embryonic signaling pathways modulate an enhancer of TCF21 Expression at the 6q23.2 coronary heart disease locus. PLoS Genet. 9:e1003652. 10.1371/journal.pgen.1003652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Haas U., Diaz R., Leeper N. J., Kundu R. K., Patlolla B., et al. (2014). Coronary heart disease-associated variation in TCF21 Disrupts a miR-224 binding site and miRNA-Mediated regulation. PLoS Genet. 10:e1004263. 10.1371/journal.pgen.1004263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L., Kelly R. G. (2013). Organogenesis of the vertebrate heart. Wiley Interdiscip. Rev. Dev. Biol. 2 17–29. 10.1002/wdev.68 [DOI] [PubMed] [Google Scholar]
- Molkentin J. D., Antos C., Mercer B., Taigen T., Miano J. M., Olson E. N. (2000). Direct activation of a GATA6 cardiac enhancer by Nkx2.5: evidence for a reinforcing regulatory network of Nkx2.5 and GATA transcription factors in the developing heart. Dev. Biol. 217 301–309. 10.1006/dbio.1999.9544 [DOI] [PubMed] [Google Scholar]
- Montefiori L. E., Sobreira D. R., Sakabe N. J., Aneas I., Joslin A. C., Hansen G. T., et al. (2018). A promoter interaction map for cardiovascular disease genetics. eLife 7:e35788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Noguchi H., Horii T., Nakabayashi K., Kimura M., Okamura K., et al. (2016). Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat. Biotechnol. 34 1060–1065. 10.1038/nbt.3658 [DOI] [PubMed] [Google Scholar]
- Moudgil A., Wilkinson M. N., Chen X., He J., Cammack A. J., Vasek M. J., et al. (2020). Self-reporting transposons enable simultaneous readout of gene expression and transcription factor binding in single cells. Cell 182 992.e21–1008.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi K., Zare H., Dell’Orso S., Grontved L., Gutierrez-Cruz G., Derfoul A., et al. (2013). ERNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol. Cell 51 606–617. 10.1016/j.molcel.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., et al. (2015). Heart disease and stroke statistics—2015 update. Circulation 131 e29–e322. [DOI] [PubMed] [Google Scholar]
- Mumbach M. R., Rubin A. J., Flynn R. A., Dai C., Khavari P. A., Greenleaf W. J., et al. (2016). HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 13 919–922. 10.1038/nmeth.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Calof A. L., Lander A. D., Schilling T. F. (2011). Multifactorial origins of heart and gut defects in nipbl-deficient zebrafish, a model of cornelia de lange syndrome. PLoS Biol. 9:e1001181. 10.1371/journal.pbio.1001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar L., Sakabe N. J., Blanski A. A. (2010). Genome-wide discovery of human heart enhancers. Genome Res. 20 381–392. 10.1101/gr.098657.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nature Methods. (2020). Method of the Year 2019: single-cell multimodal omics. Nat. Methods 17:1. 10.1038/s41592-019-0703-5 [DOI] [PubMed] [Google Scholar]
- Nelson C. P., Goel A., Butterworth A. S., Kanoni S., Webb T. R., Marouli E., et al. (2017). Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat. Genet. 49 1385–1391. [DOI] [PubMed] [Google Scholar]
- Nicole Ritter A., Ali T., Kopitchinski N., Dimmeler S., Grote Correspondence P. (2019). The lncRNA locus handsdown regulates cardiac gene programs and is essential for early mouse development. Dev. Cell 50 644–657. 10.1016/j.devcel.2019.07.013 [DOI] [PubMed] [Google Scholar]
- Nikpay M., Goel A., Won H.-H., Hall L. M., Willenborg C., Kanoni S., et al. (2015). A comprehensive 1000 genomes–based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47 1121–1130. 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega M. A., Ovcharenko I., Afzal V., Rubin E. M. (2003). Scanning human gene deserts for long-range enhancers. Science 302 413–413. 10.1126/science.1088328 [DOI] [PubMed] [Google Scholar]
- Nord A. S., Blow M. J., Attanasio C., Akiyama J. A., Holt A., Hosseini R., et al. (2013). Rapid and pervasive changes in genome-wide enhancer usage during mammalian development. Cell 155 1521–1531. 10.1016/j.cell.2013.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda M., Peterkin T., Simões F. C., Patient R., Schneider M. D. (2011). Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circ. Res. 108 129–152. 10.1161/circresaha.110.223792 [DOI] [PubMed] [Google Scholar]
- Odom D. T., Dowell R. D., Jacobsen E. S., Gordon W., Danford T. W., MacIsaac K. D., et al. (2007). Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat. Genet. 39 730–732. 10.1038/ng2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. (2006). Gene regulatory networks in the evolution and development of the heart. Science 313 1922–1927. 10.1126/science.1132292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill L. P., VerMilyea M. D., Turner B. M. (2006). Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat. Genet. 38 835–841. 10.1038/ng1820 [DOI] [PubMed] [Google Scholar]
- Osterwalder M., Barozzi I., Tissières V., Fukuda-Yuzawa Y., Mannion B. J., Afzal S. Y., et al. (2018). Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554 239–243. 10.1038/nature25461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder M., Speziale D., Shoukry M., Mohan R., Ivanek R., Kohler M., et al. (2014). HAND2 targets define a network of transcriptional regulators that compartmentalize the early limb bud mesenchyme. Dev. Cell 31 345–357. 10.1016/j.devcel.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounzain S., Micheletti R., Arnan C., Plaisance I., Cecchi D., Schroen B., et al. (2015). CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J. Mol. Cell. Cardiol. 89 98–112. 10.1016/j.yjmcc.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Ounzain S., Pezzuto I., Micheletti R., Burdet F., Sheta R., Nemir M., et al. (2014). Functional importance of cardiac enhancer-associated noncoding RNAs in heart development and disease. Curr. Ther. Res. Clin. Exp. 76 55–70. 10.1016/j.yjmcc.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige S. L., Thomas S., Stoick-Cooper C. L., Wang H., Maves L., Sandstrom R., et al. (2012). A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 151 221–232. 10.1016/j.cell.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik D. T., Cho S., Tian L., Chang H. Y., Wu J. C. (2020). Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat. Rev. Cardiol. 17 457–473. 10.1038/s41569-020-0359-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pane L. S., Fulcoli F. G., Cirino A., Altomonte A., Ferrentino R., Bilio M., et al. (2018). Tbx1 represses Mef2c gene expression and is correlated with histone 3 deacetylation of the anterior heart field enhancer. DMM Dis. Model. Mech. 11:dmm029967. 10.1242/dmm.029967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Shan J., Qiao Y., Ma L., Qin X., Wanyan H., et al. (2012). Genetic and functional analysis of the NKX2-5 gene promoter in patients with ventricular septal defects. Pediatr. Cardiol. 33 1355–1361. 10.1007/s00246-012-0346-0 [DOI] [PubMed] [Google Scholar]
- Paralkar V. R., Taborda C. C., Huang P., Yao Y., Kossenkov A. V., Prasad R., et al. (2016). Unlinking an lncRNA from its associated cis element. Mol. Cell 62 104–110. 10.1016/j.molcel.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris M., Kaplan T., Li X. Y., Villalta J. E., Lott S. E., Eisen M. B. (2013). Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet. 9:e1003748. 10.1371/journal.pgen.1003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan R. P., Hiatt J. B., Witten D. M., Kim M. J., Smith R. P., May D., et al. (2012). Massively parallel functional dissection of mammalian enhancers in vivo. Nat. Biotechnol. 30 265–270. 10.1038/nbt.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M., Kedzierska K. Z., Migdal M., Nahia K. A., Ramilowski J. A., Bugajski L., et al. (2019). Dynamics of cardiomyocyte transcriptome and chromatin landscape demarcates key events of heart development. Genome Res. 29 506–519. 10.1101/gr.244491.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio L. A., Ahituv N., Moses A. M., Prabhakar S., Nobrega M. A., Shoukry M., et al. (2006). In vivo enhancer analysis of human conserved non-coding sequences. Nature 444 499–502. 10.1038/nature05295 [DOI] [PubMed] [Google Scholar]
- Perry M. W., Boettiger A. N., Bothma J. P., Levine M. (2010). Shadow enhancers foster robustness of Drosophila gastrulation. Curr. Biol. 20 1562–1567. 10.1016/j.cub.2010.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar-Oliver A., Gersbach C. A. (2019). The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20 490–507. 10.1038/s41580-019-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijuan-Sala B., Wilson N. K., Xia J., Hou X., Hannah R. L., Kinston S., et al. (2020). Single-cell chromatin accessibility maps reveal regulatory programs driving early mouse organogenesis. Nat. Cell Biol. 22 487–497. 10.1038/s41556-020-0489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmann R. E., Gittenberger-de Groot A. C. (2019). Development and evolution of the metazoan heart. Dev. Dyn. 248 634–656. 10.1002/dvdy.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma A. V., Bezzina C. R., Christoffels V. M. (2015). Genetics of congenital heart disease: the contribution of the noncoding regulatory genome. J. Hum. Genet. 61 13–19. 10.1038/jhg.2015.98 [DOI] [PubMed] [Google Scholar]
- Pott S. (2017). Simultaneous measurement of chromatin accessibility. DNA methylation, and nucleosome phasing in single cells. eLife 6:e23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva E. M., Kubo N., Loukinov D., Tajmul M., Kang S., Kovalchuk A. L., et al. (2020). CTCF mediates chromatin looping via N-terminal domain-dependent cohesin retention. Proc. Natl. Acad. Sci. U.S.A. 117 2020–2031. 10.1073/pnas.1911708117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife-Ryan G. A., Sim C. B., Ziemann M., Kaspi A., Rafehi H., Ramialison M., et al. (2017). Multicellular transcriptional analysis of mammalian heart regeneration. Circulation 136 1123–1139. 10.1161/circulationaha.117.028252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinodoz S. A., Ollikainen N., Tabak B., Palla A., Schmidt J. M., Detmar E., et al. (2018). Higher-Order inter-chromosomal hubs shape 3D genome organization in the nucleus. Cell 174 744.e24–757.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racedo S. E., Hasten E., Lin M., Devakanmalai G. S., Guo T., Ozbudak E. M., et al. (2017). Reduced dosage of β-catenin provides significant rescue of cardiac outflow tract anomalies in a Tbx1 conditional null mouse model of 22q11.2 deletion syndrome. PLoS Genet. 13:e1006687. 10.1371/journal.pgen.1006687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racioppi C., Wiechecki K. A., Christiaen L. (2019). Combinatorial chromatin dynamics foster accurate cardiopharyngeal fate choices. eLife 8:e49921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S. A., Flynn R. A., Wysocka J. (2011). A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470 279–283. 10.1038/nature09692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. S. P., Huntley M. H., Durand N. C., Stamenova E. K., Bochkov I. D., Robinson J. T., et al. (2014). A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159 1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reecy J. M., Li X., Yamada M., DeMayo F. J., Newman C. S., Harvey R. P., et al. (1999). Identification of upstream regulatory regions in the heart-expressed homeobox gene Nkx2-5. Development 126 839–849. [DOI] [PubMed] [Google Scholar]
- Richter F., Morton S. U., Kim S. W., Kitaygorodsky A., Wasson L. K., Chen K. M., et al. (2020). Genomic analyses implicate noncoding de novo variants in congenital heart disease. Nat. Genet. 52 769–777. 10.1038/s41588-020-0652-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A., De Val S., Heidt A. B., Xu S. M., Bristow J., Black B. L. (2005). Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development 132 3405–3417. 10.1242/dev.01913 [DOI] [PubMed] [Google Scholar]
- Rojas A., Schachterle W., Xu S. M., Black B. L. (2009). An endoderm-specific transcriptional enhancer from the mouse Gata4 gene requires GATA and homeodomain protein-binding sites for function in vivo. Dev. Dyn. 238 2588–2598. 10.1002/dvdy.22091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Garrido M., Chapski D. J., Schmitt A. D., Kimball T. H., Karbassi E., Monte E., et al. (2017). High-resolution mapping of chromatin conformation in cardiac myocytes reveals structural remodeling of the epigenome in heart failure. Circulation 136 1613–1625. 10.1161/circulationaha.117.029430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A. J., Parker K. R., Satpathy A. T., Qi Y., Wu B., Ong A. J., et al. (2019). Coupled single-Cell CRISPR screening and epigenomic profiling reveals causal gene regulatory networks. Cell 176 361.e17–376.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlén P., Abdullayev I., Ramsköld D., Matskova L., Rilakovic N., Lötstedt B., et al. (2015). Genome-wide mapping of promoter-anchored interactions with close to single-enhancer resolution. Genome Biol. 16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani N. J., Schunkert H. (2008). Chromosome 9p21 and CARDIOVASCULAR DISEase. Circ. Cardiovasc. Genet. 1 81–84. 10.1161/circgenetics.108.832527 [DOI] [PubMed] [Google Scholar]
- Sanjana N., Montalbano A., Deng J., MéndezMancilla A., Wessels H.-H., Moss N. G., et al. (2020). Scalable pooled CRISPR screens with single-cell chromatin accessibility profiling. bioRxiv [Preprint]. 10.1101/2020.11.20.390971 [DOI] [Google Scholar]
- Santos R., Kawauchi S., Jacobs R. E., Lopez-Burks M. E., Choi H., Wikenheiser J., et al. (2016). Conditional creation and rescue of nipbl-deficiency in mice reveals multiple determinants of risk for congenital heart defects. PLoS Biol. 14:e2000197. 10.1371/journal.pbio.2000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachterle W., Rojas A., Xu S. M., Black B. L. (2012). ETS-dependent regulation of a distal Gata4 cardiac enhancer. Dev. Biol. 361 439–449. 10.1016/j.ydbio.2011.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaukowitch K., Joo J. Y., Liu X., Watts J. K., Martinez C., Kim T. K. (2014). Enhancer RNA facilitates NELF release from immediate early genes. Mol. Cell 56 29–42. 10.1016/j.molcel.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D., Schwalie P. C., Ross-Innes C. S., Hurtado A., Brown G. D., Carroll J. S., et al. (2010a). A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 20 578–588. 10.1101/gr.100479.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D., Wilson M. D., Ballester B., Schwalie P. C., Brown G. D., Marshall A., et al. (2010b). Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science 328 1036–1040. 10.1126/science.1186176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. F., Larsen B. D., Loft A., Mandrup S. (2016). Cofactor squelching: artifact or fact? BioEssays 38 618–626. 10.1002/bies.201600034 [DOI] [PubMed] [Google Scholar]
- Schmidt S. F., Larsen B. D., Loft A., Nielsen R., Madsen J. G. S., Mandrup S. (2015). Acute TNF-induced repression of cell identity genes is mediated by NFκB-directed redistribution of cofactors from super-enhancers. Genome Res. 25 1281–1294. 10.1101/gr.188300.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A. D., Hu M., Jung I., Xu Z., Qiu Y., Tan C. L., et al. (2016). A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Rep. 17 2042–2059. 10.1016/j.celrep.2016.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder S., Furlan-Magaril M., Mifsud B., Tavares-Cadete F., Sugar R., Javierre B. M., et al. (2015). The pluripotent regulatory circuitry connecting promoters to their long-range interacting elements. Genome Res. 25 582–597. 10.1101/gr.185272.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman O., Tanay A. (2015). Single-cell epigenomics: techniques and emerging applications. Nat. Rev. Genet. 16 716–726. 10.1038/nrg3980 [DOI] [PubMed] [Google Scholar]
- Scott I. C. (2012). Life Before Nkx2.5: Cardiovascular Progenitor Cells: Embryonic Origins and Development. Amsterdam: Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Searcy R. D., Vincent E. B., Liberatore C. M., Yutzey K. E. (1998). A GATA-dependent nkx-2.5 regulatory element activates early cardiac gene expression in transgenic mice. Development 125 4461–4470. [DOI] [PubMed] [Google Scholar]
- Serpooshan V., Liu Y. H., Buikema J. W., Galdos F. X., Chirikian O., Paige S., et al. (2017). Nkx2.5+ cardiomyoblasts contribute to cardiomyogenesis in the neonatal heart. Sci. Rep. 7 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon E., Kalma Y., Sharp A., Raveh-Sadka T., Levo M., Zeevi D., et al. (2012). Inferring gene regulatory logic from high-throughput measurements of thousands of systematically designed promoters. Nat. Biotechnol. 30 521–530. 10.1038/nbt.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shii L., Song L., Maurer K., Zhang Z., Sullivan K. E. (2017). SERPINB2 is regulated by dynamic interactions with pause-release proteins and enhancer RNAs. Mol. Immunol. 88 20–31. 10.1016/j.molimm.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. T., Priest J. R., Ovcharenko I., Ronco A., Moore R. K., Burns C. G., et al. (2005). Human-zebrafish non-coding conserved elements act in vivo to regulate transcription. Nucleic Acids Res. 33 5437–5445. 10.1093/nar/gki853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggers T., Duyzend M. H., Reddy J., Khan S., Bulyk M. L. (2011). Non-DNA-binding cofactors enhance DNA-binding specificity of a transcriptional regulatory complex. Mol. Syst. Biol. 7:555. 10.1038/msb.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonov D. R., Gowen B. G., Boontanrart M., Roth T. L., Gagnon J. D., Mumbach M. R., et al. (2017). Discovery of stimulation-responsive immune enhancers with CRISPR activation. Nature 549 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis M., Klous P., Splinter E., Moshkin Y., Willemsen R., de Wit E., et al. (2006). Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture–on-chip (4C). Nat. Genet. 38 1348–1354. 10.1038/ng1896 [DOI] [PubMed] [Google Scholar]
- Skene P. J., Henikoff S. (2017). An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M., Riley T., Liu P., Abe N., Gomez-Alcala P., Dror I., et al. (2011). Cofactor binding evokes latent differences in DNA binding specificity between hox proteins. Cell 147 1270–1282. 10.1016/j.cell.2011.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smemo S., Campos L. C., Moskowitz I. P., Krieger J. E., Pereira A. C., Nobrega M. A. (2012). Regulatory variation in a TBX5 enhancer leads to isolated congenital heart disease. Hum. Mol. Genet. 21 3255–3263. 10.1093/hmg/dds165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönmezer C., Kleinendorst R., Imanci D., Barzaghi G., Villacorta L., Schübeler D., et al. (2020). Molecular Co-occupancy identifies transcription factor binding cooperativity in vivo. Mol. Cell 81 1–13. 10.1016/s0022-2836(02)00894-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz F., Furlong E. E. M. (2012). Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 13 613–626. 10.1038/nrg3207 [DOI] [PubMed] [Google Scholar]
- Spurrell C. H., Barozzi I., Mannion B. J., Blow M. J., Fukuda-Yuzawa Y., Afzal S. Y., et al. (2019). Genome-wide fetalization of enhancer architecture in heart disease. bioRxiv [Preprint]. 10.1101/591362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimle J. D., Moskowitz I. P. (2017). TBX5: a key regulator of heart development. Curr. Top. Dev. Biol. 122 195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson A., Adams J. W., Vaccarezza M. (2017). The vertebrate heart: an evolutionary perspective. J. Anat. 231 787–797. 10.1111/joa.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagle D. A., Koop B. F., Goodman M., Slightom J. L., Hess D. L., Jones R. T. (1988). Embryonic ε and γ globin genes of a prosimian primate (Galago crassicaudatus). Nucleotide and amino acid sequences, developmental regulation and phylogenetic footprints. J. Mol. Biol. 203 439–455. 10.1016/0022-2836(88)90011-3 [DOI] [PubMed] [Google Scholar]
- Takeuchi J. K., Mileikovskaia M., Koshiba-Takeuchi K., Heidt A. B., Mori A. D., Arruda E. P., et al. (2005). Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development 132 2463–2474. 10.1242/dev.01827 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Chen Z., Bartunkova S., Yamasaki N., Izumo S. (1999). The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126 1269–1280. [DOI] [PubMed] [Google Scholar]
- Thurman R. E., Rynes E., Humbert R., Vierstra J., Maurano M. T., Haugen E., et al. (2012). The accessible chromatin landscape of the human genome. Nature 489 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkin T., Christiaen L. (2012). Development and Evolution of the Ascidian Cardiogenic Mesoderm. Amsterdam: Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Torosin N. S., Anand A., Golla T. R., Cao W., Ellison C. E. (2020). 3D genome evolution and reorganization in the Drosophila melanogaster species group. PLoS Genet. 16:e1009229. 10.1371/journal.pgen.1009229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker N. R., Chaffin M., Fleming S. J., Hall A. W., Parsons V. A., Bedi K. C., et al. (2020). Transcriptional and cellular diversity of the human heart. Circulation 142 466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton N., Swan R., Mahenthiralingam T., Pitts D., Dykes I. M. (2019). The functions of long non-coding RNA during embryonic cardiovascular development and its potential for diagnosis and treatment of congenital heart disease. J. Cardiovasc. Dev. Dis. 6:21. 10.3390/jcdd6020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard M., Smemo S., Burnicka-Turek O., Arnolds D. E., van de Werken H. J. G., Klous P., et al. (2014). A common genetic variant within SCN10A modulates cardiac SCN5A expression. J. Clin. Invest. 124 1844–1852. 10.1172/jci73140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard M., Wong L. Y. E., Tessadori F., Bakker M. L., Dreizehnter L. K., Wakker, et al. (2012). Genetic variation in T-box binding element functionally affects SCN5A / SCN10A enhancer Find the latest version. J. Clin. Invest. 122 2519–2530. 10.1172/jci62613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee R., Correard S., Wasserman W. W. (2020). Deregulated regulators: disease-causing cis variants in transcription factor genes. Trends Genet. 36 523–539. 10.1016/j.tig.2020.04.006 [DOI] [PubMed] [Google Scholar]
- van Eif V. W., Protze S., Bosada F. M., Yuan X., Sinha T., van Duijvenboden K., et al. (2020). Genome-wide analysis identifies an essential human tbx3 pacemaker enhancer. Circ. Res. 127 1522–1535. 10.1161/circresaha.120.317054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ouwerkerk A. F., Bosada F., Liu J., Zhang J., van Duijvenboden K., Chaffin M., et al. (2020). Identification of functional variant enhancers associated with atrial fibrillation. Circ. Res. 127 229–243. 10.1161/circresaha.119.316006 [DOI] [PubMed] [Google Scholar]
- van Ouwerkerk A. F., Bosada F. M., van Duijvenboden K., Hill M. C., Montefiori L. E., Scholman K. T., et al. (2019). Identification of atrial fibrillation associated genes and functional non-coding variants. Nat. Commun. 10 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoudenhove J., Yankee T. N., Wilderman A., Cotney J. (2020). Epigenomic and transcriptomic dynamics during human heart organogenesis. Circ. Res. 127 E184–E209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman C. C., Wilde A. A. M., Lodder E. M. (2015). The cardiac sodium channel gene SCN5A and its gene product NaV1.5: role in physiology and pathophysiology. Gene 573 177–187. 10.1016/j.gene.2015.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M., Kumar V. (2019). Single-Cell Epigenomics: Technology and Applications. Amsterdam: Elsevier Inc. [Google Scholar]
- Verzi M. P., McCulley D. J., De Val S., Dodou E., Black B. L. (2005). The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 287 134–145. 10.1016/j.ydbio.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Vierstra J., Lazar J., Sandstrom R., Halow J., Lee K., Bates D., et al. (2020). Global reference mapping of human transcription factor footprints. Nature 583 729–736. 10.1038/s41586-020-2528-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra J., Rynes E., Sandstrom R., Zhang M., Canfield T., Hansen R. S., et al. (2014). Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science 346 1007–1012. 10.1126/science.1246426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietri Rudan M., Barrington C., Henderson S., Ernst C., Odom D. T., Tanay A., et al. (2015). Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Rep. 10 1297–1309. 10.1016/j.celrep.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar D., Berthelot C., Aldridge S., Rayner T. F., Lukk M., Pignatelli M., et al. (2015). Enhancer evolution across 20 mammalian species. Cell 160 554–566. 10.1016/j.cell.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A., Minovitsky S., Dubchak I., Pennacchio L. A. (2007). VISTA Enhancer Browser–a database of tissue-specific human enhancers. Nucleic Acids Res. 35 D88–D92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A., Taher L., Girgis H., May D., Golonzhka O., Hoch R. V., et al. (2013). A high-resolution enhancer atlas of the developing telencephalon. Cell 152 895–908. 10.1016/j.cell.2012.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A., Zhu Y., May D., Afzal V., Gong E., Attanasio C., et al. (2010). Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 464 409–412. 10.1038/nature08801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waardenberg A. J., Ramialison M., Bouvere R., Harvey R. P. (2014). Genetic networks governing heart development. Cold Spring Harb. Perspect. Med. 4 1–24. 10.1155/2017/4135956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamstad J. A., Alexander J. M., Truty R. M., Shrikumar A., Li F., Eilertson K. E., et al. (2012). Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 151 206–220. 10.1016/j.cell.2012.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Liu D., Zhang R.-R., Yu L.-W., Zhao J.-Y., Yang X.-Y., et al. (2017). A TBX5 3′UTR variant increases the risk of congenital heart disease in the Han Chinese population. Cell Discov. 3 1–13. 10.1161/circulationaha.111.circulationaha.111.050245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Cui M., Shah A. M., Tan W., Liu N., Bassel-Duby R., et al. (2020). Cell-type-specific gene regulatory networks underlying murine neonatal heart regeneration at single-cell resolution. CellReports 33:108472. 10.1016/j.celrep.2020.108472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Z., Valdez M. R., McAnally J., Richardson J., Olson E. N., Da-Zhi W. (2001). Myogenic bHLH and MEF2 Proteins Directly Regulate the Mef2c gene - 4623.Full.pdf. Development. Available online at: https://dev.biologists.org/content/128/22/4623.short [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Zaffran S., Kuroiwa A., Higuchi H., Ogura T., Harvey R. P., et al. (2012). Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc. Natl. Acad. Sci. U.S.A. 109 18273–18280. 10.1073/pnas.1215360109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissink E. M., Vihervaara A., Tippens N. D., Lis J. T. (2019). Nascent RNA analyses: tracking transcription and its regulation. Nat. Rev. Genet. 20 705–723. 10.1038/s41576-019-0159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. S., Zheng D., Tan S. Z., Bower N. L., Garside V., Vanwalleghem G., et al. (2020). Deep conservation of the enhancer regulatory code in animals. Science 370:eaax8137. 10.1126/science.aax8137 [DOI] [PubMed] [Google Scholar]
- Woolfe A., Goodson M., Goode D. K., Snell P., McEwen G. K., Vavouri T., et al. (2005). Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol. 3:e7. 10.1371/journal.pbio.0030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Qi L. S. (2019). A CRISPR–dCas toolbox for genetic engineering and synthetic biology. J. Mol. Biol. 431 34–47. 10.1016/j.jmb.2018.06.037 [DOI] [PubMed] [Google Scholar]
- Yanai I., Peshkin L., Jorgensen P., Kirschner M. W. (2011). Mapping gene expression in two Xenopus species: evolutionary constraints and developmental flexibility. Dev. Cell 20 483–496. 10.1016/j.devcel.2011.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. H., Nadadur R. D., Hilvering C. R., Bianchi V., Werner M., Mazurek S. R., et al. (2017). Transcription-factor-dependent enhancer transcription defines a gene regulatory network for cardiac rhythm. eLife 6:e31683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Minor P. J., Zhao Y.-T., Jeong Y., Pani A. M., King A. N., et al. (2016). Cis-regulatory architecture of a brain signaling center predates the origin of chordates. Nat. Genet. 48 575–580. 10.1038/ng.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Song M., Devine P., Bruneau B. G., Scott I. C., Wilson M. D. (2018). Heart enhancers with deeply conserved regulatory activity are established early in zebrafish development. Nat. Commun. 9:4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S. (2020). Pioneer transcription factors initiating gene network changes. Annu. Rev. Genet. 54 367–385. 10.1146/annurev-genet-030220-015007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J. (2020). Seven myths of how transcription factors read the cis-regulatory code. Curr. Opin. Syst. Biol. 23 22–31. 10.1016/j.coisb.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner G. E., Tesar P. J., Scacheri P. C. (2011). Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res. 21 1273–1283. 10.1101/gr.122382.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Lupski J. R. (2015). Non-coding genetic variants in human disease. Hum. Mol. Genet. 24 R102–R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li T., Preissl S., Amaral M. L., Grinstein J. D., Farah E. N., et al. (2019). Transcriptionally active HERV-H retrotransposons demarcate topologically associating domains in human pluripotent stem cells. Nat. Genet. 51 1380–1388. 10.1038/s41588-019-0479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Liu J., Zhou C., Gao N., Rao Z., Li H., et al. (2018). In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nat. Neurosci. 21 440–446. 10.1038/s41593-017-0060-6 [DOI] [PubMed] [Google Scholar]
- Zhou P., Gu F., Zhang L., Akerberg B. N., Ma Q., Li K., et al. (2017). Mapping cell type-specific transcriptional enhancers using high affinity, lineage-specific Ep300 bioChIP-seq. eLife 6:e22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzen R. P., Girardot C., Gagneur J., Braun M., Furlong E. E. M. (2009). Combinatorial binding predicts spatio-temporal cis-regulatory activity. Nature 462 65–70. 10.1038/nature08531 [DOI] [PubMed] [Google Scholar]