Abstract
Objective
To study longitudinal relationships between type 2 diabetes mellitus (T2DM), cortical thickness, and cognitive function in older people with normal cognition, mild cognitive impairment, and Alzheimer disease (AD).
Methods
The sample was derived from the Alzheimer's Disease Neuroimaging Initiative cohort who underwent brain MRI and cognitive tests annually for 5 years. Presence of T2DM was based on fasting blood glucose ≥7.0mml/L or the use of glucose-lowering agents. We used latent growth curve modeling to explore longitudinal relationships between T2DM, cortical thickness, and cognitive function, adjusting for relevant covariates and testing for interactions.
Results
There were 124 people with T2DM (mean age 75.5 years, SD 6.2) and 693 without T2DM (mean age 75.1 years, SD 6.9) with at least 1 MRI available. AD and lower cortical thickness at study entry was associated with a lower chance of having a MRI available at each follow-up phase (all p < 0.001). T2DM was associated with lower baseline cortical thickness (p = 0.01). We found no direct effect of T2DM on decline in cortical thickness or cognitive function, but there was an indirect pathway linking T2DM and cognitive decline via baseline cortical thickness (β = −0.17, p = 0.022). There was an interaction between T2DM and education whereby the negative effect of T2DM on baseline cortical thickness was reduced in those with greater education (β = 0.34, p = 0.037). These associations changed minimally when adjusted for baseline cognitive diagnosis.
Conclusions
In an older cohort with low cerebrovascular disease burden, T2DM contributes to cognitive decline via neurodegeneration. Prior brain and cognitive reserve may protect against this effect.
Type 2 diabetes mellitus (T2DM) is associated with as much as twofold increased risk of dementia and Alzheimer disease (AD) dementia,1–3 and may present an important modifiable risk factor for reducing the population burden of dementia.4 There is limited understanding about mechanisms underlying this association, which likely involve both cerebrovascular disease and neurodegeneration.2 Most published studies,5,6 including ours,7,8 have demonstrated cross-sectional associations of T2DM with poorer cognitive function or lower brain volumes. T2DM has also been shown to be associated with greater cognitive decline9 and in a few studies with greater brain atrophy.10–12 However, there are no published studies examining brain atrophy and cognitive decline together in T2DM, and it is unknown if brain atrophy mediates the relationship between T2DM and cognitive decline.13
There are no data exploring the influence of T2DM on the trajectories of brain atrophy in the presence of mild cognitive impairment (MCI) or AD dementia, where one would expect to see acceleration of brain atrophy. In the Alzheimer's Disease Neuroimaging Initiative (ADNI) sample, which was selected to exclude those with significant cerebrovascular disease, we demonstrated lower cortical thickness in those with T2DM.7 In that analysis, although cortical thickness itself was lower in MCI and AD dementia compared with those with normal cognition, the association of T2DM with cortical thickness was independent of cognitive diagnosis.7 We aimed to build upon this work and study whether the presence of T2DM was associated with acceleration of cortical thinning and consequent cognitive decline in the ADNI sample.
Methods
The data used for this analysis were obtained from the ADNI database (ida.loni.usc.edu).14 ADNI was launched in 2003 with the primary aim of identifying MRI, PET, CSF, biochemical, clinical, and neuropsychological biomarkers of progression of MCI to AD dementia.14 ADNI aimed to recruit 800 adults between the ages of 55 and 90 years with 400 people with MCI, 200 people with early probable AD dementia, and 200 normal controls. These cognitive diagnoses were based on the National Institute of Neurologic and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association.15 Those with MCI had memory complaints16 but no significant functional impairment based on Clinical Dementia Rating.17 Participants were excluded if they were at high risk of cerebrovascular disease contributing to cognitive impairment defined by Hachinski ischemic score18 >4, were unable to undergo MRI, had other neurologic disorders, active depression, history of psychiatric diagnosis, or alcohol or substance dependence in the last 2 years, had less than 6 years of education, or were not fluent in English or Spanish.
Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all participants. Full details of ethics approval, study design, participant recruitment, and clinical testing have been published previously and are available at adni-info.org.
Clinical and genetic data
Data on demographic information, medical history, APOE ε4 (APOE4) genotype, baseline cognitive diagnosis (AD, MCI, or cognitively normal), fasting venous blood glucose levels, and medication use were downloaded from the ADNI clinical data database in August 2013. We assigned diabetes status based on fasting blood glucose ≥7.0 mml/L as per American Diabetes Association guidelines19 or the use of glucose-lowering agents. APOE4 genotyping was performed on venous blood-derived DNA at the ADNI Biomarker Core Laboratory (University of Pennsylvania) and participants deemed APOE4 positive if they carried at least one APOE4 allele.
MRI scans and image processing
The process for MRI acquisition has been described previously in ADNI publications.20,21 In brief, all ADNI participants had a 1.5T MRI performed at either screening or baseline visits between August 2005 and October 2007. The ADNI project offers scans that have been preprocessed (gradient warping, scaling, B1 correction, and N3 inhomogeneity correction) to correct for different scanners across sites.22 In our imaging laboratory, we used the FreeSurfer v5.3 (surfer.nmr.mgh.harvard.edu/) longitudinal processing pipeline to estimate mean cortical thickness, supratentorial brain volume, ventricular volume, and total intracranial volume. Participants with only baseline MRI were processed using the single time point template creation option (surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing), ensuring that all scans received the same processing steps.
Neuropsychological assessment
The neuropsychological assessment procedures have been described in previous ADNI publications.23 In brief, the following cognitive assessments were used in our analysis: American National Reading Test (premorbid intelligence), Rey Auditory Verbal Learning Test (short-term memory), Boston Naming Test (word retrieval), category fluency (verbal fluency), Clock Drawing Test (visuospatial function), Alzheimer’s Disease Assessment Scale–cognitive subscale, Construction Praxis Test (conceptual and motor spatial function), Digit Span forwards (attention) and backwards (working memory) tasks, Trail-Making Test A & B (motor speed and attention), Wechsler Adult Intelligence Scale–Revised (WAIS-R) symbol substitution test, and Wechsler Memory Scale–Revised (memory).24
Data analysis
Student t test and χ2 tests were applied to compare baseline demographic, clinical, and cognitive variables between T2DM and non-T2DM groups.
Confirmatory factor analysis (CFA)
CFA was conducted using the baseline cognitive data to reduce the large number of cognitive tests to a single latent variable for each timepoint. Maximum likelihood estimation with robust standard errors (MLR) was used that is robust to non-normally distributed data. The CFA model was evaluated using a set of fit indices, including χ2 statistics, its accompanying significance tests and scaling correction factor for MLR, root mean square error of approximation (RMSEA) with its 90% confidence interval, standardized root mean square residual (SRMR), Tucker-Lewis index (TLI), and comparative fit index (CFI).25 Adequate model fit was considered likely with χ2 probability p > 0.05 or scaling correction factor for MLR > 0.05, RMSEA < 0.05, SRMR < 0.05, TLI > 0.95, and CFI > 0.95.
Multiple imputation (MI)
We used MI to accommodate for the common issue of participant attrition in longitudinal studies. MI is increasingly becoming the preferred approach to handle missing values as it allows more accurate estimates, increases the statistical power to detect associations, and improves the generalizability of results.26 We performed the MI using a 2-stage sequence.27 First, missing data in the repeated measures of cortical thickness and cognitive function were imputed based on the characteristics of the nonmissing data at each time point and the covariates T2DM, sex, education, age, APOE4 status, and cognition. This was done 5 times to create 5 different plausible imputed datasets28 using Bayesian estimation. Second, the model was fitted separately to each of the 5 complete datasets (incorporating observed and imputed values). The results were combined using the MI combining rules29 to give parameter estimates and standard errors that account for the uncertainty due to the missing data.
We used a parallel process latent growth curve model (LGCM)30 to examine the relationships between covariates (i.e., T2DM, sex, education, age, APOE4), cortical thickness, and cognitive function. These associations were identified in previous work.9–12 The use of such specified models appears to be more accurate than any of the unrestricted model imputations.31
An assumption of MI is that data are missing at random (MAR). Although MAR is empirically unverifiable,28 we carried out sensitivity analyses using Diggle-Kenward selection models to explore whether missing data were ignorable.32
All analyses were conducted via statistical programs of SPSS 2533 and Mplus 8.1.34
The data used in this study are available via Laboratory of Neuroimaging Image & Data Archive on application adni.loni.usc.edu/data-samples/access-data/
Results
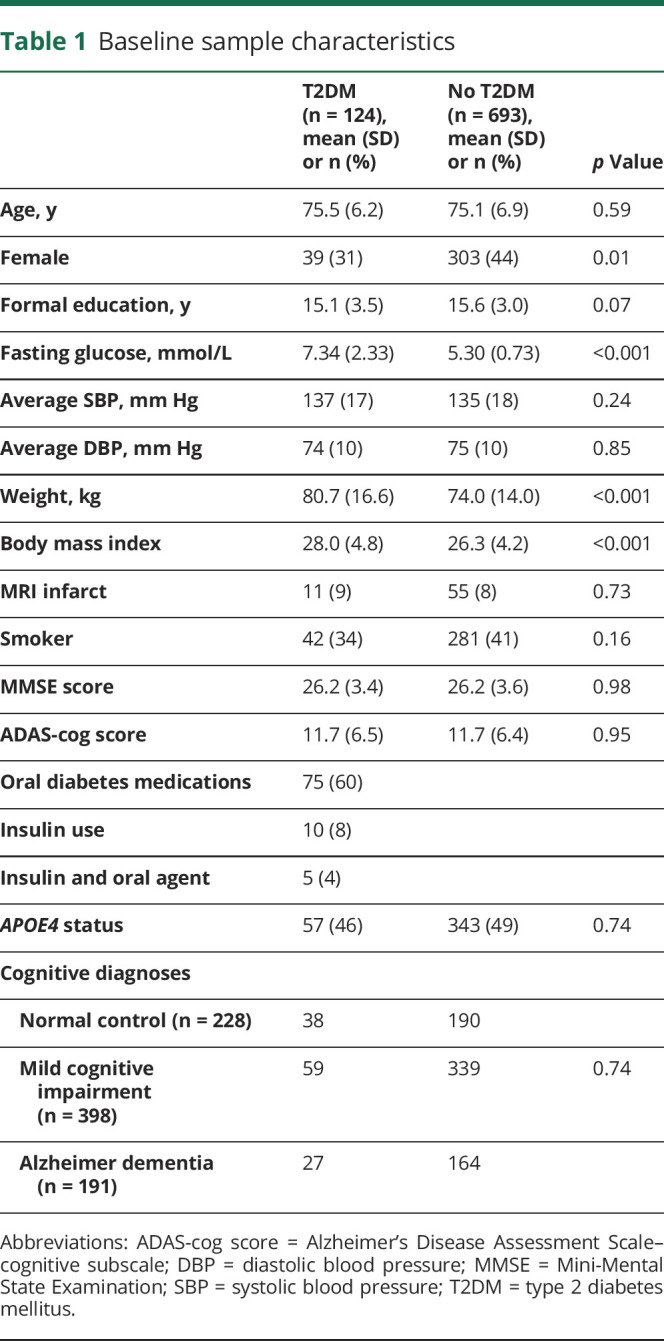
There were 817 people with at least 1 brain MRI scan available. Table 1 describes the characteristics of the study participants at study commencement. There were 124 people with T2DM (mean age 75.5 years [SD 6.2]) and 693 people without T2DM (mean age 75.1 years [SD 6.9]). Those with T2DM were more likely to be male (69%) compared with those without T2DM (male 56%). As expected, those with T2DM had greater fasting blood glucose levels and body mass index than those without T2DM. A total of 75 participants used oral hypoglycemic agents to control their T2DM and 10 used insulin (5 of whom were also on oral agents). The numbers and proportions of T2DM in each cognitive diagnostic group were 38/228 (17%) among cognitively normal controls, 59/398 (15%) among those with MCI, and 27/191 (14%) among those with AD dementia.
Table 1.
Baseline sample characteristics
Data were available for 620 participants at 12 months follow up, 487 at 24 months, 302 at 36 months, 198 at 48 months, and 56 at 60 months. The mean follow-up time was 30 (SD 20.5) months. Table 2 summarizes the numbers and characteristics of people who did and did not have MRI scans available at each time point. The number of people with brain MRI available at each time point decreased over time, more so among those with T2DM than those without T2DM (p = 0.001). There were no differences in age between those who did or did not have MRI scans available. However, the presence of AD and lower cortical thickness at study entry was associated with a greater chance of not having a brain MRI available at each follow-up phase (all p < 0.001).
Table 2.
Characteristics of participants with and without MRI scans available at each time point


CFA of cognitive measures
Factor analysis generated a single factor to summarize global cognitive function represented by 5 test scores: 30 minutes recall Auditory Verbal Reading Test, Boston Naming task, WAIS-R symbol substitution score, and category fluency. The model fitted the baseline data well, χ2 (5) = 32.14, p = 0.00, with a scaling correction factor for MLR of 0.93, SRMR = 0.02, CFI = 0.98, TLI = 0.96, RMSEA = 0.08 (0.06–0.11). All standardized factor loadings were above 0.60.
T2DM, cognition, and cortical thickness
The figure shows the standardized regression coefficients of the LGCM with the covariates T2DM, age, sex, education, and APOE4 status in explaining interindividual differences in baseline values and trajectories of cognitive function and cortical thickness. Lower baseline cortical thickness and greater loss of cortical thickness over time were associated with greater cognitive decline (p < 0.001). Greater baseline age was associated with lower baseline cortical thickness and greater loss of cortical thickness over time (p < 0.001). T2DM was associated with lower cortical thickness at baseline (p = 0.008), and there was an interaction between T2DM and education whereby the negative effect of T2DM on baseline cortical thickness was reduced in those with greater education (interaction β = 0.34, p = 0.037). There was no direct effect of T2DM on the slope of cortical thickness or cognitive function. However, there was an indirect pathway linking T2DM with greater cognitive decline via its association with lower baseline cortical thickness (β = −0.176, p = 0.022). There was a weak indirect pathway linking the T2DM × education interaction with cognitive decline via its association with lower baseline cortical thickness, not reaching statistical significance (β = 0.136, p = 0.06). Additional adjustment for baseline cognitive diagnosis did not show appreciable change these results (data not shown). There was no interaction between T2DM and baseline cognitive diagnosis with any of the outcome variables.
Figure. Change in cognitive function.
Statistically significant cross-sectional and longitudinal associations between covariables, cortical thickness, and cognitive function. β = standardized β coefficient; SE = standard error; ns = not statistically significant, i.e., p > 0.05, *p < 0.05, **p < 0.01.
In the Diggle-Kenward sensitivity analyses, examining whether data were missing at random, there were nonsignificant associations between baseline measurement and corresponding time-point outcomes where that outcome was missing: 30 minutes recall Auditory Verbal Reading Test, p = 0.27; category (animal), p = 0.60; category (vegetable), p = 0.96; WAIS-R symbol substitution score, p = 0.15; and cortical thickness, p = 0.23.
Data availability
The data used for this analysis are available on request from the ADNI database (ida.loni.usc.edu).
Discussion
In the ADNI sample selected for risk of progression to AD and relatively free of cerebrovascular disease, we did not find a direct effect of T2DM on the rate of decline in cortical thickness or cognitive function. However, T2DM was indirectly related to greater cognitive decline via its association with lower cortical thickness at baseline. This effect, together with the moderating effect of education on the T2DM–baseline cortical thickness association, suggests that the negative effect of T2DM on the brain may be dependent on brain and cognitive reserve.
To date, there have been only 3 reports of longitudinal studies describing the link between T2DM and brain structure,10–12 with variable results. In a sample of people with a similar baseline age (∼75 years) as ours, people with T2DM (n = 89) had greater brain atrophy over 3 years than those without T2DM,10 whereas in a slightly older cohort, (mean age ∼78 years) no association was found (n = 58).12 In a younger sample (mean age∼65 years), the presence of T2DM (n = 55) was associated with a greater rate of lateral ventricular volume expansion, but not whole brain volume loss, over a 4-year period.11 We confirmed the finding in prior longitudinal studies10–12 of an association of T2DM with lower baseline brain volumes, raising the possibility that the effect of T2DM on neurodegeneration may commence at an earlier age (e.g., midlife) and result in the reduction of brain reserve. Accordingly, we have previously demonstrated substantial differences in functional brain activation in twin pairs discordant for T2DM around age 60,35 which may be early signs of T2DM-related neurodegeneration. In addition, the moderating effect of education on the association between T2DM and baseline cortical thickness in the present study is also supportive of a role for cognitive reserve36 in protecting against the adverse effect of T2DM on neuronal health.
Surprisingly, in the present study, in spite of studying a sample of people at a high risk of dementia, we did not find a direct effect of T2DM on decline in cortical thickness or cognitive decline. Our sample was clearly at low cerebrovascular risk compared with previous studies10,11 (lower stroke prevalence and lower blood pressure), and this relative lack of vascular disease may have masked direct effects of T2DM on decline in cortical thickness and cognitive function. Supporting the importance of cerebrovascular disease in T2DM-related dementia, investigators of a recent large autopsy study (n = 2,365, mean age 89 years) reported that diabetes was associated with a greater burden of brain infarcts, and that the combination of diabetes and infarcts was associated with lower cognitive scores at the end of life than either infarcts or diabetes alone.37 It is thus possible that the combination of T2DM and cerebrovascular disease may be required to cause greater brain atrophy during old age even in those at high risk of AD dementia. Apart from the potential contribution of cerebrovascular disease, an interesting question that is often raised is whether the T2DM–dementia relationship is influenced by amyloid pathology, as usually seen in AD dementia. Consistent with a non-AD process, we found no interaction between T2DM and APOE4 genotype in predicting decline in cortical thickness or cognitive function. Accordingly, we7 and others38–40 have previously not found associations between T2DM and brain or CSF amyloid levels. Thus, T2DM may contribute to neuronal loss by its additive effects on age-related neurodegeneration (e.g., suspected non-Alzheimer pathophysiology or primary age-related tauopathy), by mechanisms including neuroinflammation, glycation, or insulin-signaling.41 An additional possibility is that the relationship between metabolic health and brain aging may even exist before the onset (formal diagnosis) of T2DM, the latter being a constellation of features (hyperglycemia, insulin resistance, hypertension, adiposity), each of which can have individual contributions to brain health. We could not address the contributions of these factors to cognitive decline in this study. Finally, it is also possible that those at genetic risk for T2DM are somehow primed to also develop accelerated brain aging, a scenario of shared risk rather than direct causality. Such questions could be addressed in specially designed cohorts beginning at birth or young age.
There are limitations to our study apart from the low cerebrovascular load in our sample. Sample attrition may explain some of our findings. At each time point, cognitively normal participants were more likely to have an MRI performed than those with cognitive impairment. Such attrition of the most impaired participants in longitudinal studies is well-recognized42 and this bias may have reduced the ability to detect a direct effect of T2DM on the rate of cortical thickness or cognitive decline. Hence our results do not conclusively rule out a direct contribution of T2DM to a decline in cortical thickness or cognitive function. The number of participants with T2DM was not high to begin with, and declined over time, potentially reducing study power to detect differences. To reduce the effect of attrition, we used a robust multiple imputation technique that took into account the greater risk of missing data in people with diabetes, cognitive impairment, and lower cortical thickness and performed sensitivity analyses that while not definitive, suggest that our assumption that data were missing at random was valid.43 Because of declining numbers, and the even smaller proportion of people who may have undergone CSF assays, we were unable to link changes in CSF amyloid or tau with T2DM and our other measures of interest. As the primary objectives of ADNI were not related to the study of T2DM, information regarding duration of T2DM and glucose control were unavailable, and these would have provided extra information in exploring whether diabetes management affected the reported relationships. It is also possible that people with T2DM in our study had other unmeasured factors that reduced their risk of cerebrovascular disease and as such, limit the generalizability of our results. Our study also has a number of important strengths. We used a well-characterized sample with objectively defined T2DM, and adopted established approaches to imaging analysis blinded to T2DM status. With our LGCM approach, we were able to provide a simultaneous estimate of effects of several exposures, and generate standardized coefficients to provide a comparative picture of the magnitude of contributions for each relevant exposure. For example, as shown in the figure, the strength of the association between T2DM and baseline cortical thickness was larger than that of the APOE4 genotype and baseline cortical thickness.
Our results suggest that T2DM may contribute to cognitive decline indirectly via its association with lower baseline cortical thickness, potentially because of reduction in brain reserve. Greater education, a proxy of cognitive reserve, may protect against the expression of T2DM-related cognitive decline. Finally, the expression of T2DM-related cognitive decline during old age may be more evident in people with a higher risk or load of cerebrovascular disease. Thus, enhancing reserve and preventing cerebrovascular disease may be worthwhile avenues to prevent future cognitive decline in people with T2DM.
Glossary
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- APOE4
APOE ε4
- CFA
confirmatory factor analysis
- CFI
comparative fit index
- LGCM
latent growth curve model
- MAR
missing at random
- MCI
mild cognitive impairment
- MI
multiple imputation
- MLR
maximum likelihood estimation with robust standard errors
- RMSEA
root mean square error of approximation
- SRMR
standardized root mean square residual
- T2DM
type 2 diabetes mellitus
- TLI
Tucker-Lewis index
- WAIS-R
Wechsler Adult Intelligence Scale–Revised
Contributor Information
Collaborators: Michael Weiner, Paul Aisen, Michael Weiner, Paul Aisen, Ronald Petersen, Clifford R. Jack, Jr., William Jagust, John Q. Trojanowki, Arthur W. Toga, Laurel Beckett, Robert C. Green, Andrew J. Saykin, John Morris, Enchi Liu, Robert C. Green, Tom Montine, Ronald Petersen, Paul Aisen, Anthony Gamst, Ronald G. Thomas, Michael Donohue, Sarah Walter, Devon Gessert, Tamie Sather, Laurel Beckett, Danielle Harvey, Anthony Gamst, Michael Donohue, John Kornak, Clifford R. Jack, Jr., Anders Dale, Matthew Bernstein, Joel Felmlee, Nick Fox, Paul Thompson, Norbert Schuff, Gene Alexander, Charles DeCarli, William Jagust, Dan Bandy, Robert A. Koeppe, Norm Foster, Eric M. Reiman, Kewei Chen, Chet Mathis, John Morris, Nigel J. Cairns, Lisa Taylor-Reinwald, J.Q. Trojanowki, Les Shaw, Virginia M.Y. Lee, Magdalena Korecka, Arthur W. Toga, Karen Crawford, Scott Neu, Andrew J. Saykin, Tatiana M. Foroud, Steven Potkin, Li Shen, Zaven Kachaturian, Richard Frank, Peter J. Snyder, Susan Molchan, Jeffrey Kaye, Joseph Quinn, Betty Lind, Sara Dolen, Lon S. Schneider, Sonia Pawluczyk, Bryan M. Spann, James Brewer, Helen Vanderswag, Judith L. Heidebrink, Joanne L. Lord, Ronald Petersen, Kris Johnson, Rachelle S. Doody, Javier Villanueva-Meyer, Munir Chowdhury, Yaakov Stern, Lawrence S. Honig, Karen L. Bell, John C. Morris, Beau Ances, Maria Carroll, Sue Leon, Mark A. Mintun, Stacy Schneider, Daniel Marson, Randall Griffith, David Clark, Hillel Grossman, Effie Mitsis, Aliza Romirowsky, Leyla deToledo-Morrell, Raj C. Shah, Ranjan Duara, Daniel Varon, Peggy Roberts, Marilyn Albert, Chiadi Onyike, Stephanie Kielb, Henry Rusinek, Mony J de Leon, Lidia Glodzik, Susan De Santi, P. Murali Doraiswamy, Jeffrey R. Petrella, R. Edward Coleman, Steven E. Arnold, Jason H. Karlawish, David Wolk, Charles D. Smith, Greg Jicha, Peter Hardy, Oscar L. Lopez, MaryAnn Oakley, Donna M. Simpson, Anton P. Porsteinsson, Bonnie S. Goldstein, Kim Martin, Kelly M. Makino, M. Saleem Ismail, Connie Brand, Ruth A. Mulnard, Gaby Thai, Catherine McAdams-Ortiz, Kyle Womack, Dana Mathews, Mary Quiceno, Ramon Diaz-Arrastia, Richard King, Myron Weiner, Kristen Martin-Cook, Michael DeVous, Allan I. Levey, James J. Lah, Janet S. Cellar, Jeffrey M. Burns, Heather S. Anderson, Russell H. Swerdlow, Liana Apostolova, Po H. Lu, George Bartzokis, Daniel H.S. Silverman, Neill R Graff-Radford, Francine Parfitt, Heather Johnson, Martin R. Farlow, Ann Marie Hake, Brandy R. Matthews, Scott Herring, Christopher H. van Dyck, Richard E. Carson, Martha G. MacAvoy, Howard Chertkow, Howard Bergman, Chris Hosein, Sandra Black, Bojana Stefanovic, Curtis Caldwell, Ging-Yuek Robin Hsiung, Howard Feldman, Benita Mudge, Michele Assaly, Andrew Kertesz, John Rogers, Dick Trost, Charles Bernick, Donna Munic, Diana Kerwin, Marek-Marsel Mesulam, Kristina Lipowski, Chuang-Kuo Wu, Nancy Johnson, Carl Sadowsky, Walter Martinez, Teresa Villena, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Reisa A. Sperling, Keith A. Johnson, Gad Marshall, Meghan Frey, Jerome Yesavage, Joy L. Taylor, Barton Lane, Allyson Rosen, Jared Tinklenberg, Marwan Sabbagh, Christine Belden, Sandra Jacobson, Neil Kowall, Ronald Killiany, Andrew E. Budson, Alexander Norbash, Patricia Lynn Johnson, Thomas O. Obisesan, Saba Wolday, Salome K. Bwayo, Alan Lerner, Leon Hudson, Paula Ogrocki, Evan Fletcher, Owen Carmichael, John Olichney, Charles DeCarli, Smita Kittur, Michael Borrie, T-Y Lee, Dr Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M. Carlsson, Steven G. Potkin, Adrian Preda, Dana Nguyen, Pierre Tariot, Adam Fleisher, Stephanie Reeder, Vernice Bates, Horacio Capote, Michelle Rainka, Douglas W. Scharre, Maria Kataki, Earl A. Zimmerman, Dzintra Celmins, Alice D. Brown, Godfrey D. Pearlson, Karen Blank, Karen Anderson, Andrew J. Saykin, Robert B. Santulli, Eben S. Schwartz, Kaycee M. Sink, Jeff D. Williamson, Pradeep Garg, Franklin Watkins, Brian R. Ott, Henry Querfurth, Geoffrey Tremont, Stephen Salloway, Paul Malloy, Stephen Correia, Howard J. Rosen, Bruce L. Miller, Jacobo Mintzer, Crystal Flynn Longmire, Kenneth Spicer, Elizabether Finger, Irina Rachinsky, John Rogers, Andrew Kertesz, Dick Drost, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Susan K. Schultz, Laura L. Boles Ponto, Hyungsub Shim, Karen Elizabeth Smith, Norman Relkin, Gloria Chaing, Lisa Raudin, Amanda Smith, Kristin Fargher, and Balebail Ashok Raj
Author contributions
Statistical analysis was performed by W. Wang, R. Beare, and C. Moran. C. Moran conceived the idea for the study, conducted the analysis, and wrote the original draft of the manuscript. R. Beare conducted the analysis and contributed to the writing of the manuscript. W. Wang conducted the analysis and contributed to the writing of the manuscript. M. Callisaya contributed to the writing of the manuscript. V. Srikanth conceived the idea for the study and contributed to the writing of the manuscript.
Study funding
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Disclosure
C. Moran is a recipient of an NMRC-ARC Dementia Research Development Fellowship. R. Beare is a recipient of NHMRC project grants. W. Wang reports no disclosures relevant to the manuscript. M. Callisaya is a recipient of an Alzheimer's Australia Research Foundation Grant & NHMRC Early Career Fellowship. V. Srikanth is a recipient of a National Health and Medical Research Council (NHMRC) Practitioner Fellowship and NHMRC project grants. Go to Neurology.org/N for full disclosures.
References
- 1.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262. [DOI] [PubMed] [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 3.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med 2013;369:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 5.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 2006;29:2539–2548. [DOI] [PubMed] [Google Scholar]
- 6.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012;379:2291–2299. [DOI] [PubMed] [Google Scholar]
- 7.Moran C, Beare R, Phan TG, et al. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 2015;85:1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 2013;36:4036–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganmore I, Beeri MS. Magnitude and trajectories of cognitive dysfunction in type 2 diabetes mellitus. In: Srikanth V, Arvanitakis Z, eds. Type 2 Diabetes and Dementia. London: Academic Press; 2018. [Google Scholar]
- 10.van Elderen SG, de Roos A, de Craen AJ, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 2010;75:997–1002. [DOI] [PubMed] [Google Scholar]
- 11.de Bresser J, Tiehuis AM, van den Berg E, et al. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espeland MA, Bryan RN, Goveas JS, et al. Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women's Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 2013;36:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran C, Beare R, Phan T, et al. Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. J Alzheimer's Dis 2017;59:405–419. [DOI] [PubMed] [Google Scholar]
- 14.Apostolova LG, Hwang KS, Andrawis JP, et al. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging 2010;31:1284–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 18.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32:632–637. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes A. Standards of medical care in diabetes: 2010. Diabetes Care 2010;33(suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res 2009;6:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ADNI. MRI acquisition. Available at: adni.loni.usc.edu/methods/mri-analysis/mri-pre-processing/. Accessed October 12, 2013. [Google Scholar]
- 23.ADNI. ADNI General Procedures Manual 2013. Available at: adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf. Accessed July 25, 2017. [Google Scholar]
- 24.Lezak M. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 25.Kline R. Principles and Practice of Structural Equation Modeling (4th Edition). 4th ed. New York: Guilford Press; 2015. [Google Scholar]
- 26.Stuart EA, Azur M, Frangakis C, Leaf P. Multiple imputation with large data sets: a case study of the children's mental health initiative. Am J Epidemiol 2009;169:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: a data analyst's perspective. Multivariate Behav Res 1998;33:545. [DOI] [PubMed] [Google Scholar]
- 28.Allison PD. Missing data techniques for structural equation modeling. J Abnormal Psychol 2003;112:545–557. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 30.Curran PJ, Bauer DJ. The disaggregation of within-Person and between-Person effects in longitudinal models of change. Annu Rev Psychol 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthén LK, Muthén BO. Mplus User's Guide, 6th ed. Los Angeles: Muthén & Muthén; 1998–2017. Available at: statmodel.com/ugexcerpts.shtml. Accessed August 28, 2018. [Google Scholar]
- 32.Asparouhov T, Muthén BO. Multiple Imputation With Mplus. 2010. Available at: statmodel.com/download/Imputations7.pdf. Accessed August 28, 2018. [Google Scholar]
- 33.Diggle P, Kenward MG. Informative drop-out in longitudinal data analysis. J R Stat Soc Ser C (Appl Stat) 1994;43:49–93. [Google Scholar]
- 34.IBM SPSS Statistics for Windows, version 25.0 [computer program]. Armonk: IBM; 2018. [Google Scholar]
- 35.Wood AG, Chen J, Moran C, et al. Brain activation during memory encoding in type 2 diabetes mellitus: a discordant twin pair study. J Diabetes Res 2016;2016:3978428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groot C, van Loenhoud AC, Barkhof F, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology 2018;90:e149–e156. [DOI] [PubMed] [Google Scholar]
- 37.Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer neuropathology. Alzheimers Dement 2016;12:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomita N, Furukawa K, Okamura N, et al. Brain accumulation of amyloid beta protein visualized by positron emission tomography and BF-227 in Alzheimer's disease patients with or without diabetes mellitus. Geriatr Gerontol Int 2013;13:215–221. [DOI] [PubMed] [Google Scholar]
- 39.Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology 2006;67:1960–1965. [DOI] [PubMed] [Google Scholar]
- 40.Thambisetty M, Jeffrey Metter E, Yang A, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol 2013;70:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland GT, Lim J, Srikanth V, Bruce DG. Epidemiological approaches to understanding the link between type 2 diabetes and dementia. J Alzheimer's Dis 2017;59:393–403. [DOI] [PubMed] [Google Scholar]
- 42.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol 2005;58:13–19. [DOI] [PubMed] [Google Scholar]
- 43.Spratt M, Carpenter J, Sterne JAC, et al. Strategies for Multiple Imputation in Longitudinal Studies. Am J Epidemiol 2010;172:478–487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this analysis are available on request from the ADNI database (ida.loni.usc.edu).



