Abstract
An ivory-coloured, motile, Gram-stain-negative bacterium, designated TW-1T was isolated from oil-contaminated experimental soil in Kyonggi University. The phylogenetic analysis based on 16S rRNA gene sequence revealed, strain TW-1T formed a lineage within the family Oxalobacteraceae and clustered as members of the genus Massilia. The closest members were M. pinisoli T33T (98.8% sequence similarity), M. putida 6NM-7T (98.6%), M. arvi THG-RS2OT (98.5%), M. phosphatilytica 12-OD1T (98.3%) and M. niastensis 5516S-1T (98.2%). The sole respiratory quinone is ubiquinone-8. The major cellular fatty acids are hexadeconic acid, cis-9, methylenehexadeconic acid, summed feature 3 and summed feature 8. The major polar lipids are phosphatidylethanolamine, diphosphatidylglycerol and phosphatidylglycerol. The DNA G + C content of the type strain is 66.3%. The average nucleotide identity (ANI) and in silico DNA–DNA hybridization (dDDH) relatedness values between strain TW-1T and closest members were below the threshold value for species demarcation. The genome size is 7,051,197 bp along with 46 contigs and 5,977 protein-coding genes. The genome showed 5 putative biosynthetic gene clusters (BGCs) that are responsible for different secondary metabolites. Cluster 2 showed thiopeptide BGC with no known cluster blast, indicating TW-1T might produce novel antimicrobial agent. The antimicrobial assessment also showed that strain TW-1T possessed inhibitory activity against Gram-negative pathogens (Escherichia coli and Pseudomonas aeruginosa). This is the first report of the species in the genus Massilia which produces antimicrobial compounds. Based on the polyphasic study, strain TW-1T represents novel species in the genus Massilia, for which the name Massilia antibiotica sp. nov. is proposed. The type strain is TW-1T (= KACC 21627T = NBRC 114363T).
Subject terms: Biological techniques, Drug discovery, Microbiology
Introduction
Antimicrobial-resistance (AMR) is the massive public health threat in the world1. Continuously elevated number of multidrug-resistant (MDR) strains harden the efficient treatment of infections caused by bacteria2. The infections caused by MDR strains are tremendously tough to treat and might need last resort of antibiotics1, 3. On the other hand, bacteria have been developed AMR to all antibiotics discovered to date1 and no novel antibiotics have been reported since long period. A review by Jim O’Nill estimated 700,000 deaths annually due to MDR infections caused by bacteria4. Furthermore, a study by Naylor et al. guesstimated healthcare system costs more than $90 million per year globally5. These consequences exhibited that AMR is not only accountable for public health hurdle but also pondered as economic burden. Hence, search for formidable and new antibiotics for the treatment of MDR infections caused by bacteria is extremely required.
We are continuously reconnoitring the previously uncultivated bacteria with the hope that they might produce a new bioactive molecule that may have pharmaceutical applications and might bioremediate the recalcitrant hydrocarbons. During the study of searching oil-degrading bacteria, we have surprisingly isolated a novel candidate of the genus Massilia producing antimicrobial agent that hinders the growth of Pseudomonas aeruginosa and Escherichia coli. The bioactive molecules (antimicrobial agent) from Gram-negative bacteria are scarcely reported. On the contrary, almost all the antibiotics have been reported from Gram-positive bacteria such as Streptomyces6. In this context, the report of this novel strain which possesses antimicrobial activity seems valuable.
The genus Massilia was first proposed by La Scola et al.7 with the description of Massilia timonae, which was isolated from blood of immunocompromised patient with cerebral lesion. Subsequently, the genus description has been emended by Kämpfer et al.8 and Singh et al.9. To date, 47 species of the genus Massilia with validly published names have been reported (https://lpsn.dsmz.de/genus/massilia). Members of the genus Massilia are characterized by Gram-stain-negative, rod-shaped and contain ubiquinone-8 (Q-8) as predominant isoprenoid quinone; C16:0, cyclo-C17:0, summed feature 3 (iso-C15 :0 2-OH/C16 :1ω7c), summed feature 8 (C18:1ω6c and/or C18 :1ω7c) and C14:0 as major fatty acids; phosphatidylethanolamine (PE), diphosphatidylglycerol (DPG) and phosphatidylglycerol (PG) as a principal polar lipids8–12. Although most members of the genus Massilia have been isolated from soil, others have been isolated from air, drinking water, rock surface, ice-core, glacier permafrost and human clinical samples7–21. Some members have propensity to produce bioactive substance such as dimethyl disulfide (DMDS), which could have potential for controlling plant foliar diseases10. In addition, M. chloroacetimidivorans has been reported to degrade chloroacetamide herbicide22. On the other hand, some species such as M. timonae, M. consociate, M. oculi and M. haematophila are considered as pathogenic strains as they were isolated from human clinical samples7, 8, 18. However, to our best understanding, no member of the genus Massilia has been reported yet to possess antimicrobial activity.
In this study, a novel member of the genus Massilia isolated from oil-contaminated experimental soil having promising antimicrobial activity against E. coli and P. aeruginosa (Gram-negative pathogens) has been described with its phylogenetic and taxonomic position. In addition, whole-genome analysis of strain TW-1T has been explored providing deeper insights into metabolic products.
Materials and methods
Isolation and preservation
Strain TW-1T was isolated unexpectedly during the bioremediation experiment from oil-contaminated natural soil. The oil-contaminated soil was collected form industrial site located near Jeonju City, Republic of Korea. Isolation, maintenance and preservation of strain TW-1T was carried as mentioned in previous study23.
Phylogenetic analysis
Genomic DNA of strain TW-1T was isolated by using InstaGene Matrix kit (Life Science Research; Bio-Rad) following manufacturer’s instruction. The 16S rRNA gene was amplified by using PCR (Bio-Rad) with forward and reverse primers 27F and 1492, respectively24. Applied Biosystems 3770XL DNA analyzer was used with a BigDye Terminator cycle sequencing Kit v.3.1 (Applied Biosystems, USA) for gene sequencing. After sequencing, nearly complete sequence of 16S rRNA genes was assembled using SeqMan software (DNASTAR Inc., USA). Phylogenetically closest neighbours were identified using the EzBioCloud server25 and ncbi megablast. All the 16S rRNA gene sequences of phylogenetically closest neighbours were retrieved from the ncbi GenBank database. All the retrieved sequences along with TW-1T were aligned using in silico by silva alignment (https://www.arb-silva.de/aligner/). Neighbor-joining (NJ), maximum-likelihood (ML) and maximum-parsimony (MP) phylogenetic trees were reconstructed using mega (v7.0.26) software26.
Genome analyses
For genome sequencing, extraction of genomic DNA was carried out by using DNeasy Blood and Tissue kits (Qiagen). Whole-genome shotgun sequencing of strain TW-1T was accomplished at Macrogen (Republic of Korea) using the Illumina HiSeq 2500 platform using a 150-bp × 2 paired-end kit. The whole-genome sequences were assembled by SPAdes (v3.2)27. The authenticity and legitimacy of the assembled genome were checked by comparing 16S rRNA gene sequence of strain TW-1T using ncbi Basic Local Alignment Search Tool (blastn)28. Potential contamination of genome assembly was examined in silico by ContEst16S algorithm using EzBioCloud server (https://www.ezbiocloud.net/tools/contest16s)29. Then, the whole-genome sequence of strain TW-1T was annotated using the ncbi PGAP (Prokaryotic Genome Annotation Pipeline; https://www.ncbi.nlm.nih.gov/genome/annotation_prok)30 and RAST (Rapid Annotation using Subsystem Technology; https://rast.nmpdr.org) server31. All the genome sequences of reference strains were retrieved from ncbi database. The DNA G + C content of strain TW-1T and other references used in this study were calculated based on respective whole-genome sequences. Genome-based relatedness between TW-1T and phylogenetically closest neighbours were determined based on ANI (Average Nucleotide Identity) in silico by OrthoANIu (https://www.ezbiocloud.net/tools/ani) algorithm32. The phylogenomic tree was reconstructed in silico using concatenated alignment of 92 core genes with UBCGs software33. Digital DNA-DNA hybridization (dDDH) was calculated in silico by the Genome-to-Genome Distance Calculator (GGDC 2.1) using the blast method34. The conventional DNA-DNA hybridization (DDH) was measured fluorometrically using photobiotin-labelled DNA probes and microdilution plates as recommended by Ezaki et al.35. Graphical circular map was constructed by using CGView (http://cgview.ca) server36. Transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) were analysed using tRNAscan-SE (http://lowelab.ucsc.edu/tRNAscan-SE)37 and rnammer (http://www.cbs.dtu.dk/services/RNAmmer)38 servers. The CRISPR genes and Cas clusters were determined in silico using the CRISPRCasFinder (https://crisprcas.i2bc.paris-saclay.fr) server. The anti-SMASH server was used to identify the biosynthetic gene clusters (BGCs) for various secondary metabolites39. The Clusters of Orthologous Group (COG) functional categories were allocated by digging against the KEGG (Kyoto Encyclopedia of Genes and Genomes) database40.
Physiological analyses
The cell morphology of strain TW-1T, grown on R2A agar plate for 4–5 days at 28 °C were observed by using TEM (transmission electron microscopy; Talos L120C; FEI). The colony morphology of strain of TW-1T was seen using a Zoom Stereo Microscope (SZ61; Olympus, Japan). Gram staining was performed as described previously41. The cell motility was examined in R2A (Reasoner’s Agar No. 2; MB cell; KisanBio) medium consisting 0.4% agar. Oxidase and catalase activities of strain TW-1T was examined using 1% tetra-methyl-p-phenylenediamine dihydrochloride and 3% (v/v) H2O2, respectively. Growth at various temperatures (0–45 °C) on R2A agar plates was monitored for 10 days. Growth was observed on various media including brain heart infusion agar (BHI; Oxoid), Luria–Bertani agar (LBA; Oxoid), marine agar 2216 (Becton), nutrient agar (NA; Oxoid), R2A agar, sorbitol MacConkey agar (MA; Oxoid), tryptone soya agar (TSA; Oxoid), and veal infusion agar (VIA; Becton). DNase activity of strain TW-1T was examined by using DNase agar (Oxoid). Tolerance of salt was checked in R2A broth supplemented with NaCl [Duksan Chemicals, Republic of Korea; 0–5% (w/v) at 0.5% interval]. The pH range was observed at 28 °C in R2A broth (pH 4–12 in increments of 0.5 pH units). Testing of pH after sterilization showed only minor changes. To analyse the optimum temperature, pH and NaCl, the growth curve was determined by measuring growth absorbance at 600 nm using a spectrophotometer (Biochrome Libra S4). Hydrolysis of Tweens 80, 60 and 40 were analysed as described by Smibert & Krieg42. The anaerobic growth of strain TW-1T was observed for 10 days on R2A agar at 28 °C with BD GasPak™ EZ Gas Generating Pouch System (BD). Hydrolysis of casein CM-cellulose, starch and tyrosine were assessed as mentioned in previous study43. Production of H2S and indole was checked in SIM (sulfide indole motility medium; Oxoid). Malachite green was used for spore staining. Other physiological tests were examined by using API 20NE and API ID 32GN kits (bioMérieux). The enzyme activities of strain TW-1T and other references were examined by using an API ZYM kit (bioMérieux) following the manufacturer’s instructions. All the biochemical tests including API were performed in duplicate.
For the determination of fatty acids, cells of reference strains and TW-1T were harvested from identical culture condition (at 28 °C for 4 days). Fatty acid methyl esters (FAME) of harvested cells were extracted using MIDI protocol technical note #101 (http://midi-inc.com/pdf/MIS_Technote_101.pdf). Extracted FAMEs were analysed using a HP 6890 Series GC System (Gas chromatograph; Hewlett Packard; Agilent Technologies) and the FAME compositions (percentage of totals) were identified with TSBA6 database of the Microbial Identification System44. The polar lipids and isoprenoid quinones were extracted from freeze-dried cells following the protocol of Minnikin et al.45. Isoprenoid quinone was analysed by using the HPLC (Agilent 1200 series) with following conditions. Solvent system, acetonitrile: iso-propanol (65: 35); flow rate, 1.2 mL/min; detection, 270 nm; run time, 20 min; and injection volume, 20 µL. Appropriate reagents for the spot detection were used as given by Komagata and Suzuki46.
Antimicrobial activities of strain TW-1T
Antimicrobial activities of strain TW-1T against E. coli KACC 10,185 and P. aeruginosa KEMB 121–234 were examined by disc-diffusion and spotting method, respectively. Screening were done against these Gram-negative pathogens by spotting the colonies of strain TW-1T on R2A agar plates and incubated at 28 °C for 48 h. Crude product of culture extract was prepared by the culture supernatant of strain TW-1T to evaluate disc-diffusion test. Strain TW-1T was cultured in 300 mL of R2A broth at 28 °C (180 rpm for 5 days) into a 500 mL Erlenmeyer flask. Culture supernatant of strain TW-1T was extracted by equal volume of ethyl acetate (2 ×) with pH 2.0 and 10, respectively47. Organic layer collected from extraction was completely evaporated by Rotary evaporator, (Eyela) and remained residue of crude product was dissolved in 500 µL of methanol. Then, 15 µL of crude product was diffused to a paper-disc (6 mm, Whatman) and antimicrobial activities against P. aeruginosa and E. coli KEMB were checked by measuring the inhibition zones. Trimethoprim/sulfamethaxazole (15 µg) and only methanol (15 µL) were used for positive and negative controls, respectively.
Ethics approval
This study does not describe any experimental work related to human.
Result and discussions
Phylogenetic analysis
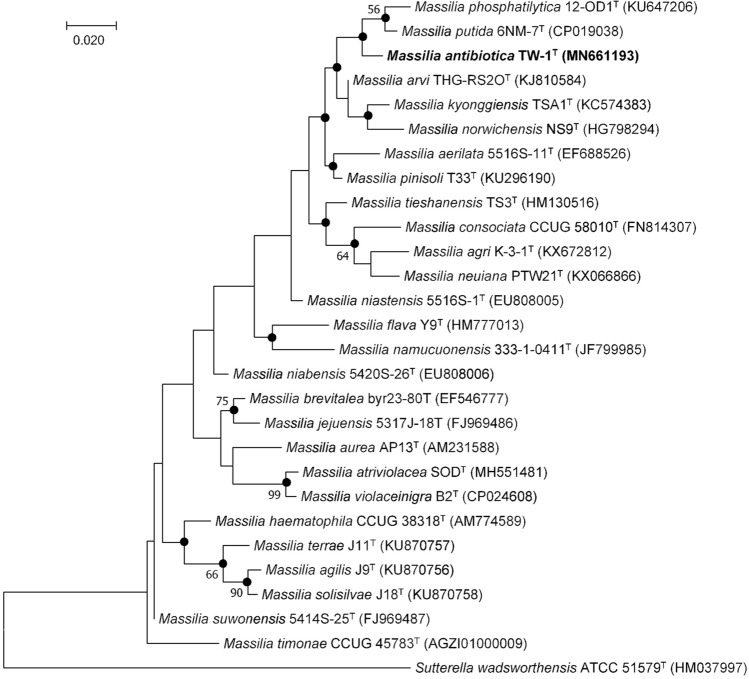
The 16S rRNA gene sequence of strain TW-1T was 1,480 bp long and has been deposited at GenBank/EMBL/DDBJ database under the accession MN661193. Phylogenetic analysis based on 16S rRNA gene sequence showed that strain TW-1T formed a lineage within the family Oxalobacteraceae and clustered as members of the genus Massilia. Strain TW-1T was closest to M. pinisoli T33T (98.8% sequence similarity) followed by M. putida 6NM-7T (98.6%), M. arvi THG-RS2OT (98.5%), M. phosphatilytica 12-OD1T (98.3%) and M. niastensis 5516S-1T (98.2%). The sequence similarities between strain TW-1T and other members of the genus Massilia were < 98%. Strain TW-1T was well clustered with M. putida, and M. phosphatilytica in both NJ and ML tress and formed a distinguish lineage in MP phylogenetic trees (Figs. 1, S1 and S2). The separate lineage formed in between M. putida and M. phosphatilytica in MP tree strongly supported the identification of strain TW-1T as a novel member in the genus Massilia (S2).
Figure 1.
Maximum-likelihood phylogenetic tree based on 16S rRNA gene sequences showing the position of strain TW-1T among the closest members of the genus Massilia. Filled circles are recovered by neighbour–joining and maximum–likelihood treeing methods. Bootstrap values (> 50%) based on 1000 bootstrap replicates are shown at branch nodes. Sutterella wadsworthensis ATCC 51579T was used as an out-group. Numbers in parentheses indicate GenBank accessions. Bar, 0.020 substitutions per nucleotide position.
Genomic analysis
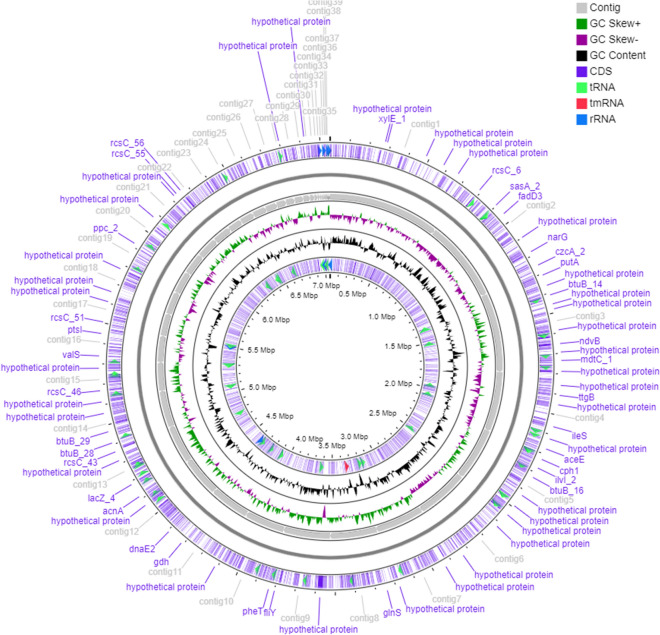
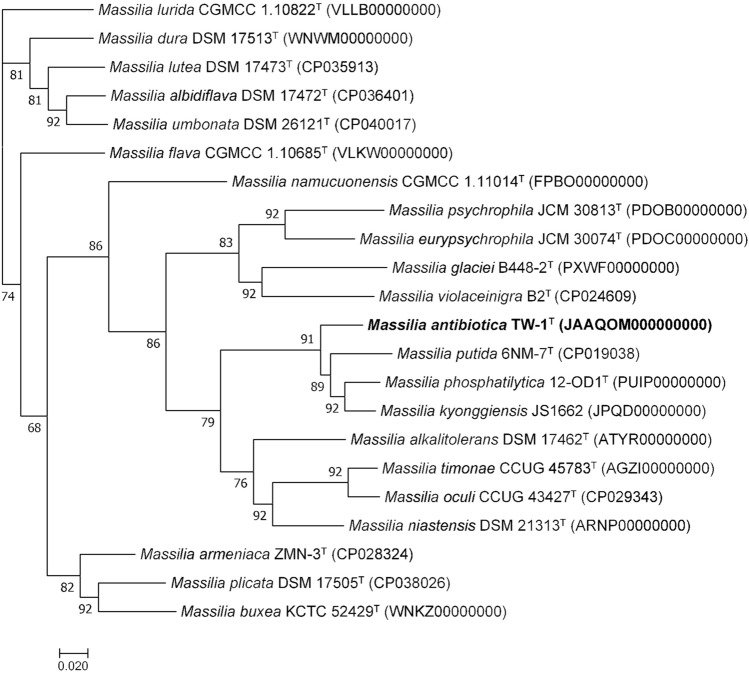
The ContEst16S analysis showed that the genome belonged to strain TW-1T and the genome has not been contaminated. Whole-genome shotgun sequence has been deposited at DDBJ/ENA/GenBank under the accession JAAQOM000000000. The genome size and N50 value of strain TW-1T are 7,051,197 bp and 309,394 bp, respectively. The genome has 46 contigs and coverage of 89.0 × (Table S1). The graphical genomic map revealed the presence of 12 rRNAs (Fig. 2). The DNA G + C content of strain TW-1T is 66.3% and within the range of Massilia species8. The ANI threshold for species delineation is recommended at 95–96%48 and ANIu between strain TW-1T and phylogenetically closest neighbours are ≤ 87.8% (Table 1). The dDDH values of ≤ 34.4% is much lower than the species threshold of 70% recommended for species demarcation34 (Table 1). Moreover, DDH relatedness between strains TW-1T, M. pinisoli KACC 18748T and M. arvi KACC 21416T were 40.2 ± 2.6 and 24.1 ± 2.1%, respectively. These data clearly show that strain TW-1T represents a novel member within the genus Massilia34. Furthermore, the phylogenomic tree constructed using UBCGs (concatenated alignment of 92 core genes) also proved that strain TW-1T was a novel member of the genus Massilia (Fig. 3).
Figure 2.
Graphical genomic map of strain TW-1T.
Table 1.
Average nucleotide identity (ANIu) and digital DNA-DNA hybridization (dDDH) between strain TW-1T and phylogenetically closest members of the genus Massilia.
| Strains | Accessions | TW-1T | |
|---|---|---|---|
| ANIu | dDDH | ||
| Massilia albidiflava DSM 17472T | CP036401 | 76.2 | 21.2 |
| Massilia alkalitolerans DSM 17462T | ATYR00000000 | 78.0 | 21.5 |
| Massilia armeniaca ZMN-3T | CP028324 | 76.5 | 21.2 |
| Massilia buxea KCTC 52429T | WNKZ00000000 | 76.2 | 21.0 |
| Massilia dura DSM 17513T | WNWM00000000 | 76.1 | 21.2 |
| Massilia eurypsychrophila JCM 30074T | PDOC00000000 | 77.1 | 20.9 |
| Massilia flava CGMCC 1.10685T | VLKW00000000 | 76.8 | 21.0 |
| Massilia glaciei B448-2T | PXWF00000000 | 76.5 | 21.3 |
| Massilia kyonggiensis JS1662 | JPQD00000000 | 87.3 | 33.3 |
| Massilia lurida CGMCC 1.10822T | VLLB00000000 | 76.5 | 20.9 |
| Massilia lutea DSM 17473T | CP035913 | 76.1 | 20.9 |
| Massilia namucuonensis CGMCC 1.11014T | FPBO00000000 | 76.3 | 20.7 |
| Massilia niastensis DSM 21313T | ARNP00000000 | 78.4 | 22.0 |
| Massilia oculi CCUG 43427T | CP029343 | 77.5 | 21.5 |
| Massilia phosphatilytica 12-OD1T | PUIP00000000 | 87.5 | 33.2 |
| Massilia plicata DSM 17505T | CP038026 | 76.2 | 20.9 |
| Massilia psychrophila JCM 30813T | PDOB00000000 | 76.2 | 20.4 |
| Massilia putida 6NM-7T | CP019038 | 87.8 | 34.4 |
| Massilia timonae CCUG 45783T | AGZI00000000 | 77.9 | 21.5 |
| Massilia umbonata DSM 26121T | CP040017 | 76.5 | 21.3 |
| Massilia violaceinigra B2T | CP024609 | 76.1 | 20.7 |
Figure 3.
Phylogenomic tree constructed using UBCGs (concatenated alignment of 92 core genes). Bar, 0.020 substitution per nucleotide position.
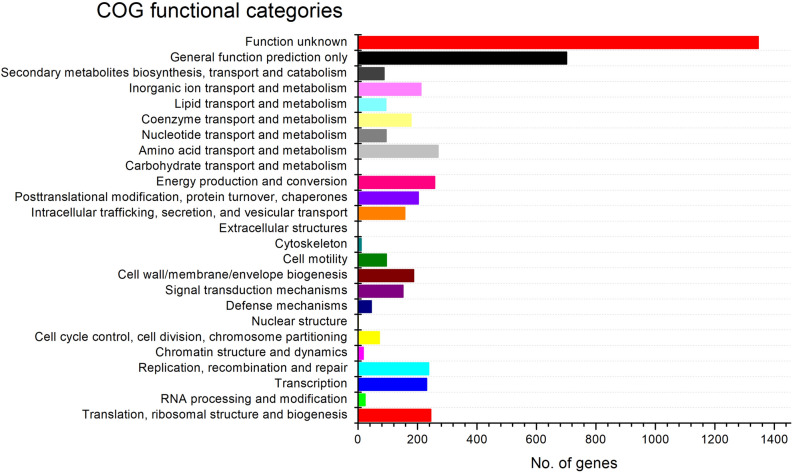
The RAST analysis revealed the presence of 339 subsystems and 4 secondary metabolisms consisting auxin biosynthesis (four, Fig. S3). The COG functional classification of proteins showed the highest and lowest number of genes were of unknown functions (1347) and extracellular structures (1) (Fig. 4). The genome of strain TW-1T consists 5 putative BGCs (terpene, siderophore, bacteriocin, acyl_amino_acid and thiopeptide) were revealed by anti-SMASH analysis (Table 2). The core and additional biosynthetic genes were predicted along with gene flaking similarities for secondary metabolite biosynthetic gene clusters (smBGCs) (Table 2). Thiopeptide BGC showed only 33% of gene similarity with Massilia putida (NZ_CP019038; 4,695,767–4,724,913) with no known clusters. Additionally, each identified gene cluster from anti-SMASH analysis were compared against the ncbi database using protein–protein blast (BlastP) (Fig. S4). The core biosynthetic genes in the thiopeptide BGC showed identified nuclear transport factor 2 family protein and OsmC domain/YcaO domain-containing proteins (Fig. S4). As no known cluster has been predicted from the thiopeptide cluster, strain TW-1T might produce unique natural products (Fig. S4). In addition, the genome contained three antibiotic biosynthesis monooxygenase (ABM; WP_166855736, WP_166862851 and WP_166864744) that are possibly responsible for biosynthesis for antibiotics.
Figure 4.
COG functional classification of proteins in the genome of strain TW-1T.
Table 2.
Numbers of predicted secondary metabolite biosynthetic gene clusters (smBGC) of TW-1T genome. The BGCs were determined using anti-SMASH (v5.1.2).
| Cluster | Gene flanking similarity (%) | smBGC type | From | To | Most similar known cluster | Core biosynthetic gene | Additional biosynthetic gene |
|---|---|---|---|---|---|---|---|
| 1 | 94 | Acyl_amino_acids | 162,489 | 223,265 | N-tetradecanoyl tyrosine (Other) (6%) | 1 | 12 |
| 2 | 13 | Thiopeptide | 272,320 | 309,699 | – | 2 | 8 |
| 3 | 100 | Bacteriocin | 82,478 | 94,127 | – | 1 | 0 |
| 4 | 100 | Terpene | 106,679 | 128,373 | – | 2 | 1 |
| 5 | 75 | Siderophore | 32,740 | 44,710 | – | 1 | 1 |
Although strain TW-1T was isolated at 28 °C, it could grow well at 4 °C. When we performed the genome mining of strain TW-1T we found the genes (CspA, CspC) related to cold shock proteins and cold-shock domain containing proteins (WP_036166698, WP_166858078, WP_166858204, WP_056448893 and WP_03616538). These proteins help the organism to adapt in cold temperatures. The genome contained arsenic resistance gene arsH (WP_166857685) and chromate resistance gene (WP_166864147) showing the strain could tolerate arsenic and chromate. The genome contained various protease genes such as rhomboid family intramembrane serine protease (WP_166860501), site-2 protease (S2P) family protein (WP_166861383), ATP-dependent Clp protease ATP-binding subunit ClpX (WP_166861695), ATP-dependent protease ATPase subunit HslU (WP_166858142), ATP-dependent protease subunit HslV (WP_166857747), trypsin-like serine protease (WP_166857912, WP_166858974), protease HtpX (WP_166861379, WP_166859769), DJ-1/PfpI/YhbO family deglycase/protease (WP_166859796, WP_166864477, WP_166859797), ATP-dependent Clp protease adapter ClpS (WP_027864722), FtsH protease activity modulator HflK (WP_166865455), and protease modulator HflC (WP_166865458). Presence of these various proteases encoding genes indicate the industrial and medical significance of strain TW-1T. Bacterial proteases are widely used in the industrial sectors for various enzymatic activities and currently these enzymes are also regarded as valuable resources for antimicrobial drug targets49, 50.
Physiological analysis
The cells of strain TW-1T are rod-shaped (Fig. S5), Gram-stain-negative, aerobic and motile with polar flagellum. The indole test is negative and strain TW-1T is non-spore-forming. Strain TW-1T hydrolysed DNA, CM-cellulose, casein, aesculin, starch, Tweens 40 and 60. Hydrolysis of chitin is negative but weakly hydrolyse Tween 80, gelatin and tyrosine. Red diffusible pigmentation was also observed while hydrolysing tyrosine. Strain TW-1T grew well but the references were unable to grow at 4 °C. Other differential physiological characteristics are given on Table 3 with phylogenetically closest species of the genus Massilia.
Table 3.
Phenotypic characteristics of strain TW-1T that differentiates with phylogenetically related species of the genus Massilia.
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Highest growth temperature (°C) | 42 | 37 | 37 | 37 | 42 | 40 | 42 |
| pH range | 4.5–10.5 | 5.5–9.0 | 5.5–8.5 | 5.0–9.0 | 6.0–8.5 | 5.5–9.0 | 5.0–9.0 |
| Highest NaCl tolerance (%, w/v) | 1 | 1 | 0.5 | 0.5 | 0.5 | 1 | 2 |
| Nitrate reduction | + | + | – | + | + | – | + |
| Urease activity | – | – | – | – | + | – | – |
| Hydrolysis of | |||||||
| Aesculin | + | + | – | – | + | + | + |
| Casein | + | – | – | – | – | – | + |
| Gelatin | w | – | + | – | – | + | – |
| Starch | + | – | + | – | + | + | + |
| Enzyme activity | |||||||
| Acid phosphatase | + | – | – | – | + | + | + |
| Alkaline phosphatase | + | + | + | + | + | + | w |
| Cystine arylamidase | + | + | + | – | + | + | – |
| Esterase (C4) | – | + | + | + | + | + | w |
| Esterase lipase (C8) | w | + | + | + | + | – | – |
| Leucine arylamidase | + | – | – | + | + | + | + |
| Lipase (C14) | w | – | – | – | – | – | – |
| N-acetyl-β-glucosaminidase | + | – | – | – | + | – | + |
| Valine arylamidase | + | – | – | + | + | + | + |
| α-galactosidase | + | + | + | + | + | – | + |
| α-glucosidase | + | + | + | – | + | – | + |
| β-galactosidase | + | + | – | – | + | + | w |
| β-glucuronidase | w | – | – | – | – | – | – |
| Assimilation from | |||||||
| 2-Ketogluconate | – | – | – | + | – | – | – |
| 3-Hydroxybenzoic acid | – | – | – | – | – | + | – |
| 3-Hydroxybutyric acid | + | – | – | – | – | + | w |
| 4-Hydroxybenzoic acid | + | – | – | – | – | + | – |
| Adipic acid | – | – | – | + | – | – | w |
| Capric acid | – | – | – | – | w | – | – |
| d-maltose | + | – | + | + | + | + | + |
| d-mannose | + | – | + | + | + | + | + |
| d-melibiose | + | – | – | – | – | – | – |
| d-saccharose (sucrose) | + | + | + | + | – | – | + |
| Glycogen | + | + | – | – | – | – | + |
| Lactic acid | + | – | – | – | – | – | w |
| l-alanine | + | – | – | – | – | + | – |
| l-arabinose | + | – | + | + | + | + | + |
| l-fucose | + | – | – | + | – | – | + |
| l-histidine | + | – | – | – | – | – | – |
| l-proline | w | – | + | – | – | + | – |
| l-rhamnose | + | – | – | – | + | + | + |
| Malic acid | – | – | + | + | – | + | + |
| N-acetyl-glucosamine | – | + | – | – | + | + | – |
| Phenylacetic acid | – | – | – | + | – | – | + |
| Potassium gluconate | – | – | + | + | + | + | w |
| Salicin | – | – | – | – | – | + | – |
| Sodium acetate | – | – | – | – | – | + | – |
| Trisodium citrate | – | – | – | + | w | – | w |
| DNA G + C content (mol%) | 66.3 | 69.4* | 64.7 | 66.2 | 63.2† | 66.3 | 66.7‡ |
Strains: 1, TW-1T; 2, M. pinisoli KACC 18748T; 3, M. putida KACC 21418T; 4, M. phosphatilytica KACC 21417T; 5, M. arvi KACC 21416T; 6, M. niastensis KACC 12599T; 7, M. kyonggiensis KACC 17471T. All data were obtained from this study except otherwise indicated. + , positive; w, weak; –, negative.
The major fatty acids of strain TW-1T were C16:0, cyclo-C17:0, summed feature 3 (iso-C15 :0 2-OH/C16 :1ω7c), summed feature 8 (C18:1ω6c and/or C18 :1ω7c) and C14:0, similar with the genus Massilia. The differences in major and minor fatty acids in addition to the presence of minor fatty acid, cyclo-C19:0 ω8c differentiate strain TW-1T from other phylogenetically related species of the genus Massilia (Table 4). The sole respiratory quinone was ubiquinone-8 (Q-8) and the major polar lipids were phosphatidylethanolamine, diphosphatidylglycerol and phosphatidylglycerol. In addition, two unidentified phospholipids (PL1–PL2), three unidentified aminolipids (AL1-AL3) and three unidentified polar lipids (L1-L3) are also seen in TLC chromatogram (Fig. S6).
Table 4.
Cellular fatty acid profiles (percentage of totals) of TW-1T and phylogenetically related reference strains of the genus Massilia.
| Fatty acid | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Saturated | |||||||
| C10:0 | 0.6 | – | – | 0.9 | 1.5 | – | – |
| C12:0 | 2.4 | 2.8 | 2.0 | 4.5 | – | 4.7 | 4.8 |
| C14:0 | 6.4 | 5.1 | 4.3 | 4.7 | 4.4 | 1.0 | 3.9 |
| C16:0 | 31.3 | 24.7 | 33.3 | 24.1 | 28.9 | 26.1 | 27.6 |
| C18:0 | 0.2 | – | – | – | 1.5 | – | – |
| Hydroxy | |||||||
| C12:0 2-OH | – | – | – | – | – | 1.9 | – |
| C14:0 2-OH | 1.2 | – | 2.7 | 6.2 | 2.9 | 0.6 | 2.9 |
| C10:0 3-OH | 3.5 | 3.4 | 5.9 | 9.8 | 6.1 | 5.3 | – |
| Branched saturated | |||||||
| Cyclo-C17:0 | 22.7 | 3.9 | 20.9 | 25.0 | 10.7 | 2.9 | 7.1 |
| Cyclo-C19:0 ω8c | 0.6 | – | – | – | – | – | – |
| Summed features* | |||||||
| 3 | 20.0 | 48.7 | 21.9 | 22.5 | 33.3 | 44.4 | 43.1 |
| 8 | 9.6 | 11.4 | 7.4 | 1.7 | 8.9 | 11.1 | 10.0 |
Strains: 1, TW-1T; 2, M. pinisoli KACC 18748T; 3, M. putida KACC 21418T; 4, M. phosphatilytica KACC 21417T; 5, M. arvi KACC 21416T; 6, M. niastensis KACC 12599T; 7, M. kyonggiensis KACC 17471T. All data were obtained from this study. Fatty acid amounting < 0.5% in all strains are not listed. –, not detected.
*Summed features represent groups of two or three fatty acids that could not be separated using the MIDI system. Summed feature 3 comprised iso-C15 :0 2-OH/C16 :1ω7c and summed feature 8 comprised C18:1ω6c and/or C18 :1ω7c.
Antimicrobial activities
Strain TW-1T showed antimicrobial activities against Gram-negative pathogens. The zone of inhibitions for P. aeruginosa KACC 10,185 and E. coli KEMB 121–234 were 17 and 18 mm, respectively (Fig. S7). This is unique result that we have isolated the bacterial strain having potent antimicrobial effects against Gram-negative pathogens from oil-contaminated soil and we report this is the first study of Massilia species producing antimicrobial compound. The determination of MIC value in addition to identification and characterization of bioactive compound are under investigation. However, based on anti-SMASH analysis, there is a high chance to get a novel antimicrobial compound from strain TW-1T as it showed thiopeptide smBGC with no known cluster blast (Fig. S4).
Based on above discussed data, strain TW-1T represents a novel species within the genus Massilia for which the name Massilia antibiotica sp. nov. is proposed.
Description of Massilia antibiotica sp. nov.
Massilia antibiotica (an.ti.bi.o’ti.ca. Gr. pref. anti against; Gr. masc. n. bios -life; N.L. fem. adj. antibiotica against life, antibiotic).
Cells (2.9–3.4 µm long and 0.9–1.0 µm wide) are rod-shaped, strictly aerobic, Gram-stain-negative and motile with polar flagellum. Colonies grow well on R2A agar plate and weakly on NA, while no growth is observed on BHI, LBA, MA, marine agar 2216, TSA and VIA. Colonies on R2A are ivory coloured, circular and convex. The colony size is 2–3 mm on R2A agar plate for 5 days at 28 °C. Colonies grow at 4–42 °C (optimum, 25–35 °C) and pH 4.5–10.5 (optimum pH, 6.5–9.0). The strain grows optimally in the absence of NaCl but tolerate 1% (w/v) of NaCl. Catalase and oxidase are positive. Hydrogen sulfide is not produced. Nitrate is reduced to nitrite. Glucose is not fermented. PNPG (4-nitrophenyl -βd-galactopyranoside) is positive. The type strain shows the following enzyme activities: positive for alkaline phosphatase, leucine arylamidase, valine arylamidase, cystine arylamidase, acid phosphatase, α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase and N-acetyl-β-glucosaminidase; weakly positive for esterase lipase (C8), lipase (C14), trypsin, α-chymotrypsin, napthol-AS-BI-phosphohydrolase and β-glucuronidase; and negative for esterase (C4), α-mannosidase and α-fucosidase. The following substrates are assimilated: 3-hydroxybutyric acid, 4-hydroxybenzoic acid, d-glucose, d-maltose, d-mannose, d-melibiose, glycogen, lactic acid, l-alanine, l-arabinose, l-fucose, l-histidine, l-proline and sucrose. The sole respiratory quinone is Q-8. The major cellular fatty acids are C16:0, cyclo-C17:0, summed feature 3 (iso-C15 :0 2-OH/C16 :1ω7c), summed feature 8 (C18:1ω6c and/or C18 :1ω7c) and C14:0. The major polar lipid are phosphatidylethanolamine, diphosphatidylglycerol and phosphatidylglycerol. The DNA G + C content of the type strain is 66.3%.
The type strain, TW-1T (= KACC 21627T = NBRC 114363T), was isolated from oil-contaminated experimental soil in Kyonggi University, Republic of Korea. The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence and the whole genome sequence of strain TW-1T are MN661193 and JAAQOM000000000, respectively.
Supplementary Information
Author contributions
R.D. wrote the main manuscript text and prepared figures 1-4 and tables 1-4 as well as supplementary materials. D.C. isolated this strain and characterized. J.K. supervised all the process of this research. All authors reviewed the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1F1A1058501).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the Result and discussions section, under the subheading ‘Description of Massilia antibiotica sp. nov.’, where, “The type strain, TW-1T 1T (= KACC 21627T = NBRC 114363T), was isolated from oil-contaminated experimental soil in Kyonggi University, Republic of Korea.“ Now reads: “The type strain, TW-1T (= KACC 21627T = NBRC 114363T), was isolated from oil-contaminated experimental soil in Kyonggi University, Republic of Korea.“
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/14/2021
A Correction to this paper has been published: 10.1038/s41598-021-92164-5
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86232-z.
References
- 1.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahal RH, Chaudhary DK. Microbial infections and antimicrobial resistance in Nepal: current trends and recommendations. Open Microbiol. J. 2018;12:230–242. doi: 10.2174/1874285801812010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terra L, et al. A novel alkaliphilic Streptomyces inhibits ESKAPE pathogens. Front. Microbiol. 2018;9:2458. doi: 10.3389/fmicb.2018.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’ Neil J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nation.s Review on Antimicrobial Resistance. London: Springer; 2014. pp. 1–16. [Google Scholar]
- 5.Naylor NR, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob. Resist. Infect. Control. 2018;7:58. doi: 10.1186/s13756-018-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genilloud O. Actinomycetes: still a source of novel antibiotics. Nat. Prod. Rep. 2017;34:1203–1232. doi: 10.1039/c7np00026j. [DOI] [PubMed] [Google Scholar]
- 7.La Scola B, Birtles RJ, Mallet MN, Raoult D. Massilia timonae gen. nov., sp. nov., isolated from blood of an immunocompromised patient with cerebellar lesions. J. Clin. Microbiol. 1998;36:2847–2852. doi: 10.1128/jcm.36.10.2847-2852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kämpfer P, Lodders N, Martin K, Falsen E. Revision of the genus Massilia La Scola et al. 200, with an emended description of the genus and inclusion of all species of the genus Naxibacter as new combinations, and proposal of Massilia consociata sp. nov. Int. J. Syst. Evol. Microbiol. 2011;61:1528–1533. doi: 10.1099/ijs.0.025585-0. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, et al. Massilia arvi sp. nov., isolated from fallow-land soil previously cultivated with Brassica oleracea, and emended description of the genus Massilia. Int. J. Syst. Evol. Microbiol. 2015;65:3690–3696. doi: 10.1099/ijsem.0.000477. [DOI] [PubMed] [Google Scholar]
- 10.Feng GD, Yang SZ, Li HP, Zhu HH. Massilia putida sp. nov., a dimethyl disulfide-producing bacterium isolated from wolfram mine tailing. Int. J. Syst. Evol. Microbiol. 2016;66:50–55. doi: 10.1099/ijsem.0.000670. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary DK, Kim J. Massilia agri sp. nov., isolated from reclaimed grassland soil. Int. J. Syst. Evol. Microbiol. 2017;67:2696–2703. doi: 10.1099/ijsem.0.002002. [DOI] [PubMed] [Google Scholar]
- 12.Altankhuu K, Kim J. Massilia pinisoli sp. nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2016;66:3669–3674. doi: 10.1099/ijsem.0.001249. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YQ, et al. Massilia dura sp. nov., Massilia albidiflava sp. nov., Massilia plicata sp. nov. and Massilia lutea sp. nov., isolated from soils in China. Int. J. Syst. Evol. Microbiol. 2006;56:459–463. doi: 10.1099/ijs.0.64083-0. [DOI] [PubMed] [Google Scholar]
- 14.Gallego V, Sánchez-Porro C, García MT, Ventosa A. Massilia aurea sp. nov., isolated from drinking water. Int. J. Syst. Evol. Microbiol. 2006;56:2449–2453. doi: 10.1099/ijs.0.64389-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, et al. Massilia violaceinigra sp. nov., a novel purple-pigmented bacterium isolated from glacier permafrost. Int. J. Syst. Evol. Microbiol. 2018;68:2271–2278. doi: 10.1099/ijsem.0.002826. [DOI] [PubMed] [Google Scholar]
- 16.Shen L, et al. Massilia eurypsychrophila sp. nov. a facultatively psychrophilic bacteria isolated from ice core. Int. J. Syst. Evol. Microbiol. 2015;65:2124–2129. doi: 10.1099/ijs.0.000229. [DOI] [PubMed] [Google Scholar]
- 17.Weon HY, et al. Massilia niabensis sp. nov. and Massilia niastensis sp. nov., isolated from air samples. Int. J. Syst. Evol. Microbiol. 2009;59:1656–1660. doi: 10.1099/ijs.0.006908-0. [DOI] [PubMed] [Google Scholar]
- 18.Kämpfer P, Lodders N, Martin K, Falsen E. Massilia oculi sp. nov., isolated from a human clinical specimen. Int. J. Syst. Evol. Microbiol. 2012;62:364–369. doi: 10.1099/ijs.0.032441-0. [DOI] [PubMed] [Google Scholar]
- 19.Sun LN, et al. Massilia buxea sp. nov., isolated from a rock surface. Int. J. Syst. Evol. Microbiol. 2017;67:4390–4396. doi: 10.1099/ijsem.0.002301. [DOI] [PubMed] [Google Scholar]
- 20.Zheng BX, Bi QF, Hao XL, Zhou GW, Yang XR. Massilia phosphatilytica sp. nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int. J. Syst. Evol. Microbiol. 2017;67:2514–2519. doi: 10.1099/ijsem.0.001916. [DOI] [PubMed] [Google Scholar]
- 21.Kim J. Massilia kyonggiensis sp. nov., isolated from forest soil in Korea. J. Microbiol. 2014;52:378–383. doi: 10.1007/s12275-014-4010-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Kim DU, Park S, Yoon JH, Ka JO. Massilia chloroacetimidivorans sp. nov., a chloroacetamide herbicide-degrading bacterium isolated from soil. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017;110:751–758. doi: 10.1007/s10482-017-0845-3. [DOI] [PubMed] [Google Scholar]
- 23.Dahal RH, Chaudhary DK, Kim J. Pinisolibacter ravus gen. nov., sp. nov., isolated from pine forest soil and allocation of the genera Ancalomicrobium and Pinisolibacter to the family Ancalomicrobiaceae fam. Nov., and emendation of the genus Ancalomicrobium Staley 1968. Int. J. Syst. Evol. Microbiol. 2018;68:1955–1962. doi: 10.1099/ijsem.0.002772. [DOI] [PubMed] [Google Scholar]
- 24.Frank JA, et al. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008;74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon SH, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- 29.Lee I, et al. ContEst16S: an algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017;67:2053–2057. doi: 10.1099/ijsem.0.001872. [DOI] [PubMed] [Google Scholar]
- 30.Tatusova T, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aziz RK, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon S-H, Ha S-M, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 33.Na SI, et al. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018;56:281–285. doi: 10.1007/s12275-018-8014-6. [DOI] [PubMed] [Google Scholar]
- 34.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezaki T, Hashimoto Y, Yabuuchi E. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Bacteriol. 1989;39:224–229. [Google Scholar]
- 36.Grant JR, Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36:W181–W184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagesen K, et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blin K, et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doetsch RN, et al. Determinative Methods of Light Microscopy. In: Gerdhardt P, et al., editors. Manual of Methods for General Bacteriology. Washington, DC: American Society for Microbiology; 1981. pp. 21–33. [Google Scholar]
- 42.Smibert RM, Krieg NR. Phenotypic Characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for General and Molecular Bacteriology. Washington, DC: American Society for Microbiology; 1994. pp. 607–654. [Google Scholar]
- 43.Dahal RH, Kim J. Fluviicola kyonggii sp. nov., a bacterium isolated from forest soil and emended description of the genus Fluviicola. Int. J. Syst. Evol. Microbiol. 2018;68:1885–1889. doi: 10.1099/ijsem.0.002759. [DOI] [PubMed] [Google Scholar]
- 44.Sasser, M. Bacterial identification by gas chromatographic analysis of fatty acid methyl esters (GC-FAME). MIDI Technical Note 101. Newark, MIDI Inc (1990).
- 45.Minnikin DE, et al. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods. 1984;2:233–241. [Google Scholar]
- 46.Komagata K, Suzuki K. 4 lipid and cell-wall analysis in bacterial systematics. Methods Microbiol. 1988;19:161–207. [Google Scholar]
- 47.Dahal RH, Chaudhary DK, Kim D-U, Pandey RP, Kim J. Chryseobacterium antibioticum sp. nov. with antimicrobial activity against Gram-negative bacteria, isolated from Arctic soil. J. Antibiot. 2021;74:115–123. doi: 10.1038/s41429-020-00367-1. [DOI] [PubMed] [Google Scholar]
- 48.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Culp E, Wright GD. Bacterial proteases, untapped antimicrobial drug targets. J. Antibiot. (Tokyo) 2017;70:366–377. doi: 10.1038/ja.2016.138. [DOI] [PubMed] [Google Scholar]
- 50.Razzaq A, et al. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019;7:110. doi: 10.3389/fbioe.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.