Abstract
Animal models are used to study neurobiological mechanisms in mental disorders. Although there has been significant progress in the understanding of neurobiological underpinnings of threat-related behaviors and anxiety, little progress was made with regard to new or improved treatments for mental disorders. A possible reason for this lack of success is the unknown predictive and cross-species translational validity of animal models used in preclinical studies. Re-translational approaches, therefore, seek to establish cross-species translational validity by identifying behavioral operations shared across species. To this end, we implemented a human open field test in virtual reality and measured behavioral indices derived from animal studies in three experiments (, , and ). In addition, we investigated the associations between anxious traits and such behaviors. Results indicated a strong similarity in behavior across species, i.e., participants in our study—like rodents in animal studies—preferred to stay in the outer region of the open field, as indexed by multiple behavioral parameters. However, correlational analyses did not clearly indicate that these behaviors were a function of anxious traits of participants. We conclude that the realized virtual open field test is able to elicit thigmotaxis and thus demonstrates cross-species validity of this aspect of the test. Modulatory effects of anxiety on human open field behavior should be examined further by incorporating possible threats in the virtual scenario and/or by examining participants with higher anxiety levels or anxiety disorder patients.
Subject terms: Anxiety, Human behaviour
Introduction
Anxiety disorders are among the most prevalent mental disorders1, have a high burden of disease2, and lead to considerable costs in the health care system3. Psychotherapy is an effective treatment for anxiety disorders4,5; however, a considerable proportion of patients does not reach remission6 and some patients experience a relapse even after successful treatment7. Likewise, although pharmacotherapy has been found effective in the treatment of anxiety disorders, meta-analyses indicate only small to moderate effect sizes in comparison to placebo8–10. One possible explanation for the limited effectiveness of current treatments is that mechanisms underlying anxiety disorders are not well understood. Indeed, although the last decades have brought immense new insights into the neurobiological underpinnings of threat-related behaviors11 and (pathological) anxiety12,13, this has not yet led to new treatments or an improvement of current treatments for anxiety disorders14,15. In the case of psychiatric drug development research, animal models are used in preclinical studies to screen for possible new compounds that affect a desired target, e.g., anxiety. Effects of drugs on targets like anxiety are usually inferred from behaviors observed in specifically designed paradigms. However, candidate compounds from preclinical studies often end up failing in clinical studies in humans (the likelihood of approval for psychiatric drugs entering Phase I trials was 6.2%, which is the second-lowest among all disease areas16). Indeed, no new drugs for anxiety disorders have been approved by the EMA or FDA in the 2010s17. One possible reason for the lack of success in translating findings from animal models to human pathological anxiety is the unknown predictive and cross-species translational validity of the animal models used in preclinical studies18,19.
Animal paradigms widely used in anxiety research are the open field test (OFT), the elevated plus-maze (EPM), and the light/dark-test, among others20–22. The OFT was introduced by Hall in 193423 to measure emotional behavior in rodents. In the test, a rodent is placed in a novel open space (e.g., a square 50 50 30 cm box) for a fixed period of time (e.g., 10 min) while its behavior is measured (e.g., center avoidance, total distance covered, defecation, grooming, or rearing)24. A central underlying assumption is that rodents avoid open spaces due to a fear of (aerial) predators25, rendering thigmotaxis–the movement along walls–an anxiety-like behavior26. The findings that anxiolytic drugs like benzodiazepines or receptor agonists typically increase the time spent in the (otherwise avoided) center of the open field27 support the assumption that behavioral OFT indices (e.g., center avoidance) are valid indicators of anxiety and have predictive validity for detecting anxiolytic effects (e.g.,26). However, the OFT is not sensitive to other drugs used in the treatment of anxiety disorders, particularly selective serotonin reuptake inhibitors27, which are currently the first-line pharmacotherapy for anxiety disorders (see, e.g., the German treatment guidelines for anxiety disorders28).
These conflicting results, together with the problems in discovering new anxiolytic medications in preclinical research15,16, challenge the validity of the rodent OFT as an adequate model for pathological anxiety18,19,27,29. As validity of animal models is essential for preclinical research, Grillon & Ernst (2016)19 suggest the use of re-translational approaches, i.e., conducting model tests in humans and comparing the results with animal studies. Cross-species translational validity of preclinical models may be achieved by “maximizing the similarity of the measures of responses across species” (19, p. 343).
First re-translational studies suggest the value of this approach. For example, Walz et al.30 realized a human open field test on a soccer field, comparing the movement behavior of agoraphobic patients vs. healthy controls and of participants high vs. low in anxiety sensitivity. In accordance with the hypotheses derived from the animal model, all participants exhibited some thigmotaxis, but agoraphobic patients and participants with high anxiety sensitivity showed more movement along the outer region of the open field (i.e., thigmotaxis), entered the center of the open field less frequently, and had a higher latency for the first center entry in comparison to their respective control group. These results suggest a cross-species similarity between animal and human (agoraphobic) open field behavior. Similarly, Biedermann et al.31 re-translated the EPM to a human virtual reality test. In accordance with the hypotheses derived from the animal model, open arms induced more anxiety than closed arms, and these anxiety ratings correlated with behavioral avoidance of the open arms. Again, results suggest a possible cross-species similarity in open arm avoidance, with the caveat that the human EPM results seemed to be affected by trait acrophobia31, an effect not in accordance with animal EPM behavior, which is thought to be independent of height32,33. Further studies have reported initial successes of such re-translational approaches (e.g.,34–40). This line of research is valuable, as human preclinical models demonstrating cross-species translational and predictive validity may successfully bridge the gap between animal preclinical research and clinical studies41.
The present study aimed at translating the Walz et al.30 human OFT to virtual reality for several reasons. Firstly, virtual reality has high ecological validity and simultaneously allows strong laboratory-like experimental control. Secondly, virtual reality allows repeated testing in the laboratory and therefore facilitates studies of underlying mechanisms based on distinct experimental manipulations. Thirdly, experimental repetitions with constant conditions allow controlled comparisons between groups, e.g., various anxiety disorders, as well as repeated testing within groups, e.g., to evaluate treatment effects. Finally, combined testing with various re-translated models assumed to measure the same construct, e.g., the OFT and EPM, would be possible, which may improve test validity.
With the virtual OFT, we investigated human movement behavior in a virtual open field in analogy to animal studies and evaluated whether anxiety traits modulate such behavior. Our hypotheses were: (1) participants prefer to stay in the outer region of the open field as indicated by behavioral indices derived from animal studies; (2) this preference for the outer region is a function of trait anxiety measures of participants, with a stronger preference for the outer region in participants with higher anxiety; (3) participants report more anxiety and arousal in the inner compared to the outer region. To this end, we conducted three experiments in virtual reality, testing different versions of the human OFT.
Materials and methods
Sample
Participants in all three experiments were recruited on a local subject recruitment platform at the University of Würzburg. The inclusion criterion was age between 18–60, the exclusion criteria were pregnancy and epilepsy (in subjects or near relatives). The samples in experiments 1, 2, and 3 consisted of participants (age: , ; 21 female), participants (age: , ; 25 female), and participants (age: , ; 58 female), respectively. No participants were excluded from data analysis. Ethical approval for the experimental procedure (virtual reality setup and subjective reports) was obtained for previous studies from the Ethics Committee of the Medical Faculty of the University of Würzburg. All participants gave their written informed consent before the experiments in accordance with the Declaration of Helsinki. Participants received either 9 EUR or course credit for their participation.
Measures
Questionnaires
State-Trait Anxiety Inventory (STAI;42,43). The STAI is a self-report questionnaire that assesses anxiety as a state and as a trait. The state anxiety subscale consists of 20 items (e.g., “I am worried”) rated on a four-point Likert scale ranging from 1 (“not at all”) to 4 (“very much so”). The trait anxiety subscale consists of 20 items (e.g., “I worry too much over something that really doesn’t matter”), rated on a four-point Likert scale ranging from 1 (“almost never”) to 4 (“almost always”). Participants are asked to rate the statements according to how they currently feel (state) or how they feel in general (trait). Scores for both subscales have a range of 20–80.
Agoraphobic Cognitions Questionnaire (ACQ;44,45). The ACQ assesses the self-reported frequency of agoraphobic cognitions on two subscales: loss of control (e.g., “I will not be able to control myself”) and physical concerns (e.g., “I am going to pass out”). The 14 items are rated on a five-point Likert scale ranging from “thought never occurs” to “thought always occurs” and subsequently means are calculated for each subscale and a total score (1–5).
Mobility Inventory (MI;45,46). The MI is a self-report measure that assesses avoidance of common situations (e.g., supermarkets, open spaces, trains) due to discomfort or anxiety. Avoidance is rated on a five-point Likert scale ranging from “never avoid” to “always avoid” for either being alone or when accompanied with a trusted person. Scores for the subscales alone and accompanied are calculated by averaging the respective ratings (1–5).
Anxiety Sensitivity Index—3 (ASI-3;47,48). The ASI-3 assess self-reported anxiety sensitivity, i.e., the tendency to interpret symptoms of fear and anxiety as harmful49. The questionnaire comprises the three subscales physical concerns (e.g., “When I feel pain in my chest, I worry that I’m going to have a heart attack”), cognitive concerns (e.g., “When my mind goes blank, I worry there is something terribly wrong with me”), and social concerns (e.g., “When I begin to sweat in a social situation, I fear people will think negatively of me”). The 18 items are rated with respect to how strong these apply to participants on a five-point Likert scale ranging from 0 (“very little”) to 4 (“very”). Sum scores for each scale are calculated from six respective items (0–24) in addition to a total score (0–54).
Ratings
Anxiety and arousal within the virtual open field were assessed by verbal reports using the questions “how anxious do you feel in this location on a scale of 0–100?” and “how aroused do you feel in this location on a scale of 0–100”. Prior to the experiment, participants were given anchors for 0 (“not at all”) and 100 (“extreme—as strong as I can imagine”). The rating scales were derived from the Subjective Units of Distress Scale (SUDS,50).
Behavior
Positioning data of participants in the virtual open field was extracted from the player position in Unreal Engine at a sample rate of 20 Hz and saved to a local text file (see https://github.com/dgromer/ue4-misc/blob/master/LogFileWriter/4.20/LogfileWriter.cpp for the implementation). From the positioning data, we calculated several behavioral indices:
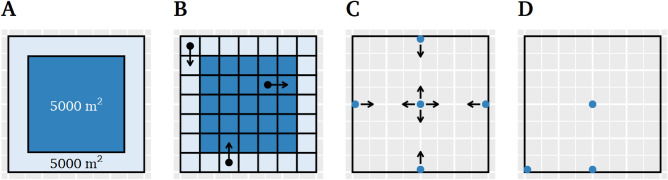
Time in outer region. For this index, the open field was subdivided into two areas: an inner and outer region. The area of the inner region was defined to be half of the total area (= 10,000 m2 / 2, resulting in a side length of m for the inner region). The outer region was defined as the remaining area (i.e., the width of the outer region was m) (see Fig. 1A). The time spent in the outer region (in seconds) was calculated as the sum of positioning data samples within the outer region divided by the sample rate (range 0–900 s). In addition to the time spent in the outer region, the total path length in meters (= the sum of Euclidean distances between each two positioning samples) and mean walking speed in m/s (= total path length divided by the time spent in the respective region) were calculated for both regions as control variables.
Figure 1.
(A) Areas of the inner and outer region of the virtual open field. (B) Binning used to calculate movement parameters based on line crossings. The arrow in the top left corner illustrates a line crossing counted as thigmotaxis. The arrow in the center region illustrates a line crossing counted as center ambulation. The arrow at the bottom illustrates a line crossing counted as center entry. (C) Starting positions. (D) Rating positions.
Proximity to wall. For this index, the distance to the nearest wall of the open field was calculated for each sample and subsequently averaged across all samples. The proximity to wall index has a range of 0–50 m.
Thigmotaxis, center entries, and center ambulation. For these indices, the open field was subdivided into a raster of 7 7 bins (side length of m each), resulting in 24 inner bins and 25 outer bins (approximating equal area of inner vs. outer regions) (see Fig. 1B). Thigmotaxis was defined as the number of line crossings within outer region bins, center entries were defined as the number of line crossings from an outer to an inner bin, and center ambulation was defined as the number of line crossings within inner region bins (see also30). As we later report the proportion of each line crossing parameter in relation to the total number of line crossings, we further calculated the fourth possible line crossing type: center leaves, defined as a line crossing from an inner to an outer bin.
Apparatus and software
The experiments ran on a Windows 10 64-bit machine (Intel Core i7-2600k, 16 GB RAM, Samsung Evo 850 SSD, NVIDIA Geforce 970 GTX) and were displayed on a HTC VIVE (HTC, New Taipei City, Taiwan) with a resolution of 1080 1200 pixels per eye. The Lighthouse tracking system of the HTC VIVE was used for positional and rotational tracking of participants. A HTC VIVE Controller was used for movement within the virtual environment. Audio instructions were presented via a HTC VIVE Deluxe Audio Strap.
The experiment and virtual open field were built in Unreal Engine 4.20 (Epic Games, Cary, North Carolina, USA) using the built-in virtual reality template and assets from the “Paragon: Agora and Monolith Environment” pack. Movement within the virtual environment was possible by either teleportation (using the technique from Unreal Engine’s virtual reality template for motion controllers) or actual walking within the space of the laboratory (about 2.5 m 3.5 m). The SteamVR Chaperone system warned participants when getting to close to a real wall.
Virtual human open field test
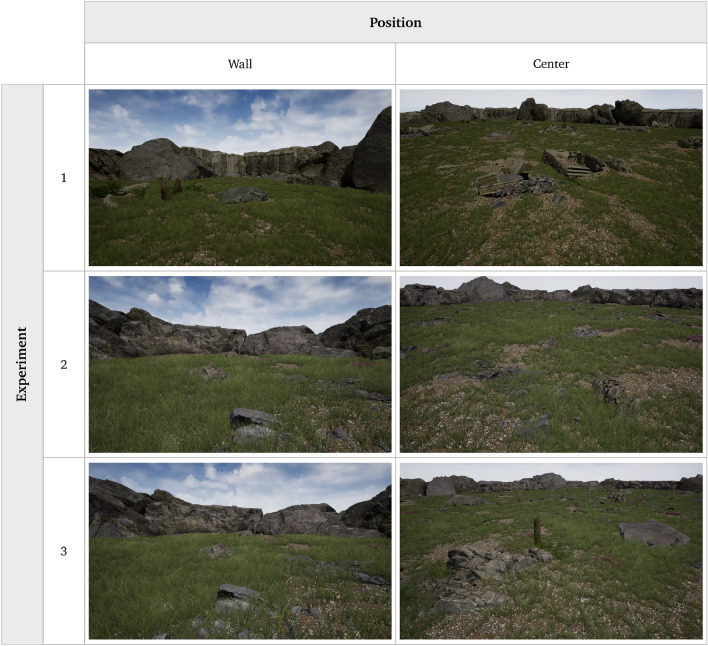
For the present study, a virtual open field was built with the goal to translate the original experimental setup to an environment appealing to human subjects. The virtual open field thus consisted of a 100 m 100 m grassland with stones and flowers spread across the area. In the three experiments, slightly different versions of the open field were used (see Fig. 2). Modifications were applied successively in an attempt to better control and standardize the effects of open field design on movement behavior. In experiment 1, the outer wall was a mix of concrete-like walls and large rocks, about 3–6 m in height, and parts of ancient concrete structures were spread across the field. In experiment 2 and 3, the outer walls were made of large rocks only, about 2.5–4.5 m in height, and the ancient concrete structures were removed. In experiment 2, only few small rocks were spread across the field. In experiment 3, more and larger rocks and some tree stumps were added across the field.
Figure 2.
Screenshots depicting the three versions of the virtual human open field test.
Experimental design and procedure
All three experiments were based on a correlational design to investigate associations between anxious traits and movement behavior in the open field.
Upon arrival at the laboratory, participants read an information letter and signed the informed consent. Next, the experimenter explained how participants could use the HTC VIVE Controller to navigate within the virtual environment. Participants were then equipped with the head-mounted display and controller. In the training phase, participants moved through a generic virtual environment collecting blue spheres to get accustomed to the virtual reality and the movement within the virtual environment. After completing the training phase, the display faded to black and participants were teleported to the virtual open field. Participants randomly started in one of eight positions: either in the center looking towards of one of the four walls, or at one of the four walls looking towards the center (see Fig. 1C). When the image faded in, participants received an audio instruction with the task to explore the virtual environment freely. Moreover, participants were asked not to stay in one position for a prolonged time. After 15 min, the display faded to black and three rating trials started. In these trials, participants were successively teleported to three locations (center of the open field, at a wall, and at a corner, see Fig. 1D) in pseudo-randomized order and asked to rate their anxiety and arousal. After the ratings, participants were helped to put off the HMD and filled in questionnaires (demographics, STAI, ACQ, MI, and ASI-3).
Data analysis
All statistical analyses were conducted with R 3.5.151. The afex package52 was used for ANOVA. Confidence intervals for Cohen’s d (95%) and partial eta-squared () (90%) were calculated with the MBESS package53,54. In the ANOVA for anxiety ratings (experiment 3), one participant had to be excluded due to missing data in one of the three ratings.
To test the hypothesis that participants prefer to stay in the outer region of the open field, we used a simulation approach to calculate expected values for the behavioral indices based on random moving agents. These expected values were then used for comparison with the participants’ actual movement behavior. In the following, we will describe the simulation approach; for the acutal implementation, see Supplementary Material 1. As participants used the teleportation technique to move within the virtual environment, such teleportation movements were simulated. The total number of simulated teleportations per run was derived from the actual movement behavior of participants in the three experiments (i.e., the median number of teleportations per participant was 367). Likewise, the teleportation distances were also sampled from the participants’ actual teleportation distances. A simulated run began in one of the eight starting positions of the open field (see Fig. 1C). Then, both a teleportation distance and direction (, i.e., straight on, ) were sampled and, if the destination was within the area of the open field, the teleportation was executed. Otherwise, a new teleportation destination was sampled. Each simulation ran until reaching the defined number of teleportations. A total of 1,000 simulation runs were conducted and expected values for the behavioral indices were then calculated from this simulated movement data.
Hypotheses were tested separately by study and not combined, because of systematic differences in movement behavior across the three experiments (see Fig. 3C).
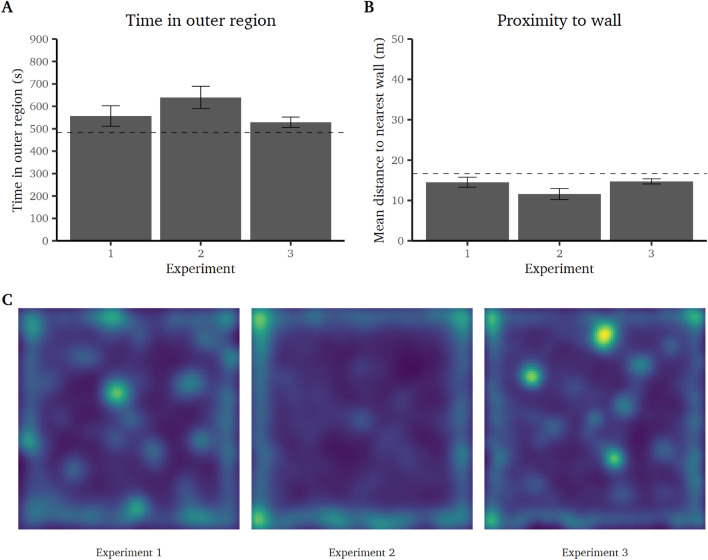
Figure 3.
(A) Mean time (in seconds) spent in the outer region of the open field in experiments 1–3 (± 95% CI; indicates significance if the CI does not include the expected value of 483.40 s (dashed line)). (B) Mean distance (in meters) to the nearest wall of the open field in experiments 1–3 (± 95% CI; indicates significance if the CI does not include the expected value of 16.67 m (dashed line)). (C) Heatmaps of the positioning data of all participant in experiments 1–3. Shading indicates the time spent in the respective position across all participants (the brighter, the more time spent in the respective position).
Results
Time in the outer region
First, we tested if participants showed a stronger preference for the outer region of the open field than would be expected by random movement. For this purpose, we conducted one-sample t-tests on the time in the outer region (against the expected value s). In all three experiments, participants spent more time in the outer region of the open field than expected, experiment 1: , , [0.20; 0.97]; experiment 2: , , [0.70; 1.63]; and experiment 3, , , [0.21; 0.67] (see Fig. 3A). Moreover, in all three experiments, participants spent more time in the outer compared to the inner region (i.e., comparing against s). In addition, we compared the walking distances and walking speeds in the inner vs. outer region of the open field using paired t-tests. The performed walking distances were larger in the outer compared to the inner region of the open field, experiment 1: , , [0.02; 0.75]; experiment 2: , , [0.53; 1.40]; and experiment 3: , , [0.30; 0.77], but the walking speed was higher in the inner compared to the outer region of the open field, experiment 1: , , [− 0.94; − 0.18]; experiment 2: , , [− 1.19; − 0.37]; and experiment 3: , , [− 0.78; − 0.31].
Proximity to wall
In addition to analyzing the time in the outer region, we tested if participants were, on average, closer to the wall then what would be expected by random movement. One-sample t-tests (against m) showed that, in experiments 2 and 3, participants preferred to stay closer to the wall, experiment 2: , , [− 1.55; − 0.64]; and experiment 3: , , [− 0.53; − 0.08]. In experiment 1, participants did not stay closer to the wall than expected, , , [− 0.68; 0.04] (see Fig. 3B).
Line crossing parameters
To compare the participants’ actual movement behavior (in terms of line crossings, see Fig. 4) with what would have been expected by random movement, we conducted a series of one-sample t-tests.
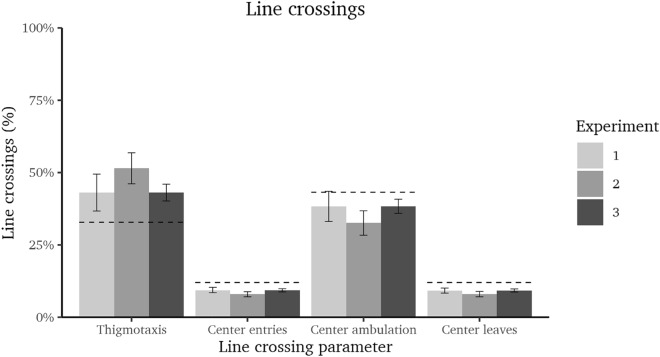
Figure 4.
Proportion of thigmotaxis, center entries, center ambulation, and center leaves in total line crossings (± 95% CI; indicates significance if the CI does not include the respective expected value (dashed line)).
For thigmotaxis, one-sample t-tests (against %) showed that, in all three experiments, participants moved more along the wall then would have been expected by chance, experiment 1: , , [0.20; 0.97]; experiment 2: , , [0.80; 1.78]; and experiment 3: , , [0.53; 1.04].
For center entries, one-sample t-tests (against ) showed that, in all three experiments, participants showed less center entries than would have been expected by random movement, experiment 1: , , [-1.46; 0.59]; experiment 2: , , [2.25; 1.12]; and experiment 3: , , [1.35; 0.80].
For center ambulation, one-sample t-tests (against ) showed that, in experiments 2 and 3, participants moved less across the center of the open field than would have been expected by random movement, experiment 2: , , [1.37; 0.50]; and experiment 3: , , [0.67; 0.21]. In experiment 1, participants center ambulation behavior did not significantly differ from the expected values, , , [0.71; 0.02].
For center leaves, one-sample t-tests (against ) showed that, in all three experiments, participants left the center less frequently than would have been expected by random movement, experiment 1: , , [1.61; 0.69]; experiment 2: , , [2.17; 1.07]; and experiment 3: , , [1.42; 0.85].
Relationships between anxiety traits and behavior
Correlational analyses were conducted to investigate associations between anxiety traits and movement patterns within the open field. As some of the behavioral parameters are related by design, we conducted the correlational analyses only with time in the outer region, thigmotaxis, and center entries. For each anxiety measure (STAI and the subscales of ASI-3, ACQ, and MI), the correlations with behavioral parameters were calculated and are reported in Tables 1, 2 and 3. Applying an experiment-wise Bonferroni correction (), non of the correlations reached significance.
Table 1.
Correlations between anxiety measures and time in outer region.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| STAI Trait | .23 | .213 | − .01 | .971 | .02 | .864 |
| ASI-3 Physical Concerns | .15 | .433 | .07 | .706 | – .06 | .570 |
| ASI-3 Cognitive Concerns | .07 | .714 | .06 | .740 | .09 | .451 |
| ASI-3 Social Concerns | .21 | .258 | − .11 | .548 | .11 | .332 |
| ACQ Physical Concerns | .06 | .745 | .06 | .771 | .27 | .014 |
| ACQ Loss of Control | .13 | .500 | .02 | .908 | − .07 | .543 |
| Mobility Inventory Accompanied | .17 | .348 | .05 | .792 | .06 | .592 |
| Mobility Inventory Alone | .06 | .758 | .07 | .721 | .14 | .207 |
STAI = State-Trait Aanxiety Inventory; ASI-3 = Anxiety Sensitivity Index—3, ACQ = Acoraphobic Cognitions Questionnaire, MI = Mobility Inventory.
Table 2.
Correlations between anxiety measures and thigmotaxis.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| STAI Trait | − .14 | .447 | − .11 | .554 | .28 | .011 |
| ASI-3 Physical Concerns | .02 | .894 | .14 | .472 | − .01 | .895 |
| ASI-3 Cognitive Concerns | − .11 | .570 | − .11 | .545 | .24 | .033 |
| ASI-3 Social Concerns | .11 | .546 | − .38 | .040 | .18 | .118 |
| ACQ Physical Concerns | .07 | .726 | .35 | .061 | .16 | .168 |
| ACQ Loss of Control | − .15 | .416 | − .24 | .199 | .04 | .720 |
| Mobility Inventory Accompanied | .04 | .844 | .04 | .839 | .08 | .482 |
| Mobility Inventory Alone | − .12 | .538 | .01 | .966 | .29 | .010 |
STAI = State-Trait Anxiety Inventory; ASI-3 = Anxiety Sensitivity Index—3, ACQ = Acoraphobic Cognitions Questionnaire, MI = Mmobility Inventory.
Table 3.
Correlations between anxiety measures and center entries.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| STAI Trait | − .49 | .005 | .01 | .976 | .30 | .008 |
| ASI-3 Physical Concerns | − .23 | .207 | .29 | .125 | − .01 | .930 |
| ASI-3 Cognitive Concerns | − .23 | .204 | .10 | .594 | .15 | .172 |
| ASI-3 Social Concerns | − .09 | .626 | .02 | .903 | − .00 | .992 |
| ACQ Physical Concerns | − .10 | .587 | .45 | .012 | − .07 | .555 |
| ACQ Loss of Control | − .25 | .180 | .06 | .752 | .09 | .437 |
| Mobility Inventory Accompanied | − .07 | .699 | .10 | .606 | .04 | .732 |
| Mobility Inventory Alone | − .26 | .165 | .21 | .260 | .24 | .033 |
STAI = State-Trait Anxiety Inventory; ASI-3 = Anxiety Sensitivity Index—3, ACQ = Acoraphobic Cognitions Questionnaire, MI = Mobility Inventory.
Looking only at the direction of the correlations, there were significantly more correlation coefficients indicating more center avoidance with higher anxiety (46 out of 72), , .
In sum, this analysis indicates that there might be an association between anxiety traits and movement behavior with in the open field; however, results were mostly not consistent across the experiments and correlation coefficients were mostly very small.
Ratings
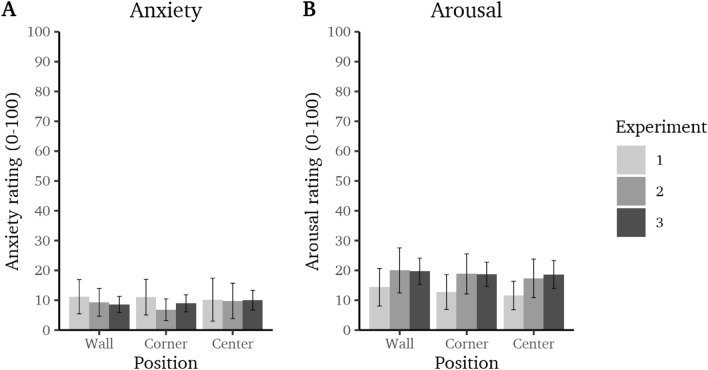
Repeated-measures ANOVA (with Greenhouse-Geisser correction of degrees of freedom if assumption of sphericity was violated) were used to compare the anxiety and arousal ratings at the center, corner, and wall positions respectively for each study. In all three experiments, the anxiety ratings did not differ between positions, experiment 1: , , [0.00; 0.04]; experiment 2: , , [0.00; 0.11]; and experiment 3: , , [0.00; 0.04] (see Fig. 5A). Likewise, the arousal ratings did not differ between positions: experiment 1, , , [0.00; 0.15]; experiment 2: , , [0.00; 0.08]; and experiment 3: , , [0.00; 0.02] (see Fig. 5B).
Figure 5.
(A) Mean ratings of anxiety and (B) arousal at the center, corner, and wall of the open field in experiments 1–3 (± 95% CI).
Discussion
The present study explored human movement behavior in a virtual OFT and investigated three research questions: firstly, whether humans—analogous to rodents—show a preference for the outer region of the open field; secondly, whether this preference is moderated by anxious traits; and thirdly, whether humans report more anxiety and arousal in the inner compared to the outer region of the open field.
Preference for the outer region
Regarding the hypothesis that humans–like rodents–spend more time in the outer compared to the center region of the open field, results from all behavioral indices (time in the outer region, average proximity to the nearest wall, thigmotaxis, center entries, and center ambulation) showed a clear preference for the outer region (with only proximity to the nearest wall and center ambulation not reaching significance in one of the three experiments). These findings were consistent across all three experiments and conform with results from rodent studies (e.g.,55–58) and a real human open field study30.
Quantitative comparisons regarding the time spent in the outer or center regions between rodent studies and the current study are, however, not trivial, as the OFT is a non-standardized test24,59 with differing center region areas (e.g., 28% of total area57, vs. 11.1% of total area58). A descriptive analysis (see Supplementary Material 2) indicated that participants in our virtual OFT tended to spend a little more time (about 7.1–9.6%) in the center region compared to rodents in animal studies56–58; however, one study55 examined rodents spending about 0.5% more time in the center region than participants in the current study. Moreover, participants in the current virtual OFT descriptively spent less time (about 15.1%) in the center region than participants in the real-life OFT30.
In sum, we conclude that thigmotaxis is a cross-species behavior, i.e., is shared among rodents and humans, which can be assessed reliably in humans with a virtual OFT paradigm. However, the quantitative comparisons suggest that the virtual OFT elicits somewhat more thigmotaxis in humans than a real OFT.
Associations between anxiety and behavior
Correlational analyses between center avoidance and trait measures of anxiety revealed only limited support for the hypothesis that anxiety modulates thigmotaxis and related behaviors in a virtual human OFT. Correlation coefficients were mostly small (and thus statistically not significant) and not consistent across the three experiments. However, we also noted that overall, most correlation coefficients indicated more center avoidance with higher anxiety. This observation suggests a possible modulatory role of anxiety on human behavior in a virtual OFT, which, however, has to be demonstrated conclusively by improved study designs. In fact, the human real-life OFT by Walz et al. (2016)30 showed large effect sizes for the comparison of agoraphobic patients vs. healthy controls and of participants high vs. low in anxiety sensitivity with regards to center avoidance measures. Several possible explanations for the weak effects in the present experiments have to be discussed.
First, the samples in the present study were not stratified for the whole range of the trait anxiety measures. This restriction of range problem can cause an underestimation of true correlation60 and is a possible reason why the present study might have failed to observe a true population effect. In addition, strong increases in thigmotaxis (or center avoidance in general) as a defensive behavior might only occur at a certain degree of anxiety. If thigmotaxis is an evolutionary-conserved adaptive response to open spaces shared across species30, it should be mediated by phylogenetically ancient circuits in the brain and increases in anxiety could lead to an activation of such circuits61,62. In the real-life OFT, where effects of anxiety on behavior could be found, Walz et al. (2016)30 examined agoraphobic patients and persons high in anxiety sensitivity, the latter with an average ASI-3 total score of 31.33 (SD = 4.54) compared to average ASI-3 total scores of 18.00 (SD = 12.94), 18.90 (SD = 12.15), and 17.48 (SD = 10.89) in the present experiments. However, by using an extreme group design, the large effect sizes reported by Walz et al. (2016) might have also overestimated the true effect63. Animal studies support the view that anxiety has only a moderate or weak effect on open field behavior as the effect of anxiolytic drugs on rodent thigmotactic behavior is limited. For example, only 56% of studies using benzodiazepines detected an effect of the drug on reducing thigmotactic behavior (27; and a more recent study57). If the population effect of anxiety on open field behavior is indeed moderate or small, the human OFT might only differentiate between high and low levels of anxiety, but not between smaller increments. Future studies should address this empirically by stratification across the whole range of trait anxiety parameters.
Second, trait anxiety likely modulates behavior via state anxiety in a given situation, i.e., individuals with high levels of trait anxiety are more likely to experience state anxiety in a potentially threatening situation, and this enhanced state anxiety motivates avoidance behavior. However, in the present study, participants reported only little anxiety or arousal even in the inner part of the virtual open field, indicating that they did not experience the situation as threatening. Thus, trait anxiety had little effects on state anxiety, and in consequence, we found only few and small associations between trait anxiety and behavior. Following the Experimental Psychopathology approach by Grillon et al. (2019)41, anxiety-induction procedures are especially valuable to investigate effects of anxiety on behavioral and cognitive operations. An example for such an anxiety-induction procedure is the threat of shock paradigm, which induces sustained anxiety by informing participants that they will receive unpleasant electrical stimuli during the experiment (e.g.,64–68). For example, Kirlic, et al. (2017)69 demonstrated that participants with high risk for affective disorder display less exploratory behavior in response to a threat of shock. Using this paradigm in the OFT would offer the possibility to investigate the effects of state anxiety on thigmotaxis as a defensive behavior. Future human OFT studies should experimentally manipulate state anxiety in order to investigate how it mediates the effects of trait anxiety on avoidance behavior. Moreover, it might be necessary to consider design changes to the human OFT to better account for human preferences and aversions. In rodents, the OFT possibly induces a state of anticipatory anxiety (pre-encounter threat)70 due to a fear of aerial predators, while the current virtual OFT for humans likely had a very low threat imminence. In rodents, brighter lighting is used to increase the aversiveness of the situation. As is commonly known, rodents are nocturnal animals, while humans are diurnal. It stands thus to reason, that the aversive effect of bright lights in rodents in the open field might rather be reproduced using darkness in humans.
Third, discrepancies between the virtual and the real-life OFT30 have to be considered, specifically, differences in locomotion and the field of view. In the present study, the participants had to use teleportation for locomotion within the virtual environment71. This method was implemented based on our experience and empirical evidence that teleportation–compared with continuous locomotion–induces relatively little simulator sickness (72–77, but see78,79). In fact, not a single participant in the current study dropped out of the experiment due to simulator sickness. However, research has shown that locomotion methods differentially affect how users navigate within virtual environments (e.g.,77,80,81). It can therefore not be ruled out that the realized “unnatural” locomotion method detrimentally affected open field behavior. In addition, the head-mounted display used in the present study had a field of view of , which does not span the whole human field of view of up to 82 (as cited in83). This restriction of peripheral vision might have influenced the perception of the expanse of the open field and thereby the behavioral response. Technological developments might overcome these shortcomings. For example, CAVE systems (e.g.,84–86) can present virtual environments with a wider field of view; and more natural locomotion methods like walking in place on an omnidirectional treadmill or redirected walking might better represent real walking71.
Fourth, although center avoidance in the open field is commonly considered an anxiety-like behavior (e.g.,55–58,87,88), this simplified interpretation has been criticized as being insufficiently validated18,59. Indeed, thigmotaxis has also been described as a spatial orientation strategy used to explore unfamiliar environments38. Assuming multiple underlying factors driving the use of thigmotaxis, the influence of anxiety on such behavior might be limited to certain situations, populations, or species. For example, the use of thigmotaxis in rodents as a strategy to avoid predators has strong face validity as staying in an open field increases the likelihood of attacks89. Likewise, avoidance of an open field center in agoraphobic patients has strong face validity as this behavior is in accordance with the DSM-5 criteria A.2 (“marked fear or anxiety about [...] [b]eing in open spaces” ) and B (“The situations are avoided [...]”)90. However, additional motivations might affect open field behavior too (e.g., foraging or mating in animals; curiosity or sensation seeking in humans), and in consequence, the effects of anxiety on open field behavior may be reduced or covered. Such divergent motivational effects covering anxiety effects might be especially prominent in healthy humans with average levels of anxiety, as examined here.
In fact, we found an influence of open field design on movement behavior (see Supplementary Material 3), which suggests that the amount of objects spread across the open field (e.g., stones, flowers) affects behavior. A possible reason for humans to spend less time in the outer region of an enriched environment may be an increased exploratory motivation or a motivation to reduce boredom. Importantly, such factors may not only be relevant for the comparison of different open field designs but likely also play an important role as inter-individual differences affecting open field behavior. Similarly, implementations of the OFT in animal studies (e.g., regarding size, shape, material, lighting, temperature, definition of zones, or definitions of behavioral parameters) vary considerably (e.g.,56,91–93, also24,59), and this might be a reason for divergent findings. We conclude that it is probably an oversimplified assumption to equate certain open field behaviors directly with anxiety (see also18). Both diverse motivations and diverse open field designs likely affect open field behavior. These factors may play a stronger role in humans than in animals; and within humans, a stronger role in healthy than in high anxious individuals or anxiety patients. Future human OFT studies should try to disentangle and assess the motivations at work besides anxiety by measuring additional state (e.g., boredom, engagement) and trait variables (e.g., sensation seeking).
Fifth, self-reported anxious traits and behaviors may only be loosely coupled, although related to the same underlying latent variable. In this regard, it would be necessary to identify and investigate intermediate processes, for example, associations between defensives behaviors and neural activation patterns with e.g., stylized game-like open field paradigms94–96. For future research, it is, however, an important goal to bridge the gap between levels of analysis of latent variables such as anxiety. We believe that virtual reality paradigms play an important role in this endeavor, as they provide the opportunity to investigate complex behaviors with high ecological validity. Until now, diagnoses of mental disorders rely on questionnaires and self-reported symptoms only. To improve diagnostics, it might be an important next step to open up the avenue for psychiatric diagnostics to consider evidence from standardized behavioral tests.
Conclusion
The present study established a human OFT in virtual reality and demonstrated a cross-species similarity in movement behavior, i.e., participants in our virtual open field exhibited thigmotaxis and showed a preference for the outer region comparable to the behavior of rodents in animal studies. However, evidence for correlations between open field behavior and anxious traits was rather weak and not unequivocal. Thus, the re-translation of the animal OFT to a human virtual OFT was successful by revealing thigmotaxis in humans. Future studies should use the approach to further elaborate what exactly the open field test measures and under which conditions anxiety modulates human open field behavior.
Supplementary information
Acknowledgements
We would like to thank A. Mykolenko, J. Kufa, and M. Falkenberg for their support during data acquisition. This study was supported by the Volkswagen Foundation (AZ 94 102). The publication of this study was supported by the Open Access Publication Fund of the University of Wuerzburg.
Author contributions
D.G., D.P.K. and P.P. contributed to the study concept and design. D.G. carried out the experiments and statistical analyses. D.G. drafted the article and D.P.K. and P.P. provided critical revisions. All authors approved the final version of the article prior to submission.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data and analysis scripts can be found at https://osf.io/7untj/.
Competing interests
P.P. is shareholder of a commercial company that develops virtual environment research systems (VTplus GmbH) for empirical studies in the field of psychology, psychiatry, and psychotherapy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-021-85678-5.
References
- 1.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Wittchen HU, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter JK, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress. Anxiety. 2018;35:502–514. doi: 10.1002/da.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatry. 2008;69:621–632. doi: 10.4088/JCP.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer KS, Levy HC, Tolin DF. Remission in CBT for adult anxiety disorders: a meta-analysis. Clin. Psychol. Rev. 2018;61:1–8. doi: 10.1016/j.cpr.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Loerinc AG, et al. Response rates for CBT for anxiety disorders: need for standardized criteria. Clin. Psychol. Rev. 2015;42:72–82. doi: 10.1016/j.cpr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Carl E, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn. Behav. Ther. 2020;49:1–21. doi: 10.1080/16506073.2018.1560358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtiss J, Andrews L, Davis M, Smits J, Hofmann SG. A meta-analysis of pharmacotherapy for social anxiety disorder: an examination of efficacy, moderators, and mediators. Expert Opin. Pharmacother. 2017;18:243–251. doi: 10.1080/14656566.2017.1285907. [DOI] [PubMed] [Google Scholar]
- 10.Roest AM, et al. Reporting bias in clinical trials investigating the efficacy of second-generation antidepressants in the treatment of anxiety disorders: a report of 2 meta-analyses. JAMA Psychiatry. 2015;72:500–510. doi: 10.1001/jamapsychiatry.2015.15. [DOI] [PubMed] [Google Scholar]
- 11.LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. Am. J. Psychiatry. 2016;173:1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- 12.Young KS, Craske MG. Survival circuits in affective disorders. Curr. Opin. Behav. Sci. 2018;24:83–88. doi: 10.1016/j.cobeha.2018.03.001. [DOI] [Google Scholar]
- 13.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 14.Zoellner LA, Foa EB. Applying research domain criteria (RDoC) to the study of fear and anxiety: a critical comment. Psychophysiology. 2016;53:332–335. doi: 10.1111/psyp.12588. [DOI] [PubMed] [Google Scholar]
- 15.Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas, D. W. et al. Clinical Development Success Rates 2006–2015. Technical Report, Biotechnology Innovation Organization (2016).
- 17.Sartori, S. B. & Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther.10.1016/j.pharmthera.2019.107402 (2019). [DOI] [PubMed]
- 18.Stanford SC. Confusing preclinical (predictive) drug screens with animal ‘models’ of psychiatric disorders, or ‘disorder-like’behaviour, is undermining confidence in behavioural neuroscience. J. Psychopharmacol. 2017;31:641–643. doi: 10.1177/0269881116689260. [DOI] [PubMed] [Google Scholar]
- 19.Grillon C, Ernst M. Gain in translation: Is it time for thigmotaxis studies in humans? Biol. Psychiatry. 2016;80:343–344. doi: 10.1016/j.biopsych.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 2001;125:141–149. doi: 10.1016/S0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 21.Litvin, Y., Pentkowski, N. S., Pobbe, R. L., Blanchard, D. C. & Blanchard, R. J. Chapter 2.5 Unconditioned models of fear and anxiety. In Blanchard, R. J., Blanchard, D. C., Griebel, G. & Nutt, D. (eds.) Handbook of Anxiety and Fear, vol. 17 of Handbook of Anxiety and Fear, 81–99. 10.1016/S1569-7339(07)00006-9 (Elsevier, 2008).
- 22.Harro J. Animals, anxiety, and anxiety disorders: How to measure anxiety in rodents and why. Behav. Brain Res. 2018;352:81–93. doi: 10.1016/j.bbr.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934;18:385–403. doi: 10.1037/h0071444. [DOI] [Google Scholar]
- 24.Walsh RN, Cummins RA. The open-field test: a critical review. Psychol. Bull. 1976;83:482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 25.Barnett SA. The Rat: A Study in Behavior. Revised. Canberra: Australian National University Press; 1976. [Google Scholar]
- 26.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol. Biochem. Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 27.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 28.Bandelow, B., Lichte, T., Rudolf, S., Wiltink, J. & Beutel, M. (eds.) S3-Leitlinie Angststörungen (Springer, Heidelberg, 2015).
- 29.Ennaceur A. Tests of unconditioned anxiety—Pitfalls and disappointments. Physiol. Behav. 2014;135:55–71. doi: 10.1016/j.physbeh.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Walz N, Mühlberger A, Pauli P. A human open field test reveals thigmotaxis related to agoraphobic fear. Biol. Psychiatry. 2016;80:390–397. doi: 10.1016/j.biopsych.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Biedermann SV, et al. An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol. 2017;15:125. doi: 10.1186/s12915-017-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol. Biochem. Behav. 1993;44:463–469. doi: 10.1016/0091-3057(93)90492-C. [DOI] [PubMed] [Google Scholar]
- 33.Falter U, Gower AJ, Gobert J. Resistance of baseline activity in the elevated plus-maze to exogenous influences. Behav. Pharmacol. 1992;3:123–128. doi: 10.1097/00008877-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Aupperle RL, Sullivan S, Melrose AJ, Paulus MP, Stein MB. A reverse translational approach to quantify approach-avoidance conflict in humans. Behav. Brain Res. 2011;225:455–463. doi: 10.1016/j.bbr.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bach DR, Korn CW, Vunder J, Bantel A. Effect of valproate and pregabalin on human anxiety-like behaviour in a randomised controlled trial. Transl. Psychiatry. 2018;8:1–10. doi: 10.1038/s41398-018-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bähner F, et al. Hippocampal–Dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a human translational imaging study. Neuropsychopharmacology. 2015;40:1674–1681. doi: 10.1038/npp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual morris water maze. J. Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kallai J, Makany T, Karadi K, Jacobs WJ. Spatial orientation strategies in Morris-type virtual water task for humans. Behav. Brain Res. 2005;159:187–196. doi: 10.1016/j.bbr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Kallai J, et al. Cognitive and affective aspects of thigmotaxis strategy in humans. Behav. Neurosci. 2007;121:21–30. doi: 10.1037/0735-7044.121.1.21. [DOI] [PubMed] [Google Scholar]
- 40.Mueller SC, et al. Impaired spatial navigation in pediatric anxiety. J. Child Psychol. Psychiatry. 2009;50:1227–1234. doi: 10.1111/j.1469-7610.2009.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grillon, C., Robinson, O. J., Cornwell, B. & Ernst, M. Modeling anxiety in healthy humans: a key intermediate bridge between basic and clinical sciences. Neuropsychopharmacology. 10.1038/s41386-019-0445-1 (2019). [DOI] [PMC free article] [PubMed]
- 42.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 43.Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Weinheim: Beltz Test GmbH; 1981. [Google Scholar]
- 44.Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear of fear in agoraphobics: The Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. J. Consult. Clin. Psychol. 1984;52:1090–1097. doi: 10.1037/0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- 45.Ehlers, A., Margraf, J. & Chambless, D. Fragebogen zu körperbezogenen Ängsten (AKV (Beltz-Test, Kognitionen und Vermeidung, 2001).
- 46.Chambless DL, Caputo GC, Jasin SE, Gracely EJ, Williams C. The mobility inventory for agoraphobia. Behav. Res. Ther. 1985;23:35–44. doi: 10.1016/0005-7967(85)90140-8. [DOI] [PubMed] [Google Scholar]
- 47.Taylor S, et al. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol. Assess. 2007;19:176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- 48.Kemper, C., Ziegler, M. & Taylor, S. ASI-3 - Angstsensitivitätsindex-3. In Leibniz-Zentrum für Psychologische Information und Dokumentation (ZPID) (ed.) Elektronisches Testarchiv (PSYNDEX Tests-Nr. 9006208). 10.23668/psycharchives.403 (ZPID (Leibniz Institute for Psychology Information), Trier, 2009).
- 49.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav. Res. Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 50.Wolpe J. Psychotherapy by Reciprocal Inhibition. Stanford, CA: Stanford University Press; 1958. [Google Scholar]
- 51.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2018).
- 52.Singmann, H., Bolker, B., Westfall, J., Aust, F. & Ben-Shachar, M. S. afex: Analysis of Factorial Experiments (2019). R package version 0.25-1.
- 53.Kelley, K. MBESS: The MBESS R Package (2019). R package version 4.6.0.
- 54.Kelley, K. Confidence intervals for standardized effect sizes: theory, application, and implementation. J. Stat. Softw.10.18637/jss.v020.i08 (2007).
- 55.He T, Guo C, Wang C, Hu C, Chen H. Effect of early life stress on anxiety and depressive behaviors in adolescent mice. Brain Behav. 2020 doi: 10.1002/brb3.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahrens S, et al. A central extended amygdala circuit that modulates anxiety. J. Neurosci. 2018;38:5567–5583. doi: 10.1523/jneurosci.0705-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson T, Grabowski-Boase L, Tarantino LM. Prototypical anxiolytics do not reduce anxiety-like behavior in the open field in C57BL/6J mice. Pharmacol. Biochem. Behav. 2015;133:7–17. doi: 10.1016/j.pbb.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mudgal J, et al. Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J. Funct. Foods. 2020 doi: 10.1016/j.jff.2019.103638. [DOI] [Google Scholar]
- 59.Stanford SC. The open field test: reinventing the wheel. J. Psychopharmacol. 2007 doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- 60.Sackett PR, Yang H. Correction for range restriction: an expanded typology. J. Appl. Psychol. 2000;85:112–118. doi: 10.1037/0021-9010.85.1.112. [DOI] [PubMed] [Google Scholar]
- 61.Qi S, et al. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc. Natl. Acad. Sci. 2018;115:3186–3191. doi: 10.1073/pnas.1712314115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mobbs D, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preacher, K. J. Extreme groups designs. In Cautin, R. L. & Lilienfeld, S. O. (eds.) The Encyclopedia of Clinical Psychology, vol. 2, 1189–1192. 10.1002/9781118625392.wbecp190 (John Wiley & Sons, Inc., Hoboken, NJ, 2015).
- 64.Bublatzky F, Riemer M, Guerra P. Reversing threat to safety: incongruence of facial emotions and instructed threat modulates conscious perception but not physiological responding. Front. Psychol. 2019 doi: 10.3389/fpsyg.2019.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kavcıoğlu FC, Bublatzky F, Pittig A, Alpers GW. Instructed threat enhances threat perception in faces. Emotion. 2019 doi: 10.1037/emo0000708. [DOI] [PubMed] [Google Scholar]
- 66.Lago TR, et al. Threat-of-shock decreases emotional interference on affective stroop performance in healthy controls and anxiety patients. Eur. J. Neurosci. 2019;00:1–10. doi: 10.1111/ejn.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn. Affect. Behav. Neurosci. 2011;11:217. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vytal K, Cornwell B, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: from impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirlic N, Aupperle RL, Misaki M, Kuplicki R, Alvarez RP. Recruitment of orbitofrontal cortex during unpredictable threat among adults at risk for affective disorders. Brain Behav. 2017 doi: 10.1002/brb3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mobbs D, Headley DB, Ding W, Dayan P. Space, time, and fear: survival computations along defensive circuits. Trends Cogn. Sci. 2020;24:228–241. doi: 10.1016/j.tics.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Boletsis C. The new era of virtual reality locomotion: a systematic literature review of techniques and a proposed typology. Multimodal Technol. Interact. 2017;1:24. doi: 10.3390/mti1040024. [DOI] [Google Scholar]
- 72.Vlahovic, S., Suznjevic, M. & Kapov, L. S. Subjective assessment of different locomotion techniques in virtual reality environments. In 2018 Tenth International Conference on Quality of Multimedia Experience (QoMEX), 1–3. 10.1109/QoMEX.2018.8463433 (2018).
- 73.Rahimi, K., Banigan, C. & Ragan, E. D. Scene transitions and teleportation in virtual reality and the implications for spatial awareness and sickness. IEEE Transactions on Visualization and Computer Graphics 1–1. 10.1109/TVCG.2018.2884468 (2018). [DOI] [PubMed]
- 74.Christou, C. G. & Aristidou, P. Steering versus teleport locomotion for head mounted displays. In De Paolis, L. T., Bourdot, P. & Mongelli, A. (eds.) Augmented Reality, Virtual Reality, and Computer Graphics, Lecture Notes in Computer Science, 431–446. 10.1007/978-3-319-60928-7_37 (Springer International Publishing, Cham, 2017).
- 75.Frommel, J., Sonntag, S. & Weber, M. Effects of controller-based locomotion on player experience in a virtual reality exploration game. In Proceedings of the 12th International Conference on the Foundations of Digital Games, FDG ’17, 1–6. 10.1145/3102071.3102082 (Association for Computing Machinery, New York, NY, USA, 2017).
- 76.Habgood, J. M. P., Moore, D., Wilson, D. & Alapont, S. Rapid, continuous movement between nodes as an accessible virtual reality locomotion technique. In 2018 IEEE Conference on Virtual Reality and 3D User Interfaces (VR), 371–378. 10.1109/VR.2018.8446130 (2018).
- 77.Coomer, N., Bullard, S., Clinton, W. & Williams-Sanders, B. Evaluating the effects of four VR locomotion methods. In Proceedings of the 15th ACM Symposium on Applied Perception - SAP 18. 10.1145/3225153.3225175 (ACM Press, 2018).
- 78.Bozgeyikli, E., Raij, A., Katkoori, S. & Dubey, R. Point and teleport locomotion technique for virtual reality. In Proceedings of the 2016 Annual Symposium on Computer-Human Interaction in Play - CHI PLAY 16. 10.1145/2967934.2968105 (ACM Press, 2016).
- 79.Clifton J, Palmisano S. Effects of steering locomotion and teleporting on cybersickness and presence in HMD-based virtual reality. Virtual Reality. 2019 doi: 10.1007/s10055-019-00407-8. [DOI] [Google Scholar]
- 80.Paris, R. et al. How video game locomotion methods affect navigation in virtual environments. In ACM Symposium on Applied Perception 2019 on-SAP 19. 10.1145/3343036.3343131 (ACM Press, 2019).
- 81.Xie, X., Paris, R. A., McNamara, T. P. & Bodenheimer, B. The effect of locomotion modes on spatial memory and learning in large immersive virtual environments: a comparison of walking with gain to continuous motion control. In Creem-Regehr, S., Schöning, J. & Klippel, A. (eds.) Spatial Cognition XI, Lecture Notes in Computer Science, 58–73. 10.1007/978-3-319-96385-3_5 (Springer International Publishing, Cham, 2018).
- 82.Werner EB. Manual of Visual Fields. New York: Churchill Livingstone; 1991. [Google Scholar]
- 83.Toet A, Jansen SEM, Delleman NJ. Effects of field-of-view restrictions on speed and accuracy of manoeuvring. Percept. Mot. Skills. 2007;105:1245–1256. doi: 10.2466/pms.105.4.1245-1256. [DOI] [PubMed] [Google Scholar]
- 84.Gromer D, et al. Height simulation in a virtual reality CAVE system: validity of fear responses and effects of an immersion manipulation. Front. Hum. Neurosci. 2018 doi: 10.3389/fnhum.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kinateder M, et al. The effect of dangerous goods transporters on hazard perception and evacuation behavior—a virtual reality experiment on tunnel emergencies. Fire Saf. J. 2015;78:24–30. doi: 10.1016/j.firesaf.2015.07.002. [DOI] [Google Scholar]
- 86.Kinateder M, et al. Social influence on route choice in a virtual reality tunnel fire. Transp. Res. Part F Traffic Psychol. Behav. 2014;26:116–125. doi: 10.1016/j.trf.2014.06.003. [DOI] [Google Scholar]
- 87.Crumeyrolle-Arias M, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 88.De Gregorio D, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136–150. doi: 10.1097/j.pain.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fanselow MS, Hoffman AN, Zhuravka I. Timing and the transition between modes in the defensive behavior system. Behav. Process. 2019 doi: 10.1016/j.beproc.2019.103890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5 edn. (American Psychiatric Publishing, 2013).
- 91.Aguilar BL, Malkova L, N’Gouemo P, Forcelli PA. Genetically epilepsy-prone rats display anxiety-like behaviors and neuropsychiatric comorbidities of epilepsy. Front. Neurol. 2018 doi: 10.3389/fneur.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rombolà L, et al. Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules. 2017;22:614. doi: 10.3390/molecules22040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weber MD, et al. The influence of microglial elimination and repopulation on stress sensitization induced by repeated social defeat. Biol. Psychiat. 2019;85:667–678. doi: 10.1016/j.biopsych.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bach DR, et al. Human hippocampus arbitrates approach-avoidance conflict. Curr. Biol. 2014;24:541–547. doi: 10.1016/j.cub.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korn CW, et al. Amygdala lesions reduce anxiety-like behavior in a human benzodiazepine-sensitive approach-avoidance conflict test. Biol. Psychiat. 2017;82:522–531. doi: 10.1016/j.biopsych.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mobbs D, et al. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. 2009;29:12236–12243. doi: 10.1523/jneurosci.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analysis scripts can be found at https://osf.io/7untj/.