Abstract
The cortical control of gait and mobility involves multiple brain regions. Therefore, one could speculate that the association between specific spatial patterns of cortical thickness may be differentially associated with different mobility domains. To test this possibility, 115 healthy participants aged 27–82 (mean 60.5 ± 13.8) underwent a mobility assessment (usual-walk, dual-task walk, Timed Up and Go) and MRI scan. Ten mobility domains of relatively simple (e.g., usual-walking) and complex tasks (i.e., dual task walking, turns, transitions) and cortical thickness of 68 ROIs were extracted. All associations between mobility and cortical thickness were controlled for age and gender. Scaled Subprofile Modelling (SSM), a PCA-regression, identified thickness patterns that were correlated with the individual mobility domains, controlling for multiple comparisons. We found that lower mean global cortical thickness was correlated with worse general mobility (r = − 0.296, p = 0.003), as measured by the time to complete the Timed Up and Go test. Three distinct patterns of cortical thickness were associated with three different gait domains during simple, usual-walking: pace, rhythm, and symmetry. In contrast, cortical thickness patterns were not related to the more complex mobility domains. These findings demonstrate that robust and topographically distinct cortical thickness patterns are linked to select mobility domains during relatively simple walking, but not to more complex aspects of mobility. Functional connectivity may play a larger role in the more complex aspects of mobility.
Subject terms: Motor cortex, Premotor cortex
Introduction
Walking abilities and mobility are critical to safe ambulation and functional independence. Conversely, alterations in gait are associated with and predictive of mortality, morbidity, and multiple adverse health outcomes1,2. Furthermore, various groups have generated models of gait that sort discrete gait characteristics into specific gait domains3–5. Interestingly, prospective clinical studies have shown that changes in some of these specific gait domains are associated with the development of disparate outcomes (e.g., Alzheimer’s disease, mild cognitive impairment, Parkinsonism, and death)1,2,6–8. In addition, these gait domains were used to provide a framework for investigating and interpreting findings related to the neural correlates of gait control9,10. Altogether, these findings suggest that the cortical control of gait and mobility and their relationship to specific brain regions and structures is not monolithic. Instead, the relationships between brain structures and walking abilities apparently depend on the specifics of the mobility domain under question.
Neuroimaging studies also support the idea that the links between gait and cortical function are dependent on the specific aspect of gait10. Alterations in gait during simple and complex walking tasks and mobility components such as turning and transitions (to and from walking to sitting) have been associated with reduced gray matter volume (GMV) across multiple brain regions11–16. Slower gait and reduced step length, two components of the “pace” domain of gait3 were associated with reduced mean global GMV11,14, reduced frontal17, occipital, and hippocampal GMV11,12,14,15, lower volumes in cerebellar regions11,15, and lower basal ganglia volumes11,13. Some studies reported that greater (worse) step-to-step variability of step time, a gait measure related to fall risk, was associated with decreased hippocampal volume18 and reduced right parietal lobe19. However, these findings were not consistently observed20. More generally, whole-brain analyses of the structures that link cortical structures to specific aspects of gait are lacking13.
One way of study these relationships is to investigate cortical thickness patterns. Until recently, the methodology of whole brain analysis approaches has been restricted to voxel-based morphometry (VBM), implemented volumetrically across the cortex or as deformation analysis in longitudinal datasets. In VBM, gray matter density and gray matter concentrations are crucial for the interpretation of the results, but voxel density at specific point is meaningless as it is a mixed measure of grey matter including cortical surface area and cortical folding. In contrast, cortical thickness is a selective measure of gray matter that may be more sensitive to cortical atrophy and a loss of gray matter21,22 and may be more robust since it does not need to be adjusted for intracranial volume. Therefore, in this study, we use a different whole brain analysis approach that measures cortical thickness across the entire cerebrum. This approach may reveal specific patterns of gray matter loss that correlate to particular gait domains.
The neuroimaging studies that have examined the relationship between gait domains and cortical regions are generally limited in two additional key areas. First, most of the extant literature evaluated gait under relatively simple, single-task walking conditions13. That is, the participants were asked to walk at their comfortable speed. In contrast, everyday mobility often takes place as the participant moves around while simultaneously performing one or more tasks23,24. In contrast to single-task, “usual-walking”, dual-task walking requires additional cognitive resources, especially those associated with executive function and attention, which involves and calls into play additional brain areas25–27. Second, the relationships between GMV and turns and transitions (i.e., moving to and from sitting to walking), putatively two relatively complex tasks, have not been well-studied. While the cortical control that regulates these two important aspects of mobility (i.e., turns and transitions) is apparently different from that of other aspects of gait28, little is known about their association with gray matter volumes in specific brain regions.
The present analyses were designed to better understand the relationships between specific domains of gait and mobility and brain structures. As summarized in Table 1, we evaluated 10 domains of mobility, adapting previous classifications3 to cover usual-walking, dual-task walking, turns, and transitions. Rather than evaluating GMV, we used measures of cortical thickness since previous work has suggested that it may afford superior measurement properties29. As detailed below, we used a novel whole brain approach to study the relationship between cortical thickness patterns and mobility domains. Based on the prospective clinical studies that demonstrated that specific gait domains are related to disparate adverse health outcomes1,2,6–8 and based on parallel studies of cortical thickness in cognitive function29, we hypothesized that distinct cortical thickness patterns would be selectively associated with specific mobility domains. Furthermore, we hypothesized that these associations would depend on the complexity of the task for example, more frontal regions involved during dual-task walking compared to usual-walking.
Table 1.
The gait and mobility measures used to assess each task and their grouping into ten mobility domains.
| Task | Measures | Domains | Z-score | |
|---|---|---|---|---|
| Usual walk | Stride time (m/s) | 1.12 ± 0.01 | Rhythm usual | 0.04 ± 0.09 |
| Gait speed (m/s) | 0.97 ± 0.01 | Pace usual | − 0.29 ± 0.07 | |
| Stride length (m) | 1.20 ± 0.02 | |||
| Stride regularity | 0.76 ± 0.01 | Variability usual | − 0.01 ± 0.03 | |
| Stride time variability | 1.54 ± 0.06 | |||
| Step regularity | 0.63 ± 0.01 | Symmetry usual | − 0.20 ± 0.08 | |
| Step symmetry | 0.81 ± 0.02 | |||
| Dual-task (DT) walk | Stride time (m/s) | 1.23 ± 0.01 | Rhythm DT | 0.04 ± 0.10 |
| Gait speed (m/s) | 0.84 ± 0.01 | Pace DT | − 0.24 ± 0.07 | |
| Stride length (m) | 1.15 ± 0.01 | |||
| Stride regularity | 0.61 ± 0.01 | Variability DT | − 0.03 ± 0.04 | |
| Stride time variability (%) | 2.67 ± 0.21 | |||
| Step regularity | 0.55 ± 0.01 | Symmetry DT | − 0.13 ± 0.07 | |
| Step symmetry | 0.92 ± 0.03 | |||
| Transitions | Ant-post range (g) | 1.94 ± 0.25 | Transition | − 0.02 ± 0.03 |
| Ant-post jerk (g/s) | 1.39 ± 0.62 | |||
| Pitch (deg/s) | − 79.58 ± 4.04 | |||
| Roll (deg/s) | 50.63 ± 2.85 | |||
| Turns | Yaw amplitude (deg/s) | 168.96 ± 4.30 | Turns | 0.03 ± 0.05 |
| Yaw duration (s) | 1.72 ± 0.06 | |||
Methods
We followed all related tenets of the Declaration of Helsinki and all procedures in this work were approved by the local Columbia University Medical Center Institutional Review Board. All included participants gave their informed written consent.
Participants
The present analysis is based on a larger cohort study of the whole adult lifespan sample, evaluating the relationship of different cognitive domains with fMRI and various structural features25. In this study community-living, healthy participants were recruited using established market mailing procedures to equate the recruitment procedures of young and old adults30. Participants who responded were screened over the telephone to ensure that they met basic inclusion criteria (e.g., right-handed, English speaking, no psychiatric or neurological disorders that can affect cognition, normal or corrected-to-normal vision, and not taking any CNS-targeting medications). At initial recruitment, general exclusion criteria included MRI contraindications, hearing impairment, and objective cognitive or functional impairment. All subjects were required to be right handed native English speakers. Exclusion criteria included uncontrolled high blood pressure, current or recent non-skin neoplastic disease or melanoma (but not prostatic carcinoma), active hepatic disease or primary renal disease requiring dialysis, primary untreated endocrine diseases (Well-treated hypothyroidism was not excluded), HIV infection or other medical disorders judged by neurologist to interfere with study, pregnant or lactating (participation allowed 3 months after ceasing lactation), and medications that target the CNS taken within the last month. Subjects with psychiatric issues were excluded if they had a history of psychosis, ECT, current or recent major depressive disorder, bipolar disorder or anxiety disorder. Neurological exclusions included brain disorder such as stroke, tumor, infection, epilepsy, multiple sclerosis, degenerative diseases, head injury (LOC > 5 min), mental retardation, imaged cortical stroke or large subcortical lacunae or infarct or space-occupying lesion (≥ 2 cubic cm), and diagnosed learning disability, dyslexia, or ADHD25. Eligible individuals were further screened in person and a Mattis Dementia Rating Scale score of at least 130 was required for inclusion in the study31.
Gait and mobility measures
A small, lightweight sensor that includes a three-axis accelerometer and a three-axis gyroscope (DynaPort; McRoberts, The Hague, the Netherlands) was used for assessment of mobility. The device was worn on the lower back of the participants to assess gait and Timed Up and Go (TUG) test32,33. The protocol included walking along a 20-m-long corridor for one minute under two conditions: (1) preferred, usual-walking speed, and (2) dual-tasking; reciting words that start with the letter “A” (cognitive task) while walking. The dual-task walking condition was considered a more complex task that requires motor and cognitive brain resources. The spatio-temporal gait measures obtained from mean values of multiple steps included stride time, gait speed, and stride length. Dynamic features of gait that reflect within subject changes over time during the walk included stride time variability, stride regularity, step regularity, and step symmetry, as previously described34,35. These seven gait measures were grouped into four domains based on previous studies3–5,10: pace encompassing stride length and gait speed, rhythm including stride time, variability including stride regularity and stride time variability, and symmetry encompassing step regularity and step symmetry. This was performed separately for straight-line, single-task, usual-walking and for straight-line dual-task walking, altogether generating 8 domains (Table 1).
The TUG test consisted of standing up from a chair, walking 3 m at a normal pace, turning around, walking back, and sitting back down in the chair. Acceleration signals were derived from three axes: vertical, mediolateral, and anterior–posterior. Angular velocities were derived from the gyroscope as yaw (rotation around the vertical axis), pitch (rotation around the mediolateral axis), and roll (rotation around the anterior–posterior axis) to generate quantitative measures for three subtasks: sit-to-stand and stand-to-sit transitions and turning36. The transition domain (9th domain) included four measures: anterior–posterior range, anterior–posterior jerk, pitch, and roll, while the turn domain (10th domain) included the yaw amplitude and yaw duration5. These measures were calculated only from the TUG tests and added two more mobility domains. Before creating these 10 domains (pace simple, single-task/dual-task, rhythm simple, single-task/dual-task, variability simple single-task/ dual-task, symmetry simple/dual-task, transitions, turns), all the mobility measures were normalized by subtracting their means and by then dividing by their standard deviations. After normalizing each of the measures, we averaged the normalized measures within each domain. For example, the pace domain score was calculated by averaging the normalized gait speed and the normalized stride length, giving each of the components similar weight. We hypothesized that each of these gait domains that represents a combined variables reflect independent neuroanatomical substrates that would be quantified using different patterns of cortical thickness.
MRI acquisition and cortical thickness measures
MRI images were acquired in a 3.0 T Philips Achieva Magnet using a standard quadrature head coil. A T1-weighted scout image was acquired to determine the participant’s position. T1-weighted images of the whole brain were acquired for each participant with an MPRAGE sequence with 180 contiguous 1 mm thick axial slices using the following parameters: TR 6.5 ms, TE 3 ms; flip angle 8°, acquisition matrix 256 × 256, and 256 mm field of view37. A neuroradiologist reviewed anatomical scans, and any with potentially clinically significant findings, such as abnormal neural structure, was entirely removed from the sample prior to the current analysis. Cortical thickness was measured using the FreeSurfer analysis package (Version 5.1, https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation) and resulted in 68 regions of interest (ROIs) and mean global cortical thickness of the entire brain (not just of the 68 ROIs) for all 115 participants37,38.
Statistical analyses
Demographics and mobility measures
All variables were evaluated for normality and homogeneity using box plots and scatter plots. Demographic and general mobility measures are reported as mean ± standard deviation for continuous variables and percent for categorical variables. Pearson correlations (not adjusted for age and gender) were performed to examine associations between demographic variables, gait speed and TUG duration as proxies for general mobility and mean global cortical thickness.
Subprofile scaling modeling of adjusted thickness mobility relationships
Since cortical thickness very often shows strong associations with age and gender39, we residualized both the mobility outcome and the cortical thickness array with respect to age, gender, and their interaction, before performing Subprofile Scaling Modeling (SSM), i.e. a form of PCA (Principal Component Analysis) regression, on the residual data. SSM’s starting point is the following decomposition which would generically hold for any multivariate technique:
Here, Y is the cortical thickness array, with rows indexing ROIs and columns indexing participants. This matrix was normalized such that both within-row sums across columns and within-column sums across rows yield zero, i.e. the whole brain mean of each participant and the mean image across participants were removed from the data array. V is a matrix of principal components with rows indexing ROIs. W is a matrix of component scores with the rows indexing participants. Both V and W have N − 1 columns, where N is the number of participants or observations. (Our normalization of Y removed one degree of freedom.) Before performing the SSM analysis, we included an extra step and removed the influence of several nuisance covariates that included age, gender and age by gender interaction from Y.
If we write our array as nuisance covariates as
where age and gender are mean-centered column vectors of chronological age and gender (1 = men, 2 = women) with as many rows as participants.
The nuisance covariates can be removed from the pattern scores with linear regression:
A residualized data array can be written as
This array is free of any associations with the nuisance covariates; thus patterns derived from it will yield pattern scores whose correlation with the nuisance covariates is zero by design. We similarly adjusted the mobility outcomes and remove the influence of the nuisance covariates.
The adjusted mobility outcomes and cortical thickness arrays were then submitted to standard SSM analysis. First, a PCA was performed on the cortical thickness volumes, then a brain-behavioral regression determined the best fitting set of PCs to predict the gait/mobility measure. The best-fitting PC-set was determined according to the AIC (Akiake’s Information Criterion) as it provides a more inclusive sets of PCs and do not enforce sparsity implicitly like BIC. If a PC does not contribute sufficiently, the bootstrap procedure would down-weight the influence accordingly. The purpose of using the PCA was to account for correlation in the regional thickness values, rather than performing the analysis on a region-wise level, which would lead to unduly conservative multiple-comparison corrections.
Inferential robustness of the ROI-loadings in the derive patterns was assessed with bootstrap resampling. If we write the whole derivation process as a function:
where rMob refers to the residualized mobility outcome, we can obtain bootstrap samples
where rMob* and rY* refer to resampled data arrays for which participants were sampled with replacement while keeping the assignment of mobility outcome to thickness intact. We generated 10,000 bootstrap samples, enabling us to plot the coverage interval [2.5‰ 97.5‰] of the bootstrap distribution.
There was no apriori sample size calculation since this would require a prior effect size and some Monte-Carlo simulations. However, Type-I error was tightly controlled as too few observations would have degraded the stability of the loadings: one could find a pattern but hardly localized it as the loadings switch signs too much under bootstrap procedure. This was an inherent protection against Type-I errors of commission in identifying significant loadings.
Results
Participants
One hundred and thirty participants were included in the current analyses. Participant characteristics are summarized in Table 2 and mobility measures in Table 1. The age of the participants ranged from 27 to 82 years; 64 (55.7%) were women. TUG duration ranged from 6 to 19 s and gait speed ranged from 71 to 140 cm/s. In univariate associations, higher age was associated with lower levels of mean global cortical thickness and slower gait speed, higher (i.e., worse) stride time variability, and longer (i.e., worse) durations of the TUG. Participants with lower values of mean global cortical thickness took longer to complete the TUG and had higher stride time variability. These associations did not persist after adjusting for age and sex. Mean global cortical thickness was not associated with the other gait measures specified in Table 2.
Table 2.
Participant characteristics and univariate correlations.
| Pearson’s correlation (r) | |||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Min–max | Mean global thickness | Gait speed | Stride time CV | TUG | |
| Age (years) | 60.45 | 13.75 | 27–82 | − 0.462** | − 0.154 | 0.203* | 0.304* |
| Gender (% female) | 55.7 | ||||||
| Mean global thickness (mm) | 2.49 | 0.12 | 2.16–2.83 | – | 0.027 | − 0.210* | − 0.296* |
| Gait speed (m/s) | 0.97 | 0.19 | 0.71–1.40 | 0.027 | – | − 0.259* | − 0.231* |
| Stride time variability (%) | 1.54 | 0.67 | 0.46–4.09 | − 0.210* | − 0.259* | – | 0.215* |
| TUG duration (s) | 11.76 | 2.31 | 6–19 | − 0.296* | − 0.231* | 0.215* | – |
*p < 0.05, **p < 0.001.
Principal component analysis of cortical thickness
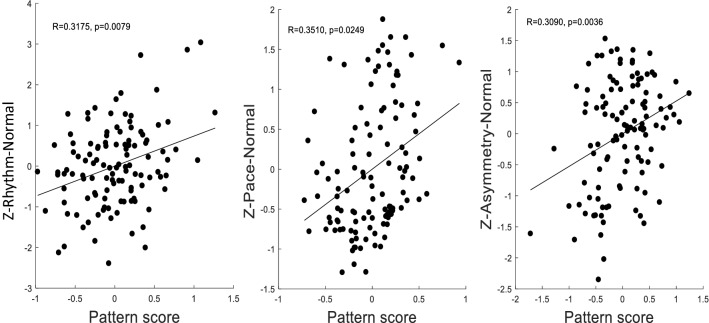
Principal components analysis was applied to the thickness measures across all ROIs and 67 principal components (PCs) were derived. We assume invariance across the group and look for differences in degree along one pattern established by a PCA across the whole group. After controlling for age, gender, and age*gender by removing the mean pattern across participants from the data array, three mobility domains were significantly associated with three different subsets of PCs. During single-task, usual-walking, a linear combination of PC1–3 best fit the rhythm domain (R = 0.3175), a linear combination of PC1–6 best fit the pace domain (R = 0.3510), and a linear combination of PC1–2 best fit the gait symmetry domain (R = 0.3090) (Fig. 1). All R-values are bivariate Pearson correlations. No PC combinations were significantly correlated with these domains during dual-task walking or with gait variability, turns or transitions.
Figure 1.
The three patterns which were derived such that their pattern scores correlate maximally with the mobility variables.
The specific brain regions underlying the PC combinations
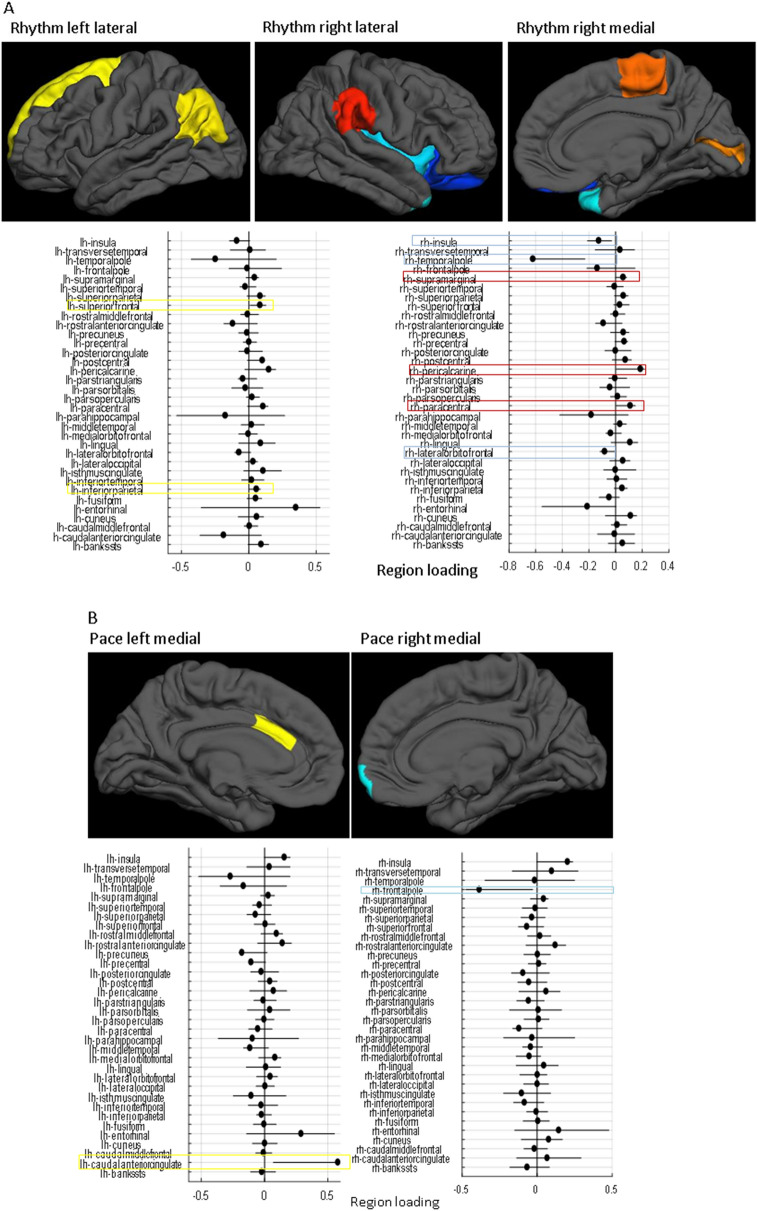
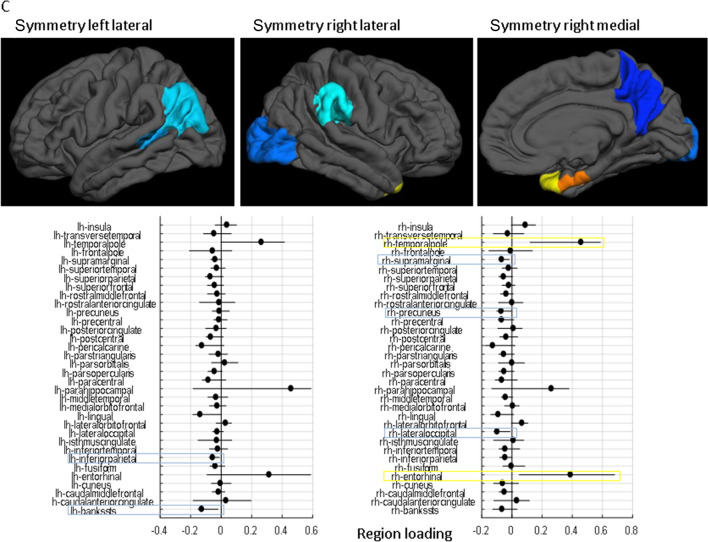
Gait rhythm during single-task, usual-walking was correlated with higher positive loading in the left inferior-parietal, right pericalcarine, and right paracentral. Negative loadings were observed in the right insula and right temporal pole (Fig. 2A). Pace during singe-task, usual-walking was associated with higher positive loading in the left caudal anterior cingulate and negative loading in the right frontal pole (Fig. 2B). Lastly, gait symmetry during single-task, usual-walking was correlated with higher positive loadings in the right entorhinal, and right temporal pole and negative loadings in the left inferior parietal, left bankssts (i.e., cortical areas around the superior temporal sulcus), right lateral occipital, right inferior parietal, left pars-opercularis, and right supramarginal (Fig. 2C).
Figure 2.
The loading of each brain region in the linear combination of PCs that best fit each of the mobility variables. The top surface plots show regions that were deemed robust in the bootstrap procedure, with hot colors (red shades) indicating positive loadings, and cold colors (blue shades) indicating negative loadings according to the left bars (lh = left hemisphere, rh = right hemisphere). The horizontal line plots show point estimates for the loadings as black dots for each region and indicate the coverage interval [5‰ 95‰] of the 10,000 bootstrap iterations. (A) the cortical-thickness pattern constructed from PCs1–3, which is associated with usual-walking gait rhythm, (B) the cortical-thickness pattern constructed from PCs 1–6, which is associated with usual-walking gait pace, and (C) the specific brain regions constructed from PCs 1–2 which is associated with usual-walking gait symmetry. Positive loadings indicate a relatively thicker regional cortex with the gait measures, while negative loadings indicate a relatively thinner regional cortex.
Discussion
In this study, we evaluated the relationship between spatial patterns in cortical thickness and mobility measured in multiple domains. Our novel approach revealed that three distinct patterns of cortical thickness were associated with three different gait domains during simple, single-task usual-walking: pace, rhythm, and symmetry. In contrast, no associations were observed between cortical thickness and more complex mobility measures such as dual-task walking, transitions, or turns.
Several previous studies have shown correlations between changes in brain structural variables and gait impairments10,14,16,19. However, our approach of using whole-brain analyses with principal component regression to optimally identify specific brain area patterns associated with different mobility modalities has not been previously applied. The present findings demonstrate selective associations between cortical thickness patterns and mobility domains during relatively simple, single-task walking after controlling for age and gender. Different combinations of six patterns of cortical thickness (PC1–6), each based on 68 ROIs, were correlated to three mobility domains (Supplementary Table 1). Interestingly, specific and different brain regions underlying the PC combinations correlated with rhythm, pace, and symmetry. These correlations included positive and negative associations. The negative associations indicate that better performance is associated with relatively lesser thickness at some ROIs with co-varying greater thickness at other ROIs. In general, this finding of negative and positive associations parallels the select relationships between cognitive domains and cortical thickness patterns that were previously reported29. For example, higher positive loadings in the post central area and negative loadings in the parahippocampal area and insula correlated with higher global cognitive function29. Together, these findings support the idea that specific cognitive and motor functions are differentially associated with distinct cortical thickness patterns.
The present findings indicate that rhythm was positively correlated with the left inferior parietal lobule, an area that is important for the interpretation of sensory information40, right pericalcarine that relates to primary visual areas, and right paracentral that relates to the primary motor cortex. At the same time, rhythm was negatively correlated with the right insula, a region that plays a role in processing bodily sensations41, and the right temporal pole, a region that has been linked to semantic memory42. In general, these findings indicate that rhythm is associated with greater thickness in sensorimotor brain areas along with lower thickness in ventral areas related to memory and sensation. More generally, these associations indicate that a specific cortical thickness pattern is associated with this domain of gait during usual-walking.
In line with previous studies that examined the correlation between pace and gray matter volume (GMV)10, pace (i.e., higher walking speed) was positively correlated with the left caudal-anterior cingulate. This is a brain region that is active during highly demanding tasks that require cognitive control43. Pace was also negatively correlated with the right frontal pole region, which acts as a supervisory attentional control system44. Interestingly, both regions are part of the higher-order cognitive system and opposite correlations with pace during simple walking may indicate that each play a different role; perhaps the frontalpole is not called into play when the demands of the task are comparatively low as in relatively simple walking. This finding stands somewhat in contrast to other work in older adults and patients with neurodegeneration that has shown the important role of the prefrontal cortex during gait, even during usual-walking17,26,45,46. One possible explanation is that for older populations their simple, usual-walking is already challenging, making further demands on higher-order cognitive control. However, if this were the entire explanation, one would expect to see correlations under the dual-task walking condition, which we did not observe. Further work is needed to better understand these findings.
Higher (i.e., better) symmetry was positively correlated with the right temporal pole, a region that is involved in learning and remembering visual-spatial information47 and the right entorhinal that play a role in navigation48. In contrast, higher symmetry was negatively associated with the left inferior parietal and right supramarginal cortex, regions that are related to body image49. Right lateral occipital gyrus and left inferior parietal lobule that are both part of the perception network50,51, while the left pars-operuclaris that is important for action observation and imitation52. These co-varying thicknesses in brain regions may reflect the complex interaction between different brain networks during gait10. Interestingly, symmetry is relatively understudied in the literature in comparison to other measures of gait10 and more research needs to be conducted to confirm and elaborate on this interpretation.
Cortical thickness is a volumetric measure that has been considered as a proxy for GMV that does not need to be adjusted for intracranial volume12. The advantage of cortical thickness relies on its selectivity to detect changes in gray matter that may be more sensitive to cortical atrophy, compared to other GMV analysis. This can explain the fact that while gait disturbances, mainly from the pace domain, were previously associated with reduced GMV in various brain regions11,15,16, our results demonstrate that cortical thickness patterns involve both positive and negative associations with distinct brain regions. While the negative loadings are somewhat counter-intuitive, similar findings were observed in studies of the association between thickness and cognition29. In line with studies that used GMV, we found lower thickness in occipital areas11, hippocampal areas53, and limbic regions12,15. However, it is important to emphasize that the specific changes we observed are not an absolute difference in thickness but a relative difference in the context of a covariance pattern. In other words, just observing the thickness of each area separately will not give the same results. Previous GMV studies showed reduced volume in the prefrontal cortex, supplementary motor cortex, and sensorimotor cortex that were associated with worse gait performance12,15. In contrast, we observed that greater thickness in the paracentral gyrus was related to a better rhythm of gait (shorter stride time). These contradictory findings may suggest that GMV and cortical thickness are complementary volumetric measures that do not necessarily reflect identical brain properties.
The complexity of the associations between mobility and cortical thickness patterns supports the idea that gait is a multifaceted task that can be viewed as a process that requires "higher-level" cognitive control45,46,54,55. Two main locomotor pathways have been identified involving multiple brain areas for the control of gait: the dorsal pathway of cognitive locomotor control and the ventral pathway for emotional locomotor control56. The distinct associations that we observed show that the relationships between cortical thickness and gait are not necessarily local and probably involve patterns of correlations and connectivity between different vertices in various spatial dimensions. In addition, different patterns of cortical thickness are apparently linked to specific aspects of gait and the level of task difficulty. Unique patterns were related to pace, rhythm, and symmetry3 likely because each represents different gait characteristics12 that have distinctions in their cognitive control.
Measures of the performance of more complex aspects of mobility such as dual-task walking, turns, and transitions were not correlated with any patterns of structural cortical thickness. This negative finding is in contrast to our hypothesis that they will be highly correlated with frontal areas associated with cognition. Recent models suggest that the executive locomotor control is activated to supplement automaticity during more complex walking tasks57,58. Therefore, with aging and disease, a loss of automaticity increases the reliance on additional cognitive resources59. As a consequence, subcortical neural activation is compensated for by more cortical areas59–61. As such, it is possible that measures of dual-task walking would correlate with changes in subcortical gray matter that cannot be assessed using methods of cortical thickness. This may explain our negative finding with dual-task walking and the correlation only with usual walking.
In addition, no correlations between cortical thickness patterns and dual task walking may indicate that these facets of mobility are more dependent on functional activity of the brain26,62, while structural measures may not be enough to explain their performance. Various functional neuroimaging studies support our observation by showing increased activation and/or connectivity with greater task complexity, irrespective of age and gray matter volume26,62,63. The fact that patterns of cortical thickness were not associated with these complex mobility tasks can also be explained in light of the important role of functional brain measures, consistent with the cognitive reserve theory64. According to this theory, the performance of a relatively simple task can be within the normal range despite alterations in cortical thickness patterns, as long as sufficient functional activity, considered as a compensatory mechanism, takes place64. Alternatively, once task complexity exceeds functional capacity, changes in structural patterns of cortical thickness can become evident and mobility deficits will be observed65. Of course, functional connectivity also likely plays a role in the more simple, usual-walking; while cortical thickness patterns were significantly associated with these gait domains, much of the variance was not explained by cortical thickness, suggesting that other factors, like involvement of subcortical areas, connectivity, and perhaps peripheral structure and function, also play an important role.
This study has several limitations. For example, the dual-task included only one type of cognitive task (i.e., reciting words that start with the letter “A”) and measures of dual-task cost were not included in the analysis. Future studies should explore the impact of different cognitive tasks and evaluate the association between dual-task costs and cortical thickness patterns. In addition, in this study, we aimed to filter out the age effects. In the future, it may be of interest to focus on the effects of aging.
In conclusion, applying whole-brain analyses with PCA yielded topographically distinct cortical thickness patterns that were related to selective, relatively simple gait domains. Furthermore, the negative findings with respect to other mobility domains and more challenging conditions underscore the specificity of our findings. The present results motivate the investigation of functional connectivity analyses to evaluate their role in the more complex mobility domains. More generally, these findings emphasize the idea that applying different methodological approaches can reveal unique aspects of the involvement of different cortical regions that can advance our knowledge of how the brain functions to facilitate mobility. In addition, the results support the idea that the cortical control of gait depends on the specific domain.
Supplementary Information
Acknowledgements
This work was supported by the National Institute on Aging (Grant Numbers R01 AG026158, RF1AG038465, and F32AG053035). We thank the research participants and the research assistants for their time and effort.
Author contributions
I.M., C.G.H., J.M.H., A.M., and Y.S. contributed to the concept and rationale for the study. C.G.H. and I.M. contributed to data analysis. I.M., C.G.H., J.M.H., A.M., and Y.S. contributed to the interpretation of the results and to the drafting of the manuscript. All authors participated to the approval of the final manuscript and take responsibility for the content and interpretation of this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85058-z.
References
- 1.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verghese J, Wang C, Holtzer R. Relationship of clinic-based gait speed measurement to limitations in community-based activities in older adults. Arch. Phys. Med. Rehabil. 2011;92:844–846. doi: 10.1016/j.apmr.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 4.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J. Neurol. Neurosurg. Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verlinden VJ, van der Geest JN, Hoogendam YY, Hofman A, Breteler MM, Ikram MA. Gait patterns in a community-dwelling population aged 50 years and older. Gait Posture. 2013;37:500–505. doi: 10.1016/j.gaitpost.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Buchman AS, Dawe RJ, Leurgans SE, Curran TA, Truty T, Yu L, Barnes LL, Hausdorff JM, Bennett DA. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:1176–1183. doi: 10.1093/gerona/glz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Coelln R, Dawe RJ, Leurgans SE, Curran TA, Truty T, Yu L, Barnes LL, Shulman JM, Shulman LM, Bennett DA, Hausdorff JM, Buchman AS. Quantitative mobility metrics from a wearable sensor predict incident parkinsonism in older adults. Parkinsonism Relat. Disord. 2019;65:190–196. doi: 10.1016/j.parkreldis.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Q, Chastan N, Bair WN, Resnick SM, Ferrucci L, Studenski SA. The brain map of gait variability in aging, cognitive impairment and dementia—a systematic review. Neurosci. Biobehav. Rev. 2017;74:149–162. doi: 10.1016/j.neubiorev.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson J, Allcock L, Mc AR, Taylor JP, Rochester L. The neural correlates of discrete gait characteristics in ageing: a structured review. Neurosci. Biobehav. Rev. 2019;100:344–369. doi: 10.1016/j.neubiorev.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS ONE. 2014;9:e84909. doi: 10.1371/journal.pone.0084909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Laat KF, Reid AT, Grim DC, Evans AC, Kotter R, van Norden AG, de Leeuw FE. Cortical thickness is associated with gait disturbances in cerebral small vessel disease. Neuroimage. 2012;59:1478–1484. doi: 10.1016/j.neuroimage.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Dumurgier J, Vercruysse O, Paquet C, Bombois S, Chaulet C, Laplanche JL, Peoc'h K, Schraen S, Pasquier F, Touchon J, Hugon J, Lehmann S, Gabelle A. Intersite variability of CSF Alzheimer's disease biomarkers in clinical setting. Alzheimers Dement. 2013;9:406–413. doi: 10.1016/j.jalz.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology. 2015;57:851–861. doi: 10.1007/s00234-015-1536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stijntjes M, de Craen AJ, van der Grond J, Meskers CG, Slagboom PE, Maier AB. Cerebral microbleeds and lacunar infarcts are associated with walking speed independent of cognitive performance in middle-aged to older adults. Gerontology. 2016;62:500–507. doi: 10.1159/000444583. [DOI] [PubMed] [Google Scholar]
- 17.Allali G, Montembeault M, Brambati SM, Bherer L, Blumen HM, Launay CP, Liu-Ambrose T, Helbostad JL, Verghese J, Beauchet O. Brain structure covariance associated with gait control in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:705–713. doi: 10.1093/gerona/gly123. [DOI] [PubMed] [Google Scholar]
- 18.Beauchet O, Launay CP, Annweiler C, Allali G. Hippocampal volume, early cognitive decline and gait variability: which association? Exp. Gerontol. 2015;61:98–104. doi: 10.1016/j.exger.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Beauchet O, Annweiler C, Celle S, Bartha R, Barthelemy JC, Roche F. Higher gait variability is associated with decreased parietal gray matter volume among healthy older adults. Brain Topogr. 2014;27:293–295. doi: 10.1007/s10548-013-0293-y. [DOI] [PubMed] [Google Scholar]
- 20.Beauchet O, Launay CP, Barden J, Liu-Ambrose T, Chester VL, Szturm T, Grenier S, Leonard G, Bherer L, Annweiler C, Helbostad JL, Verghese J, Allali G. Association between falls and brain subvolumes: results from a cross-sectional analysis in healthy older adults. Brain Topogr. 2017;30:272–280. doi: 10.1007/s10548-016-0533-z. [DOI] [PubMed] [Google Scholar]
- 21.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Del DS, Galna B, Godfrey A, Bekkers EMJ, Pelosin E, Nieuwhof F, Mirelman A, Hausdorff JM, Rochester L. Analysis of free-living gait in older adults with and without Parkinson's disease and with and without a history of falls: identifying generic and disease-specific characteristics. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:500–506. doi: 10.1093/gerona/glx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillel I, Gazit E, Nieuwboer A, Avanzino L, Rochester L, Cereatti A, Croce UD, Rikkert MO, Bloem BR, Pelosin E, Del DS, Ginis P, Giladi N, Mirelman A, Hausdorff JM. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur. Rev. Aging Phys. Act. 2019;16:6. doi: 10.1186/s11556-019-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li KZH, Bherer L, Mirelman A, Maidan I, Hausdorff JM. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front. Neurol. 2018;9:913. doi: 10.3389/fneur.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Deutsch JE, Hausdorff JM, Mirelman A. Altered brain activation in complex walking conditions in patients with Parkinson's disease. Parkinsonism Relat. Disord. 2016;25:91–96. doi: 10.1016/j.parkreldis.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwhof F, Bloem BR, Reelick MF, Aarts E, Maidan I, Mirelman A, Hausdorff JM, Toni I, Helmich RC. Impaired dual tasking in Parkinson's disease is associated with reduced focusing of cortico-striatal activity. Brain. 2017;140:1384–1398. doi: 10.1093/brain/awx042. [DOI] [PubMed] [Google Scholar]
- 28.Mirelman A, Weiss A, Buchman AS, Bennett DA, Giladi N, Hausdorff JM. Association between performance on timed up and go subtasks and mild cognitive impairment: further insights into the links between cognitive and motor function. J. Am. Geriatr. Soc. 2014;62:673–678. doi: 10.1111/jgs.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Habeck C, Razlighi Q, Salthouse T, Stern Y. Selective association between cortical thickness and reference abilities in normal aging. Neuroimage. 2016;142:293–300. doi: 10.1016/j.neuroimage.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern Y, Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, Shaked D, Salthouse T. The reference ability neural network study: motivation, design, and initial feasibility analyses. Neuroimage. 2014;103:139–151. doi: 10.1016/j.neuroimage.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattis, S. Dementia Rating Scale professional manual. Odessa, FL: Psychological Assessment Resources, Inc (1988).
- 32.Del DS, Hickey A, Hurwitz N, Mathers JC, Rochester L, Godfrey A. Measuring gait with an accelerometer-based wearable: influence of device location, testing protocol and age. Physiol. Meas. 2016;37:1785–1797. doi: 10.1088/0967-3334/37/10/1785. [DOI] [PubMed] [Google Scholar]
- 33.Trojaniello D, Cereatti A, Della CU. Accuracy, sensitivity and robustness of five different methods for the estimation of gait temporal parameters using a single inertial sensor mounted on the lower trunk. Gait Posture. 2014;40:487–492. doi: 10.1016/j.gaitpost.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Mirelman A, Heman T, Yasinovsky K, Thaler A, Gurevich T, Marder K, Bressman S, Bar-Shira A, Orr-Urtreger A, Giladi N, Hausdorff JM. Fall risk and gait in Parkinson's disease: the role of the LRRK2 G2019S mutation. Mov. Disord. 2013;28:1683–1690. doi: 10.1002/mds.25587. [DOI] [PubMed] [Google Scholar]
- 35.Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, Hausdorff JM. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil. Neural Repair. 2013;27:742–752. doi: 10.1177/1545968313491004. [DOI] [PubMed] [Google Scholar]
- 36.Weiss A, Herman T, Plotnik M, Brozgol M, Giladi N, Hausdorff JM. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol. Meas. 2011;32:2003–2018. doi: 10.1088/0967-3334/32/12/009. [DOI] [PubMed] [Google Scholar]
- 37.Habeck C, Gazes Y, Razlighi Q, Stern Y. Cortical thickness and its associations with age, total cognition and education across the adult lifespan. PLoS ONE. 2020;15:e0230298. doi: 10.1371/journal.pone.0230298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stern Y, Gazes Y, Razlighi Q, Steffener J, Habeck C. A task-invariant cognitive reserve network. Neuroimage. 2018;178:36–45. doi: 10.1016/j.neuroimage.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creze M, Versheure L, Besson P, Sauvage C, Leclerc X, Jissendi-Tchofo P. Age- and gender-related regional variations of human brain cortical thickness, complexity, and gradient in the third decade. Hum. Brain Mapp. 2014;35:2817–2835. doi: 10.1002/hbm.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig AD. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 42.Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, Krieger-Redwood K, Bernhardt BC, Smallwood J, Jefferies E. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage. 2016;137:165–177. doi: 10.1016/j.neuroimage.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray JR, Braver TS. Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cogn. Affect. Behav. Neurosci. 2002;2:64–75. doi: 10.3758/CABN.2.1.64. [DOI] [PubMed] [Google Scholar]
- 44.Orr JM, Smolker HR, Banich MT. Organization of the human frontal pole revealed by large-scale DTI-based connectivity: implications for control of behavior. PLoS ONE. 2015;10:e0124797. doi: 10.1371/journal.pone.0124797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain. 2011;134:3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt-Hieber C, Hausser M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 2013;16:325–331. doi: 10.1038/nn.3340. [DOI] [PubMed] [Google Scholar]
- 49.Vocks S, Busch M, Gronemeyer D, Schulte D, Herpertz S, Suchan B. Neural correlates of viewing photographs of one's own body and another woman's body in anorexia and bulimia nervosa: an fMRI study. J. Psychiatry Neurosci. 2010;35:163–176. doi: 10.1503/jpn.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora A, Weiss B, Schurz M, Aichhorn M, Wieshofer RC, Perner J. Left inferior-parietal lobe activity in perspective tasks: identity statements. Front. Hum. Neurosci. 2015;9:360. doi: 10.3389/fnhum.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagy K, Greenlee MW, Kovacs G. The lateral occipital cortex in the face perception network: an effective connectivity study. Front. Psychol. 2012;3:141. doi: 10.3389/fpsyg.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb. Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maidan I, Nieuwhof F, Bernad-Elazari H, Reelick MF, Bloem BR, Giladi N, Deutsch JE, Hausdorff JM, Claassen JA, Mirelman A. The role of the frontal lobe in complex walking among patients with Parkinson's disease and healthy older adults: an fNIRS study. Neurorehabil. Neural Repair. 2016;30:963–971. doi: 10.1177/1545968316650426. [DOI] [PubMed] [Google Scholar]
- 55.Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017;115:41–46. doi: 10.1016/j.bandc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Takakusaki K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017;10:1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montero-Odasso M, Perry G. Gait disorders in Alzheimer's disease and other dementias: there is something in the way you walk. J. Alzheimers Dis. 2019;71:S1–S4. doi: 10.3233/JAD-190790. [DOI] [PubMed] [Google Scholar]
- 58.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov. Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E. Freezing of gait in Parkinson's disease: disturbances in automaticity and control. Front. Hum. Neurosci. 2012;6:356. doi: 10.3389/fnhum.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark DJ, Rose DK, Ring SA, Porges EC. Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Front. Aging Neurosci. 2014;6:217. doi: 10.3389/fnagi.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat. Rev. Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson DS, Pickett KA, Duncan RP, Perlmutter JS, Earhart GM. Brain activity during complex imagined gait tasks in Parkinson disease. Clin. Neurophysiol. 2014;125:995–1005. doi: 10.1016/j.clinph.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Connell MA, Basak C. Effects of task complexity and age-differences on task-related functional connectivity of attentional networks. Neuropsychologia. 2018;114:50–64. doi: 10.1016/j.neuropsychologia.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Stern Y, Barnes CA, Grady C, Jones RN, Raz N. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol. Aging. 2019;83:124–129. doi: 10.1016/j.neurobiolaging.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16:1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.