Abstract
Context:
Disordered functional architecture of brain networks may contribute to the well-documented increased risk for psychiatric disorders in offspring of patients with schizophrenia.
Objective:
To investigate aberrant interactions between regions associated with affective processing in children and adolescent offspring of patients with schizophrenia (HR-SCZ group) and healthy control subjects using dynamic causal modeling of functional magnetic resonance imaging data.
Design:
Subjects participated in a continuous affective processing task during which positive, negative, and neutral valenced faces were presented. Interactions between regions in the brain’s face- and emotion-processing network were modeled using dynamic causal modeling. Multiple competing models were evaluated by a combinatorial approach and distinguished at the second level using Bayesian model selection before parameter inference.
Setting:
Participants were recruited from the community.
Participants:
Twenty-four controls with no family history of psychosis (to the second degree) and 19 children and adolescent offspring of a parent with schizophrenia (age range, 8 to 20 years).
Results:
Bayesian model selection revealed a winning model, the architecture of which revealed bidirectional frontolimbic connections that were modulated by valence. Analyses of parameter estimates revealed that HR-SCZ group members were characterized by (1) decreased driving inputs to the visual cortex; (2) decreased intrinsic coupling, most robustly between frontolimbic regions; and (3) increased modulatory inhibition by negative valence of frontolimbic connections (all P<.01, Bonferroni corrected).
Conclusions:
These results are the first demonstration of network analyses techniques for functional magnetic resonance imaging data in children and adolescents at risk for schizophrenia. Dysfunctional interactions within the emotional processing network provide evidence of latent vulnerabilities that may confer risk for disordered adolescent development and eventually the emergence of the manifest disorder.
NEURODEVELOPMENTAL characterizations of schizophrenia1–3 suggest that the emergence of this disorder is preceded by a premorbid or vulnerable phase in adolescence. Critical alterations to the normal cognitive and affective developmental program that may be driven by a combination of environmental and genetic factors may characterize this vulnerable phase.4 First-degree relatives, particularly unaffected children and adolescent offspring of a parent with schizophrenia at significantly increased risk for schizophrenia (HR-SCZ subjects) constitute an important group in whom to assess heritable factors of premorbid dysfunction before the typical age of illness onset.5
Deficits in affective functioning and social interaction have long been considered cardinal features of schizophrenia.6 Longitudinal cohort studies have indicated that social and affective deficits emerge in the premorbid stages of the disorder in adolescence.7,8 Impairments in social and affective function have also been documented in HR-SCZ subjects, particularly in the domains of social adjustment, withdrawal, and interpersonal competence.9,10 These deficits may result from disordered interactions between corticolimbic regions subserving emotional processing, although the mechanisms of these alterations are poorly understood. Studying network interactions during emotional processing in adolescent HR-SCZ subjects by effective connectivity modeling may provide important insights on latent network vulnerabilities in the (genetically predisposed) adolescent brain. These vulnerabilities in turn may constitute an emergent risk for schizophrenia or other disorders. In this study, we used functional magnetic resonance imaging (fMRI) and dynamic causal modeling (DCM) to characterize and compare corticolimbic interactions in HR-SCZ and control subjects with no family history of psychosis.
Normal social and emotional function relies on the ability to successfully appraise and respond to social cues, such as the affect signaled by a face,11,12 and is mediated by a network of visual, limbic, and prefrontal regions.13–15 This basic emotion-processing network (eFigure 1; http://www.archgenpsychiatry.com) is initiated by stimulus processing and feature extraction in visual areas.11,16 These regions feed into core limbic circuitry, including the amygdala,17–19 projections of which to ventral, medial, and dorsal prefrontal regions may be essential to transmitting affective information to the higher-order heteromodal cortex. In turn, frontal control of the amygdala20 may be essential in modulating the deliberative (as opposed to the automatic) responses driven by corticoamygdala interactions.21 All these intrinsically present connections may be subject to modulatory or contextual effects. For example, top-down modulation of the amygdala by the frontal cortex may be mediated by the task or judgment on the face that is required, or stimulus properties may provide important bottom-up modulation of individual regions.
Regions within this corticolimbic circuit and their interplay mature rapidly through adolescence,22 and derailment of these interactions may be an underlying cause of the emergence of psychiatric disorders in late adolescence.6,7,11 Given that alterations of neural circuits for social-emotional processing in schizophrenia may be genetically mediated,23 capturing emergent properties of network interactions may help characterize differences in affective processing in HR-SCZ subjects and in the eventual identification of markers of precursors of the illness.
Dynamic causal modeling is an important method for inferring effective connectivity from neuroimaging data.24–27 Dynamic causal modeling uses an explicit model of neural dynamics to capture causal interactions between regions within the network (intrinsic connections), modulation of intrinsic connections by the experimental context (eg, valence of a face), and driving inputs to regions (eg, visual stimulation driving face-processing regions). Using Bayesian methods, DCM selects from among competing models or hypotheses that best capture network interactions during the examined task.28 Parameter estimates of interregional interactions derived from winning models reflect measures of effective connectivity that can be compared to assess differences between groups.
Herein, we present the first application of DCM to fMRI data collected in children and adolescent offspring of a parent with schizophrenia who underwent scanning during a continuous affective paradigm. The analyses aimed to understand alterations in the connective architecture of the corticolimbic circuit underlying emotional processing in adolescent HR-SCZ subjects and to investigate whether evidence of dysconnection that is hypothesized to characterize the schizophrenia diathesis29 may also be evident in young individuals at increased risk for the illness. We hypothesized that this would be revealed particularly by reduced intrinsic coupling between corticolimbic regions evident in the DCM.
METHODS
SUBJECTS
Forty-three subjects gave informed consent or assent to participate in the fMRI studies (approved by the Human Investigative Committee at Wayne State University). For assenting subjects, the parent provided consent. Of these, 19 were HR-SCZ subjects and 24 were healthy controls with no family history of psychosis (to the second degree) group matched for age (range, 8–20 years) and full-scale IQ.30 Exclusion criteria included (1) DSM-IV diagnosis of mental retardation, (2) DSM-IV diagnosis of substance dependence or significant use in the past month, (3) significant history or presence of a current medical or neurological illness, (4) significant head injury, (5) current or recent use of any psychotropic medication, and (6) pregnancy. Of the 43 subjects, 2 were left-handed (1 each in the HR-SCZ and control groups).
Subjects were recruited from the greater Detroit area through advertisements and in-patient services at Wayne State University. All subjects underwent screening through telephone and personal interviews as well as questionnaires. Diagnoses for parents were reached using the Structured Clinical Interview for DSM-IV Axis II Personality Disorders for schizophrenia administered in person.31 All control and HR-SCZ subjects underwent clinical evaluation in person using the Schedule for Affective Disorders and Schizophrenia for School-age Children–Present and Lifetime Version32 and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders and were free of psychotropic medication at the time of assessments. Assessments were administered by a trained interviewer. Demographic information is depicted in Table 1. All HR-SCZ subjects were healthy apart from the following comorbidities: separation anxiety (n=1), attention-deficit/hyperactivity disorder (n=2), and social phobia (n=1). Groups did not differ by age (t=0.97; P=.51), full-scale IQ (t=0.26; P=.80), education (t = 1.11; P = .13), or sex distribution (Yates χ2 = 0.007; P = .91). The HR-SCZ group had significantly lower scores on the Global Assessment of Function subscale of the Structured Interview for Prodromal Symptoms33 (t=3.36; P=.006), suggestive of a subtle increase in psychopathology.
Table 1.
Characteristics of Study Groupsa
| HR-SCZ Group (n = 19) | Control Group (n = 24) | |
|---|---|---|
| Sex, No. | ||
| Male | 12 | 16 |
| Female | 7 | 8 |
| Age, range, y | 8–20 | 10–20 |
| Age, y | 14.3 (3.1) | 14.6 (2.6) |
| Full-scale IQ | 96.2 (13.7) | 92.0 (15.9) |
| Educational level, y | 8.4 (2.6) | 9.5 (2.8) |
| GAF score | 76.2 (10.7) | 85.8 (6.5) |
Abbreviations: GAF, Global Assessment of Function subscale of the Structured Interview for Prodromal Symptoms; HR-SCZ, children and adolescent offspring of patients with schizophrenia.
Groups did not differ in terms of age, full-scale IQ, education, or distribution of sex. As a group, HR-SCZ subjects showed lower GAF33 scores than controls, suggesting on average transient or slight impairments in social or occupational function. Unless otherwise indicated, data are expressed as mean (SD).
EXPERIMENTAL PARADIGM AND ANALYSIS
Functional MRI was performed with an emotional n-back paradigm using a jittered event-related design.34 During the paradigm, normatively rated faces conveying expressions from 3 valence categories—positive (happy), negative (sadness, fear, or anger), and neutral35—were interspersed with pixilated control images and presented in a pseudorandom order. Subjects indicated (by pressing a button) when the valence category signaled on the current trial was the same as on the previous trial (regardless of identity). Stimuli were displayed for 3 seconds, with the stimulus interval between events randomly jittered between 3 to 5 seconds (0.5-second increments; an example is given in eFigure 2). A total of 96 stimuli (80 faces across the 3 valence categories and 16 control stimuli) were presented during the course of the experiment. Across the task and for each affective category, 25% of the trials (ie, 24 of the total 96 trials) were target trials (requiring a response of “same”). Task demand was maintained at a constant throughout the course of the experiment by virtue of the continuous stimulus presentation.
Functional MRIs were acquired on a 4.0-T full-body scanner with an 8-channel head coil (MedSpec; Bruker Biospin). Gradient echoplanar imaging was continuously acquired during an 11.5-minute scan time (repetition time, 2000 milliseconds; echo time, 30 milliseconds; matrix, 64×64; 24 sections; field of view, 240 mm; voxel size, 3.8×3.8×4.0 mm; 345 scans). Stimuli were projected from a computer onto a screen mounted over the subject’s head and viewed through a mirror. Responses were provided using an MRI-compatible 2-button box. Foam padding was packed around subjects’ heads to minimize movement, and earplugs were used to reduce noise. Stimuli were presented and responses collected using the Presentation computer program.36
The MRIs were preprocessed and analyzed using commercially available software (SPM; Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience). Images were realigned to correct for head movement, spatially normalized to the Montreal Neurological Institute template brain, and smoothed spatially by means of a gaussian filter of 8-mm full-width half-maximum. First-level general linear model analysis used 4 regressors to represent sensory and affective processes and modeled as 3-second boxcar vectors (representing individual events) convolved with a canonical hemodynamic reference wave form. The regressors included (1) distorted images, (2) negative faces, (3) neutral faces, and (4) positive faces. The 6 head-motion parameters were included as covariates of no interest. Data were detrended with a high-pass filter (cut-off, 1/128 second). An autoregressive AR(1) model was used to account for serial correlation.
DCM ANALYSES
Dynamic causal modeling enables the investigation of effective connectivity within a neural system and the context-dependent changes thereof induced by stimuli or task.28 The brain is modeled as a deterministic bilinear system whose inputs (perturbations) are experimental manipulations and whose outputs are hemodynamic signals measured by fMRI. Changes in neuronal states (x) over time (t) are modeled according to the following equation, with A representing the intrinsic coupling; B(j), context-dependent changes by input uj; and C, direct (driving) inputs:
The intrinsic connectivity matrix (A) thus represents the task-independent component of interregional interactions, whereas task-dependent modulations in B represent the changes in coupling strength brought on by a particular stimulus or task. Activity is induced in the system by the direct effects (C). Model parameters are estimated from comparison between predicted (using a biophysically validated forward model)37 and observed blood oxygenation level–dependent signals using Bayesian inversion, yielding rate constants for the modeled system (units of 1/s or Hertz).
Assessment of effective connectivity using DCM requires evaluation and comparison of models representing competing hypotheses on the connective architecture of the investigated neural system.24,38–40 To explore a comprehensive combination of intrinsic and modulatory interactions between corticolimbic regions, we constructed a factorial model space (Figure 1) based on previous studies on face and emotion processing (described in eFigure 1).
Figure 1.
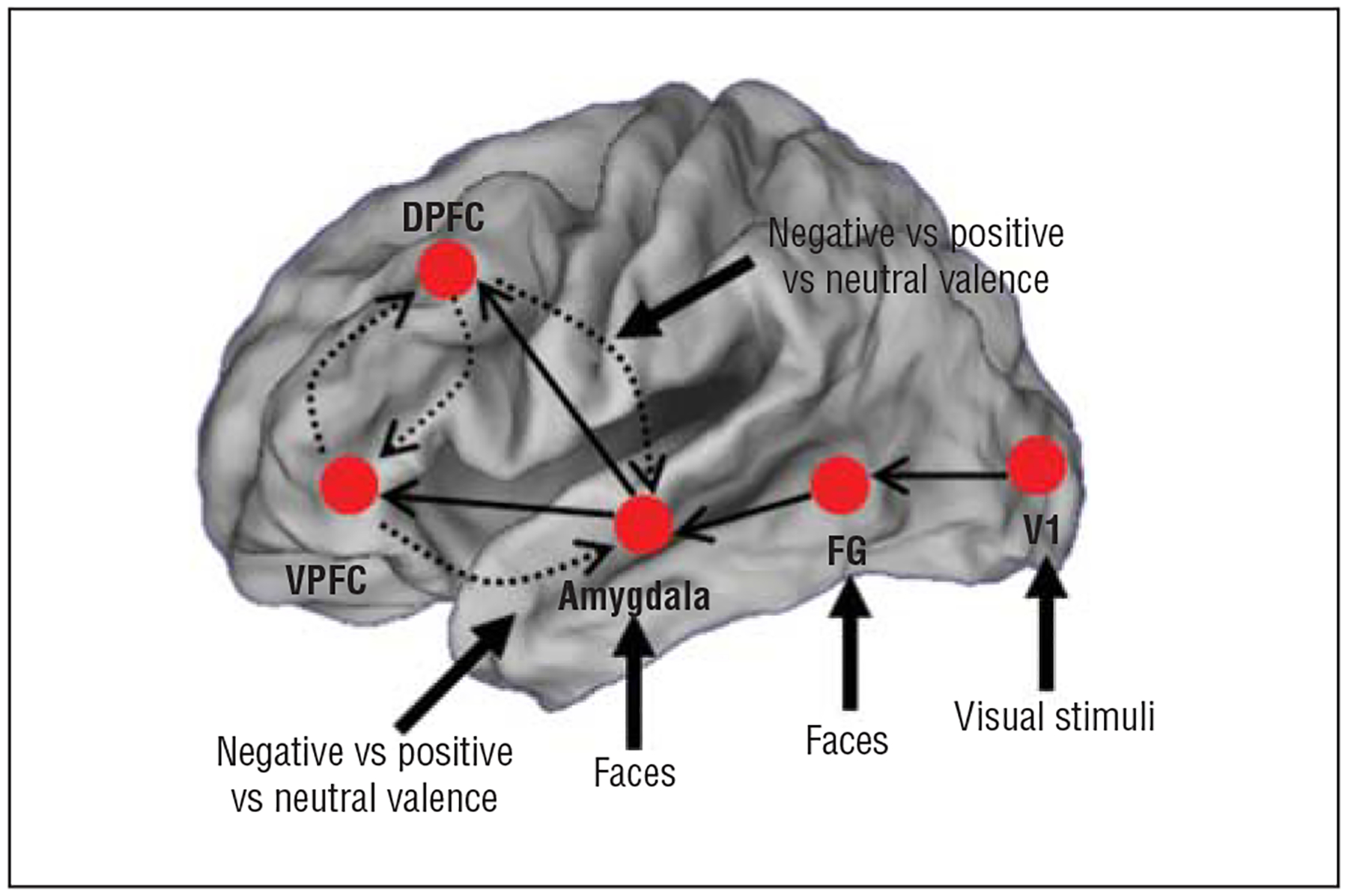
Summary of the general model architecture from which the dynamic causal models were constructed, based on a schematic network summarized in eFigure 1. Driving inputs to specific regions of interest were fixed across all models evaluated. All intrinsic connections are directional. Solid arrows depict fixed connections across all evaluated models. Dashed arrows depict conditions that were varied across models to assess the necessity of frontolimbic connections. Contextual modulation of frontolimbic connections by facial valence was also varied across models (all combinations of positive, neutral, and negative valences) in models in which intrinsic connections exist (see the “Methods” section for details). DPFC indicates dorsal prefrontal cortex; FG, fusiform gyrus; V1, primary visual cortex; and VPFC, ventral prefrontal cortex.
MODEL SPECIFICATION
Five regions of the corticolimbic system central to affective face processing and generation of automatic and deliberative emotional responses28–32 were included in the model: primary visual cortex (V1), fusiform gyrus (FG), amygdala, ventral prefrontal cortex (VPFC), and dorsal prefrontal cortex (DPFC). Because visual stimuli drive activity in the V1 and affective information may bypass early visual processing via direct thalamic input into the FG and amygdala,21,23 these regions were defined as input regions throughout alternative models. The forward intrinsic connections from the V1 to the FG and subsequently the amygdala were based on primate and in vivo imaging studies.41 In addition, intrinsic connections from the amygdala to the VPFC and DPFC were included, reflecting the bottom-up flow of information within frontolimbic neurocircuitry. These connections were a common intrinsic framework and present across all evaluated models.
The necessity of frontolimbic connections and modulatory inputs were evaluated by varying the following additional factors and modulators independently, building a factorial model space:
The inclusion of intrinsic connections from the VPFC and DPFC to the amygdala was varied to investigate the necessity of frontal modulation of the limbic system;
The necessity of modulatory effects on intrinsic frontolimbic connections was investigated using models without modulation and using those for which the intrinsic connections were modulated by all possible combinations of each valence class (positive, neutral, and negative faces);
Finally, interactions between the VPFC and DPFC were investigated by varying intrinsic connections between these regions. This combinatorial approach yielded 136 evaluated DCMs.
MODEL ESTIMATION
Modeling was conducted using DCM, version 8, in SPM, version 8. For modeling, time series from regions of interest were extracted within spheres of 5-mm radii centered on the peak for the effects-of-interest F contrast (P<.05, adjusted for effects of no interest). Regions were defined in stereotactic space using anatomic criteria.41 Each of the 136 DCMs was estimated for each subject. To identify the most likely generative model across subjects, a random-effects Bayesian model selection procedure was used.42 This variational Bayesian method by Stephan and colleagues42 treats the model as a random variable and estimates the parameters of a Dirichlet distribution describing the probabilities for all models being considered. Because these probabilities define a multimodal distribution over model space, it is possible to compute the exceedance probability of one model being more likely than any other. All models were compared against each other by this random-effects analysis as implemented in SPM8 and additionally using the traditional fixed-effects model comparison. Bayesian averages of parameter estimates (intrinsic, modulatory, and driving inputs) were subsequently analyzed to uncover potential differences in connectivity between the control and HR-SCZ groups.28
RESULTS
BEHAVIORAL RESULTS
Discrimination sensitivity during the task was assessed using d′, an established metric of discriminability or sensitivity in signal detection theory43,44 reflecting the ability of the subject to discriminate targets from distracters. The metric incorporates the hit rate (eg, the rate of responding “same” to successively presented stimuli in the same valence category) and the false-alarm rate (eg, the rate of responding “different” to successively presented stimuli in different valence categories) and is based on the difference between the inverse function of the cumulative gaussian distribution applied to each. A higher d′ reflects greater sensitivity to the task.
An analysis of covariance with group as the single factor and age and sex as covariates revealed no significant differences in d′ between groups (F1,40=0.27; P=.60), indicating that overall discrimination performance between the control group (mean d′=2.45) and the HR-SCZ group (mean d′=2.31) was comparable. Response bias measures based on the bias criterion metric c45 did not differ either (F1,40=0.84; P=.37). The analyses of behavioral data demonstrated that the cognitive component of the task was comparable across groups.
fMRI ACTIVITY DATA
Random-effects inference was performed by a full factorial analysis that used group and valence as factors and age and sex as covariates. Analyses (cluster level P<.05, familywise error) did not reveal significant differences in activation. A lack of differences in activation was also supported in a conjunction analysis46 that revealed significant overlap in activation for each contrast of interest (all, negative, positive, and neutral faces demonstrated greater activation than distorted images). Significant clusters from the conjunction analyses are depicted in Figure 2 and Table 2. These results suggest overlapping substrates of activation involved in emotional processing for faces and across valence category, highlighting the value of assessing network interactions with DCM.
Figure 2.
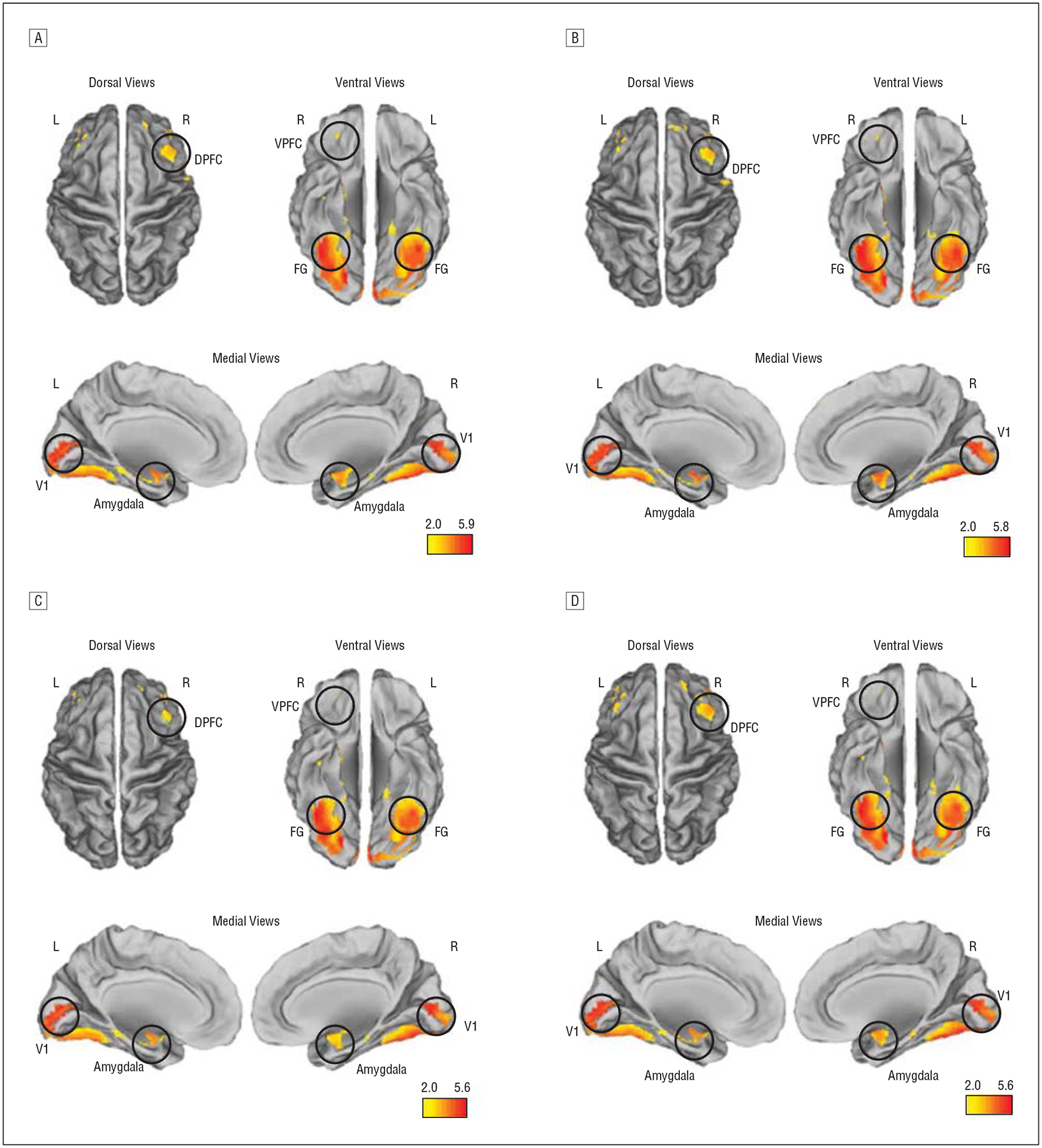
Results of a conjunction analysis in control subjects and children and adolescent offspring of patients with schizophrenia (HR-SCZ subjects) depict widespread overlap in activation in both groups in our network of interest. A, All faces. B, Negative valence. C, Neutral valence. D, Positive valence. Significant clusters (P<.05, corrected at cluster level) are depicted on dorsal, ventral, and medial surface projections. DPFC indicates dorsal prefrontal cortex; FG, fusiform gyrus; L, left; R, right; V1, primary visual cortex; and VPFC, ventral prefrontal cortex. The color bars represent the conjunction statistic and are unitless. Table 2 provides statistical information.
Table 2.
Results of Conjunction Analyses With the Minimum Inference Statistic of First-Level Contrasts Across Both Study Groupsa
| Conjunction | Region | Cluster, kE | Statistic | x, y, z MNI Coordinates |
|---|---|---|---|---|
| All faces | V1 | 1105 | 5.63 | −2, −78, 8 |
| FG | 1120 | 5.83 | −38, −58, −22 | |
| Amygdala | 168 | 3.73 | 28, 0, −24 | |
| VPFC | 40 | 1.73 | 44, 44, 22 | |
| DPFC | 541 | 3.39 | 24, 46, −14 | |
| Negative | V1 | 1115 | 5.55 | 14, −80, 2 |
| FG | 1156 | 5.89 | −40, −56, −22 | |
| Amygdala | 168 | 4.16 | 30, −4, −12 | |
| VPFC | 51 | 1.97 | 24, 46, −14 | |
| DPFC | 570 | 3.48 | 42, 48, 20 | |
| Neutral | V1 | 1083 | 5.65 | −2, −78, 8 |
| FG | 1055 | 5.28 | −38, −60, −22 | |
| Amygdala | 166 | 2.72 | 30, 2, −26 | |
| VPFC | 41 | 1.32 | 44, 44, 22 | |
| DPFC | 410 | 2.86 | 24, 46, −14 | |
| Positive | V1 | 1097 | 5.12 | −2, −82, 2 |
| FG | 1086 | 5.20 | −38, −60, −22 | |
| Amygdala | 164 | 3.60 | 30, −4, −12 | |
| VPFC | 68 | 1.71 | 42, 44, 26 | |
| DPFC | 585 | 3.67 | 22, 44, −12 |
Abbreviations: DPFC, dorsal prefrontal cortex; FG, fusiform gyrus; MNI, Montreal Neurological Institute; VPFC, ventral prefrontal cortex; V1, primary visual cortex.
Results reveal significant overlap in activation across regions of interest used in the study (P<.05, familywise error). Statistics are explained in Nichols et al.46
DCM: BAYESIAN MODEL SELECTION
The random-effects and fixed-effects analyses applied across subjects yielded identical evidence of a single winning model. Figure 3 depicts exceedance probabilities from the results of the random-effects analysis applied to all 136 models and 43 subjects, expressed as a fraction of relative a priori chance, with the single winning model representing the best fit within the evaluated model space.40 An additional Bayesian model selection procedure performed after excluding HR-SCZ subjects with comorbidities confirmed these results.
Figure 3.
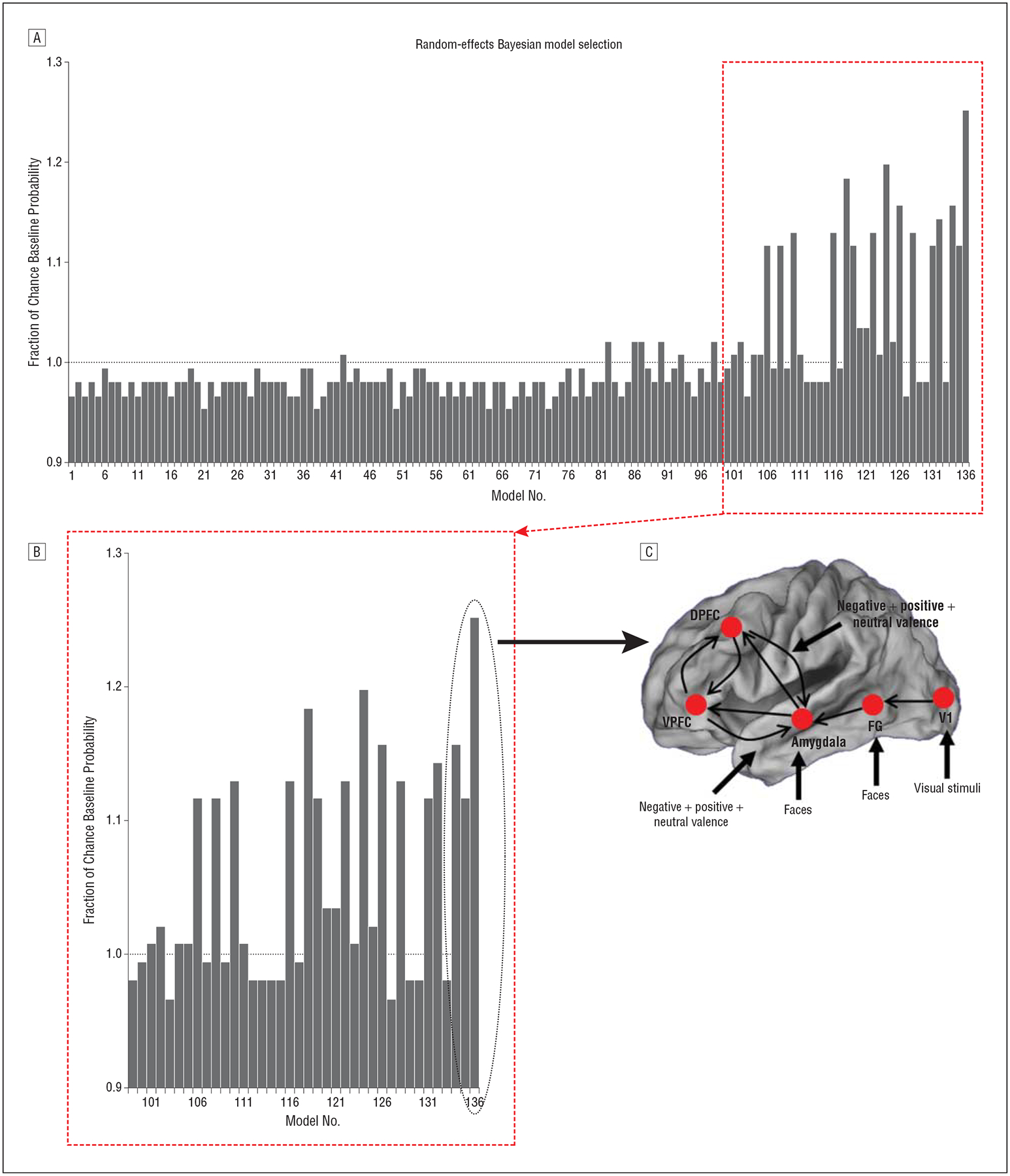
The winning model, the exceedance probabilities across models as a fraction of change, and a magnification of the winning model exceedance probability. A, The observed exceedance probabilities (EP; ie, the relative likelihood that a given model is the generative model for the observed functional magnetic resonance imaging data) across all 136 models are depicted relative to the flat a priori probability. Given 136 models in the space, the expected random a priori chance EP is .007 (given flat prior probabilities on the model space). When observed EPs are expressed relative to this chance EP value, the a priori probability represents unity (dotted line). Models with fractional values higher than 1 are more likely relative to their competition in the space (ie, the posterior is higher than the flat prior probability). B, The inset in part A is magnified to emphasize the winning model. The winning model is approximately 25% higher than chance and higher than its closest competitors in the space. C, The winning model structure is depicted with the observed driving inputs, intrinsic connections, and contextual modulatory effects. DPFC indicates dorsal prefrontal cortex; FG, fusiform gyrus; V1, primary visual cortex; and VPFC, ventral prefrontal cortex.
Intrinsic connections that had been varied as factors in model assessment (described in the “Methods” section) emerged as part of a common intrinsic network architecture in the winning model. The winning model intrinsic architecture evinced connections from the VPFC and DPFC to the amygdala and between the VPFC and DPFC. Frontolimbic connections were also modulated by valence. In the following paragraphs, we comprehensively consider the model parameters associated with driving inputs, intrinsic connections, and contextual modulation in the winning model.
DRIVING INPUTS INTO THE SYSTEM
Figure 4 depicts driving inputs observed in each of the groups for the winning model. Significantly reduced driving inputs to the V1 were observed in the HR-SCZ group relative to the control group (t statistics are provided in eTable 1).
Figure 4.
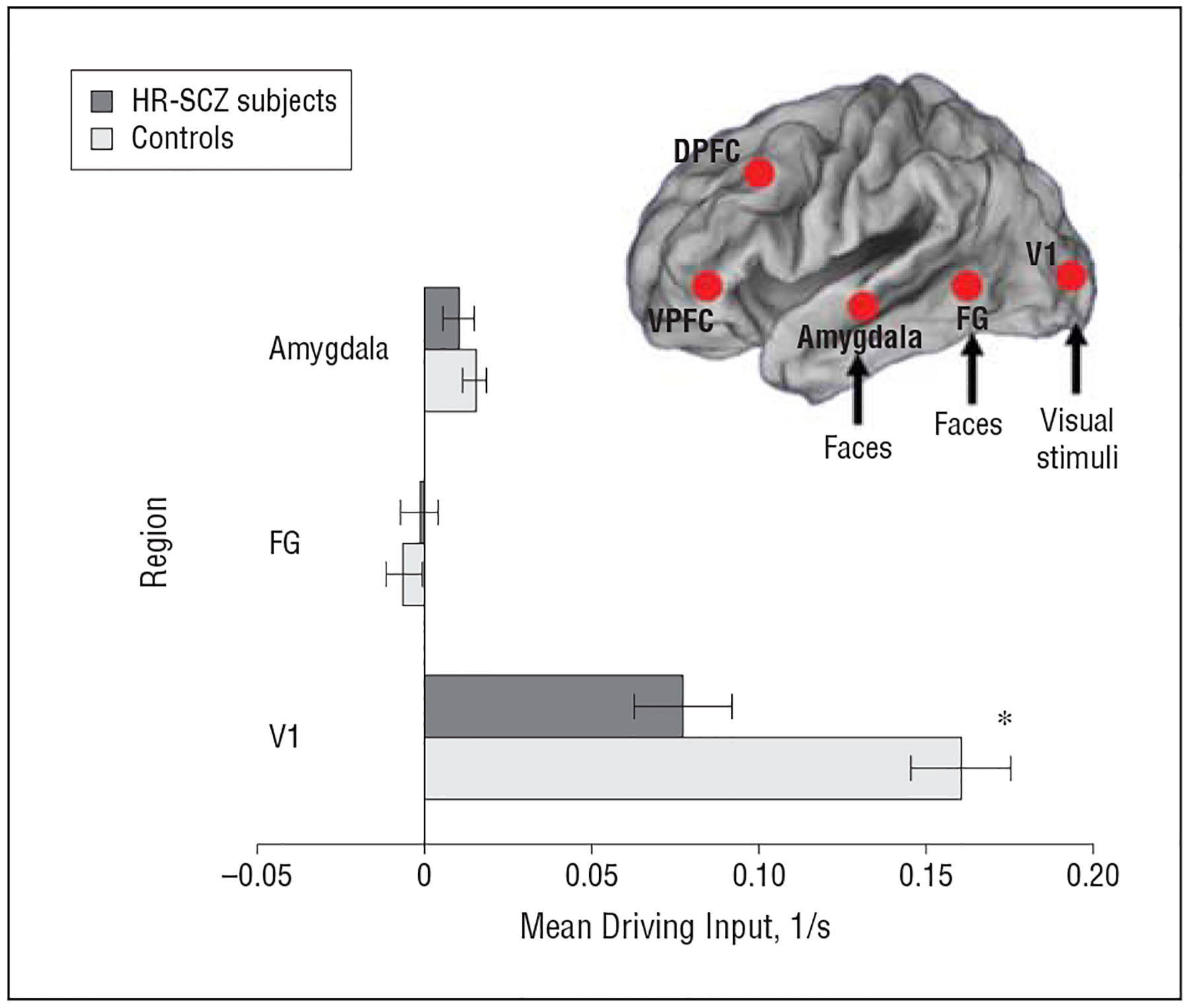
Mean driving inputs for control subjects and children and adolescent offspring of patients with schizophrenia (HR-SCZ subjects) for the winning model depicted in Figure 3C. Differences in coupling parameter estimates between the control group and HR-SCZ group are depicted. *P=.01, Bonferroni-corrected familywise error rate. The t statistics are provided in eTable 1. Error bars represent standard deviations derived from Bayesian parameter averages. DPFC indicates dorsal prefrontal cortex; FG, fusiform gyrus; V1, primary visual cortex; and VPFC, ventral prefrontal cortex.
INTRINSIC CONNECTIONS BETWEEN AREAS
In general, HR-SCZ subjects showed significantly reduced intrinsic connectivity across nodes of the assessed network, with the magnitude of these reductions significantly pronounced in the unidirectional and bidirectional coupling between frontolimbic (DPFC, VPFC, and amygdala) regions (Figure 5 and eTable 2).
Figure 5.
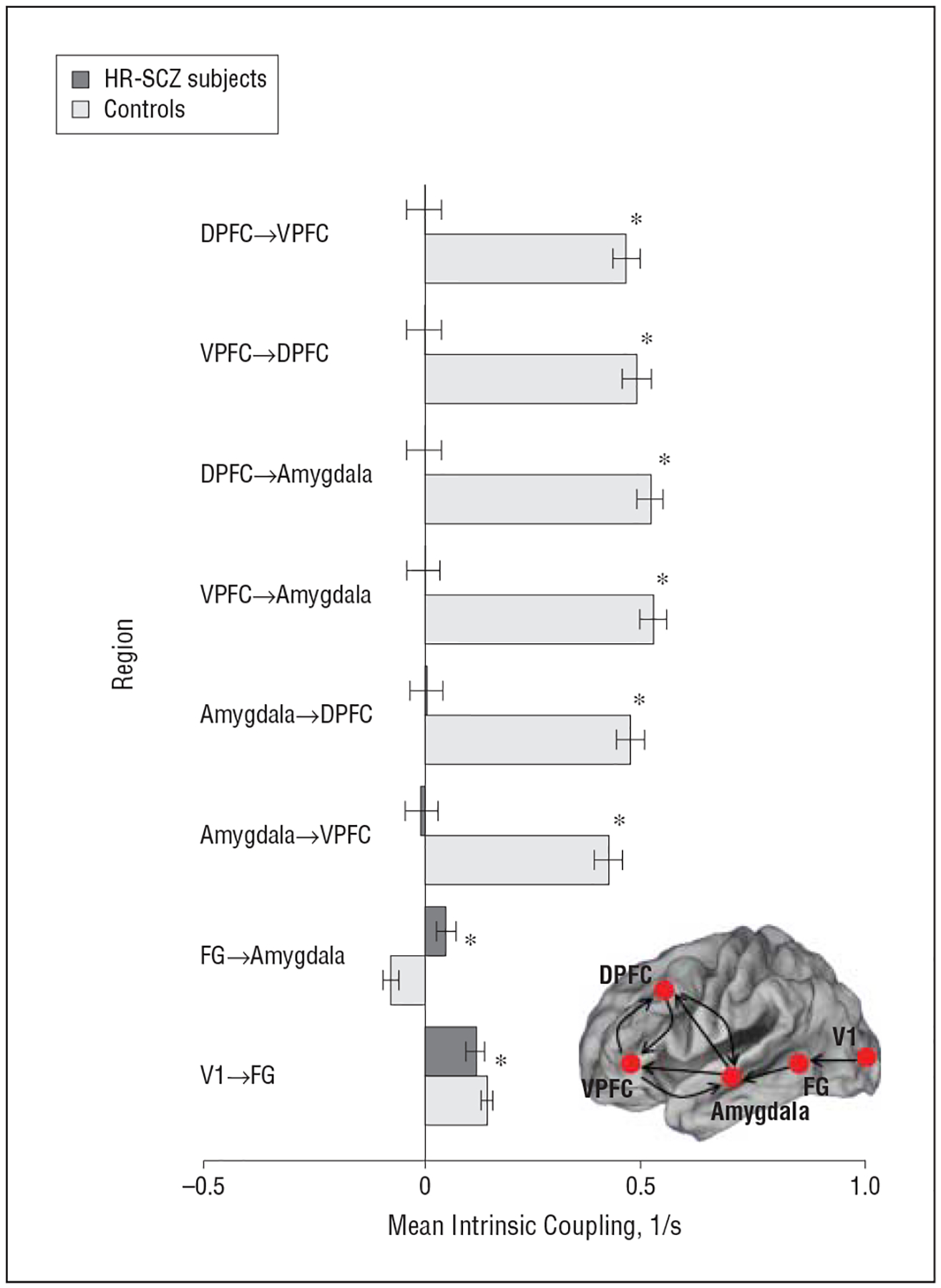
Mean intrinsic coupling for control subjects and children and adolescent offspring of patients with schizophrenia (HR-SCZ subjects) for the winning model depicted in Figure 3C. Significantly reduced intrinsic connectivity is observed in the HR-SCZ subjects with reduced excitatory connectivity particularly notable in frontolimbic pathways, that is, in the affective core of the circuit. *P=.01, Bonferroni corrected (eTable 2). Error bars represent standard deviations derived from Bayesian parameter averages. DPFC indicates dorsal prefrontal cortex; FG, fusiform gyrus; V1, primary visual cortex; and VPFC, ventral prefrontal cortex.
CONTEXTUAL MODULATION OF INTRINSIC CONNECTIVITY
Figure 6 depicts observed contextual (ie, valence-related) modulation of frontolimbic conditions for each valence category. Positive values indicate that the valence context enhances coupling from the source to the target regions, whereas negative values indicate that the context inhibits it. The following differences were observed: (1) differences between groups were specific to the non–positive valence stimuli; (2) negative valence served to inhibit coupling from frontal regions to the amygdala with significantly greater inhibition observed in HR-SCZ subjects; and (3) an isolated result was the significantly increased excitation of the DPFC-to-AMYG pathway by neutral valence in HR-SCZ compared with control subjects. Across subjects, individual coupling parameters for the winning model for each subject were not correlated with the effects of sex or age (for all, P=.15) (t statistics are provided in eTable 3).
Figure 6.
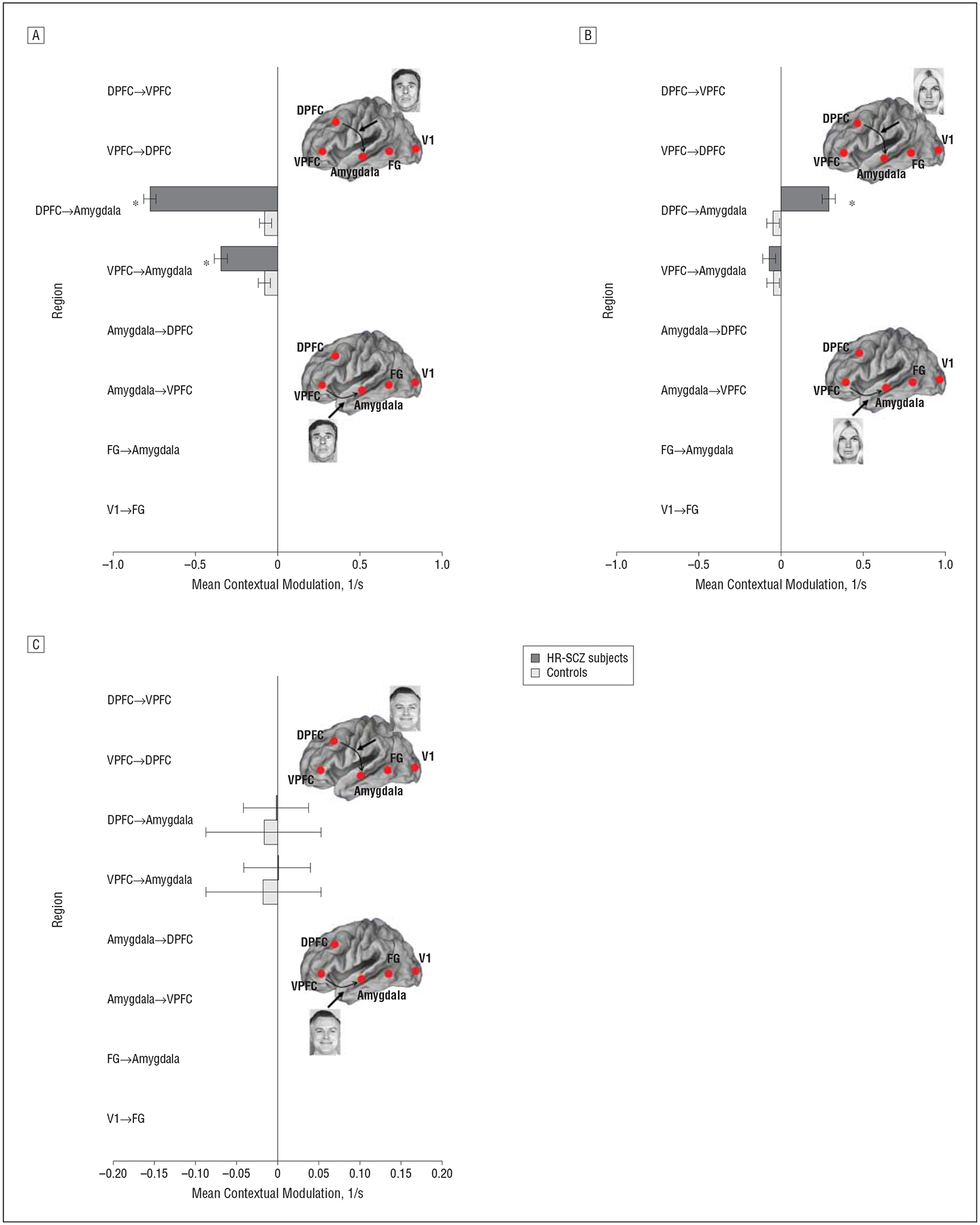
Contextual modulation by valence of frontolimbic pathways for control subjects and children and adolescent offspring of patients with schizophrenia (HR-SCZ subjects) for the winning model depicted in Figure 3C. A, Negative valence. B, Neutral valence. C, Positive valence. Denoted coupling parameters of the controls and HR-SCZ subjects are significantly different (*P=.01, Bonferroni corrected) (t statistics are provided in eTable 3). In the HR-SCZ subjects, we observed significantly increased inhibition by negative valence of DPFC-to-amygdala and VPFC-to-amygdala connections. No intergroup differences are observed for positive valence. Significantly increased excitation of the DPFC-to-amygdala pathway is observed for neutral valence. DPFC indicates dorsal prefrontal cortex; FG, fusiform gyrus; V1, primary visual cortex; and VPFC, ventral prefrontal cortex.
COMMENT
We examined corticolimbic connectivity during affective processing in the adolescent offspring of schizophrenia patients and in controls with no family history of psychosis (to the second degree) using fMRI and DCM. Our principal results included the following:
The control and HR-SCZ groups performed similarly during the task, indicating that HR-SCZ subjects were not impaired in discriminating between affective faces;
No differences in regional activation between the HR-SCZ and control groups were observed. This absence of significant regional effects highlights the unique value of investigating effective connectivity in distinguishing healthy but at-risk adolescent groups from controls;
DCM analyses revealed a winning model that validated hypotheses on the presence of frontolimbic intrinsic connections and their modulation by valence;
Systematic differences in driving inputs and coupling parameters were observed between the groups. The HR-SCZ group showed reduced driving inputs to the V1. Furthermore, the HR-SCZ group showed decreased intrinsic excitatory coupling between the frontolimbic regions that form the core of the affective-processing circuit. Finally, the HR-SCZ group showed increased modulatory ventral and dorsal frontal-to-limbic inhibition in response to neutral and negative faces, respectively, with the contextual modulation to negative faces being hyperinhibitory.
A notable cognitive component of the task is the presence of stable working memory demands (related to the temporary maintenance of the affective category of the stimulus) throughout the course of the experiment. These working memory demands provide a parallel but consistent task demand driving the primary cognitive component of the paradigm. The choice of the paradigm was driven by 2 competing considerations. First, working memory itself is an established intermediate phenotype in the schizophrenia diathesis.47 In young high-risk individuals, behavioral impairments during oculomotor delayed match-to-sample tasks have been reported in large samples,48,49 and disordered brain interactions during verbal n-back tasks have been demonstrated using fMRI.50 Second, emotion-processing deficits have been hypothesized to characterize the risk state for schizophrenia.51 Therefore, a task that combines these attributes offers unique value to the study of risk samples that is different from previously used affective n-back paradigms. For example, emotional facial stimuli have been paired with verbal stimuli during verbal n-back paradigms (to examine the interference of affective distracters on the primary task),52 or some tasks require the identity of faces (rather than signaled affect) to be remembered during a facial n-back.53 These paradigms assess implicit affective processing mechanisms, whereas the current paradigm makes the explicit assessment of the valence itself the primary target of the task. Because there were no detectable differences in sensitivity on the task between groups, we believe that differences in fMRI and DCM results can be interpreted as being associated with the affect-related components of the neural response.
These results suggest a fundamental disordering in corticolimbic interactions subserving affective processing in HR-SCZ subjects despite similar regional activation patterns. In other words, DCM proved to be highly sensitive in uncovering emergent impairments in functional brain organization that were not apparent in regional brain activation patterns or behavioral performance.
EMOTIONAL-PROCESSING NETWORK
The model of affective processing yielded by the Bayesian model selection procedure is well in line with the current conceptual framework of affective processing (see eFigure 1). First, reciprocal intrinsic connections among the VPFC, DPFC, and amygdala observed in all models were consistent with hypothesized connectivity in frontolimbic circuits.41,54,55 Second, valence (and in particular negative valence) resulted in top-down modulation of the amygdala by the VPFC and DPFC, confirming the hypothesized role of frontal structures in sending regulatory inputs to the amygdala.56,57 In the healthy brain, these regulatory inputs should serve to appropriately inhibit the amygdala’s response in the face of threat or fear.58 Positive faces did not differentially modulate frontolimbic connections, presumably reflecting the heterogeneous response of the frontolimbic circuit to positive valence16 and/or a reward-based response as opposed to responses to negative-valence (or threat-based) faces.17,59
The core regions of the VPFC, DPFC, and amygdala are central to the evaluation, experience, and modulation of emotion. The amygdala appears highly sensitive to emotional or affective novelty and salience,12,60 explaining its sensitivity to affective stimuli in general and faces in particular during active and passive viewing.61 Its sensitivity to negative valence, supported by animal studies showing amygdala responsivity to threat and fear,62 provides a strong measure of cross-species convergence with regards to amygdala function.63 TheDPFCisthoughttostoregoalstatestoward which effortful regulation of affect and related behaviors aredirected.64 Appraisalandreappraisalstudiessuggestthat these frontal areas are associated with the modulation of the labile amygdala response to emotional stimuli. Thus, when subjects must reappraise a previous response to negative emotional stimuli, the balance of activity between the frontal and limbic systems changes, leading to increased frontal (particularly VPFC) activity and decreased amygdala activity.65 Notably, thiseffectofreappraisalislessfocusedwithin the frontal cortex in children and adolescents (compared with adults), suggesting immature systems for emotional control.66 This immaturity is compounded by a relative (to adults)poverty of frontostriatal maturation and connections in adolescence.67,68 This immaturity may render the affective system particularly vulnerable in adolescents at risk for psychiatric disorders.
(DYS-)CONNECTIVITY IN HR-SCZ SUBJECTS AND CONTROLS
The combination of differences among driving, intrinsic, and modulatory interactions provides evidence of differences in how visual and affective information “flows” through corticolimbic circuits in HR-SCZ subjects compared with controls, even without widespread regional differences in activation. These collective results indicate that the integration of information during affective processing is altered in this at-risk population.
DIFFERENCES IN DRIVING INPUTS
Differences between the control and HR-SCZ groups were observed in driving inputs (visual or faces), with inputs to the V1 by visual stimuli being significantly reduced in the HR-SCZ group. Previous evidence suggests that schizophrenia is characterized by deficits in basic perception revealed inlongerprocessingtimesforstimulionperceptiontasks,69 impairments in tasks involving perceptual grouping,70,71 and reduced P1 amplitude in occipital leads during visual processing tasks.72,73 Evidence of deficits in risk populations is variable; recent electrophysiological studies in individuals showing psychotic symptoms and therefore at ultrahigh risk for schizophrenia indicate similar perceptual deficits.72 Reductions in driving inputs to the V1 in HR-SCZ subjects may reflect this latent deficit in perceptual processing or attention deficits that characterize risk groups.
DIFFERENCES IN INTRINSIC CONNECTIVITY
The observed pattern of reduced intrinsic coupling observable in the HR-SCZ group suggests a reduction in excitatory coupling between frontolimbic regions. These regions lie within the affective core of the face-processing circuit and are central to mediating the affective or emotional response.11,74 Reduced intrinsic coupling within this network is consistent with the general idea of reduced information flow or dysconnection in schizophrenia,75–77 thus constituting an emergent connective dysfunction within affective neurocircuitry in a population under genetic risk. Frontolimbic connections rapidly mature in adolescence,78 and maturation is strongly correlated with the development of face and emotion processing and social development.79,80 Reductions in intrinsic coupling within corticolimbic circuits may impair the affective response and mediate emergence of impairments in social behavior and adjustment in vulnerability for schizophrenia.8
DIFFERENCES IN MODULATORY INFLUENCES
The largely increased inhibitory modulation of frontolimbic pathways in HR-SCZ subjects (Figure 6) must be considered together with evidence of task-independent hypocoupling between frontolimbic regions (Figure 5). Significantly reduced frontolimbic connections suggest that the basic intrinsic architecture within the affective circuit is impaired and potentially less efficient in HR-SCZ subjects. In this context, the exaggerated inhibition of frontolimbic connections by nonpositive stimuli may reflect systemic attempts to regulate emergent hypersensitivity of the limbic system to negative emotional stimuli (which are known to characterize frank adolescent psychosis81). For instance, in clinical symptoms such as anhedonic depression, aberrantly increased regulatory inputs may be exerted to mediate the effects of negative symptoms.82–84 Therefore, in a system impaired by reduced intrinsic coupling and aberrant salience due to inefficient (thalamic) filtering, exaggerated inhibition may reflect a compensatory response by the frontal lobes to maintain affective homeostasis within the brain’s affective circuit.85 A characteristic of the gradual onset of psychiatric disorders may be the eventual imbalance between the excitatory (amygdala) and inhibitory (frontal) regions of frontolimbic circuitry leading to a persistent pattern of emotion dysregulation that is thought to characterize not only schizophrenia but also other disorders seen more frequently in HR-SCZ subjects than in controls, such as mood, anxiety, and stress-related disorders.86–88
As our own results suggest, activation differences (or lack thereof) may neglect important mechanistic features of (dysfunctional) processing emerging from interactions between the different elements of brain networks. The emphasis on an overall system’s approach is significant because the understanding of neural contributions to health, disease, and vulnerability or risk must be framed within the context of the system’s biologic mechanisms.25 By distinguishing among driving (sensory-based), intrinsic (context-independent), and modulatory (context-dependent, psychologically relevant) contributions to interregional interactions, DCM allows for the assessment of (aberrant) network interactions. In particular, the corticolimbic architecture in HR-SCZ subjects appears hypersensitive (to driving input), intrinsically hypoconnected, and hyperinhibitory (with respect to frontolimbic interactions). These dysfunctional network interactions should reflect genetic- and environment-related neurodevelopment deficits that may or may not resolve under adaptive mechanisms through adolescence.2 Eventually, they may result in disordered autonomic emotional responses that have characterized schizophrenia itself.89
CONCLUSIONS
Adolescence is a complex period within the overall developmental arc of the brain.90 Mechanisms of cognitive and emotional control are evolving, leading to the well-documented evidence of motivational, social, and reward-based behavioral vulnerabilities during this time,91 as well as to the increased incidence of psychiatric disorders.92
The study of HR-SCZ subjects presents advantages and significant conceptual challenges. Because of their medication-naïve status, studying HR-SCZ subjects can provide some insight into the schizophrenia diathesis independent of the significant confound of medication effects and provide significant advantages. However, these individuals are not characterized by a specific illness but are instead at elevated risk for Axis I psychopathology in general and schizophrenia in particular.93 Although many possible premorbid precursors of schizophrenia have been identified in this risk population,94 potential aberrations of corticolimbic interactions have remained unassessed. Our results now provide direct evidence of dysfunctional interactions within the corticolimbic network in this vulnerable state that may reflect the effects of vulnerability genes for schizophrenia and that in turn could alter the molecular biologic features of the synapse, resulting in disordered neural coupling in the schizophrenia brain.23 Large-scale longitudinal studies, however, will provide information on how latent vulnerabilities are (or are not) translated into more classic phenotypes of schizophrenia or other psychiatric disorders. Furthermore, in HR-SCZ subjects, it will be important to assess whether brain networks subserving domains such as attention and working memory (both of which are impaired in schizophrenia) also show dysfunction using DCM. Such analyses will reveal whether dysconnection is domain specific or domain general.
Supplementary Material
Funding/Support:
This study was supported by grant MH 68680 from the National Institute of Mental Health; the Children’s Research Center of Michigan; the National Alliance for Research on Schizophrenia and Depression (Dr Diwadkar); the Joe Young Sr. Fund (Department of Psychiatry and Behavioral Neurosciences, Wayne State University); a Global Grants Award from the Office of the Associate Vice President, Wayne State University (Dr Diwadkar); grant R01-MH074457-01A1 from the Human Brain Project (Dr Eickhoff); the Helmholtz-Initiative on Systems Biology (“Human Brain Model”); and the German Research Foundation (Integrated Research Training Group 1328).
Role of the Sponsors: The funding agencies played no role in the design, analysis, or interpretation of this study.
Footnotes
Previous Presentations: This study was presented in part at the Meeting of the Society for Biological Psychiatry; May 21, 2010; New Orleans, Louisiana; at the Meeting of the Organization on Human Brain Mapping; June 28, 2011; Quebec City, Quebec; and as a poster presentation at the Meeting of the Wisconsin Symposium on Emotion; April 21–22, 2010; Madison, Wisconsin.
Online-Only Material: The eFigures and eTables are available at http://www.archgenpsychiatry.com.
REFERENCES
- 1.Chua SE, Murray RM. The neurodevelopmental theory of schizophrenia: evidence concerning structure and neuropsychology. Ann Med. 1996;28(6):547–555. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. [DOI] [PubMed] [Google Scholar]
- 4.Jones P, Murray RM. The genetics of schizophrenia is the genetics of neurodevelopment. Br J Psychiatry. May 1991;158:615–623. [DOI] [PubMed] [Google Scholar]
- 5.Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophr Res. 2005;79(1):45–57. [DOI] [PubMed] [Google Scholar]
- 6.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts,” 4: clinical features and conceptualization. Schizophr Res. 2009;110(1–3):1–23. [DOI] [PubMed] [Google Scholar]
- 7.Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizo-phreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59(5):449–456. [DOI] [PubMed] [Google Scholar]
- 8.Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM. Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am J Psychiatry. 1997;154(11):1544–1550. [DOI] [PubMed] [Google Scholar]
- 9.Erlenmeyer-Kimling L Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet. 2000;97(1): 65–71. [DOI] [PubMed] [Google Scholar]
- 10.Hans SL, Auerbach JG, Auerbach AG, Marcus J. Development from birth to adolescence of children at-risk for schizophrenia. J Child Adolesc Psychopharmacol. 2005;15(3):384–394. [DOI] [PubMed] [Google Scholar]
- 11.Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nat Rev Neurosci. 2005;6(8):641–651. [DOI] [PubMed] [Google Scholar]
- 12.Adolphs R What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191(1):42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17(10):2400–2406. [DOI] [PubMed] [Google Scholar]
- 14.Ishai A Let’s face it: it’s a cortical network. Neuroimage. 2008;40(2):415–419. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. 1999;9(2):228–234. [DOI] [PubMed] [Google Scholar]
- 16.Posamentier MT, Abdi H. Processing faces and facial expressions. Neuropsychol Rev. 2003;13(3):113–143. [DOI] [PubMed] [Google Scholar]
- 17.Barbour T, Murphy E, Pruitt P, Eickhoff SB, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophr Res. 2010;123(2–3):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29(37):11 614–11 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398(3):431–458. [DOI] [PubMed] [Google Scholar]
- 20.Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25(32):7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. [DOI] [PubMed] [Google Scholar]
- 22.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence [published corrections appear in Mol Psychiatry. 2005;10(4)420 and Mol Psychiatry. 2005;10(8):804]. Mol Psychiatry. 2005;10(1):40–68; image 5. [DOI] [PubMed] [Google Scholar]
- 24.Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41(4):1382–1394. [DOI] [PubMed] [Google Scholar]
- 25.Stephan KE. Ontheroleofgeneralsystemtheoryforfunctionalneuroimaging. JAnat. 2004;205(6):443–470.15610393 [Google Scholar]
- 26.Stephan KE, Harrison LM, Penny WD, Friston KJ. Biophysical models of fMRI responses. Curr Opin Neurobiol. 2004;14(5):629–635. [DOI] [PubMed] [Google Scholar]
- 27.Stephan KE, Penny WD, Marshall JC, Fink GR, Friston KJ. Investigating the functional role of callosal connections with dynamic causal models. Ann N Y Acad Sci. 2005;1064:16–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephan KE,Penny WD,Moran RJ,den Ouden HE,Daunizeau J,Friston KJ. Tensimple rules for dynamic causal modeling. Neuroimage. 2010;49(4):3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D Wechsler Memory Scale–Revised. New York, NY: Psychological Corp; 1987. [Google Scholar]
- 31.First MD, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York, NY: Biometrics Research Dept, NYSPI; 1997. [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 33.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 34.Josephs O, Henson RN. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philos Trans R Soc Lond B Biol Sci. 1999; 354(1387):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekman P, Oster H. Facial expressions of emotion. Annu Rev Psychol. 1979;30:527–554. [Google Scholar]
- 36.Presentation [computer program]. Albany, CA: Neurobehavioral Systems Inc; 2005. [Google Scholar]
- 37.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003; 19(4):1273–1302. [DOI] [PubMed] [Google Scholar]
- 38.Grefkes C, Wang LE, Eickhoff SB, Fink GR. Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb Cortex. 2010;20(4):783–797. [DOI] [PubMed] [Google Scholar]
- 39.Eickhoff SB, Heim S, Zilles K, Amunts K. A systems perspective on the effective connectivity of overt speech production. Philos Transact A Math Phys Eng Sci. 2009;367(1896):2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eickhoff SB, Dafotakis M, Grefkes C, Shah NJ, Zilles K, Piza-Katzer H. Central adaptation following heterotopic hand replantation probed by fMRI and effective connectivity analysis. Exp Neurol. 2008;212(1):132–144. [DOI] [PubMed] [Google Scholar]
- 41.Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300 (4):549–571. [DOI] [PubMed] [Google Scholar]
- 42.Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46(4):1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickens TD. Elementary Signal Detection Theory. Oxford, England: Oxford University Press; 2001. [Google Scholar]
- 44.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York, NY: Wiley; 1966. [Google Scholar]
- 45.Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- 46.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. [DOI] [PubMed] [Google Scholar]
- 47.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999; 46(5):650–661. [DOI] [PubMed] [Google Scholar]
- 48.Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Axelson D, Rajarathinem R, Haddad L, Amirsadri A, Zajac-Benitez C, Rajan U, Keshavan MS. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: comparing vulnerability markers. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diwadkar VA, Sweeney JA, Boarts D, Montrose DM, Keshavan MS. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectr. 2001;6(11):899–903. [DOI] [PubMed] [Google Scholar]
- 50.Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res. 2011;45(8):1067–1076. [DOI] [PubMed] [Google Scholar]
- 51.Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophr Bull. 2008;34(5):888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, Ruf B, Saricicek A, Pearlson GD, Bhagwagar Z, Phillips ML. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012; 42(1):29–40. [DOI] [PubMed] [Google Scholar]
- 53.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage. 2008; 39(3):1396–1407. [DOI] [PubMed] [Google Scholar]
- 55.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. [DOI] [PubMed] [Google Scholar]
- 56.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry. 2003; 54(5):515–528. [DOI] [PubMed] [Google Scholar]
- 57.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. [DOI] [PubMed] [Google Scholar]
- 58.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. [DOI] [PubMed] [Google Scholar]
- 59.Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57(6): 624–632. [DOI] [PubMed] [Google Scholar]
- 60.Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49(3):2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Mald-jian J, Gur RE. Brain activation during facial emotion processing. Neuroimage. 2002;16(3, pt 1):651–662. [DOI] [PubMed] [Google Scholar]
- 62.LeDoux J The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003; 23(4–5):727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73(1):39–48. [DOI] [PubMed] [Google Scholar]
- 64.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. [DOI] [PubMed] [Google Scholar]
- 65.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008; 63(6):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lévesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–369. [DOI] [PubMed] [Google Scholar]
- 67.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20(9):2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McClure RK. The visual backward masking deficit in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(2):301–311. [DOI] [PubMed] [Google Scholar]
- 70.Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM. Perceptual grouping in disorganized schizophrenia. Psychiatry Res. 2006;145(2–3):105–117. [DOI] [PubMed] [Google Scholar]
- 71.Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26(31):8168–8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeap S, Kelly SP, Sehatpour P, Magno E, Garavan H, Thakore JH, Foxe JJ. Visual sensory processing deficits in schizophrenia and their relationship to disease state. Eur Arch Psychiatry Clin Neurosci. 2008;258(5):305–316. [DOI] [PubMed] [Google Scholar]
- 73.Donohoe G, Morris DW, De Sanctis P, Magno E, Montesi JL, Garavan HP, Robertson IH, Javitt DC, Gill M, Corvin AP, Foxe JJ. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry. 2008;63(5):484–489. [DOI] [PubMed] [Google Scholar]
- 74.Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51(1):59–67. [DOI] [PubMed] [Google Scholar]
- 75.Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–125. [DOI] [PubMed] [Google Scholar]
- 76.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. [DOI] [PubMed] [Google Scholar]
- 77.Érdi P, Flaugher B, Jones T, Ujfalussy B, Zalányi L, Diwadkar VA. Computational approach to the schizophrenia: disconnection syndrome and dynamical pharmacology. In: Ricciardi LM, Buonocore A, Pirozzi E, eds. BIOCOMP. Melville, NY: American Institute of Physics; 2007:65–87. [Google Scholar]
- 78.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010; 3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herba C, Phillips M. Annotation: development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. J Child Psychol Psychiatry. 2004;45(7):1185–1198. [DOI] [PubMed] [Google Scholar]
- 80.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. [DOI] [PubMed] [Google Scholar]
- 81.Pauly K, Seiferth NY, Kellermann T, Backes V, Vloet TD, Shah NJ, Schneider F, Habel U, Kircher TT. Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008; 47(11):1299–1310. [DOI] [PubMed] [Google Scholar]
- 82.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48 (8):813–829. [DOI] [PubMed] [Google Scholar]
- 83.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58 (11):843–853. [DOI] [PubMed] [Google Scholar]
- 84.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. [DOI] [PubMed] [Google Scholar]
- 85.Barbas H Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211(2):237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77(5):283–298. [DOI] [PubMed] [Google Scholar]
- 87.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci. 1999;877:383–396. [DOI] [PubMed] [Google Scholar]
- 88.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. [DOI] [PubMed] [Google Scholar]
- 89.Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, Skerrett D, Phillips ML,David AS,Peduto A,Gordon E. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161(3):480–489. [DOI] [PubMed] [Google Scholar]
- 90.Paus T Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–68. [DOI] [PubMed] [Google Scholar]
- 91.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities: keynote address. Ann N Y Acad Sci. 2004;1021:1–22. [DOI] [PubMed] [Google Scholar]
- 92.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Erlenmeyer-Kimling L, Adamo UH, Rock D, Roberts SA, Bassett AS, Squires-Wheeler E, Cornblatt BA, Endicott JJ, Pape S, Gottesman II. The New York High-Risk Project: prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Arch Gen Psychiatry. 1997;54(12):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keshavan MS, Gilbert AR, Diwadkar VA. Developmental hypotheses of schizophrenia. In: Lieberman JA, Stroup TS, Perkins DO, eds. Textbook of Schizophrenia. Arlington, VA: American Psychiatric Publishing; 2006:69–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


