Abstract
Background: The immunomodulatory enzyme, indoleamine 2,3-dioxygenase (IDO) facilitates tryptophan catabolism at the rate-limiting step of the kynurenine (Kyn) pathway. IDO expression and elevations in Kyn metabolites are associated with immunosuppressive tumor microenvironment including T cell proliferative arrest and generation of regulatory T cells (Tregs) which can favor tumor progression. However, the extent of the role of IDO in acute myeloid leukemia (AML) is currently ill-defined. This study reviews the role of IDO-driven Treg function in AML and evaluates the current body of evidence implicating IDO in AML pathogenesis.
Method: Studies related to IDO in AML were identified through a systematic review of PubMed and Scopus. Data extracted described sample analysis, IDO expression, IDO in prognosis, techniques used in Treg phenotypic studies, and the effect of IDO inhibitors.
Results: Twenty studies were included in the systematic review. Expression of IDO was identified in a range of cells in AML, both inducible and constitutive. Seven studies indicated an association between elevated expression and poor clinical prognosis. Six studies suggested a positive correlation between IDO expression and Treg induction, with FoxP3 being the prominent Treg phenotypic marker. Of eight studies investigating IDO inhibition, some reported reductions in Treg frequency and enhanced effector T cell proliferation.
Conclusion: This review highlights that IDO expression in AML is associated with poor prognosis and measurement of IDO and its Kyn metabolites may offer utility as prospective prognostic markers. Pharmacological inhibition of IDO using novel drugs may hold promise for the treatment of AML.
Keywords: acute myeliod leukemia; regulatory T cells; indoleamine 2,3-dioxygenase; prognosis; 1-Methyltryptophan
Introduction
Acute myeloid leukemia (AML) is a hematological malignancy which interferes with the normal haematopoietic production of blood cells. It is characterized by the accumulation and expansion of immature myeloid cells (blasts) within the bone marrow that proliferate rapidly and inhibit differentiation of the normal progenitor cells (1). AML is primarily a disease of adulthood and comprises ~80% of hematological malignancies observed in patients over 60 (2, 3). AML is typically treated by a standard induction chemotherapy of a 7 + 3 regimen of cytarabine with an anthracycline (daunorubicin or idarubicin), genetic risk profiles then determine follow up treatment of consolidation chemotherapy or allogeneic hematopoietic stem cell transplantation (4). The efficacy of standard induction chemotherapy is limited and is followed by AML relapse and mortality in a majority of patients over 60 (4, 5).
The “two-hit” model of AML leukaemogenesis proposes that two mutation classes drive the disease, those that augment proliferation and cell survival (e.g., FLT3) and those which disrupt haemopoietic differentiation and apoptosis (e.g., CBFβ-MYH11) (6). Recent development of therapies targeting such mutations including the FLT3 inhibitors, lestaurtinib, and midostaurin have improved patient outlook (7). These have been shown to produce clinical responses, but they are often transient and subject to resistance mechanisms (8). Overall, current standard of care regarding chemotherapeutics is limited, with prognosis remaining poor for many patients, warranting the need for detailed biological understanding of disease pathogenesis to develop future therapies (9). A deeper understanding of the molecular mechanisms underlying AML pathogenesis may offer new avenues to successful targeted therapies.
The heterogeneity of immune response between patients has made it difficult to characterize the landscape of AML tumor microenvironment. Several components within the tumor microenvironment interact to reprogram tumor initiation, progression, and response to therapies. Malignancies like AML deploy a plethora of immune escape mechanisms, including evasion of immunosurveillance systems, whereby cancer cells are able to avoid destruction (10). Such escape mechanisms in AML include aberrant expression of immune checkpoint receptors, deregulation of tumor necrosis receptor families and ligands which regulate T cell and natural killer cell responses, and immunomodulatory enzymes which abrogate anti-tumoral T cell effector function (11, 12).
Indoleamine 2,3-dioxygenase (IDO) is a haem-containing, immunomodulatory enzyme that catalyzes the oxidative cleavage of the indole ring of the essential amino acid tryptophan (Trp), producing immunoregulatory metabolites, collectively known as kynurenines [Kyn(s)] (13). IDO was originally described to promote immune tolerance in mammalian pregnancy, chronic infection, autoimmunity and allergic inflammation, but it has now become evident that it plays an important role in suppressing antitumour responses, and promoting tumor resistance (14). Some of the Kyn metabolites of tryptophan catabolism are known to inhibit T cell proliferation by arresting the cell cycle and inducing apoptosis (15). Direct action of Kyn has been associated with the generation of regulatory T cells (Tregs) (15). Natural Tregs, characterized by their constitutive expression of surface markers, such as CD25, CTLA-4, and the forkhead box P3 (FoxP3) transcription factor, are able to aid evasion of immune surveillance and suppress cytotoxic CD8+ T cell proliferation, favoring tumor progression (16).
Defining the tumor microenvironment and immune escape mechanisms of AML will support the development of new therapeutic strategies. IDO is of particular interest in this regard as studies report IDO expression in both bone marrow and in peripheral blood AML blasts, but the role it plays in disease progression remains unclear (12). Research aimed at defining tumor resistance and identifying immunosuppressive mechanisms in AML is expanding, but there is a lack of consolidated review that aims to systematically decipher the role of this immunosuppressive enzyme. Therefore, this study will review the role of IDO-driven Treg function in AML and evaluate the current body of evidence implicating IDO in AML pathogenesis, to determine its indication in prognosis and as a potential therapeutic opportunity.
Methods
A systematic review of literature was performed to identify the role of IDO AML progression. The review was conducted according to the guidelines outlined in the PRISMA statement (Supplementary Table 1) (17).
Search Strategy
A systematic search of PubMed (performed in October 2020) was conducted to identify appropriate studies. The following keyword combinations were used:
“Indoleamine 2,3-dioxygenase” and “AML”
“Indoleamine 2,3-dioxygenase” and “Acute Myeloid Leukemia”
“1-Methyltryptophan” and “Acute Myeloid Leukemia”
“1-Methyltryptophan” and “AML”
“1-MT” and “Acute Myeloid Leukemia”
“1-MT” and “AML”
“IDO” and “Acute Myeloid Leukemia”
“Tryptophan” and “Acute Myeloid Leukemia”
An additional search was conducted in the Scopus database using the keywords “Indoleamine 2,3-dioxygenase” and “AML”. Reference lists of identified papers were also scrutinized to identify additional sources.
Inclusion criteria for the systematic review were: Studies based on human cells and/or cell lines; Studies that assessed the role of IDO in AML; Studies published in English.
Exclusion criteria for the review were: Review/Systematic review articles, case studies, letters to the editors, books, and abstracts; Studies focusing on other cancer types, such as chronic myeloid leukemia (CML), acute lymphocytic leukemia (ALL) etc.; Studies primarily based on animal models of AML and AML studies that did not investigate IDO.
Data Extraction
Studies satisfying the inclusion criteria were independently assessed and a data extraction was conducted to obtain the following data: Authors, journal, publication year, sample size, sample type, sample analysis method, IDO expression, role of IDO in prognosis, Treg markers identified, role of Tregs in AML, and the effect of IDO inhibitors. Due to the heterogenous nature of findings and variations in study designs, number of samples and methods employed for quantitation of IDO, meta-analysis of the results was not feasible.
Results
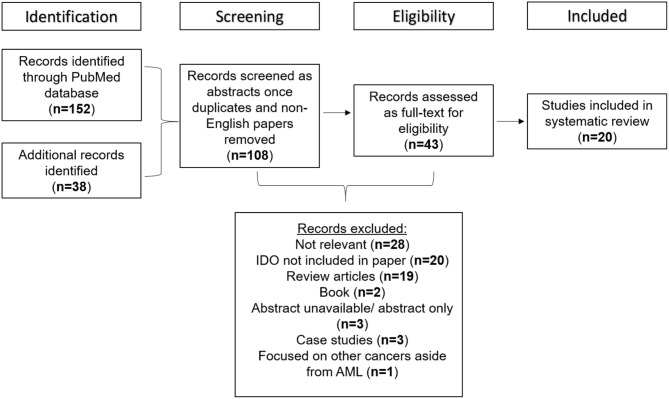
Of 190 papers identified through PubMed and Scopus search, 108 met the inclusion criteria once duplicates and non-English papers were removed. When full texts were screened, a final 20 studies were found to be suitable for inclusion in the study. The reasons for manuscript exclusions were those that had no mention of both IDO and AML (n = 28), manuscripts that did not tackle the role of IDO in AML (n = 20), those with unavailable abstracts or only abstracts were available (n = 20), review articles (n = 19), books (n = 2), those based on case studies alone (n = 3), and those focused on other types of cancer aside from AML (n = 1). A flow diagram detailing the screening process used to determine the studies suitable for inclusion in the systematic review is shown in Figure 1.
Figure 1.
Flow diagram detailing the studies retrieved for the systematic review.
Data Summary
The basic characteristics of the studies included in this review are summarized in Table 1.
Table 1.
Summary of the basic characteristics of the acute myeloid leukemia (AML) study samples included in the systematic review.
| Study | Type of sample | Sample number |
|---|---|---|
| Arandi et al. (12) | Peripheral blood | 37 de novo Cytogenetically normal-AML 22 Healthy controls |
| Mansour et al. (18) | Bone marrow | 12 de novo adult AML patients 8 Healthy controls |
| Folgiero et al. (19) | Peripheral blood Bone marrow |
37 child AML patients |
| Fukuno et al. (20) | Bone marrow | 62 de novo adult AML patients |
| Corm et al. (21) | Blast cells Sera |
184 adult AML patients |
| Mangaonkar et al. (22) | Bone marrow | 40 adult AML patients |
| EL Kholy et al. (23) | Peripheral blood | 25 adult AML patients 25 Healthy controls |
| Curti et al. (24) | Bone marrow Peripheral blood |
76 AML patients |
| Mabuchi et al. (25) | Bone marrow | 48 adult AML patients |
| Curti et al. (26) | Peripheral blood | 8 AML patients |
| Hara et al. (27) | Bone marrow | 29 adult AML patients |
| Martine et al. (28) | Bone marrow Peripheral blood |
356 AML patients |
| Wang et al. (29) | Bone marrow Peripheral blood Serum |
55 de novo AML patients 45 Healthy controls |
| Chen et al. (30) | Bone marrow Peripheral blood |
49 AML patients 11 ALL patients 15 Healthy controls |
| Curti et al. (31) | Bone marrow Primary AML cells |
76 AML patients |
| Trabanelli et al. (32) | Peripheral blood Buffy coats |
Unspecified number of AML patients and healthy controls |
| Folgiero et al. (33) | Bone marrow Buffy coats |
Unspecified number of child AML patients and healthy controls |
| Zhou et al. (34) | Heparinized blood samples | 102 AML patients 102 Healthy controls |
| Lecciso et al. (35) | Bone marrow Peripheral blood |
23 AML patients Unspecified number of healthy controls |
| Iachininoto et al. (36) | HL-60 cells | – |
A substantial majority of the studies selected focused on human samples alone (12, 18–23, 25–34). One study assessed human samples and incorporated some aspects of murine AML biology (24), one study looked at human samples, a murine model and HL-60 cell lines (35) whilst one exclusively HL-60 cells (36). Of the studies focusing on human samples, 10 were based on samples taken from adults (≥18 years) (12, 18, 20–23, 25, 27, 31, 35), one from child samples (19), whilst eight did not specify subject age (24, 26, 28–30, 32–34). Some of the studies used AML classification systems; 11 studies applied the French-American-British (FAB) classification (12, 19, 20, 23, 25, 27–31, 35) and five used cytogenic risk categories (22, 27–29, 31). In eight of the studies AML type was categorized as either de novo AML (12, 18, 20, 25, 27, 29), cytogenetically normal AML (12) and AML where complete remission (CR) has been achieved (32).
In relation to the methodologies employed by the studies for the quantitation of IDO, seven studies focused directly on IDO1 (19, 22, 26, 32, 33, 35, 36), whilst the remaining studies were non-specific in the type of IDO. None of the studies focused on IDO2 alone. The most common method, used in 15 of the 20 studies, to detect IDO mRNA expression was polymerase-chain reaction (12, 19–24, 26–28, 30–32, 34, 36). IDO protein expression was detected using western blotting in seven studies (21, 26, 28, 31, 33, 35, 36), immunohistochemistry in three studies (18, 22, 30), and immunocytochemistry in two studies (24, 31). Nine studies focused on IDO activity by measuring Trp and Kyn levels using high-performance liquid chromatography (HPLC) (19, 21, 24–27, 29, 33, 36). Some additional methods used in the studies included the measurement of IDO activity by colorimetric assay (Bradford method) (23), spectrophotometric analysis (32), and fluorescent detection (34).
Of the 20 studies identified, eight investigated the role of Tregs in relation to IDO (12, 18, 19, 24, 26, 32, 35, 36). The main methodology used was flow cytometry in five of the studies (18, 19, 32, 35, 36), with T cell surface staining (19, 36), standard mixed lymphocyte reaction (24, 26), polymerase-chain reaction (12), phenotypic and functional assays (26), and mixed tumor lymphocyte cultures (19, 36).
Role of IDO in AML
Methodologies used and the proposed role of IDO within AML are summarized in Table 2.
Table 2.
Summary of the methods for detection of indoleamine 2,3-dioxygenase (IDO) and regulatory T cell (Treg) markers, and the suggested role of IDO and Tregs in AML progression and prognosis.
| Study | Method for detecting IDO | Suggested role of IDO in AML | Method for detecting Tregs | Treg markers | Association between Tregs and IDO |
|---|---|---|---|---|---|
| Arandi et al. (12) | Real-time PCR | High IDO expression in peripheral blood mononuclear cells is associated with the promotion of disease progression, poor immune response, and poor prognosis. | Real-time PCR | FoxP3 | FoxP3 expression is upregulated in AML patients. Positive correlation observed between IDO and FoxP3 expression |
| Mansour et al. (18) | Immunohistochemistry | IDO expression in mesenchymal stem cells has a positive correlation with bone marrow blasts. Modulating the immune system may improve patient survival in AML. |
Flow cytometry | CD4 CD25 |
IDO expression in mesenchymal stem cells positively correlated with percentage of Tregs |
| Folgiero et al. (19) | Real-time quantitative PCR, reverse phase HPLC | IDO protein and activity is not constitutively expressed. Ability to increase IDO expression is exclusively assigned to FAB-M4/M5 subtypes. |
T cell staining, Flow cytometry | FoxP3 | IDO-expressing AML blasts favor the emergence of FoxP3-expressing Tregs |
| Fukuno et al. (20) | Real-time reverse transcription PCR | IDO expression is not significantly different between FAB subtypes and cytogenic risk profiles but is associated with poor prognosis. | – | – | – |
| Corm et al. (21) | Real-time quantitative PCR, WB, HPLC | There is no correlation between IDO mRNA and Fab subtypes. IDO may be expressed constitutively in come patients, but blast stimulation may be required in others. |
|||
| Magaonkar et al. (22) | Real-time quantitative PCR, Immunohistochemistry, HPLC | IDO mRNA has a negative correlation with overall survival and high levels IDO found to be an independent predictor of poor outcomes. | – | – | – |
| El Kholy et al. (23) | Colorimetric assay, reverse transcriptase PCR | Mononuclear cells expressing IDO mRNA also have functional activity. Increased IDO activity is a negative prognostic sign in AML. |
– | – | – |
| Curti et al. (24) | Real-time PCR, Immunocytochemistry, HPLC | IDO can be constitutively expressed. Production of IDO in AML cells directly increases the number of Treg cells. |
Flow cytometry, SMR | CD4 CD25 |
Treg cells in IDO-expressing AML are induced by the conversion of CD4+CD25− T cells into CD25+ T cells |
| Mabuchi et al. (25) | HPLC | High serum concentrations of Kyn are associated with poor outcomes. | – | – | – |
| Curti et al. (26) | Real-time quantitative PCR, WB, reverse phase HPLC | IDO is expressed in both immature and mature dendritic cells, but marked upregulation is seen in the later stages of dendritic cell generation. | SMR, phenotypic and functional assays | CD4 CD25 |
AML-like dendritic cells have increased percentage Tregs which reduce T cell proliferation in culture |
| Hara et al. (27) | PCR, HPLC | Combination of elevated Kyn levels and IDO mRNA are associated with poor outcomes in AML. | – | – | – |
| Martine et al. (28) | Real-time quantitative PCR, WB | No difference in IDO mRNA between FAB subtypes and cytogenic risk profiles. High IDO expression is associated with poor outcomes. |
– | – | – |
| Wang et al. (29) | UHPLC-Q-TOFMS | AML patients exhibit higher levels of Kyn, suggesting IDO expression increases cancer burden. | |||
| Chen et al. (30) | Immunohistochemistry, Real-time fluorescence PCR | IDO mRNA expression is highest in AML-M5 subtypes and is associated with poor prognosis. | – | – | – |
| Curti et al. (31) | Immunocytochemistry, WB, Reverse transcriptase PCR | AML samples can show constitutive expression of IDO. AML-M5 has higher, but non-significant, expression in IDO positive cells. |
– | – | |
| Trabanelli et al. (32) | Real-time quantitative PCR, Spectrophotometric analysis | Presence of PGE2 can upregulate IDO mRNA in dendritic cells suggesting modulation role in the expression and function of IDO. | T cell staining, FC | CD4 CD25 FoxP3 |
Addition of an IDO inhibitor reduced Tregs suggesting a IDO1 mediated induction of Tregs in dendritic cells, in the presence of PGE2 |
| Folgiero et al. (33) | Reverse phase HPLC, WB | IDO positive AML blasts can down-regulate natural killer cell degranulation, thus favoring immune escape. | – | – | – |
| Zhou et al. (34) | Real-time PCR, fluorescence detection | Redox protein, thioredoxin, correlates with IDO and could contribute to aggressive tumor growth. | – | – | – |
| Lecciso et al. (35) | WB | ATP release from daunorubicin chemotherapy treatment may promote the IDO pathway. | FC | CD4 CD25 FoxP3 |
ATP-mediated upregulation of IDO1 in mature dendritic cells correlated with the generation of Tregs |
| Iachininoto et al. (36) | Real-time quantitative PCR, Reverse phase HPLC | Cox-2 pathway may regulate IDO expression in AML cells. Inhibition of the Cox-2/PGE2 pathway may constrain the leukemia-induced immune response. |
T cell staining, FC | FoxP3 | Conversion of CD4+ T cells to bona fide Tregs promoted in IFN-γ challenged HL-60 cells |
PCR, polymerase-chain reaction; HPLC, high-performance liquid chromatography; WB, western blot; SMR, standard mixed lymphocyte reaction; UHPLC-Q-TOFMS, ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry.
IDO expression was identified in peripheral blood mononuclear cells (12, 23, 31), but not in bone marrow-derived mononuclear cells (31). Its expression was also identified in bone marrow-derived mesenchymal stem cells (18) and dendritic cells (DCs) (21, 25, 26). Four of the 20 studies reported elevated IDO expression in AML patients compared to healthy controls (12, 18, 23, 30). One study identified elevated Kyn levels in AML patients compared to healthy controls (29).
Three studies identified constitutive IDO expression by leukemic cells of some AML patients (21, 24, 31). One study found no constitutive IDO expression on leukemic blasts from peripheral blood and bone marrow but identified induction by IFN-γ (19). This was supported by two studies indicating the ability of IFN-γ to induce IDO at the molecular level in samples which lacked constitutive expression of IDO (21, 31).
Correlations between IDO expression and FAB types were investigated in seven of the studies reviewed (19–21, 25, 28, 30, 31). Four suggested no significant difference in IDO expression or Kyn levels between subtypes (20, 21, 25, 28). Of the remaining studies, one identified higher IDO expression in the FAB-M5 subgroup compared to non-M5 and healthy controls (1.3- and 3.1-fold, respectively) (30), one found upregulated IDO expression in FAB-M4/M5 subgroups compared to no upregulation in FAB-M1/M2 groups (19). One final study identified higher IDO-positive expression in the FAB-M5 subgroup, although this was not statistically significant (31).
Three studies assessed correlations between cytogenic risk profile and IDO expression, with all concluding that IDO expression has no correlation with cytogenic risk profile (19, 20, 28). Additional factors influencing the IDO pathway were assessed in five of the studies (32–36). Two studies identified the key role of the cyclooxygenase-2/Prostaglandin E2 (COX-2/PGE2) pathway in the upregulation of IDO expression and function in AML cells (32, 36), suggesting modulation of IDO can occur as a result of the environmental cytokine composition (32). These studies suggest that inhibition of the COX-2/PGE2 pathway (36) and DC based vaccines (32) may constrain leukemia-induced immune suppression.
Seven studies investigated the correlation between IDO expression and the percentage of blasts in either bone marrow or peripheral blood (12, 18, 20, 23, 25, 27, 31). One study identified a positive correlation between IDO expression and bone marrow blast cell percentage in bone-marrow derived mesenchymal cells (18), whilst four studies found no significant correlation between IDO expression and blast percentage in bone marrow (12, 20, 23, 27), and one established no correlation between IDO expression and mean percentage of peripheral blood blasts (12). Two further studies found no significant correlation between Kyn levels and peripheral blood and bone marrow blast percentage (25) and comparable circulating blast cell counts between IDO-positive and IDO-negative patients (31).
One study identified that IDO positive expression on AML blasts reduces natural killer cell degranulation, thereby promoting immune escape (33). Another study investigated the involvement of oxidative stress in relation to IDO expression, finding a positive correlation between IDO and the redox protein, thioredoxin (34). The final study found that ATP release from chemotherapy treatments (especially daunorubicin) can promote the IDO pathway, suggesting that current chemotherapy treatments may drive the immune suppressive environment in AML via IDO upregulation (35).
IDO and Prognosis
Regarding prognosis, seven studies associated high IDO expression/activity (and its related elevated Kyn levels) with poor prognosis (12, 20, 23, 25, 27, 28, 30). This poor outcome was linked specifically to IDO mRNA expression in two studies (27, 30). In terms of survival, overall survival (OS) was reduced in IDO-positive groups compared to IDO-negative groups in three studies (19, 20, 27) (Table 3). However, one paper did not correlate IDO mRNA with OS (21).
Table 3.
Overall survival data (%) for IDO-positive and IDO-negative AML samples.
Fewer IDO-positive patients achieved CR than IDO-negative patients in three of the studies reviewed (20, 27, 28). One study found that CR was similar between the IDO-positive and -negative groups, but did identify relapse cases consisted of mainly IDO-positive patients (19). CR was lower in patients with high Kyn levels in one study (27), but there was no significant difference in CR based on Kyn levels in another (25). However, 3-year OS associated with these studies showed that high Kyn levels (≥2.4 μM) resulted in lower overall OS (25, 27).
Five of the studies investigating the effect of IDO and its consequent elevation of Kyn levels also identified key prognostic markers for patient outcomes (20, 25, 27–29). One study identified IDO expression as a useful prognostic factor (28) and a further three studies specifically identified IDO mRNA as a potential candidate (20, 27, 29). However, one suggested that IDO mRNA could be used independently from existing molecular markers (20), whilst another suggested this marker is not independent of other factors (29). Measurement of Kyn levels were also identified as a useful prognostic factor in two studies (25, 27), with one of the studies indicating that the prognostic value of both IDO mRNA and measure of Kyn levels in combination can be associated with poor outcomes (27).
Relationship Between IDO Expression and Treg Abundance
The link between IDO expression and Treg abundance was addressed in eight studies (12, 18, 19, 24, 26, 32, 35, 36). The methods, markers and roles used are summarized in Table 2. FoxP3 was the predominant Treg phenotypic marker, used as a sole marker in three studies (12, 19, 36), or in combination with CD4 and CD25 in two studies (32, 35). The remaining studies identified Tregs through the expression of the markers CD4 and CD25 (18, 24, 26). One study identified Treg subpopulations using a combination of the following markers: CD3/CD4/CD25/CD127/CD15s/CD45A/FoxP3/PD-1/Ki67 (35).
An increase in Treg abundance in AML patients compared to control groups was identified in three studies (12, 18, 24), whilst six studies identified a positive correlation between IDO expression and Treg percentage (12, 18, 19, 24, 26, 35). This correlation was shown in mesenchymal stem cells (18), IFN-γ activated AML blasts (19), and AML-like DCs (26). Generation of Tregs was also suggested in DCs via an IDO mediated mechanism in the presence of PGE2 (32). One study showed that the CD4+CD25+ Treg phenotype significantly reduced T cell proliferation in culture (26), and another suggested IDO elevation may be associated with an increased Treg frequency (12). One study also found that tumor microenvironment enriched with IDO-expressing AML cells could convert CD4+CD25− T cells into CD4+CD25+ T cells via tryptophan catabolism (24).
Effect of IDO Inhibition
Of the 20 studies, eight included experiments using an IDO inhibitor (21, 23, 24, 26, 31, 32, 35, 36), whilst three studies suggested IDO inhibition could be used therapeutically in AML treatment (22, 27, 28). Six of these studies included the IDO inhibitor 1-methyl-tryptophan (1-MT) (23, 24, 26, 31, 32, 35). 1-MT reduced Treg frequency (24, 32, 35) and Kyn production in IDO-expressing DCs (26) and increased T cell proliferation in IDO-positive cells but not IDO-negative cells (31). One study explored the effect of 1-MT in combination with the chemotherapeutic agent Adriamycin, establishing that the combination was significantly more effective in reducing blast cell proliferation than Adriamycin alone (23).
Other IDO inhibitors used included pyrrolidine dithiocarbamate, abrogated Kyn production (21) and the cyclooxygenase-2 inhibitor, Nimesulide, which abrogated Kyn release and inhibited Treg differentiation to a lesser extent than 1-MT (36). Overall, two of the studies suggested IDO inhibition in adjuvant to chemotherapy may be a means to clear residual leukemic cells and mount an effective anti-tumoral immune response (21, 23).
Discussion
IDO has been shown to play an important role in the maintenance of immune tolerance, whilst also being implicated in several autoimmune inflammatory diseases, chronic infections and cancers (37). Its activity is majorly attributed to the enzymatic catabolism of the essential amino acid Trp, producing metabolites which arrest T cell proliferation, induce T cell apoptosis, and generate Tregs, producing an immune suppressive environment able to evade immunosurveillance systems. In AML, there is little biological understanding of disease pathogenesis resulting in high mortality rates despite efforts to refine current techniques and the development of targeted therapies. IDO and its Treg-inducing environment are not well-characterized in AML and so the clinical outlook for patients expressing IDO remains unclear.
This systematic review evaluates current knowledge on the role of IDO in AML, aiming to decipher its contribution to disease progression, prognosis, and potential therapeutic interventions. It is clear from the majority of the studies that IDO can be expressed on mononuclear cells of the peripheral blood, bone-marrow derived mesenchymal cells as well as leukemic blasts themselves (12, 18, 19, 21, 23–26, 31). Methodologies across these studies were consistent, focusing of peripheral blood and bone marrow, and utilizing PCR techniques. Only mesenchymal cell IDO expression was identified using bone marrow-derived samples with immunohistochemistry. This consistency across methodologies and samples used suggests that the expression of IDO is variable across multiple cell types. However, there occurs to be some discrepancy whether IDO is constitutively expressed (21, 24, 31) or induced (19, 21, 31). Consensus stands that whilst a subset of patients may have constitutive expression of IDO, others require it to be induced. These findings indicate an inter-patient variability in IDO expression in AML and that further studies should assess expression on a case-by-case basis to overcome the discrepancy. It also suggests that constitutive expression of IDO is not necessary to the progression of AML, but that it may favor progression.
In this review, a controversy was also highlighted regarding IDO expression relating to FAB subtypes. Some of the studies suggested that higher IDO expression is observed in the FAB-M5 subtype (19, 30, 31). The FAB-M5 subtype is associated with leukemia derived from a myelomonocytic lineage (38), and as aforementioned, IDO expression on mononuclear cells, including monocytes and monocyte-derived DCs has been found. As FAB-M5 is leukemia of monocytic origin and IDO can be expressed by these cells, it could account for this correlation. Contrasting this, four of the studies suggested that there was no significant difference in IDO expression between the FAB subtypes (20, 21, 25, 28). The methodologies used in these studies and those identifying a difference in IDO expression between subtypes were consistent. However, the sample size of those finding no difference were typically much larger (n = 650), providing more reliable statistical data. Accordingly, FAB subtypes should not be used to classify the likelihood of IDO expression.
AML mortality rates remain high despite attempts to refine treatment and develop more targeted techniques. Prognostic markers stratifying disease progression, such as the FLT3 mutation, have thus far only had limited effects on patient outcomes. Among the reviewed articles, seven studies assessed IDO expression prognosis (12, 20, 23, 25, 27, 28, 30). All associated high expression of IDO with poor prognosis, with some finding lower OS and CR attainment in IDO-positive groups. Across some of the studies, the value of IDO mRNA and Kyn levels as prognostic factors were assessed, finding that both hold potential as prognostic markers in AML (20, 25, 27, 29). Measurement of Kyn levels in patient samples are relatively easier than IDO mRNA measurements as this can be done in serum. However, a combination of both is likely to be most beneficial as a prognostic marker.
The Kyn metabolites produced by Trp cleavage can support Treg generation. The presence of Treg within the tumor environment is known to promote the development and progression of tumors by curtailing effective anti-tumor immune response, denoting poor survival in various types of cancers (39). Eight of the studies in the present review attempted to establish the role of Tregs in relation to IDO expression in AML (12, 18, 19, 24, 26, 32, 35, 36). These studies agreed that a positive correlation exists between IDO expression and Treg induction (12, 18, 19, 24, 26, 35), suggesting that in the case of AML, the immune suppressive environment may be resultant of the Treg accumulation induced by the expression of IDO. The most common CD4 Treg markers used across the studies were FoxP3 and CD25. However, they may both be transiently expressed by activated T cells in human and may not represent bonafide Tregs (40). CD127, is consistently expressed in high levels in activated T cells, but low or absent in Tregs, and therefore identification of CD25+FoxP3+CD127low/− CD4 T cells may be more indicative of a Treg phenotype (41).
Pharmacological inhibition of IDO has been shown to break maternal immune tolerance in pregnancy, spontaneously aborting fetus, and precipitate autoimmunity in susceptible strains in mice (42, 43). Such findings provided proof-of-concept that breaking tolerance to tumor antigens in cancers where IDO is used as a route to immune escape may hold promise for cancer immunotherapy. Several studies included in this review also used the IDO inhibitor 1-MT in vitro. In samples from AML patients, 1-MT reduces Treg induction and Kyn levels, and increases effector T cell proliferation in vitro (23, 24, 26, 31, 32, 35). The 1-MT-D isoform, Indoximod, is an IDO inhibitor which exhibits a range of oral bioavailability across species, a good safety profile and the ability to slow tumor growth in preclinical studies (44, 45). In clinical trials, Indoximod has been tested as a monotherapy and in combination with chemotherapy agents where efficacy has been identified but primary endpoints have failed to be achieved, resulting in the termination of trials (45). Other IDO inhibitors that were not amongst the studies of this review, including the more potent Epacadostat, have also been trialed as therapies in many cancers but they lacked distinct objective responses (46). Preclinically, it has been suggested that a combination of IDO with checkpoint inhibitors may produce a synergistic effect that restores T cell deficits with cancer (45, 47). The ECHO 301 trial is the latest development exploring this clinically, testing Epacadostat in combination with anti-PD-1 antibody pembrolizumab in metastatic melanoma patients. This trial failed to demonstrate improved progression free survival and OS in the treatment combination compared to pembrolizumab monotherapy and thus terminated early (48).
Failure of the clinical trials to date raises questions as to where IDO inhibition could be incorporated into AML therapy, but it is important to note that although trials have taken place in other cancer types, few have focused on IDO inhibition in AML. Recently, a trial combining Indoximod with standard (7 + 3) chemotherapy was conducted in AML patients (NCT02835729) which has yet to report any findings (49). Results of this study could provide valuable insights into the rationale for continuation of trials investigating the clinical relevance of IDO inhibition therapies in AML. Nevertheless, early-mortality of AML patients with the highest IDO expression may highly likely benefit from IDO-inhibitor drugs as a component of their treatment regimen (22).
The present review is subject to limitations as only English papers could be included and a meta-analysis was not suitable due to the heterogeneity of data. It is also acknowledged that many of the papers came from the same authors and research groups/institutions therefore findings potentially could be subject to bias. The papers included in this review investigated IDO with no delineation of its isoforms or focused entirely on IDO1. It must be noted that IDO has two isoforms, IDO1 and IDO2, which exhibit high sequence homology but exhibit kinetic, functional and expression differences, with IDO2 expression limited to subsets including antigen-presenting cells (50). Current evidence suggests that IDO2 may serve an important role within immune control by supporting IDO1-dependent Treg function as part of the adaptive immune system (51). Therefore, it is important that the contribution of IDO2 in AML pathogenesis relating to Treg abundance and prognosis is explored in further studies.
To the best of our knowledge, this is the first systematic review highlighting the importance of IDO in AML progression and prognosis. IDO can be expressed in various cell types involved in AML pathogenesis and this expression can be either inducible or constitutive, differing between patients. The review identified the potential of IDO and Kyn levels as prospective prognostic markers as IDO expression correlates with worse patient outcomes. However, more robust CD4 Treg (CD4+CD25+CD127low/−) markers may be required to delineate the contribution of bonafide Treg within the tumor microenvironment where IDO activity is functionally relevant. Furthermore, the role of other similar immunosuppressive enzymes, such as Tryptophan 2,3-dioxygenase may offer novel insights into the contribution of immunomodulatory enzymatic cascade in AML pathogenesis and prognosis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GW performed the search, screening and data extraction, interpreted the results, and wrote the manuscript. PK contributed to the results interpretation and writing the manuscript. LD designed the study, checked the accuracy of the data extraction, and contributed to the results interpretation and writing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- IDO
indoleamine 2,3-dioxygenase
- AML
acute myeloid leukemia
- Treg
regulatory T cell
- Trp
Tryptophan
- Kyn
Kynurenine
- FAB
French-American-British
- CR
complete remission
- DC
dendritic cell
- OS
overall survival
- 1-MT
1-methyl-tryptophan.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.651687/full#supplementary-material
References
- 1.Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. (2017) 31:63–76. 10.1016/j.blre.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 2.Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. (2006) 107:2099–107. 10.1002/cncr.22233 [DOI] [PubMed] [Google Scholar]
- 3.Brown G, Marcinkowska E. Acute myeloid leukaemia: new targets and therapies. Int J Mol Sci. (2017) 18:2577. 10.3390/ijms18122577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis JR, Benjamin DJ, Jonas BA. New and emerging therapies for acute myeloid leukaemia. J Invest Med. (2018) 66:1088. 10.1136/jim-2018-000807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. (2013) 381:484–95. 10.1016/S0140-6736(12)61727-9 [DOI] [PubMed] [Google Scholar]
- 6.Reilly JT. Pathogenesis of acute myeloid leukaemia and inv(16)(p13;q22): a paradigm for understanding leukaemogenesis? Br J Haematol. (2005) 128:18–34. 10.1111/j.1365-2141.2004.05236.x [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, Burnett A, Lo-Coco F, Löwenberg B. FLT3 inhibition as a targeted therapy for acute myeloid leukemia. Curr Opin Oncol. (2009) 21:594–600. 10.1097/CCO.0b013e32833118fd [DOI] [PubMed] [Google Scholar]
- 8.Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. (2017) 16:991–1001. 10.1158/1535-7163.Mct-16-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. (2006) 24:4499–506. 10.1200/jco.2006.06.5037 [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Crit Rev Oncol Hematol. (2016) 103:62–77. 10.1016/j.critrevonc.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 12.Arandi N, Ramzi M, Safaei F, Monabati A. Overexpression of indoleamine 2,3-dioxygenase correlates with regulatory T cell phenotype in acute myeloid leukemia patients with normal karyotype. Blood Res. (2018) 53:294–8. 10.5045/br.2018.53.4.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol. (2012) 42:1932–7. 10.1002/eji.201242572 [DOI] [PubMed] [Google Scholar]
- 14.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. (2003) 24:242–8. 10.1016/S1471-4906(03)00072-3 [DOI] [PubMed] [Google Scholar]
- 15.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. (2012) 72:5435. 10.1158/0008-5472.CAN-12-0569 [DOI] [PubMed] [Google Scholar]
- 16.Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. (2018) 96:21–33. 10.1111/imcb.1003 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour I, Zayed RA, Said F, Latif LA. Indoleamine 2,3-dioxygenase and regulatory T cells in acute myeloid leukemia. Hematology. (2016) 21:447–53. 10.1080/10245332.2015.1106814 [DOI] [PubMed] [Google Scholar]
- 19.Folgiero V, Goffredo BM, Filippini P, Masetti R, Bonanno G, Caruso R, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget. (2014) 5:2052–64. 10.18632/oncotarget.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuno K, Hara T, Tsurumi H, Shibata Y, Mabuchi R, Nakamura N, et al. Expression of indoleamine 2,3-dioxygenase in leukemic cells indicates an unfavorable prognosis in acute myeloid leukemia patients with intermediate-risk cytogenetics. Leuk Lymphoma. (2015) 56:1398–405. 10.3109/10428194.2014.953150 [DOI] [PubMed] [Google Scholar]
- 21.Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients' sera by HPLC and is inducible by IFN-γ. Leuk Res. (2009) 33:490–4. 10.1016/j.leukres.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 22.Mangaonkar A, Mondal AK, Fulzule S, Pundkar C, Park EJ, Jillella A, et al. A novel immunohistochemical score to predict early mortality in acute myeloid leukemia patients based on indoleamine 2,3 dioxygenase expression. Sci Rep. (2017) 7:12892. 10.1038/s41598-017-12940-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Kholy NM, Sallam MM, Ahmed MB, Sallam RM, Asfour IA, Hammouda JA, et al. Expression of indoleamine 2,3-dioxygenase in acute myeloid leukemia and the effect of its inhibition on cultured leukemia blast cells. Med Oncol. (2011) 28:270–8. 10.1007/s12032-010-9459-6 [DOI] [PubMed] [Google Scholar]
- 24.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. (2006) 109:2871–7. 10.1182/blood-2006-07-036863 [DOI] [PubMed] [Google Scholar]
- 25.Mabuchi R, Hara T, Matsumoto T, Shibata Y, Nakamura N, Nakamura H, et al. High serum concentration of L-kynurenine predicts unfavorable outcomes in patients with acute myeloid leukemia. Leuk Lymphoma. (2016) 57:92–8. 10.3109/10428194.2015.1041388 [DOI] [PubMed] [Google Scholar]
- 26.Curti A, Trabanelli S, Onofri C, Aluigi M, Salvestrini V, Ocadlikova D, et al. Indoleamine 2,3-dioxygenase-expressing leukemic dendritic cells impair a leukemia-specific immune response by inducing potent T regulatory cells. Haematologica. (2010) 95:2022–30. 10.3324/haematol.2010.025924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T, Matsumoto T, Shibata Y, Nakamura N, Nakamura H, Ninomiya S, et al. Prognostic value of the combination of serum l-kynurenine level and indoleamine 2,3-dioxygenase mRNA expression in acute myeloid leukemia. Leuk Lymphoma. (2016) 57:2208–11. 10.3109/10428194.2015.1128541 [DOI] [PubMed] [Google Scholar]
- 28.Martine EDC, Arjan AvdL, Corine JH, Jeroen JWMJ, Adri Z, Ruud D, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. (2008) 93:1894–8. 10.3324/haematol.13112 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Tan G, Wang H, Chen P, Hao J, Wang Y. Identification of novel serum biomarker for the detection of acute myeloid leukemia based on liquid chromatography-mass spectrometry. J Pharma Biomed Anal. (2019) 166:357–63. 10.1016/j.jpba.2019.01.022 [DOI] [PubMed] [Google Scholar]
- 30.Chen XL, Guo JM, Zhang Y, Niu XN, Pei XH, Zhang WH. Expression of indoleamine 2,3-dioxygenase in acute leukemic cells and the clinical significance. Int J Clin Exp Med. (2016) 9:8605–9. [Google Scholar]
- 31.Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia. (2007) 21:353–5. 10.1038/sj.leu.2404485 [DOI] [PubMed] [Google Scholar]
- 32.Trabanelli S, Lecciso M, Salvestrini V, Cavo M, Očadlíková D, Lemoli RM, et al. PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response. J Immunol Res. (2015) 2015:253191. 10.1155/2015/253191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folgiero V, Cifaldi L, Li Pira G, Goffredo BM, Vinti L, Locatelli F. TIM-3/Gal-9 interaction induces IFNγ-dependent IDO1 expression in acute myeloid leukemia blast cells. J Hematol Oncol. (2015) 8:36. 10.1186/s13045-015-0134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou FL, Zhang WG, Wei YC, Meng S, Bai GG, Wang BY, et al. Involvement of oxidative stress in the relapse of acute myeloid leukemia. J Biol Chem. (2010) 285:15010–5. 10.1074/jbc.M110.103713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lecciso M, Ocadlikova D, Sangaletti S, Trabanelli S, De Marchi E, Orioli E, et al. ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front Immunol. (2017) 8:1918. 10.3389/fimmu.2017.01918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iachininoto MG, Nuzzolo ER, Bonanno G, Mariotti A, Procoli A, Locatelli F, et al. Cyclooxygenase-2 (COX-2) inhibition constrains indoleamine 2,3-dioxygenase 1 (IDO1) activity in acute myeloid leukaemia cells. Molecules. (2013) 18:10132–45. 10.3390/molecules180910132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Z, Yue L, Shi J, Shao M, Wu T. Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J Cancer. (2019) 10:2771–82. 10.7150/jca.31727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Z, Hu K, Tian L, Dai Y, Pang Y, Cui W, et al. Clinical and biological implications of mutational spectrum in acute myeloid leukemia of FAB subtypes M4 and M5. Cancer Gene Ther. (2018) 25:77–83. 10.1038/s41417-018-0013-6 [DOI] [PubMed] [Google Scholar]
- 39.Facciabene A, Motz GT, Coukos G. T-Regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. (2012) 72:2162. 10.1158/0008-5472.CAN-11-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. (2013) 13:461–7. 10.1038/nri3464 [DOI] [PubMed] [Google Scholar]
- 41.Yu N, Li X, Song W, Li D, Yu D, Zeng X, et al. CD4+CD25+CD127low/− T cells: a more specific Treg population in human peripheral blood. Inflammation. (2012) 35:1773–80. 10.1007/s10753-012-9496-8 [DOI] [PubMed] [Google Scholar]
- 42.Dahal LN, Hall LS, Barker RN, Ward FJ. Indoleamine 2,3 dioxygenase contributes to transferable tolerance in rat red blood cell inducible model of experimental autoimmune haemolytic anaemia. Clin Exp Immunol. (2013) 173:58–66. 10.1111/cei.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. (1998) 281:1191. 10.1126/science.281.5380.1191 [DOI] [PubMed] [Google Scholar]
- 44.Jia L, Schweikart K, Tomaszewski J, Page JG, Noker PE, Buhrow SA, et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: absence of toxicity due to saturating absorption. Food Chem Toxicol. (2008) 46:203–11. 10.1016/j.fct.2007.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yentz S, Smith D. Indoleamine 2,3-dioxygenase (IDO) inhibition as a strategy to augment cancer immunotherapy. BioDrugs. (2018) 32:311–7. 10.1007/s40259-018-0291-4 [DOI] [PubMed] [Google Scholar]
- 46.Günther J, Däbritz J, Wirthgen E. Limitations and off-target effects of tryptophan-related IDO inhibitors in cancer treatment. Front Immunol. (2019) 10:1801. 10.3389/fimmu.2019.01801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. (2013) 210:1389–402. 10.1084/jem.20130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. (2019) 20:1083–97. 10.1016/S1470-2045(19)30274-8 [DOI] [PubMed] [Google Scholar]
- 49.Institute NC . A Study of Indoximod in Combination With (7+3) Chemotherapy in Patients With Newly Diagnosed Acute Myeloid Leukemia. (2016). Available online at: https://www.clinicaltrials.gov/ct2/show/results/NCT02835729?term=indoximod&cond=acute$+$myeloid$+$leukemia&draw=2&rank=1&view=results
- 50.Merlo LM, Mandik-Nayak L. IDO2: a pathogenic mediator of inflammatory autoimmunity. Clin Med Insights Pathol. (2016) 9:21–8. 10.4137/CPath.S39930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LM, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. (2014) 26:357–67. 10.1093/intimm/dxt073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.