Abstract
Coccidiosis is an important intestinal parasitic disease that causes great economic losses to the global poultry production industry. Circular RNAs (circRNAs) are long non-coding RNAs that play important roles in various infectious diseases and inflammatory responses. However, the expression profiles and functions of circRNAs during Eimeria tenella (E. tenella) infection remain unclear. In this study, high-throughput sequencing was carried out to detect circRNAs in chicken cecal tissues from the control (JC), resistant (JR), and susceptible (JS) groups on day 4.5 postinfection (pi), respectively. A total of 104 circRNAs were differentially expressed, including 47 circRNAs between the JS and JC groups, 38 between the JR and JS groups, and 19 between the JR and JC groups. Functional analyses indicated that these differentially expressed circRNAs were involved in pathways related to E. tenella infection; the adaptive immune response was enriched in the JS vs JC group, the NF-kappa B signaling and natural killer cell-mediated cytotoxicity pathways were enriched in the JS vs JC and JR vs JC groups, while the B cell receptor signaling pathway was enriched in only the JR vs JC group. Moreover, the coexpression network of differentially expressed circRNAs and mRNAs suggested that circRNA2202 and circRNA0759 associated with DTX1 in the JS vs JC group, circRNA4338 associated with VPREB3 and CXCL13L3 in the JR vs JC group, and circRNA2612 associated with IL8L1 and F2RL2 in the JR vs JS group were involved in the immune response upon E. tenella infection. In conclusion, our results provide valuable information on the circRNAs involved in the progression of chicken E. tenella infection and advance our understanding of the circRNA regulatory mechanisms of host resistance and susceptibility to E. tenella infection in chickens.
Keywords: chicken, circRNAs, E. tenella, cecum, expression profiles
Introduction
Avian coccidiosis is an intestinal parasitic disease caused by Eimeria protozoa and hinders the development of the global poultry industry (Shirley and Lillehoj, 2012). The global poultry industry has annual economic losses of up to $3 billion due to coccidiosis. Seriously, the subclinical economic losses caused by infected chickens are even more severe, including the impact on weight gain and egg production (Sharman et al., 2010; Blake and Tomley, 2014). To date, there are seven species of Eimeria that have been recognized worldwide, and each genus of coccidia is parasitic in a specific region. E. tenella parasitizes the chicken cecum, mainly causing bleeding of the cecum epithelium and bloody stools (Yu et al., 2019). In addition, due to the high tolerance and survival rate of coccidia oocysts in the environment, E. tenella is common in the poultry industry. At present, the prevention strategies for coccidiosis are mainly divided into three types: anticoccidial drugs, vaccines, and strict feeding hygiene management (Swaggerty et al., 2015). Drug resistance issues and violations of antibiotic bans have attracted much attention (Xie et al., 2020). In this regard, selecting breeds and lines with natural resistance to coccidiosis based on the genetic variability of chickens and genes related to resistance is an effective, long-term prevention strategy (Kim et al., 2009). Genetically distinct lines of broilers have shown differences in resistance or susceptibility to Salmonella enteritidis (Swaggerty et al., 2005), Campylobacter jejuni (Li et al., 2008), and E. tenella (Swaggerty et al., 2011) infections. Broiler breeders with an efficient innate immune response are more resistant to E. tenella (Swaggerty et al., 2011), and a novel selected mean based on a higher phenotype of some pro-inflammatory mediators was formed to produce broilers that are naturally more resistant to E. tenella (Swaggerty et al., 2015).
Circular RNAs (circRNAs) are a special type of endogenous non-coding RNA that are widespread in animals and plants (Li et al., 2019). Compared to linear RNA, circRNA is formed through the back-splicing of covalently bound 3′-and 5′-ends. Therefore, they are more highly conserved and stable (Jeck et al., 2013). Previous studies have shown that circRNAs can play a regulatory role as miRNA sponges and can also act as posttranscriptional regulators and templates for translating proteins to perform biological functions (Hansen et al., 2013; Jeck et al., 2013; Memczak et al., 2013). Increasing evidence has shown that circRNAs play an important regulatory role in pathological processes such as Alzheimer’s disease, cancer, and viral infections (Li et al., 2015; Li et al., 2017; Cervera-Carles et al., 2020). However, the expression and regulatory mechanisms of circRNAs during E. tenella infection are unclear. In this study, we first investigated the expression profile and function of circRNAs in cecal tissues of broilers infected with E. tenella in different groups (the control, resistant, and susceptible groups) to understand the complex mechanisms of resistance and susceptibility to E. tenella mediated by circRNAs.
Materials and Methods
Samples and Challenge
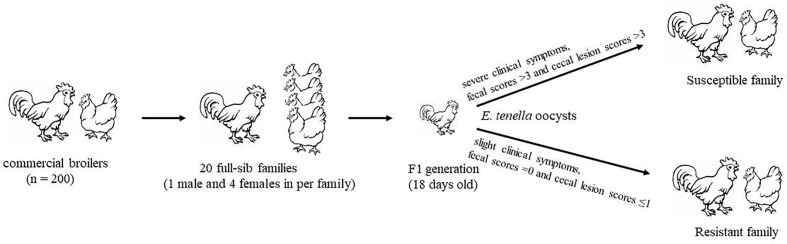
A total of 20 full-sib family broilers were selected from 200 Jinghai Yellow Chickens at Jinghai Yellow Chicken Resource Farm in Jiangsu Province, China. One male and four female parents in each family were artificially fertilized to produce the F1 generation (the number of F1 generations of each family was not less than 10). The offspring of each family were raised separately in oocyst-free cages and fed antibiotic-free feed and water. At 18 days old, F1 individuals from each family were randomly divided into two groups: the treatment group and the control group. Each chicken in the treatment group was orally infected with the same dose of sporulated E. tenella oocysts (3.5 × 104 oocysts/bird). In this study, the clinical symptoms (emaciation, drooping wings, and dying death), fecal scores (Morehouse and Baron, 1970), and cecal lesion scores (Johnson and Reid, 1970) were used together to discriminate susceptible (JS, severe clinical symptoms, fecal scores >3, and cecal lesion scores >3) from resistant (JR, slight clinical symptoms, fecal scores = 0, and cecal lesion scores ≤1) birds. Thus, the most resistant and most susceptible families were selected for subsequent sequencing ( Figure 1 ). Each chicken in the control group of the resistant family received the same amount of normal saline (JC).
Figure 1.
Workflow of sample selection.
Parasite oocysts were collected from the Department of Parasitology, College of Veterinary Medicine, Yangzhou University (Jiao et al., 2018). On the 4.5th day postinfection [the point time to identify the resistance and susceptibility chickens according to whether cecum bleeding or not (Rose and Hesketh, 1976)], the cecal tissues of the three broilers in the JS, JR, and JC groups were collected, snap-frozen, and preserved at −80°C for later use. All animal protocols were approved by the Animal Welfare Committee of Yangzhou University, and all efforts were made to minimize the suffering of the chickens.
Library Construction and High‐Throughput Sequencing Analysis
Total RNA from cecal samples in each group was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The purity of RNAs was checked using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). The concentration and integrity of RNAs were determined using the Qubit® RNA Assay Kit in Qubit® 2.0 Flurometer (Life Technologies, CA, USA) and Bioanalyzer 2100 system (Agilent Technologies, CA, USA), respectively. The quality of the RNA samples met the experimental requirements (RIN> = 7 28S/18S> = 0.7) and were used for RNA sequencing.
Ribosomal RNA was removed using the Ribo-Zero Gold rRNA Removal Kit (Illumina), and the remaining RNA was fragmented by a fragmentation reagent. Then, the libraries were constructed using TruSeq Stranded Total RNA with Ribo-Zero Gold (Illumina, Cat. no. RS-122-2301) according to the manufacturer’s instructions. Whole transcriptome sequencing, including circRNA sequencing and mRNA sequencing, was carried out on an Illumina HiSeq 2500 (OE Biotech, Shanghai, China), and the reading length was 2 × 150 BP (pe150).
Differentially Expressed circRNA and mRNA Analysis
The clean reads were aligned to a reference genome using HISAT2 (Kim et al., 2015). Then, CIRI (v2.0.3) was employed to detect and identify the circRNAs (Gao et al., 2015). The expression of circRNAs was calculated using spliced reads per million mapped reads (RPM). For mRNAs, Cufflinks (Trapnell et al., 2010) was used to calculate the fragments per kilobase of transcript per million mapped reads (FPKM) value (Roberts et al., 2011) of expression of each gene, and the read counts of each gene were obtained by HTSeq-count (Anders et al., 2015). The DESeq (2012) R package was also used to perform differential expression analysis. Finally, the differentially expressed circRNAs and mRNAs were screened based on fold change (FC) > 2, and the P-value was <0.05.
CircRNA Functional Analysis
Gene Ontology (GO) analysis was performed to determine the basic functions of the host genes of differentially expressed circRNAs, including three classes: biological process (BP), cellular component (CC), and molecular function (MF). The Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to analyze the functional pathways of the host genes of the circRNAs. GO terms and KEGG pathways were considered to be significantly enriched based on a P-value <0.05.
Construction of the circRNA-mRNA Coexpression Network
We used the Pearson correlation coefficient (PCC) to calculate the correlation between differentially expressed circRNAs and differentially expressed mRNAs and constructed a coexpression network. CircRNA-mRNA interaction pairs were extracted with the value of PCC ≥0.8 and P-value <0.05, and the top 100 coexpression of circRNA-mRNA network map was then constructed using the R network package.
Real-Time PCR
Total RNA was extracted from original cecal tissues and used to synthesize cDNA using an RT-PCR Kit (TaKaRa Biotech, Dalian). Seven circRNAs were selected to verify the reliability of RNA-Seq, including six circRNAs, and β-actin was used as an internal reference gene. Primer sequences ( Table 1 ) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Then, qRT-PCR was performed using SYBR Green (TaKaRa Biotech, Dalian). The reactions were carried out as follows: initial denaturation at 94°C for 30 s, followed by 40 cycles of denaturation for 5 s at 95°C and annealing for 34 s at 60°C. Data at multiple points were collected for dissolution curve analysis, and the procedure was as follows: 95°C for 15 s, 60°C for 1 min, 95°C for 15 s, and 60°C for 15 s. Each sample was analyzed in triplicate. The qRT-PCR results were analyzed using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Primer sequences for differentially expressed circRNAs.
| circRNAs | Primer sequences (5’-3’) | Length/bp | |
|---|---|---|---|
| β-actin | F: CCTGAACCTCTCATTGCCA R: GAGAAATTGTGCGTGACATCA |
152 | |
| circRNA_4375|Chr31:5251588_5267684_- | F: CTTAGAAACAACGAGGGACGA R: TCAGGACTCACAGTGATGGGA |
183 | |
| circRNA_6530|ChrZ:38536561_38537174_+ | F: AGTCACACCTCCCGATCAAGG R: GGTGCGGAGTGAGAGGTACA |
121 | |
| circRNA_6300|ChrW:4831150_4838596_+ | F: TGCAGACCAGATACGGGGTA R: CCCAAGCACTGGAAGGGCTA |
136 | |
| circRNA_0759|Chr1:130466378_130493459_- | F: GGACATTGCTATGTTTGCAGG R: ACATCACCACCTCTCGAGTTC |
162 | |
| circRNA_4340|Chr31:2362269_2405166_- | F: CCATAAAAGCACCGGCTCTC R: CATATTCCAACCCCTTGCCG |
250 | |
| circRNA_1909|Chr15:8218348_8230982_- | F: GGGTATAAAAGGGCATCGAG R: AGCCATAACCATAGCCACTG |
227 | |
bp, base pair; circRNA, circular RNA; F, forward; R, reverse.
Results
Identification of circRNAs in Cecal Tissues by RNA-Seq
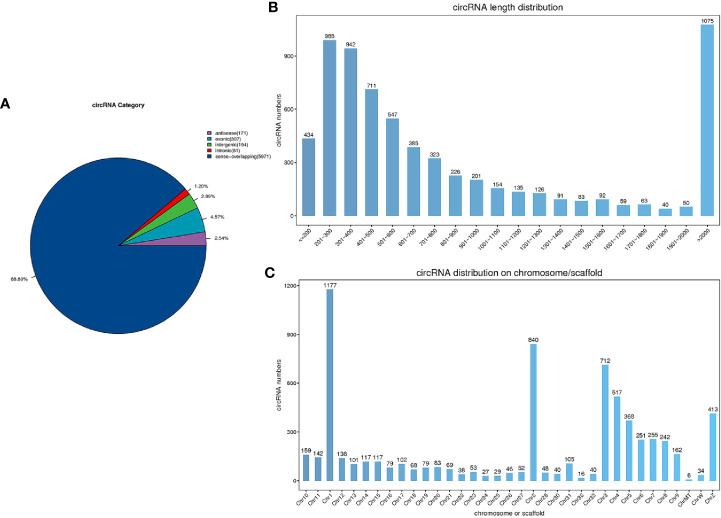
We obtained 1000.44 M raw reads from nine libraries by Illumina sequencing. After removing adapter reads and low-quality reads, a total of 967.44 M clean reads were used for the identification of circRNAs. The accuracy of base recognition (Q30) in each sample was greater than 95% ( Table 2 ). A total of 6,725 circRNAs were identified in all nine cecal samples of broilers (JR vs JC, 5,439; JR vs JS, 5,752; JS vs JC, 5,352). The majority of them were sense-overlapping circRNAs (88.80%), while a small proportion (1.20%) of circRNAs contained intronic sequences ( Figure 2A ). In addition, the lengths of these circRNAs were mostly concentrated at 200–2,000 nt ( Figure 2B ), which was consistent with the reported length distribution of circRNAs. Most of the circRNAs were distributed on chromosome 1, followed by distribution on chromosomes 2 and 3 ( Figure 2C ).
Table 2.
Sequencing data and quality parameters.
| Sample | Raw reads | Clean reads | Multiple mapped (%) | Uniquely mapped (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| JC1 | 107.54 M | 104.92 M | 6299668 (6.00) | 93010583 (88.65) | 96.13 | 47.73 |
| JC2 | 109.24 M | 105.23 M | 13308196 (12.65) | 84687626 (80.48) | 95.89 | 53.21 |
| JC3 | 111.36 M | 107.18 M | 8417074 (7.85) | 93783447 (87.50) | 95.86 | 48.32 |
| JR1 | 110.64 M | 107.46 M | 7548912 (7.02) | 94792432 (88.21) | 95.81 | 48.05 |
| JR1 | 102.58 M | 100.09 M | 6540337 (6.53) | 88766317 (88.69) | 96.21 | 47.62 |
| JR1 | 117.57 M | 114.35 M | 9002737 (7.87) | 99731881 (87.22) | 96.01 | 47.96 |
| JS1 | 115.24 M | 110.56 M | 8323574 (7.53) | 97330051 (88.03) | 95.55 | 47.73 |
| JS2 | 110.87 M | 106.66 M | 7483362 (7.02) | 93909225 (88.04) | 95.77 | 47.61 |
| JS3 | 115.40 M | 110.99 M | 9413810 (8.48) | 96283860 (86.75) | 95.77 | 48.21 |
M, the size of the data volume; Q30, the percentage of bases with a Qphred value greater than 30 in Raw base to the total base; GC, the percentage of the total number of G and C in CleanBase to the total number of bases.
Figure 2.
Characteristics of circRNAs in chicken cecal tissues during E. tenella infection. (A) circRNA category. (B) The length distribution of circRNAs. (C) Chromosomal distribution of circRNAs.
Differentially Expressed circRNAs in Chicken Cecal Tissues During E. tenella Infection
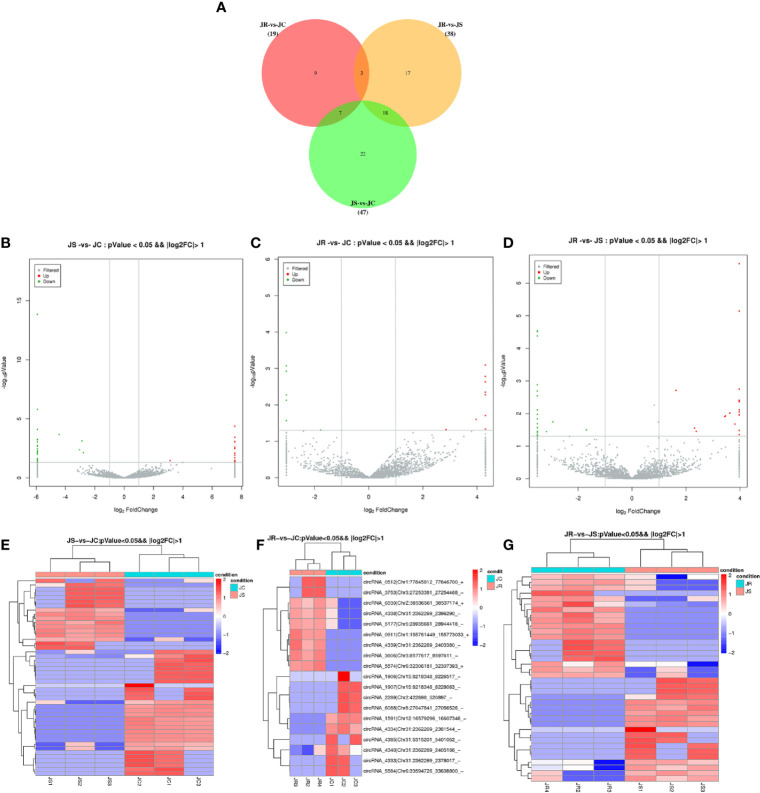
To further investigate the regulatory role of these circRNAs in cecal tissues challenged with E. tenella, we analyzed the differential expression of circRNAs from circRNA expression profiling based on a fold change (FC) > 2 and a P-value <0.05. A total of 47 (17 upregulated and 30 downregulated), 38 (19 upregulated and 19 downregulated), and 19 (9 upregulated and 10 downregulated) differentially expressed circRNAs were identified in the JS vs JC, JR vs JS, and JR vs JC groups, respectively ( Figures 3B–D , Supplementary Table 1 ). Three circRNAs showed differential expression in the JR vs JS and JR vs JC groups, seven circRNAs showed differential expression in the JS vs JC and JR vs JC groups, and 18 circRNAs showed differential expression in the JR vs JS and JS vs JC groups ( Figure 3A ). Hierarchical clustering analysis showed that the expression patterns of significantly differentially expressed circRNAs were different between the control group and the treatment group ( Figures 3E–G ).
Figure 3.
Expression profiles of differentially expressed circRNAs in chicken cecal tissues of different groups during E. tenella infection. (A) Venn diagram of differentially expressed circRNAs. (B–D) Volcano plots of differentially expressed circRNAs in the JS vs JC group (B), JR vs JC group (C), and JR vs JS group (D). (E–G) Hierarchical clustering plots of differentially expressed circRNAs in the JS vs JC group (E), JR vs JC group (F), and JR vs JS group (G).
GO Enrichment and KEGG Pathway Analyses of Differentially Expressed circRNAs
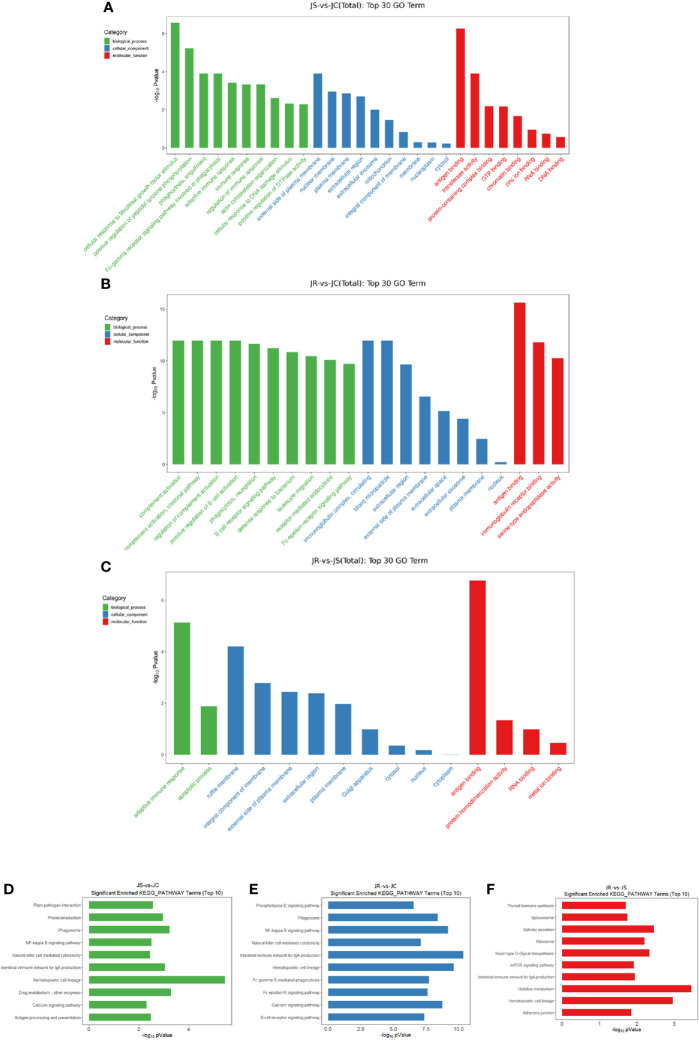
Because most circRNAs are derived from middle exons of protein-coding genes, the processing of circRNAs can affect splicing of their precursor transcripts, resulting in changes in the expression of linear host genes (Li et al., 2018). To explore the functions of differentially expressed circRNAs, host genes of these circRNAs were used for GO and KEGG enrichment analyses. The list of host genes is shown in Supplementary Table 1 . The top 30 significantly enriched GO terms are shown in Figure 3 ( Figures 4A–C , Supplementary Table 2 ). Biological processes, adaptive immune response, immune response, and regulation of immune response were enriched in the JS vs JC group. Positive regulation of B cell activation and the B cell receptor signaling pathway were enriched in the JR vs JC group. The adaptive immune response and apoptotic process were enriched in the JR vs JS group. For the cellular component, the immunoglobulin complex, circulating and blood microparticle were enriched in the JR vs JC group. Regarding molecular function, antigen binding was enriched in the JS vs JC group, antigen binding and immunoglobulin receptor binding were enriched in the JR vs JC group, and antigen binding was enriched in the JR vs JS group.
Figure 4.
Functional enrichment analysis of differentially expressed circRNAs in chicken cecal tissues of different groups during E. tenella infection. (A–C) GO term analysis of differentially expressed circRNAs in the JS vs JC group (A), JR vs JC group (B), and JR vs JS group (C). (D–F) KEGG pathway analysis of differentially expressed circRNAs in the JS vs JC group (D), JR vs JC group (E), and JR vs JS group (F).
Some signaling pathways that are potentially involved in the regulation of E. tenella infection and host resistance were significantly (P < 0.05) enriched in the top 10 KEGG pathways in different groups, such as the NF-kappa B signaling pathway and natural killer cell-mediated cytotoxicity in the JS vs JC and JR vs JC groups, the B cell receptor signaling pathway in the JR vs JC group, the intestinal immune network for IgA production and hematopoietic cell lineage in all three groups ( Figures 4D–F , Supplementary Table 3 ).
Validation of Differentially Expressed circRNAs
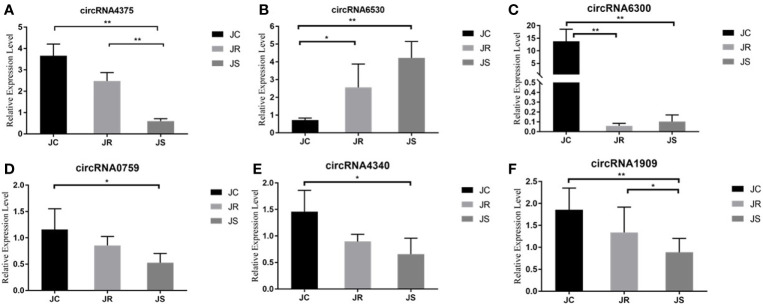
To further verify the reliability of the RNA sequencing results, six circRNAs with high fold changes and a high number of junctions from high-throughput sequencing were randomly selected for qRT-PCR. The qRT-PCR results suggested that circRNA expression using qRT-PCR was consistent with the RNA sequencing results ( Figure 5 ).
Figure 5.
Validation of differentially expressed circRNAs in chicken cecal tissues of different groups during E. tenella infection. Six circRNAs are in the following order: circRNA4375 (A), circRNA6530 (B), circRNA6300 (C), circRNA0759 (D), circRNA4340 (E) and circRNA1909 (F). Values are the mean ± SD (n = 3 per group), and qRT-PCR analysis was conducted in triplicate. *P < 0.05, **P < 0.01.
The Interaction Network of Differentially Expressed circRNA-mRNA Networks
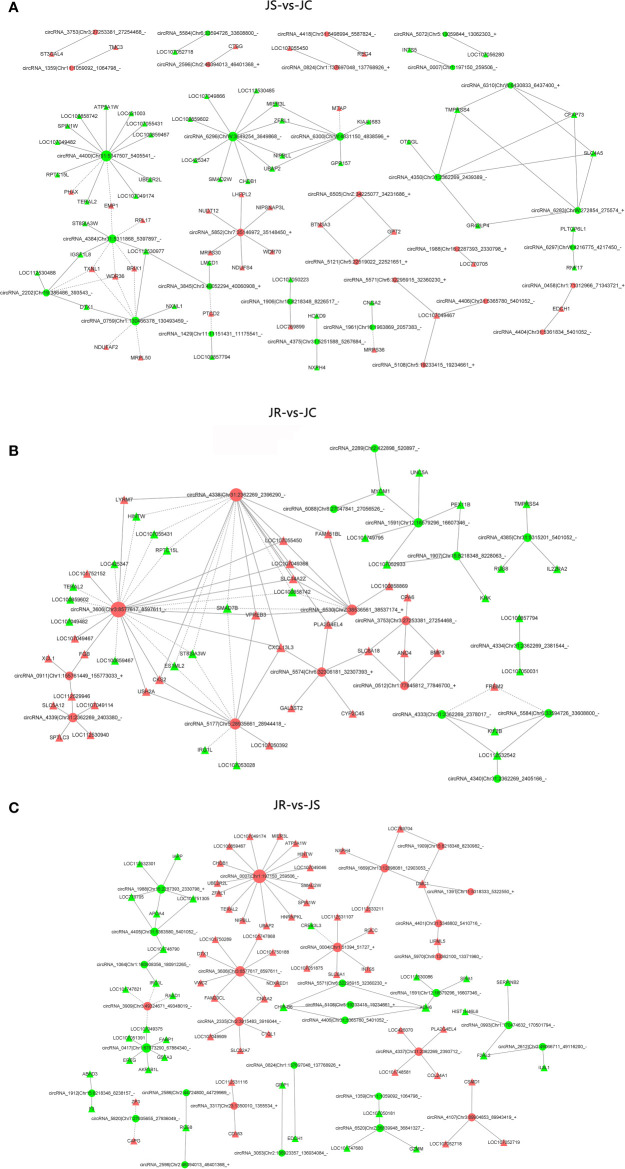
To reveal the function of circRNAs, a coexpression network map between differentially expressed circRNAs and mRNAs was constructed using the P-value of hypergeometric distribution. In the top 100 coexpressed circRNA-mRNA network maps, a total of 32 circRNAs and 74 mRNAs were identified in the JS vs JC group ( Figure 6A , Supplementary Table 4 ). Eighteen circRNAs and 55 mRNAs in the JR vs JC group were coexpressed in the cecums of E. tenella-infected chickens ( Figure 6B , Supplementary Table 5 ), and 30 circRNAs and 79 mRNAs were coexpressed in the JR vs JS group ( Figure 6C , Supplementary Table 6 ). Among them, in the JS vs JC group, circRNA2202 and circRNA0759 were positively correlated with DTX1 expression. Moreover, circRNA6300 was positively correlated with the expression of RORC and CD101. CircRNA4338 was coexpressed with VPREB3 and CXCL13L3 in the JR vs JC group, and circRNA2612 was coexpressed with IL8L1 and F2RL2 in the JR vs JS group.
Figure 6.
The coexpression network of differentially expressed circRNAs-mRNAs. (A) JS vs JC group. (B) JR vs JC group. (C) JR vs JS group. The red and green represent upregulated and downregulated circRNAs, respectively, while the circles represent the circRNAs, and the triangles represent the mRNAs.
Discussion
Research on the selection of broiler chicken breeds or strains naturally resistant to coccidiosis has been on the way. Johnson and Edgar (1982) reported early that the genetic variability of chickens could affect the resistance and susceptibility to acute cecal coccidiosis (ACC) and confirmed that resistant and susceptible lines of Auburn Strain Leghorn resulted in a sixfold difference in the ACC mortality rate. Later, Quist et al. (1993) showed that coccidia infection in the host cells of the Auburn Strain Leghorn-resistant line was more serious than in the host cells of the resistant line in vitro. In addition, Marek’s disease and Necrotizing Enteritis-resistant and -susceptible strains have been reported (Heidari et al., 2020; Pham et al., 2020). Therefore, the resistance of chickens to pathogens are related to genetic variability. Swaggerty et al. (2011) selected broiler lines with strong innate immunity and showed stronger natural resistance to coccidia, as demonstrated by lower lesion scores and higher weight gain. The coccidial resistance of chickens also depend on the immune capacity of the host. In this study, the chicken groups that are naturally resistant and susceptible to E. tenella were selected from different families to explore the molecular mechanism of the differences between susceptible groups and resistant groups.
CircRNAs not only participate in the posttranscriptional regulation of genes functioning as miRNA molecular sponges but also interact with RNA binding proteins (Du et al., 2016). Although the functions of most circRNAs remain largely unexplored, as circRNA functions are being gradually revealed, it is becoming increasingly important to study the circRNA mechanisms in infection and diseases. In previous studies, the expression pattern and regulation of circRNA have been described in porcine endemic diarrhea virus (PEDV) (Chen et al., 2019b), bovine viral diarrhea virus (BVDV) (Li et al., 2019), and crucian carp Carassius auratus gibelio (Hu et al., 2019). In poultry, Chen et al. (2019a) found that five differentially expressed circRNAs were upregulated in chicken ammonia poisoning. Liu et al. (2020) reported that 27 differentially expressed circRNAs were involved in the immune response to infectious bursal infection in chickens. Fan et al. (2020) performed transcriptome sequencing on the small intestine tissues of chickens infected with E. necatrix. and showed that 13 differentially expressed circRNAs, such as circRNA2673, circRNA3106, and circRNA1579, played an important role in the process of E. necatrix infection. In this study, E. tenella infection induced the significant alteration of these circRNAs expression. Further, we found that the functional enrichment of these differentially expressed circRNAs were significantly enriched in the adaptive immune response and the B cell receptor signaling pathways. Adaptive immunity is specific and regulates the antigen-specific immune responses to prevent colonization and growth of the pathogen inside the host and the adaptive immune response plays a dominant role in anticoccidial protective immunity (Kim et al., 2019). B cells are the important components of adaptive immune responses in birds (Girard et al., 1997). When coccidia begins to infect the host, B lymphocytes can express antigen-specific surface immunoglobulin molecules that bind to the antigen, and then B cells with the same surface immunoglobulin continue to proliferate and differentiate to participate in host immunity (Lillehoj, 1998). In addition, the natural killer cell-mediated cytotoxicity was the pathway that differentially expressed circRNAs significantly were enriched. Studies have shown that natural killer (NK) cells are involved in defense against invasion of the gut mucosa by coccidia and associated with the innate immunity of coccidial infection (Lillehoj, 1998; Min et al., 2013). Additionally, this study also revealed the potential role of NF-kappa B signaling and the intestinal immune network for IgA production pathways in the process of coccidia infection. These results indicated that these circRNAs are involved in the coccidial infection through affecting chicken innate and adaptive immune response.
To date, a large number of circRNAs have been identified in various species. However, the ways of most circRNAs function are various. Construction of a circRNA-mRNA coexpression network is an effective method to predict circRNA function. In this study, we analyzed the network of circRNA-mRNA coexpression. In the JS vs JC group, circRNA2202 and circRNA0759 were positively correlated with the expression of Deltex1 (DTX1). DTX1 is an important Notch signaling target and participates in T cell anergy (Matsuno et al., 1998). DTX1 lack enhances T cell activation, enhances autoantibody production, and promotes inflammation (Hsiao et al., 2009). Moreover, DTX1 controls the stability of Tregs at another level in vivo by maintaining the protein expression of Foxp3 in Tregs (Hsiao et al., 2015). Therefore, circRNA2202 and circRNA0759 would participate in the immune response of coccidia infection by regulating the DTXI gene. In the JR vs JC group, circRNA4338 was positively coexpressed with VPREB3 and CXCL13L3. CXCL13 is a member of the chemokine superfamily CXC subtype and is also known as a B cell chemokine 1 or B lymphocyte chemotactic agent (Gunn et al., 1998). VPREB3 belongs to the immunoglobulin (Ig) superfamily and regulates the assembly of the light chain proteins VPREB1 and IGLL1 to form the pre-B-cell receptor (pre-BCR) (Rosnet et al., 2004). The complete pre-BCR plays a vital role in the development of mammalian B lymphocytes (Rodig et al., 2010). Felizola et al. (2015) showed that VPREB3 is expressed in B cell differentiation and mature B lymphocyte subsets. Therefore, circRNA4338 would be involved in the immune response to coccidia infection. In the JR vs JS group, circRNA2612 was coexpressed with IL8L1 and F2RL2. Interleukin-8 (IL-8), known as chemokine CX-CL8 (CXCL8), is a proinflammatory factor closely related to the occurrence and development of various inflammatory diseases. IL-8 exerts various biological functions by binding to the receptors CXCR1 and CXCR2, including pro-inflammatory cell chemotaxis, inducing neutrophils to release lysosomal enzymes to eliminate pathogens (Coussens and Werb, 2002), activating immune responses and promoting infected tissue healing (Wang et al., 2020). As a G-protein-coupled protease-activated receptor, F2RL2 plays an important role in the coagulation cascade (Kaufmann and Hollenberg, 2012). Research by Liu et al. (2019) showed that the expression level of F2RL2 was downregulated during infection with BVDV, suggesting that the complement system may play a key role in BVDV infection. In this study, circRNA2612 would participate in the immune response of the chicken host against E. tenella infection by associating with IL8L1 and F2RL2. At present, there are related reports on the functional verification of circRNAs involved in disease and the inflammatory response through regulatory genes (Ma et al., 2018; Zhang et al., 2019). However, few studies have been reported on the functional test of circRNA regulatory genes involved in the immune response to coccidiosis. Therefore, functional verification experiments on the screened circRNAs in vivo and in vitro are the next research work of our group.
In conclusion, the results of this study demonstrate for the first time that circRNAs are differentially expressed in the cecal tissues of chickens infected with E. tenella and may be involved in host responses to E. tenella infection in chickens. These findings also provide valuable data for understanding the circRNA complex mechanisms of E. tenella resistance and susceptibility in chickens. However, the action mechanisms of circRNAs and mRNAs remain to be clarified.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI; PRJNA678759.
Ethics Statement
The animal study was reviewed and approved by the Animal Welfare Committee of Yangzhou University.
Author Contributions
HY and GD designed the study and wrote the manuscript. HY, CM, and QW performed the experiments and analyzed the data. HY and WZ performed the transcriptome data and prepared the figures. TZ, GZ, KX, HS, and JW reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Jiangsu Agricultural Industry Technology System (JATS[2020]437), the National Sci-Tech Support Plan (2014BAD13B02), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the China Agriculture Research System (CARS-41-G23).
Conflict of Interest
HS was employed by Jiangsu Jinghai Poultry Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all members of this work for their advice and technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.628667/full#supplementary-material
References
- Anders S., Pyl P. T., Huber W. (2015). HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. P., Tomley F. M. (2014). Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 30, 12–19. 10.1016/j.pt.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Cervera-Carles L., Dols-Icardo O., Molina-Porcel L., Alcolea D., Cervantes-Gonzalez A., Muñoz-Llahuna L., et al. (2020). Assessing circular RNAs in Alzheimer’s disease and frontotemporal lobar degeneration. Neurobiol. Aging 92, 7–11. 10.1016/j.neurobiolaging.2020.03.017 [DOI] [PubMed] [Google Scholar]
- Chen D., Miao Z., Peng M., Xing H., Zhang H., Teng X. (2019. a). The co-expression of circRNA and mRNA in the thymuses of chickens exposed to ammonia. Ecotoxicol. Environ. Saf. 176, 146–152. 10.1016/j.ecoenv.2019.03.076 [DOI] [PubMed] [Google Scholar]
- Chen J., Wang H., Jin L., Wang L., Huang X., Chen W., et al. (2019. b). Profile analysis of circRNAs induced by porcine endemic diarrhea virus infection in porcine intestinal epithelial cells. Virology 527, 169–179. 10.1016/j.virol.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L. M., Werb Z. (2002). Inflammation and cancer. Nature 420, 860–867. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W. W., Yang W., Liu E., Yang Z., Dhaliwal P., Yang B. B. (2016). Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 44, 2846–2858. 10.1093/nar/gkw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. C., Liu T. L., Wang Y., Wu X. M., Wang Y. X., Lai P., et al. (2020). Genome-wide analysis of differentially expressed profiles of mRNAs, lncRNAs and circRNAs in chickens during Eimeria necatrix infection. Parasites Vectors 13, 167. 10.1186/s13071-020-04047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felizola S. J. A., Katsu K., Ise K., Nakamura Y., Arai Y., Satoh F., et al. (2015). Pre-B lymphocyte protein 3 (VPREB3) expression in the adrenal cortex: precedent for non-immunological roles in normal and neoplastic human tissues. Endocr. Pathol. 26, 119–128. 10.1007/s12022-015-9366-7 [DOI] [PubMed] [Google Scholar]
- Gao Y., Wang J., Zhao F. (2015). CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 16, 4. 10.1186/s13059-014-0571-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard F., Fort G., Yvore P., Quere P. (1997). Kinetics of specific immunoglobulin A, M and G production in the duodenal and caecal mucosa of chickens infected with Eimeria acervulina or Eimeria tenella. Int. J. Parasitol. 27, 803–809. 10.1016/S0020-7519(97)00044-1 [DOI] [PubMed] [Google Scholar]
- Gunn M. D., Ngo V. N., Ansel K. M., Ekland E. H., Cyster J. G., Williams L. T. (1998). A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391, 799–803. 10.1038/35876 [DOI] [PubMed] [Google Scholar]
- Hansen T. B., Jensen T. I., Clausen B. H., Bramsen J. B., Finsen B., Damgaard C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- Heidari M., Zhang L., Zhang H. (2020). MicroRNA profiling in the bursae of Marek’s disease virus-infected resistant and susceptible chicken lines. Genomics 112, 2564–2571. 10.1016/j.ygeno.2020.02.009 [DOI] [PubMed] [Google Scholar]
- Hsiao H. W., Liu W. H., Wang C. J., Lo Y. H., Wu Y. H., Jiang S. T., et al. (2009). Deltex1 is a target of the transcription factor NFAT that promotes T cell energy. Immunity 31, 72–83. 10.1016/j.immuni.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Hsiao H. W., Hsu T. S., Liu W. H., Hsieh W. C., Chou T. F., Wu Y. J., et al. (2015). Deltex1 antagonizes HIF-1α and sustains the stability of regulatory T cells in vivo. Nat. Commun. 6, 6353. 10.1038/ncomms7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Dai Y., Zhang X., Dai K., Liu B., Yuan R., et al. (2019). Identification and characterization of novel type of RNAs, circRNAs in crucian carp Carassius auratus gibelio. Fish Shellfish Immunol. 94, 50–57. 10.1016/j.fsi.2019.08.070 [DOI] [PubMed] [Google Scholar]
- Jeck W. R., Sorrentino J. A., Wang K., Slevin M. K., Burd C. E., Liu J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA (New York N.Y.) 19, 141–157. 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J., Yang Y., Liu M., Li J., Cui Y., Yin S., et al. (2018). Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Vet. Parasitol. 254, 172–177. 10.1016/j.vetpar.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Johnson L. W., Edgar S. A. (1982). Responses to prolonged selection for resistance and susceptibility to acute cecal coccidiosis in the auburn strain single comb white leghorn. Poult. Sci. 61, 2344–2355. 10.3382/ps.0612344 [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. (1970). Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28, 30–36. 10.1016/0014-4894(70)90063-9 [DOI] [PubMed] [Google Scholar]
- Kaufmann R., Hollenberg M. D. (2012). “Proteinase-activated receptors (PARs) and calcium signaling in cancer,” in Calcium Signaling. Ed. Islam M. S. (Springer: Dordrecht, Netherlands; ), 979–1000. [DOI] [PubMed] [Google Scholar]
- Kim D. K., Kim C. H., Lamont S. J., Keeler C. L., Lillehoj H. S. (2009). Gene expression profiles of two B-complex disparate, genetically inbred Fayoumi chicken lines that differ in susceptibility to Eimeria maxima. Poult. Sci. 88, 1565–1579. 10.3382/ps.2009-00012 [DOI] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. H., Chaudhari A. A., Lillehoj H. S. (2019). Involvement of T cell immunity in avian coccidiosis. Front. Immunol. 10, 2732. 10.3389/fimmu.2019.02732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Swaggerty C. L., Kogut M. H., Chiang H., Wang Y., Genovese K. J., et al. (2008). The paternal effect of campylobacter jejuni colonization in ceca in broilers. Poult. Sci. 87, 1742–1747. 10.3382/ps.2008-00136 [DOI] [PubMed] [Google Scholar]
- Li P., Chen S., Chen H., Mo X., Li T., Shao Y., et al. (2015). Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin. Chim. Acta 444, 132–136. 10.1016/j.cca.2015.02.018 [DOI] [PubMed] [Google Scholar]
- Li X., Liu C. X., Xue W., Zhang Y., Jiang S., Yin Q. F., et al. (2017). Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol. Cell 67, 214–227.e217. 10.1016/j.molcel.2017.05.023 [DOI] [PubMed] [Google Scholar]
- Li X., Yang L., Chen L. (2018). The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell. 71, 428–442. 10.1016/j.molcel.2018.06.034 [DOI] [PubMed] [Google Scholar]
- Li C., Li X., Hou X., Ni W., Zhang M., Li H., et al. (2019). Comprehensive analysis of circRNAs expression profiles in different periods of MDBK cells infected with bovine viral diarrhea virus. Res. Vet. Sci. 125, 52–60. 10.1016/j.rvsc.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S. (1998). Role of T lymphocytes and cytokines in coccidiosis. Int. J. Parasitol. 28, 1071–1081. 10.1016/S0020-7519(98)00075-7 [DOI] [PubMed] [Google Scholar]
- Liu C., Liu Y., Liang L., Cui S., Zhang Y. (2019). RNA-Seq based transcriptome analysis during bovine viral diarrhoea virus (BVDV) infection. BMC Genom. 20, 774. 10.1186/s12864-019-6120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Song J., Liu X., Shan H. (2020). Research Note: Circular RNA expressing in different developmental stages of the chicken bursa of Fabricius. Poult. Sci. 99, 3846–3852. 10.1016/j.psj.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Ma X., Zhao X., Zhang Z., Guo J., Guan L., Li J., et al. (2018). Differentially expressed non-coding RNAs induced by transmissible gastroenteritis virus potentially regulate inflammation and NF-κB pathway in porcine intestinal epithelial cell line. BMC Genom. 19, 747. 10.1186/s12864-018-5128-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K., Eastman D., Mitsiades T., Quinn A. M., Carcanciu M. L., Ordentlich P., et al. (1998). Human deltex is a conserved regulator of Notch signalling. Nat. Genet. 19, 74–78. 10.1038/ng0598-74 [DOI] [PubMed] [Google Scholar]
- Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- Min W., Kim W. H., Lillehoj E. P., Lillehoj H. S. (2013). Recent progress in host immunity to avian coccidiosis: IL-17 family cytokines as sentinels of the intestinal mucosa. Dev. Compar. Immunol. 41, 418–428. 10.1016/j.dci.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Morehouse N. F., Baron R. R. (1970). Coccidiosis: evaluation of coccidiostats by mortality, weight gains, and fecal scores. Exp. Parasitol. 28, 25–29. 10.1016/0014-4894(70)90062-7 [DOI] [PubMed] [Google Scholar]
- Pham T. T., Ban J., Hong Y., Lee J., Vu T. H., Truong A. D., et al. (2020). MicroRNA gga-miR-200a-3p modulates immune response via MAPK signaling pathway in chicken afflicted with necrotic enteritis. Vet. Res. 51:8. 10.1186/s13567-020-0736-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist K. L., Taylor R.L. Jr., Johnson L. W., Strout R. G. (1993). Comparative development of Eimeria tenella in primary chick kidney cell cultures derived from coccidia-resistant and -susceptible chickens. Poult. Sci. 72, 82–87. 10.3382/ps.0720082 [DOI] [PubMed] [Google Scholar]
- Roberts A., Trapnell C., Donaghey J., Rinn J. L., Pachter L. (2011). Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 12, R22. 10.1186/gb-2011-12-3-r22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodig S. J., Kutok J. L., Paterson J. C., Nitta H., Zhang W., Chapuy B., et al. (2010). The pre-B-cell receptor associated protein VpreB3 is a useful diagnostic marker for identifying c-MYC translocated lymphomas. Haematologica 95, 2056–2062. 10.3324/haematol.2010.025767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. (1976). Immunity to coccidiosis: stages of the life-cycle of Eimeria maxima which induce, and are affected by, the response of the host. Parasitology 73, 25–37. 10.1017/S0031182000051295 [DOI] [PubMed] [Google Scholar]
- Rosnet O., Blanco-Betancourt C., Grivel K., Richter K., Schiff C. (2004). Binding of free immunoglobulin light chains to VpreB3 inhibits their maturation and secretion in chicken B cells. J. Biol. Chem. 279, 10228–10236. 10.1074/jbc.m312169-a200 [DOI] [PubMed] [Google Scholar]
- Sharman P. A., Smith N. C., Wallach M. G., Katrib M. (2010). Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 32, 590–598. 10.1111/j.1365-3024.2010.01209.x [DOI] [PubMed] [Google Scholar]
- Shirley M. W., Lillehoj H. S. (2012). The long view: a selective review of 40 years of coccidiosis research. Avian Pathol. 41, 111–121. 10.1080/03079457.2012.666338 [DOI] [PubMed] [Google Scholar]
- Swaggerty C. L., Ferro P. J., Pevzner I. Y., Kogut M. H. (2005). Heterophils are associated with resistance to systemic Salmonella enteritidisinfections in genetically distinct chicken lines. FEMS Immunol. Med. Microbiol. 43, 149–154. 10.1016/j.femsim.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Swaggerty C. L., Genovese K. J., He H., Duke S. E., Pevzner I. Y., Kogut M. H. (2011). Broiler breeders with an efficient innate immune response are more resistant to Eimeria tenella. Poult. Sci. 90, 1014–1019. 10.3382/ps.2010-01246 [DOI] [PubMed] [Google Scholar]
- Swaggerty C. L., Pevzner I. Y., Kogut M. H. (2015). Selection for pro-inflammatory mediators produces chickens more resistant to Eimeria tenella. Poult. Sci. 94, 37–42. 10.3382/ps/peu053 [DOI] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., van Baren M. J., et al. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. H., Yu H. L., Zou W. B., Mi C. H., Dai G. J., Zhang T., et al. (2020). Study of the relationship between polymorphisms in the IL-8 gene promoter region and coccidiosis resistance index in Jinghai Yellow Chickens. Genes 11, 476–488. 10.3390/genes11050476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Huang B., Xu L., Zhao Q., Zhu S., Zhao H., et al. (2020). Comparative transcriptome analyses of drug-sensitive and drug-resistant strains of Eimeria tenella by RNA -sequencing. J. Eukaryot. Microbiol. 67, 406–416. 10.1111/jeu.12790 [DOI] [PubMed] [Google Scholar]
- Yu H., Zou W., Xin S., Wang X., Mi C., Dai G., et al. (2019). Association analysis of single nucleotide polymorphisms in the 5’ regulatory region of the IL-6 gene with Eimeria tenella resistance in Jinghai Yellow Chickens. Genes 10, 890–897. 10.3390/genes10110890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang T., Feng R., Huang H., Xia T., Sun C. (2019). CircARF3 alleviates mitophagy-mediated inflammation by targeting miR-103/TRAF3 in mouse adipose tissue. Mol. Ther.: Nucleic Acids 14, 192–203. 10.1016/j.omtn.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI; PRJNA678759.