Abstract
OBJECTIVES:
To develop a prognostic model for hospital admissions over a 1-year period among community-dwelling older adults with self-reported hearing and/or vision impairments based on readily obtainable clinical predictors.
DESIGN:
Retrospective cohort study.
SETTING:
Medicare Current Beneficiary Survey from 1999 to 2006.
PARTICIPANTS:
Community-dwelling Medicare beneficiaries, aged 65 years and older, with self-reported hearing and/or vision impairment (N = 15,999).
MEASUREMENTS:
The primary outcome was any hospital admission over a predefined 1-year study period. Candidate predictors included demographic factors, prior healthcare utilization, comorbidities, functional impairment, and patient-level factors. We analyzed the association of all candidate predictors with any hospital admission over the 1-year study period using multivariable logistic regression. The final model was created using a penalized regression method known as the least absolute shrinkage and selection operator. Model performance was assessed by discrimination (concordance statistic (c-statistic)) and calibration (evaluated graphically). Internal validation was performed via bootstrapping, and results were adjusted for overoptimism.
RESULTS:
Of the 15,999 participants, the mean age was 78 years and 55% were female. A total of 2,567 participants (16.0%) had at least one hospital admission in the 1-year study period. The final model included seven variables independently associated with hospitalization: number of inpatient admissions in the previous year, number of emergency department visits in the previous year, activities of daily living difficulty score, poor self-rated health, and self-reported history of myocardial infarction, stroke, and nonskin cancer. The c-statistic of the final model was 0.717. The optimism-corrected c-statistic after bootstrap internal validation was 0.716. A calibration plot suggested that the model tended to overestimate risk among patients at the highest risk for hospitalization.
CONCLUSION:
This prognostic model can help identify which community-dwelling older adults with sensory impairments are at highest risk for hospitalization and may inform allocation of healthcare resources.
Keywords: sensory impairment, hearing impairment, vision impairment, hospitalization, prediction model
INTRODUCTION
Sensory impairments are highly prevalent in older adults, with around one in nine adults aged 80 years and older reporting hearing and vision impairment combined.1 Hearing and vision impairments alone and in combination are independently associated with numerous adverse health outcomes, including reduced quality of life, higher rates of cognitive impairment, poor physical functioning, and increased risk of hospitalization.1–4 Hospitalization often represents a sentinel event among older adults that precipitates functional decline, which is particularly true among those with sensory impairments who are at high risk of hospital-associated delirium.5 Therefore, promoting interventions, such as treatment of hearing and/or vision loss, that may help to reduce hospitalization is important to prevent these adverse outcomes and promote healthy aging.6 Risk prediction models can guide clinical decision-making and allocation of healthcare resources by providing estimates of which patients are at highest risk for hospitalization. Multiple models have been developed to predict risk of hospital admission among community-dwelling older adults, although none has specifically looked at a population with sensory impairments.7–11 This is largely because there are few prospective cohorts with sufficient information about sensory impairments that also have adequate data on outcomes related to healthcare utilization. To address this issue, we developed a prognostic model for hospital admissions among participants with self-reported hearing and/or vision impairment using data from the Medicare Current Beneficiary Survey (MCBS).
METHODS
Participants
The MCBS is a continuous survey of a nationally representative sample of Medicare beneficiaries that links to healthcare utilization claims data.12 Participants included in this study were community dwelling, aged 65 years and older, and had self-reported hearing and/or vision impairment at the start of the study period. We defined hearing impairment based on two self-reported questions: (1) Which statement best describes your hearing (with a hearing aid, if you use one)? (no trouble, a little trouble, or a lot of trouble) and (2) Do you use a hearing aid (yes, no, or deaf)? If participants reported “a little trouble” or “a lot of trouble” or if they used hearing aids or indicated deafness, they were classified as hearing impaired. Vision impairment was defined based on one self-reported question: “How much trouble do you have with your vision?” (no trouble, little trouble, or a lot of trouble). Participants who reported “little trouble” or “a lot of trouble” were classified as visually impaired. We did not define vision impairment based on use of glasses or contacts because 83% of participants in the MCBS cohort reported using glasses, suggesting poor measure sensitivity. The final cohort included 15,999 participants of a total population of 24,009 participants interviewed between 1999 and 2006.
Measures
We defined periods of 2-year units between the index years of 1999 to 2006, where the first year of the 2-year study units allowed us to look back on certain predictors, such as emergency department (ED) visits and hospital admissions. Our primary outcome was whether the participant had any hospital admission during the second year of the 2-year study units. The primary outcome was verified by Centers for Medicare & Medicaid Services claims codes. Candidate predictors were chosen based on prior research suggesting an association between sensory impairments and hospital admissions.7,10 These included sociodemographic factors (age, sex, race, education, and income), healthcare use (hospital admissions and ED visits in the first year), self-reported comorbidities (myocardial infarction, stroke, diabetes mellitus, nonskin cancer, dementia, osteoporosis, hypertension, emphysema, osteoarthritis, Parkinsonʼs disease, and rheumatoid arthritis), functional impairment (difficulty with activities of daily living (ADLs) and instrumental ADLs), and patient-level factors (barriers to receiving care, satisfaction with health care received, and self-rated health). To measure ADL difficulty, participants were asked questions about having any difficulty doing the following tasks by themselves and without special equipment: eating, toileting, dressing, bathing/showering, walking, and getting in or out of bed/chairs. The number of items that participants had difficulty with were added up to create an ADL difficulty score (range = 0–6). Self-rated health was assessed as a single item by asking participants to compare their health to others of the same age. Responses were classified as fair or poor versus excellent, very good, or good. For additional details on how variables were defined, see Supplementary Appendix S1.
Statistical Analysis
Descriptive statistics comparing participants with sensory impairments who did and did not have a hospital admission were presented as means for continuous variables or frequencies for categorical variables. To develop our prediction model, we analyzed the association of all a priori selected candidate variables with any hospital admission during the second year of the 2-year study unit using multivariable logistic regression.13,14 We then developed a parsimonious model by using a penalized regression method known as the least absolute shrinkage and selection operator (LASSO).15 We used 500 bootstrap samples and included those predictors that were retained in more than 60% of the bootstrapped samples.16 As a separate analysis, we repeated this process in subsets stratified by type of sensory impairment (vision, hearing, and dual sensory impairment). Our results did not differ substantially, so we present the model in the full cohort, which had better precision due to larger sample size.
Model performance was assessed through discrimination and calibration. Discrimination was measured with the concordance statistic (c-statistic), which is equivalent to the area under the receiver operating characteristic curve. Calibration refers to the agreement between the observed risk of hospitalization and predicted risk. This was visually assessed through a calibration plot with the predicted proportion of hospitalization on the x axis and observed proportion of hospitalization on the y axis.14 Model validation was performed to quantify any optimism in the prediction model. Internal validation using bootstrapping calculated the apparent performance as measured by the c-statistic on the bootstrap samples.13 A Web application was built using R Shiny to calculate hospital admission risk based on a patientʼs specific characteristics.17 All statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Inc). Further details of the design are provided in the supplement.
RESULTS
The baseline characteristics of the 15,999 participants included in this study are summarized in Table 1. The mean age was 78 years, and 55% were female. Of the total sample, 2,567 participants (16.0%) had at least one hospital admission in the second year of the 2-year study unit. Compared with those who were not hospitalized in the second year, participants who were hospitalized were more likely to have a hospitalization in the first year (mean = 0.51 vs 0.10), have an ED visit in the first year (mean = 0.76 vs 0.42), have higher ADL difficulty score (mean = 0.83 vs 0.50), report poor self-rated health (35.5% vs 21.9%), and report more comorbidities.
Table 1.
Baseline Characteristics of Participants with Self-Reported Hearing and/or Vision Impairment Stratified by Those Hospitalized and Not Hospitalized in the 1-Year Study Period
| Characteristic | Hospitalized (n = 2,567) | Not hospitalized (n = 13,432) | P value |
|---|---|---|---|
| Age, mean (SD), y | 78.7 (7.2) | 77.3 (7.2) | <.001 |
| Female, % | 54.1 | 55.8 | .12 |
| Black race, % | 12.2 | 12.4 | .73 |
| Annual income <$10,000, % | 25.1 | 22.0 | .007 |
| Never graduated from high school, % | 36.3 | 31.1 | <.001 |
| Lives alone, % | 36.2 | 36.0 | .88 |
| Medicaid insurance coverage eligible, % | 15.5 | 12.1 | <.001 |
| No. of inpatient admissions in the first of the 2-y study period, mean (SD) | 0.51 (0.91) | 0.10 (0.40) | <.001 |
| No. of ED visits in the first of the 2-y study period, mean (SD) | 0.76 (2.47) | 0.42 (1.16) | <.001 |
| ADL difficulty score, mean (SD) (range = 0–6) | 0.83 (1.13) | 0.50 (0.91) | <.001 |
| IADL difficulty score, mean (SD) (range = 0–6) | 1.09 (1.26) | 0.73 (0.71) | <.001 |
| Poor self-rated health, % | 35.5 | 21.9 | <.001 |
| Self-reported trouble getting health care, % | 6.0 | 5.6 | .46 |
| Dissatisfied with quality of health care received, % | 4.3 | 5.4 | .02 |
| Self-reported comorbidities, % | |||
| Myocardial infarction | 26.7 | 14.3 | <.001 |
| Stroke | 20.5 | 12.6 | <.001 |
| Cancer (excluding skin cancer) | 26.8 | 19.8 | <.001 |
| Dementia | 6.2 | 4.1 | <.001 |
| Diabetes mellitus | 26.6 | 19.8 | <.001 |
| Osteoporosis | 21.9 | 21.3 | .49 |
| Hypertension | 69.7 | 64.0 | <.001 |
| Emphysema | 20.4 | 15.1 | <.001 |
| Osteoarthritis | 69.4 | 63.9 | <.001 |
| Parkinson’s disease | 2.5 | 1.8 | .02 |
| Rheumatoid arthritis | 13.7 | 11.4 | .001 |
| Type of sensory impairment | |||
| Vision impairment only | 16.4% Hospitalized | ||
| Hearing impairment only | 13.8% Hospitalized | ||
| Vision and hearing impairment | 17.8% Hospitalized |
Abbreviations: ADL, activity of daily living; ED, emergency department; IADL, instrumental ADL; SD, standard deviation.
The final model after using the LASSO technique included seven variables: number of inpatient admissions in the previous year, number of ED visits in the previous year, ADL difficulty score, poor self-rated health, and self-reported history of myocardial infarction, stroke, and nonskin cancer (Table 2). The c-statistic was 0.717. After bootstrap internal validation was performed, the optimism-corrected estimate of the c-statistic was 0.716. A calibration plot is shown in Supplementary Figure S1. Sample calculations are provided in Figure 1 that demonstrate the range of risk for patients with hypothetical baseline characteristics. To allow for individualized predictions, a Web application (https://mcbspredictionmodel.shinyapps.io/shinyapp/) and Excel spreadsheet are provided as supplements (Supplementary Figure S2).
Table 2.
Multivariable-Adjusted Prognostic Factors for 1-Year Inpatient Admission After Using the Least Absolute Shrinkage and Selection Operator
| Factor | β Coefficient | Odds ratio | 95% CI |
|---|---|---|---|
| No. of inpatient admissions in previous year | .93 | 2.53 | 2.35–2.72 |
| No. of ED visits in previous year | .07 | 1.08 | 1.05–1.11 |
| History of myocardial infarction | .56 | 1.75 | 1.57–1.95 |
| History of cancer (other than skin cancer) | .31 | 1.36 | 1.23–1.51 |
| History of stroke | .26 | 1.29 | 1.14–1.45 |
| ADL difficulty score (range = 0–6) | .17 | 1.18 | 1.13–1.24 |
| Poor self-rated health | .36 | 1.43 | 1.23–1.57 |
| Note: The concordance statistic (c-statistic; area under the receiver operating characteristic curve) = 0.717. | |||
| Optimism-corrected c-statistic after bootstrap validation = 0.716. | |||
Abbreviations: ADL, activity of daily living; ED, emergency department.
Figure 1.
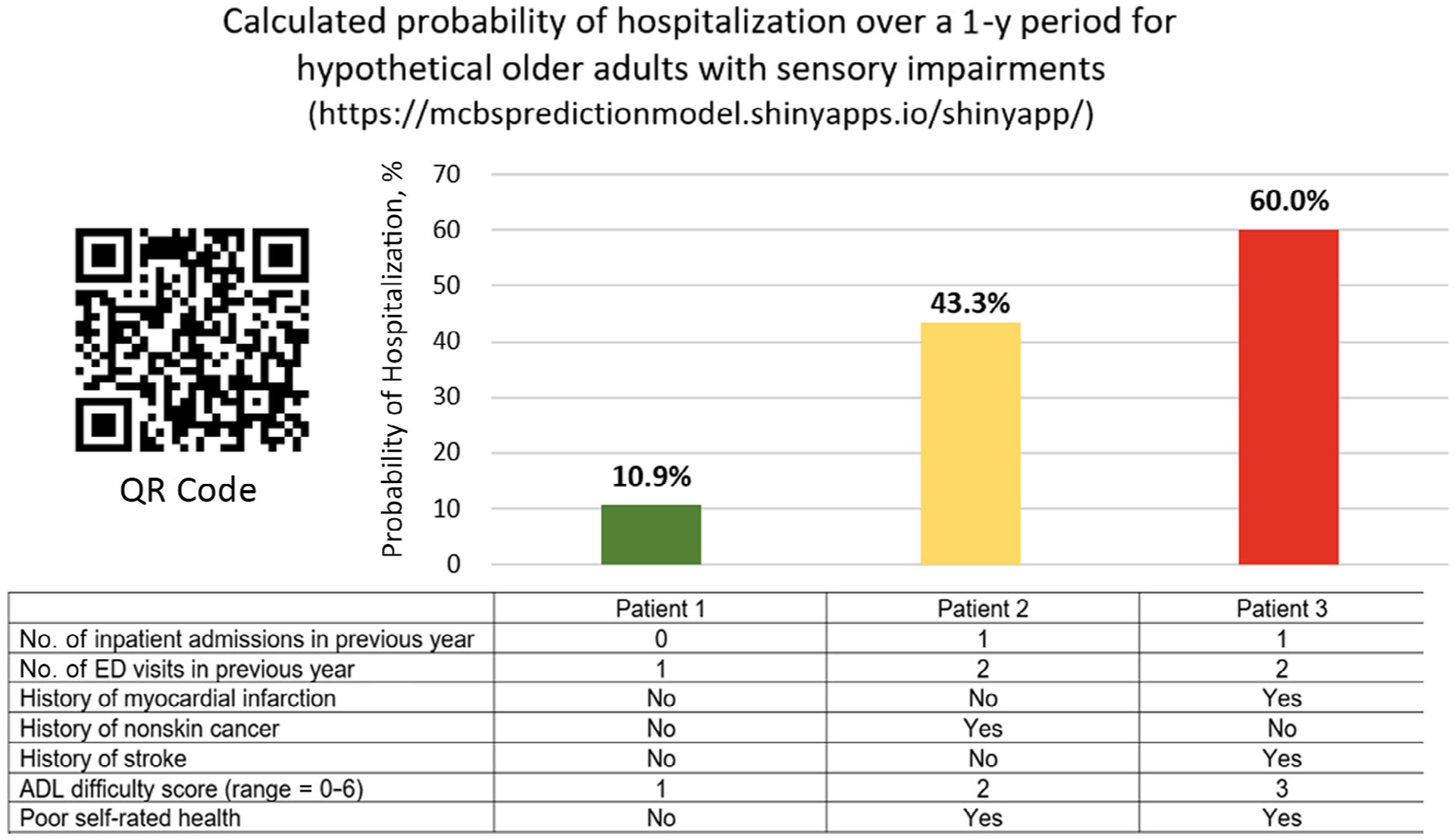
Hypothetical patient examples with the prediction modelʼs calculated probability of hospitalization over a 1-year period. ADL, activity of daily living; ED, emergency department.
DISCUSSION
We developed a prognostic model for risk of 1-year inpatient admission in a cohort of community-dwelling older adults with self-reported hearing and/or vision impairment. In our final model, predictors of inpatient admissions included number of inpatient admissions in the previous year, number of ED visits in the previous year, ADL difficulty score, self-rated health, and comorbidities, including self-reported history of myocardial infarction, stroke, and nonskin cancer. Many of the variables included in our final model are consistent with previous studies, particularly those related to healthcare utilization and specific medical diagnoses.7,10 For example, one commonly used score that estimates hospitalization risk is the Probability of Repeated Admission score, which includes age, sex, poor self-rated health, availability of an informal caregiver, history of coronary artery disease, diabetes mellitus in the previous year, hospital admission during previous year, and more than six physician visits during previous year.11,18 Our model also looked at several variables less frequently included in prediction models, including those related to self-reported functional scores, access to care, and social support.7,10 We included these because older adults with sensory impairments represent a particularly vulnerable patient population as they often have higher rates of social isolation and depression, increased physical disability, and higher healthcare utilization.19–22 We found that ADL difficulty score and self-rated health contributed meaningfully to overall model performance, highlighting the importance of measuring patient-level and functional variables in vulnerable patient populations.
The model had good discrimination, with a c-statistic of 0.717, which is similar to other models of hospital admission.9,10 The primary advantage of using this model relates to an intentional difference in case mix, meaning that the predicted probabilities will apply specifically to community-dwelling older adults with sensory impairments who are underrepresented in general models. There was little evidence of overoptimism in bootstrap validation. The calibration plot suggests that the model tends to overestimate risk among patients at the highest risk for hospitalization.
Given the dramatic impact of hospitalizations on healthcare spending and functional decline, an emphasis has been placed on identifying interventions that can help meet the complex needs of older adults with multimorbidity. In community settings, interventions that target specific risk factors or functional abilities may be more effective at improving patient-reported and functional outcomes compared with broad organizational interventions.23,24 Among those with sensory impairments, sensory restorative services (e.g., hearing aids or cataract surgery) and sensory rehabilitative services (e.g., customization and counseling) have been associated with improved sensory-specific and general health-related quality of life.25–27 However, many older adults do not receive these interventions due to a lack of screening for sensory deficits, perceptions among patients and healthcare professionals that sensory impairments are a normal part of aging, limited access to services, and often high out-of-pocket cost.26,28,29 Tools such as this prediction model, which can readily be calculated through a patient or caregiver interview, may help inform efforts to get these therapies to people who stand to benefit the most. In turn, sensory restorative procedures, rehabilitation programs, and increased care coordination at home can help to improve function and independence to hopefully prevent adverse health outcomes, such as hospitalizations or ED visits.30 For this tool to work optimally, efforts to increase screening for sensory impairments and reducing barriers to accessing sensory services will be needed.
There are a few important limitations in the development and validation of this prognostic model. First, sensory impairments and comorbidities were based on self-report rather than objective measurements. Subjective measures of sensory impairment are important in their own right because they capture the perceived quality of sensory impairment that likely impacts healthcare outcomes. Second, information on certain sensory-specific elements, such as the severity of sensory impairment, access to corrective devices, and utilization of sensory rehabilitation services, was not available. Consideration of these factors might enhance future predictive models. Third, we only included community-dwelling older adults in our population sample. Therefore, this model does not apply to those in assisted living or long-term care facilities. Fourth, although this model was internally validated, future research should focus on externally validating the model using a different cohort of older adults with sensory impairments to determine its generalizability. In addition, a competing risk analysis could be performed that incorporates time to death, which may help with obtaining more accurate predictions among those in the highest-risk strata.
In summary, older adults with sensory impairments are an increasingly prevalent and underrecognized vulnerable population that are at high risk for adverse health outcomes and contribute greatly to healthcare cost and utilization. Our model suggests that many of the previously identified predictors of hospital admissions among community-dwelling older adults apply to those specifically with sensory impairments. We hope that this tool will help provide clinicians and patients with prognostic information that can guide clinical decisions and inform allocation of healthcare resources.
Supplementary Material
Supplementary Appendix S1: A supplemental file includes additional details on the methods used to develop the prediction model, a calibration plot, and screenshots of the Web application and Excel sheet that can be used to calculate the probability of 1-year hospital admission.
Supplementary Figure S1: Calibration plot of the final prediction model. Predicted outcome probabilities (on the x axis) are plotted against observed outcome frequencies (on the y axis).
Supplementary Figure S2: A Web application and Excel sheet were created to allow users to calculate the probability of 1-year hospital admission using the final prediction model. This can be accessed at: https://mcbspredictionmodel.shinyapps.io/shinyapp/. The Excel file can be provided on request.
ACKNOWLEDGMENTS
Financial Disclosure: This work is supported by the Duke Claude D. Pepper Older American Independence Center (P30AG028716), the Physical Resilience Indicators and Mechanisms in the Elderly Collaborative (UH2AG056925), the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002553), and the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham Veterans Affairs Health Care System.
Sponsor’s Role: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Conflict of Interest: None of the authors has conflicts of interest, including financial interests, activities, relationships, and affiliations.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 2013;173(4):312–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genther DJ, Betz J, Pratt S, et al. Association between hearing impairment and risk of hospitalization in older adults. J Am Geriatr Soc. 2015;63(6):1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bal S, Kurichi JE, Kwong PL, et al. Presence of vision impairment and risk of hospitalization among elderly Medicare beneficiaries. Ophthalmic Epidemiol. 2017;24(6):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huddle MG, Deal JA, Swenor B, Genther DJ, Lin FR. Association between dual sensory impairment, hospitalization, and burden of disease. J Am Geriatr Soc. 2016;64(8):1735–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudi E, Zazove P, Meade M, McKee MM. Association between hearing aid use and health care use and cost among older adults with hearing loss. JAMA Otolaryngol Head Neck Surg. 2018;144(6):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAna JF, Crawford AG, Novinger BW, et al. A predictive model of hospitalization risk among disabled Medicaid enrollees. Am J Manag Care. 2013; 19(5):e166–e174. [PubMed] [Google Scholar]
- 9.Inouye SK, Zhang Y, Jones RN, et al. Risk factors for hospitalization among community-dwelling primary care older patients: development and validation of a predictive model. Med Care. 2008;46(7):726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace E, Stuart E, Vaughan N, Bennett K, Fahey T, Smith SM. Risk prediction models to predict emergency hospital admission in community-dwelling adults: a systematic review. Med Care. 2014;52(8):751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817. [DOI] [PubMed] [Google Scholar]
- 12.Adler GS. A profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev. 1994;15(4):153–163. [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. New York, NY: Springer; 2015. [Google Scholar]
- 14.Steyerberg E Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2009. [Google Scholar]
- 15.Tibshirani R Regression shrinkage and selection via the LASSO. J R Stat Soc Ser B Methodol. 1994;58:267–288. [Google Scholar]
- 16.Austin PC, Tu JV. Bootstrap methods for developing predictive models. Am Stat. 2004;58:131–137. [Google Scholar]
- 17.Chang W, Cheng J, Allaire J, et al. Shiny: Web Application Framework for R. Version 1.4.0.2. Vienna, Austria: R Project for Statistical Computing. http://CRAN.R-project.org/package=shiny. Accessed May 11, 2020. [Google Scholar]
- 18.Wallace E, Hinchey T, Dimitrov BD, Bennett K, Fahey T, Smith SM. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61(3):357–364. [DOI] [PubMed] [Google Scholar]
- 19.National Academies of Sciences, Engineering, and Medicine. In: Blazer DG, Domnitz S, Liverman CT, eds. Hearing Health Care for Adults: Priorities for Improving Access and Affordability. Washington, DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 20.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health. In: Welp A, Woodbury RB, M A M C, Teutsch SM, eds. Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington, DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 21.Davidson JGS, Guthrie DM. Older adults with a combination of vision and hearing impairment experience higher rates of cognitive impairment, functional dependence, and worse outcomes across a set of quality indicators. J Aging Health. 2019;31(1):85–108. [DOI] [PubMed] [Google Scholar]
- 22.Prager AJ, Liebmann JM, Cioffi GA, Blumberg DM. Self-reported function, health resource use, and total health care costs among Medicare beneficiaries with glaucoma. JAMA Ophthalmol. 2016;134(4):357–365. [DOI] [PubMed] [Google Scholar]
- 23.Boult C, Reider L, Leff B, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med. 2011;171(5):460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Wallace E, OʼDowd T, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris D, Fraser SG, Gray C. Cataract surgery and quality of life implications. Clin Interv Aging. 2007;2(1):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed NS, Lin FR, Willink A. Hearing care access?: focus on clinical services, not devices. JAMA. 2018;320(16):1641–1642. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson MA, Kitterick PT, Chong LY, et al. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev. 2017;9: CD012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacala JT, Yueh B. Hearing deficits in the older patient: “I didnʼt notice anything.”. JAMA. 2012;307(11):1185–1194. [DOI] [PubMed] [Google Scholar]
- 29.Lin FR, Hazzard WR, Blazer DG. Priorities for improving hearing health care for adults: a report from the National Academies of Sciences, Engineering, and Medicine. JAMA. 2016;316(8):819–820. [DOI] [PubMed] [Google Scholar]
- 30.Deal JA, Goman AM, Albert MS, et al. Hearing treatment for reducing cognitive decline: design and methods of the aging and cognitive health evaluation in elders randomized controlled trial. Alzheimers Dement (N Y). 2018; 4:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix S1: A supplemental file includes additional details on the methods used to develop the prediction model, a calibration plot, and screenshots of the Web application and Excel sheet that can be used to calculate the probability of 1-year hospital admission.
Supplementary Figure S1: Calibration plot of the final prediction model. Predicted outcome probabilities (on the x axis) are plotted against observed outcome frequencies (on the y axis).
Supplementary Figure S2: A Web application and Excel sheet were created to allow users to calculate the probability of 1-year hospital admission using the final prediction model. This can be accessed at: https://mcbspredictionmodel.shinyapps.io/shinyapp/. The Excel file can be provided on request.


