Summary
The protocol provided here describes methodologies for making a highly cost-effective, chemically defined medium for culturing hiPSCs we call B8 medium. The typical cost of B8 medium is US$10 per liter, which with modifications included here is more affordable than standard media. We provide simple protocols for making B8 supplement aliquots, making the basal media DMEM/F12, Matrigel-coated plates, thawing, passaging, culturing, and cryopreserving hiPSCs. We show typical differentiation results and provide a comprehensive troubleshooting guide.
For complete details on the use and execution of this protocol, please refer to Kuo et al. (2020).
Subject areas: Cell Culture, Stem Cells
Graphical abstract
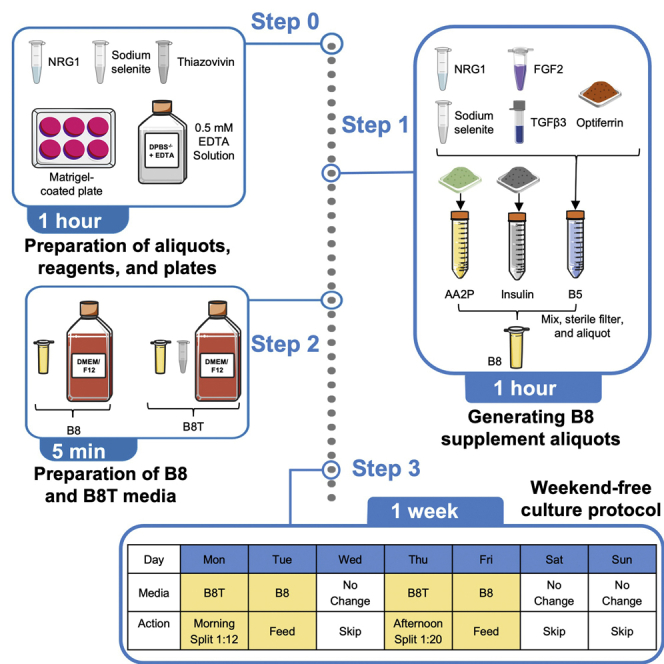
Highlights
-
•
The protocol describes a cost-effective, chemically defined B8 medium for culturing hiPSCs
-
•
The typical cost for B8 medium is US$10 per liter, which is more affordable than existing media
-
•
Protocols for B8 supplement aliquots, basal media DMEM/F12, and Matrigel-coated plates
-
•
We also describe the process of thawing, passaging, culturing, and cryopreserving hiPSCs
The protocol provided here describes methodologies for making a highly cost-effective, chemically defined medium for culturing hiPSCs we call B8 medium. The typical cost of B8 medium is US$10 per liter, which with modifications included here is more affordable than standard media. We provide simple protocols for making B8 supplement aliquots, making the basal media DMEM/F12, Matrigel-coated plates, thawing, passaging, culturing, and cryopreserving hiPSCs. We show typical differentiation results and provide a comprehensive troubleshooting guide.
Before you begin
Human induced pluripotent stem cells (hiPSCs) should can be obtained from existing academic hiPSC labs or from commercial sources such as WiCell (wicell.org) or Coriell (coriell.org). hiPSC use should follow institutional guidelines including institutional review board approval and relevant material transfer agreements. We grow our hiPSCs in 5% O2 (hypoxic) incubators, this hypoxic environment is not essential for this protocol to be successful, but has previously been demonstrated to improve genome stability (Kuijk et al., 2020; Thompson et al., 2020), reprogramming efficiency (Mathieu et al., 2014; Yoshida et al., 2009), and maintenance of the pluripotent state (Forristal et al., 2010; Guo et al., 2013; Mathieu et al., 2013; Narva et al., 2013). All cell culture steps should be performed in a Class II or higher biosafety cabinet such as a Labconco Logic+. All solutions should be kept sterile, and proper sterile technique should be used throughout.
The protocol detailed here has been in use in our lab for a number of years and validated with hundreds of cell lines. It is imperative to recognize that cells must be cultured on a strict repetitive manner, typically passaging every 3, 3.5, or 4 days. Changing the number of days of culture based on visual inspection is not appropriate, rather cells should be split on schedule and plated at different ratios, typically 1:15 to 1:20 until they typically reach 70%–80% confluent and rhythm has been established. The protocol provided here uses the “low-cost, easier to make” formula variant of B8 medium with lower levels of insulin and transferrin described in (Kuo et al., 2020) (Figure S5C). NRG1 and TGFB3 are used at very low concentrations in this formula, and we have found it not to be cost-effective to make these proteins in house due to comparative poor yield when generating these ourselves. This is likely to be the case for all but the highest use academic labs (>1,000 L per year).
Preparation of DMEM/F12
Timing: 1 h
We identified DMEM/F12 as the optimal basal media to use in combination with B8 supplement aliquots for the culture of hiPSCs. Now that the cost of growth factors in the B8 supplement aliquots have been reduced, the major cost of B8 medium is the DMEM/F12, which represents 65% of the total cost. This cost of DMEM/F12 varies considerably across laboratories and we have found that our comparably cheap cost ($6.50/L) is not the common case, with the list price of the commonly used Gibco DMEM/F12 ∼10× higher ($64.60/L). In addition, the availability, length of time, and cost of delivery may vary across locations. To combat this and due to our own changing needs as scale increases, where the cost of DMEM/F12 has become prohibitive, we have developed a protocol for the simple generation of DMEM/F12 in 20 L batches from powder. The total cost of powders and a sterile filter for this protocol is $5.22/L.
Note: We have found the most cost-effective version of DMEM/F12 in powder form to be from Corning. This version comes without L-glutamine, HEPES, or sodium bicarbonate which must be added.
-
1.
Fill a clean and autoclaved 20 L polycarbonate carboy with 19 L of Milli-Q water. Place on a high capacity stirrer with a Magnetic PVDF Carboy Stirrer.
-
2.
Slowly add 2 bottles of DMEM/F12 powder replacing the stirrer occasionally to agitate powder at the bottom of the carboy.
-
3.
Once the DMEM/F12 is dissolved add 48.8 g of sodium bicarbonate, 71.5 g of HEPES, and 73 g of L-glutamine, and continue to mix until fully dissolved.
-
4.
Top up to 20 L with Milli-Q water.
Note: Check pH and osmolarity which typically come out at pH 7.1 and 310 osm/L.
Note: When checking the pH and osmolarity, separate media in 50 mL tubes for each measurement. Use an osmometer to check the osmolarity and correct the value by adding cell culture grade water until the desired value is reached. For pH, corrections should be made using sodium hydroxide (NaOH) or hydrochloric acid (HCl) solutions. Then, scale up the values of cell culture grade water, HCl, and/or NaOH for the appropriate final volume of media being made.
-
5.
Place carboy on a cart and wheel next to a cell culture hood.
-
6.
Attach the carboy to a peristaltic pump into a PES sterile filtration system at <75 psi.
-
7.
Inside the cell culture hood, use the system to fill 20 clean and autoclaved 1 L polycarbonate bottles with DMEM/F12. Cap the bottles and store at 4°C until use.
Note: We wash our polycarbonate bottles in a standard scientific glassware washer and autoclave empty on the lowest cycle (15 min, 120°C). Bottles must be allowed to cool before the caps are tightened to prevent loss of shape. Bottles can be autoclaved ∼30 times before the plastic becomes brittle.
Note: Once made, bottles of DMEM/F12 can be kept at 4°C for at least 1 year.
Preparation of Matrigel-coated plates
Timing: 30 min
Note: The use of a low concentration Matrigel (a 1:800 dilution) was optimized and found to work successfully without sacrificing cell proliferation or differentiation capacity (Kuo et al., 2020). Dilutions up to 1:1,000 are still suitable for hiPSC culture, however 1:800 is preferred to avoid batch-to-batch variability in the supplier concentrations and operator error when filling the wells.
-
1.
Thaw a bottle of growth factor-reduced Matrigel overnight at 4°C.
Note: The bottle of Matrigel can be stored at 4°C for over six months in a high-performance refrigerator that is able to maintain a constant temperature. There is no need to aliquot it or place on ice.
-
2.
Set out 42 × 6-well cell culture plates in a cell culture hood.
-
3.
Set a P1000 pipettor to 625 μL and have a sterile 1,000 μL tip ready.
-
4.
Place a 500 mL bottle of 4°C DMEM in cell culture hood.
-
5.
Briefly take Matrigel bottle out of the 4°C and add 625 μL Matrigel to the DMEM bottle for a dilution of 1:800.
CRITICAL: Hold the Matrigel bottle by the neck when transporting the bottle to the cell culture to minimize heat transfer that could cause gelation of the Matrigel.
Note: Any concentration of Matrigel in within the typically suppled range of 8 to 12 mg/mL (see product insert) is suitable for use at 1:800 dilution.
Note: It is possible to coat a smaller number of plates by just transferring the appropriate amount of DMEM to a sterile bottle and mixing with the correct volume of Matrigel to maintain the desired 1:800 dilution. For example, to coat only 21 plates, use 250 mL of DMEM and 312.5 μL of Matrigel. Diluted Matrigel solution should not be stored and reused.
-
6.
Immediately return the Matrigel bottle to 4°C
CRITICAL: Minimize the time the bottle will be kept out of the 4°C in order to avoid gelation of the Matrigel.
-
7.
Mix the Matrigel/DMEM solution by inversion.
-
8.
For 6 well plates, add 2 mL of Matrigel solution per well.
Note: The addition of 2 mL of Matrigel solution per well of a 6-well plates, instead of a smaller volume, minimizes the likelihood of the wells drying out.
-
9.
Place the plates in a 37°C incubator for at least 30 min before use.
Note: Plates can be kept in the incubator for up to 4 weeks without risk of wells drying out.
CRITICAL: If the wells have dried out, the plate should be discarded.
Alternatives: Instead of Matrigel, plates can be coated with Geltrex or Cultrex at the same 1:800 dilution (Kuo et al., 2020), or defined proteins such as recombinant laminin-521, Synthemax II-SC, and vitronectin (Burridge et al., 2014).
Alternatives: As an alternative to DMEM, a Matrigel diluent solution can be made from Milli-Q water with 6 mg/L phenol red sodium salt, 140 mg/L sodium phosphate monobasic monohydrate, and 1,200 mg/L sodium bicarbonate and filter sterilized as above.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| DMEM/F12 powder | Corning | 90-091-PB |
| Sodium bicarbonate | Sigma-Aldrich | S8875 |
| HEPES | Fisher Bioreagents | BP310-5 |
| L-Glutamine | Sigma-Aldrich | G3126 |
| Matrigel growth factor-reduced basement membrane matrix (10 mL) | Corning | 354230 |
| DMEM with L-glutamine and 4.5 g/L D-glucose | Corning | 10-017-CV |
| L-ascorbic acid 2-phosphate trisodium salt | Wako | 321-44823 |
| Recombinant human insulin | Gibco | A11382IJ |
| Hydrochloric acid (1 N) | Sigma-Aldrich | H9892 |
| Recombinant human transferrin (Optiferrin) | InVitria | 777TRF029-10G |
| Recombinant human FGF2-G3 (solution, 1 mg/mL) | Made by a recombinant protein production core | N/A |
| Sodium selenite | Sigma-Aldrich | S5261-10G |
| Recombinant human NRG1 | Peprotech | 100-03-250UG |
| 0.5 M EDTA | Invitrogen | 15575020 |
| Recombinant human TGFβ3 (comes as a solution at 250 μg/mL, 100 μg) | Shenandoah Biotechnology | 8000-07-100ug |
| Dimethylsulfoxide (DMSO) | Fisher Bioreagents | BP231-100 |
| Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+ (DPBS−/−) | Corning | 21-031-CV |
| Thiazovivin (powder) | LC Labs | T9753 |
| Sodium hydroxide (1 N) | Sigma-Aldrich | S2770 |
| Other | ||
| Polycarbonate bottles and caps | Nalgene | 362015-1000 + 362150-4384 |
| 6-well tissue culture treated plates (5-pack) | Greiner | 657165 |
| 15 mL conical tubes | Corning Falcon | 352097 |
| 50 mL conical tubes | Corning Falcon | 352098 |
| CoolCell LX | Corning | 432002 |
| Cryovials | Greiner | 122261 |
| 5 mL pipettes sterile, individually wrapped | Fisher Basix | 14-955-232 |
| 10 mL pipettes sterile, individually wrapped | Fisher Basix | 14-955-234 |
| 25 mL pipettes sterile, individually wrapped | Fisher Basix | 14-955-235 |
| 2 mL aspiration pipettes sterile, individually wrapped | Corning Falcon | 357558 |
| Cell culture incubators with 5% CO2/5% O2 | Thermo Scientific | Heracell VIOS 160i |
| Class II, type A2 or better biosafety cabinet | Labconco | Purifier Logic+ |
| Liquid nitrogen (liquid N2) | Thermo | CryoPlus 2 |
| Centrifuge | Thermo Sorvall | ST40 |
| Aspirator vacuum | N/A | N/A |
| Microscope | Nikon | Eclipse Ts2 |
| 2 mL sterile microtubes | Axygen | MCT-200-C-S |
| Analytical balance | Mettler Toledo | ML204 |
| pH meter | Mettler Toledo | SevenCompact S220 |
| 20 L polycarbonate carboy | Nalgene | 2317-0050PK |
| High capacity stirrer | Cimarec | 50098760 |
| Magnetic PVDF carboy stirrer | Nalgene | DS22270020 |
| FlexiPump Peristaltic Pump FlexiPro | FlexiPump | 562000 |
| Millipore Steripak-GP | Millipore | SPGPM10RJ |
Alternatives: Commercial FGF2 is available from suppliers such as Peprotech, (100-18B, US$ 350/1 mg) which works for the standard non-weekend-free protocol at 100 ng/mL. Northwestern’s protein production core (http://rppc.mccormick.northwestern.edu) generates FGF2-G3 for external academic use at a cost of approximately US$ 1290/100 mg. Many institutions with protein production core facilities can likely achieve similar yield and production costs, and plasmids in suitable expression E. coli are available from Addgene (https://www.addgene.org/135521/). Other stable versions of FGF2 are available such as Gibco Heat Stable Recombinant Human bFGF (PHG0360) although cost may be prohibitive (US$ 2124/1 mg).
Materials and equipment
These are supplementary reagents and solutions used throughout the protocol and should be prepared ahead of time. Their preparation time is minimal, approximately 1 h.
| Reagent | Final concentration | Amount |
|---|---|---|
| EDTA (0.5 M) | 0.5 mM | 500 μL |
| Dulbecco’s phosphate-buffered saline without Ca2+ or Mg2+ (DPBS−/−) | n/a | 500 mL |
| Total | n/a | 500.5 mL |
Note: Make this solution inside a cell culture hood. The solution can be stored at room temperature indefinitely.
| Reagent | Final concentration | Amount |
|---|---|---|
| Thiazovivin | 10 mM | 100 mg |
| DMSO | n/a | 32 mL |
| Total | n/a | 32 mL |
Note: After dilution, make 200 μL aliquots and store at −20°C. Aliquots are stable for 1 year.
Alternatives: Thiazovivin can be replaced by other ROCK inhibitors such as Y27632 and HA-1077, with appropriate adjustments in concentrations. Thiazovivin was selected for this protocol due to its effectiveness at lower concentrations (2 μM) and therefore lower cost, compared to Y27632 (Kuo et al., 2020; Xu et al., 2010) or HA-1077 (Gao et al., 2019; So et al., 2020).
| Reagent | Final concentration | Amount |
|---|---|---|
| NRG1 | 1.42 μM | 250 μg |
| Sterile Milli-Q Water | n/a | 2.5 mL |
| Total | n/a | 2.5 mL |
Note: Make 100 μL aliquots, and store at −20°C. Aliquots are stable for 1 year.
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium selenite | 11.6 mM | 100 mg |
| Sterile Milli-Q Water | n/a | 50 mL |
| Total | n/a | 50 mL |
CRITICAL: Sodium selenite is toxic by ingestion, inhalation, and skin absorption. Handle in a fume hood, wear appropriate PPE such as a face mask, googles, and long-sleeved lab coat.
Note: Make 1 mL aliquots, and store at −20°C. Aliquots are stable for ≥1 year.
Preparation of DMEM/F12
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM/F12 Powder | n/a | 2 bottles |
| HEPES | 15 mM | 71.5 g |
| Sodium bicarbonate | 29 mM | 48.8 g |
| L-glutamine | 2.5 mM | 73 g |
| Sterile Milli-Q water | n/a | 20 L |
| Total | n/a | 20 L |
Note: Check pH and osmolarity which typically come out at pH 7.1 and 310 Osm/L.
Note: When checking the pH and osmolarity, separate media in 50 mL tubes for each measurement. Use an osmometer to check the osmolarity and correct the value by adding cell culture grade water until the desired value is reached. For pH, corrections should be made using sodium hydroxide (NaOH) or hydrochloric acid (HCl) solutions. Then, scale up the values of cell culture grade water, HCl, and/or NaOH for the appropriate final volume of media being made.
Note: After mixing the powders, the media should be sterile filtered and transferred to appropriate bottles for future use. The medium bottles can be stored 4°C for at least 1 year.
Note: We wash our polycarbonate bottles in a standard scientific glassware washer and autoclaved. Bottles must be allowed to cool before the caps are tightened to prevent loss of shape. Bottles can be autoclaved ∼30 times before the plastic becomes brittle.
Step-by-step method details
Generating B8 supplement aliquots
Timing: 1 h
Preparation of B8 supplement aliquots
Note: All the values in this section are to prepare B8 supplement aliquots for 100 L of B8 medium. To prepare other volumes, these values can be scaled up and down accordingly.
-
1.Prepare AA2P solution.
-
a.Add 35 mL of sterile Milli-Q water and 7 mL of HCl (1 N) into a 50 mL conical tube.
-
b.Weight 20 g of L-ascorbic acid 2-phosphate and add to the Falcon tube.
-
c.Mix vigorously and place on a rocker at 50 rpm for 10 min or until dissolved.
-
a.
Note: Final volume should be 50 mL and pH should be 7.1.
CRITICAL: Always add the AA2P powder to the water. Otherwise, a hydrogel will be formed when the water starts being added to the salt, resulting in an increased difficulty of obtaining a homogenous solution.
Note: Only the tri sodium salt version of L-ascorbic acid 2-phosphate (also called 2-phospho-L-ascorbic acid trisodium salt) can be made at his high concentration.
-
2.Prepare insulin solution.
-
a.Place 48 mL of sterile Milli-Q water into a 50 mL conical tube.
-
b.Weight 0.5 g of insulin and add into the conical tube.
-
c.Add 400 μL of HCl (1 N) and mix by inversion until solution is clear (approximately 1 min).
-
d.Add 550 μL of NaOH (1 N), and mix by inversion until solution is clear.
-
a.
Note: Insulin is relatively insoluble in water at physiological pH, but solubilizes at acidic pH. Once in solution, it is possible to increase the pH without precipitation of the solute. As pH increases with the addition of NaOH, solution becomes cloudy again. However, simply mixing by inversion is enough to homogenize the tube and obtain a crystal clear solution.
Note: Final pH should be 7.1.
-
3.Prepare B5 solution.
-
a.Place 47 mL of sterile Milli-Q water into a 50 mL conical tube.
-
b.Weight 0.5 g of transferrin, add into the tube, and mix by inversion until solution is clear.
-
c.Add 1 mL of 2 mg/mL sodium selenite solution.
-
d.Add 1 mL of 4 mg/mL FGF2 solution.
-
e.Add 40 μL of 250 μg/mL TGFB3 solution (comes as a solution).
-
f.Add 100 μL of 100 μg/mL NRG1 solution.
-
g.Mix by inversion until solution is clear.
-
a.
-
4.Mixing the components made on steps 1–3 and making the B8 supplement aliquots.
-
a.In a cell culture hood, add the three tubes (AA2P, insulin, and B5) along with 50 mL of sterile Milli-Q water to a sterile bottle and mix.
-
b.Add the 200 mL solution to a 250 mL bottle-top PES filter to sterilize.
-
c.Make 2 mL B8 supplement aliquots in sterile 2 mL microtubes. Freeze at −20°C.
-
a.
Note: B8 supplement aliquots can be stored at −20°C for up to 1 year.
Preparation of B8 medium
Timing: 10 min
-
5.
In a cell culture hood, thaw one B8 supplement aliquot.
-
6.
Add the aliquot to 1 L of 4°C DMEM/F12.
-
7.
Mix by inversion.
-
8.
Keep the media at 4°C and avoid exposure to light.
-
9.
Media should be used within 4 weeks.
CRITICAL: If using a DMEM/F12 basal medium from supplier other than Corning, make sure that the concentrations of salts and the chemical properties (pH and Osmolarity) of the basal medium are the same as those presented in (Kuo et al., 2020). For example, for 1 L of Gibco DMEM/F12 (11330-032), a separate aliquot of 15 mL at 1 M of sodium bicarbonate would be needed, which would also require correction of pH and osmolarity of the final solution.
Preparation of B8T medium
Timing: 10 min
-
10.
In a cell culture hood, thaw one B8 supplement aliquot and one aliquot of thiazovivin.
-
11.
Add the aliquots to 1 L of 4°C DMEM/F12.
-
12.
Mix by inversion.
-
13.
Keep the media at 4°C and avoid exposure to light.
-
14.
Media should be used within 4 weeks.
Note: Both B8 and B8T media are needed for the following steps of the protocol. B8 medium is used in the daily media exchange steps, whereas B8T medium is used for thawing and passaging of hiPSCs.
Thawing and initial plating of hiPSCs
Timing: 15–20 min
Note: hiPSCs should preferentially be generated in house. All lines generated by other laboratories, including commercially available lines, must be mycoplasma tested (Lonza MycoAlert Plus).
Note: We do not prewarm media to room temperature or 37°C before use due to concerns of the stability of the FGF2 in the media. All media used in all steps is at 4°C. We have not noted any negative effects on the growth. Additionally, avoiding placing media bottles in water baths reduces potential for contamination.
-
15.
Transfer a 6-well Matrigel-coated plate from the incubator to the cell culture hood.
-
16.
Aspirate the Matrigel solution from 3 of the wells, add 2 mL of B8T medium into each of them.
-
17.
Transfer a vial of hiPSCs from liquid nitrogen into a 37°C water bath.
-
18.
Keep the cells into the water bath until only a sliver of ice remains.
-
19.
Use a 10 mL pipette to remove the contents from the vial. Fill the pipette with 10 mL of B8T medium, and use this to remove the contents of the vial. Transfer approximately 5 mL to a 15 mL conical tube, then wash out the vial with approximately 1 mL of medium, and transfer the remainder to the conical tube.
Note: The use of a thiazovivin-containing medium (B8T) improves cell survival after dissociation, improving plating consistency.
-
20.
Centrifuge 3 min at 200 × g. Aspirate supernatant. Resuspend pellet in 2 mL B8T and transfer to the three B8T-containing wells of the Matrigel-coated 6-well plate from step 16 at a 1:2 (1 mL of cell suspension), 1:3 (670 μL of cell suspension), and 1:6 ratios (330 μL of cell suspension).
-
21.
Return the plate to the incubator. Medium should be exchanged with B8 medium every 24 h, until wells become 70%–80% confluent.
Note: Ideally, the cells should reach 70%–80% confluence in 3–4 days. Adjust the split ratio to 1:12 to 1:20 to achieve this result, as higher split ratios are associated with more uniform distribution of cells across the well and result in more efficient differentiations into cardiomyocytes. For other desired cell types, split ratios might be changed to improve differentiation efficiency.
Pause Point: When cells reach 70%–80% confluence, they could be passaged (steps 22–28), frozen (steps 29–38), or differentiated (your choice of differentiation protocol according to specific cell needs and experimental design (Burridge et al., 2014; Cyganek et al., 2018; Hogrebe et al., 2020)). For a weekend-free culture of hiPSCs, check steps 39–45.
Passage of hiPSCs with EDTA
Timing: 20–30 min
Note: All the volumes here are for one well of a 6-well plate. For other types of plates, the volumes need to be changed accordingly.
Note: EDTA solution is kept at room temperature.
CRITICAL: This protocol is designed to keep the hiPSCs in the logarithmic growth phase. Therefore, the number of days of culture should be kept consistent and cells should not be allowed to become more than 90% confluent. If cells overgrow, becoming more than 90% confluent, they become contact inhibited, which results in a slow lag phase growth after passaging.
-
22.
Aspirate cell culture medium.
-
23.
Add 1 mL of 0.5 mM EDTA per well and incubate for 6 min at room temperature (inside the cell culture hood).
Note: The chosen incubation duration should be set so that the cells come off easily during step 26. We use 6 min for all cell lines. Longer incubation times will result in the cells detaching from the wells before the aspiration of the EDTA solution, leading to preventable loss of cells during the following steps.
Note: We use EDTA for cell passaging to preserve small clumps of cells when passaging, instead of isolated single cells as would be the case if TrypLE was used.
-
24.
While hiPSCs are still incubating in EDTA, aspirate Matrigel solution from two new 6-well plates and replace it with 1 mL of B8T medium per well.
-
25.
When the 6 min are finished, aspirate EDTA from the well from step 23.
-
26.
With a P1000 tip, add 1 mL of B8T medium to the well from step 25, and blast medium against cell surface to dissociate cells and detach them from the Matrigel layer. Cells should come off easily after pipetting about 5 times.
CRITICAL: Avoid pipetting cells too much as it decreases the cell survival after passaging.
-
27.
Top up well with B8T medium for the desired split ratio. For 1:12 split ratio, top up well with 11 mL of B8T medium. For 1:15, use 14 mL of B8T medium. For 1:20, remove 250 μL, then top up well with 14.25 mL of B8T medium. For 1:24 remove 400 μL, then top up well with 14.4 mL of B8T medium.
-
28.
Add 1 mL of cell solution per well to two new Matrigel-coated 6-well plates to a final volume of 2 mL per well.
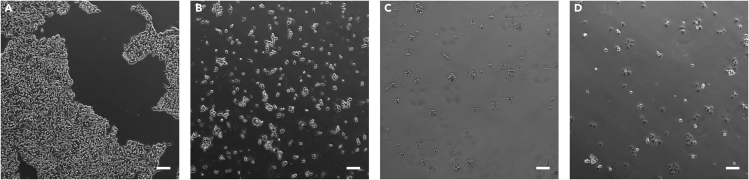
Note:Figure 1 presents representative phase contrast images of hiPSCs during steps 24, 26, and 28 (10 and 30 min post seeding).
Figure 1.
Dissociation with EDTA and plating of hiPSCs using B8T
Phase-contrast images of hiPSCs: (A) during EDTA treatment; (B) in suspension in B8T immediately after dissociation; (C) 10 min; and (D) 30 min post seeding in a Matrigel-coated plate. Scale bar, 100 μm.
Freezing of hiPSCs
Timing: 25–26 h
-
29.
To prepare the freezing solution, combine 5 mL of DMSO with 45 mL B8T medium in a 50 mL Falcon tube to a final concentration of 10% (v/v) DMSO. Mix the solution by inverting. This should be prepared fresh each time, immediately before use.
-
30.
Start with cells at 70%–80% confluence.
-
31.
Aspirate culture medium.
-
32.
Add 1 mL of 0.5 mM EDTA per well and incubate for 6 min at room temperature (inside the cell culture hood).
-
33.
After 6 min, aspirate EDTA from the wells.
-
34.
With a P1000 tip, add 1 mL of B8T medium to the wells, and blast medium against cell surface to dissociate cells and detach them from the Matrigel layer. Cells should come off easily after pipetting about 5 times. Transfer the cells to a 15 mL conical tube.
CRITICAL: Avoid pipetting cells too much as it decreases the cell survival after passaging.
-
35.
Centrifuge the tube(s) at 200 × g for 3 min, then aspirate supernatant.
-
36.
Use 1 mL of freezing solution to resuspend the cells for each well, transferring each cell solution to a cryovial.
-
37.
Place the cryovial(s) in a Coolcell (or other cell-freezing container) and move it to a −80°C freezer.
CRITICAL: After addition of freezing media to the cells and their transfer to the cryovial, move them to −80°C freezer as quickly as possible. Viability of cells decay with their long-term maintenance in freezing medium at room temperature.
-
38.
After 2–24 h in –80°C, transfer the cells to liquid nitrogen storage.
CRITICAL: Long-term storage in the −80°C freezer decreases the viability of the cells post thawing.
Note: Freezing one well of a 6 well plate will result in about 1 million cells per vial.
Weekend-free culture of hiPSCs
Timing: 1 week
The following protocol provides a strategy for a 7 days hiPSC culture schedule, with two passaging steps (days 1 and 4) and 3 days (day 3, day 6, and day 7) without need of media change. If day 1 is chosen to be on a Monday, no media changes will be required on Wednesday and during the weekend.
Note: Cell culture can be transitioned to the weekend-free culture protocol after hiPSCs have been split at least four consecutive times at 1:15 ratio in a 4 day schedule (passage (d0), media exchange (d1), media exchange (d2), media exchange (d3), passage (d4)).
Note: A medium volume of 2 mL per well of a 6-well plate or equivalent is maintained for all steps.
-
39.
On the morning of day 1 (Monday), passage the cells following steps 22–28, and seed them at 1:12–1:15 ratio in B8T medium.
Note: This ratio can be further optimized for different cell lines in order to have the cells ready for a new passage after 3.5 days.
-
40.
On the morning of day 2 (Tuesday), change the media to B8 medium, adding 2 mL per well.
-
41.
Skip media change on day 3 (Wednesday).
-
42.
On the afternoon of day 4 (Thursday), split the cells and seed them at 1:20 ratio in B8T medium.
Note: Similar to the previous passage, the ratio mentioned here could be optimized for each cell line in order to have cells ready for passage after 3.5 days.
-
43.
On the afternoon of day 5 (Friday), change media to B8 medium. Add 2 mL per well.
-
44.
Skip media change on day 6 (Saturday) and day 7 (Sunday).
-
45.
On the morning of day 8/day 1 (Monday), passage the cells again to 1:20 ratio in B8T medium.
Expected outcomes
A successful production of B8 medium will allow the generation and maintenance of high quality hiPSCs, also preventing their spontaneous differentiation (Kuo et al., 2020). B8 medium provides a cost-effective alternative for commonly used hiPSC culture media such as E8 (Chen et al., 2011) and mTeSR (Ludwig and Thomson, 2007). After identifying the proper split ratio, hiPSCs can be maintained at a weekend-free media exchange routine minimizing the number of interventions during culture and saving time for the researcher. Our protocol has been thoroughly validated on different hiPSC lines, including genetically-engineered knockout lines, without loss of culture quality or differentiation performance.
Transitioning of live cells cultured in E8, Essential-8 (Gibco), or TeSR-E8 (Stem Cell Technologies) can be performed directly without any need for a transition period. Equally, cells cultured in these media then cryopreserved can be thawed in to B8 medium. Like E8, B8 medium results in cells growing less densely than mTeSR1, hence we recommend that cells cultured in mTeSR1 are split to multiple densities (1:3, 1:6, 1:12) then transitioned to B8 medium 1 day after split. We do not recommend thawing cells cultured in mTeSR1 (or on MEF) directly in to B8 medium, rather recover them in their original medium, then transition to B8 medium.
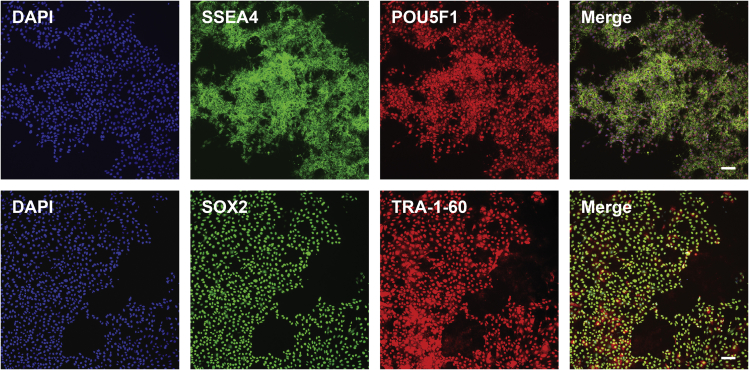
hiPSC cultured long-term in B8 medium will express high levels of the pluripotency markers SSEA4, POU5F1, SOX2, TRA-1-60 (Figure 2). Once dissociated, cells will quickly adhere, form colonies before forming a near monolayer after 3 days of growth (Figure 3). Cells should not be allowed to overgrow and achieve ≥90% confluency (Figure 3E) as this will result in cells leaving the logarithmic growth phase and the culture quality might substantially decrease. In this case, hiPSCs will present greater heterogeneity in the cell cycle phases, which will impact their differentiation potential (Laco et al., 2018). Post-passage overgrown cells will also take longer to expand, might have decreased survivability, and will present unreliable differentiation for some passages.
Figure 2.
Representative images of hiPSCs maintained in B8 medium stained for pluripotency markers
These cells were generated and expanded in B8 medium for 94 passages, and were cultured in B8 medium for 3 days before fixing and staining. Scale bar, 100 μm.
Figure 3.
Representative phase-contrast images of hiPSCs kept in B8 at different time points post plating
(A) 4 h, (B) 24 h, (C) 48 h, (D) 72 h; (E) 90 h. Scale bar, 100 μm.
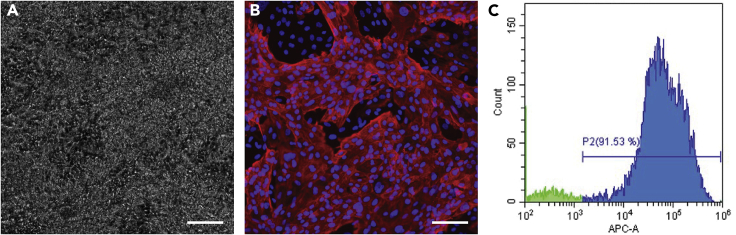
hiPSCs grown in B8 medium have been successfully differentiated into cardiomyocytes (Figure 4), skeletal muscle, and endothelial cells (data not shown) in our lab. Figure 4A presents a phase-contrast image of hiPSC-CMs, day 17, differentiated following a version of the CDM3 protocol (Burridge et al., 2014). Figure 4B presents hiPSC-CMs immunostained for TNNT2 (red) and DAPI (blue), and Figure 4C presents flow cytometry data indicating the purity of the cardiomyocytes obtained with a combination of our B8 and CDM3 protocols.
Figure 4.
hiPSC-derived cardiomyocytes obtained from hiPSCs cultured in B8 following the protocols described here
(A) Phase-contrast image of day 15 cardiomyocytes.
(B) hiPSC-CMs stained for TNNT2 (red) and DAPI (blue). Scale bar, 100 μm.
(C) Flow cytometry plot indicating the purity of our hiPSC-CMs.
Limitations
Readers may be concerned of batch-to-batch variability, we typically make batches of 100–200 B8 supplement aliquots every month and despite using this methodology over 5 years have not experienced a “bad batch,” suggesting the protocol is robust.
Quality control is completed by comparing the previous batch to a new batch using our 12-well Prestoblue assay, detailed in (Kuo et al., 2020). New batches of FGF2-G3 (which we have made once per year) should be assessed for potency using the same assay using 0–100 ng/mL and compared to the published data. Although we have not completed any planned stability tests, anecdotally we have not seen any issues with stability, and sodium selenite, NRG1, TGFB3, and FGF2-G3 aliquots have all been kept for ≥1 year. We have not assessed the long-term stability of the B8 supplement aliquots as these are typically used in ≤6 months.
The protocol described here utilizes FGF2-G3 generated by a core facility or in house and available for purchase from Northwestern as described in (Kuo et al., 2020), 2020. The use of commercial FGF2 increased the cost of the media by more than 4×. Conversely, we found that production of TGFB3 and NRG1 resulted in much lower yields than FGF2-G3 and, in the case of NRG1 require the 6xHis tag to be cleaved further reducing yield. For the small amounts of these two proteins to be used, we have not found it cost-effective to make them in house.
Our protocol has been successfully tested with 100s of different cell lines, either commercial or derived in house. As the number of passages increase, we have found that hiPSCs do start to grow faster in all types of pluripotent culture media, especially over passage 100, and can potentially lose their differentiation potential. The proposed B8 and B8T media in addition to the suggested culture procedures in this protocol cannot revert this biological process.
Troubleshooting
Problem 1
When preparing B8 supplement aliquots, L-ascorbic acid 2-phosphate does not dissolve in water at the concentration mentioned in the protocol.
Potential solution
Verify if the L-ascorbic acid 2-phosphate being used is the L-ascorbic acid 2-phosphate trisodium salt, as only this salt can be used at high concentration. If using other salts, change the concentrations accordingly, and pH.
Problem 2
Significant level of cell death is observed after thawing.
Potential solution
Make sure that the quality of the frozen cells was high before freezing, as this will affect their survival post thawing. Avoid freezing cells that left the logarithmic growth phase (Figure 3E), passage them 2–3 times to allow them to return logarithmic growth before freezing. Additionally, vials should be transferred to liquid nitrogen within 2–24 h.
Problem 3
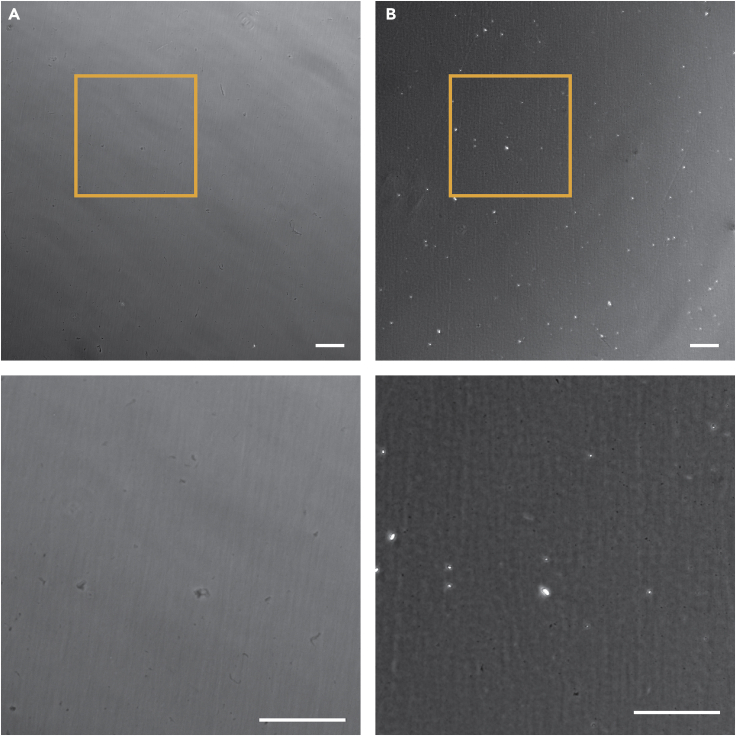
Cells are not attaching to the Matrigel-coated plates when passaged.
Potential solution
Ascertain the quality of the Matrigel-coated plates, verifying if they have not dried out (see Figure 5). If they have (Figure 5B), discard them and coat new plates before passaging the hiPSCs. Make sure the cells were not more than 80% confluent at the time of passaging (Figure 3D). Overgrown cells (Figure 3E) will be contact inhibited and will present decrease quality and variability in their cell cycle stage. This can lead to decreased attachment to the plates, in addition to slow growth after attachment/passaging. Make sure to keep the cells in EDTA for at most 6 min. Keeping cells in EDTA for longer will result in cells detaching from the plate during passaging and being aspirated when replacing the solution with B8T medium. A reduced number of cells will decrease the number of cells that will successfully attach to the plates after passaging, hindering the growth of the hiPSCs. Lastly, as before, check the media date. If B8 and B8T media are more than 1 month old, discard them, and make new.
Figure 5.
Visual assesment of quality of Matrigel-coated plates
Representative images of (A) Matrigel layer in good condition; (B) Matrigel layer that has dried out. Bottom row: zoomed in versions of inserts in the top images. Scale bar, 100 μm.
Problem 4
hiPSCs are growing slowly after plated.
Potential solution
If hiPSCs overgrew before passaging (>90% confluency, Figure 3E), they will have left the logarithmic growth phase and will require couple passages before returning to it. If the cells were passaged too early (<60% confluency, Figure 3C), were passaged at too high split ratio (1:15 or 1:20) when they were already growing slowly, or were kept in EDTA for a long interval (>6 min) the number of plated cells will be too small at the beginning of the culture and will take longer for them to return to the correct growth ratio. Therefore, make sure to keep a consistent passaging schedule (number of days) to avoid this process. Make sure that the media exchange schedule is consistent with the desired outcomes and protocols (e.g., weekend-free culture as proposed here). Similar to before, if the Matrigel-coated plates have dried out (Figure 5B), the cells will not attach properly or not attach at all, leading to poor growth. Make sure to always use good quality Matrigel-coated plates. Lastly, ascertain that the B8 and B8T media are not more than a month old. If that is the case, make a new set.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Paul W. Burridge (paul.burridge@northwestern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze new datasets/code.
Additional resources
Up-to-date versions of this protocol are available at: https://labs.feinberg.northwestern.edu/burridge/b8/index.html
Acknowledgments
This work was supported by NIH NCI grant R01 CA220002, American Heart Association Transformational Project Award 18TPA34230105, and the Foundation Leducq (P.W.B.).
Author contributions
Conceptualization, P.W.B.; Writing – Original Draft, D.M.L.; Writing – Review & Editing, D.M.L., H.F., and P.W.B.; Figures, D.M.L., H.F., and M.G.; Funding Acquisition, P.W.B.
Declaration of interests
The authors declare no competing interests.
References
- Burridge P.W., Matsa E., Shukla P., Lin Z.C., Churko J.M., Ebert A.D., Lan F., Diecke S., Huber B., Mordwinkin N.M. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyganek L., Tiburcy M., Sekeres K., Gerstenberg K., Bohnenberger H., Lenz C., Henze S., Stauske M., Salinas G., Zimmermann W.H. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3:e99941. doi: 10.1172/jci.insight.99941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forristal C.E., Wright K.L., Hanley N.A., Oreffo R.O., Houghton F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Nath S.C., Jiao X., Zhou R., Nishikawa S., Krawetz R., Li X., Rancourt D.E. Post-passage rock inhibition induces cytoskeletal aberrations and apoptosis in Human embryonic stem cells. Stem Cell Res. 2019;41:101641. doi: 10.1016/j.scr.2019.101641. [DOI] [PubMed] [Google Scholar]
- Guo C.W., Kawakatsu M., Idemitsu M., Urata Y., Goto S., Ono Y., Hamano K., Li T.S. Culture under low physiological oxygen conditions improves the stemness and quality of induced pluripotent stem cells. J Cell Physiol. 2013;228:2159–2166. doi: 10.1002/jcp.24389. [DOI] [PubMed] [Google Scholar]
- Hogrebe N.J., Augsornworawat P., Maxwell K.G., Velazco-Cruz L., Millman J.R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020;38:460–470. doi: 10.1038/s41587-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijk E., Jager M., van der Roest B., Locati M.D., Van Hoeck A., Korzelius J., Janssen R., Besselink N., Boymans S., van Boxtel R. The mutational impact of culturing human pluripotent and adult stem cells. Nat. Commun. 2020;11:2493. doi: 10.1038/s41467-020-16323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H.H., Gao X., DeKeyser J.M., Fetterman K.A., Pinheiro E.A., Weddle C.J., Fonoudi H., Orman M.V., Romero-Tejeda M., Jouni M. Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Reports. 2020;14:256–270. doi: 10.1016/j.stemcr.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laco F., Woo T.L., Zhong Q., Szmyd R., Ting S., Khan F.J., Chai C.L.L., Reuveny S., Chen A., Oh S. Unraveling the Inconsistencies of Cardiac Differentiation Efficiency Induced by the GSK3β Inhibitor CHIR99021 in Human Pluripotent Stem Cells. Stem Cell Reports. 2018;10:1851–1866. doi: 10.1016/j.stemcr.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T., Thomson J.A. Defined, feeder-independent medium for human embryonic stem cell culture. Curr. Protoc. Stem Cell Biol. 2007;Chapter 1 doi: 10.1002/9780470151808.sc01c02s2. Unit 1C 2. [DOI] [PubMed] [Google Scholar]
- Mathieu J., Zhang Z., Nelson A., Lamba D.A., Reh T.A., Ware C., Ruohola-Baker H. Hypoxia induces re-entry of committed cells into pluripotency. Stem Cells. 2013;31:1737–1748. doi: 10.1002/stem.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J., Zhou W., Xing Y., Sperber H., Ferreccio A., Agoston Z., Kuppusamy K.T., Moon R.T., Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592–605. doi: 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narva E., Pursiheimo J.P., Laiho A., Rahkonen N., Emani M.R., Viitala M., Laurila K., Sahla R., Lund R., Lahdesmaki H. Continuous hypoxic culturing of human embryonic stem cells enhances SSEA-3 and MYC levels. PLoS One. 2013;8:e78847. doi: 10.1371/journal.pone.0078847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So S., Lee Y., Choi J., Kang S., Lee J.Y., Hwang J., Shin J., Dutton J.R., Seo E.J., Lee B.H. The Rho-associated kinase inhibitor fasudil can replace Y-27632 for use in human pluripotent stem cell research. PLoS One. 2020;15:e0233057. doi: 10.1371/journal.pone.0233057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O., von Meyenn F., Hewitt Z., Alexander J., Wood A., Weightman R., Gregory S., Krueger F., Andrews S., Barbaric I. Low rates of mutation in clinical grade human pluripotent stem cells under different culture conditions. Nat. Commun. 2020;11:1528. doi: 10.1038/s41467-020-15271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhu X., Hahm H.S., Wei W., Hao E., Hayek A., Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl. Acad. Sci. U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Takahashi K., Okita K., Ichisaka T., Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze new datasets/code.