Abstract
This project was aimed to formulate and characterize mucoadhesive buccal tablets of aceclofenac, utilizing different proportions of three polymers carbopol 934, hydroxypropyl methylcellulose, and sodium carboxymethylcellulose. Twelve batches of buccoadhesive aceclofenac were prepared by the direct compression method. The compressed tablets were then evaluated for physicochemical parameters such as hardness, thickness, weight variation, drug content, friability, swelling index, surface pH, and ex vivo mucoadhesion. In vitro dissolution test was conducted for 12 h according to Indian Pharmacopeia 2018, using the rotating paddle method in phosphate buffer of pH 7.4. Physiochemical parameters like weight variation (231.25–268.75 mg), hardness (8.32–11.56 kg), friability (0.04–0.2%), diameter (9.00 mm), thickness (3.8–4.05 mm), and drug content ((97.67–102.25%) were within the acceptable limit as per Indian Pharmacopeia 2018. The swelling index was reported to be in the range of 112.93–450.19%, at 8 h. The surface pHs of all the batches were in between 6.72 to 6.96. The mucoadhesive strengths (40.5–50 g) varied with the change in polymer concentrations especially of carbopol 934. The dissolution profile of all the batches varied greatly, with a maximum release of 109.41% (in batch 12 at 6 h) to a minimum release of 44.82% (in batch 3 at 12 h). Among them, only batch 1 ensured sustained and effective drug release (88.34% at 12 h) with appropriate swelling index (112.93%) and mucoadhesive strength (40 g). Fourier Transform Infrared Spectroscopy analysis showed no evidence of drug excipients interaction. Hence, the results concluded that buccal mucoadhesive aceclofenac tablets can be formulated. Furthermore, the property of the tablet not only depends on the concentration but also the behavior of the polymers used.
Keywords: Aceclofenac, Buccal tablets, Mucoadhesion, Swelling index
Aceclofenac; Buccal tablets; Mucoadhesion; Swelling index
1. Introduction
The oral route of drug administration is the most common and preferred route for drug delivery, as it enables easy ingestion, self-medication, accurate dosage, flexible and controlled dosing schedule, and patient compliance with a low chance of administration difficulty [1, 2]. It also has some major disadvantages such as the first-pass effect, gastrointestinal enzymatic degradation, and slow onset of action [3]. To overcome these disadvantages, mucoadhesive drug delivery and sublingual drug delivery could be better alternatives [4].
Mucoadhesive dosage forms are specially designed to adhere to the mucosal surface, thus intensifying retention of the drug at the site of application, while providing a controlled rate of drug release for better therapeutic outcome [5]. To mention, a few mucoadhesive drug delivery systems are adhesive patches, adhesive gels, adhesive tablets, adhesive films, adhesive discs, etc. [6]. Several regions such as the gastrointestinal (GI) tract, the urogenital tract, the ear, the nasal route, and the airways in the body are lined by the mucosal layer. These are either single-layered epithelium found in the GI tract, bronchi, and intestines or multilayered stratified epithelium found in the esophagus, vagina, and cornea and are the potential sites where mucoadhesive drug delivery systems can be useful [6, 7].
Buccal mucosa is one of such mucosal site which has a high extent of vascularization and enables direct drain of blood flow into the jugular vein, which helps to avoid the possible metabolism of drugs by the gastrointestinal route and liver [8]. The buccal delivery thus implies the absorption of medication through the mucosal lining of the buccal cavity. Easier drug administration, the possibility of prompt termination in the condition of unpredicted side effects and emergencies, the possibility of incorporating enzyme inhibitor/permeation enhancer, etc. are other major advantages of this drug delivery system [9, 10].
Various mucoadhesive polymers (natural, semi-synthetic, and synthetic) used in this delivery system become adhesive on hydration [11], therefore can be used for targeting a drug to a particular region of the body. Initially, when the mucoadhesive product is in contact with the mucosal membrane, it swells and spreads, initializing deep contact with the mucosal layer and then mucoadhesive materials (polymers) are activated by the presence of moisture and drug releases slowly [12].
On the other hand, aceclofenac is a potent cyclooxygenase-2 (COX-2) inhibitor, a newer non-steroidal anti-inflammatory drug (NSAID) with good anti-inflammatory, analgesic, and anti-pyretic activity, most commonly used for the treatment of osteoarthritis, rheumatoid arthritis, dental pain, and other rheumatoid disorders. It is an aryl acetic acid derivative, insoluble in water and highly permeable. It is characterized as a biopharmaceutical classification system (BCS) class II drug [13, 14]. It is highly protein-bound and possesses a short biological half-life of 4–4.3 h. The usual dose of aceclofenac is 100 mg twice or thrice daily [15]. The conventional dosage form of aceclofenac leads to a lot of inconvenience and fluctuations in therapy, with some adverse effects like gastrointestinal disturbances, peptic ulceration, and gastrointestinal bleeding. Thus, devising sustained-release medication is a good alternative for reducing its dosing frequency, for prolonged effect with improved bioavailability, while also improving safety and efficacy of the medication [13]. This study was designed to formulate the different batches of mucoadhesive aceclofenac tablets by using different polymers like carbopol 934, hydroxypropyl methylcellulose, and sodium carboxymethylcellulose along with their quality control evaluation.
2. Materials and methods
2.1. Drug and chemicals
Aceclofenac (99.97% pure with loss on drying 0.34%) was obtained as a gift from Time Pharmaceuticals Pvt. Ltd, Nepal. Carbopol 934 (CP) was purchased from Himedia Laboratories India. Hydroxypropyl methylcellulose (HPMC) and sodium carboxymethylcellulose (SCMC) were purchased from Loba Chemie Pvt. Ltd, Mumbai. Magnesium stearate, micro crystalline cellulose powder 200 (MCCP 200), and talc were purchased from Sigma-Aldrich, Inc. (St Louis, MO, USA). All the chemicals and reagents used were of analytical grade.
2.2. Instruments
High Performance Liquid Chromatography (HPLC) (prominence-i LC-2030, Shimadzu, Japan), FTIR Spectrophotometer (Perkin-Elmer FTIR, Perkin-Elmer, USA), Dissolution apparatus and digital hardness tester (Electrolab India), Friability tester (Toshiba, India), UV spectrophotometer and vernier caliper (Shimadzu, Japan), laboratory water purification system (HiTech Instruments Co. Ltd, China), Tablet compression machine (punch) 10 station (Shiva Pharma Engineering India).
2.3. Formulation of aceclofenac mucoadhesive tablets
Mucoadhesive tablets were prepared by adopting a previously established method with slight modification [16]. Direct compression technique was applied for the tablet compression, using varying proportions of different grades of polymer. All the powders in pure form were accurately weighed. Aceclofenac was then mixed with CP. The remaining polymers were mixed with talc in a separate pouch. These two mixtures were then mixed for 5 min after passing through a 40 mesh sieve. MCCP 200 and aerosil were mixed in a separate pouch for 2 min. Then it was mixed with the previous mixture for 5 min. Finally, magnesium stearate was added and the resultant mixtures were mixed and the blend was then compressed into tablets having an average weight of 250 mg, using a ten station tablet punch. Twelve batches were prepared and coded from B1 to B12. The details of the composition of each batch were obtained from the previous study [17], with some modifications as given in Table 1.
Table 1.
Composition of various batches of mucoadhesive buccal tablets of aceclofenac.
| B1 (mg) | B2 (mg) | B3 (mg) | B4 (mg) | B5 (mg) | B6 (mg) | B7 (mg) | B8 (mg) | B9 (mg) | B10 (mg) | B11 (mg) | B12 (mg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aceclofenac | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CP | 25 | 30 | 35 | 40 | 25 | 30 | 35 | 40 | 25 | 30 | 35 | 40 |
| HPMC | 60 | 55 | 50 | 45 | - | - | - | - | 30 | 27.5 | 25 | 22.5 |
| SCMC | - | - | - | - | 60 | 55 | 50 | 45 | 30 | 27.5 | 25 | 22.5 |
| Magnesium stearate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| MCCP 200 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Talc | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Aerosil | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Grand total | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
2.4. Evaluation of tablet properties
Different quality control parameters of all the batches of mucoadhesive aceclofenac tablets were analyzed by adopting the method described in Indian Pharmacopeia 2018 [18].
2.4.1. Weight variation
Twenty tablets (n = 20) from each batch were weighed using electronic balance and their average weight was calculated.
2.4.2. Friability
Twenty tablets (n = 20) of each batch were weighed and put into the friabilator drum. After 100 revolutions of friabilator, tablets were recovered. The tablets were then freed from dust and weighed. Friability was calculated from the Eq. (1).
| (1) |
2.4.3. Hardness
Twenty tablets (n = 20) were taken for the hardness test using a hardness tester. The tablet was placed between the two probes, of which, one is a movable probe and another is an immovable probe of the hardness tester. Then the force was applied from the movable probe. The force to break the tablet was recorded, which was taken as the hardness of the tablet.
2.4.4. Drug content
2.4.4.1. HPLC chromatographic condition for drug content determination
For the drug content assay of newly formulated batches, a reverse phase HPLC system was used. The chromatographic condition for the analysis was selected from pharmacopeial assay for aceclofenac tablets (IP 2018) [18]. The system consisted of a UV-visible detector set at 275 nm, and an autosampler set at 20 μL injections with a 1.5 mL/min flow rate. The output signal of the UV-visible detector was recorded by using data-based lab solution software. The chromatographic separation was carried out using a C18 column (Shimadzu, 4.6 mm i.d, 5.0 μm particle size, 150 mm length). The mobile phase consisted of the isocratic elution of solution (A). The elution of the solvent system was continued up to 1.5 times of the retention time for the standard aceclofenac. To confirm the system suitability, standard solution was injected 5 times consecutively, and then average tailing factor, average number of theoretical plate (NTP), and RSD of the area were calculated [19].
Solution A- A mixture of 55 volumes of buffer solution prepared by adding 1.0 mL of glacial acetic acid in 1000 mL of water and 45 volumes of acetonitrile.
2.4.4.2. Preparation of standard and sample solution for HPLC
For the assay, 1 mg/mL of aceclofenac standard stock solution was prepared by dissolving 100 mg of standard in 100 mL HPLC grade acetonitrile, with the help of ultrasonic water bath. The stock solution was subjected for 10 fold dilution in 50 mL volumetric flask by using solvent mixture (55 volumes of acetonitrile and 45 volumes of water). Similarly, to prepare the sample solution, 20 tablets from each batch were crushed into very fine powder in a dried mortar and pestle. Drug powder equivalent to 100 mg of active material (around 250 g drug powders) was weighed and transferred into 100 mL of volumetric flask. Around 60 mL of acetonitrile was poured and all samples were subjected to sonication for 30 min, volume was diluted up to 100 mL. After the filtration, the sample solution was again diluted 10 fold same as the standard solution. Finally, both sample and standard solutions were filtered by using 0.22 μm filters (PTFE filters, Thermo Scientific, Waltham, MA, USA) for the injection. The assay of each batch was determined by Eq. (2).
| (2) |
(Abbreviations- sp: sample, std: standard, Wsp: weight of sample powder taken, Wstd: weight of standard drug taken, LOD: loss on drying for standard, Avg wt: average weight of 20 tablets.)
2.4.5. Mucoadhesion test
Porcine buccal mucosa was used as a model mucosal surface for bioadhesion test. Immediately after slaughter, the buccal mucosa was removed from the pig and transported to the laboratory in tyrode solution and kept at room temperature. Mucoadhesive forces of the tablets (n = 3) were determined utilizing modified balance using strips of the porcine buccal mucosa washed with tyrode solution.
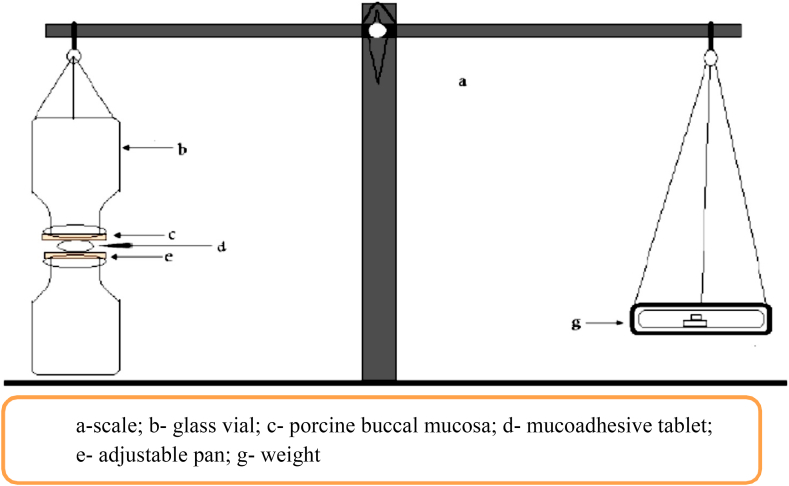
The mucoadhesive forces of the tablets were determined by the modified pan balance as shown in Figure 1. The porcine buccal mucosa was cut into the appropriate size pieces and washed with tyrode solution. During the test, a section of buccal mucosa (c) was fitted on the upper glass vial (b) using a rubber band. The exposed mucosa had a diameter of 1 cm. The vial with buccal mucosa (b) was stored in the tyrode solution for 10 min at room 37 °C. Then, the vial with buccal mucosa (b) and another vial (e) were fixed on adjusted height which was equal to the thickness of the tablet. To the lower vial, the tablet was placed with the help of bilayered adhesive tape. The position of both vials was adjusted so that the adhesive tape and the buccal mucosa get attached. A constant force was applied to the upper vial to get the tablets attached to buccal mucosa uniformly for 2 min, and then the upper vial was connected to the balance. Then the weight on the right pan was slowly increased by 0.5 g until two vials get detached from each other. The total weight (g), to detach was recorded as the measure of mucoadhesive strength [20].
Figure 1.
General lay out of modified pan balance used for the determination of mucoadhesion force of newly formulated tablets [20].
2.4.6. Swelling test
From each batch, three tablets were individually weighed (W1) and placed separately in petri dishes with 5 mL phosphate buffer of pH 6.8. At the time interval of 1, 2, 4, and 8 h, they were taken out from the petri dish and excess water was removed by using filter paper. The swollen tablets were reweighed (W2) and the percentage of hydration was calculated for each tablet, using the Eq. (3) [21].
| (3) |
2.4.7. In vitro dissolution studies
In vitro dissolution was conducted by using the method, specified in the Indian Pharmacopoeia 2018 [18]. The rotating paddle method was used to study drug release from the tablets. Six tablets (n = 6) were taken for the dissolution study. The dissolution medium consisted of 900 mL of phosphate buffer of pH 7.4. The test was performed at 37 °C ± 0.5 °C at a rate of 50 rpm. Total 5 mL samples were withdrawn at every hour and the same volume was replaced with a fresh medium. Samples withdrawn were diluted to 50 mL with the buffer. The samples were filtered and analyzed by using an ultraviolet spectrophotometer at 273 nm. The percentage of drug release was calculated using the calibration curve of the standard drug [18]. For the calibration curve, the stock solution of aceclofenac was prepared in phosphate buffer pH 7.4, at a concentration of 32 mg/mL. The stock solution was diluted to prepare the solution of different concentrations from 0.25 μg/mL to 13 μg/mL and the absorbance was measured using a UV spectrophotometer at a wavelength of 273 nm.
2.4.8. Compatibility study
For the drug excipient compatibility study, infrared (IR) spectroscopy was conducted using a FTIR spectrophotometer and the spectrum was recorded in the wavelength region of 1950 to 400 cm−1. The procedure consisted of dispersing a sample (drug alone or mixture of drug and excipients) in potassium bromide and compressed into discs by applying a pressure of 5 tons for 5 min in a hydraulic press. The pellet was placed in the light path and the spectrum was obtained [13].
2.4.9. Surface pH studies
The pHs of three tablets (n = 3) from each batch were determined. The tablets were placed in distilled water maintained at pH 6.8 and allowed to swell up to 2 h. The surface pH of the tablet was determined by using a pH meter electrode [21].
2.4.10. Determination of release kinetics
Dissolution data obtained were fitted to zero-order, first-order, Higuchi, Hixson Crowell, and Korsmeyer-Peppas equations to understand the rate and mechanism of aceclofenac release from the prepared batches. The zero-order release rate describes the system where the drug release rate is independent of its concentration. The first-order release rate describes the release from the system as concentration-dependent, which shows log cumulative percent drug remaining versus time. Higuchi's model describes the release of the drug from an insoluble matrix as a square root of a time-dependent process based on Fickian diffusion. Higuchi's root kinetics shows the cumulative percentage drug release versus the square root of time. Hixson Crowell model describes the drug release from the system where there is a change in surface area and diameter of particles or tablets. R2 is a statistical measure of how close the data are to the fitted regression line. The value close to 1 was considered as the most preferred one [13, 22].
2.5. Statistical analysis
Values were expressed as mean ± SD. Post Hoc Tukey test followed by one way-ANOVA was used for statistical analysis of mucoadhesive strength of different batches. P-value less than 0.05 (p˂0.05) was considered to be statistically significant. For kinetic studies Kinet DS 3.0 software was used.
3. Results
3.1. Optimization of chromatographic conditions and calibration curve for standard
The chromatographic condition described by the IP 2018 for the analysis of aceclofenac tablet was adopted for the quantitative analysis of newly formulated batches of aceclofenac mucoadhesive tablets. The method was verified for the analysis, after confirming the system suitability. The analysis time was extended up to 15 min. In the system suitability examination, average tailing factor, average number of theoretical plate (NTP), and RSD of the area were found to be 1.61,7231, and 0.39 respectively, which comply with the given criteria of IP 2018.
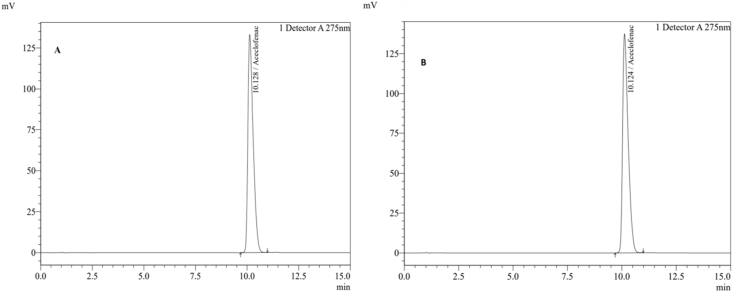
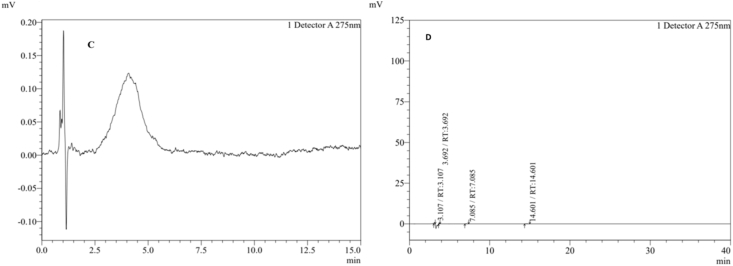
Figure 2 represent the chromatograms of standard and test sample. Similarly, the chromatogram of the blank and placebo sample is given in Figure 3. As shown in Figure 3, no significant additional peaks were observed in the blank and placebo sample. Furthermore, the chromatographic patterns of all the formulated batches (B1–B12) were almost similar to the standard drug chromatogram (Figure 2).
Figure 2.
HPLC Chromatogram of the standard and test sample (A: standard drug Aceclofenac, B: Batch sample).
Figure 3.
HPLC chromatogram of the blank and placebo (C: Blank, D: Placebo).
Similarly, to calculate the dissolution profiles of different batches, the calibration curve equation (Y = 0.0239X+0.0043) was achieved by plotting absorbance versus concentration of the standard. Since the correlation coefficient (R2) of the standards was 0.9984, linearity was proved.
3.2. Evaluation of tablet properties
3.2.1. Weight variation, hardness, thickness, friability, diameter, and surface pH of the tablets
Numerical values of all the quality control parameters, investigated for 12 different batches are depicted in Table 2. In the weight variation study, the weight of the tablet varied between 249.6 ± 2.39 to 253.75 ± 4.01 mg which was within the Indian Pharmacopeial limit i.e. 231.25–268.75 mg (7.5% deviation).
Table 2.
Numerical data for the different quality control evaluation parameters of the newly formulated 12 different batches.
| Batch | Wt. Variation |
Hardness |
Friability |
Diameter |
Thickness |
Surface pH |
Drug content |
|---|---|---|---|---|---|---|---|
| (mg± SD) | (Kg± SD) | (%) | (mm) | (mm± SD) | ± SD | (%± SD) | |
| B1 | 249.7 ± 2.20 | 9.22 ± 1.52 | 0.20 | 9 | 3.93 ± 0.09 | 6.96 ± 0.2 | 99.44 ± 1.2 |
| B2 | 249.6 ± 2.39 | 8.32 ± 1.36 | 0.14 | 9 | 4 ± 0.00 | 6.72 ± 0.4 | 97.67 ± 0.56 |
| B3 | 250.45 ± 1.98 | 10.57 ± 1.38 | 0.04 | 9 | 3.94 ± 0.06 | 6.85 ± 0.1 | 98.95 ± 1.26 |
| B4 | 250.75 ± 1.97 | 11.14 ± 1.07 | 0.07 | 9 | 4.03 ± 0.04 | 6.88 ± 0.25 | 98.00 ± 1.36 |
| B5 | 252.55 ± 2.03 | 8.99 ± 0.65 | 0.09 | 9 | 4.05 ± 0.05 | 6.84 ± 0.1 | 103.03 ± 0.76 |
| B6 | 251.75 ± 1.91 | 9.41 ± 0.81 | 0.05 | 9 | 4 ± 0.00 | 6.86 ± 0.2 | 101.16 ± 2.01 |
| B7 | 249.85 ± 1.98 | 9.50 ± 0.86 | 0.13 | 9 | 3.8 ± 0.00 | 6.86 ± 0.32 | 99.96 ± 0.87 |
| B8 | 249.7 ± 2.55 | 11.25 ± 1.34 | 0.11 | 9 | 3.9 ± 0.00 | 6.80 ± 0.15 | 98.90 ± 1.13 |
| B9 | 251.2 ± 2.33 | 9.17 ± 0.81 | 0.15 | 9 | 4 ± 0.00 | 6.88 ± 0.23 | 99.32 ± 1.43 |
| B10 | 253.75 ± 4.01 | 10.29 ± 0.56 | 0.15 | 9 | 4 ± 0.00 | 6.95 ± 0.32 | 99.86 ± 0.32 |
| B11 | 249.85 ± 3.97 | 9.78 ± 1.33 | 0.09 | 9 | 4 ± 0.00 | 6.84 ± 0.43 | 102.21 ± 2.13 |
| B12 | 251.35 ± 3.71 | 11.56 ± 1.36 | 0.07 | 9 | 4 ± 0.00 | 6.82 ± 0.19 | 101.53 ± 1.76 |
Tablet thickness was almost uniform in all the batches and found to be between 3.8 ± 0.00 to 4.05 ± 0.05 mm. Also, the diameter was found to be uniform (9 ± 00 mm) in all batches. Similarly, the friability of tablets ranged from 0.04 to 0.2%, which was within the acceptable range as mentioned in IP i.e. below 1%, indicating that the tablets of all batches are having good compactness and showing enough resistance to mechanical shock and abrasion. In the hardness test, the harnesses for different batches were found to be between 8.32 ± 1.36 to 11.56 ± 1.36 kg that indicates the hardness of the tablets was within the pharmacopoeial limit i.e. above 5 kg. Moreover, the surface pH of all the batches were in between 6.72 to 6.96, which was close to the neutral pH and all the batches were in the acceptable range of buccal pH 6.5 to 7.5.
3.2.2. Drug content
As shown in Table 2, the drug content varied between 97.67 to 103.03% which reflects good uniformity in drug content among different batches.
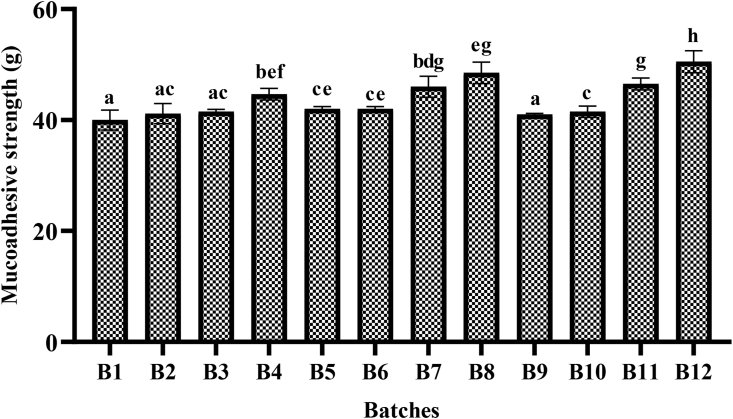
3.2.3. Mucoadhesive strength
The in vitro mucoadhesive strength study was performed and results are shown in Figure 4. On the modified pan balance, the force required to detach the tablet from the porcine buccal mucosa was recorded. The mucoadhesive properties were reported to be influenced by the nature and amount of bioadhesive polymers used in the formulation. In this study, the mucoadhesive strength of the formulations was reported to be prominently influenced by the concentration of CP. The lowest value was reported in B1 (40 g) in which the lowest proportion of CP was incorporated. Similarly highest value was reported in B12 (50.5 g) which has the highest amount of CP, among other batches.
Figure 4.
Mucoadhesive strength of different batches calculated by using modified pan balance. Note: Different alphabet (a–h) indicate significance difference among the batches (p < 0.05).
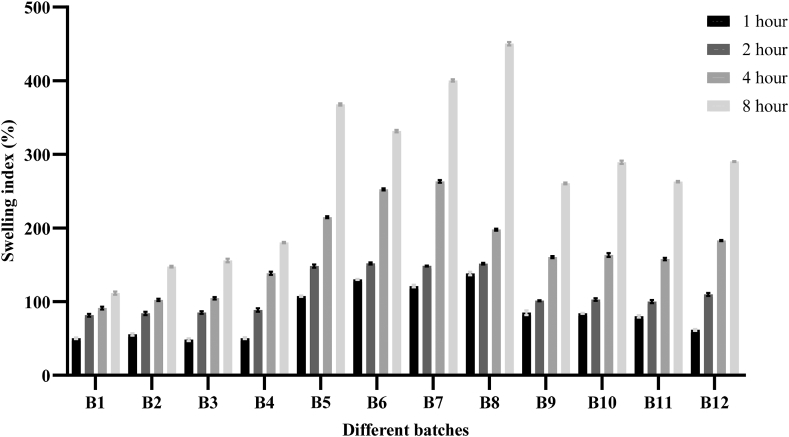
3.2.4. Swelling index
The swelling property of all the batches was performed by evaluating the swelling index at different time intervals (1, 2, 4, and 8 h) and the results are depicted in Figure 5. All the formulations showed an appreciable increase in swelling index, proportional to the time increased, and achieving maximum swelling effect at 8 h. The tablets did not show any significant change in their morphological shape and form, throughout the study. The highest swelling was shown by the batch B8 containing CP and SCMC i.e. 450.19% whereas the lowest swelling behavior was shown by the batch B1 (112.23%) containing HPMC and CP.
Figure 5.
Comparative study of swelling index for 12 batches of aceclofenac mucoadhesive tablets at different time intervals.
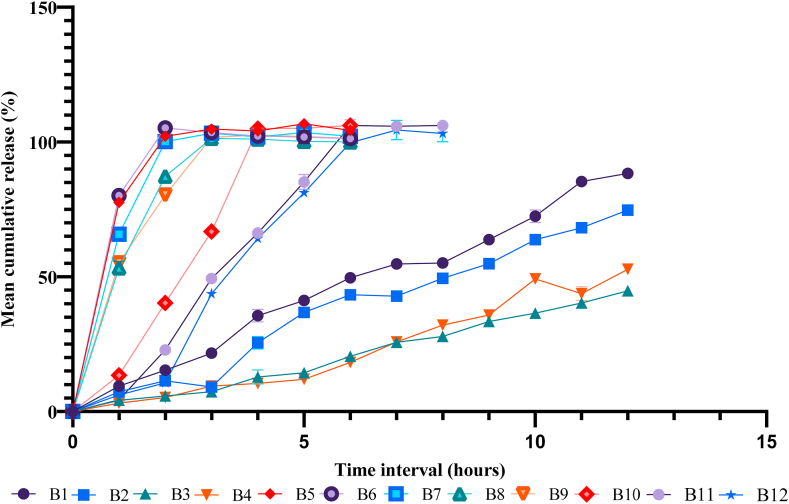
3.2.5. In vitro dissolution studies
To explore the effect of polymer composition and proportion in drug release behavior, the in vitro dissolution study of formulated batches of mucoadhesive tablets was carried out and the results are presented in Figure 6. The highest and lowest drug release was observed in B12 (106.14%) and B3 (44.82%) respectively. In the case of batches with CP and HPMC (B1–B4), the drug release was found to be relatively low even after 12 h, i.e. 88.34%, 74.72%, 44.82%, and 52.82%, respectively. Similarly, the higher drug release patterns were shown by the combination of CP and SCMC (B5–B8), where the drug release was reported to be 104.82%, 103.5%, 103.24%, and 101.34%, respectively within 3 h. Furthermore, an almost similar drug release pattern was observed in the batches having the combination of all three polymers (B9–B12). In batches B9 and B10, the drug release was found to be 102.57%, 104.96%, respectively in 4 h and it reached up to 106.14% in 6 h for B11 and the drug release of batch B12 was 104.43% in 7 h.
Figure 6.
Dissolution profile of different batches of aeclofenac mucoadhesive tablets.
3.2.6. Determination of release kinetics
The data obtained from in vitro dissolution studies were fitted into mathematical models. The R2 and n values for zero-order, first-order, Higuchi, Hixson Crowell, and Korsmeyer-Peppas models were illustrated in Table 3. In the kinetics study, the order of drug release from all the batches was studied by plotting the log percentage cumulative retained versus time curve. Observation of higher R2 values for zero-order and first-order indicated that B1 and B2 seems to fit better with first order kinetics whereas the release pattern of all other batches seem to be suitable with zero order kinetics. From Table 3, it is confirmed that the batches B1, B2, and B4 follow Hixson-Crowell kinetic model i.e. they release by erosion mechanism [20]. The remaining batches B3 and B5–B12 follow Korsmeyer-Peppas models i.e their drug release mechanism cannot be described exactly or more than one type of release is involved [10].
Table 3.
Release kinetics data of all the batches.
| Batch | Zero order |
First order |
Higuchi |
Hixson-Crowell |
Korsmeyer-Peppas |
|
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | |
| B1 | 0.9345 | 0.9780 | 0.3690 | 0.9872 | 0.9110 | 1.1758 |
| B2 | 0.9719 | 0.9757 | 0.5643 | 0.9884 | 0.9329 | 0.9568 |
| B3 | 0.9877 | 0.9120 | 0.6058 | 0.9699 | 0.9882 | 1.0244 |
| B4 | 0.9629 | 0.9370 | 0.4562 | 0.9745 | 0.9638 | 1.1678 |
| B5 | 0.4286 | 0.4286 | -6.5375 | 0.4286 | 0.6572 | 0.1227 |
| B6 | 0.4286 | 0.4286 | -8.8953 | 0.4286 | 0.6572 | 0.1085 |
| B7 | 0.4286 | 0.4286 | -1.1191 | 0.4286 | 0.6572 | 0.2178 |
| B8 | 0.6113 | 0.5803 | 0.3054 | 0.5907 | 0.8069 | 0.3320 |
| B9 | 0.6917 | 0.5984 | 0.7734 | 0.6341 | 0.8480 | 0.7384 |
| B10 | 0.7364 | 0.6406 | 0.6937 | 0.6823 | 0.8775 | 0.9517 |
| B11 | 0.9175 | 0.7322 | 0.6178 | 0.8166 | 0.9334 | 1.2080 |
| B12 | 0.8911 | 0.7259 | 0.6551 | 0.8028 | 0.9366 | 1.1242 |
n = release exponent.
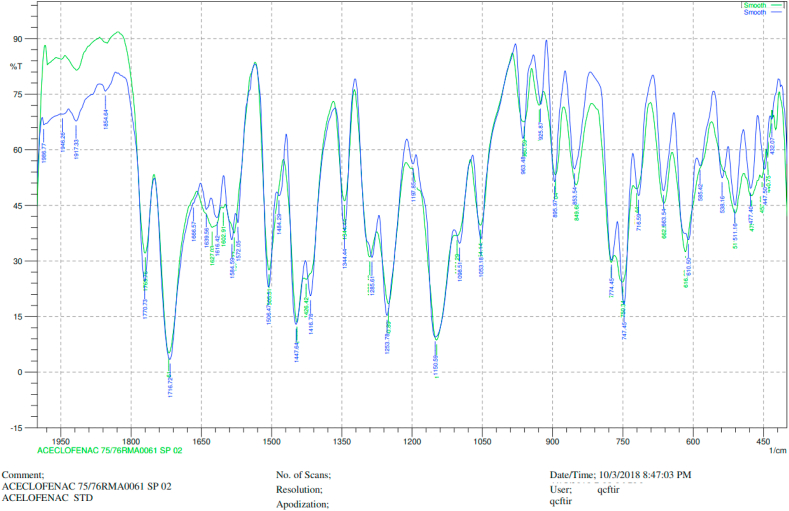
3.2.7. Compatibility studies
Drug excipient compatibility studies were carried out by an IR spectrophotometer. The IR spectra of pure aceclofenac and the drug with polymers are shown in Figure 7. There was no interaction between drug and polymers.
Figure 7.
Chromatogram of FTIR for Aceclofenac (blue color: FTIR chromatogram of standard drug Aceclofenac, green color: FTIR chromatogram of the Aceclofenac mucoadhesive tablet containing HPMC, Carbopol 934, and Sodium CMC.
4. Discussion
An ideal mucoadhesive tablet should ensure immediate and sustained drug release, to exhibit an instantaneous but prolonged pharmacological effect as well as it must have sufficient residence time in the buccal cavity [23]. To achieve these requirements, aceclofenac mucoadhesive buccal tablets were formulated and investigated for different physicochemical characters.
The tablets of all the batches were smooth, flat-faced, white, and circular with no visible cracks. The variation in weight, hardness, friability, and diameters of the entire batch was within pharmacopeial limit, ensuring formulated tablets were of standard quality. Hardness was reported to be increased with an increased proportion of CP [23]. The drug content of the batches was found to be in the range of 97.67 % (B2) to 102.25 % (B11), which falls within the prescribed limit of the pharmacopeia.
Mucoadhesive strength signifies the extent of adherence between the epithelial surface and/or mucus and a polymeric substance present in the formulation. Three major stages in the mucoadhesion process are wetting of polymer, interpenetration, and mechanical interconnection between polymer and mucus. Strength of mucoadhesion is highly affected by residence time with mucus, type of biological membrane used, swelling behavior of the polymer, average molecular weight, concentration, and composition of the polymer being used [10, 16, 22]. In our study, all the formulated batches exhibited satisfactory mucoadhesive strength ranging from 40 g to 50.5 g. The statistical analysis (one-way ANOVA followed by posthocTukey test) suggested that there was a significant difference (p < 0.05) in mucoadhesive strength, among most of the batches (Figure 4). In this study, an increased amount of CP has led to the increased mucoadhesive force proportionally, in all the batches. A similar effect was observed in previous studies also [16, 22, 24]. CP can form secondary bioadhesion bonds on the mucin. Also, high-density polymeric chains present in it can result in greater entanglement and interpenetration at the interfacial region of mucosa. This might be a possible reason for its maximum mucoadhesive effect [22, 25]. While analyzing the effect of SCMC, mucoadhesive strength was decreased with its increased amount. The same result was recorded during the formulation of the buccal bioadhesive ondansetron tablet [26]. The tendency of SCMC to disintegrate in the water might be the possible reason for this effect [27]. Besides that polymeric groups required for bioadhesion process are occupied by the water molecules and higher swelling of this polymer creates a strain of hydrogen bond and other forces [28]. However, SCMC has a better mucoadhesive effect than HPMC due to its higher viscosity [26]. Therefore, the combination of carbopol 934 and SCMC resulted in more bioadhesive strength as compare to the combination of CP and HPMC.
The surface pH of the tablets was in the range of 6.72–6.96, which was almost similar to the pH of the buccal cavity. Therefore, all the batches do not produce mucosal irritation and discomfort and improve patient compliance [29, 30].
Swelling of the mucoadhesive tablet to the optimum extent is very crucial to ensure the prolonged and steady release of the drug with successful mucoadhesion. In this study, the swelling index of all the batches was improved with the increase in time. This is due to the gradual absorption of water by the hydrophilic polymers used [4]. As shown in Figure 5, the swelling index for the batches containing CP and SCMC (B5–B8) was found to be a maximum. Additionally, the swelling behavior of all the batches was improved by increasing the proportion of CP. A similar effect was observed in the previous studies [24, 31]. Contradictorily, another study concluded that the swelling index is inversely proportional to the amount of CP used in the formulation [20]. The swelling index is correlated with the hydrophilicity of cellulose derivatives. This usually varies according to the degree of substitution and to some extent with the viscosity of polymers. The hydrophilic polymer SCMC increased the surface wettability and consequently water penetration within the matrix. The SCMC is a hydrophilic polymer and can absorb water, so maximum swelling was seen with batches containing a high proportion of SCMC and CP [12, 26]. Moreover, the batches containing CP and HPMC (B1–B4) showed the least swelling index. Perioli et al. reported that due to the slow hydrating nature, the swelling index of the tablet decreases with an increase in HPMC proportion [3]. The swelling index of bioadhesive polymer is substantially associated with its mucoadhesive character. Polymers having the highest initial rate of swelling also may have the highest mucoadhesion strength [ 28]. Also in this study, there is a linear correlation between mucoadhesive strength and swelling index. Thus, the swelling index of the polymer might be the primary factor contributing to mucoadhesive strength. So, further research is necessary to confirm this result.
The in vitro drug release studies revealed that the release of aceclofenac depends upon the nature and proportion of polymers used. In the case of the batches containing CP and HPMC (B1–B4), the percentage of drug release was comparatively low even after 12 h. It has been reported that the drug release profile is decreased with increasing the proportion of HPMC [23, 26]. Slowly hydrating nature and comparatively low viscosity of the HPMC may lower the rate of dissolution [27]. HPMC can facilitate a prolonged drug release, as it swells steadily to form a gel which is then dissolved in the water releasing the drugs [8]. Similarly, the rate of drug release is inversely proportional to the amount of CP present in the formulation. When CP comes in contact with water, it swells well and its viscosity becomes very high which ultimately hinders the drug release [16]. The lower rate of drug release in the batches B3 and B4 (having the highest amount of CP) also supported this statement. Also, in the case of the batches containing CP and SCMC (B5–B8) the percentage of drug release was very high (101.34–104.82%) even within 3 h. This can be credited to the higher extent of swelling of SCMC. This result was further proved by the swelling studies results, where the maximum swelling behavior was also shown by the batches having a high dissolution profile. Interestingly, we reported that there was a gradual decrease in dissolution rate from B5 (104.82%) to B8 (101.34 %), as the proportion of SCMC was gradually reduced.
This signifies that the drug release profile is mainly influenced by the concentration of SCMC. It is expected that the property of SCMC to uptake a higher amount of water may result in significant swelling of the polymer matrix, enabling the drug to release out rapidly [26]. In the case of the batches containing all the 3 polymers (B9–B12), the amount of CP was increased linearly whereas the proportion of HPMC and SCMC were decreased gradually. However, the change in drug release pattern was not linear. The reason behind this drug release pattern is unclear, so further study is necessary. It is to be noted that the addition of HPMC and reduction in the amount of SCMC in the batches B9–B12 had extended the time for complete release of drug as compared to B5–B8. In batches B9 and B10, the drug was released completely within 4 h whereas it took 6 and 7 h respectively for the batches B11 and B12. The reason behind the fast release from the batch B5–B12 was due to the presence of SCMC [23].
The values of n were estimated by linear regression of log (Mt/M∞) versus log (t), and these values indicated that the release of aceclofenac was found to be a Fickian diffusion (B5– B8) non-Fickian diffusion (B9), and super case II transport (B1– B4 and B10– B12).
Drug excipient compatibility studies were carried out by an IR spectrophotometer. The IR spectra of pure Aceclofenac and the drug with polymers are shown in Figure 7 respectively. The major characteristic bands on the spectra of both pure compound and formulated tablet at 1771.69 cm−1, 1717.68 cm−1, 1508.40 cm−1, 1256.68 cm−1, 1149.62 cm−1, 1056.07 cm−1, and 750.34 cm−1 were found to be similar [14]. Besides, the absence of other peaks in the tablet spectra justified that there is no interaction.
In summary, the mucoadhesive strength of the aceclofenac tablet was mainly attributed to the amount of CP present. The CP and SCMC are major contributing ingredients for the swelling index, where SCMC is a more powerful swelling agent than CP. Moreover, the dissolution profile was mainly influenced by SCMC concentration. To achieve the desired characters of the mucoadhesive tablet, the proper combination of all the polymers is crucial. Among 12 different batches, B5–B12 exhibited rapid drug release. Due to this reason, these batches could not meet the sustained release criteria. Also, they had a relatively higher swelling index than the normal [24]. Thus, further modification and study of these batches is necessary to achieve desired characters. The batches containing CP and HPMC (B1–B4), showed the effective sustain release property but the drug release was ineffective in B2–B4. Interestingly, B1 satisfied the condition of mucoadhesive strength, sustained and effective release [26, 32]. Thus this formulation was considered to be effective to meet all the criteria of mucoadhesive tablet. Moreover, this study also suggested that HPMC can play a significant role to regulate the swelling behavior, bioadhesion force, and drug release rate of the tablet. Although it has moderately swelling property, it enables steady entry and entrapment of liquid in the polymeric network, which is very significant to achieve sustained release of the drug. Thus many researchers prefer the combination of HPMC/CP mixture as a bioadhesive material [25, 28].
5. Conclusion
The study was conducted to formulate and evaluate mucoadhesive buccal tablets of aceclofenac with a sustained release property, to achieve patient compliance for the management of different types of pain. Among 12 different batches, B1 showed sustained and effective drug release, swelling index as well as mucoadhesive strengths. Its physicochemical properties also complied with the pharmacopoeial standards. The results also demonstrate that CP has a major role to increase the mucoadhesive strength. The swelling behavior of the formulation can be optimized by changing the proportion of CP and SCMC. However higher concentration of SCMC can result in abrupt release of the drugs. Therefore HPMC can play a significant role to check the swelling behavior and drug release rate. However, extensive research in suitable polymers and drug candidates is indispensable. Moreover, the formulation of an aceclofenac mucoadhesive tablet can be an effective alternative route to prevent the first-pass effect and to improve the bioavailability of aceclofenac through the mucosal membrane. It can also enhance patient compliance by fascinating extended release of the drug.
Declarations
Author contribution statement
Santosh Koirala: Performed the experiment; Analyzed and interpreted the data; Wrote the paper.
Prabin Nepal: Performed the experiments; Analyzed and interpreted the data.
Govinda Ghimire; Rojina Basnet; Ishwori Rawat: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Aashma Dahal: Performed the experiments.
Jitendra Pandey: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kalpana Parajuli-Baral: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are very much thankful to Dr. Raj Kumar Thapa and the Department of Pharmaceutical Sciences, School of Health and Allied Sciences. We are also grateful to the Time Pharmaceutical Pvt. Ltd for providing all the technical support.
References
- 1.Gilhotra R.M., Ikram M., Srivastava S., Gilhotra N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014;28(2):81–97. doi: 10.7555/JBR.27.20120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi A. Mouth dissolving tablets: a new venture in modern formulation technology. Pharma Innov. 2012;1(8):14–31. [Google Scholar]
- 3.Bhowmik D., Chiranjib B., Pankaj K., Chandira R.M. Fast dissolving tablet: an overview. J. Chem. Pharm. 2009;1(1):163–177. [Google Scholar]
- 4.Reddy P.C., Chaitanya K.S., Rao Y.M. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. DARU J. Pharm. Sci. 2011;19(6):385–403. [PMC free article] [PubMed] [Google Scholar]
- 5.Shaikh R., Singh T.R.R., Garland M.J., Woolfson A.D., Donnelly F.D. Mucoadhesive drug delivery systems. J. Pharm. Bioall. Sci. 2011;3(1):89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander A., Ajazuddin S., Tripathi D.K., Verma T., Mayura J., Patel S. Mechanism responsible for mucoadhesion of mucoadhesive drug delivery system: a review. Int. J. Appl. Biol. Pharmaceut. Technol. 2011;2(1):434–445. [Google Scholar]
- 7.Asane G.S., Nirmal S.A., Rasal K.B., Naik A.A., Mahadik M.S., Rao Y.M. Polymers for mucoadhesive drug delivery system: a current status. Drug Dev. Ind. Pharm. 2008;34(11):1246–1266. doi: 10.1080/03639040802026012. [DOI] [PubMed] [Google Scholar]
- 8.Madhav N.V., Shakya A.K., Shakya P., Singh K. Orotransmucosal drug delivery systems: a review. J. Contr. Release. 2009;140(1):2–11. doi: 10.1016/j.jconrel.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Smart J.D. Buccal drug delivery. Expet. Opin. Drug. Deliv. 2005;2(3):507–517. doi: 10.1517/17425247.2.3.507. [DOI] [PubMed] [Google Scholar]
- 10.Shanker G., Kumar C.K., Gonugunta C.S.R., Kumar B.V., Veerareddy P.R. Formulation and evaluation of bioadhesive buccal drug delivery of tizanidine hydrochloride tablets. AAPS PharmSciTech. 2009;10(2):530–539. doi: 10.1208/s12249-009-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Çelik B. Risperidone mucoadhesive buccal tablets: formulation design, optimization and evaluation. Drug Des. Dev. Ther. 2017;11:3355–3365. doi: 10.2147/DDDT.S150774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boddupali B.M., Mohammed Z.N.K., Nath R.A., Bhanji D. Mucoadhesive drug delivery system: an overview. J. Adv. Pharm. Technol. Res. 2010;1(4):381–387. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannan S., Manivannan R., Ganesan K., Nishad P.K., Kumar N.S. Formulation and evaluation of sustained release tablets of aceclofenac using hydrophilic matrix system. Int. J. Pharm. Tech. Res. 2010;2(3):1775–1780. [Google Scholar]
- 14.Kabir A.K.L., Halder S., Shuma M.L., Rouf A.S.S. Formulation development and in vitro evaluation of drug release kinetics from sustained release aceclofenac matrix tablets using hydroxypropyl methyl cellulose. Dhaka Univ. J. Pharm. Sci. 2012;11(1):37–43. [Google Scholar]
- 15.Legrand E. Aceclofenac in the management of inflammatory pain. Expet Opin. Pharmacother. 2004;5(6):1347–1357. doi: 10.1517/14656566.5.6.1347. [DOI] [PubMed] [Google Scholar]
- 16.Patel V.M., Prajapati B.G., Patel M.M. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS PharmSciTech. 2007;8(1):1–8. doi: 10.1208/pt0801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakhat P.D., Kondawar A.A., Rathi L.G., Yeole P.G. Development and in-vitro evaluation of buccoadhesive tablets of metoprolol tartarate. Ind. J. Pharm. Sci. 2008;70(1):121–124. doi: 10.4103/0250-474X.40349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Government of India . vol. II. controller of publications; Delhi: 2018. pp. 1143–1144. (Indian Pharmacopoeia). [Google Scholar]
- 19.Poudel A., Pandey J., Lee H.-K. Geographical discrimination in curcuminoids content of turmeric assessed by rapid UPLC-DAD validated analytical method. Molecules. 2019;24(9):1805. doi: 10.3390/molecules24091805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhanja S., Ellaiah P., Martha S.K., Sahu P.K., Tiwari S.P., Panigrahi B.B., Das D. Formulation and in vitro evaluation of mucoadhesive buccal tablets of timolol maleate. Int. J. Pharm. Biomed. Res. 2010;1(4):129–134. [Google Scholar]
- 21.Manivannan R., Balasubramaniam A., Anand D.C.P., Sandeep G., Rajkumar N. Formulation and in vitro evaluation of mucoadhesive buccal tablets of diltiazem hydrochloride. Res. J. Pharm. Technol. 2008;1(4):478–480. [Google Scholar]
- 22.Nirosha M., Badarinath A.V., Hyndavi M. Design and evaluation of esomeprazole mucoadhesive buccal tablets. Int. Res. J. Pharmaceut. Appl. Sci. 2017;7(5):42–49. [Google Scholar]
- 23.Vaidya V.M., Manwar J.V., Mahajan N.M., Sakarkar D.M. Design and in- vitro evaluation of mucoadhesive buccal tablets of terbutaline sulphate. Int. J. PharmTech Res. 2009;1(3):588–597. [Google Scholar]
- 24.Kumar B.P., Kavitha P., Devi K.J. Formulation design and evaluation of mucoadhesive buccal tablets of nitroglycerin. Int. J. Pharm. Pharmaceut. Sci. 2014;6(7):251–259. [Google Scholar]
- 25.Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive delivery systems. II. Formulation and in-vitro/in-vivo evaluation of buccal mucoadhesive tablets containing water-soluble drugs. Drug Dev. Ind. Pharm. 2004;30(9):995–1004. doi: 10.1081/ddc-200037226. [DOI] [PubMed] [Google Scholar]
- 26.Hassan N., Khar R.K., Ali M., Ali J. Development and evaluation of buccal bioadhesive tablet of an anti-emetic agent ondansetron. Aaps Pharmscitech. 2009;10(4):1085–1092. doi: 10.1208/s12249-009-9304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perioli L., Ambrogi V., Rubini D., Giovagnoli S., Ricci M., Blasi P. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J. Contr. Release. 2004;95(3):521–533. doi: 10.1016/j.jconrel.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev. Ind. Pharm. 2004;30(9):985–993. doi: 10.1081/ddc-200037245. [DOI] [PubMed] [Google Scholar]
- 29.Peddapalli H., Bakshi V., Boggula N. Formulation, in vitro and ex vivo characterization of mucoadhesive buccal tablets for antihypertensive drug. Asian J. Pharmaceut. Clin. Res. 2018;11(8):402–411. [Google Scholar]
- 30.Aframian D.J., Davidowitz T., Benoliel R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006;12(4):420–423. doi: 10.1111/j.1601-0825.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 31.Balamurugan M., Saravanan V.S., Ganesh P., Senthil S.P., Hemalatha P.V., Pandya S. Development and in-vitro evaluation of mucoadhesive buccal tablets of domperidone. Res. J. Pharm. Technol. 2008;1(4):377–380. [Google Scholar]
- 32.Bahri-Najafi R., Rezaei Z., Peykanpour M., Shabab L., Solooki R., Akbari P. Formulation of nicotine mucoadhesive tablet for smoking cessation and evaluation of its pharmaceuticals properties. Adv. Biomed. Res. 2013;2(4):1–9. doi: 10.4103/2277-9175.122515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.