Abstract
Background
Chemotherapy remains to be the method of choice used by clinicians to treat acute myeloid leukemia (AML) patients. However, the most common problem usually faced in the course of treatment is multidrug resistance (MDR). Nowadays, combination therapy involving natural products as adjuvant therapy to chemotherapy and radiotherapy has been used for many of health problems. Coumarin is a natural compound with known chemotherapeutic activity, as well as other pharmacological properties. We focused on the combination of coumarin and doxorubicin in overcoming of drug-resistance in acute myeloid leukemia.
Methods
Cell viability, Apoptotic and necrotic cell death with FACS, oxidative stress detection, and protein expression analysis were used in this study.
Results
Coumarin as a single drug exerts a significant cell death on Human acute myeloid leukemia (HL60); however, it does not show the same effect on drug-resistant acute myeloid leukemia (HL60/ADR). Comparing the effects of doxorubicin and coumarin as single drugs versus a combination of coumarin and doxorubicin showed a significant apoptotic cell death.
Conclusion
In AML patients, the development of multiple drug resistance (MDR) is the biggest challenge in treating AML patients. Combination therapy with coumarin may be a good choice to overcome the drug resistance in AML patients.
Keywords: Anticancer drugs, Apoptosis, Natural medicine, FACS assay, Cell viability
Anticancer drugs; Apoptosis; Natural medicine; FACS assay; Cell viability
1. Introduction
Cancer is a major cause of death worldwide, according to World Health Organization (WHO) reports. More than 13 million cancer patients will die by 2030 [1,2]. It was reported that 20% of the people would suffer from some form of cancer before being 75 years old [3]. Despite the number of clinical trials carried out and advances made in diagnosis and treatment, some cancer patients do not respond normally to the established cancer treatments. However, chemotherapy remains to be the method of choices in treating cancer patients. Drug resistance is considered the main obstacle for many of the anticancer agents, which subsequently requires the administration of higher doses of the chemotherapeutic agents to overcome drug resistance [4, 5]. Acute myeloid leukemia (AML) or acute non-lymphocytic leukemia is a complicated disease associated with abnormal differentiation and high cellular proliferative rate of hematopoietic stem cells [6]. Clinical drug resistance is generally recognized as being conferred by one of the following aspects: cellular drug resistance, leukemia regrowth, and drug pharmacokinetics [7, 8, 9, 10]. Previously published results treated certain cell lines with drugs that possess cytotoxic effect. These agents include actinomycin, colchicine, and Adriamycin. However, cell lines tested developed resistance to those agents [11, 12]. The mechanism by which cancerous cells develop multidrug resistance is because of less presence of the drug intracellularly after prolonged adminstration, which subsequently allows the cells to develop a better efflux system for these molecules [13]. ATP-binding cassette (ABC) transporters such as P-glycoprotein (P-gp) and MRP1 mediate the active drug efflux system [14, 15].
Natural medicine is one of the most commonly used complementary and alternative therapies by cancer patients [16, 17]. Coumarin (1,2-benzopyrone) is a natural drug found in many resources as a higher plant, like Umbelliferae and Rutaceae, some essential oils like cassia leaf oil, cinnamon bark oil, and lavender oil are also rich in microorganisms as well, like novobiocin and coumermycin from Streptomyces and aflatoxins from Aspergillus species but also synthetic drug [18, 19]. Coumarin has a wide spectrum of biological effects as anti-inflammatory, antioxidant, antinociceptive, anti–cancer, antiasthmatic, antidepressant, antituberculosis, anti- Alzheimer, and antihyperlipidemic [20, 21, 22, 23, 24, 25]. The anticancer activities of coumarins are well established; coumarin acts as a kinase inhibitor, suppresses cancer cell proliferation via cell cycle arrest, and exerts an antimitotic activity and affecting the P-gp of the cancer cell. It also inhibits angiogenesis, heat shock protein 90 (Hsp90), telomerase, carbonic anhydrase, monocarboxylate transporters, aromatase, and sulfatase [26, 27, 28].
2. Material and methods
2.1. Cell lines
Both cell lines used (Acute human myeloid leukemia (AML) HL60 and HL60/ADR) were purchased from ATCC (American Tissue Culture Collection). The two cell lines were cultured in RPMI cell culture medium and supplemented with 10% inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin antibiotic solution. Incubation conditions were fixed at 37 °C and 5% CO2 in humid conditions for the following experiments. In all experiments, the control plates were given the complete growth culture media only and subsequently under normal culture conditions, cells are able to divide and increase in number.
2.2. Cell viability assay
To study the cell viability of HL60 and HL60/ADR cells in coumarin and doxorubicin treatment, both HL60 and HL60/ADR cells were cultured in RPMI provided with 10% FBS and 1% penicillin/streptomycin. The initial cell number (4 × 105 cell/mL) and treated with (100, 250, 500, and 1000 μg/mL coumarin) with or without 100 ng/mL doxorubicin and incubated for 24 h, positive and negative controls (100 ng/mL doxorubicin and normal growth media), respectively were involved. Viable cells were detected by trypan blue dye exclusion method.
2.3. Apoptosis and necrosis using FACS analysis
The apoptotic and necrotic cell death by coumarin and doxorubicin were measured using Annexin V and 7AAD. Briefly, each cell line (HL60 or HL60/ADR) was cultured in 10 cell culture dishes with an initial seeding count of 4 × 105 cell/mL in RPMI growth media. Cells were treated with (100, 250, 500 and 1000 μg/mL coumarin) with or without 100 ng/mL doxrubcin and incubated for 24 h, positive and negative control (100 ng/mL doxorubicin and normal growth media) were involved. Then, the cells washed once with PBS, suspended in 100 μL 1X Annexin V binding buffer, add 5μL FITC Annexin V. Cells were allowed to react at room temperature for 15 min. After the allowed time, 5 μL of 7AAD were added to each tube. Finally, 400 μL of 1X Annexin V binding buffer were added to each tube and analyzed by flow cytometer.
2.4. ROS detection
HL60/ADR cells were cultured in RPMI growth medium containing (100, 250, 500, and 1000 μg/mL coumarin) with or without 100 ng/mL doxorubicin and incubated for 24 h. Positive and negative controls (100 ng/mL doxorubicin and normal growth media) were involved and ROS inducer (pyocyanin) as a positive control was involved at a concentration of 500 μM for 30 min. After treatment, cells were centrifuged and suspended in 200 μL of ROS detection Mix (ROS-ID® Hypoxia/Oxidative Stress Detection Kit, ENZ-51042-K500) for 30 min at normal cell culture conditions. After washing twice with PBS, cells were attached to the glass slides using the cytospin, and Images were obtained with confocal microscopy using 490/525 nm filter and μM scale bar.
2.5. Western blot analysis
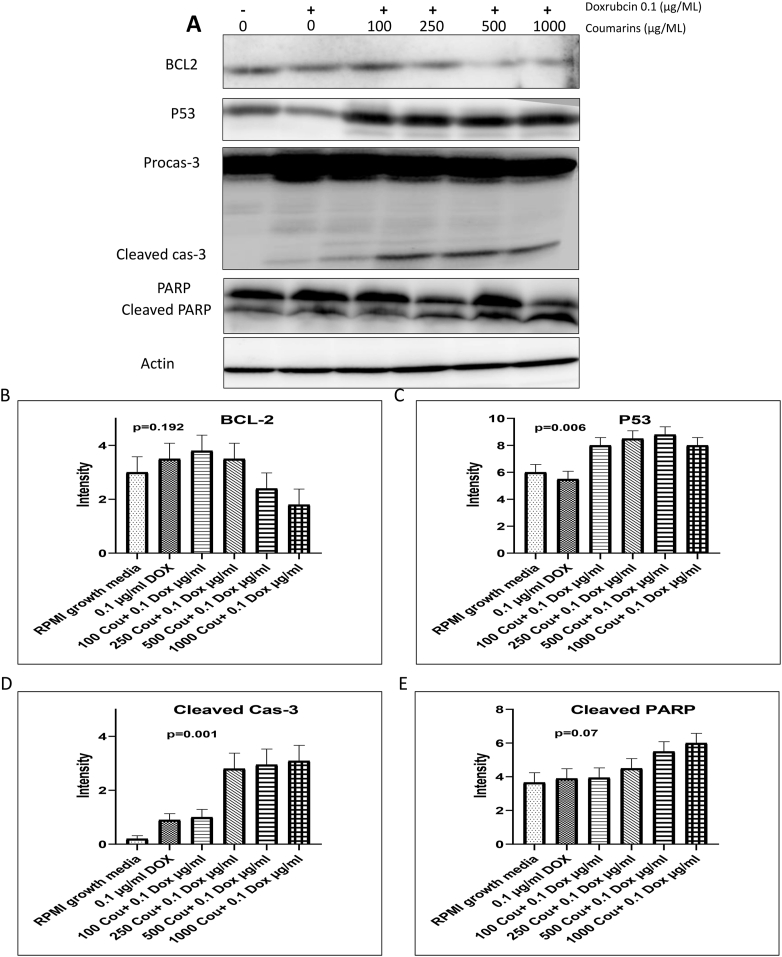
The effect of combining coumarin and doxorubicin on the apoptotic cell death was investigated in HL60/ADR cells using Western blot analysis (Figure 1 A). The cells were treated with (100, 250, 500, and 1000 μg/mL) concentrations of coumarin combined with doxorubicin at 100 ng/mL for 24 h. After treatment, cells were harvested in a proper amount of RIPA buffer, collected in Eppendorf tubes, and left for 10 min on ice with regular vortexing. Then the tubes were centrifuged at 12000 rpm for 20 min at 4 °C. The supernatants were transferred to another Eppendorf and kept at -80 °C to be used for protein electrophoresis using 30 μg protein from each sample. Protein samples were loaded in SDS-PAGE and the proteins were transferred to nitrocellulose membranes. Membranes were processed for blocking and incubated overnight in 4 °C with anti Bcl-2, p53, Caspase-3, PARP, and actin (cell signal 1:1000) primary antibody and then incubated with the proper secondary antibody for 1 h at 25 °C. Finally, the proteins were visualized by enhanced chemiluminescence luminal (ECL) solution. The membranes were then scanned on C-Digits scanner using Image Studio Digits version 3.1.
Figure 1.
Western bloting of Cas-3, P53, PARP and Actin in HL60/ADR after co-treatment of 0.1 μg/ml of doxirubcin with (100, 250, 500, and 1000 μg/ml) coumarin) and (B-E) a quantification of western blot data using Image J softrware.
2.6. Statistical analysis
The quantitative data were expressed as averages ±SD. Data were then plotted on graphs and analyzed statistically. Statistical differences were investigated using One-way analysis of variance (ANOVA) followed by Student's t-test.
3. Results
3.1. Coumarin induces HL60 cell death
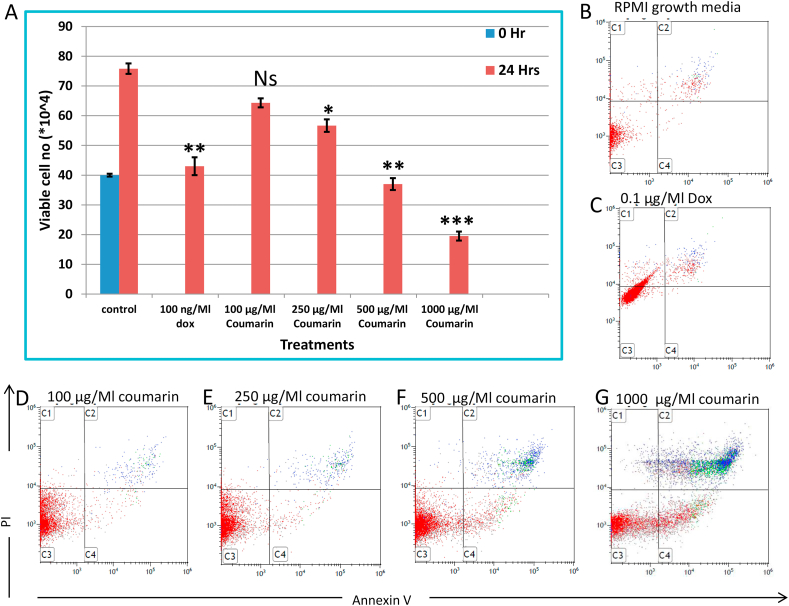
Cell viability was investigated in HL60 under different concentrations of coumarin (0, 100, 250, 500, and 1000 μg/mL), using trypan blue assay after 24 h. Quantitatively, it was shown that HL60 cells exert significant cell death with (250, 500, and 1000 μg/mL) with P < 0.05, 0.01, and 0.001, respectively; comparing with 100 ng/mL (P < 0.01) as a positive control as shown in Figure 2A. Analyzing cell viability, FACS results confirm HL60 cell death under the same experimental conditions using Annexin V and 7AAD as in Figure 2 B–G.
Figure 2.
(A) cytotoxic effect of coumarin and doxrubcin treatment on HL60 cells using trypane blue exclusion dye. Apoptotic and necrotic cell death were assessed in HL60 using Annexin V and PI staining and analyzed using flow cytometer in ( B) negative control, (C) treated with 0.1 μg/ml of doxirubcin as a positive control, and (D -G) treated with (100, 250, 500, and 1000 μg/ml) coumarin.
3.2. The combination of coumarin and doxorubicin show marked cell death in drug-resistant acute myeloid leukemia
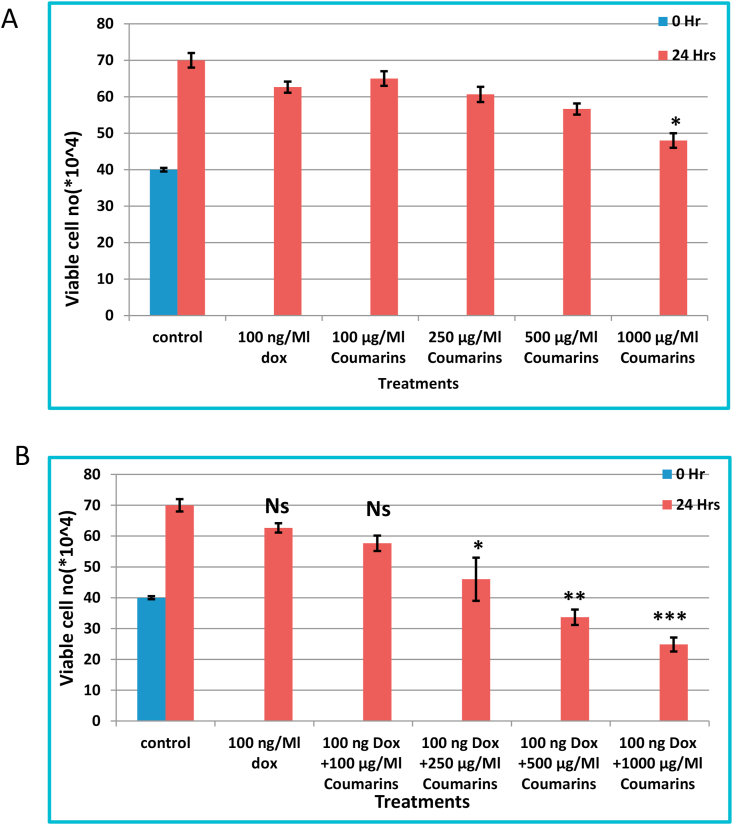
Cell death was investigated in HL60/ADR cells under different concentrations of coumarin (0, 100, 250, 500, and 1000 μg/mL) with or without doxorubicin (100 ng/mL) using trypan blue exclusion method after 24 h of treatment. Quantitatively, coumarin as a single drug show significant reduction in cell viability at 1000 μg/mL (P < 0.05) as shown in Figure 3A, while it was shown that HL60/ADR cells exert significant cell death with (250, 500, and 1000 μg/mL) with P < 0.05, 0.01, and 0.001, respectively, comparing with 100 ng/mL (P < 0.01) as a positive control as shown in Figure 3B.
Figure 3.
Cell viability was assessed in HL60/ADR cells using trypane blue exclusion dye; (A) treated with different coumarin concentrations and (B) co-treated with 100 ng/ml doxrubcin with different coumarin concentrations.
3.3. Apoptotic cell death in drug-resistant acute myeloid leukemia was observed with a combination of coumarin and doxorubicin
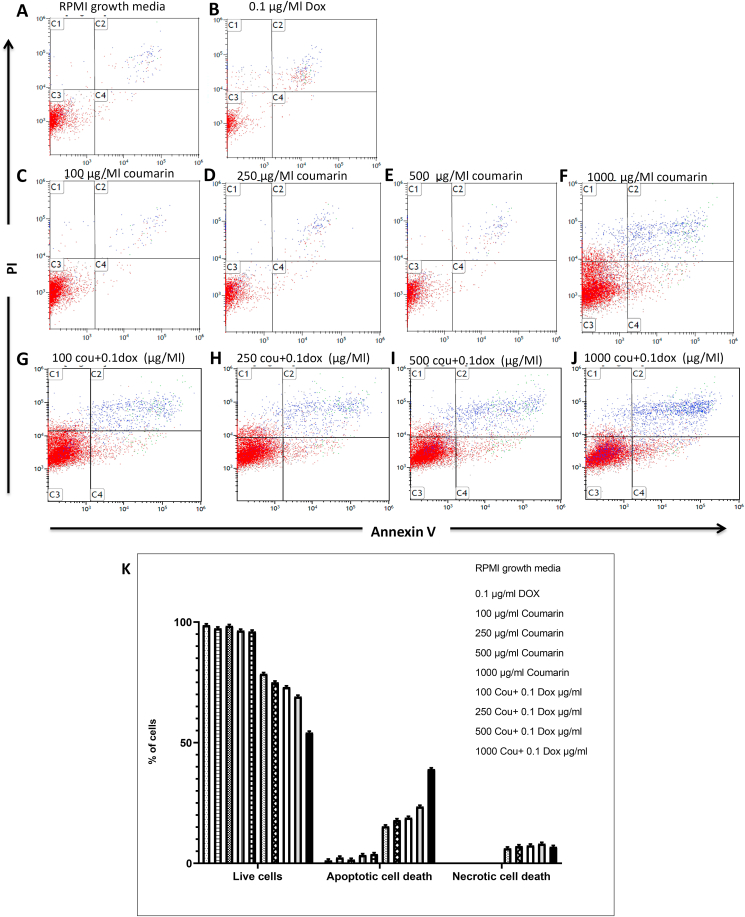
Consistent with cell viability results, flow cytometry analysis with Annexin V and 7AAD of HL60/ADR treated with coumarin (0, 100, 250, 500, and 1000 μg/mL) with or without doxorubicin (100 ng/mL) reveal that coumarin as a single drug show high apoptotic cell only at 1000 μg/mL (P < 0.001), while a similar trend of markedly apoptotic cell death with (100, 250, 500, and 1000 μg/mL) comparing with 100 ng/mL as a positive control and RPMI normal growth media as shown in Figure 4(A–J).
Figure 4.
Apoptotic and necrotic cell death were assessed in HL60/ADR cells using Annexin V and PI staining and analyzed using flow cytometer in ( B) (negative control), (C-F) HL60/ADR cells treated with (100, 250, 500, and 1000 μg/ml) coumarin , and (G-J) combination of 0.1 μg/ml of doxirubcin with (100, 250, 500, and 1000 μg/ml) coumarin and (K) quantification of Flowcytometry results.
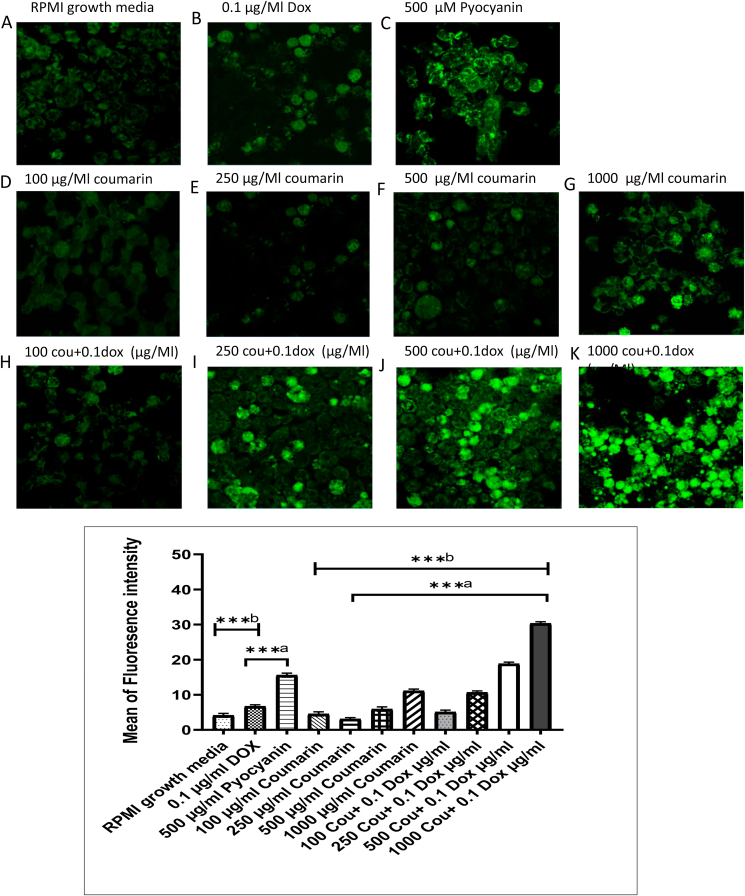
3.4. ROS generation considerably increased with the combination of doxorubicin (100 ng/ML) with the increasing concentration of coumarin
Increasing the level of oxidative stress was markedly observed with the combination of coumarin and doxorubicin through an increase of the ROS signal. The ROS signal was dramatically increased with coumarin concentration (100, 250, 500, 1000 μg/mL) with doxorubicin (100 ng/mL) versus treatment of coumarin alone. The ROS signal of the combination treatment was also high comparing with the positive ROS inducer (500 μM pyocyanin) as shown in Figure 5 (A–K).
Figure 5.
Confocal imaging of ROS in HL60/ADR after treatment with; (A) negative control, (B) 0.1 μg/ml of doxirubcin, (C) 500 μM Pyocyanin, (D-G) treated with (100, 250, 500, and 1000 μg/ml) coumarin, and (H-K) combination of 0.1 μg/ml of doxirubcin with (100, 250, 500, and 1000 μg/ml) coumarin and (L) quantification of the intensity of fluorescence using imge J software.
3.5. Coumarin and doxorubicin repressed Bcl-2 and increased p53, cleaved casp-3, and PARP protein expressions
Western blot observations demonstrated that there is a significant decrease in the expression of apoptotic inhibitor protein Bcl-2 with the combination of coumarin (500 and 1000 μg/mL) with 100 ng/mL doxorubicin. On the contrary, the combinations of coumarin (100, 250, 500 and 1000 μg/mL) with 100 ng/mL doxorubicin produced a significant increment of apoptotic-induced protein p53. Cleaved caspase-3 and cleaved PARP were markedly observed with the combination of coumarin (250, 500 and 1000 μg/mL) with 100 ng/mL doxorubicin.
4. Discussion
Acute myeloid leukemia (AML) as a clonal disorder is characterized by the increase of an immature, abnormally differentiated myeloid cell population in the bone marrow and peripheral blood. AML is the most common acute leukemia in adults aged above 45 years [29]. About one-third of the AML patients do not attain a complete remission with conventional chemotherapy treatment developed drug-resistant AML, which is considered a highly heterogeneous disease showing aggressive behavior toward all of the normal strategies of treatment therapy [29, 30]. Natural compounds and their derivatives as enhancer drugs play an important role in cancer treatment. Natural compounds and/or their derivatives mainly contribute with 60% of therapeutic drugs compositions used in cancer treatment [31]. In addition to the high cytotoxic effect of the chemotherapeutic agents towards the normal rapidly proliferating cells, drug resistance is the main obstacle in blood cancer treatment. In order to mitigate the side effects and drug resistance, modified therapeutic regimens such as combination therapy have been introduced [32, 33, 34]. Therefore, our research focused on the combination of coumarin as adjuvant natural therapy with doxorubicin to study the enhancement role of coumarin in overcoming doxorubicin resistance in AML patients. Coumarin and its derivatives have been reported by many studies to show cell cycle arrest, inhibit cell proliferation, and induce apoptotic cell death in chronic myeloid leukemia and histiocytic lymphoma [35, 36]. Inconsistent with the previous studies, our cell viability results show the high cytotoxic effect of coumarin on HL60 cells at (250, 500, 1000 μg/mL) with P < 0.05, 0.01, 0.001, respectively; in comparing with doxorubicin (100 ng/mL), with P < 0.01. In parallel with cytotoxicity results, FACS analysis with Annexin V and 7AAD show that coumarin at 500, 1000 μg/mL exert marked apoptotic cell death.
Drug resistance is one of the main problems in AML treatment. In the AML patients, the occurrence of resistance to chemotherapeutic agents is called multidrug resistance (MDR). The HL60/ADR is known to be resistant to many chemotherapeutic agents used solely. Many research articles have used combination of chemotherapeutic agents to gain the benefit of synergistic effects of both agents used together [37, 38]. In a previous research [37], high doses of Adriamycin were used in combination to overcome the multiple drug resistance in HL60 cells. MDR is caused by multidrug resistance protein1 (MDR1) gene product, known as P-glycoprotein (P-gp) which is an important ATP-dependent efflux pump of the cell membrane that pumps many drugs out of cells [39]. Treatment failure in AML patients and the generation of cellular resistance to a wide variety of anti-AML drugs is due to overexpression of P-gp [40]. DOX is one of the most potent intracellular ROS inducer anti-cancer drugs and causes acute cardiac arrhythmias and chronic cumulative cardiomyopathy as a side effect [41, 42, 43, 44]. Inconsistent to cell death results, intracellular oxidative stress increased by ROS generation, which increased with the combination of doxorubicin and coumarin and it was interestingly observed that the ROS induction was increased by an increase of coumarin concentration, while single treatment of coumarin exhibits no significant change in ROS generation compared with positive and negative controls. ROS are mediators of cell death and caspase-dependent apoptosis inducers [45]. Both DOX and coumarin have no significant cell death effect on adriamycin-resistant human acute myeloid leukemia (HL60/ADR) compared with HL60 cells as shown in cell viability and apoptotic cell death with flow cytometry results. Inconsistent with cell viability results, doxorubicin combination with coumarin showed an observed cell death in HL60/ADR cells and the rate of cell death markedly increased with the increase of coumarin concentration, which might be explained by the role of coumarin in inhibition of extracellular efflux of DOX.
Many studies reported the important role of p53 tumor suppressor protein as apoptotic cell death regulator [46, 47]. It was showed that DOX induces p53 activation and apoptosis [48]. In addition to cell viability, ROS and flow cytometry results, our Western blot results show that combination of DOX with different doses of coumarin has a positive effect on p53 protein expression in HL60/ADR. It was demonstrated that DOX has a negative effect on the anti-apoptotic Bcl-2 protein expression and affected oxidative stress by increasing hydrogen peroxide production in breast cancer cells [49]. Inconsistent with the published data, our results show a negative effect of combination between DOX and coumarin on Bcl-2 protein expression. The caspase-3-like activity is increased through a protease cascade during apoptosis in the early stages [50]. It has been reported that DOX induces apoptosis through activation of caspase-3 [51]. In accordance with the previous results, the combination of DOX and coumarin induce apoptosis through the activation of caspase-3 and PARP proteins.
5. Conclusion
In summary, in this study, we demonstrate that single treatment with DOX or coumarin has a marked cytotoxic effect on human acute myeloid leukemia, but has no effect on drug-resistant acute myeloid leukemia. However, the combination of coumarin and doxorubicin shows a significant apoptotic cell death in drug-resistant acute myeloid leukemia.
Declarations
Author contribution statement
Nouf A-Abbas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nehad Al-Shaer: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. CA cancer. J. Clin. 2011;61:69e90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kim S.-K. Springer International Publishing; Switzerland: 2015. Handbook of Anticancer Drugs from Marine Origin. [Google Scholar]
- 3.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. International Agency for Research on Cancer; Lyon, France: 2010. GLOBOCAN 2008 v1.2. "Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.10. [Google Scholar]
- 4.Solyanik G.I. Multifactorial nature of tumor drug resistance. Exp.Oncol. 2011;32:181–185. [PubMed] [Google Scholar]
- 5.Vijayaraghavalu S., Peetla C., Lu S., Labhasetwar V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol. Pharm. 2012;9:2730–2742. doi: 10.1021/mp300281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghoneum A., Sharma S., Gimzewski J. Nano-hole induction by nanodiamond and nanoplatinum liquid, DPV576, reverses multidrug resistance in human myeloid leukemia (HL60/AR) Int. J. Nanomed. 2013;8:2567–2573. doi: 10.2147/IJN.S43417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klumper E., Ossenkoppele G.J., Pieters R. In vitro resistance to cytosine arabinoside, not to daunorubicin, is associated with the risk of relapse in de novo acute myeloid leukaemia. Br. J. Haematol. 1996;93:903–910. doi: 10.1046/j.1365-2141.1996.d01-1742.x. [DOI] [PubMed] [Google Scholar]
- 8.Preisler H.D. Multidrug resistance is more than MDR1 activity. Leuk. Res. 1995;19:429–431. doi: 10.1016/0145-2126(95)00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Löwenberg B., van Putten W.L., Touw I.P., Delwel R., Santini V. Autonomous proliferation of leukemic cells in vitro as a determi- nant of prognosis in adult acute myeloid leukemia. N. Engl. J. Med. 1993;328:614–619. doi: 10.1056/NEJM199303043280904. [DOI] [PubMed] [Google Scholar]
- 10.Raza A., Preisler H.D., Day R. Direct relationship between remission duration in acute myeloid leukemia and cell cycle kinet- ics: a leukemia intergroup study. Blood. 1990;76:2191–2197. [PubMed] [Google Scholar]
- 11.Biedler J.L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radiographie and cytogenetic studies. Cancer Res. 1970;30:1174–1184. [PubMed] [Google Scholar]
- 12.Ling V., Thompson L.H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J. Cell. Physiol. 1974;S3:103–111. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- 13.Inaba M., Kobayashi H., Sakurai V., Johnson R.K. Active efflux of daunorubicin and Adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979;39:2200–2203. [PubMed] [Google Scholar]
- 14.Kasaian J., Mosaffa F., Behravan J., Masullo M., Piacente S., Ghandadi M., Iranshahi M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpenecoumarins. Fitoterapia. 2015;103:149–154. doi: 10.1016/j.fitote.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Wei J., Zhou Y., Jiang G.Q., Xiao D. Silencing of ETS1 reverses adriamycin resistance in MCF-7/ADR cells via downregulation of MDR1. Cancer Cell Int. 2014;14:22. doi: 10.1186/1475-2867-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo M., Spagnuolo C., Tedesco I., Russo G.L. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins (Basel) 2012;2:517–551. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad S., Aggarwal B.B. Chronic diseases caused by chronic inflammation require chronic treatment: anti-inflammatory Role of dietary spices. Clin Cellular Immunol. 2014;5:1–11. [Google Scholar]
- 18.Lacy A., O´Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004;10:3797–3811. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 19.Jain P.K., Joshi H.,J. Coumarin: chemical and pharmacological profile. App. Pharm. Sci. 2012;2(6):236–240. [Google Scholar]
- 20.Lee S.J., Lee U.S., Kim W.J., Moon S.K. Inhibitory effect of esculetin on migration, invasion and matrix metalloproteinase-9 expression in TNF-α-induced vascular smooth muscle cells. Mol. Med. Rep. 2011;4:337–341. doi: 10.3892/mmr.2011.420. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Wang L., Gonzalez-Molleda L., Wang Y., Xu J., Yuan Y. Antiviral activity of (+)-Rutamarin against Kaposi's sarcoma-associated herpesvirus by inhibition of the catalytic activity of human topoisomerase II. Antimicrob. Agents Chemother. 2014;58:563–573. doi: 10.1128/AAC.01259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostova I., Bhatia S., Grigorov P., Balkansky S., Pramar V.S., Prasad A.K., Saso L. Coumarins as antioxidants. Curr. Med. Chem. 2011;18:3929–3951. doi: 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- 23.Anand P., Singh B., Singh N. Bioorg. Med. Chem. 2012;20:1175–1180. doi: 10.1016/j.bmc.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Recillas A., Navarrete-Vazquez G., Hidalgo-Figueroa S., Rios M.Y., Ibarra-Barajas M., Estrada-Soto S. Semisynthesis, ex vivo evaluation, and SAR studies of coumarin derivatives as potential antiasthmatic drugs. Eur. J. Med. Chem. 2014;77:400–408. doi: 10.1016/j.ejmech.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Sashidhara K.V., Modukuri R.K., Singh S., Rao K.B., Teja G.A., Gupta S., Shukla S. Design and synthesis of new series of coumarin–aminopyran derivatives possessing potential anti-depressant-like activity. Bioorg. Med. Chem. Lett. 2015;15:337–341. doi: 10.1016/j.bmcl.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Chia-Hsiung C., Ku-Chung L., Wai-Theng W., Yen-Fang X., Lin C. Induction of ROS-independent JNK-activation-mediated apoptosis by a novel coumarin derivative, DMAC, in human colon cancer cells. Chem. Bio. Int. 2014;218:42–49. doi: 10.1016/j.cbi.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Saidu N., Valente S., Emilie B., Gilbert K., Denyse B., Mathias M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioog. Med. Chem. 2012;4:1584–1593. doi: 10.1016/j.bmc.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Jurgen G., Hironobu S., Shiuan C., Atul P. Steroid sulfatase inhibitors: promising new tools for breast cancer therapy? J. Steroid Biochem. Mol. Biol. 2011;125:39–45. doi: 10.1016/j.jsbmb.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Sonneveld P. Multidrug resistant cells with high proliferative capacity determine response to therapy in acute myeloid leukemia,identification and modulation of drug resistance in acute myeloid leukemia. 2014;9(1025):63. [PubMed] [Google Scholar]
- 30.De Kouchkovsky I., Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Canc. J. 2016;6(7):e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H., Jiang W., Xie M. Flavonoids: recent advances as anticancer drugs. Recent Pat. Anti-Cancer Drug Discov. 2010;5(2):152–164. doi: 10.2174/157489210790936261. [DOI] [PubMed] [Google Scholar]
- 32.Ebermann R., Alth G., Kreitner M., Kubin A. Natural products derived from plants as potential drugs for the photodynamic destruction of tumor cells. J. Photochem. Photobiol. B Biol. 1996;36(2):95–97. doi: 10.1016/s1011-1344(96)07353-8. [DOI] [PubMed] [Google Scholar]
- 33.Lacy A., O'Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharmaceut. Des. 2004;10(30):3797–3811. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 34.Pan J., Zhang Q., Zhao C., Zheng R. Redifferentiation of human hepatoma cells induced by synthesized coumarin. Cell Biol. Int. 2004;28(5):329–333. doi: 10.1016/j.cellbi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Zwergel C., Valente S., Salvato A., Xu Z., Talhi O., Mai A., Silva A., Altucci L., Kirsch G. Novel benzofuran–chromone and –coumarin derivatives: synthesis and biological activity in K562 human leukemia cells. Med. Chem. Commun. 2013;4:1571–1579. [Google Scholar]
- 36.Seidel C., Schnekenburger M., Zwergel C., Gaascht F., Mai A., Dicato M., Kirsch G., Valente S., Diederich M. Novel inhibitors of human histone deacetylases: Design, synthesis and bioactivity of 3-alkenoylcoumarines. Bioorg. Med. Chem. Lett. 2014;24(16):3797–3801. doi: 10.1016/j.bmcl.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L., Wang Z., Khishignyam T., Chen T., Zhou C., Zhang Z., Jin M., Wang R., Qiu Y., Kong D. In vitro anti-leukemia activity of dual PI3K/mTOR inhibitor Voxtalisib on HL60 and K562 cells, as well as their multidrug resistance counterparts HL60/ADR and K562/A02 cells. Biomed. Pharmacother. = Biomedecine & pharmacotherapie. 2018;103:1069–1078. doi: 10.1016/j.biopha.2018.04.089. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q., Chen Y., Zhang L., Zhong Y., Zhang Z., Wang R., Jin M., Gong M., Qiu Y., Kong D. Antiproliferative effect of ZSTK474 alone or in combination with chemotherapeutic drugs on HL60 and HL60/ADR cells. Oncotarget. 2017;8(24):39064–39076. doi: 10.18632/oncotarget.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirose M., Hosoi E., Hamano S., Jalili A. Multidrug resistance in hematological malignancy. J. Med. Invest. 2003;50(3–4):126–135. [PubMed] [Google Scholar]
- 40.van der Kolk D.M., de Vries E.G., Muller M., Vellenga E. The role of drug effl ux pumps in acute myeloid leukemia. Leuk. Lymphoma. 2002;43(4):685–701. doi: 10.1080/10428190290016773. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.Y., Kim S.J., Kim B.J., Rah S.Y., Chung S.M., Im M.J., Kim U.H. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp. Mol. Med. 2006;38(5):535. doi: 10.1038/emm.2006.63. [DOI] [PubMed] [Google Scholar]
- 42.Sinha B.K., Mimnaugh E.G., Rajagopalan S., Myers C.E. Adriamycin activation and oxygen free radical formation in human breast tumor cells: protective role of glutathione peroxidase in adriamycin resistance. Cancer Res. 1989;49:3844–3848. [PubMed] [Google Scholar]
- 43.Ubezio P., Civoli F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic. Biol. Med. 1994;16:509–516. doi: 10.1016/0891-5849(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 44.Gouaze V., Mirault M.E., Carpentier S., Salvayre R., Levade T., Andrieu-Abadie N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol. Pharmacol. 2001;60:488–496. [PubMed] [Google Scholar]
- 45.Holze C., Michaudel C., Mackowiak C., Haas D.A., Benda C., Hubel P.,., Leung D.W. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018;19(2):130. doi: 10.1038/s41590-017-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal M.L., Taylor W.R., Chernov M.V., Chernova O.B., Star k G.R. The p53 network. J. Biol. Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Ko J.L., Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 48.Wang S., Konorev E.A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms intermediacy of H2O2-and p53-dependent pathways. J. Biol. Chem. 2004;279(24):25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- 49.Pilco-Ferreto N., Calaf G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016;49(2):753–762. doi: 10.3892/ijo.2016.3558. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson D.W., Thornberry N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 51.Ueno M., Kakinuma Y., Yuhki K.I., Murakoshi N., Iemitsu M., Miyauchi T., Yamaguchi I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J. Pharmacol. Sci. 2006;101(2):151–158. doi: 10.1254/jphs.fp0050980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.