Introduction
Corynebacterium Striatum is a gram positive bacillus. It is part of normal flora of skin and mucus membranes in humans. It is included in non-diphtheroid Corynebacterium group and is generally considered nonpathogenic in humans [1]. Given its ubiquitous nature in the environment and on human body surfaces, a positive blood culture is commonly presumed to be a contaminant. However, there are some case reports of C striatum species causing nosocomial infections, especially as an opportunistic pathogen in immuno-compromised hosts [1]. Recently cases have been described of infections in immuno-competent hosts [2,3]. There is also some evidence that this bacteria can be multi drug resistant [4]. Rarely, C striatum can be associated with diseases like chronic obstructive pulmonary disease exacerbation [5], skin infections like cellulitis or deep tissue infections, osteomyelitis [6], prosthetic device infections [7], meningitis [8] and infective endocarditis with native or prosthetic valve involvement and device associated Infective endocarditis [9]. We present two cases of infective endocarditis with abscess formation and conduction abnormalities caused by C striatum. Case 1 was device associated and Case 2 had native valve involvement.
Case 1
A 55 year old male patient with medical history of coronary artery disease status post percutaneous coronary intervention, sick sinus syndrome status post permanent pacemaker placement in 2018, congestive heart failure with preserved ejection fraction, obstructive sleep apnea, chronic obstructive pulmonary disease (on home noninvasive ventilation), diabetes mellitus, obesity, history of cocaine and alcohol abuse, end stage renal disease on hemodialysis, hypertension and dyslipidemia was sent to the hospital from the hemodialysis center for worsening shortness of breath which started during outpatient hemodialysis session. His initial blood pressure was 125/78 mm Hg, heart rate 73/min, afebrile and pulse oxygen saturation of 98 % on 6 L supplemental oxygen.
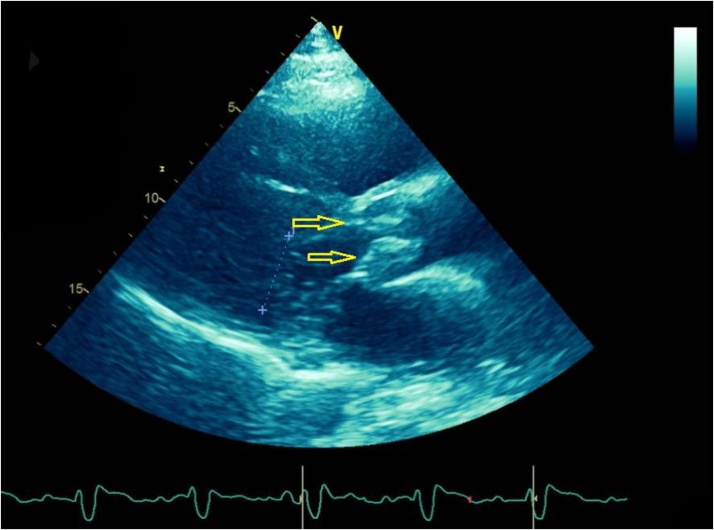
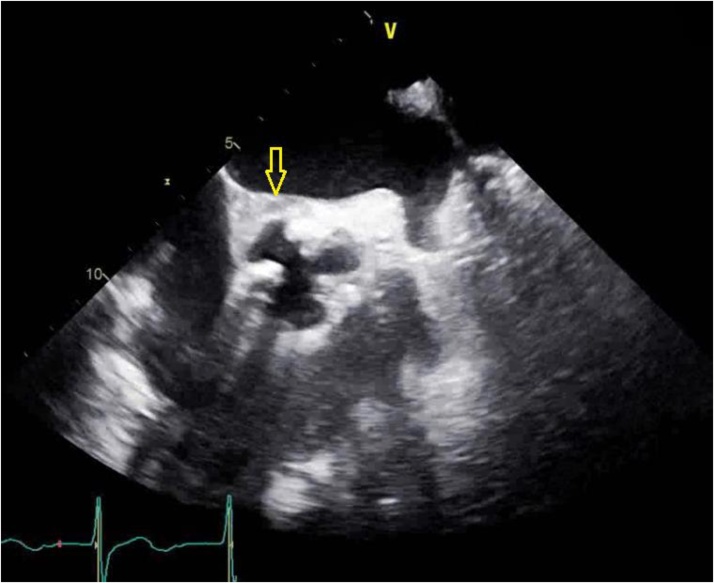
On physical examination patient had diffuse wheezing and crackles along with 2+ bilateral lower extremity edema. Chest X ray showed congestion in bilateral lung fields from volume overload. He required non-invasive ventilation. Patient was given intravenous (IV) furosemide and was taken for urgent hemodialysis which resulted in some improvement of his symptoms. Patient required another session of hemodialysis the following day with ultrafiltration. His symptoms improved markedly and he was planned to be discharged with outpatient continuation of hemodialysis. However, patient could not be discharged due to social and behavioral problems. He had history of bipolar mood disorder and substance abuse. He developed socially inappropriate behavior towards his family members and hospital staff. He was evaluated by psychiatrist who recommended haloperidol as needed for agitation. His hospital course was further complicated by development of fever to 101 F and acute on chronic anemia with a drop in hemoglobin to 6.1 gm/dl from a baseline of 8 mg/dl along with melena on hospital day 15. Patient received 4 units of packed red blood cell transfusions. He also developed thrombocytopenia with platelet count of 20,000 and received 1 unit of platelet transfusion. He had negative heparin induced thrombocytopenia panel and his fibrinogen levels were within normal limits. Patient continued to develop low grade fevers and two sets of blood cultures resulted in growth of C striatum. Repeat blood cultures were drawn subsequently which remained positive for growth of C striatum on hospital day 16, 17, 18 and 19. He had transthoracic echocardiogram on hospital day 17 which showed non-mobile vegetations on right and left cusps of aortic valve (Fig. 1). This was a new finding as compared to the prior echocardiogram performed one month ago.
Fig. 1.
Para-sternal long axis view from transthoracic echocardiogram. The yellow arrows are pointing towards aortic valve vegetations.
Patient was initially given intravenous (IV) Vancomycin to empirically cover for Methicillin Resistant Staphylococcus Aureus (MRSA). Since the patient was obese and required high doses of intravenous (IV) Vancomycin, 4 days later he was switched to intravenous (IV) Daptomycin and Rifampin due to concerns for intravenous (IV) Vancomycin toxicity.
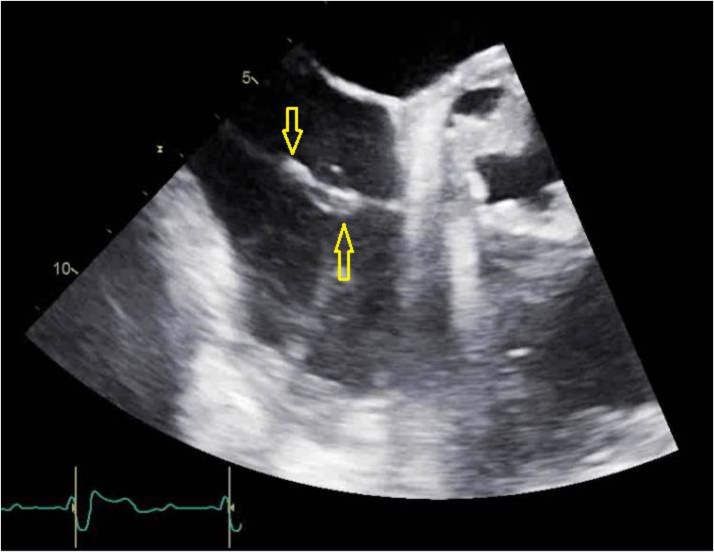
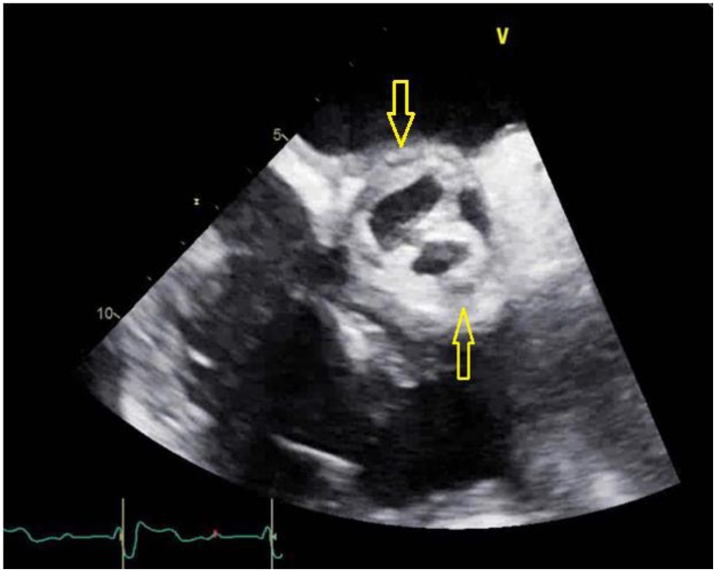
Schistocytes were present on peripheral blood smear which may represent vegetation induced hemolysis. Haptoglobin levels were elevated. It is suspected that the patient might have stress ulcers from bacteremia causing gastrointestinal bleeding leading to a drop in hemoglobin and thrombocytopenia from consumption of platelets. Hematology team was consulted for anemia and thrombocytopenia. Thrombotic thrombocytopenic purpura was not suspected. Gastroenterology team was also consulted for possible blood loss anemia, secondary to melena, and supportive care with resuscitation was recommended. Patient was more dependent on noninvasive mechanical ventilation during this time due to shortness of breath, secondary to symptomatic anemia and fluid overload from multiple transfusions. However, on hospital day 21 the patient had altered mental status and required endotracheal intubation. His tunneled dialysis catheter was removed as a possible source of infection and further hemodialysis were carried out via arterio-venous (A/V) fistula. Patient had impaired flow through his A/V fistula about 1 month prior to these events which warranted placement of tunneled dialysis catheter and surgical intervention for restoration of A/V fistula. An electrocardiogram showed new onset of first degree atrio-ventricular block with prolonged PR interval. A trans-esophageal echocardiogram (TEE) revealed multiple mobile densities on the right ventricular lead of pacemaker (Fig. 2). It also confirmed aortic valve vegetation along with aortic root abscess and aortic regurgitation (Fig. 3).
Fig. 2.
Trans-esophageal echocardiogram image of mid-esophageal aortic valve, short axis view at 30 degrees. Arrows are pointing toward vegetations on the pacemaker lead.
Fig. 3.
Trans-esophageal echocardiogram image of mid-esophageal aortic valve, short axis view at 30 degrees. Arrows are pointing toward the aortic abscesses.
On hospital day 26, his permanent pacemaker was removed and pacemaker lead associated vegetation was sent for pathology and microbiology, this also confirmed C striatum infection. After a prolonged hospital course, on day 30 of hospitalization the patient developed complete Atrio-Ventricular block and had cardiopulmonary arrest. Cardio Pulmonary Resuscitation (CPR) was done as per ACLS protocol and patient achieved return of spontaneous circulation (ROSC) after 8 min. He had repeat TEE done which showed severe aortic insufficiency, moderate mitral regurgitation and tricuspid regurgitation. A transvenous temporary pacemaker was placed for complete heart block.
The next day, patient had cardiac arrest again requiring CPR and ROSC was achieved in 2 min. Patient developed ventricular tachycardia post CPR and had 2 failed defibrillation attempts. At that point, in line with patient’s prior wishes, his family made a surrogate decision to stop any further escalation of care or resuscitative efforts. No further resuscitation efforts were done and the patient expired.
Case 2
An 82-year-old male that was brought in by his family to the emergency department (ED) for a change in his mental status that gradually developed over a 2 day period. The patient’s daughter who lives with him stated that his symptoms started with him getting lost around the house. He then started having visual hallucinations and lost orientation to person and place. His medical history was remarkable for coronary artery disease, peripheral artery disease, colorectal carcinoma, hypertension, and chronic normocytic anemia. He had no past surgical history, and no allergies. He had no smoking history, and consumed alcohol occasionally.
On presentation, he had a temperature of 102.7 F, heart rate of 111 bpm, blood pressure of 100/56 mmHg and a respiratory rate of 20 per minute. Laboratory work up was significant for white blood cell count of 17,000 × 10^3 cells/mm3 with 85 % polymorphic neutrophils and a lactic acid of 2.5. His urinalysis was negative. Chest x-ray showed no opacities and his respiratory viral panel including influenza was negative. His serum pro-calcitonin level was 0.35. A computed tomography (CT) scan showed no acute intracranial pathology and a lumbar puncture was attempted three times without success.
The patient was empirically started on broad spectrum coverage with intravenous (IV) Cefepime and Vancomycin therapy. On hospital day 1, blood cultures obtained at the time of admission and 4 h later from a different site grew gram positive rods in aerobic bottle. Due to this the empiric antibiotic coverage was now switched to intravenous (IV) Ampicillin and Vancomycin to cover for Listeria. Later cultures showed growth of C striatum in aerobic bottles. Transthoracic echocardiogram showed a large, mobile mass on the anterior mitral valve leaflet consistent with a vegetation and associated mild to moderate regurgitation of the aortic valve. His bacteremia was deemed to be secondary to native valve endocarditis and antibiotic coverage was narrowed down to intravenous (IV)Vancomycin only.
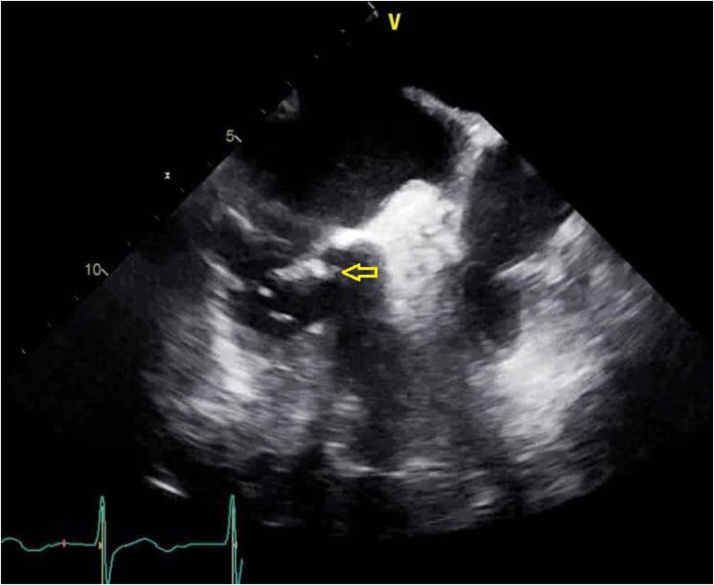
After a total of 5 days of intravenous (IV) Vancomycin, repeat blood cultures showed no growth in either the aerobic or the anerobic bottles. However, electrocardiogram showed PR interval prolongation to 350 milliseconds from baseline PR interval of approximately 200 milliseconds. A transesophageal echocardiogram showed a multi-lobar large vegetation (Fig. 4) on the ventricular aspect at the base of the anterior mitral leaflet and paravalvular abscess (Fig. 5). Mild mitral regurgitation was also present. Prolonged PR interval on electrocardiogram was most likely secondary to the para-valvular abscess leading to conduction abnormalities. Despite the resolution of his bacteremia the patient's hospital course was complicated by aspiration pneumonia and persistent sepsis. After further discussion with palliative care team and the patient’s family, the decision was made to initiate comfort measures. Patient later on expired on day 10 of hospitalization.
Fig. 4.
Trans-esophageal echocardiogram image at mid-esophageal level at 78 degrees. Arrows is pointing towards the vegetation.
Fig. 5.
Trans-esophageal echocardiogram image of mid-esophageal aortic valve short axis view at 30 degrees. The arrows is pointing towards the aortic abscesses.
Discussion
C striatum was described for the first time by Chester in 1901 and then Eberson in 1918, and since then numerous Corynebacterium species have been identified and reclassified [10]. C striatum is a gram positive rod that is found extensively in the environment and is part of normal flora of skin and mucus membranes in humans. It is usually considered non-virulent in humans. However recently there have been cases documented in the literature with infections caused by C striatum specially in the nosocomial settings [[10], [11], [12]]. Positive culture growth of C striatum from different sites can sometimes be erroneously rendered contaminant as it is freely found in the environment and frequently colonizes human body surfaces. However, recent data provides some evidence that it can be virulent in humans and cause opportunistic infections. Some clinical manifestations can be life threatening such as infective endocarditis and osteomyelitis [[9], [10], [11]]. Persistently positive blood cultures with C striatum should raise concern for underlying infection as it is shown to be pathogenic in certain clinical scenarios. The pathogenesis of infective endocarditis or prosthetic device associated infections can be explained by the ability of this bacteria to form biofilms.
Based on a literature review by Hyo-Lim Hong and colleagues in 2016 [10], 23 cases with infective endocarditis from C striatum were identified. Number of cases with native valve endocarditis were 18/22 (82 %). More than half of these cases were hospital-acquired infections and 11 cases (50 %) were associated with medical devices; including 4 cases of prosthetic valves, 3 cases involving pacemaker leads, 3 cases of vascular access for hemodialysis and 1 case of a ventriculoatrialshunt infection. Valve replacements were performed in one quarter of the cases and overall mortality approached 27 %.
Another significant factor contributing to the virulence of C striatum is antibiotic resistance. This bacteria is characteristically resistant to penicillin class of antibiotics. There is some evidence that it may respond to intravenous (IV) Vancomycin, although there have been some case reports of intravenous (IV) Vancomycin resistance as well [10]. The proposed rationale for resistance is that given the ubiquitous nature of the organism and being part of commensal flora, this bacteria is constantly exposed to commonly used antibiotics in the hospital setting which can lead to acquired resistance via spontaneous mutations [15].
Some authors also postulate thatC striatum in itself is not the infective organism but rather it is isolated in blood cultures as a part of polymicrobial infection causing IE where it just colonizes prior vegetations. However there is not enough evidence in the literature to support this claim. The vegetations isolated from the pacemaker lead of case 1 failed to reveal organisms other than C striatum in the final cultures.
Based on some studies mortality associated with IE from C striatum could be as high as 26–40 % [10,14]. Both the cases presented above had extremely poor outcomes. The echocardiogram findings exhibited destruction of the valves with abscesses formation. This calls for increased awareness of infections secondary to C striatum in the medical community. Prompt aggressive measures should be taken with appropriate antibiotic therapy and interventions to achieve source control. As evidenced if this bacteria is not treated aggressively early on in the disease course it can lead to abscess formation which is associated with grave prognosis.
Conclusion
C striatum is part of normal flora of skin and mucus membranes in humans and when isolated in the blood culture is thought to represent a contaminant. However, it can be an opportunistic pathogen and cause disease. Persistently positive blood cultures should raise a high suspicion for infective endocarditis or prosthetic device associated infections. As the mortality associated with IE from C striatum could be high [9,13], it warrants the diagnosis to be made early so that appropriate antibiotic therapy can be administered along with the achievement of strict source control such as removal of any prosthetic device.
References
- 1.Funke G., von Graevenitz A., Clarridge J.E., 3rd, Bernard K.A. Clinical microbiology of coryneform bacteria. ClinMicrobiol Rev. 1997;10(1):125–159. doi: 10.1128/CMR.10.1.125-159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavin S.E., Leonard R.B., Briselden A.M., Coyle M.B. Evaluation of the rapid CORYNE identification system for Corynebacterium species and other coryneforms. J ClinMicrobiol. 1992;30(7):1692–1695. doi: 10.1128/JCM.30.7.1692-1695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMullen A.R., Anderson N., Wallace M.A., Shupe A., Burnham C.A. When good bugs go bad: epidemiology and antimicrobial resistance profiles of Corynebacterium striatum, an emerging multidrug-resistant, opportunistic pathogen. Antimicrob Agents Chemother. 2017;61(11):e01111–01117. doi: 10.1128/AAC.01111-17. Published 2017 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn W.O., Werth B.J., Butler-Wu S.M., Rakita R.M. Multidrug-resistant Corynebacterium striatum associated with increased use of parenteral antimicrobial drugs. Emerg Infect Dis. 2016;22(11):1908–1914. doi: 10.3201/eid2211.160141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renom F., Gomila M., Garau M. Respiratory infection by Corynebacterium striatum: epidemiological and clinical determinants. New Microbes New Infect. 2014;2(4):106–114. doi: 10.1002/nmi2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma R., Kravitz Gr. Corynebacterium striatum empyema and osteomyelitis in a patient with advanced rheumatoid arthritis. Case Rep. 2016;2016 doi: 10.1136/bcr-2016-214691. bcr2016214691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo G., Kim J., Uh Y., Lee H.G., Hwang G.Y., Yoon K.J. Multidrug-resistant Corynebacterium striatum bacteremia: first case in Korea. Ann LabMed. 2015;35:472–473. doi: 10.3343/alm.2015.35.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss K., Labbé A.C., Laverdière M. Corynebacterium striatum meningitis: case report and review of an increasingly important Corynebacterium species. Clin Infect Dis. 1996;23:1246–1248. doi: 10.1093/clinids/23.6.1246. [DOI] [PubMed] [Google Scholar]
- 9.Hascoet S., Mauri L., Claude C., Fournier E., Lourtet J., Riou J.Y. Infective endocarditis risk after percutaneous pulmonary valve implantation with the melody and Sapien valves. JACC CardiovascInterv. 2017;10:510. doi: 10.1016/j.jcin.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Hong H.L., Koh H.I., Lee A.J. Native valve endocarditis due to Corynebacterium striatum confirmed by 16S ribosomal RNA sequencing: a case report and literature review. Infect Chemother. 2016;48(3):239–245. doi: 10.3947/ic.2016.48.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houghton T., Kaye G.C., Meigh R.E. An unusual case of infective endocarditis. Postgrad Med J. 2002;78:290–291. doi: 10.1136/pmj.78.919.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syed M.A., Ashcherkin N., Sundhu M., Hakam L., Gul S. Recurrent bacteremia with Corynebacterium striatum after prosthetic valve replacement: a case report. Cureus. 2019;11(5) doi: 10.7759/cureus.4670. Published 2019 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen M., Mohlin A.W., Nilson B. From contamination to infective endocarditis-a population-based retrospective study of Corynebacterium isolated from blood cultures. Eur J ClinMicrobiol Infect Dis. 2020;39(1):113–119. doi: 10.1007/s10096-019-03698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belmares J., Detterline S., Pak J.B. Corynebacteriumendocarditis species-specific risk factors and outcomes. BMC Infect Dis. 2007;7:4. doi: 10.1186/1471-2334-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alibi S., Ferjani A., Boukadida J. Occurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital. Sci Rep. 2017:9704. doi: 10.1038/s41598-017-10081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]