Abstract
Background
Myocardial toxicity is a common side effect of chemotherapy and is associated with adverse outcomes in cancer patients. Sufficient prediction of chemotherapy-induced myocardiotoxicity (CIMC) is desirable. Therefore, we sought to develop a feasible scoring system to predict CIMC in cancer patients undergoing non-anthracycline chemotherapy.
Methods
We determined a scoring system, the “Cardiotoxicitiy Score” (the CardTox-Score), by multivariable regression of the parameters considered relevant to the development of CIMC, based on previously published data and current guidelines. Variables of the risk model consist of clinical (age, presence of cardiovascular risk conditionsconditions), blood tests (NT-proBNP), and echocardiographic parameters (left ventricular (LV) ejection fraction, LV strain analysis). The CardTox-Score was examined in an internal validation cohort by use of ROC and regression analysis.
Results
We prospectively investigated 225 patients (58.21 ± 6.3 years, 52.8% female) who received non-anthracycline myocardiotoxic anticancer agent as a derivation cohort. All patients underwent echocardiography before, during and after anticancer therapy. The mean follow-up duration was 25 ± 4 months. We found the CardTox-Score (>6 points) to be a strong independent predictor (AUC: 0.983, OR: 6.38, 95% CI: 1.6 2.8, p < 0.001) for the development of CIMC with high sensitivity (100%) and specificity (84.2%) in the validation cohort (n = 30, 59.2 ± 6.5 years, 57% female). Moreover, the CardTox-Score appropriately predicted all-cause mortality with high specificity (93.7%) and sensitivity (92.9%) as well (OR: 4.85, AUC: 0.978, p = 0.01).
Conclusion
The CardTox-Score offers a promising, feasible, and easy-to-handle scoring system for predicting CIMC in cancer patients undergoing non-anthracycline regimes, independent from the type of cancer.
Keywords: Chemotherapy, Cardiotoxicity, Cardiomyopathy, Risk assessment, Strain analysis
Abbreviations: AUC, Area under the curve; CI, Confidence interval; CK-MB, Creatine kinase isoenzyme MB; FU, Follow-up; LDL, Low-density lipoprotein; LV-GLS, Left-ventricular global longitudinal strain; LV-EF, Left-ventricular ejection fraction; NT-proBNP, N terminal pro-brain natriuretic peptide; OR, Odds ratio; ROC, Receiver operating characteristic
1. Introduction
Oncological mortality has decreased worldwide over the years, due to recent developments in early cancer detection and advanced treatment modalities, with a consequence of an increased prevalence of therapy-related adverse events [1], [2]. Cardiotoxicity is a common side effect of anticancer therapies, with an estimated incidence of up to 20%, which leads to premature mortality and unfavourable clinical outcomes in cancer patients [3], [4]. Chemotherapy-induced myocardiotoxicity (CIMC) is the most frequent type of cardiotoxicities through anticancer agents and is considered to have three main pathomechanisms. Firstly, chemotherapeutic agents might damage the myocardium and the cardiomyocytes directly, which leads to “left ventricular dysfunction” and is usually caused by anthracyclines (dose-dependent), alkylating agents (dose-dependent), anti-metabolites, anti-microtubule agents, monoclonal antibodies, tyrosine kinase inhibitors, and mTOR inhibitors. Secondly, anticancer agents might induce premature coronary artery disease, owing to increased atherosclerosis, followed by impaired coronary perfusion and myocardial ischemia – commonly through fluoropyrimidines, cisplatin, VEGF inhibitors, radiotherapy. Thirdly, chemotherapy and radiotherapy have been correlated with increased thromboembolic events, which can lead to vascular complications that cause impaired tissue perfusion – anthracyclines, taxanes, platins and VEGF inhibitor [5], [6], [10]. Taken together, all anticancer therapy modalities except cancer surgery could lead to myocardiotoxicity through various pathomechanisms – not only directly but also secondary-, followed by the development of acute heart failure due to incurred therapy-induced cardiomyopathy, which is associated with a reduced quality of life, decreased functional capacity, more frequent dysrhythmias, and increased mortality in cancer patients.
Apart from the different pathological profile of the chemotherapeutic drug and its dosage, the side effects depend on individual patients' cardiovascular history and dispositions. To maintain favourable outcomes in cancer patients, cardiotoxicity should be prevented or at least detected early and treated sufficiently. All pathways of CIMC can be interrupted or even reversed in most patients. In this context, there are specific therapeutic strategies for anticancer therapy-associated cardiovascular diseases [7], [8], [9]. The current guidelines suggest early initiation of optimised heart-failure therapy, dose reduction and/or changing of the anticancer agent in case of proven cardiotoxicity, and regular clinical and echocardiographic controls in patients with CIMC or undergoing cardiotoxic anticancer therapy [10], [11], [12]. Risk factors considered relevant to the development of CIMC have previously been described [12], [13], [21]. Left-ventricular (LV) strain analysis is a validated tool for early recognition of myocardial injury in patients with various cardiovascular diseases [14].
However, there is no reliable and feasible scoring system for the risk assessment of myocardial toxicity prior to initiation of chemotherapy in cancer patients, which might be useful for therapy planning, patient selection and prevention of CIMC, including increasing favourable clinical outcomes and reducing cardiovascular mortality in cancer patients. Generally, there are two different pathogenesis of anticancer agent-related myocardial toxicity – type 1: dose-dependent, anthracyclines and type 2: dose-independent, non-anthracyclines. To get reproducible results and make conceivable statistical analysis, we aimed to develop a scoring system for the pretherapeutic risk assessment of the development of CIMC in patients who receive type 2 –non-anthracyclines dose-independent– chemotherapy agents.
2. Methods
2.1. Patients, follow-up, endpoints
2.1.1. Derivation cohort
We prospectively and randomly investigated adult patients (≥18 years) who received cardiotoxic anticancer agents at the University Hospital Bonn without pre-existing chemotherapy-related cardiotoxicity between March 2018 and May 2019. All patients underwent comprehensive echocardiography at baseline before initiating chemotherapy and routinely at scheduled follow-ups (FUs) – at a frequency of every 6–8 weeks as an intermediate evaluation or more frequent in case of clinical suspicion or presence of cardiotoxicity. All included patients were followed for at least one year after the last anticancer therapy application for the complete assessment of survival and quality of life. A comprehensive blood test was performed prior to initiation of anticancer treatment and included serum levels of NT-proBNP, troponin I, triglyceride, LDL-cholesterol, and creatinine. An invasive coronary angiography and electrocardiogram were performed in all patients with developed CIMC to exclude underlying cardiovascular geneses (dysrhythmias and coronary artery disease) as a reason for clinical and echocardiographic impairment. The exclusion criteria were as follows; patients who previously received anticancer therapy, patients presented with pre-existing chemotherapy-induced cardiotoxicity at baseline, patients with pre-existing heart failure with either preserved ejection fraction or reduced ejection fraction due to cardiovascular genesis, and patients with infaust prognosis (expected survival < 12 months). Furthermore, we excluded the patients who received anthracyclines to make a homogenous cohort regarding pathomechanism of myocardiotoxicity– only anticancer agents which lead to dose-independent cancer therapeutics-related cardiac dysfunction.
2.1.2. Validation cohort
We retrospectively searched patients who had received non-anthracycline myocardiotoxic chemotherapy regimens from January 2018 to December 2018 from our internal patients' database. We randomly chose 30 patients with at least two years FU from those searched patients as a validation cohort. Other inclusion and exclusion criteria were similar to the derivation cohort.
2.1.3. Endpoints, ethical issues
We defined the primary endpoint as the development of CIMC within the FU period, which describes a 10% reduction in LV ejection fraction (LV-EF) to a value below 50% or a 15% relative reduction of the LV global longitudinal strain (LV-GLS) from baseline within FU, according to the current recommendations [10]. All clinically relevant LV function changes were confirmed by a repeated echocardiography two weeks after the initial diagnosis. All-cause mortality was selected as the secondary endpoint.
This observational study was in accordance with the Declaration of Helsinki. All patients signed written informed consent for using clinical data for research purposes. All patient data were pseudonymised before being transferred into the study database or used for study purposes.
2.2. Conventional and speckle tracking echocardiography
All echocardiographic examinations were performed at a frequency of every six to eight weeks or more frequent (every two to three weeks) in case of detection of CIMC. According to our internal basic echocardiography protocol, all images were acquired with a frame rate of 20–30 pgs, without additional pre-processing adjustments. The endocardial border was manually traced in the apical 2- and 4-chamber view in end-diastole and end-systole to calculate LV-EF according to Simpsons' formula from volumes obtained by the summation of a stack of elliptical discs. Conventional transthoracic echocardiography was performed with commercially available echocardiographic systems (iE 33, Philips Medical Systems, Andover, Massachusetts; GE Vivid E9, GE Health Medical, Horten, Norway) and echocardiography probes (X5-1 and M5Sc-D), following the current recommendations and guidelines [20]. The echocardiographer who performed the baseline and FU evaluation was blinded to the patients' characteristics and procedural outcomes.
Speckle tracking echocardiography was performed to analyse LV deformation from an apical four-chamber view using strain analysis. The images were analysed using a post-processing workflow of dedicated software (TomTec Image Arena, 4D LV-Analysis, Munich, Germany) to assess LV-EF and deformation (LV-GLS). The endocardium border of the LV was manually traced using the point-and-click technique. Endocardium borders were semi-automatically assessed at end-diastole and end-systole. A re-adjustment was done in the case of suboptimal alignment. Following this, the offline measurements of the time-to-peak analysis – strain values and strain rates– were automatically generated.
2.3. The cardiotoxicity score – CardTox-score
We developed a scoring system, “Cardiotoxicity Score (CardTox-Score),” by using multiple logistic regression analyses and confidence interval estimation of known parameters that were considered relevant to the development of cancer therapeutics-related cardiac dysfunction based on previously published data and the current guidelines [10], [13], [15], [16], [21]. The investigated variables of the risk model consist of clinical (age ≥ 60 years, BMI > 25 kg/m2, presence of other cardiovascular risk conditions such as arterial hypertension, diabetes mellitus, nicotine consumption, hyperlipidaemia, coronary artery disease), laboratory (troponin I > 0.04 pg/ml, NT-proBNP > 400 pg/ml), and echocardiographic parameters (LV-EF ≤ 50%, LV diastolic function ≥ grade 1, LV-GLS < -20%). The continuous variables were changed into categorical variables prior to being used for the further analysis of the CardTox-Score, according to the above-mentioned cut-off values by reference to the current guidelines and previously published meta-analyses with a large number of patients [10], [12], [17], [21]. Each risk factor was evaluated firstly with a univariate logistic regression analysis concerning the development of CIMC. To avoid unstable and biased prediction due to the limited number of events (CIMC, n = 25) and to get an easy-to-handle score system, we re-arranged the parameters – which stayed statistically significant after the univariate logistic regression analysis– in three main categories as follows: age (one point for ≥ 60 years), cardiovascular risk parameters (two points for each factor), and baseline impaired LV function assessed by echocardiography (LV-EF ≤ 50%, LV-GLS<- 20%) or NT-proBNP > 400 pg/dl (three points for each situation). We performed a multivariate logistic regression analysis to allocate the points based on regression coefficients, ranging from 0 to 12 points. The scale of the CardTox is presented in the supplementary figure Figure Supplementary figure 1.
2.4. Statistical analysis
The normal distribution of continuous variables was examined using the Kolmogorov–Smirnov test or the Shapiro–Wilk test. Continuous data were expressed as mean values ± the standard deviation if normally distributed. The non-normally distributed continuous variables were presented as median values with the interquartile range. The Student's two-sample t-test or the Man–Whitney U test was performed to compare continuous variables. Categorical data were presented as frequencies and percentages. Fisher's exact test or the Chi-square test was used to compare categorical data. The Kruskal–Wallis test was performed to compare the incidence of CIMC according to cancer type. The univariate, as well as multivariate logistic regression analysis, were performed to investigate the candidate predictors for the estimation of CIMC, to assess the point allocation of the CardTox-Score, and to estimate all-cause mortality with the following covariates: age, LV-EF, history of arterial hypertension and diabetes mellitus, serum level of NT-proBNP at baseline. The ROC curve analysis was used to identify the discriminative power of the CardTox-Score with sensitivity and specificity and to determine the cut-off value. Two-tailed p-values were considered to be significant if ranging below 0.05. Statistics were performed using SPSS (PASW statistic, Version 25.0.0.0, SPSS Inc., Chicago, Illinois, USA) and MedCalc Statistical Software (Version 19.2, MedCalc Software Ltd, Ostend, Belgium).
3. Results
3.1. Derivation cohort
3.1.1. Patients' characteristics
We included 225 patients (58.21 ± 6.3 years, 52.8% female) as the derivation cohort with normal baseline LV function who received cardiotoxic anticancer therapy at our centre. The most considerable portion of patients of the derivation cohort presented with breast cancer (36.4%, n = 82), followed by haematological cancer (26.2%, n = 59), gastrointestinal cancer (13.7%, n = 31), and lung cancer (7.5%, n = 17). 16% (n = 36) of the derivation cohort had other neoplastic diseases such as gynaecological tumours, skin tumours, or other endocrinological malignancies (Table 1).
Table 1.
Comparison of the baseline characteristics in the derivation cohort stratified by the developed CIMC.
| No CIMC (N = 200) | With CIMC (N = 25) | p-Value | |
|---|---|---|---|
| Age, (years), mean ± SD | 57 ± 13 | 62 ± 8.7 | 0.3 |
| Age ≥ 60 (years), %(n) | 40 (80) | 68 (17) | 0.05 |
| BMI, (kg/m2), mean ± SD | 23.8 ± 1.2 | 24.2 ± 3.6 | 0.4 |
| BMI > 25 (kg/m2), % (n) | 46 (92) | 64 (16) | 0.09 |
| Gender, (female), %(n) | 58 (116) | 52 (13) | 0.8 |
| Arterial hypertension, %(n) | 27 (54) | 52 (13) | 0.0099 |
| Hyperlipidaemia, %(n) | 10 (20) | 48 (12) | 0.001 |
| Diabetes mellitus, %(n) | 8 (16) | 20 (5) | 0.02 |
| Nicotine consumption, %(n) | 16 (32) | 36 (9) | 0.02 |
| History of stroke, %(n) | 3 (6) | 12 (3) | 0.03 |
| Peripheral artery disease, %(n) | 5 (10) | 8 (2) | 0.6 |
| Coronary artery disease, % (n) | 8 (16) | 16 (4) | 0.09 |
| Creatinine, (mg/dl), median (IQR) | 0.82 (0.74 0.86) | 0.81 (0.69 1.1) | 0.9 |
| Troponin I, (pg/ml), median (IQR) | 0.02 (0.02 0.04) | 0.42 (0.08 0.87) | 0.001 |
| Triglyceride, (mg/dl), median (IQR) | 90 (79.16 151.69) | 80 (93.63 125.06) | 0.3 |
| LDL-cholesterol, (mg/dl), median (IQR) | 72 (52 116.92) | 82 (65.56 140.43) | 0.2 |
| NT-proBNP, (pg/ml), median (IQR) | 89 (41.94 363.13) | 176 (101.76 366.48) | 0.01 |
| Type of cancers, % (n) | |||
| Breast cancer | 39 (78) | 16 (4) | |
| Haematological cancer | 27 (54) | 20 (5) | |
| Gastrointestinal cancer | 14 (28) | 12 (3) | |
| Lung cancer | 6 (12) | 20 (5) | |
| Other | 14 (28) | 32 (8) | |
CIMC: chemotherapy-induced myocardiomyopathy, LDL: low-density lipoprotein, NT-proBNP: N terminal pro-brain natriuretic peptide, SD: standard deviation.
Concerning anticancer agents, 48.8% (n = 110) of the derivation cohort was treated with alkylating agents (cyclophosphamide, ifosfamide, chlorambucil, bendamustine, melphalan, busulfan, dacarbazine), 47.3% (n = 106) of patients had monoclonal antibodies as an anticancer therapy, such as trastuzumab (Anti-HER2), bevacizumab (Anti-VEGF), denosumab (Anti-RANKL), rituximab (Anti-CD20), panitumumab (Anti-EGF), elotuzumab (Anti-CD319), cetuximab (Anti-EGFR), and pertuzumab (Anti-HER2), and 9.3% (n = 21) had immune checkpoint inhibitors - ipilimumab (Anti-CTLA-4), nivolumab (Anti-PD1), atezolizumab (Anti-PD-L1), pembrolizumab (Anti-PD1). Anti-microtubule agents (docetaxel, paclitaxel, vincristine, etoposide, irinotecan) were given in 26.2% (n = 59) of patients, and 36% (n = 81) of patients got anti-metabolites and fluoropyrimidines (clofarabine, 5-FU, capecitabine, gemcitabine, pemetrexed). Protein kinase inhibitors—trametinib (MEK inhibitor), binimetinib (MEK inhibitor), dabrafenib (BRAF inhibitor), encorafenib (BRAF inhibitor), sorafenib (RAF and VEGFR-2/3 inhibitor), everolimus (mTOR inhibitor), temsirolimus (mTOR inhibitor), sirolimus (mTOR inhibitor)—were applicated in 51.5% (n = 116) of patients. In addition to chemotherapeutic agents, radiotherapy was performed in 148 patients (65.7%) with a mean cumulative dose of 18 Gy/m2 as primary or symptomatic therapy. 70.22% (n = 158) of patients underwent cancer surgery without any persistent cardiovascular complications.
3.1.2. Comparison of the groups
11.1% (n = 25) of the patients with normal baseline LV function developed CIMC (62 ± 8.7 years, 52% female) within the FU period. The mean duration of development of CIMC was 3.2 months after the first application of non-anthracycline anticancer therapy. Changes in LV function during FU are as follows –divided our cohort into two groups –: with developed CIMC (LV-EFBaseline 57.2 ± 12.1% to LV-EFFollow-up 39.2 ± 12.5%, p < 0.0001; LV-GLSBaseline − 18.5 ± 7.5% to LV-GLSFollow-up − 9.1 ± 4.5%, p < 0.0001) or without CIMC (LV-EFBaseline 62.3 ± 7.3% to LV-EFFollow-up 61.3 ± 7.3%, p = 0.24; LV-GLSBaseline − 19.7 ± 4.2% to LV-GLSFollow-up − 18.4 ± 2.1%, p = 0.5).
Demographic characteristics, including age, gender, and body mass index (BMI), were not significantly different between the groups. Patients who developed CIMC within FU more often showed cardiovascular risk factors, such as arterial hypertension (27% vs 52%, p = 0.0099), hyperlipidaemia (10% vs 48%, p = 0.001), nicotine consumption (16% vs 36%, p = 0.02), diabetes mellitus (8% vs 20%, p = 0.02), and history of stroke (3% vs 12%, p = 0.03) at baseline. In contrast, there were no significant differences concerning the incidence of coronary artery disease (8% vs 16%, p = 0.09) and peripheral artery disease (5% vs 8%, p = 0.6) between the groups. Baseline blood tests showed significantly higher serum levels of troponin I (0.02 pg/ml [0.02 0.04] vs. 0.42 pg/ml [0.08 0.87], p = 0.001), and NT-proBNP (89 pg/ml [41.94 to 363.13] vs. 176 pg/ml [101.76 to 366.48], p = 0.01) in patients with CIMC. Demographical and baseline characteristics are presented in Table 1. There was no significant difference concerning cardioprotective agents – also in doses–at baseline in both groups (beta-blockers: 18% vs 25%, p = 0.1; angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: 10% vs 18%, p = 0.3; aspirin: 8% vs 15%, p = 0.1; statins: 15% vs 35% p = 0.07).
Prior to anticancer therapy initiation, echocardiography showed significantly more often diastolic dysfunction ≥ grade 1 (56% vs 96%, p = 0.0012) in the CIMC group. The remaining echocardiographic parameters were comparable in both groups of the derivation cohort (Table 2). There were no significant differences in the incidence of radiotherapy – no CIMC: 66.5% (n = 133) vs with CIMC: 60% (n = 15%)– and cancer surgery – no CIMC: 70.5% (n = 141) and with CIMC: 68% (n = 17)– in both groups of the derivation cohort.
Table 2.
Comparison of baseline echocardiographic characteristics between the groups of the derivation cohort.
| No CIMC (N = 200) | With CIMC (N = 25) | p-Value | |
|---|---|---|---|
| LV-EF, % (mean ± SD) | 62.3 ± 7.3 | 57.2 ± 12.1 | 0.1 |
| LV-SV, ml (mean ± SD) | 57.5 ± 19.3 | 48.1 ± 14.3 | 0.08 |
| LV-EDV, ml (mean ± SD) | 92.9 ± 34.6 | 103.1 ± 39.2 | 0.2 |
| LV-ESV, ml (mean ± SD) | 36.2 ± 21.2 | 45.1 ± 21.3 | 0.1 |
| E/É ratio (mean ± SD) | 8.8 ± 4.1 | 8.5 ± 1.5 | 0.7 |
| LV diastolic dysfunction, %(n) | 0.0012 | ||
| none | 44 (88) | 4 (1) | |
| Grade 1 | 55 (110) | 84 (21) | |
| Grade 2 | 1 (2) | 12(3) | |
| LV- GLS, % (mean ± SD) | −19.7 ± 4.2 | −18.5 ± 7.5 | 0.7 |
| RVSP, mmHg (mean ± SD) | 19.3 ± 13 | 21.2 ± 5.3 | 0.6 |
| TAPSE, cm (mean ± SD) | 2.3 ± 0.4 | 2 ± 0.3 | 0.1 |
CIMC: chemotherapy-induced myocardiomyopathy, EDV: end-diastolic volume, EF: ejection fraction, ESV: end-systolic volume, GLS: global longitudinal strain, LV: left ventricle, RVSP: right-ventricular systolic pressure, SD: standard deviation, SV: stroke volume, TAPSE: tricuspid annular presystolic excursion.
3.1.3. Predictors for prognosis
The mean FU duration was 25 ± 4 months. In this time, 29.8% (n = 67) of patients in the derivation cohort died: twelve patients (17.9%) due to progression of cancer or oncological complications, sixteen patients (23.8%) due to cardiovascular causes in the context of chemotherapy-associated heart failure, and thirty-nine (58.2%) due to septic shock and multiple organ failure.
We found a strong positive linear relationship between delta (FU to baseline) LV-EF and delta LV-GLS according to Pearson's (p = 0.014) and Spearman (p = 0.019) analysis, with a correlation coefficient of 0.23 (95% CI: 0.058 0.393) (Fig. 1).
Fig. 1.
Scatter diagram of the positive linear correlation between delta LV-EF (DeltaEF) and delta LV-GLS (DeltaGLS) with a trend line.
3.1.4. Derivation of the CardTox-Score
All candidate risk parameters known to be relevant for the development of CIMC were compared using the students' t-test in consideration of the development of CIMC (Table 1, Table 2). Nineteen parameters were found to be relevant (p < 0.20) for this risk model. The continuous variables were converted into the categorical variables according to previously published cut-off values [11], [15], [16] and were subsequently added into a univariate logistic regression analysis. According to results from the univariate regression analysis based on the development of CIMC, we found the following relevant risk factors; age ≥ 60 years (OR: 1.5, 95% CI: 0.84–2.12, p = 0.01), presence of coronary artery disease (OR: 2.0, 95% CI: 0.48–4.41, p = 0.01), presence of arterial hypertension (OR: 1.4, 95% CI: 0.56–2.32, p = 0.03), presence of hyperlipidaemia (OR: 1.8, 95% CI: 0.65–2.85, p = 0.02), presence of diabetes mellitus (OR: 1.8, 95% CI: 0.58–3.12, p = 0.03), serum level of NT-proBNP > 400 pg/ml (OR: 2.24, 95% CI: 1.13–4.46, p = 0.001), LV-EF to ≤ 50% or LV-GLS < 20% (OR: 3.1, 95% CI: 1.51–5.85, p = 0.0001) (Table 3). A further evaluation for the point allocation was performed by a multivariate regression analysis of those parameters – age, coronary artery disease, arterial hypertension, hyperlipidaemia, diabetes mellitus and baseline LV function, which were found to be statistically significant according to the univariate regression analysis. The point distribution based on the results from the multivariate analysis is presented in Table 4.
Table 3.
Univariate logistic regression analysis of the different risk factors concerning development of CIMC.
| OR | 95% Cl | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age ≥ 60 years | 1.5 | 0.84 2.12 | 0.01 |
| BMI > 25 kg/m2 | 1.2 | 0.65 1.83 | 0.1 |
| Medical history | |||
| Coronary artery disease | 2.0 | 0.48 3.41 | 0.01 |
| Arterial hypertension | 1.4 | 0.56 2.32 | 0.03 |
| Hyperlipidemia | 1.8 | 0.65 2.85 | 0.02 |
| Diabetes mellitus | 1.8 | 0.58 3.12 | 0.03 |
| Nicotine consumption | 1.7 | 0.54 2.85 | 0.08 |
| Laboratory | |||
| Troponin I > 0.04 pg/ml | 1.3 | 0.35 2.06 | 0.12 |
| NT-proBNP > 400 pg/ml | 2.24 | 1.13 4.46 | 0.001 |
| Echocardiography | |||
| LV-EF ≤ 50% or LV-GLS < 20% | 3.1 | 1.51 5.85 | 0.0001 |
| LV diastolic dysfunction ≥ grade 1 | 1.1 | 0.21 1.45 | 0.09 |
BMI: body mass index, CI: confidence interval, CIMC: chemotherapy-induced myocardiotoxicity, EF: ejection fraction, GFR: glomerular filtration rate, GLS: global longitudinal strain, LDL: low-density lipoprotein, LV: left ventricle, NT-proBNP: N terminal pro-brain natriuretic peptide, OR: odds ratio.
Table 4.
Point distribution according to multivariate logistic regression analysis of risk parameters concerning the development of CIMC.
| Categories | Factors | OR | 95% Cl | p-Value | points |
|---|---|---|---|---|---|
| Age | |||||
| Age ≥ 60 vs < 60 years | 1 | 1.17 | 0.33 1.32 | 0.05 | 1 |
| Cardiovascular risk | |||||
| Coronary artery disease (yes/no) | 1 | 1.43 | 0.52 2.66 | 0.03 | 2 |
| Arterial hypertension(yes/no) | 2 | 3.32 | 1.78 9.32 | 0.001 | 42 |
| Hyperlipidemia(yes/no) | ≥3 | 5.54 | 2.56 15.43 | <0.001 | 62 |
| Diabetes mellitus(yes/no) | |||||
| NT-proBNP > 400 vs ≤ 400 pg/ml | |||||
| LV function | |||||
| LV-EF ≤ 50% or LV-GLS<-20% | 1 | 2.35 | 1.87 5.01 | 0.006 | 3 |
| Total | 12 |
EF: ejection fraction, GLS: global longitudinal strain, LV: left ventricle, NT-proBNP: N terminal pro-brain natriuretic peptide.
3.2. Validation cohort
3.2.1. Patients' characteristics
The validation cohort (n = 30) consisted of retrospectively and randomly selected patients (59.2 ± 6.5 years, 57% females) who received non-anthracycline myocardiotoxic anticancer agents. They presented with a similar distribution of the type of cancers compared to the derivation cohort – 36.6% (n = 11) breast cancer, 16.7% (n = 5) haematological cancer, 13.3% (n = 4) gastrointestinal cancer, 10% (n = 3) lung cancer, and other cancer entities were 23.3% (n = 7). Compared to the derivation cohort, there were no significant differences in baseline and demographical characteristics (Table 5).
Table 5.
Comparison of the cohorts; derivation vs validation.
| Derivation cohort (n = 225) | Validation cohort (n = 30) | p-Value | |
|---|---|---|---|
| Age, (years), mean ± SD | 58.41 ± 9.1 | 59.2 ± 6.5 | 0.7 |
| BMI, (kg/m2), mean ± SD | 23.9 ± 5.5 | 24.3 ± 2.2 | 0.3 |
| Gender, (female), %(n) | 57 (1 2 9) | 57 (17) | 0.9 |
| Arterial hypertension, %(n) | 30 (67) | 43 (13) | 0.09 |
| Hyperlipidaemia, %(n) | 14 (32) | 17 (5) | 0.4 |
| Diabetes mellitus, %(n) | 9 (21) | 10 (3) | 0.3 |
| Nicotine consumption, %(n) | 18 (41) | 20 (6) | 0.2 |
| History of stroke, %(n) | 4 (9) | 7 (2) | 0.1 |
| Peripheral artery disease, %(n) | 5 (12) | 3 (1) | 0.5 |
| Coronary artery disease, % (n) | 9 (20) | 10 (3) | 0.6 |
| Creatinine, (mg/dl), median (IQR) | 0.81 (0.67–1.09) | 0.78 (0.63–0.98) | 0.8 |
| Troponin I, (pg/ml), median (IQR) | 0.04 (0.02–0.23) | 0.03 (0.01–0.12) | 0.9 |
| Triglyceride, (mg/dl), median (IQR) | 130 (88–203) | 128 (67–212) | 0.6 |
| LDL-cholesterol, (mg/dl), median (IQR) | 125.5 (89.5–143) | 115 (69 138) | 0.2 |
| NT-proBNP, (pg/ml), median (IQR) | 175.6 (41.94 363.13) | 143 (96.5 310) | 0.08 |
| Type of cancers, % (n) | 0.1 | ||
| Breast cancer | 35.1 (79) | 36.6 (11) | |
| Haematological cancer | 28 (63) | 16.7 (5) | |
| Gastrointestinal cancer | 14.2 (32) | 13.3 (4) | |
| Lung cancer | 5.3 (12) | 10 (3) | |
| Other | 14.6 (39) | 23.3 (7) |
CIMC: chemotherapy-induced myocardiomyopathy, LDL: low-density lipoprotein, NT-proBNP: N terminal pro-brain natriuretic peptide, SD: standard deviation.
Echocardiographic examinations prior to the initiation of myocardiotoxic anticancer therapy in the validation cohort showed –comparable to the derivation cohort– normal LV and RV function without any clinically relevant pathologies (LV-EDV: 90.5 ± 29.3 ml, LV-ESV: 33.4 ± 21.4 ml, LV-EF: 59.9 ± 5.6%, LV-SV: 55.4 ± 14.2 ml, LV-GLS: −20.4 ± 4.3%, TAPSE: 2.5 ± 0.2 cm, RVSP: 17.5 ± 11.3 mmHg). The comparison of the pretherapeutic echocardiographic characteristics of both cohorts – derivation vs validation– is presented in Table 6.
Table 6.
Comparison of baseline echocardiographic characteristics between the cohorts; derivation vs validation.
| Derivation cohort (n = 225) | Validation cohort (n = 30) | p-Value | |
|---|---|---|---|
| LV-EF, % (mean ± SD) | 61.7 ± 8.5 | 59.9 ± 5.6 | 0.5 |
| LV-SV, ml (mean ± SD) | 57.1 ± 19.3 | 55.4 ± 14.2 | 0.7 |
| LV-EDV, ml (mean ± SD) | 93.6 ± 35.2 | 90.5 ± 29.3 | 0.3 |
| LV-ESV, ml (mean ± SD) | 36.6 ± 19.4 | 33.4 ± 21.4 | 0.4 |
| E/É ratio (mean ± SD) | 8.8 ± 4.1 | 8.4 ± 3.2 | 0.1 |
| LV diastolic dysfunction, %(n) | 0.9 | ||
| none | 39.5 (89) | 46.7 (14) | |
| Grade 1 | 58.2 (131) | 50 (15) | |
| Grade 2 | 0.3 (5) | 0.3 (1) | |
| LV- GLS, % (mean ± SD) | −19.5 ± 4.1 | −20.4 ± 4.3 | 0.8 |
| RVSP, mmHg (mean ± SD) | 19.6 ± 12.8 | 17.5 ± 11.3 | 0.4 |
| TAPSE, cm (mean ± SD) | 2.3 ± 0.4 | 2.5 ± 0.2 | 0.2 |
CIMC: chemotherapy-induced myocardiomyopathy, EDV: end-diastolic volume, EF: ejection fraction, ESV: end-systolic volume, GLS: global longitudinal strain, LV: left ventricle, RVSP: right-ventricular systolic pressure, SD: standard deviation, SV: stroke volume, TAPSE: tricuspid annular presystolic excursion.
36.7% (n = 11) of the validation cohort had become a diagnosis of CIMC during FU with a mean time-to-diagnosis period of 4.1 ± 1.2 months from the initiation of anticancer agents. All-cause mortality within a FU with a mean duration of 19 ± 3 months was 46.7% (n = 14) in our internal validation cohort.
3.2.2. Validation of the CardTox-Score
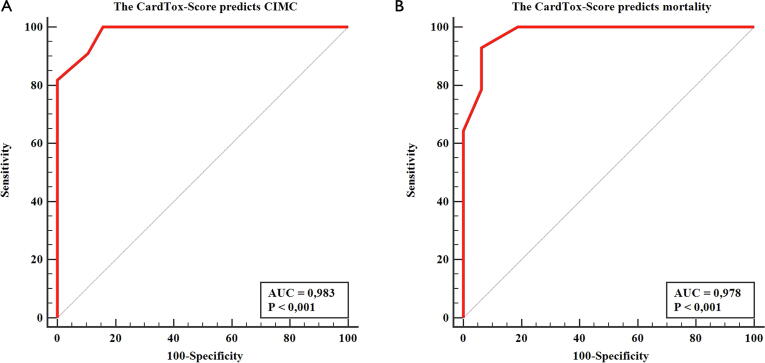
According to the ROC analysis, we found a high predictive value of the CardTox-Score both for the development of CIMC, with a high specificity (84.2%) and sensitivity (100%) (cut-off value: 6 points, AUC: 0.983, 95% CI: 0.854–1.000, p < 0.001), and for all-cause mortality (cut off value: > 6 points, AUC: 0.978, p < 0.001), also with high specificity (93.7%) and sensitivity (92.9%) in the validation cohort (Fig. 2).
Fig. 2.
A: ROC curve of the CardTox-Score for the prediction of the development of CIMC in the validation cohort (AUC: 0.983, sensitivity: 100%, specificity: 84.2%, p < 0.0001). B: ROC curve of the CardTox-Score for the prediction of all-cause mortality in the validation cohort (AUC: 0.978, sensitivity: 92.9%, specificity: 93.7%, p < 0.001).
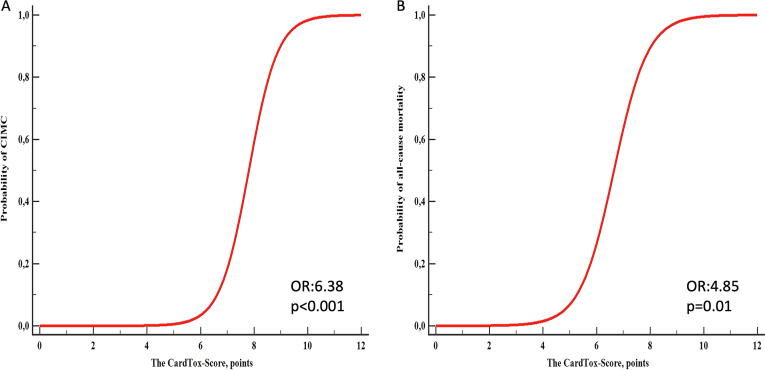
The logistic regression analysis confirmed that the CardTox-Score adequately and highly significantly predicts not only the development of CIMC (OR: 6.38, 95% CI: 1.21–33.52, p < 0.001), but also all-cause mortality (OR: 4.85, 95% CI: 1.39 16.86, p = 0.01) (Fig. 3). A further correlation analysis revealed that the presence of CIMC shows a positive and significant correlation with all-cause mortality in the validation cohort (r: 0.81, 95% CI: 0.64 to 0.90, p < 0.001).
Fig. 3.
A: Graphical depiction of the relationship between the CardTox-Score and probability of CIMC in the validation cohort (OR: 6.38, p < 0.001). B. Graphical depiction of the relationship between the CardTox-Score and probability of all-cause mortality in the validation cohort (OR: 4.85, p = 0.01).
3.3. Relationship to the type of cancer and anticancer therapy
Of note, we found no relevant differences in the type of cancer between the cohorts, using the Kruskal–Wallis test (p = 0.1) (Table 5). Moreover, there were no statistically significant differences concerning anticancer therapy regimes between the cohorts – the incidence of radiotherapy (65.7% vsvs 63.3%, p = 0.7), the incidence of cancer surgery (70.2% vs 60%, p = 0.1), the distribution of pharmaceutical anticancer modalities (p ≥ 0.1) (Table 7). Furthermore, there was no significant association between the presence of CIMC and the type of cancer in the validation cohort, according to the Kruskal-Wallis test (p = 0.9).
Table 7.
Anti-cancer therapy modalities.
| Derivation cohort (n = 225) | Validation cohort (n = 30) | p-Value | |
|---|---|---|---|
| Cytotoxic therapy | |||
| Alkylating agents, %(n) | 48.8(110) | 53.3 (16) | 0.3 |
| Anti-metabolites, %(n) | 36(81) | 40 (12) | 0.7 |
| Anti-microtubule agents, %(n) | 26.2(59) | 30 (9) | 0.8 |
| Targeted therapy | |||
| Monoclonal antibodies, %(n) | 47.3(106) | 46.6 (14) | 0.9 |
| Protein kinase inhibitors, %(n) | 51.5(116) | 43.3 (13) | 0.2 |
| Immunotherapy | |||
| Checkpoint inhibitors, %(n) | 9.3(21) | 13.3 (4) | 0.2 |
| Radiotherapy, %(n) | 65.7(1 4 8) | 63.3 (19) | 0.7 |
| Cancer surgery, %(n) | 70.2(158) | 60 (18) | 0.1 |
CIMC: chemotherapy-induced myocardiotoxicity.
4. Discussion
The major findings of the present study are as follows:
-
1.
The CardTox-Score was found to be a highly discriminative predictor for the development of CIMC (100% sensitivity and 84.2% specificity) as well as for all-cause mortality (92.9% sensitivity and 93.7% specificity) in our internal validation cohort who underwent dose-independent cardiotoxic anticancer agents, with a cut-off value of six points.
-
2.
CIMC was an independent predictor for all-cause mortality in our internal validation cohort.
-
3.
In our internal validation cohort, the CardTox-Score estimates the development of CIMC independently from the type of cancer.
Heart failure, both acute and chronic, describes an additional challenging problem in cancer patients undergoing anticancer modalities and is associated with unfavourable effects on their outcomes. The current guidelines recommend cardiovascular prevention and risk assessment prior to the initiation of cardiotoxic anticancer therapy regimes, regular clinical FU during chemotherapy, and early initiation of secondary cardioprotective therapy, including beta-blockers and RAS blockers, when cardiotoxicity is found [10], [21]. Risk factors for the development of CIMC have previously been published [10], [21]. However, there is no proper scoring system for the risk assessment of CIMC in cancer patients who are going to receive cardiotoxic agents. It might prevent or at least decrease the incidence of CIMC and lead to more favourable outcomes in cancer patients.
In a meta-analysis of 14 studies with a total of 1350 patients, Totzeck et al. showed that LV-GLS detects myocardial damage earlier than the conventional echocardiographic assessment of LV-EF [9]. In line with this finding, we found a significant reduction of LV-GLS without a concomitant distinct decrease in LV-EF in 15 patients from our derivation cohort. The CardTox-Score includes LV-EF and LV-GLS as echocardiographic risk parameters to make a more precise evaluation of myocardial mechanics and pathology, which may lead us to detect subtle myocardial changes and subsequently give a more decisive prediction of CIMC.
Rydzek et al. showed the unfavourable effects of impaired baseline cardiovascular status on mortality in a study with 326 subjects with breast or lung cancer [17]. In concordance with these findings, we also found the CardTox-Score correlates with all-cause mortality in our internal validation cohort, a large proportion of whom suffered from breast and lung cancer (46.6%). Moreover, we found a highly significant linear correlation between CIMC and all-cause mortality in the validation cohort. Pre-existing advanced cancer should be considered to be a high competing risk for all-cause mortality.
Concerning cardiovascular laboratory diagnostic tests, serum levels of troponin and NT-proBNP have been shown by Michel et al. to be a proper screening parameter for chemotherapy-related LV dysfunction in a meta-analysis of 61 trials with a total of 5691 cancer patients [16]. We consistently included the serum levels of troponin I and NT-proBNP in the CardTox-Score as a surrogate laboratory parameter for myocardial injury, which was found to be a strong predictor for CIMC.
Hamirani et al. showed that the presence of arterial hypertension, hyperlipidaemia, and coronary artery disease correlates with reduced LV function in 549 patients after anthracycline or trastuzumab therapy [18]. In agreement with this study, we found that the CardTox-Score – which includes five cardiovascular risk factors – showed high predictive value for the development of CIMC in our small internal validation cohort. As a relevant difference, our validation cohort only consists of patients who underwent dose-independent myocardiotoxic anticancer agents.
Furthermore, a cardiovascular score called FRESCO consisting of age, sex, smoking, and body mass index was shown by Ferraro et al. to be an independent predictor for developing CIMC in 130 patients with B-cell lymphoma, five years after anthracycline therapy [19]. In line with this finding, the CardTox-Score adequately predicts CIMC with high sensitivity and specificity. In contrast to the FRESCO score, the CardTox-score consists of not only demographic parameters but also clinical cardiovascular risk conditions (arterial hypertension, hyperlipidaemia, presence of coronary artery disease), echocardiographic parameters (LV-EF, LV-GLS), and laboratory parameters (NT-proBNP). Therefore, the CardTox-Score could lead to an adequate prediction of CIMC according to a comprehensive assessment of the pre-existing cardiovascular status. On the other hand, we performed the validation analysis in a cohort that consists of patients who underwent non-anthracycline cardiomyotoxic anticancer agents.
Taken together, all of the cited studies have been performed in patients with selected types of cancer—mostly breast or haematological cancers. On the contrary, we found that the CardTox-Score is a promising tool for adequate risk assessment of CIMC in patients with various cancer types (36.6% breast cancer, 16.7% haematological cancer, 13.3% gastrointestinal cancer, 10% lung cancer, 23.3% others) who underwent non-anthracycline anticancer regimes, irrespective of the cancer type.
Besides, it should be mentioned that anticancer therapy-related cardiotoxicity is a huge complex of various adverse events, including cardiomyotoxicity (direct and indirect) and vascular complications – coronary and periphery-, dysrhythmias, and thromboembolic events. We excluded other possible cardiotoxicity aetiologies and only included patients who developed impaired LV function during FU without any relevant concomitant coronary artery disease, dysrhythmias or thromboembolic events.
To the best of our knowledge, it is the first study that presents a scoring system for pretherapeutic primary risk assessment of cancer therapeutics-related myocardial toxicity in patients undergoing non-anthracycline cardiomyotoxic anticancer agents independent from the type of cancer disease.
4.1. Limitations
This single-centre prospective observational study has several limitations. We included only a limited number of cancer patients (n = 255) with many different variations of anticancer therapy –a derivation cohort (n = 225) and a validation cohort (n = 30). The study's nature– randomly inclusion, exclusion of patients who underwent anthracyclines, retrospective inclusion of the validation cohort– and the lack of randomisation should be considered possible bias factors. On the other hand, our data may not sufficiently present a real-world situation. The impact of the type of anticancer agent on the development of CMIC cannot be excluded. Besides, our echocardiographic examinations were not adjudged by an independent core laboratory. Due to insufficient image quality for strain analysis from all apical views in some patients, we performed the LV strain analysis from a single apical four-chamber view, which might cause misestimation of myocardial deformation. We analysed the CardTox-Score in a validation cohort with a small number of patients; therefore, the CardTox-Score still needs to be proven in multicentric prospective validation studies with a large number of patients.
5. Conclusion
The CardTox-Score offers a promising, elementary, easy-to-handle, feasible scoring system for pretherapeutic primary risk assessment of chemotherapy-induced myocardial toxicity in cancer patients who receive non-anthracycline anticancer regimes, irrespective of the type of cancer. Our preliminary findings should be validated by a multicentric prospective study with a much larger number of patients and events.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Dr Meghan Campbell (scientific coordinator in the Heart Center Bonn, University Hospital Bonn, Germany) for proofreading the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100751.
Contributor Information
Can Öztürk, Email: can.oeztuerk@ukbonn.de.
Dayanat Validyev, Email: dvalidyev@yahoo.com.
Ulrich Marc Becher, Email: ulrich-marc.becher@ukbonn.de.
Marcel Weber, Email: marcel.weber@ukbonn.de.
Georg Nickenig, Email: georg.nickenig@ukbonn.de.
Vedat Tiyerili, Email: vedat.tiyerili@ukbonn.de.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Ferlay J., Colombet M., Soerjomataram I. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale D., Ciceri F., Latini R., Franzosi M.G., Sandri M.T., Civelli M. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the international cardiooncology society-one trial. Eur. J. Cancer. 2018;94:126–137. doi: 10.1016/j.ejca.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Seidman A., Hudis C., Pierri M.K., Shak S., Paton V., Ashby M. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2002;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 5.Henning R.J., Harbison R.D. Cardio-oncology: cardiovascular complications of cancer therapy. Future Cardiol. 2017;13:379–396. doi: 10.2217/fca-2016-0081. [DOI] [PubMed] [Google Scholar]
- 6.Snipelisky D., Park J.Y., Lerman A. How to develop a cardio-oncology clinic. Heart Fail. Clin. 2017;13:347–359. doi: 10.1016/j.hfc.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Barish R., Lynce F., Unger K., Barac A. Management of cardiovascular disease in women with breast cancer. Circulation. 2019;139:1110–1120. doi: 10.1161/CIRCULATIONAHA.118.039371. [DOI] [PubMed] [Google Scholar]
- 8.Gulati G., Heck S.L., Ree A.H. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomised, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Totzeck M., Schuler M., Stuschke M. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., Aboyans V. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. J. Heart Fail. 2017;19:9–42. doi: 10.1002/ejhf.654. [DOI] [PubMed] [Google Scholar]
- 11.Armenian S.H., Lacchetti C., Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline summary. J. Oncol. Pract. 2017;13:270–275. doi: 10.1200/JOP.2016.018770. [DOI] [PubMed] [Google Scholar]
- 12.Michel L., Rassaf T. Cardio-oncology: need for novel structures. Eur. J. Med. Res. 2019;24:1–4. doi: 10.1186/s40001-018-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slivnick J., Vallakati A., Addison D. Personalized approach to cancer treatment-related cardiomyopathy. Curr. Heart Fail. Rep. 2020;17:43–55. doi: 10.1007/s11897-020-00453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thavendiranathan P., Poulin F., Lim K.-D. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J. Am. Coll. Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 15.Al-Kindi S.G., Oliveira G.H. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo. Clin. Proc. 2016;91:81–83. doi: 10.1016/j.mayocp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Michel L., Mincu R.I., Mahabadi A.A. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur. J. Heart Fail. 2020;22:350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 17.Rydzek J., Gąsior Z.T., Dąbek J. Assessment of risk factors for mortality in patients with cardiovascular disease and a history of treatment for malignancy. Kardiol. Pol. 2015;73:730–739. doi: 10.5603/KP.a2015.0071. [DOI] [PubMed] [Google Scholar]
- 18.Hamirani Y., Fanous I., Kramer C.M. Anthracycline- and trastuzumab-induced cardiotoxicity: a retrospective study. Med. Oncol. 2016;33:82–85. doi: 10.1007/s12032-016-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferraro M.P., Gimeno-Vazquez E., Subirana I. Anthracycline-induced cardiotoxicity in diffuse large B-cell lymphoma: NT-proBNP and cardiovascular score for risk stratification. Eur. J. Haematol. 2019;102:509–515. doi: 10.1111/ejh.13234. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the american society of echocardiography (2019) 1–64. 10.1016/j.echo.2018.06.004. [DOI] [PubMed]
- 21.Lyon A.R., Dent S., Stanway S., Earl H., Brezden-Masley C., Cohen-Solal A. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the cardio-oncology study group of the heart failure association of the european society of cardiology in collaboration with the international cardio-oncology society. Eur. J. Heart Fail. 2020;22(15):242. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]