Abstract
Agroforestry is increasingly being identified as an integrated land use enhancing plant diversity while reducing habitat loss and fragmentation. This paper examined species diversity, composition, structure and management in agroforestry systems. Two Kebeles (Kachabira and Mesafe) were purposively selected for this study. Then, farmers who dominantly practiced agroforestry practices such as home garden, parkland and live fence were stratified based on wealth categories. Ten percent of the sample households were randomly selected from each wealth category. Accordingly, a total of 83 households were selected. Inventories of plant species were done by sampling one plot of each farm management type. A total of 59 plant species, belonging to 56 genera and 36 families were recorded across the home gardens, parklands and live fences in the study area. Among the plant species, trees constituted 42%, shrubs 27%, herbs 29% and climber 2%. From recorded plant species, 66% were native and the remainders 34% were introduced species. From the native species recorded in this study, Lippia adoensis and Millettia ferruginea were endemic to Ethiopia. The mean Shannon diversity index of rich, medium and poor households in the three different agroforestry practices were 1.75, 1.57 and 1.62 in home garden, 0.36, 0.30 and 0.49 in parkland and 0.84, 0.99 and 1.00 in live fence respectively. The largest tree basal area was recorded in the live fence (14.7 m2ha-1), followed by home garden and parkland. The study revealed that agroforestry plays an important role in the conservation of biodiversity, and also by providing food, income and a wide range of other products such as fuel wood, construction material, fodder, spices and medicinal plants. Farm household landholding size, species preference and management found to be the most important influencing factors that affect the diversity of plant species. Further detailed study of explicit examining of the factors such as socio-ecological effects that determine species diversity and the contribution of different functional groups to livelihood is needed to fully understand the agroforestry system.
Keywords: Biodiversity, Home garden, Live fence, Parkland, Species diversity
Biodiversity; Home garden; Live fence; Parkland; Species diversity
1. Introduction
Ethiopia is a tropical country with varied macro and micro-climatic conditions with diverse cultural and farming practices that have contributed to the formation of diverse ecosystems inhabited with a great diversity of life forms of both plants and animals [1, 2, 3].
Agroforestry is the production of trees and of non-tree crops or animals on the same piece of land [4]. It is increasingly viewed as providing ecosystem services, environmental benefits, and economic commodities as part of multifunctional working landscapes [5]. Agroforestry can be particularly important for smallholder farmers and the multifunctional role of agroecosystems has also been emphasized by [6] as it generates diverse products and services. Agroforestry has been practiced for a very long time in many parts of the world. Among the agroforestry systems used by farmers, home gardens, parklands and live fences are the most dominant practices in Ethiopia [7, 8].
Degradation of environment and other threats to components of ecological systems is the most serious environmental problem in Ethiopia [2, 3, 9, 10]. Flora diversities on the natural and human modified ecosystem are declining over time and space due to induced human pressures and other climatic factors [10].
Agroforestry is increasingly being identified as an integrated land use that can directly enhance plant diversity while reducing habitat loss and fragmentation. The integration can be either in a spatial mixture or in a temporal sequence. In agroforestry systems, there are both ecological and economical interactions between the different components [11, 12]. The mixing of different crops and woody species in agroforestry allows niche diversification and some of the combinations complement each other. Farmers usually use such trees for shade and as a facility for social gatherings of the villagers demonstrating the special regard given to trees in the traditional rural life [13].
Biodiversity in agricultural ecosystems provides for our food and the means to produce it. The variety of plants and animals that constitute the food we eat are obvious parts of agricultural biodiversity [14]. This entails the importance of on-farm conservation [15]. Farmers, especially in developing countries are responsible for managing agricultural biodiversity in agricultural ecosystems as a critical resource for providing them with food security, nutrition and sustenance of their livelihoods [16].
Framers in Kachabira district of Southern Ethiopia have been practicing different agroforestry practices for several decades. They have accumulated a wealth of indigenous knowledge on the management of farming system particularly non-crop and crop plant diversity on the agricultural landscape. Studies on agroforestry have been focusing only on one of the landuse practices and the little explicit study was executed on home garden, parkland and live fence. However, there were inadequate empirical scientific studies on how farmers manage agroforestry and the role it plays for biodiversity conservation in the studied district. Thus, this study attempts to assess and compare floristic compositions, woody species diversity and structure in home garden, parkland and live fence, to describe agroforestry management practices and challenges in the study area.
2. Materials and methods
2.1. The study area
The study was conducted in Kachabira district, Kembata Tembaro Zone, Southern Nations, Nationalities and Peoples' Regional State (SNNPRS). Geographically, the district is located between 7o6′0″ N to 7o20′0″ N latitude and 37o40′0″ E to 37o56′0″ E longitude [Figure 1]. The district capital Shinshicho is located 327 km away from Addis Ababa, the capital city of Ethiopia. The altitude of the district varies from 1900-2800 m above sea level. The mean monthly minimum and maximum temperature of the district is 18 °C and 31 °C respectively. The mean total annual rainfall ranges from 1200 mm to 1500 mm. The study area has two major agroecological zones, Dega (highland) and Weyna-dega (midland) [17].
Figure 1.
Map of the study area.
2.2. Study site selection and sampling techniques
A preliminary reconnaissance survey was conducted in September 2015 to have an insight into the survey sites and identify agroforestry practices. From the district, two representative Kebeles were selected purposively based on the existence and extensive practice of agroforestry, namely, Kachabira and Mesafe for this study. Then, from each selected Kebeles, two villages were randomly selected. A total of four villages (Hebokota and Kodade from Kachabira and Bosona and Shenko from Mesafe) were used for this study. Districts are the third-level administrative divisions of Ethiopia. They are further subdivided into a number of kebele or neighborhood associations, which are the smallest local administrative unit in Ethiopia.
Then, farmers practicing the three agroforestry practices (home gardens, parkland and live fence) were stratified based on wealth category. The stratification of households into wealth category was done using key informants. Twenty (five key informants per village) were used for this study. The key informants were selected using snowball method [18]. Farm size, number of cattle, purchasing agricultural input, providing food for family and mature Enset stems were major criterias for the classification of households into different wealth categories (Table 1).
Table 1.
Major local criteria for the classification of households into different wealth categories.
| Key informants' criteria | Wealth class |
||
|---|---|---|---|
| Rich | Medium | Poor | |
| Land holding (ha) | 1–2 | 0.5–1 | 0.25–0.5 |
| Ox number | ≥2 pairs | 2 pair | 1or 0 |
| Cow number | >10 | 2 | 1 or 0 |
| Goat & sheep number | >5 | ≥2 | 1 or 0 |
| Ability to send their children to school | 100% | ≥50 | ≤50% |
| Ability to purchase Agricultural inputs | without credit | 50% credit | 100% credit |
| Food self-sufficiency throughout the year | sufficient | moderate sufficient | insufficient |
| Enset plants (mature stem number) | 75–100 | 40–60 | 20–40 |
The sample household's size for the study accounted for 10% of the total households in each village [19]. Accordingly, 28 sample households from the poor, 40 from medium and 15 from rich wealth category were randomly selected, adding the total number to 83 sample households.
The plot size was 10 m × 10 m for home garden [20], 20 m × 20 m for parkland [21] and 10 m × 5 m live fence [22] to conduct plant species inventory.
2.3. Inventory of plant species
Plant species inventory was performed between October 15, 2015 and December 10, 2015 and data were recorded on the plant uses, type, number and abundance of species from the farms of sampled households selected for the socioeconomic survey. Inventory of home garden, parkland and live fence were taken place a sample plot per farm across sampled households. Figure 2 showing photos of studied habitats in the study area selected with their characteristic vegetation; home garden (A), parkland (B) and live fence (C).
Figure 2.
Features of the three studied agroforestry practices: home garden (A), parkland (B) and live fence (C). Photo taken by the corresponding author Abayneh Legesse (2015) and comes from their personal collection.
All woody species, Diameter at Breast Height (DBH) ≥ 5 cm and height ≥1.5 m was measured. Diameter measurement of trees was taken at breast height (1.3 m above the ground) and for shrub at a stamp height (DSH at 30 cm height from the ground). For tree species forked below 1.3 m, individual stems were separately measured and treated as two trees [23]. For woody species with DBH below 5 cm, only stem count was made to abundances but not measured (DBH) [23]. In the case of multi-stemmed shrub each stem was measured, and the diameter equivalent of the plant calculated as the square root of the sum of the diameters of all stems per plant [24]. The diameter equivalent was calculated using Eq. (1):
| (1) |
where d = diameter equivalent height, di = diameter of the ith stem at the measurement height (cm).
The local name of the plant species found in the sample plots was identified and recorded with the help of key informants and scientific nomenclature was carried out using volumes of Flora of Ethiopia and Eritrea [25, 26, 27, 28, 29, 30, 31, 32] and Natural Database for Africa (NDA) Version 2.
2.4. Data analysis
Before the data analysis was run, a check was conducted for the normal distribution and homogeneity of residuals from plots for species diversity index variable, stem number and basal area. The generalized linear model (GLM) was used to analyze the effect of wealth and households' plant use types on species richness, stem number and basal area in three agroforestry practices. The data obtained from the diversity indices, stem number and basal area were compared using one-way ANOVA when the results are normal. When the ANOVA showed significant differences, we used the results of the Tukey's test (HSD) for multiple comparisons of means. If the data presented abnormality and heterogeneity, the non-parametric test, the Chi-squared test was applied using the Kruskal-Wallis H test. The data obtained in this study were analyzed using the SPSS Statistics software package, Version 20.0 (IBM Corporation, USA) at p ˂ 0.05 as probability levels.
2.5. Diversity indices
Measurement of diversity is needed to quantify and characterize farm practices according to the degree of diversity and to examine the relationship between wealth categories and species diversity. Based on individual farms, the mean numbers of tree/shrub species per farm practices were estimated for each wealth category. Although several quantitative descriptions are available for characterizing species diversity, the Shannon-Wiener and Shannon equitability (Evenness) [33, 34, 35, 36] are commonly used and considered in this study. Richness and diversity of each farm practices types were calculated as the number of species, Shannon and evenness indices.
2.5.1. Shannon-Wiener Diversity Index (H)
Shannon diversity index (H) was used to evaluate the plant species diversity among the three agroforestry practices.
The Shannon diversity index was calculated using Eq. (2) as follows:
| (2) |
where, H= Shannon-Wiener Diversity Index, S = number of species, i = 1, 2, 3…s, pi = Proportion of individuals or the abundance of the ith species expressed as a proportion of the total cover and ln is the natural logarithm (log to the base of e).
2.5.2. Evenness (equitability) index
Evenness (Shannon equitability) index (E) was calculated to estimate the homogeneous distribution of woody species on farms. Evenness (Shannon equitability) index (E) was calculated by using Eq. (3) as follows:
| (3) |
where, S = the number of species, pi = proportion of individuals of the ith species or the abundance of the ith species expressed as a proportion of the total abundance.
2.5.3. Simpson's diversity index (D)
The Simpson's diversity index was derived from probability theory and it is the probability of picking two organisms at random which are of different species. Simpson's diversity (D) was calculated by using Eq. (4) as follows:
| (4) |
where, D = Simpson's diversity index, pi = proportion of individuals of the ith species or the abundance of the ith species expressed as a proportion of the total abundance.
2.6. Basal area and stem density of woody species
Inventory data gathered from three practices were computed for the number of woody species and basal area per hectare (ha). The total number of all individuals of woody species in all plots was calculated.
Basal area is the cross-sectional area of tree stems at diameter at breast height. It is a measure of dominance and calculated using the following Eq. (5):
| (5) |
where, BA = Basal area in m2 per hectare,
d = diameter at breast height (m),
Hence the number of woody species and basal area per hectare is used as an estimate of the relative standing yield of wood resources.
2.7. Importance value index (IVI)
The IVI indicates the importance of each tree/shrub species in the three agroforestry practices. IVI is the sum-up of the relative frequency relative density and relative dominance and it was calculated using the following Equations (6), (7), (8), (9), (10), (11) and (12):
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
2.8. Species frequency
Frequency is defined as the probability of a chance of finding a species in a given sample area or plot [34]. The frequency of each woody species was calculated by determining the proportion of the plot on which that species was encountered.
2.9. Ethics statement
Informed consent was obtained from the participants. The purpose of the study was the collection and analyzation of data for an M.Sc. thesis was explained in advance. Ethical approval for the study was received from Hawassa University, Wondo Genet College of Forestry and Natural Resources Graduate Council (GC), Department Council (DC) and Academic Council (AC). The research team got permission from the Kachabirra district Administration to conduct the study.
3. Results and discussion
3.1. Households' and socio-economic characteristics
There was a significant difference (p < 0.05) across wealth categories in terms of age class (Table 2). The majority of the poor category households (60.71%) and some proportion of the medium (40%) households' aged from 31 to 40 years old and 33.33% of rich aged in both 41 to 50 and 51 to 60 years-old, showing that availability of more productive working force for agroforestry management in the poor households than medium and rich households. Old age (>60 years-old) was only limited to medium and rich households, accounting 2.5% and 20%, respectively (Table 2).
Table 2.
Age of the respondent among wealth category (n = 83).
| Age class | Wealth category |
2 | p-value | ||
|---|---|---|---|---|---|
| Poor % | Medium % | Rich % | |||
| 20–30 | 3.57 | 2.5 | - | 18.28 | 0.001∗∗ |
| 31–40 | 60.71 | 40 | 13.33 | ||
| 41–50 | 25 | 25 | 33.33 | ||
| 51–60 | 10.71 | 30 | 33.33 | ||
| >60 | - | 2.5 | 20 | ||
∗∗Indicate significant deference at p < 0.05.
Agriculture was the primary occupation of all of the households' (100%) and among them, 7.2% of them were involved in other income-generating activities in addition to agriculture such as petty trading and labor work. The mean land holding size of the poor, medium and rich was estimated to 0.41, 0.68 and 1.43 ha, respectively (Table 3). The overall mean farm size of the sampled households at the study site was 0.84 ha, which is lower than the national average of 1.02 ha [37]. There was a significant difference in landholding size among the wealth categories (p < 0.05; Table 3). Households with small land owned were positively associated with the adoption of farmland agroforestry [38]. Small farm size as the main barrier to tree-planting and increasing of species diversity [39]. Other studies also reported positive effects of farm size on tree planting elsewhere in Ethiopia [40, 41].
Table 3.
Family size and landholding size (ha) of the respondent among wealth category (n = 83).
| Categories | Wealth category | Mean (±SD) | p-value | |
|---|---|---|---|---|
| Family size | Poor | 6.46 ± 1.84 | 10.59 | 0.34 |
| Medium | 6.45 ± 1.78 | |||
| Rich | 6.80 ± 1.37 | |||
| Landholding size (ha) | Poor | 0.41 ± 0.19 | 52.5 | 0.001∗∗ |
| Medium | 0.68 ± 0.38 | |||
| Rich | 1.43 ± 0.69 |
∗∗Indicate significant deference at p < 0.05.
Family size of the household was not significantly differed among the wealth categories (p > 0.05; Table 3). The highest family size was recorded for rich (6.80 persons, ranging 4 to 9 members), followed by poor (6.46 persons, ranging 3 to 10 members) and medium (6.45 persons, range 2–10 members) wealth categories (Table 3). The overall average family size was 6.57 in the study area. The higher family size for rich and poor households than the medium could be an indicator of more labour availability for agroforestry management in the former two wealth categories than the later one. This is in line with the notion that large family size is normally connected with a higher labour gift, which would enable a household to accomplish various agricultural tasks particularly during peak seasons [42].
3.2. Floristic composition of plant species
Altogether, a total of 59 plant species that belong to 56 genera and 36 families were recorded in the home garden, parkland and live fence of the study area (Table 4). Among the plant species, trees constituted 42%, shrubs 27%, herbs 29% and climber 2% (Table 4). From the recorded plant species, 66% were native (endemic and indigenous) species and the remainders 34% were introduced species (Table 4). This result is comparable with findings of [19] who found 40 woody species in the different agro-ecosystems in the Tigray region. However, it was higher than that of [43] who recorded 32 woody species in the three agroforestry practices at Gununo in Wolayitta Zone.
Table 4.
A List of the recorded species scientific name, local names, families, life form, origin and uses in the study area.
| Scientific name | Local name | Family | Life form | Origin | Uses |
|---|---|---|---|---|---|
| Acacia abyssinica Hochst. | Odora | Fabaceae | Tree | Indigenous | Bh, Fo, Fw, Lf, Sh, Ch |
| Albizia gummifera (J. F. Gmel.) C. A. Sm. | Matichu | Fabaceae | Tree | Indigenous | Bh, Cm, Sh |
| Brucea antidysenterica J. F. Mill. | Duketa | Simaroubaceae | Shrub | Indigenous | Fw, Lf, M |
| Cajanus cajan (L.) Mill | Atara | Fabaceae | Herb | Introduced | Fd, Fw, Lf |
| Calpurina aurea (Ait) | Chea | Fabaceae | Tree | Indigenous | Fw, Bh, M, |
| Carica papaya L. | Papaya | Caricaceae | Tree | Introduced | Fr, Is |
| Casimiroa edulis La Llave | Kasimira | Rutaceae | Tree | Introduced | Bh, Fr, Fw, Lf, Is |
| Casuarina equisetifolia L. | Shiwashiwe | Casuarinaceae | Tree | Introduced | Cm, Fw |
| Catha edulis (Vahl) Forssk. ex Endl. | Chata | Celastraceae | Shrub | Indigenous | Is, M |
| Celtis africana Burm. | Sutichu | Ulmaceae | Tree | Indigenous | Cm, Fw |
| Coffea arabica L. | Buna | Rubiaceae | Shrub | Indigenous | Is, Fw |
| Colocasia esculenta (L.) | Gebiza | Araceae | Herb | Introduced | Fd, Is |
| Cordia africana Lam. | Wanja | Boraginaceae | Tree | Indigenous | Bh, Cm, Fw, Sh |
| Coriandrum sativum L. | Wodimamu | Apiaceae | Herb | Introduced | Sp, Is |
| Croton macrostachyus Del. | Mesena | Euphorbiaceae | Tree | Indigenous | Bh, Cm, Ch,Fw, Sh |
| Cucurbita pepo L. | Debakula | Cucurbitaceae | Herb | Introduced | Fd, Is |
| Cupressus lusitanica Mill. | Ferenji homa | Cupressaceae | Tree | Introduced | Cm, Fw, Lf |
| Dovyalis abyssinica (A. Rich.) Warb. | Koshima | Flacourtiaceae | Shrub | Indigenous | Fw, Lf |
| Ehretia cymosa Thonn. | Ulagichu | Boraginaceae | Shrub | Indigenous | Cm, Fw |
| Ensete ventricosum (Welw.) | Wesita | Musaceae | Herb | Indigenous | Fd, Is |
| Entanda abyssinica Steud.Ex A.Rich. | Gorta | Mimosaceae | Shrub | Indigenous | Fw, Lf |
| Eragrostis tef (Zucc.) Trotter | Taffa | Poaceae | Herb | Indigenous | Fd, Fo, Is |
| Erythrina abyssinica Lam. ex DC. | Welechu | Fabaceae | Tree | Indigenous | Cm, Fw, Fo, Lf, Sf |
| Euphorbia tirucalli L. | Matuta | Euphorbiaceae | Shrub | Indigenous | Lf |
| Ficus vasta Forssk. | Odechuta | Moraceae | Tree | Indigenous | Cm, Fw, Sh, Bh |
| Grevillea robusta R. Br. | Gravila | Proteaceae | Tree | Introduced | Cm, Fw |
| Hypoestes forskaolii (Vahl) R.Sch. | Omoruta | Acanthaceae | Herb | Indigenous | M, Is |
| Ipomoea batatas (L.) Lam. | Shukarita | Convolvulaceae | Herb | Introduced | Fd, Is |
| Juniperus procera Hochst. ex Endl. | Abeshi homa | Cupressaceae | Tree | Indigenous | Cm, Fw, Lf, Sf |
| Justicia schimperiana (Hochst. ex Nees) T. Anders. | Gulibana | Acanthaceae | Shrub | Indigenous | Fw, M, Lf |
| Lippia adoensis Hochst. ex Walp. | Kosoretita | Verbenaceae | Shrub | Endemic | Is, Sp |
| Lycopersicon esculentum Mill. | Timatima | Solanaceae | Herb | Introduced | Fd, Is |
| Maesa lanceolata Forssk. | Gewada | Myrsinaceae | Tree | Indigenous | Fw, Lf |
| Mangifera indica L. | Manguta | Anacardiaceae | Tree | Introduced | Fr, Fw, Is, Sh |
| Millettia ferruginea (Hochst.) Bak. | Hengezena | Fabaceae | Tree | Endemic | Cm, Fw, M, Lf |
| Musa x-paradisiaca L. | Muza | Musaceae | Herb | Indigenous | Fr, Is |
| Ocimum americanum L. | Besobila | Lamiaceae | Herb | Introduced | Sp, Is |
| Olea europaea L. | Wera | Oleaceae | Tree | Indigenous | Cm, Lf, Sh, Bh |
| Persea americana Mill. | Abukatuta | Lauraceae | Tree | Introduced | Fr, Bh, Is, Sh |
| Phaseolus vulgaris L. | Wokita | Fabaceae | Climber | Introduced | Fd, Is, Sf |
| Pinus patula Schiede ex Schltdl. | Pachula | Pinaceae | Tree | Indigenous | Cm, Fw, Lf |
| Podocarpus falcatus (Thunb.) Mirb. | Zagishu | Podocarpaceae | Tree | Indigenous | Cm, Fw, Sh, Bh |
| Prunus africana (Hook.f.) Kalkm. | Gerbichu | Rosaceae | Tree | Indigenous | Cm, Bh, Fw, Lf |
| Psidium guajava L. | Zayituna | Myrtaceae | Tree | Introduced | Fr, Bh, Lf |
| Rhamnus prinoides L'Herit. | Gesha | Rhamnaceae | Shrub | Indigenous | Is, Fw,Lf |
| Ricinus communis L. | Chena | Euphorbiaceae | Shrub | Indigenous | Lf, Is |
| Rosa x richardii Rehd. | Tsigereda | Rosaceae | Shrub | Indigenous | Lf |
| Ruta chalepensis L. | Telechuta | Rutaceae | Herb | Indigenous | M, Is |
| Saccharum officinarum L. | Shenkora | Poaceae | Herb | Introduced | Fd, Fo, Is |
| Solanum incanum L. | Maheta | Solanaceae | Shrub | Indigenous | Fw, Lf |
| Solanum macrocarpon poir. | Bulita | Solanaceae | Shrub | Endemic | M |
| Solanum tuberosum L. | Dinicha | Solanaceae | Herb | Introduced | Fd, Is |
| Sorghum bicolor (L.) Moench | Beshinka | Poaceae | Herb | Indigenous | Fd, Fw, Is |
| Syzygium guineense (Wild.) DC. | Goteta | Myrtaceae | Tree | Indigenous | Cm, Bh, Fw, Lf |
| Trichilia dregeana Sond. | Bonga | Meliaceae | Tree | Indigenous | Cm, Fw, Sh |
| Vernonia amygdalina Del. | Heba | Asteraceae | Shrub | Indigenous | Fw, Fo, Lf, M |
| Vernonia auriculifera Hiern. | Reja | Asteraceae | Shrub | Indigenous | Fw, Lf, M |
| Vicia faba L. | Bakela | Fabaceae | Herb | Introduced | Fd, Fo, Fw, Is |
| Zea mays L. | Bokola | Poaceae | Herb | Introduced | Fd, Fo, Fw, Is |
Key: Bh = bee hive, Ch = charcoal, Cm = constriction material, Fo = fodder, F = food, Fr = fruit, Fw = fuel wood, Is = income source, M = medicine, Lf = live fence, Sf = soil fertility, Sh = shade, Sp = spice.
From the plant families, Fabaceae was the most dominant family represented by 8 species. From the 36 plant families assessed in studied agroforestry, Fabaceae was the most dominant one and the most likely reason for this might be that the households' preference is inclined towards growing of leguminous plant species and medicinal plants in their farm land.
Plant species in the study area were composed of native (indigenous and endemic) and introduced species. From recorded plant species, 66% were native and the remainders 34% were introduced (Table 4). From the native species recorded in this study, Lippia adoensis and Millettia ferruginea were endemic to Ethiopia. The result indicated the effectiveness of the conservation of larger proportion of native flora in the agroforestry practices.
Farmers in the study area planted or retained different plant species in their land holdings to fulfill the demands of various products and service such as construction material, food, shade, bee hive, soil fertility improvement, shade, fuel wood, medicine, income source and fruit (Table 4) depending on the availability of the space, compatibility with agricultural crops and household needs [40, 44].
3.3. Plant species diversity
The highest mean species richness was recorded in the home garden agroforestry practice of rich (8.40) and the least in the parkland of medium (1.45) Table 5. The highest mean Shannon diversity was recorded in home garden of rich households. The highest mean Shannon diversity was recorded in parkland and live fence of poor households' (Table 5). The Shannon diversity index of home garden and parkland of this study was lower than other studies elsewhere in Ethiopia [43]. The species richness in home garden of the present study was lower than that of reported [13] in different agroecological zones of Ethiopia and the findings of [45] in Sidama Zone, Southern Ethiopia. The present study showed that variations in plant species diversity across wealth category within practices, but it was not significantly differed (p > 0.05) across the wealth categories within practices (Table 5). This is due to the farm household landholding size, species preference, management, and the opportunity that afford the farmer to maintain the plant species on their farm. The mean value of Evenness (E) index was the highest for the home garden agroforestry practices. The mean Evenness value for home garden was 0.82, 0.79 and 0.83 for poor, medium and rich respectively indicating that the uniformity in the distribution in the composition of species was 82% for poor, 79% for medium and about 83% for rich (Table 5).
Table 5.
Mean (SD) Species richness, Shannon, Simpson diversity indices and Evenness (Equitability) of plant species in home garden, parkland and live fence and wealth categories of Kachabira district, Southern Ethiopia (poor n = 28, medium n = 40, rich n = 15).
| Agroforestry practices | Wealth category | Species richness | Shannon index | Simpson | Equitability |
|---|---|---|---|---|---|
| Home garden | Poor | 7.50 ± 3.95 | 1.62 ± 0.61 | 0.70 ± 0.22 | 0.82 ± 0.19 |
| Medium | 7.52 ± 2.75 | 1.57 ± 0.48 | 0.68 ± 0.18 | 0.79 ± 0.18 | |
| Rich | 8.40 ± 2.64 | 1.75 ± 0.47 | 0.74 ± 0.18 | 0.83 ± 0.17 | |
| p-value | 0.625 | 0.516 | 0.687 | 0.614 | |
| Parkland | Poor | 1.75 ± 1.62 | 0.49 ± 0.53 | 0.30 ± 0.29 | 0.49 ± 0.47 |
| Medium | 1.45 ± 1.20 | 0.30 ± 0.40 | 0.19 ± 0.25 | 0.33 ± 0.42 | |
| Rich | 1.53 ± 1.30 | 0.36 ± 0.44 | 0.22 ± 0.26 | 0.41 ± 0.45 | |
| p-value | 0.673 | 0.251 | 0.32 | 0.254 | |
| Live fence | Poor | 3.71 ± 1.80 | 1 ± 0.45 | 0.54 ± 0.21 | 0.76 ± 0.25 |
| Medium | 4.17 ± 2.15 | 0.99 ± 0.50 | 0.51 ± 0.22 | 0.72 ± 0.25 | |
| Rich | 3.40 ± 2.35 | 0.84 ± 0.65 | 0.43 ± 0.32 | 0.59 ± 0.39 | |
| p-value | 0.413 | 0.583 | 0.142 | 0.397 |
3.4. Importance value index (IVI)
Cordia africana was the most important woody species in home garden, followed by Coffea arabica, Persea americana and Mangifera indica. Cordia africana was also the most important woody species in parkland, followed by Coffea arabica, Croton macrostachyus and Persea americana. While Vernonia amygdalina is the most important woody species in the live fence, followed by Cupressus lusitanica, Juniperus procera and Erythrina abyssinica (Table 6). The IVI values of the species describe their importance of the species to various uses for the farm household. Of these species, Cordia africana maintained due to its importance for shade, improving soil fertility and positive association or compatibility with food crops.
Table 6.
Importance value index (IVI, %) of three agroforestry practices in Kachabira district.
| Species Name | Home garden | Parkland | Live fence |
|---|---|---|---|
| Acacia abysinica | ─ | 3.1 | 1.1 |
| Albizia gummifera | 2.3 | 3.1 | 0.9 |
| Calpurnia aurea | ─ | ─ | 2.6 |
| Casimiroa edulis | ─ | ─ | 1 |
| Catha edulis | 1 | ─ | ─ |
| Coffea arabica | 26.9 | 10.9 | ─ |
| Cordia africana | 39.8 | 54.7 | 6.5 |
| Croton macrostachyus | 1.5 | 9.5 | 9.3 |
| Cupressus lusitanica | ─ | ─ | 20 |
| Ehretia cymosa | ─ | ─ | 0.5 |
| Erythrina abyssinica | 1.6 | 6.7 | 10 |
| Ficus vasta | ─ | 0.7 | ─ |
| Grevillea robusta | ─ | 1.5 | 2.6 |
| Juniperus procera | ─ | ─ | 14.9 |
| Mangifera indica | 4.9 | 1.4 | ─ |
| Olea africana | ─ | ─ | 1.7 |
| Persea americana | 18.8 | 7.8 | ─ |
| Podocarpus falcatus | 0.5 | ─ | 1.7 |
| Prunus africana | ─ | 0.6 | 0.5 |
| Ricinus communis | 2.7 | ─ | ─ |
| Trichilia dregeana | ─ | ─ | 2 |
| Vernonia amygdalina | ─ | ─ | 24.7 |
Note: “─” indicate absence.
3.5. Estimation of wood resources
3.5.1. Number of stems
The inventory results indicated that the density of the stem varied among the three agroforestry practices and wealth categories (Table 7). The stem density significantly differed among the three agroforestry practices, but it was not significantly differed (p > 0.05) across the wealth categories in home garden and parkland but there was a significance difference (p < 0.05) in live fence within wealth categories (Table 7). The highest stems density was recorded in the live fence (1459 stems ha−1). The stem density was highest for medium households in home garden (411 stems ha−1), parkland (65 stems ha−1) and live fence (1714 stems ha−1). Regarding wealth categories, the high number of stems per hectare was found on gardens of medium farmers indicating the ability of medium farmers to intensively utilize their farms. The present finding was not in agreement with [46] who reported the high number of stems per hectare was found in gardens of poor farmers in home garden in Gimbo district, South West Ethiopia. The number of tree species retained was influenced by the number of active labor units, land size, adoption of agricultural technologies, access to credit and the presence of a nursery was reported by [38, 47] in Ethiopia.
Table 7.
Mean (±SD) number stems of woody species (stems ha−1) in Kachbira District, Southern Ethiopia (n = 83).
| Practices | Poor | Medium | Rich | Overall mean |
|---|---|---|---|---|
| Home garden | 353 ± 412a | 411 ± 379a | 392 ± 486a | 389 ± 413b |
| Parkland | 53 ± 39a | 65 ± 76a | 44 ± 27a | 58 ± 60a |
| Live fence | 1312 ± 1908b | 1714 ± 2235c | 1118 ± 1397a | 1459 ± 1970c |
Different letters indicate differences and similar letters indicate non-significant differences among agroforestry practices and wealth category (p < 0.05).
3.5.2. Basal area
The inventory results indicated that the basal area varied among the three agroforestry practices and wealth categories (Table 8). There was a significant difference in basal area between poor, medium and rich wealth categories in the live fence (p < 0.05) but didn't show a significant difference for home garden and parkland among wealth category (p > 0.05). The highest basal area was recorded in the live fence (14.7 m2ha-1). The basal area was highest for rich households when compared to the poor and medium wealth categories in three practices (Table 8). The possible reason for this might be mainly due to the rich farmers might have retained a number of trees/shrubs as their farm size is relatively larger than the others. Besides, they might have some income at hand to save the wood resources on their farm. The result of this finding for home garden was higher than [46] who reported a mean basal area 5.128 m2ha-1 in home garden of Gimbo district, South West Ethiopia.
Table 8.
Mean (±SD) basal area of woody species (m2 ha−1) across three agroforestry practices and wealth categories in Kachabira district, Southern Ethiopia (n = 83).
| Practices | Poor | Medium | Rich | Overall mean |
|---|---|---|---|---|
| Home garden | 5.1 ± 5.4a | 5.1 ± 7.7a | 10.2 ± 17.9a | 6.3 ± 10.8b |
| Parkland | 1.3 ± 1.5a | 0.98 ± 0.8a | 1.8 ± 2.9a | 1.2 ± 1.5a |
| Live fence | 9.2 ± 10.6a | 16.1 ± 23b | 18.0 ± 17.7c | 14.7 ± 19.2c |
Different letters indicate differences and similar letters indicate non-significant differences among agroforestry practices and wealth category (p < 0.05).
3.6. Woody species preference and management
3.6.1. Woody species preference
Woody species preference ranking was done with a relative score. Thus, respondents were asked to rank the five most important woody species among the species they plant and/or retain. Woody species preference was in the order of importance: Coffea arabica, Cordia africana, Persea americana, Mangifera indica, Croton macrostachys, Rhamnus prinoides, Acacia abyssinica, Vernonia amygdalina, Erythrina abyssinica and Albizia gummifera (Table 9). Reason for preference were multiple uses, such as fuel wood, construction, income, food, shade, bee keeping and soil fertility improvement. The finding of this study is in line with the findings of [40, 46] who reported reasons for retaining different woody species to depend on the tangible uses and services that they render to the household.
Table 9.
Respondents' woody species preference ranking according to their benefit in the study area (n = 83).
| Species | Respondents |
Relative score |
Total score | Rank | Uses/Reason of preference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 1st | 2nd | 3rd | 4th | 5th | ||||
| Acacia abyssinica | 1 | - | 2 | 5 | 1 | 0.01 | - | 0.05 | 0.5 | 0.03 | 0.59 | 7th | 1,3, 6 and 8 |
| Albizia gummifera | - | - | 1 | 1 | 1 | - | - | 0.01 | 0.02 | 0.02 | 0.05 | 10th | 1 and 6 |
| Coffea arabica | 49 | 20 | 14 | - | - | 28.93 | 5.4 | 2.61 | - | - | 36.94 | 1st | 3,4 and 5 |
| Cordia africana | 25 | 30 | 24 | - | - | 7.53 | 12.16 | 7.68 | - | - | 27.37 | 2nd | 1,2,3,5 and 6 |
| Croton macrostachys | - | 1 | 3 | 4 | 8 | - | 0.01 | 0.12 | 0.31 | 1.37 | 1.81 | 5th | 1,2,5 and 6 |
| Erythrina abyssinica | - | - | - | 2 | 1 | - | - | - | 0.08 | 0.03 | 0.11 | 9th | 1,2,6,7 and 8 |
| Mangifera indica | 2 | 1 | 14 | 10 | 17 | 0.05 | 0.01 | 2.61 | 1.92 | 7.81 | 12.4 | 4th | 3 and 4 |
| Persia americana | 6 | 20 | 16 | 25 | 2 | 0.43 | 5.4 | 3.41 | 12.01 | 0.11 | 21.36 | 3rd | 1,3 and 4 |
| Rhamnus prinoides | - | 2 | 1 | 5 | 4 | - | 0.05 | 0.01 | 0.48 | 0.43 | 0.97 | 6th | 1, 3 and 8 |
| Vernonia amygdalina | - | - | - | - | 3 | - | - | - | - | 0.24 | 0.24 | 8th | 1 and 8 |
| Total | 83 | 74 | 75 | 52 | 37 | ||||||||
Note: Relative score was calculated by multiplying the number of respondents in each rank by its proportion (e.g. (49 × 49/83) = 28.93)).
Reason of preference, 1 = Fuel wood; 2 = Construction materials; 3 = Income; 4 = Food; 5 = Shade; 6 = Bee keeping; 7 = Soil fertility improvement; 8 = Fodder.
3.6.2. Management of woody species and herbaceous plant
The most common management practices used by the farmers in the study area included pruning, pollarding, coppicing and thinning for woody species (Table 10). Similar woody species management practices were reported in other parts of Ethiopia [40, 46, 48]. Farmers not only have the knowledge of different woody species management practices but also they have the knowledge of which woody species require the different set of management practices and appropriate time. Digging, weeding, composting and harvesting were the most common management practices used in the study area. Almost all respondents used animal manure, kitchen and house wastes, ash and litter in their home garden to increase soil fertility. Similar findings reported [46] organic households wastes and animal manure application in home gardens elsewhere in Ethiopia.
Table 10.
Response of surveyed households (%) on management practices of some woody species recorded in different practice on the study area (n = 83).
| Woody species | Pruning | Pollarding | Coppicing | Lopping | Thinning | Reason for management |
|---|---|---|---|---|---|---|
| Acacia abyssinica | 6 | 12 | 12 | 12 | 4 | 4 and 5 |
| Albizia gummifera | 4 | 2 | 4 | 4 | 1 | 1 and 4 |
| Coffea arabica | 24 | - | 20 | - | 24 | 1 and 2 |
| Cordia africana | 66 | - | 66 | 67 | - | 1,2,3 and 4 |
| Croton macrostachys | 11 | 18 | 19 | 19 | - | 1,3 and 4 |
| Erythrina abyssinica | 12 | 4 | 12 | - | 5 | 1,4 and 5 |
| Grevillea robusta | 25 | - | 25 | 5 | - | 4 |
| Juniperus procera | - | 2 | - | - | 2 | 4 |
| Mangifera indica | - | 5 | - | 5 | - | 1, and 3 |
| Persia americana | 12 | - | - | 5 | - | 1,3 and 4 |
| Prunus africana | 1 | 1 | 1 | - | - | 3 and 4 |
| Rhamnus prinoides | 2 | - | 5 | - | - | 1 and 4 |
| Vernonia amygdaline | 28 | 15 | 60 | - | - | 3,4 and 5 |
| Vernonia auriculifera | 26 | - | 35 | - | - | 2 and 4 |
Note: Reason for management, 1 = for growth, 2 = to reduce competition, 3 = to reduce shade, 4 = for fuel wood, and 5 = for fodder.
3.6.3. Criteria for selection of tree species to integrate with crops
Trees were an integral part of the farming system and farmers have long experience in integrating trees as their farming field in the study area. Woody species in farmland were both native and exotic species, and important assets since they are vital for farmers' day to day life. The first and second species to integrate with crops in the study area were Cordia africana and Erythrina abyssinica, respectively. Farmers select tree species suitable to each tree growing niche and density of planting to minimize the effect of trees on crops and on other and reduce competitions between tree species. For instance, trees that contribute positively to agricultural crops are grown dispersed in crop fields, while trees that compete with crops are planted separately to reduce the effect. In order to integrate trees on farmlands, farmers apply a number of criteria, including fast growth, compatibility with crops, multipurpose use-value, increase soil fertility, low branch volume, fast decomposing ability. Similar findings were reported by [49] in crop- livestock- trees mixed systems in Lemo district Southern Ethiopian.
3.7. Challenges for managing agroforestry practices in the study area
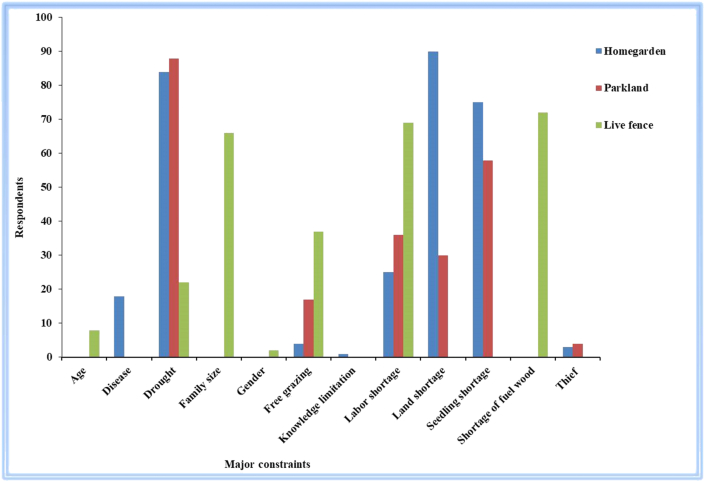
Respondents were asked for the major constraint that they encounter in the process of home garden, parkland and live fence management. Twelve constraints were identified in three studied practices (Figure 3). The nature of the constraints depends on the practices. Majority of the respondents (respectively 90% and 84%) stated that land shortage and drought were major constraints for managing home garden, while drought (88%) and seedling shortage (58%) for managing parkland and shortage of fuel wood (72%) and labor shortage (69%) were major constraints for managing live fence (Figure 3) in the study area. This finding is supported by [38, 47, 50] who reported the diversity of plant species in agroforestry is influenced by factors such as socio-economic status, garden size, rainfall pattern and management system and [48] who reported scarcity of arable land, free grazing, shortage of seedling, pest and cultural preferences and personal preferences are major factors of agroforestry practices.
Figure 3.
Major challenges of farmers in managing homegarden, parkland and live fence plant species in Kachabira district, Southern Ethiopia (n = 83).
4. Conclusions
The inventory of the vegetation data indicated the presence of different species which includes tree, shrub, herb and climber in the studied area. Fabaceae was the most species rich family owing to the households' species preference in their farmland. Among the recorded plant species, native species comprise a larger portion than introduced species. The highest mean number of stems per hectare was observed on gardens of the medium and rich households. The highest mean basal area was observed in rich households when compared to the poor and medium wealth categories across the three studied practices. Farmers in the study area accumulated a wealth of knowledge for woody species management practices. Farm household landholding size, species preference and management found to be the most important influencing factors that affect the diversity of plant species. Woody species preference depends on the contribution to household livelihoods and compatibility with food crops. A further detailed study of explicit examining socio-ecological factors determining plant species diversity and the contribution of different functional groups to livelihood is needed.
Declarations
Author contribution statement
A. Legesse, M. Negesh: Conceived and designed the analysis; Analyzed and interpreted the data; Contributed analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Ethiopian Biodiversity Institute and Hawassa University, Wondo Genet College of Forestry and Natural Resources.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to thank Mr. Belayneh Lemage, Mr. Berhanu Sumamo and Mr. Desalegn Ergena for their assistance during data collection. We also would like to extend our gratitude to Kachabira and Mesafe farmers', district and Kebele experts for their valuable contributions to this study. We would like to thank Dr. Gemedo Dalle, Dr. Maria Johansson, Mr. Getachew Tadesse, Mr. Dinsa Doboch, Mr. Mulatu Simeon, Mr. Melese Ungamo, Mr. Ephrem Berhanu and Mr. Behailu Bogale for their comments, assistance and encouragement during this study.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bishaw B., Asfaw Z. Hydrological and related aspects of deforestation and degradation of woody vegetation. In: Kloos H., Legesse W., editors. Water Resources Management in Ethiopia Implications for the Nile Basin. Cambria press; New York: 2010. [Google Scholar]
- 2.Leipzig H. Genetic Resource Centre; Addis Ababa, Ethiopia: 1996. Ethiopia: Country Report to the FAO International Technical Conference on Plant Genetics Resource. [Google Scholar]
- 3.Ethiopian Biodiversity Institute . 2014. Ethiopia’s Fifth National Report to the Convention on Biological Diversity May 2014 Addis Ababa, Ethiopia. [Google Scholar]
- 4.Motis T. 2007. Agroforestry Principles. Echo. Pobrano Z.http://www.echonet.org [Google Scholar]
- 5.Jose S. Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor. Syst. 2009;76:1–10. [Google Scholar]
- 6.Millennium Ecosystem Assessment (MEA) World Resources Institute; Washington, DC: 2005. Ecosystems and Human Well-Being: Biodiversity Synthesis. [Google Scholar]
- 7.Tadesse E., Abdulkedir A., Khamzina A., Son Y., Noulèkoun F. Contrasting species diversity and values in home gardens and traditional parkland agroforestry systems in Ethiopian sub-humid lowlands. Forests. 2019;10(3):266. [Google Scholar]
- 8.Worku M., Bantihun A. Review on woody species and socio-economic roles of traditional agroforestry practices in Ethiopia. J. Fund. Renew. Energy. 2017;7:246. [Google Scholar]
- 9.FAO . FAO (Food and Agriculture Organization of the United Nations); Rome: 2015. Global forest Resource Assessment. [Google Scholar]
- 10.Newbold T., Hudson L.N., Hill S.L., Contu S., Lysenko I., Senior R.A., Börger L., Bennett D.J., Choimes A., Collen B., Day J. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- 11.Nair P.R. 1993. An Introduction to Agroforestry. Kluwer Academic Publishers Dordrecht/Boston/London in Cooperation with International Centre for Research in Agroforestry. [Google Scholar]
- 12.Garrett H.E.G. USDA-SCS; Fort Collins: 1997. Agroforestry: an Integrated Land-Use Management System for Production and farmland Conservation. United States Department of Agriculture Soil Conservation Service (USDASCS) Report 68-3A7S-3-1341. [Google Scholar]
- 13.Asfaw Z., Nigatu A. Homegardens in Ethiopia: characteristics and plant diversity. SINET, Ethiop. J. Sci. 1995;18:235–266. [Google Scholar]
- 14.Jarvis D.I., Padoch C., Cooper H.D. Columbia University Press; New York: 2007. Managing Biodiversity in Agricultural Ecosystems. Bioversity International. [Google Scholar]
- 15.Regmi B.R., Sthapit B.R., Upadhyay M.P., Shrestha P. Mainstreaming good practices of on farm conservation in context of Western Terai landscape complex project (WTLCP) In: Sthapit B.R., Gauchan D., Subedi A., Jarvis D., editors. On-farm Management of Agricultural Biodiversity in Nepal: Lessons Learned, Proceeding of the National Symposium, 18-19 July 2006, Kathmandu, Nepal. 2008. pp. 211–217. [Google Scholar]
- 16.Sundar I. Food security through biodiversity conservation. Int. Conf. Asia Agric. Anim. IPCBEE. 2011;13:131. [Google Scholar]
- 17.KDARDO . 2015. Kachabira District Agricultural and Rural Development Office Report. [Google Scholar]
- 18.Goodman L.A. 1961. Snowball Sampling. The Annals of Mathematical Statistics; pp. 148–170. [Google Scholar]
- 19.Guyassa E., Raj A.J. Assessment of biodiversity in cropland agroforestry and its role in livelihood development in dryland areas: a case study from the Tigray region, Ethiopia. J. Agric. Sci. Technol. 2013;9:829–844. [Google Scholar]
- 20.Hailu H., Asfaw Z. Homegardens and agrobiodiversity conservation in Sabata town, Oromia regional state, Ethiopia. SINET: Ethiop. J. Sci. 2011;34(1):1–16. [Google Scholar]
- 21.Molla A., Kewessa G. Woody species diversity in traditional agroforestry practices of Dellomenna District, Southeastern Ethiopia: implication for maintaining native woody species. Int. J. Biodivers. 2015;2015:1–13. [Google Scholar]
- 22.Scherr S.J., Roger J.H., Oduol P.A. Evaluating alley-cropping and tree border technologies in Western Kenya. Agrofor. Syst. 1990;11:259–280. [Google Scholar]
- 23.Abed T., Stephens N.C. Tree measurement manual for farm foresters. In: Parsons M., editor. second ed. National Forest Inventory, Bureau of Rural Sciences; Canberra: 2003. [Google Scholar]
- 24.Snowdon P., Raison J., Keith H., Ritson P., Grierson P., Adams M. 2002. Protocol for Sampling Tree and Stand Biomass. [Google Scholar]
- 25.Edwards S., Hedberg I. National Herbarium, Addis Ababa University; 2000. Flora of Ethiopia and Eritrea. Vol. 2. Part 1, Magnoliaceae to Flacourtiaceae. [Google Scholar]
- 26.Edwards S., Tadesse M., Hedberg I. The National Herbarium, Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University; Uppsala: 1995. Flora of Ethiopia and Eritrea, Vol. 2, Part 2. [Google Scholar]
- 27.Hedberg I., Edwards S. The National Herbarium, Addis Ababa and Uppsala University; Uppsala: 1989. Flora of Ethiopia: Pittosporaceae to Araliaceae. Vol. 3. [Google Scholar]
- 28.Hedberg I., Edwards S., Nemomissa S. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany; Uppsala, Sweden: 2003. Flora of Ethiopia and Eritrea. Apiaceae to Dipsacaceae. Vol. 4, Part 1. [Google Scholar]
- 29.Hedberg I., Friis I., Edwards S. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany; Uppsala, Sweden: 2004. Flora of Ethiopia and Eritrea. Asteraceae. Vol. 4, Part 2. [Google Scholar]
- 30.Hedberg I., Kelbessa E., Edwards S., Demissew S., Persson E. Vol. 5. Addis Ababa, Ethiopia and Uppsala, Sweden: the National Herbarium, Addis Ababa University; 2006. Flora of Ethiopia and Eritrea. Gentianaceae to Cyclocheilaceae. [Google Scholar]
- 31.Edwards S., Tadesse M., Hedberg I. Vol. 6. The National Herbarium, Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University; Uppsala: 1995. Flora of Ethiopia and Eritrea. [Google Scholar]
- 32.Hedberg I., Edwards S. Vol. 7. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany; Uppsala, Sweden: 1995. Flora of Ethiopia and Eritrea. Poaceae. [Google Scholar]
- 33.Pielou E.C. Wiley; London: 1975. Ecological Diversity. [Google Scholar]
- 34.Magurran A.E. Croom Helm Limited; London: 1988. Ecological Diversity and its Measurement. [Google Scholar]
- 35.Kent M., Kent P., Coker P. Belhaven Press; London: 1992. Vegetation Description and Analysis: A Practical Approach. [Google Scholar]
- 36.Zhang J.T. China Science and Technology Press; Beijing: 1995. Quantitative Methods in Vegetation Ecology. [Google Scholar]
- 37.Ethiopian Economic Association . Ethiopian Economic Association and Ethiopian Economic Policy Research Institute; 2002. A Research Report on Land Tenure and Agricultural Development in Ethiopia. [Google Scholar]
- 38.Amare D., Wondie M., Mekuria W., Darr D. Agroforestry of smallholder farmers in Ethiopia: practices and benefits. Small-scale For. 2019;18(1):39–56. [Google Scholar]
- 39.Omati J.M., Parton K.A., Sinden J.A., Ehui S.K. Monitoring changes in the land-use practices following agrarian de-collectivization in Ethiopia. Agric. Ecosyst. Environ. 1999;72(2):111–118. [Google Scholar]
- 40.Lemage B., Legesse A. Management and socioeconomic determinants of woody species diversity in parkland agroforestry in Tembaro District, Southern Ethiopia. Biodivers. Int. J. 2018;2(5):456–462. [Google Scholar]
- 41.Abiyu A., Shete M., Gratzer G. 2012. Spatial Patterns and Determinants of Smallholder Tree Planting in Northwest Highlands of Ethiopia. [Google Scholar]
- 42.Croppenstedt A., Demeke M., Meschi M. Technology adoption in the presence of constraints: the case of fertilizer demand in Ethiopia. Rev. Dev. Econ. 2003;7(1):58–70. [Google Scholar]
- 43.Bajigo A., Tadesse M. Woody species diversity of traditional agroforestry practices in Gununo Watershed in Wolayitta zone, Ethiopia. J. For. Res. 2015;4:1–7. [Google Scholar]
- 44.Lemessa D., Legesse A. Non-crop and crop plant diversity and determinants in homegardens of Abay Chomen District, Western Ethiopia. Biodivers. Int. J. 2018;2(5):433‒439. [Google Scholar]
- 45.Abebe T. Dissertation, University of Wageningen; 2005. Diversity in Homegarden Agroforestry Systems in Southern Ethiopia. [Google Scholar]
- 46.Yakob G., Asfaw Z., Zewdie S. Wood production and management of woody species in homegardens agroforestry: the case of smallholder farmers in Gimbo District, South West Ethiopia. Int. J. Nat. Sci. Res. 2014;2:165–175. [Google Scholar]
- 47.Abiyu A., Teketay D., Gratzer G., Shete M. Tree planting by smallholder farmers in the upper catchment of Lake Tana Watershed, Northwest Ethiopia. Small-scale For. 2016;15(2):199–212. [Google Scholar]
- 48.Agidie A., Ayele B., Wassie A., Hadgu K.M., Aynekulu E., Mowo J. Agroforestry practices and farmers’ perception in Koga Watershed, upper blue nile basin, Ethiopia. J. Agric. 2013;59:75–89. [Google Scholar]
- 49.Kuria A., Lamond G., Pagella T., Gebrekirstos A., Hadgu K., Sinclair F.L. SNNPR Region; Ethiopian highlands: 2014. Local Knowledge of Farmers on Opportunities and Constraints to Sustainable Intensification of Crop- Livestock- Trees Mixed Systems in Lemo Woreda. [Google Scholar]
- 50.Seta T., Demissew S., Asfaw Z. Home gardens of Wolayta, Southern Ethiopia: an ethnobotanical profile. Acad. J. Med. Plants. 2013;1 14-13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.