Abstract
The cancer stem cell (CSC) hypothesis was proposed over 4 decades ago and states that tumor growth is maintained by a small subset of cancer cells analogous to normal tissue stem cells in terms of self-renewal and differentiation capacity. Advances in CSC isolation were initially achieved in hematological malignancies and later in solid tumors, including hepatocellular carcinoma (HCC), the major histological type of liver cancer. Increasing evidence suggests the importance of liver CSCs for tumor growth, metastasis, and chemo/radiation resistance in HCC, but the application of the liver CSC concept for the clinical diagnosis and treatment of HCC has not yet been achieved to the extent initially expected. Furthermore, the heterogeneity and plasticity of liver CSCs has recently been noted and might be related to drug resistance and the rapid growth and/or metastasis of the tumor after treatment. Here, we introduce our recent advancement in liver CSC research and discuss the clinical implications, which may lead to the development of improved diagnostics and treatment in HCC.
Keywords: Alpha-fetoprotein, Cancer stem cells, CD90, EpCAM, Hepatocellular carcinoma, Laminin gamma 2 monomer
Abbreviations: AFP, alpha-fetoprotein; CSC, cancer stem cells; HCC, hepatocellular carcinoma; LG2m, laminin γ2 monomer
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the second leading cause of cancer death worldwide [1]. The majority of HCCs arise from a background of chronic liver diseases caused by hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, and a high incidence has historically been observed in Asia and Africa due to high prevalence of these viruses [2,3]. Currently, several guidelines are available for HCC screening in high-risk patients infected with HBV or HCV. Moreover, increasing numbers of HCC cases have been observed in most industrialized countries, potentially related to the high incidence of obesity and underlying metabolic diseases known as non-alcoholic fatty liver diseases (NAFLD). There is an urgent need for the development of improved diagnostics and treatment of HCC in these populations.
HCC is known as a heterogeneous disease in terms of morphology, cellular behavior, responses to treatment, and clinical outcome [[4], [5], [6]]. Traditionally, these heterogeneities have been explained by differences in genetic/epigenetic changes accumulated during the clonal evolution of cancer cells [7,8]. In contrast, cancer cells have similar capacities to stem cells, including self-renewal, differentiation, and limitless division. These observations resulted in the proposal of the cancer stem cell (CSC) hypothesis, which states that a subset of tumor cells possess stem cell features, enabling them to self-renew and divide asymmetrically in order to generate heterogenous cell populations [9]. Recent evidence suggests the existence of CSCs in HCC, and they are considered highly tumorigenic, metastatic, and chemo/radiation resistant, indicating the importance of targeting liver CSCs for the development of improved diagnostics and treatment of HCC [5,10]. Here we summarize the evidence surrounding liver CSCs by focusing on their heterogeneity and clinical application.
2. Heterogeneity of liver CSCs
The major characteristics of CSCs are self-renewal and differentiation capacity in isolated cells, experimentally confirmed by in vitro clonogenicity and in vivo tumorigenicity with the formation of cellular heterogeneity in the tumor [11]. Therefore, cell isolation methods for enriching tumorigenic cells with differentiation ability have been extensively explored to identify liver CSCs [12]. Thus far, various cell surface markers have been reported to enrich liver CSCs, including epithelial cell adhesion molecule (EpCAM), CD133, CD90 (Thy 1), CD44, CD24, and CD13 [13]. Most of these markers are also known to be expressed in hepatoblasts or hepatic progenitor cells but not in adult mature hepatocytes.
The characteristics of normal hepatic progenitor cells isolated using different markers might be distinct. For example, studies evaluating the characteristics of oval cells suggested different natures of EpCAM-positive and CD90-positive cells in chemically injured rat liver [14,15]. EpCAM-positive cells express classical oval cell markers including AFP, OV-1, and CK19, whereas CD90-positive cells express desmin and a-SMA but not AFP, OV-1, or CK19, indicating that CD90+ populations are more likely to be mesenchymal cells.
Similarly, we found that EpCAM-positive and CD90-positive cells isolated from HCC are distinct in terms of gene and protein expression patterns in both fresh primary HCC tissues and cell lines (Fig. 1) [16]. Alpha-fetoprotein (AFP), a well-known oncofetal protein and the most utilized tumor marker for the screening and evaluation of HCC, was produced mainly in EpCAM-positive cancer cells. EpCAM-positive HCC cells show polygonal epithelial cell morphology and highly tumorigenic capacity. In contrast, CD90-positive HCC cells show spindle-like mesenchymal cell shapes and are highly metastatic. To date, no tumor markers have been available to reflect the presence of CD90-positive cancer cells; however, we recently identified that a specific form of basement membrane component, laminin γ2 monomer (termed LG2m), is produced in abundance in CD90-positive HCC cells (see section 3) [17].
Fig. 1.
Two distinct liver cancer stem cells. Liver cancer stem cells are a heterogenous population in terms of cellular phenotypes. EpCAM-positive liver cancer stem cells show polygonal epithelial cell shape, express genes associated with epithelial cell function, produce alpha-fetoprotein (AFP), and are highly tumorigenic. In contrast, CD90-positive liver cancer stem cells show mesenchymal cell shape, express genes associated with mesenchymal cell function, produce laminin γ2 monomer (LG2m), and are highly metastatic.
Our recent studies suggested that these heterogeneous liver CSCs interact together to orchestrate tumor growth and metastasis [18]. For example, the presence of CD90-positive cells is a risk for distant metastasis in HCC [16]. We found that CD90-positive CSCs are not only metastatic to distant organs by themselves, but also help in the metastasis of CD90-negative cells, including EpCAM-positive cells, which originally have no distant metastatic capacity, through activation of TGF-β signaling (Fig. 2) [16]. Sorafenib is a receptor tyrosine kinase inhibitor that suppresses c-Kit signaling, and we found that inhibition of c-Kit signaling had no effect on primary tumor growth but suppressed distant organ metastasis mediated by CD90-positive CSCs [16,19]. EpCAM-positive CSCs produced DKK1, one of the target genes activated by Wnt signaling like EpCAM, suggesting that Wnt signaling is activated in EpCAM-positive CSCs [20,21]. However, DKK1 is also known to be a suppressor of Wnt signaling, and the role of DKK1 on HCC development needs to be investigated further. Oncostatin M (OSM) is an interleukin-6-related cytokine known to enhance hepatocytic differentiation, and OSM treatment in EpCAM-positive CSCs resulted in an increase of non-CSCs through OSM receptor signaling, thereby facilitating 5-fluorouracil (5-FU) sensitivity [22].
Fig. 2.
Plasticity and interaction of hepatocellular carcinoma cells. Heterogenous liver cancer stem cells collaborate to orchestrate the development of hepatocellular carcinoma. EpCAM-positive liver cancer stem cells show activation of Wnt signaling and resistance to sorafenib, especially when tumor suppressor capicua is inactivated. Oncostatin M (OSM) induces hepatocytic differentiation of EpCAM-positive liver cancer stem cells to non-cancer stem cells. EpCAM-positive liver cancer stem cells alone cannot metastasize to distant organs but acquire metastatic ability in the presence of CD90-positive liver cancer stem cells, potentially through paracrine TGF-β signaling. CD90-positive liver cancer stem cells express c-Kit, and sorafenib has no effect on EpCAM-positive HCC cells but suppresses de novo metastasis of hepatocellular carcinoma mediated by CD90-positive liver cancer stem cells, potentially through c-Kit signaling inhibition. Cellular stresses induced by chemotherapeutic reagents such as 5-fluorouracil (5-FU), epirubicin, or transcatheter arterial chemoembolization induce de-differentiation of non-cancer stem cells or transdifferentiation of EpCAM-positive liver cancer stem cells to CD90-positive liver cancer stem cells.
Our recent studies also suggested that heterogeneous liver CSCs and non-CSCs might show cellular plasticity in response to genotoxic or molecularly targeted reagents. Sorafenib treatment resulted in an increase of EpCAM-positive CSCs in HCC cell lines [19,23]. We found that capicua, encoded by CIC, was mutated and functionally abrogated in acquired sorafenib-resistant HCC in humans [23]. Capicua is a regulator of receptor tyrosine kinase signaling and works as a tumor suppressor, whereas its inactivation induces extracellular signal-regulated kinase (ERK) signaling activation and an increase of EpCAM-positive cells. Furthermore, we found genotoxic stresses induced by 5-FU or epirubicin treatment results not only in enrichment of EpCAM-positive cells but also in de novo generation of CD90-positive and CD105-positive mesenchymal liver CSCs originally not found in EpCAM-positive HCC cell lines (Fig. 2) [24]. Indeed, CD105-positive HCC cells were detected in human HCC specimens surgically resected after transcatheter arterial chemoembolization (TACE) treatment, suggesting that mesenchymal liver CSCs expressing CD90 or CD105 are resistant to hypoxia and cytotoxic reagents, and are potentially generated de novo after cellular stresses from epithelial CSCs or non-CSCs. These data illustrate the complexity of HCC development organized by liver CSCs, and even after successful treatment targeting the specific liver CSC population, CSCs might arise de novo from a non-CSC population.
3. Application of liver CSC concept for clinical diagnosis
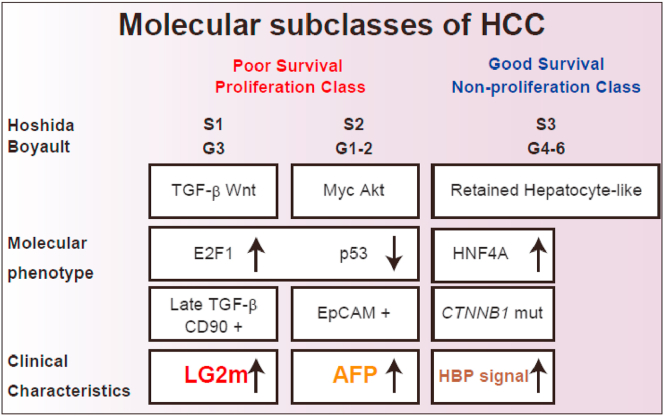
Several staging systems are currently available for HCC classification, including Barcelona Clinic Liver Cancer (BCLC) staging, and are based on tumor number and size, vascular invasion, metastatic status, hepatic reserve, and performance status. These systems can provide an approximate estimate of patient survival, but patients diagnosed at the same disease stage sometimes show a different prognosis, potentially due to the malignant phenotype of the tumor [25]. Extensive efforts to understand the nature of HCC at the molecular level through transcriptome analysis led to the definition of the molecular subclasses of HCC related to patient prognosis [[26], [27], [28]]. Hoshida's S1–3 subclasses [29] or Boyault's G1–6 subclasses [30] are based on microarray data and could be utilized for prognosis (Fig. 3). However, the application of microarray data still needs to be validated prior to its implementation in clinical practice. Thus far, AFP is the only marker clinically available for detecting HCC with poor prognosis, which is closely related to the presence of EpCAM-positive CSCs [31].
Fig. 3.
Molecular hepatocellular carcinoma subclasses and prognosis. Hepatocellular carcinoma (HCC) is molecularly categorized into several subclasses, roughly divided into a poor survival proliferation class or a good survival non-proliferation class. Hoshida's S1 and Boyault's G3 subclass is molecularly associated with the activation of TGF-β/Wnt signaling, presence of CD90-positive liver cancer stem cells, and elevation of serum laminin γ2 monomer (LG2m). Hoshida's S2 and Boyault's G1-2 subclass is molecularly associated with the activation of Myc/Akt signaling, EpCAM-positive liver cancer stem cells, and elevation of serum alpha-fetoprotein (AFP). Some HCCs show high intensity in hepatobiliary phase (HBP) images of Gd-EOB-DTPA-enhanced MRI. Molecularly, these HCCs have CTTNB1 mutations with activation of HNF4A, and patients show good survival outcomes.
Metastasis determines the prognosis of cancer, but no markers are clinically available for diagnosing the metastatic nature of the tumor before it metastasizes. Given that the presence of CD90-positive CSCs is closely associated with distant organ metastasis, biomarkers reflecting the existence of CD90-positive CSCs could help to predict the metastatic nature of HCC. Recently, we found that LG2m, a monomeric form of γ2 chain of laminin-332 in basement membrane [32], is actively produced in CD90-positive HCC cells [17]. LG2m is cancer specific extracellular matrix and promotes malignant behaviors of cancer [33,34]. Accordingly, we evaluated the prognostic value of LG2m measurement in HCC patients. Microarray data of HCC cell lines clearly indicated that LG2m elevation is a marker of HCC corresponding to the Hoshida's S1 and Boyault's G3 subclasses. No correlation was observed between serum LG2m and AFP nor serum LG2m and des-gamma-carboxy prothrombin (DCP), indicating that LG2m is a novel distinct HCC marker. LG2m elevation correlated with poor overall patient survival in two independent HCC cohorts. Furthermore, we found that neither AFP nor DCP but rather LG2m elevation was closely associated with the risk of later development of extrahepatic spread. Thus, we concluded that LG2m is a novel serum marker produced by CD90-positive CSCs that is elevated in Hoshida's S1 and Boyault's G3 subclasses and is associated with distant organ metastasis and a poor prognosis. As far as we know, LG2m is the first tumor marker that correlates with the risk of metastasis to be identified, and efforts to measure serum LG2m in the clinical setting are currently ongoing.
Molecular profiling of HCC also provides information on HCC subclasses with better survival rates (Fig. 3). This HCC subclass is characterized by gene expression patterns reminiscent to mature hepatocytes. Gd-EOB-DTPA-enhanced MRI has been used to evaluate liver tumors in Japan since 2008. Gd-EOB-DTPA is characterized by its rapid and specific uptake by hepatocytes via organic anion transporting polypeptides (OATPs) expressed in the sinusoidal membrane. Therefore, Gd-EOB-DTPA uptake at hepatobiliary phase (HBP) in the liver is considered to reflect hepatocyte function [35]. We found that HCC with high Gd-EOB-DTPA uptake capacity at HBP showed good overall survival without elevation of AFP [31]. Gene signature analysis suggested that this specific HCC is characterized by the activation of transcription factor HNF4A, a master regulator of mature hepatocyte function, in HCC [36,37]. Furthermore, this HCC subclass showed strong nuclear β-catenin staining or CTNNB1 mutations [37,38], and lower expression of EpCAM.
Taken together, our studies demonstrate the utility of serum AFP, LG2m, and Gd-EOB-DTPA-enhanced MRI findings for reflecting the presence or absence of liver CSCs and patient prognosis (Fig. 3). Importantly, AFP and Gd-EOB-DTPA-MRI findings are currently available in the clinical setting, and the addition of LG2m would further supplement the information about the nature and clinical outcome of HCC in the near future.
4. Conclusions
Accumulating evidence suggests that the diversity of liver CSCs might explain the heterogeneous nature of HCC in terms of growth, metastasis, treatment responses, and prognosis. Efforts to apply these basic research data to the clinical setting will foster the development of novel diagnostic and therapeutic approaches to defeat HCC.
Declaration of competing interest
All authors declare that they have no competing financial interests regarding this manuscript.
Acknowledgments
We would like to thank Drs. Naohiko Koshikawa (Tokyo Institute of Technology) and Motoharu Seiki (Institute of Medical Science, The University of Tokyo), inventors of the method to detect serum LG2m, for their fruitful discussions to prepare this review article. The preparation of this review article was supported by grants from the Japan Agency for Medical Research and Development (Grant No. JP19ae0101075) and JSPS KAKENHI (JP18H02792).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita T., Honda M., Kaneko S. Molecular mechanisms of hepatocarcinogenesis in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2011;26(6):960–964. doi: 10.1111/j.1440-1746.2011.06723.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T., Nault J.C. Stemness of liver cancer: from hepatitis B virus to Wnt activation. J Hepatol. 2016;65(5):873–875. doi: 10.1016/j.jhep.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T., Honda M., Takatori H., Nishino R., Minato H., Takamura H. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol. 2009;50(1):100–110. doi: 10.1016/j.jhep.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T., Wang X.W. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123(5):1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishino R., Honda M., Yamashita T., Takatori H., Minato H., Zen Y. Identification of novel candidate tumour marker genes for intrahepatic cholangiocarcinoma. J Hepatol. 2008;49(2):207–216. doi: 10.1016/j.jhep.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Nowell P.C. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 8.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 9.Jordan C.T., Guzman M.L., Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T., Kaneko S. Orchestration of hepatocellular carcinoma development by diverse liver cancer stem cells. J Gastroenterol. 2014;49(7):1105–1110. doi: 10.1007/s00535-014-0951-1. [DOI] [PubMed] [Google Scholar]
- 11.Visvader J.E., Lindeman G.J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Canc. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 12.Nio K., Yamashita T., Kaneko S. The evolving concept of liver cancer stem cells. Mol Canc. 2017;16(1):4. doi: 10.1186/s12943-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi N., Yamashita T., Kaneko S. Molecular biology of liver cancer stem cells. Liver cancer. 2014;3(2):71–84. doi: 10.1159/000343863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yovchev M.I., Grozdanov P.N., Zhou H., Racherla H., Guha C., Dabeva M.D. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47(2):636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- 15.Yovchev M.I., Zhang J., Neufeld D.S., Grozdanov P.N., Dabeva M.D. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology. 2009;50(2):601–611. doi: 10.1002/hep.23012. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T., Honda M., Nakamoto Y., Baba M., Nio K., Hara Y. Discrete nature of EpCAM(+) and CD90(+) cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57(4):1484–1497. doi: 10.1002/hep.26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T., Koshikawa N., Shimakami T., Terashima T., Nakagawa M., Nio K. Serum laminin γ2 monomer as a novel diagnostic and predictive biomarker for hepatocellular carcinoma. Hepatology. 2021 doi: 10.1002/hep.31758. Epub 2021/02/21; In press. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita T., Kaneko S. Orchestration of hepatocellular carcinoma development by diverse liver cancer stem cells. J Gastroenterol. 2014;49(7):1105–1110. doi: 10.1007/s00535-014-0951-1. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M., Yamashita T., Okada H., Oishi N., Nio K., Hayashi T. Sorafenib suppresses extrahepatic metastasis de novo in hepatocellular carcinoma through inhibition of mesenchymal cancer stem cells characterized by the expression of CD90. Sci Rep. 2017;7(1):11292. doi: 10.1038/s41598-017-11848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita T., Budhu A., Forgues M., Wang X.W. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Can Res. 2007;67(22):10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T., Ji J., Budhu A., Forgues M., Yang W., Wang H.Y. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita T., Honda M., Nio K., Nakamoto Y., Takamura H., Tani T. Oncostatin m renders epithelial cell adhesion molecule-positive liver cancer stem cells sensitive to 5-Fluorouracil by inducing hepatocytic differentiation. Can Res. 2010;70(11):4687–4697. doi: 10.1158/0008-5472.CAN-09-4210. [DOI] [PubMed] [Google Scholar]
- 23.Hashiba T., Yamashita T., Okada H., Nio K., Hayashi T., Asahina Y. Inactivation of transcriptional repressor capicua confers sorafenib resistance in human hepatocellular carcinoma. Cellular and molecular gastroenterology and hepatology. 2020;10(2):269–285. doi: 10.1016/j.jcmgh.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomura Y., Yamashita T., Oishi N., Nio K., Hayashi T., Yoshida M. De novo emergence of mesenchymal stem-like CD105+ cancer cells by cytotoxic agents in human hepatocellular carcinoma. Translational oncology. 2017;10(2):184–189. doi: 10.1016/j.tranon.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva A., Hoshida Y., Toffanin S., Lachenmayer A., Alsinet C., Savic R. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Canc Res : an official journal of the American Association for Cancer Research. 2010;16(19):4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita T., Forgues M., Wang W., Kim J.W., Ye Q., Jia H. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Can Res. 2008;68(5):1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita T., Hashimoto S., Kaneko S., Nagai S., Toyoda N., Suzuki T. Comprehensive gene expression profile of a normal human liver. Biochem Biophys Res Commun. 2000;269(1):110–116. doi: 10.1006/bbrc.2000.2272. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T., Honda M., Kaneko S. Application of serial analysis of gene expression in cancer research. Curr Pharmaceut Biotechnol. 2008;9(5):375–382. doi: 10.2174/138920108785915102. [DOI] [PubMed] [Google Scholar]
- 29.Hoshida Y., Nijman S.M., Kobayashi M., Chan J.A., Brunet J.P., Chiang D.Y. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Can Res. 2009;69(18):7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyault S., Rickman D.S., de Reynies A., Balabaud C., Rebouissou S., Jeannot E. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45(1):42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita T., Kitao A., Matsui O., Hayashi T., Nio K., Kondo M. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014;60(5):1674–1685. doi: 10.1002/hep.27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshikawa N., Moriyama K., Takamura H., Mizushima H., Nagashima Y., Yanoma S. Overexpression of laminin gamma 2 chain monomer in invading gastric carcinoma cells. Can Res. 1999;59(21):5596–5601. [PubMed] [Google Scholar]
- 33.Yasuda H., Nakagawa M., Kiyokawa H., Yoshida E., Yoshimura T., Koshikawa N. Unique biological activity and potential role of monomeric laminin-gamma 2 as a novel biomarker for hepatocellular carcinoma: a review. Int J Mol Sci. 2019;20(1) doi: 10.3390/ijms20010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rousselle P., Scoazec J.Y. Laminin 332 in cancer: when the extracellular matrix turns signals from cell anchorage to cell movement. Semin Canc Biol. 2020;62:149–165. doi: 10.1016/j.semcancer.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Kanki A., Tamada T., Higaki A., Noda Y., Tanimoto D., Sato T. Hepatic parenchymal enhancement at Gd-EOB-DTPA-enhanced MR imaging: correlation with morphological grading of severity in cirrhosis and chronic hepatitis. Magn Reson Imaging. 2012;30(3):356–360. doi: 10.1016/j.mri.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Kitao A., Matsui O., Yoneda N., Kozaka K., Kobayashi S., Koda W. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology. 2012;265(3):780–789. doi: 10.1148/radiol.12120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitao A., Matsui O., Yoneda N., Kozaka K., Kobayashi S., Sanada J. Hepatocellular carcinoma with beta-catenin mutation: imaging and pathologic characteristics. Radiology. 2015;275(3):708–717. doi: 10.1148/radiol.14141315. [DOI] [PubMed] [Google Scholar]
- 38.Kitao A., Matsui O., Yoneda N., Kozaka K., Kobayashi S., Koda W. Gadoxetic acid-enhanced magnetic resonance imaging reflects co-activation of beta-catenin and hepatocyte nuclear factor 4 alpha in hepatocellular carcinoma. Hepatol Res. 2018;48(2):205–216. doi: 10.1111/hepr.12911. [DOI] [PubMed] [Google Scholar]