Abstract
Treatment of burn injuries with Mesenchymal stem cells (MSCs) is a great promise due to their unique properties. As two natural and functional wound dressing, Chitosan and Aloe-Vera gel assist wound regeneration by providing a proper environment. In the current study, we aimed to compare the effect of encapsulated BMSCs in Chitosan-based gel and Aloe-Vera gel on the healing of grade-II burn injuries compared to other groups in the rat. After creation of a 2 × 2 cm grade-II burn on dorsal skin of rats, treatments were performed for each group. The wound closure rate and healing properties were evaluated by histopathological analysis on 7, 14, 21 and, 28 days post-treatment. The expression rate of VEGF, Collagen-I and Collagen-III genes was also assessed on days 3, 7, 14, 21 and 28 performing qRT-PCR. The full wound healing with inconsiderable scar formation was achieved for Aloe-vera/BMSCs and Chitosan/BMSCs group on 28th day post-treatment. Pathological results also demonstrated the highest angiogenesis and granulation tissue formation for Aloe-vera/BMSCs and Chitosan/BMSCs groups respectively. The expression level of VEGF, Collagen-I, and Collagen-III genes was significantly higher in these groups on days 14 and 21, compared to other groups. Results demonstrated the synergistic effect of BMSCs when combined with Chitosan or Aloe-vera gel enhanced the healing process of wound healing more than chitosan gel treatment. Therefore, this gel can be considered as effective approaches for treatment of burn injuries.
Keywords: Aloe vera gel, Burn, Chitosan, Mesenchymal stem cells, Wound healing
Abbreviations: ASCs, (Adipose-derived stem cell); bFGF, (basic fibroblast growth factor); BMSCs, (Bone marrow-derived-MSCs); DMEM-LG, (Dulbecco's Modified Eagle Medium-low glucose); FGF, (Fibroblast growth factor); IFN-γ, (Interferon-γ); IL-1, (Interleukin-1); MSCs, (Mesenchymal stem cells); TGF-β, (Transforming growth factor-β); TNF-α, (Tumor necrosis factor-α); VEGF, (Vascular endothelial growth factor)
1. Introduction
Burn wounds are of serious devastating traumas with significant cost for both individuals and the health care system [1]. Debridement, dressing and skin grafting are of traditional and usual treatments for burn treatment. However, these approaches come with delayed wound healing, tissue necrosis and scar formation [2]. Nowadays, stem cell-based regenerative medicine has become a growing interest among scientists. It's been reported that Mesenchymal stem cells (MSCs) have a great potential in tissue repair to regenerate many damaged tissues such as myocardium, blood vessels, cartilage, bone, and skin [3]. MSCs are self-renewal cells with a wide range of differentiation capacity and multi-potency to differentiate into mesoderm lineage including osteocyte, adipose, and chondrocyte [4,5]. MSCs contribute in each phase of normal wound healing including inflammation, proliferation and remodeling phase. In brief, MSCs contribute to wound healing mostly by differentiation into keratinocytes, fibroblasts and, epithelial cells. Also, their well-known paracrine activities decrease inflammation and enhance angiogenesis, re-epithelialization and, collagen synthesis [6]. One of the main sources for MSCs is bone marrow (BMSCs), however similar populations are also obtainable from the umbilical cord, peripheral blood, adipose tissue etc. [7].
The therapeutic effects of herbal drugs have been reported for many years. In comparison to synthetic medicines, Herbal medicines/products are less expensive comprising moderate efficiency without toxicity or low toxicity [8]. Aloe vera is a tropical plant from the Liliaceae family, growing easily in hot and dry climates. In the center of each leaf, there is mucilaginous tissue which is called Aloe vera gel. Aloe vera gel has widely been used for cosmetic, medical and, even general health purposes for centuries. Wound and burn treatment is one of the major Aloe vera indications. It has been reported that Aloe vera possesses anti-inflammation, anti-microbial, and anti-oxidant properties which accelerates wound healing. Furthermore, the bradykinin and thromboxane found in Aloe vera relieve pain and by increasing the dead cells fall-off, they accelerate wound healing [9].
Chitin is a natural biopolymer that is the basic component of the crustacean exoskeleton. The N-deacetylation of chitin creates a cationic polysaccharide known as chitosan. Chitosan is a conspicuous biopolymer due to its unique properties including being non-toxic, non-antigenic, biologically adhesive, biocompatible, biodegradable with a hemostatic effect [10,11]. Furthermore, chitosan possesses anti-microbial functions by its poly-cationic character which facilitates interaction with negatively-charged microbial cell-wall and membrane [12]. Chitosan also increases the synthesis of collagen type-III and the recruitment of fibroblast cells to promote granulation tissue formation. It also offers some immunological properties such as inhibition of pro-inflammatory cytokines [13]. Taken together, these and other positive properties such as promoting wound contraction and cell differentiation make chitosan-based hydrogels as suitable candidate for stem cell-based wound therapy [14]. Since advances in MSCs delivery to the burn site using biological scaffolds have opened up new avenues to manipulate the functionality and the fate of stem cells; we selected Aloe vera and chitosan gel as two unique scaffolds with the ability to encapsulate cells with the aim of wound healing accelerants. Therefore, we aimed to compare the therapeutic effects of 6 treatment groups including a) Aloe vera/BMSCs, b) Chitosan/BMSCs, c) BMSCs alone, d) Aloe vera gel alone, e) Chitosan-based gel alone and, f) Control (Vaseline gauze) on the regeneration of grade-II burn wounds in the experimental rat model.
2. Methods
2.1. BMSCs isolation, culture, and characterization
In brief, animals were anesthetized before sacrificing then both femur excised and after removing surrounding tissues, each bone marrow was aspirated by a syringe containing 2 ml Dulbecco's Modified Eagle Medium-low glucose (DMEM-LG) (Sigma, USA) supplemented with 10% FBS (Gibco, Life Technologies, USA) and 100 U/ml Penicillin (Sigma, USA), 100 μg/ml streptomycin (Sigma, USA) and 25 ng/ml Amphotericin (Sigma, USA) and introduced to a culture flask. Cell culture was performed with the same media and kept at 37 °C in a 5% CO2 incubator. Cultured cells were monitored for adherent cells regularly and non-adherent cells were removed. When cells reached 90% confluency, cell passages were carried out using Trypsin–EDTA solution (Gibco, USA).
2.2. Preparation of Aloe vera gel
Fresh Aloe vera was prepared from a commercial plant nursery and the Aloe vera gel was extracted from fresh leaves and after homogenizing with blender, the pH was adjusted to 7.4 ± 0.2 and stored at 4 °C refrigerator.
2.3. Preparation of chitosan-based gel
The 5% chitosan (Sigma–Aldrich, USA) solution in 43% acetic acid was prepared and placed on stirrer with a magnet for 48 h to achieve a homogenous gel.
2.4. Animals and experimental groups
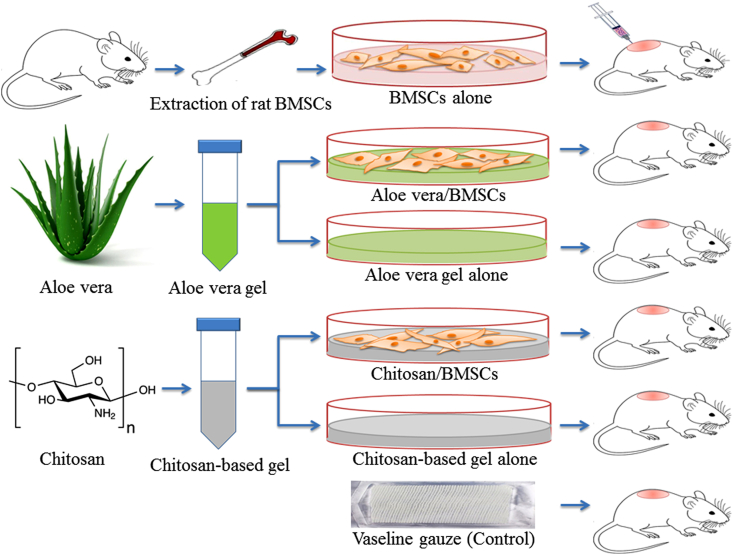
A total of 48 Wistar-albino male rats (300–340 g weighting and 3–4 months old) were purchased from the animals’ nests of Shahrekord University of Medical Sciences for making grade-II burn wounds. The rats used in current study were housed for 1 week under an air-conditioned environment with a temperature at 22–26 °C and relative humidity of 40–60%. This research was approved by the Shahrekord University of Medical Sciences and carried out according to the ethical guidelines. All animals included in the study were clinically healthy and were randomly divided into 6 groups (each one contained 8 rats) including (A) Aloe vera/BMSCs (receiving 40 g Aloe vera gel+ 2 × 106 cells), (B) Aloe vera (receiving 40 g Aloe vera gel alone), (C) Chitosan/BMSCs (receiving 40 g Chitosan-based gel+ 2 × 106 cells), (D) Chitosan (receiving 40 g Chitosan-based gel alone), (E) BMSCs (Injection of 2 × 106 Cell around the wound) and, (F) Control (receiving routine treatment including Vaseline gauze) (Fig. 1).
Fig. 1.
Schematic diagram of experimental groups for treatment of grade-II burn injury in the rat model. All groups received their treatment plus Vaseline gauze while the control group received Vaseline gauze only. BMSCs: Bone marrow-derived Mesenchymal Stem Cells.
2.5. Creation of grade-II burn wound
All groups were kept under the same condition such as free access to food and water before or after making the burn wound. Animals were anesthetized using intraperitoneal (IP) injection of xylazine (Sigma Aldrich) (10 mg/kg) and ketamine hydrochloride (Sigma, Germany) (50–100 mg/kg). A grade-II burn wound was created using hot plate (diameter: 2 × 2 cm) at 190 °C temperature which placed for 20 s on the dorsal skin of rats with equal pressure [15].
2.6. Wound healing assessment
For evaluation of wound closure rate and healing efficacy, the wound site of each treatment group was imaged by a digital camera on days 7, 14, 21 and, 28 post-treatment. The images were evaluated by Image J software (NIH Image J Software) and the percentage of wound closure was calculated based on wound size at any interval using the following equation:
| %Wound closure = (AOW-AAW)/AOW × 100% |
Whereas AOW is the area of original wound size and AAW is the area of actual wound size at each interval.
2.7. Histopathological assessments
Rats were sacrificed on days 7, 14, 21 and, 28 post-treatment and the wound site with approximately 1 × 1 cm of surrounding skin were harvested and fixed in 10% formalin (Sigma-Aldrich) [16]. The paraffin-embedded tissue sections were prepared on a rotary microtome and stained with Hematoxylin–Eosin as standard protocols [17]. All sections were examined by a pathologist under light microscopy for the survey of inflammation, epithelialization, angiogenesis, and formation of granulated tissue.
2.8. Gene expression analysis by Real-Time qPCR
The expression rate of VEGF (vascular endothelial growth factor) and collagen genes (type 1 and 3) was examined by Real-Time qPCR [18]. Total RNA was extracted from burned skin tissues which were taken on days (3, 7, 14, 21 and, 28) post-treatment for qRT-PCR section. RNA extraction was performed using Trizol Reagent (YTA, Iran) according to the manufacturer's protocol. The purity and concentration of the extracted RNA were determined by NanoDrop 2000 Spectrophotometer (Thermo Scientific, Germany), and the RNA was treated with DNase-I (EN0521, Fermentas, Germany) for elimination of probable DNA contamination. The cDNA was synthesized according to the Takara kit (TaKaRa, Japan) protocol and the qRT-PCR was carried out using a Real-time PCR devise (Corbett Rotor-Gene 6000, Germany). The sequences of utilized primers are presented in Table 1. The GAPDH gene was used as control and fold changes were calculated by 2˗ΔΔCt method.
Table 1.
Designed and utilized primers in Real-time qPCR.
| Gene | Primer Sequence (5′ to 3′) | TM˚C | Product Size (bp) | |
|---|---|---|---|---|
| VEGF | F | ATCAAACCTCACCAAAGCC | 55.65 | 76 |
| R | TCTTTGGTCTGCATTCACATCT | 57.97 | ||
| Collagen-I | F | CAAGAACGGAGATGATGGGGAA | 60.09 | 146 |
| R | CACCATCCAAACCACTGAAACC | 59.96 | ||
| Collagen-III | F | GTAATAGACCTCAAGGCCCCAA | 59.49 | 128 |
| R | TGATTCACAGATTCCAGGGGAG | 59.49 | ||
| GAPDH | F | GGCAAGTTCAATGGCACAGT | 59.32 | 151 |
| R | TGGTGAAGACGCCAGTAGACTC | 61.71 | ||
2.9. Statistical analysis
All data were expressed as means ± SD. All statistical analysis was performed by SPSS software version 22 (SPSS, Chicago, IL) using two-way ANOVA for multiple group comparisons. Graphs were created by Graph pad Prism Software, version 8 (Graphpad Software, California, USA). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Wound closure rate
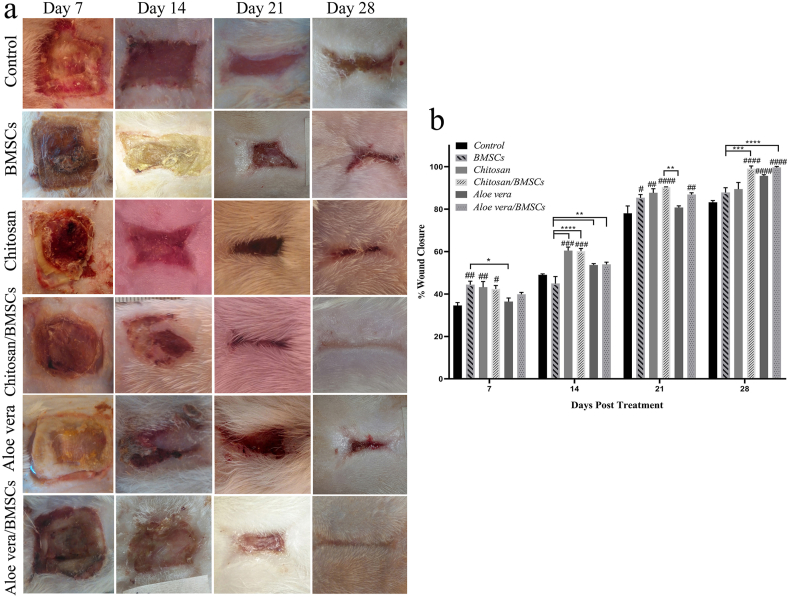
The images of wound sites demonstrated that all treatment groups accelerate the wound closure rate compared to the control group receiving only Vaseline gauze. However, among treatment groups, the Chitosan, Chitosan/BMSCs, Aloe vera and, Aloe vera/BMSCs groups had a higher wound healing effect on days 21 and 28 post-treatment. The full wound healing with negligible scar formation was seen for Chitosan/BMSCs and Aloe vera/BMSCs on day 28 post-treatment (Fig. 2a). The statistical analysis of wound closure also confirmed the highest wound closure rate of these two groups after treatment compared to other groups (Fig. 2b).
Fig. 2.
The wound closure rate of burn wounds treated with BMSCs, Chitosan, Chitosan/BMSCs, Aloe vera, Aloe vera/BMSCs and, Control on days 7, 14, 21 and, 28 post-treatment. (a) Macroscopic images of wound sites. (b) Analytical graph of wound closure rate: all treatment groups had a higher wound healing effect compared to the control group (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001). The highest wound closure rate was seen for Chitosan/BMSCs and Aloe vera/BMSCs on days 21 and 28. Error bars refer to SD. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
3.2. Histopathological assessments
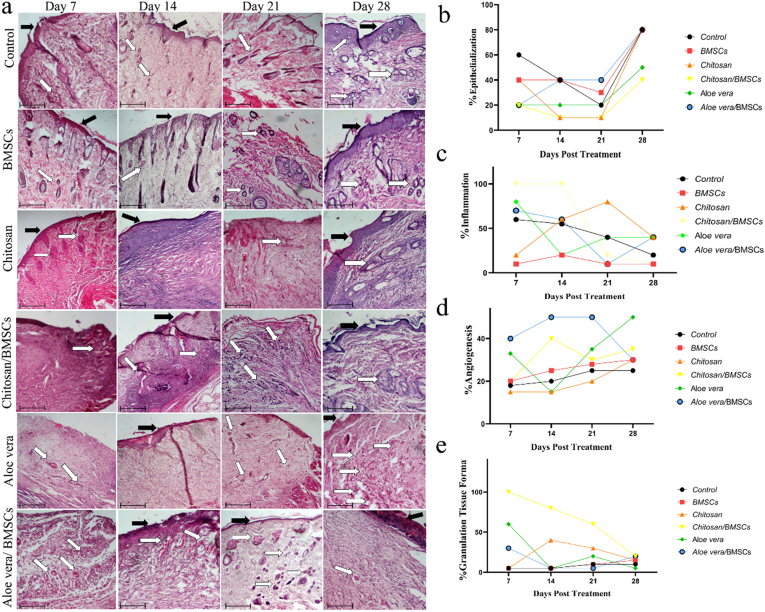
All H&E stained samples were evaluated by a pathologist who was blinded for treatment groups. The parameters of, inflammation, epithelialization, granulation tissue formation and, angiogenesis were evaluated. All groups were almost similar in terms of inflammation and had a moderate level of inflammation especially during the first days post-treatment. However, more inflammation was seen for Chitosan/BMSCs and Chitosan groups, and the lowest inflammation was seen for BMSCs alone group (Fig. 3a and c). In regards to epithelialization, samples were almost similar, but a relatively ascending rate was detected for Aloe vera/BMSCs from day 7–28 post-treatment (Fig. 3a and b). On the contrary, the angiogenesis and granulated tissue formation rate was different between groups. The newly formed endothelial tubes are corresponding to angiogenesis and the higher number of them was seen for Aloe vera/BMSCs and Aloe vera group (Fig. 3a and d). On the other hand, the thicker granulated tissue has been formed for Chitosan/BMSCs and Chitosan groups (Fig. 3a and e). Taken together, the pathological images demonstrated an improved structurally formed epidermis for Chitosan, Chitosan/BMSCs and, Aloe vera/BMSCs post-treatment (Fig. 3).
Fig. 3.
The H&E images of treated wounds with BMSCs, Chitosan, Chitosan/BMSCs, Aloe vera, Aloe vera/BMSCs and, Control groups after 7, 14, 21 and, 28 days post-treatment. Scale bar = 100 μm (a). Scoring of Epithelialization (b), Inflammation (c), Angiogenesis (d) and, Granulation tissue formation (e) extracted from the H&E results which represent a quantitative and qualitative assessment of burn wound healing. The black arrows demonstrate the epithelialization and the white arrows show angiogenesis. A thicker granulated tissue was seen for Chitosan/BMSCs and a higher angiogenesis rate was seen for Aloe vera and Aloe vera/BMSCs groups compared to other groups.
3.3. The expression rate of VEGF, Collagen-I and, Collagen-III genes
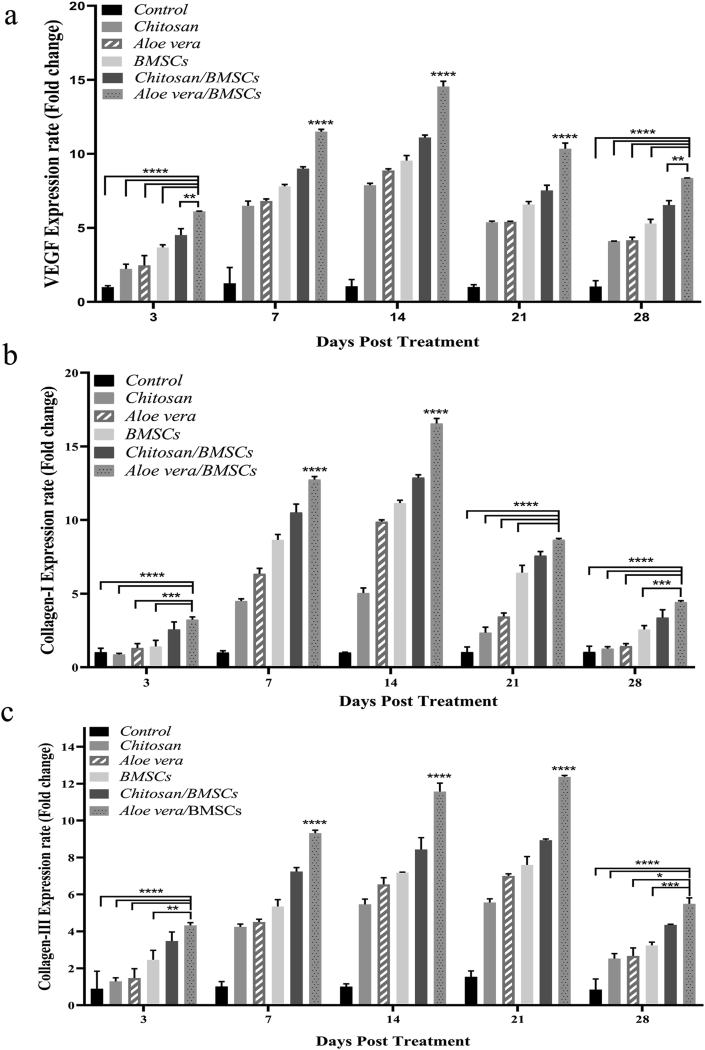
The expression rate of VEGF, Collagen-I and, Collagen-III genes demonstrated almost a similar pattern. As shown in Fig. 4a, the peak expression rate of VEGF was seen in Aloe vera/BMSCs group on day 14 post-treatment (fold change: 14.5 ± 0.4) and Chitosan/BMSCs on day 14 (fold changes: 11.1 ± 0.2) (p < 0.0001) (Fig. 4a). After that, the Chitosan/BMSCs group showed a higher VEGF expression rate among groups (p < 0.001, not shown in the figure). Collagen-I gene had also the higher rate in Aloe vera/BMSCs on day 14 (fold change: 16.5 ± 0.3) and Chitosan/BMSCs on day 14 (fold change: 12.9 ± 0.2) (p < 0.0001) (Fig. 4b). On the other side, the Collagen-III gene almost showed an ascending rate from day 3 to day 21 post-treatment and the peak expression rate of it was seen on day 21 for the Aloe vera/BMSCs group (fold change: 12.4 ± 0.1) (p < 0.0001) (Fig. 4c). After 28 days post-treatment, all gene expressions were decreased, however, the higher expression of each gene was still detected for the Aloe vera/BMSCs group compared to the other groups.
Fig. 4.
The gene expression rate of VEGF (a), Collagen-I (b) and, Collagen-III (c) of wounds treated with BMSCs, Chitosan, Chitosan/BMSCs, Aloe vera, Aloe vera/BMSCs and, Control groups after 3, 7, 14, 21 and, 28 days post-treatment. The most expression rate of VEGF and Collagen-I was seen on day 14 and the peak expression of Collagen-III was seen on day 21 for Aloe vera/BMSCs group. Error bars refer to SD. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
4. Discussion
Skin is the greatest organ in our body with numerous functions such as thermoregulation, fluid hemostasis, metabolic functions, and protecting against pathogens. One of the severe skin damages with high mortality, morbidity, and costs are burn injuries. Therefore, many scientists and researchers investigate to achieve an efficient approach to accelerate the wound healing process and decrease the risk of infection [19]. In this study, we evaluated the therapeutic effects of 6 treatment groups including bone marrow-derived-MSCs (BMSCs), Chitosan-based gel, Aloe vera gel, encapsulated BMSCs in chitosan-based gel, encapsulated BMSCs in Aloe vera and Vaseline gauze as the control group on the healing of grade-II burn wound in a rat model (Fig. 1). For the last years, researchers had focused on the application of a different sources of Mesenchymal stem cells which effectively accelerates the wound healing through its unique characteristics. MSCs possess anti-inflammatory and immunomodulatory properties, they also increase the expression and secretion of growth factors such as VEGF (Vascular endothelial growth factor), bFGF (basic fibroblast growth factor), TGF-β (transforming growth factor-β), FGF (fibroblast growth factor) etc, which enhances angiogenesis and formation of granulation tissue [20]. Rasulov et al. were the first who used BMSCs for the treatment of burn wounds in humans. They applied BMSCs on the surface of burns which accelerated the wound healing through increasing angiogenesis [21]. Results of the application of BMSCs on a rat model of burn injury demonstrated reduced cell infiltration and, increased angiogenesis which is similar to our discoveries [22]. Our result showed that the injection of BMSCs around the wound site increases the wound closure rate compared to the control group (Fig. 2). The pathological results also confirmed the anti-inflammatory function of BMSCs. The BMSCs alone group had a lower level of inflammation and a relatively high percent of epithelialization among groups (Fig. 3). It also assists wound healing by increasing VEGF, Collagen-I and Collagen-III expression rates especially on days 7 and 14 post-treatment (Fig. 4). Since the application of natural substrates such as Chitosan and Aloe vera gel has shown promising results in wound treatment, the major aim of this study was to combine BMSCs with these substances to compare their beneficial effects.
Chitosan-based gels possess unique properties such as being antimicrobial, biocompatible, non-toxic, biodegradable and, having biological activities that make them ideal materials for accelerating the wound healing process [16]. It has been shown that chitosan-based gels promote wound healing mostly by reducing high levels of inflammation and wound infection [23]. In a recent study, Shabunin AS et al., designed and utilized a wound dressing based on chitin and chitosan nano fibers for treatment of grade-III burn injury. This scaffold increased epithelialization significantly within 28 days as well as elevated levels of microvesicles [24]. El Sadik et al. compared the effect of chitosan gel either with systemic and intradermal injection of MSCs for the treatment of the full-thickness wound. They reported accelerated wound healing for intradermal injection of MSCs rather than other groups. The full healed wound was seen for this group by 15th day post-treatment [25]. Combining MSCs with Chitosan-based gel was one of our goals in this study; however Chitosan-based gel and BMSCs (in the form of direct injection around the wound) groups were also investigated separately. Based on our results, both treatments increased wound closure rate similarly but pathological assessments showed differences in terms of inflammation, angiogenesis and, the formation of granulated tissue (Fig. 3). The Chitosan group had the highest inflammation rate while the BMSCs group showed the lowest inflammation level. We suggest that, when the BMSCs are injected directly into the wound margins, the wound receives a high percentage of cells which benefits wound healing through modulation of inflammation. This is possibly due to the anti-inflammatory and immunomodulatory function of MSCs through interaction with immune cells and proinflammatory factors such as TNF-α (tumor necrosis factor-α), IL-1 (interleukin-1) and, IFN-γ (interferon-γ) [26]. Also, our results showed higher angiogenesis and formation of granulated tissue in the combination of BMSCs with Chitosan-based gel than the application of each group alone. One of the suggested reasons is the higher viability rate of BMSCs in Chitosan-based gel which is also investigated by Soriano-Ruiz JL et al. They reported that MSCs viability in a chitosan/glycosaminoglycan scaffold reached to approximately 100% by day 5 and 7, indicating high cell viability [14]. Our results also showed a high expression rate of Collagen-I and Collagen-III genes in Chitosan/BMSCs group which is consistent with another study by Maged A et al., who applied a rosuvastatin-loaded chitosan scaffold in combination with MSCs [27]. This method enhanced fibroblast proliferation and promoted wound healing by regulating collagen deposition and distribution in the epidermis. Therefore, in this section, we conclude that the combination of BMSCs with Chitosan-based gel enhances the therapeutic effects of BMSCs and Chitosan rather than the application of each one alone.
To discuss Aloe vera, it is noteworthy to mention multiple studies that have investigated and reported the effects of Aloe vera gel and/or extract in wound treatment. Akhoondinasab MR et al. compared Aloe vera extract and silver sulfadiazine for treatment of grade-III burn injury in a rat model. Their result showed the shorter healing time in the Aloe vera group than silver sulfadiazine [8]. Chrubasik JE et al. who had reviewed the clinical trials on the application of Aloe vera, confirmed the shorter healing time in the Aloe vera treatment group by approximately 8 days than control groups [28]. In a recent study, Rahman MS et al. designed a new study to combine Aloe vera extract and amnion membrane to prepare a gel for the treatment of burn injuries. This combination resulted in increased wound closure, wound recovery time, re-epithelialization, epidermis thickness, proliferating of keratinocytes and, angiogenesis rate than control groups [29]. In another study, the honey-milk-Aloe vera ointment was utilized which led to higher angiogenesis, wound closure rate and the density of collagen fibers as well as reduction of inflammation and scar formation [29]. All these studies are similar to our findings in the application of Aloe vera gel. We found that Aloe vera gel/BMSCs treatment increases the wound closure rate, as the wound is fully healed on the 28th day with a negligible scar (Fig. 2a). Also, based on our pathology assessments, the Aloe vera/BMSCs group had a higher rate of angiogenesis and a relatively ascending rate of re-epithelialization among groups (Fig. 3). This was also in agreement with the peak expression rate of VEGF, Collagen-I and, Collagen-III genes on day 14 at the wound site (Fig. 4) which is similar to another study by Oryan A et al. They reported that combination of Aloe vera gel with adipose-derived stem cell (ASCs) reduces inflammatory response by decreasing expression of TGF-β1 and IL-1β genes at day 7 post-treatment. They also detected the higher angiogenesis and re-epithelialization rate on day 14 compared to the other groups [30].
5. Conclusion
The present work suggested that wound healing could be accelerated by a method (BMSCs combined with Chitosan or Aloe-vera gel). Chitosan gel was found to enhance wound healing. However, BMSCs showed even higher promotion of wound healing as compared to the acceleration effect of chitosan. Our results indicated the synergistic effects of Chitosan and Aloe vera gel when combined with BMSCs for the treatment of grade-II burn injuries. Both composite groups acted much better than the application of each treatment alone. They remarkably promoted wound healing through increasing wound closure rate, angiogenesis and, the formation of granulated tissue. Although discovering the exact mechanism of wound healing by these composites needs further investigations, this study introduces Chitosan/BMSCs and Aloe vera/BMSCs as two effective composites for the treatment of burn injuries.
Ethics approval and consent to participate
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals' prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86–23, Revised 1985). The Ethics code of IR. SKUMS.REC.1396.223 was also received by Ethics Committee of University of Medical Sciences for Use and Care of Laboratory Animals.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Funding
This research was funded by University of Medical Sciences
Authors' contributions
ES, KAD, and MC conceived and designed the study; MC, AFK, and FYN conducted the research; ES, KAD, and MC performed the experiments. MC Analyzed the data. MC wrote the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express their sincere appreciation to the Medical Herbs Research Center of Shahrekord University of Medical Sciences for their supports.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Lumenta D.B., Hautier A., Desouches C., Gouvernet J. Mortality and morbidity among elderly people with burns — evaluation of data on admission. Burns. 2008;34:965–974. doi: 10.1016/j.burns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Qu W., Li R., Zheng C., Zhang L. Efficacy of autologous platelet-rich gel in the treatment of deep grade II burn wounds. Int J Clin Exp Med. 2018;11(3):2654–2659. [Google Scholar]
- 3.Wu Y., Wang J., Scott P.G., Tredget E.E. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15(Suppl 1):S18–S26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 4.Satija N.K., Singh V.K., Verma Y.K. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13(11–12):4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding D.-C., Shyu W.-C., Lin S.-Z. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 6.Abo-elkheir W., Hamza F., Elmofty A.M., Emam A., Abdl-moktader M. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: a case-control prospective study. Am J Stem Cell. 2017;6(3):23–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Caplan A. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Akhoondinasab M.R., Akhoondinasab M., Saberi M. Comparison of healing effect of Aloe vera extract and silver sulfadiazine in burn injuries in experimental rat model. World J Plast Surg. 2014;3(1):29–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Hai Z., Ren Y., Hu J., Wang H., Qin Q., Chen T. Evaluation of the treatment effect of Aloe vera fermentation in burn injury healing using a rat model. Mediat Inflamm. 2019;2019 doi: 10.1155/2019/2020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azevedo M.A., Bourbon A.I., Vicente A.A., Cerqueira M.A. International Journal of Biological Macromolecules Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B 2. Int J Biol Macromol. 2014;71:141–146. doi: 10.1016/j.ijbiomac.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Nosrati H., Pourmotabed S., Sharifi E. A review on some natural biopolymers and their applications in angiogenesis and tissue engineering. J Appl Biotechnol Reports. 2018;5(3):81–91. [Google Scholar]
- 12.Banerjee M., Mallick S., Paul A., Chattopadhyay A., Ghosh S.S. Heightened reactive oxygen species generation in the antimicrobial activity of a three component iodinated chitosan?silver nanoparticle composite. Langmuir. 2010;26(8):5901–5908. doi: 10.1021/la9038528. [DOI] [PubMed] [Google Scholar]
- 13.Friedman A.J., Phan J., Schairer D.O. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: a targeted therapy for cutaneous pathogens. J Invest Dermatol. 2013;133(5):1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano-ruiz J.L., Gálvez-martín P., López-ruiz E., Suñer-carbó J. Design and evaluation of mesenchymal stem cells seeded chitosan/glycosaminoglycans quaternary hydrogel sca ff olds for wound healing applications. Int J Pharm. 2019;570(August):118632. doi: 10.1016/j.ijpharm.2019.118632. [DOI] [PubMed] [Google Scholar]
- 15.Afzali L., Mirahmadi-Babaheydari F., Shojaei-Ghahrizjani F., Rahmati S., Shahmoradi B., Banitalebi-Dehkordi M. The effect of encapsulated umbilical cord-derived mesenchymal stem cells in PRPCryogel on regeneration of grade-II burn wounds. Regen Eng Transl Med. 2020:1–11. doi: 10.1007/s40883-020-00188-6. [DOI] [Google Scholar]
- 16.Chehelgerdi M., Doosti A. Effect of the cagW-based gene vaccine on the immunologic properties of BALB/c mouse: an efficient candidate for Helicobacter pylori DNA vaccine. J Nanobiotechnol. 2020;18:1–6. doi: 10.1186/s12951-020-00618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A.H., Jacobson K.A., Rose J., Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb Protoc. 2008;5 doi: 10.1101/pdb.prot4986. pdb.prot4986 (2008) [DOI] [PubMed] [Google Scholar]
- 18.Taherzadeh-Soureshjani P., Chehelgerdi M. Algae-meditated route to cuprous oxide (Cu2O) nanoparticle: differential expression profile of MALAT1 and GAS5 LncRNAs and cytotoxic effect in human breast cancer. Cancer Nanotechnol. 2020;11(1):1–34. [Google Scholar]
- 19.Safarpoor Dehkordi F., Tirgir F., Valizadeh Y. Effects of Guajol® ointment synthesized from medicinal smoke condensate of jennet feces on burn wound healing on Wistar rat. Vet Res Forum Int Q J. 2017;8(3):215–221. [PMC free article] [PubMed] [Google Scholar]
- 20.Nourian Dehkordi A., Mirahmadi Babaheydari F., Chehelgerdi M., Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10(1) doi: 10.1186/s13287-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasulov M.F., Vasil’chenkov A.V., Onishchenko N.A. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139(1):141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 22.Rasulov M.F., Vasilenko V.T., Zaidenov V.A., Onishchenko N.A. Cell transplantation inhibits inflammatory reaction and stimulates repair processes in burn wound. Bull Exp Biol Med. 2006;142(1):112–115. doi: 10.1007/s10517-006-0306-x. [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Wang C., Li C. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018 doi: 10.1039/c7ra13510f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shabunin AS, Yudin VE, Dobrovolskaya IP et al. Chitin/chitosan Nanofibers: processing and biomedical applications.
- 25.El Sadik A.O., El Ghamrawy T.A., Abd El-Galil T.I. The effect of mesenchymal stem cells and chitosan gel on full thickness skin wound healing in albino rats: histological, immunohistochemical and fluorescent study. PLoS One. 2015;10(9):1–19. doi: 10.1371/journal.pone.0137544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., Zhao Y., Hao H., Han W., Fu X. Mesenchymal stem cell–based therapy for nonhealing wounds: today and tomorrow. Wound Repair Regen. 2015;23(4):465–482. doi: 10.1111/wrr.12304. [DOI] [PubMed] [Google Scholar]
- 27.Maged A., Abdelkhalek A.A., Mahmoud A.A., Salah S., Ammar M.M., Ghorab M.M. European Journal of Pharmaceutical Sciences Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur J Pharmaceut Sci. 2019;127(October 2018):185–198. doi: 10.1016/j.ejps.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Chrubasik J.E., Roufogalis B.D., Wagner H., Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7–8):568–579. doi: 10.1016/j.phymed.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Rahman M.S., Islam R., Rana M.M. Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Compl Alternative Med. 2019;19(1):1–15. doi: 10.1186/s12906-019-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oryan A., Alemzadeh E., Mohammadi A.A., Moshiri A. Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019;377(2):215–227. doi: 10.1007/s00441-019-03015-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.