Key Points
Question
Among critically ill patients undergoing tracheal intubation worldwide, how common are major adverse events during the peri-intubation period?
Findings
In this prospective observational study that included 2964 patients from 197 sites across 29 countries from October 2018 to July 2019, at least one major clinical event occurred after intubation in 45.2% of patients, including cardiovascular instability in 42.6%, severe hypoxemia in 9.3%, and cardiac arrest in 3.1%.
Meaning
Among an international sample of critically ill patients undergoing tracheal intubation, major cardiopulmonary events occurred frequently.
Abstract
Importance
Tracheal intubation is one of the most commonly performed and high-risk interventions in critically ill patients. Limited information is available on adverse peri-intubation events.
Objective
To evaluate the incidence and nature of adverse peri-intubation events and to assess current practice of intubation in critically ill patients.
Design, Setting, and Participants
The International Observational Study to Understand the Impact and Best Practices of Airway Management in Critically Ill Patients (INTUBE) study was an international, multicenter, prospective cohort study involving consecutive critically ill patients undergoing tracheal intubation in the intensive care units (ICUs), emergency departments, and wards, from October 1, 2018, to July 31, 2019 (August 28, 2019, was the final follow-up) in a convenience sample of 197 sites from 29 countries across 5 continents.
Exposures
Tracheal intubation.
Main Outcomes and Measures
The primary outcome was the incidence of major adverse peri-intubation events defined as at least 1 of the following events occurring within 30 minutes from the start of the intubation procedure: cardiovascular instability (either: systolic pressure <65 mm Hg at least once, <90 mm Hg for >30 minutes, new or increase need of vasopressors or fluid bolus >15 mL/kg), severe hypoxemia (peripheral oxygen saturation <80%) or cardiac arrest. The secondary outcomes included intensive care unit mortality.
Results
Of 3659 patients screened, 2964 (median age, 63 years; interquartile range [IQR], 49-74 years; 62.6% men) from 197 sites across 5 continents were included. The main reason for intubation was respiratory failure in 52.3% of patients, followed by neurological impairment in 30.5%, and cardiovascular instability in 9.4%. Primary outcome data were available for all patients. Among the study patients, 45.2% experienced at least 1 major adverse peri-intubation event. The predominant event was cardiovascular instability, observed in 42.6% of all patients undergoing emergency intubation, followed by severe hypoxemia (9.3%) and cardiac arrest (3.1%). Overall ICU mortality was 32.8%.
Conclusions and Relevance
In this observational study of intubation practices in critically ill patients from a convenience sample of 197 sites across 29 countries, major adverse peri-intubation events—in particular cardiovascular instability—were observed frequently.
This international cohort study describes the incidence and nature of cardiovascular instability, severe hypoxemia, and cardiac arrest surrounding endotracheal intubation.
Introduction
Tracheal intubation is a high-risk procedure commonly performed in critically ill patients, yet relatively little is known about adverse peri-intubation events.1 Underlying shock, respiratory failure, metabolic acidosis, and other pathophysiological changes substantially increase the risk of adverse peri-intubation events in critically ill patients compared with patients undergoing intubation in the operating room.1,2,3,4,5,6 Small prospective studies, retrospective analyses, or national level studies7,8,9 suggest that up to 28% of critically ill patients undergoing tracheal intubation may experience a life-threatening complication such as severe hypoxemia or hemodynamic instability and 2.7% of procedures are complicated by cardiac arrest.7,10 In 2011, the Fourth National Audit Project launched in the UK was the first attempt to assess intubation-related morbidity and mortality at a national level.9 It reported major gaps in clinical practice such as poor identification of at-risk patients, poor planning, unavailability of skilled clinicians and equipment especially during off hours, and lack of—or failure to correctly interpret—capnography. In addition, the current incidence and consequences of adverse peri-intubation events on either short-term or long-term patient survival have never been investigated before in a large international prospective cohort. Systematic evaluation of routine clinical practice and occurrence of adverse events could establish the baseline for investigating higher-priority interventions to reduce this risk.
Given these important knowledge gaps, the International Observational Study to Understand the Impact and Best Practices of Airway Management in Critically Ill Patients (INTUBE) study was developed with the following objectives: to assess incidence and types of major adverse peri-intubation events in critically ill patients, to examine factors associated with these events and to determine the association of adverse peri-intubation events with outcomes among critically ill patients.
Methods
Study Design
This was an international multicenter, prospective, cohort study. The enrollment window consisted of 8 consecutive weeks as selected by each center from October 1, 2018, to July 31, 2019. Different scientific societies and networks of critical care that had endorsed the study recruited centers by public announcement (eAppendix 1 in the Supplement). Separate ICUs from the same hospital were considered as different sites. The ethics committee of the coordinating center (Comitato Etico Brianza, No 1420 of July 31, 2018) approved the study. All participating centers obtained local ethics committee approval before study start (when required according to local regulation), with either the patient’s written consent or waiver of consent for participation. National coordinators and local investigators were responsible for the integrity and validity of data collection (eAppendix 2 and 3 in the Supplement).
Patients
Investigators included all consecutive critically ill adult patients (≥18 years) with a life-threatening impairment of the cardiovascular, respiratory, or neurological system requiring in-hospital intubation during the enrollment period at each center. Local investigators screened and reported all in-hospital emergency intubations occurring in the emergency department, ICU, and wards during the study period. Patients who underwent out-of-hospital tracheal intubation, intubation following a cardiac arrest, and patients intubated for the purposes of general anesthesia were excluded (study protocol is included in eAppendix 4 of the Supplement).
Data Collection
Local investigators screened all consecutive emergency intubations performed in a participating center during the study period. Reasons for exclusion were requested for nonenrolled patients. Centers were advised that data should be recorded in real time by an investigator not directly involved in the airway management procedure on either the paper or electronic version of the case report form on the REDCap cloud.
The case report form (eAppendix 5 in the Supplement) consisted of 7 sections: (1) enrollment; (2) demographic data and clinical characteristics; (3) intubation setting; (4) patient’s physiological parameters before intubation; (5) details of the intubation procedure; (6) outcome of intubation; and (7) status at ICU discharge.
Outcomes
The primary outcome of the study was to determine the incidence and profile of major adverse peri-intubation events, defined as the occurrence of at least 1 of the following within 30 minutes from the start of the intubation procedure: (1) severe hypoxemia (oxygen saturation as measured by pulse oximetry Spo2 <80%), (2) cardiac arrest; and (3) cardiovascular instability (systolic arterial pressure <65 mm Hg recorded at least once or systolic arterial pressure <90 mm Hg for >30 minutes; new requirement for or increase of vasopressors; or fluid bolus >15 mL/kg to maintain the target blood pressure). Patients meeting a criterion before the start of laryngoscopy (eg, Spo2 <80% at the end of preoxygenation) were not included in the outcome calculation because this was a preexisting event, not a peri-intubation event.
The secondary outcomes included the incidence of cardiac arrhythmia; difficult intubation; a cannot-intubate or cannot-oxygenate scenario; emergency front-of-neck airway; pulmonary aspiration of gastric contents; esophageal intubation; pneumothorax or pneumomediastinum; airway injury; dental injury; and ICU mortality. We calculated 28-day mortality as a post hoc analysis.
The eMethods in the Supplement provide detailed definitions and describe the procedures for data quality control.
Centers Enrollment and Sample Size Calculation
The aim of the study was to collect data on at least 1000 major adverse peri-intubation events. From a previously published report, the expected incidence of at least 1 major event (ie, severe hypoxemia, cardiovascular instability, and cardiac arrest) was 28%.7 Therefore, it was originally planned to collect data from a convenience sample of 3600 procedures. In each center, a mean intubation rate ranging from 0.5 to 2 intubations per day was considered based on the differing workloads (eg, total hospital beds, number of ICUs, and number of ICU beds) and local policies. To avoid over representation from centers with a higher admission rate, the total number of enrolled patients was limited to the first 20 consecutive cases during the enrollment window. The study plan was to recruit at least 180 centers worldwide to achieve this target.
Statistical Analysis
The characteristics of the cohort were described using a mean SD or a median interquartile range (IQR) as appropriate, if variables were continuous or with frequency and percentages if variables were categorical. Bivariable analyses were conducted using χ2 or Fisher exact test for categorical variables, and the Mann-Whitney or t test for continuous variables. A complete case analysis, with no assumptions made for missing data, was performed.
The following post hoc explorative analyses were performed to evaluate any variable associated with the development of major adverse events: a multivariable logistic regression model was performed including as covariates variables with P values <.05 in the bivariable analyses or with a clinically relevant meaning (ie, a clinically important parameter likely to lead to a physician implementing a corrective action to address it); a multivariable model of factors associated with first-pass intubation success was also developed; in addition, 28-day mortality in patients with major adverse peri-intubation events compared with those not experiencing these events was assessed. A Kaplan-Meier estimate of the survival to day 28 from the intubation was performed in order to account for censoring. The association between major adverse events and mortality adjusted for age, sex, heart failure, hematologic malignancy, ischemic heart disease, solid neoplasm, and Sequential Organ Failure Assessment score was also evaluated through a multivariable logistic regression model.
To account for clustering due to the presence of a site effect, all the analyses were implemented using a mixed-model with a random intercept for the site. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical analyses were performed with R 3.6.2 (http://www.R-project.org). All P values were 2-sided, with P values <.05 considered statistically significant.
Results
Participating ICUs and Enrolled Patients
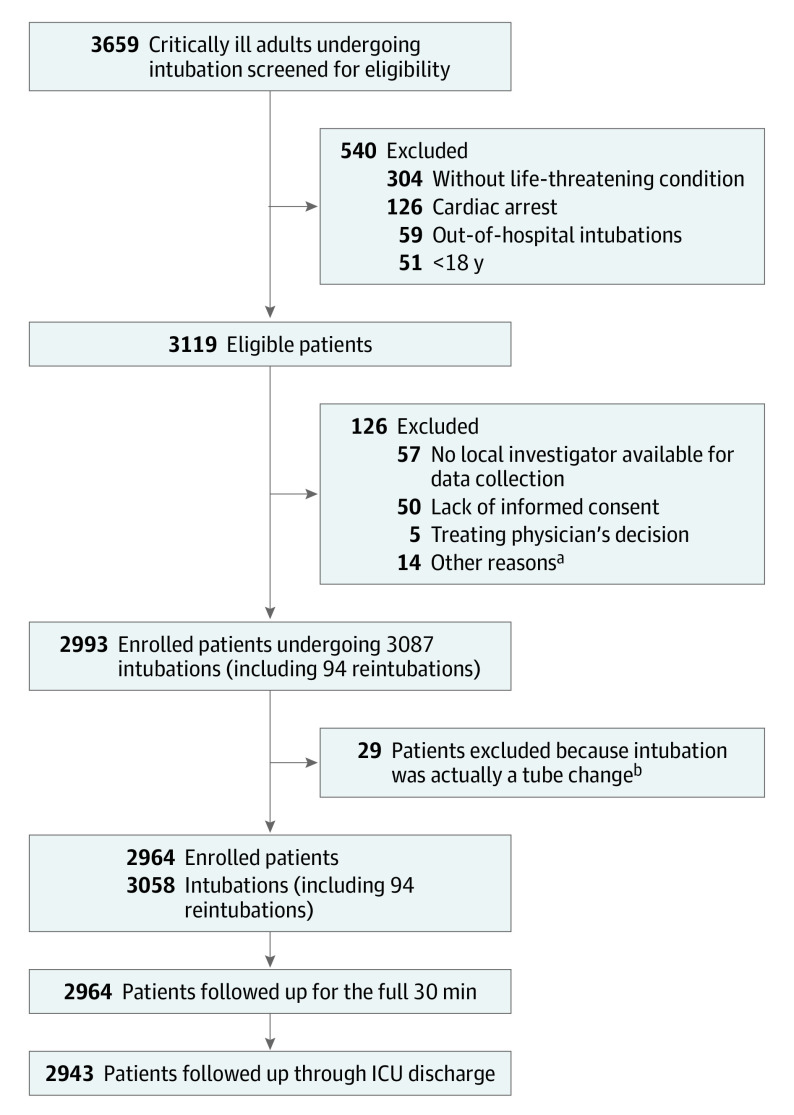
A total of 226 centers registered for participation. Following data verification and elimination of nonrecruiting sites, data from 197 centers across 29 countries worldwide were included in the final analysis (eAppendix 3 in the Supplement). Of the 3659 screened patients, 2964 were included (Figure 1). Ninety-four intubations corresponding to previously enrolled patients were excluded from the primary analysis of patients’ characteristics and peri-intubation outcomes because they were reintubations (eTable 1 in the Supplement). Table 1 and eTable 2 describe the main characteristics of included patients. The study included 1857 men (62.6%) and 1107 women (37.4%). Median age of included patients was 63 years (IQR, 49-74 years).
Figure 1. INTUBE Study Patients Flow Through Screening, Enrollment, and Follow-up .
aTen patients were excluded because their situation was too urgent for data collection; 2 required venoarterial extracorporeal membrane oxygenation; and 2 were prisoners.
bPatients were not reintubated but underwent tube change (eg, for tube obstruction or cuff rupture) using a tracheal tube exchange catheter.
Table 1. Baseline Patients’ Characteristics and Reasons for Intubation.
| Variable | No. (%) (n = 2964) |
|---|---|
| Age, median (IQR), y (n = 2963) | 63.0 (49.0-74.0) |
| Sex | |
| Men | 1857 (62.6) |
| Women | 1107 (37.4) |
| Weight, median (IQR), kg (n = 2956) | 71.2 (60.0-84.0) |
| BMI, median (IQR) (n = 2946) | 25.4 (22.5-29.4) |
| SOFA score, median (IQR)a | 7.0 (4.8-10.0) |
| Respiratory infection during previous 30 d | 288 (9.7) |
| Radiological finding | |
| Lung opacities | |
| Bilateral | 826 (27.9) |
| Unilateral | 364 (12.3) |
| Pleural effusion | 403 (13.6) |
| Otherb | 266 (9.0) |
| Respiratory support prior to intubation (n = 2443) | |
| Standard oxygen | 1509 (61.8) |
| Noninvasive ventilation | 521 (21.3) |
| High-flow nasal cannula | 313 (12.8) |
| Continuous positive airway pressure | 100 (4.1) |
| Pao2/Fio2, median (IQR) (n = 1780) | 165.0 (100.0-265.0) |
| Spo2/Fio2, median (IQR) (n = 2372)c | 165.7 (105.6-261.1) |
| Receiving vasopressor or inotropic support | 769 (25.9) |
| Fluid bolus 30 min before intubation, No./total (%)d | 1065/2827 (37.7) |
| Blood pressure, mean (SD), mm Hg (n = 2959) | |
| Systolic | 126.3 (35.7) |
| Diastolic | 70.0 (20.7) |
| Heart rate, mean (SD), beats/min (n = 2960) | 103.7 (26.2) |
| Location of intubation | |
| ICU | 1992 (67.2) |
| Emergency department | 623 (21.0) |
| Medical ward | 186 (6.3) |
| Surgical ward | 69 (2.3) |
| Othere | 94 (3.2) |
| Reason for intubation (n = 2960) | |
| Respiratory failure | 1548 (52.3) |
| Neurological impairment | 902 (30.5) |
| Cardiovascular instability | 277 (9.4) |
| Airway obstruction | 137 (4.6) |
| Emergency or urgent procedure | 29 (1.0) |
| Otherf | 67 (2.2) |
| Degree of emergency (n = 2962) | |
| Tracheal intubation required | |
| Without any delay | 1536 (51.9) |
| <1 h | 1065 (35.9) |
| ≥1 h | 361 (12.2) |
| ≥1 Anatomical reason to anticipate a difficult airway (n = 2798) | 1308 (46.8) |
| MACOCHA score ≥3g | 426 (14.4) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; Fio2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; Spo2, oxygen saturation as measured by pulse oximetry.
Scores were calculated with last values before intubation, and missing data omitted were adjusted accordingly.
Included pulmonary contusion, rib fracture(s), pneumothorax, hemothorax, signs of pulmonary hyperinflation or emphysema, and pulmonary congestion or cardiomegaly.
Reported only when Spo2 was 98% or less.
Any fluid bolus administered 30 minutes preceding intubation to reach or maintain the hemodynamic goals according to clinical judgment.
Included the postoperative recovery room, cardiology, radiology, and endoscopy interventional rooms.
Included inadequate reversal of neuromuscular block and self-extubation.
Predicts difficult intubation in the ICU. Its calculation includes Mallampati score III and IV (5 points), obstructive sleep apnea syndrome (2 points), reduced mobility of the cervical spine (1 point), limited mouth opening less than 3 cm (1 point), coma (1 point), severe hypoxemia (1 point), nonanesthesiologist operator (1 point) (range, 0, easy intubation-12, very difficult intubation).11
Tracheal Intubation Setting and Clinician’s Characteristics
The main reason for tracheal intubation was respiratory failure in 1548 patients (52.3%), followed by neurological impairment in 902 patients (30.5%), and cardiovascular instability in 277 cases (9.4%; Table 1)
Resident physicians intubated 1536 patients (51.9%), and anesthesiologists intubated 1601 patients (54.0%; eTable 3 in the Supplement).
Primary Outcome
Of the critically ill patients undergoing tracheal intubation, 1340 (45.2%) experienced at least 1 major adverse peri-intubation event (Table 2; eTable 4 in the Supplement).
Table 2. Peri-intubation Adverse Events.
| Adverse events | No./Total (%) |
|---|---|
| Major adverse events (primary outcome) | 1340/2964 (45.2) |
| Cardiovascular instability | 1172/2753 (42.6) |
| New need or increase of vasopressors | 1053/1172 (89.9) |
| Systolic pressure <90 mm Hg for >30 min | 252/1026 (24.6) |
| Fluid bolus >15 mL/kg | 151/1163 (13.5) |
| Systolic pressure <65 mm Hg | 157/1163 (13.5) |
| Severe hypoxia (lowest Spo2<80%) | 272/2916 (9.3) |
| Cardiac arrest | 93/2964 (3.1) |
| With return of spontaneous circulation | 49/93 (52.7) |
| With death | 44/93 (47.3) |
| Cause of cardiac arresta | |
| Hypovolemia or hemodynamic instability | 34/92 (36.9) |
| Hypoxia | 23/92 (25.0) |
| Thrombosis (coronary or pulmonary) | 19/92 (20.6) |
| Hypokalemia or hyperkalemia | 3/92 (3.3) |
| Cardiac tamponade | 3/92 (3.3) |
| Toxins | 2/92 (2.2) |
| Tension pneumothorax | 2/92 (2.2) |
| Otherb | 6/92 (6.5) |
| Other adverse events | |
| Esophageal intubation | 167/2959 (5.6) |
| New onset cardiac arrhythmia | 167/2960 (5.6) |
| Atrial fibrillation | 48/167 (28.7) |
| Ventricular tachycardia | 41/167 (24.6) |
| Bradycardia | 38/167 (22.8) |
| Otherc | 40/167 (23.9) |
| Difficult intubationd | 138/2957 (4.7) |
| Aspiration of gastric contentse | 116/2960 (3.9) |
| Dental injury | 28/2960 (1.0) |
| Pneumothorax | 22/2963 (0.7) |
| Airway injury | 21/2959 (0.7) |
| Tracheal laceration | 5/21 (23.8) |
| Bronchial laceration | 1/21 (4.8) |
| Laryngeal laceration | 7/21 (33.3) |
| Otherf | 8/21 (38.1) |
| Pneumomediastinum | 8/2960 (0.3) |
Abbreviation: Spo2, oxygen saturation as measured by pulse oximetry.
Cause of cardiac arrest described which, according to clinical judgment, was the main reason for the cardiac arrest.
Included severe cardiomyopathy and unknown cause.
Included supraventricular tachyarrhythmia and multiple ventricular ectopic beats.
Defined as a procedure requiring more than 2 laryngoscopy attempts before success. See Results section for difficult intubation definition.
Inhalation of oropharyngeal or gastric contents into the larynx and the respiratory tract within the first 24 hours after intubation according to clinical and/or radiographic findings.
Included pharyngeal injury and bleeding through the tracheal tube from unclear origin.
The primary outcome was observed in 778 of 1548 patients (50.3%) intubated for respiratory failure; 178 of 277 (64.3%) for hemodynamic instability (absolute difference with respect to respiratory failure, 14.0%; 95% CI, 7.6% to 20.4%) and in 295 of 902 (32.7%) with neurological impairment (absolute difference with respect to respiratory failure, −17.6%; 95% CI, −21.6% to −13.5%). Data for the composite outcome calculation was available for all patients. eTable 5 in the Supplement shows missing data for each single major adverse event.
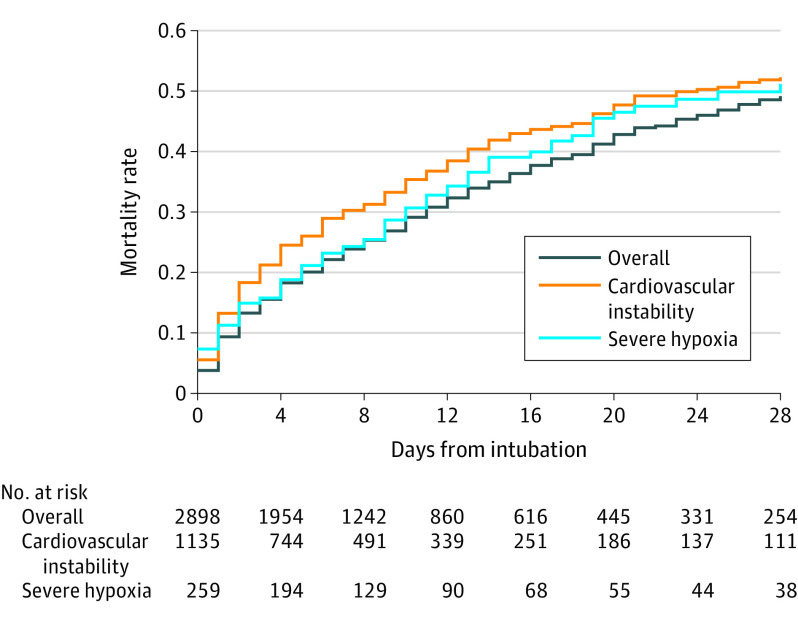
Cardiovascular instability accounted for the majority of the events, occurring in 1172 (42.6%) of all patients. Severe hypoxemia was the second most common event, observed in 272 patients (9.3%). Ninety-three patients (3.1%) had a cardiac arrest following tracheal intubation (Figure 2). Of these, 49 patients (52.7%) had a sustained return of spontaneous circulation, and 44 (47.3%) died following cardiac arrest. The main reported reason for cardiac arrest was hypovolemia or hemodynamic instability in 34 patients (36.9%), followed by hypoxemia in 23 (25.0%).
Figure 2. Mortality Rate by Days From Intubationa.
aMedian time of observation in the overall population was 6.0 days (interquartile range, 2.0-13.0 days).
Secondary Outcomes
Of the secondary outcomes, 167 patients (5.6%) had an esophageal intubation; 167 (5.6%), new onset cardiac arrhythmia; 138 (4.7%), difficult intubation; and 116 (3.9%), aspiration of gastric contents (Table 2; eFigure 1 in the Supplement). A total of 2943 patients were followed up through ICU discharge. The overall ICU mortality was 32.8% (966 of 2943). Of those who experienced a major adverse peri-intubation event, 40.7% of patients (541 of 1328) died vs 26.3% (425 of 1615) who did not experience an adverse peri-intubation event (absolute risk difference, 14.4%; 95% CI, 10.9%-17.9%; P < .001).
Tracheal Intubation Practices and Success Rates
Of the study patients, 1847 (62.4%) received preoxygenation by a bag-valve mask, the most frequently used method. Noninvasive ventilation was used for 344 patients (11.6%) and high-flow nasal cannula for 160 patients (5.4%; Table 3; eTable 2 in the Supplement).
Table 3. Techniques, Medications, and Confirmations of Intubations.
| Variable | No. (%) (n = 2964) |
|---|---|
| Application of an airway management protocol | |
| Standard protocol | |
| In place and used | 1510 (51.0) |
| In place and not useda | 443 (15.0) |
| No standard protocol in place | 1009 (34.0) |
| Preoxygenation method (n = 2960) | |
| Bag-valve mask | 1847 (62.4) |
| Standard facemask | 389 (13.2) |
| Noninvasive ventilation | 344 (11.6) |
| High-flow nasal cannula | 160 (5.4) |
| Anesthesia breathing circuitb | 56 (1.9) |
| Continuous positive airway pressure | 51 (1.7) |
| Venturi system | 47 (1.6) |
| Nasal cannula | 47 (1.6) |
| Otherc | 19 (0.6) |
| Apneic oxygenation, No./total (%)d | 308/2959 (10.4) |
| Rapid sequence induction, No./total (%)e | 1727/2777 (62.2) |
| Cricoid pressure, No./total (%) | 1120/2956 (37.9) |
| Induction agent, No./total (%)f | 2774/2964 (93.6) |
| Propofol | 1230 (41.5) |
| Midazolam | 1079 (36.4) |
| Etomidate | 527 (17.8) |
| Ketamine | 421 (14.2) |
| Muscle relaxant use, No./total (%) | 2095/2776 (75.5) |
| Rocuronium | 1239 (41.8) |
| Succinylcholine | 646 (21.8) |
| Vecuronium | 95 (3.2) |
| Cisatracurium | 85 (2.9) |
| Opioid use for intubation, No./total (%) | 1415/2776 (51.0) |
| Method of laryngoscopy (n = 2963) | |
| Direct laryngoscopy with Macintosh or Miller blade | 2416 (81.5) |
| Video laryngoscopy | 505 (17.1) |
| Other methodg | 42 (1.4) |
| Use of intubation adjuncts (n = 1055) | |
| Stylet | 816 (77.4) |
| Bougie | 230 (21.8) |
| Otherh | 9 (0.8) |
| First method used to confirm intubation (n = 2956) | |
| Auscultation | 1711 (57.9) |
| Waveform capnographyi | 758 (25.6) |
| Colorimetric carbon dioxide detectionj | 222 (7.5) |
| Capnometryk | 138 (4.7) |
| None | 7 (0.2) |
| Otherl | 120 (4.1) |
| Success, No./total (%) | |
| First pass | 2360/2958 (79.8) |
| Second pass | 460/2958 (15.6) |
| Emergency front-of-neck accessm | 4 (0.13) |
Standard protocol was not used in intensive care unit (57.3%), emergency department (26.6%), ward (11.5%), and other places (4.51%), including recovery, cardiology, radiology, and endoscopy interventional rooms.
Anesthesia breathing circuits (eg, Mapleson C) are used outside the operating room in some centers instead of self-inflating bags (bag-valve mask). While they require a source of oxygen to work, they provide a lower resistance alternative in spontaneously breathing patients.
Included invasive mechanical ventilation (for patients with self-extubation) and preoxygenation via bag-valve and an extraglottic airway device.
Oxygen administration during laryngoscopy or fiberoscopy.
Rapid onset induction without positive pressure ventilation between induction and laryngoscopy.
Proportion of patients receiving each subcategory of induction agent. Some patients received more than 1 induction drug while others received an opioid as induction agent or underwent awake fiberoptic intubation under local anesthesia.
Included direct laryngoscopy with McCoy blade and fiberoptic intubation. Nasotracheal intubations were performed in 0.8% of patients.
Included tube exchange catheter, lighted stylet, and Magill forceps.
Monitor provided the graphic measurement of exhaled carbon dioxide plotted against time.
Device uses a photochemical reaction to detect the presence of carbon dioxide in the exhaled air.
Provides only the absolute value of carbon dioxide concentration in the exhaled air.
Included chest x-ray and fiberoscopy.
One cricothyroidotomy, 1 percutaneous tracheostomy, and 2 surgical tracheostomies.
Of the study patients, 1727 (62.2%) underwent rapid sequence induction (ie, no ventilation between induction and laryngoscopy). Propofol, the most frequently used induction agent, was given to 1230 patients (41.5%). When administered to patients with concurrent hemodynamic instability, propofol was significantly associated with cardiovascular instability among 128 of 201 patients (63.7%) compared with 47 of 95 patients (49.5%) receiving etomidate (absolute difference, 14.2%; 95% CI, 1.4%-27.0%; P = .02).
A neuromuscular blocking agent was administered to 2095 patients (75.5%), with rocuronium, the most commonly administered drug, administered to 1239 patients (41.8%), followed by succinylcholine to 646 patients (21.8%).
A video laryngoscope was used as the primary device for tracheal intubation for 505 patients (17.1%), of those 302 (59.8%) who had undergone video laryngoscopy had at least 1 predictor of difficult airway management identified prior to intubation.
First-pass intubation success was achieved for 2360 of 2958 patients (79.8%). A second attempted intubation was achieved for 460 patients (15.6%), and 133 patients (4.5%) required more than 2 attempts. Of the 5 patients (0.17%) who were not able to be intubated, 1 patient required supraglottic airway insertion and 4 patients required front-of-neck airway (1 cricothyroidotomy, 1 percutaneous tracheostomy, and 2 emergency surgical tracheostomies).
The rate of major adverse events was significantly lower with first-pass intubation success than it was for patients requiring 2 attempts (43.2% vs 51.5%; absolute difference, 8.4%; 95% CI, 3.3%-13.5%; P = .001) and for patients requiring 3 or more attempts (43.2% vs 58.0%; absolute difference, 14.2%; 95% CI, 5.2%-23.1%; P < .001; eFigure 2 in the Supplement).
Waveform capnography was used for 1025 patients (34.5%) during intubation. Of those, 797 (40.0%) were in the ICU, 145 (23.3%) in the emergency department, and 18 (6.9%) in a ward. The absolute difference between those treated in the ICU vs those in the emergency department was −16.7% (95% CI, −20.8% to −12.7%) and between those in the ICU and those in a ward, −33.1% (95% CI, −37.1% to −29.1%). Capnography was not used for 115 patients (68.9%) who had undergone esophageal intubation.
Post Hoc Exploratory Analyses
In a multivariable analysis, several patient and setting-related variables significantly associated with major adverse peri-intubation events were identified (eTable 6 in the Supplement): older age (odds ratio [OR], 1.01; 95% CI, 1.01-1.02), past history of heart failure (OR, 1.52; 95% CI, 1.06-2.18), hematologic malignancy (OR, 1.61; 95% CI, 1.01-2.56), lower systolic arterial pressure (OR, 0.991; 95% CI, 0.986-0.995), administration of a fluid bolus before intubation (OR, 1.26; 95% CI, 1.005-1.572), higher heart rate (OR, 1.01; 95% CI, 1.00-1.01), cardiovascular instability as reason for tracheal intubation (OR, 1.87; 95% CI, 1.22-2.86), high risk of pulmonary aspiration (OR, 1.39; 95% CI, 1.04-1.86), use of a video laryngoscope (OR, 1.36; 95% CI, 1.00-1.84), and lower Spo2/Fio2 ratio (OR, 0.998; 95% CI, 0.997-0.999). First-pass intubation success was significantly associated with a reduced likelihood of major adverse peri-intubation events (OR, 0.59; 95% CI, 0.45-0.76).
In another post hoc multivariable analysis, variables significantly associated with first-pass intubation failure were identified (eTable 7 in the Supplement): being an attending physician or consultant vs being in training (OR, 0.52; 95% CI, 0.40-0.69), having anesthesia as primary specialty (OR, 0.53; 95% CI, 0.41-0.69), and the use of a video laryngoscope (OR, 0.60; 95% CI, 0.42-0.85) were significantly associated with a reduced likelihood of first-pass intubation failure. In contrast, first-pass intubation failure was significantly associated with Mallampati class III and IV (OR, 1.55; 95% CI, 1.03-2.33), reduced mouth opening (OR, 2.27; 95% CI, 1.56-3.31), neck stiffness (OR, 2.01; 95% CI, 1.31-3.09), presence of beard (OR, 1.77; 95% CI, 1.12-2.79), high risk of aspiration of gastric contents (OR, 1.40; 95% CI, 1.04-1.88), past surgery or radiotherapy on neck and airways (OR, 6.83; 95% CI, 2.72-17.2), presence of any other predictor of difficult airway management (OR, 1.91; 95% CI, 1.13-3.22), and the degree of urgency of intubation—intubation required in less than 1 hour vs intubation required as soon as possible after presentation—(OR, 1.28; 95% CI, 1.00-1.63).
In multivariable regression, after adjusting for baseline patient characteristics, the occurrence of a major adverse peri-intubation event was significantly associated with ICU mortality with an adjusted OR of 1.52 (95% CI, 1.26-1.83, P < .001), eTable 8 in the Supplement.
Data on 28-day mortality was available for 2942 patients. Overall 28-day mortality was 30.5% (897 of 2942) of patients, with a mortality of 37.7% (500 of 1327) of patients with a major adverse peri-intubation event and 24.6% (397 of 1615) of patients without events for an absolute difference of 13.1% (95% CI, 9.5%-16.3%, P < .001, eFigure 3 in the Supplement). In multivariable regression, after adjustment for baseline patient characteristics (reported in 2899 cases), the occurrence of a major adverse peri-intubation event was significantly associated with 28-day mortality with an adjusted OR of 1.44 (95% CI, 1.19-1.74; P < .001; eTable 9 in the Supplement).
Discussion
In this international observational study of intubation practices and peri-intubation morbidity in 197 sites across 29 countries, major complications—in particular cardiovascular instability—were observed frequently.
A key finding of this study was the identification of cardiovascular instability as the most frequent adverse event following intubation. Evidence for interventions aiming to achieve cardiovascular stability before tracheal intubation is currently limited. In a before-and-after study investigating the effectiveness of a 10-item bundle of interventions to reduce complications from intubation, the authors reported that its implementation (intervention period) was associated with a relative reduction of 50% of cardiovascular collapse and severe hypoxemia compared with their incidence registered during the baseline period. This bundle comprised the preinduction administration of 500 mL of crystalloids and early start of norepinephrine in case of low diastolic pressure following intubation.12 It was not possible, however, to evaluate the contribution of the individual hemodynamic components of the bundle given the concurrent implementation of other interventions. In a recent study, Janz and colleagues13 randomized critically ill patients to receive a 500-mL bolus of crystalloids or no bolus before intubation. The trial was stopped for futility, and the authors did not identify any benefit from crystalloids administration. A benefit was detected, instead, in the subgroup of patients receiving positive pressure ventilation (either via bag-mask ventilation or noninvasive ventilation), while potential harm was detected in the rest of the population. Further studies are required to investigate interventions to limit peri-intubation cardiovascular instability.
This study confirmed the importance of achieving first-pass intubation success given the higher incidence of adverse events associated with repeated intubation attempts.14 The need for multiple intubation attempts increased the risk of severe hypoxia and cardiac arrest. A high level of expertise in tracheal intubation is paramount to reduce the need for repeated intubation attempts. Indeed, staff physicians and anesthesiologists more frequently achieved first-pass intubation success than did residents and other clinicians with different specialty backgrounds. These findings emphasize the importance of experience in airway management and of measures to enhance tracheal intubation skills, along with proficiency in hemodynamic optimization.15
The role of video laryngoscopy to facilitate tracheal intubation in critically ill patients remains unclear. A recent meta-analysis16 of randomized studies comparing video laryngoscopy with direct laryngoscopy for intubation in critically ill patients, showed that video laryngoscopy did not shorten the time to intubation nor improve first-pass success rates, irrespective of the operator’s experience. Trials evaluating the effectiveness of video laryngoscopy in critically ill patients, the use of associated devices (bougie or stylet), the glottic view achieved (full vs partial), and appropriate patient position during tracheal intubation may more clearly define its role in this high-risk setting.17
Ketamine and etomidate have been recommended as the induction agent of choice for intubation of critically ill patients, given their more favorable hemodynamic effect.18 In this study, however, ketamine and etomidate were seldom used, with propofol still representing the most commonly used induction agent. Another finding of the present study was the low use of waveform capnography as standard monitoring during tracheal intubation. In 68.9% of patients with tracheal tube accidentally placed in the esophagus, waveform capnography was not in place, so clinicians relied on inaccurate clinical signs such as auscultation or chest movement for detection of esophageal intubation.19,20 In the National Audit Project 4 report, a national audit of the complications of airway management in hospitals across the UK, capnography was not used in 75% to 100% of unrecognized esophageal intubations and contributed to 77% avoidable deaths related to tracheal intubation among critically ill patients.9 The present study focused on major adverse peri-intubation events and no deaths could be directly attributed to lack of capnography. Nevertheless, 10 years after the publication of the National Audit Project 4 report, this study reported an underuse of capnography to confirm tracheal intubation in the critically ill.9,21
Limitations
This study has several limitations. First, direct access to the source data was not available. Given the self-reporting nature of adverse events, this may have led to an overestimation of some conditions (eg, patient’s severity or airway management difficulties) or alternatively underestimation of procedure-related adverse events. Second, not all patients may have been enrolled leading to a selection bias. Third, a selection bias in participating centers may have occurred, limiting generalizability of findings. However, participating hospitals were representative of different levels of care and geography. Fourth, interpretation of results may be biased by residual or unmeasured confounders. Despite adjustment for disease severity, it may be possible that residual confounders influenced the higher incidence and severity of adverse events in some subgroups of critically ill patients. Fifth, this study did not collect information on direct long-term consequences of adverse peri-intubation events on specific patient’s outcomes (eg, hypoxic brain injury). However, the aim of the study was to prospectively collect data on immediate adverse events.
Conclusions
In this observational study of intubation practices in critically ill patients from a convenience sample of 197 sites across 29 countries, major adverse peri-intubation events—in particular, cardiovascular instability—were observed frequently.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
eMethods. Variables and outcomes definitions, data quality control
eTable 1. Outcomes of patients’ undergoing reintubations
eTable 2. Patients’ characteristics, reason for intubation and procedure description in patients with and without peri-intubation major adverse events
eTable 3. Center, operator’s characteristics and intubation setting
eTable 4. Outcomes distribution according to the geographical areas of participating centers
eTable 5. Missing values for the study outcomes
eFigure 1. Incidence of peri-intubation major adverse events and other peri-intubation events
eFigure 2. Incidence of peri-intubation adverse events according to the number of attempts
eFigure 3. Kaplan-Meier survival curves by peri-intubation major adverse events, cardiovascular instability, cardiac arrest, and severe hypoxia
eTable 6. Multivariable analysis of organizational, operator, and patient factors associated with major peri-intubation adverse events
eTable 7. Multivariable analysis of patient and operator factors associated with first pass intubation failure
eTable 8. Adjusted ICU mortality
eTable 9. Adjusted mortality at 28 days after intubation
eAppendix 1. List of endorsing Scientific Societies/Networks endorsing the study
eAppendix 2. National Coordinators of INTUBE Study
eAppendix 3. List of INTUBE study centers with local investigators.
eAppendix 4. Study protocol.
eAppendix 5. Case report form
References
- 1.Russotto V, Myatra SN, Laffey JG. What’s new in airway management of the critically ill. Intensive Care Med. 2019;45(11):1615-1618. doi: 10.1007/s00134-019-05757-0 [DOI] [PubMed] [Google Scholar]
- 2.Russotto V, Cortegiani A, Raineri SM, Gregoretti C, Giarratano A. Respiratory support techniques to avoid desaturation in critically ill patients requiring endotracheal intubation: a systematic review and meta-analysis. J Crit Care. 2017;41:98-106. doi: 10.1016/j.jcrc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Sakles JC, Pacheco GS, Kovacs G, Mosier JM. The difficult airway refocused. Br J Anaesth. 2020;125(1):e18-e21. doi: 10.1016/j.bja.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Mosier JM. Physiologically difficult airway in critically ill patients: winning the race between haemoglobin desaturation and tracheal intubation. Br J Anaesth. 2020;125(1):e1-e4. doi: 10.1016/j.bja.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Mosier JM, Sakles JC, Law JA, Brown CA III, Brindley PG. Tracheal intubation in the critically ill. where we came from and where we should go. Am J Respir Crit Care Med. 2020;201(7):775-788. doi: 10.1164/rccm.201908-1636CI [DOI] [PubMed] [Google Scholar]
- 6.Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017;43(2):226-228. doi: 10.1007/s00134-016-4426-0 [DOI] [PubMed] [Google Scholar]
- 7.Jaber S, Amraoui J, Lefrant J-Y, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006;34(9):2355-2361. doi: 10.1097/01.CCM.0000233879.58720.87 [DOI] [PubMed] [Google Scholar]
- 8.Griesdale DEG, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34(10):1835-1842. doi: 10.1007/s00134-008-1205-6 [DOI] [PubMed] [Google Scholar]
- 9.Cook TM, Woodall N, Harper J, Benger J; Fourth National Audit Project . Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society, II: intensive care and emergency departments. Br J Anaesth. 2011;106(5):632-642. doi: 10.1093/bja/aer059 [DOI] [PubMed] [Google Scholar]
- 10.De Jong A, Rolle A, Molinari N, et al. Cardiac arrest and mortality related to intubation procedure in critically ill adult patients: a multicenter cohort study. Crit Care Med. 2018;46(4):532-539. doi: 10.1097/CCM.0000000000002925 [DOI] [PubMed] [Google Scholar]
- 11.De Jong A, Molinari N, Terzi N, et al. ; AzuRéa Network for the Frida-Réa Study Group . Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med. 2013;187(8):832-839. doi: 10.1164/rccm.201210-1851OC [DOI] [PubMed] [Google Scholar]
- 12.Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36(2):248-255. doi: 10.1007/s00134-009-1717-8 [DOI] [PubMed] [Google Scholar]
- 13.Janz DR, Casey JD, Semler MW, et al. ; PrePARE Investigators; Pragmatic Critical Care Research Group . Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): a randomised controlled trial. Lancet Respir Med. 2019;7(12):1039-1047. doi: 10.1016/S2213-2600(19)30246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jong A, Rolle A, Pensier J, Capdevila M, Jaber S. First-attempt success is associated with fewer complications related to intubation in the intensive care unit. Intensive Care Med. 2020;46(6):1278-1280. doi: 10.1007/s00134-020-06041-2 [DOI] [PubMed] [Google Scholar]
- 15.Mosier JM, Malo J, Sakles JC, et al. The impact of a comprehensive airway management training program for pulmonary and critical care medicine fellows: a three-year experience. Ann Am Thorac Soc. 2015;12(4):539-548. doi: 10.1513/AnnalsATS.201501-023OC [DOI] [PubMed] [Google Scholar]
- 16.Cabrini L, Landoni G, Baiardo Redaelli M, et al. Tracheal intubation in critically ill patients: a comprehensive systematic review of randomized trials. Crit Care. 2018;22(1):6-9. doi: 10.1186/s13054-017-1927-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaber S, De Jong A, Pelosi P, Cabrini L, Reignier J, Lascarrou J-B. Videolaryngoscopy in critically ill patients. Crit Care. 2019;23(1):221-227. doi: 10.1186/s13054-019-2487-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgs A, McGrath BA, Goddard C, et al. ; Difficult Airway Society; Intensive Care Society; Faculty of Intensive Care Medicine; Royal College of Anaesthetists . Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120(2):323-352. doi: 10.1016/j.bja.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 19.Holland R, Webb RK, Runciman WB. The Australian Incident Monitoring Study. Oesophageal intubation: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21(5):608-610. doi: 10.1177/0310057X9302100519 [DOI] [PubMed] [Google Scholar]
- 20.Mort TC. Esophageal intubation with indirect clinical tests during emergency tracheal intubation: a report on patient morbidity. J Clin Anesth. 2005;17(4):255-262. doi: 10.1016/j.jclinane.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 21.Cook TM, Woodall N, Frerk C. A national survey of the impact of NAP4 on airway management practice in United Kingdom hospitals: closing the safety gap in anaesthesia, intensive care and the emergency department. Br J Anaesth. 2016;117(2):182-190. doi: 10.1093/bja/aew177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Variables and outcomes definitions, data quality control
eTable 1. Outcomes of patients’ undergoing reintubations
eTable 2. Patients’ characteristics, reason for intubation and procedure description in patients with and without peri-intubation major adverse events
eTable 3. Center, operator’s characteristics and intubation setting
eTable 4. Outcomes distribution according to the geographical areas of participating centers
eTable 5. Missing values for the study outcomes
eFigure 1. Incidence of peri-intubation major adverse events and other peri-intubation events
eFigure 2. Incidence of peri-intubation adverse events according to the number of attempts
eFigure 3. Kaplan-Meier survival curves by peri-intubation major adverse events, cardiovascular instability, cardiac arrest, and severe hypoxia
eTable 6. Multivariable analysis of organizational, operator, and patient factors associated with major peri-intubation adverse events
eTable 7. Multivariable analysis of patient and operator factors associated with first pass intubation failure
eTable 8. Adjusted ICU mortality
eTable 9. Adjusted mortality at 28 days after intubation
eAppendix 1. List of endorsing Scientific Societies/Networks endorsing the study
eAppendix 2. National Coordinators of INTUBE Study
eAppendix 3. List of INTUBE study centers with local investigators.
eAppendix 4. Study protocol.
eAppendix 5. Case report form