Key Points
Question
What effect does continued treatment with 2.4 mg of subcutaneous semaglutide have on the maintenance of body weight loss in adults with overweight or obesity without diabetes?
Findings
In this randomized clinical trial of adults with overweight or obesity, 803 participants completed a 20-week run-in of weekly treatment with subcutaneous semaglutide, 2.4 mg, with a mean weight loss of 10.6%, and were randomized to continued treatment with subcutaneous semaglutide vs placebo for an additional 48 weeks. At the end of this time, mean weight change was −7.9% vs +6.9%, respectively, a difference that was statistically significant.
Meaning
Among adults with overweight or obesity completing a 20-week run-in period, maintaining treatment with subcutaneous semaglutide compared with switching to placebo resulted in continued weight loss.
Abstract
Importance
The effect of continuing vs withdrawing treatment with semaglutide, a glucagon-like peptide 1 receptor agonist, on weight loss maintenance in people with overweight or obesity is unknown.
Objective
To compare continued once-weekly treatment with subcutaneous semaglutide, 2.4 mg, with switch to placebo for weight maintenance (both with lifestyle intervention) in adults with overweight or obesity after a 20-week run-in with subcutaneous semaglutide titrated to 2.4 mg weekly.
Design, Setting, and Participants
Randomized, double-blind, 68-week phase 3a withdrawal study conducted at 73 sites in 10 countries from June 2018 to March 2020 in adults with body mass index of at least 30 (or ≥27 with ≥1 weight-related comorbidity) and without diabetes.
Interventions
A total of 902 participants received once-weekly subcutaneous semaglutide during run-in. After 20 weeks (16 weeks of dose escalation; 4 weeks of maintenance dose), 803 participants (89.0%) who reached the 2.4-mg/wk semaglutide maintenance dose were randomized (2:1) to 48 weeks of continued subcutaneous semaglutide (n = 535) or switched to placebo (n = 268), plus lifestyle intervention in both groups.
Main Outcomes and Measures
The primary end point was percent change in body weight from week 20 to week 68; confirmatory secondary end points were changes in waist circumference, systolic blood pressure, and physical functioning (assessed using the Short Form 36 Version 2 Health Survey, Acute Version [SF-36]).
Results
Among 803 study participants who completed the 20-week run-in period (with a mean weight loss of 10.6%) and were randomized (mean age, 46 [SD, 12] years; 634 [79%] women; mean body weight, 107.2 kg [SD, 22.7 kg]), 787 participants (98.0%) completed the trial and 741 (92.3%) completed treatment. With continued semaglutide, mean body weight change from week 20 to week 68 was −7.9% vs +6.9% with the switch to placebo (difference, −14.8 [95% CI, −16.0 to −13.5] percentage points; P < .001). Waist circumference (−9.7 cm [95% CI, −10.9 to −8.5 cm]), systolic blood pressure (−3.9 mm Hg [95% CI, −5.8 to −2.0 mm Hg]), and SF-36 physical functioning score (2.5 [95% CI, 1.6-3.3]) also improved with continued subcutaneous semaglutide vs placebo (all P < .001). Gastrointestinal events were reported in 49.1% of participants who continued subcutaneous semaglutide vs 26.1% with placebo; similar proportions discontinued treatment because of adverse events with continued semaglutide (2.4%) and placebo (2.2%).
Conclusions and Relevance
Among adults with overweight or obesity who completed a 20-week run-in period with subcutaneous semaglutide, 2.4 mg once weekly, maintaining treatment with semaglutide compared with switching to placebo resulted in continued weight loss over the following 48 weeks.
Trial Registration
ClinicalTrials.gov Identifier: NCT03548987
This randomized trial compares the effects of continuing weekly treatment with subcutaneous semaglutide vs switching to placebo on change in body weight over 48 weeks among adults with overweight or obesity who completed a 20-week run-in period with semaglutide.
Introduction
Obesity is a chronic, relapsing disease with a substantial burden on individuals, society, and the economy.1 Maintaining long-term weight loss is challenging because of metabolic adaptation2 and the difficulty of adhering to lifestyle interventions,3 with weight regain often following weight loss.4
Sustained weight loss of 5% to 15% is advised to improve many conditions associated with overweight/obesity, with adjunctive pharmacotherapy recommended to help achieve this goal.5,6 However, approved antiobesity medications have only moderate efficacy (3%-8% body weight reduction beyond lifestyle intervention alone).7,8,9 Short-term treatment (3-6 months) fails to produce long-term health benefits,5 while several agents also have safety concerns.7,8,9 Well-tolerated new therapies that can produce substantial, sustained weight loss, when used long term, are therefore needed.7,10
Subcutaneous semaglutide is a glucagon-like peptide 1 (GLP-1) receptor agonist approved for the treatment of type 2 diabetes at doses of 1.0 mg or less once weekly.11 Weight loss with semaglutide is believed to stem from improved appetite control, and consequent reduced energy intake, via effects in the hypothalamus and area postrema of the brain.12,13 Once-weekly subcutaneous semaglutide, 2.4 mg, is being investigated for the treatment of overweight/obesity in the global phase 3 Semaglutide Treatment Effect in People With Obesity (STEP) program.10 This dose, which is greater than what is currently approved for type 2 diabetes, was chosen based on a phase 2 clinical trial, in which greater weight loss was seen with once-daily semaglutide, 0.4 mg (equal to 2.8 mg/wk), vs current approved medical therapy.14 Proposed to be more clinically convenient, weekly administration was supported by a tolerability trial and pharmacokinetic modeling.10,15
The STEP 4 withdrawal trial was conducted to compare the effect of continuing once-weekly treatment with subcutaneous semaglutide, 2.4 mg, vs switching to placebo (both with lifestyle intervention) on body weight in participants with overweight/obesity who reached a semaglutide treatment dosage of 2.4 mg once weekly during an initial 20-week run-in.
Methods
Trial Design and Oversight
This trial was a 68-week, randomized, double-blind, placebo-controlled withdrawal study conducted at 73 sites in 10 countries (eAppendix 1 in Supplement 1) from June 2018 to March 2020. The protocol and amendments (see trial protocol in Supplement 2 and statistical analysis plan in Supplement 3) were approved by an independent ethics committee or institutional review board at each site. The study was conducted according to the International Council for Harmonisation Good Clinical Practice Guideline and the Declaration of Helsinki16; all participants provided written informed consent.
Participants
Adults (≥18 years old) with at least 1 self-reported unsuccessful dietary effort to lose weight and with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 30 or higher or a BMI of 27 or higher with at least 1 treated or untreated weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, cardiovascular disease; type 2 diabetes was excluded) were enrolled. Key exclusion criteria were a hemoglobin A1c of 6.5% (48 mmol/mol) or greater and a self-reported change in body weight of more than 5 kg within 90 days of screening. Full eligibility criteria are shown in eAppendix 2 in Supplement 1. To meet regulatory requirements, race and ethnicity were recorded in this study and were determined by each participant according to fixed selection categories (with the option of answering “other,” “not applicable,” or “unknown”). Participants eligible for randomization at week 20 had to have attained the target maintenance dose of semaglutide (2.4 mg once weekly) by week 16 and have continued taking this dose until week 20.
Procedures
All participants initially received open-label once-weekly subcutaneous semaglutide, 0.25 mg, increased every 4 weeks to the maintenance dose of 2.4 mg once weekly by week 16, and continued to week 20 (run-in period; eFigure 1 in Supplement 1). Participants receiving semaglutide, 2.4 mg, at week 20 were randomized in a 2:1 ratio using a blocking schema (block size of 6) in a double-blind manner, via an interactive web-based response system, to continue this treatment or switch to matching placebo for 48 weeks (weeks 20-68; randomized period), with a 7-week follow-up. Participants unable to tolerate semaglutide, 2.4 mg/wk, during the randomized period were permitted to receive 1.7 mg/wk at the treating investigator’s discretion and were recommended to make at least 1 attempt to reescalate.
All participants received a lifestyle intervention from week 0 to week 68, including monthly counseling by qualified health care professionals, in person or by telephone. Participants were prescribed a reduced-calorie diet (500-kcal/d deficit relative to estimated energy expenditure calculated at week 0) and increased physical activity (150 min/wk), recorded daily by participants (using paper diaries, apps, or other tools) and reviewed during counseling visits.
Outcomes
The primary end point was percent change in body weight from randomization (week 20) to week 68. Confirmatory secondary end points (in hierarchical testing order) were change from week 20 to week 68 in waist circumference, systolic blood pressure, and physical functioning score on the Short Form 36 Version 2 Health Survey, Acute Version (SF-36; eAppendix 3 in Supplement 1). Supportive secondary end points were changes from week 20 to week 68 in absolute body weight (in kilograms), hemoglobin A1c, fasting plasma glucose, fasting serum insulin, diastolic blood pressure, lipid levels, and the SF-36 physical and mental component summary scores (other than physical functioning, changes in domain scores are not reported); whether participants achieved the SF-36 physical functioning responder threshold (data not reported) and gained weight from week 20 to week 68; and total overall and categorical weight loss from week 0 to week 68. Exploratory end points included changes in antihypertensive and lipid-lowering medication use (see eAppendix 4 in Supplement 1 for a full list).
Adverse event assessments included the number of treatment-emergent and serious adverse events during run-in (weeks 0-20) and from randomization to trial end (weeks 20-75); adverse events were recorded for the randomized period if onset was after randomization. Additional safety-related end points are listed in eAppendix 4 in Supplement 1. Cardiovascular events, acute pancreatitis events, and deaths were reviewed by an independent external event adjudication committee.
Sample Size Calculation
A sample size of 750 randomized participants (assuming a 5% permanent discontinuation rate from week 20 to week 68 and available data for 60% of these participants at week 68) was calculated to provide 95% power for the primary and confirmatory secondary end points, tested in a predefined hierarchal order. The calculation included assumed differences between treatment groups of 8.7 percentage points in body weight change, 5.8 cm in waist circumference change, 4 mm Hg in systolic blood pressure change, and 3.9 points in SF-36 physical functioning score change, based on data from the phase 2 trial of semaglutide for obesity.14 At least 900 participants were needed to start the trial intervention to ensure that at least 750 were randomized.
Statistical Analysis
Efficacy end points were analyzed using the full analysis set (ie, all participants randomly assigned to a treatment group regardless of whether they initiated treatment); assessments of adverse events and laboratory parameters used the safety analysis set (all participants exposed to ≥1 dose of study treatment). Observation periods included the in-trial period (time from week 0 to date of last contact with trial site) and the on-treatment period (administration of any dose of trial product within the prior 14 days [prior 49 days for adverse event evaluation]). All results from statistical analyses were accompanied by 2-sided 95% confidence intervals and corresponding P values (statistical significance defined as P < .05). Because of the potential for type I error due to multiple comparisons, findings for analyses of supportive secondary end points should be interpreted as exploratory. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Two estimands (treatment policy estimand and trial product estimand) were used to evaluate treatment efficacy from different perspectives10,17 and accounted for intercurrent events and missing data differently.18,19,20 All analyses in the statistical hierarchy (eTable 1 in Supplement 1) were based on the treatment policy estimand (the primary estimand; similar to an intention-to-treat analysis), which quantified the average treatment effect regardless of adherence to treatment or initiation of rescue interventions (antiobesity medications or bariatric surgery) between week 20 and week 68. Continuous end points were analyzed using analysis of covariance, with randomized treatment as a factor and baseline (week 20) end point value as a covariate. Categorical end points were analyzed using logistic regression, with the same factor and covariate (baseline body weight in kilograms was used for the analysis of participants who gained weight).10 A multiple imputation approach21 was used in which missing data were imputed from week 68 measurements from participants in the same treatment group. One thousand complete data sets were generated and analyzed, and the results were combined using the Rubin formula22 to obtain overall estimates. To account for the multicenter study design, a post hoc mixed-effects regression analysis of the primary end point was performed, with study site as a random effect.
The trial product estimand (the secondary estimand) quantified the average treatment effect modeled to assume participants continued taking randomized treatment for the planned study duration without medication discontinuation or rescue interventions between week 20 and week 68. Continuous end points were assessed using a mixed model for repeated measurements (MMRM), with randomized treatment as a factor and baseline (week 20) end point value as a covariate,10 all nested within visit. An unstructured covariance matrix for measurements within the same participant was used. For the analysis of participants who gained weight, the MMRM (with baseline body weight in kilograms as the covariate) was used to classify participants according to whether they gained weight or not. This classification was then analyzed using logistic regression, with the same factor and covariate as the MMRM.
Two further estimands (the tertiary and quaternary estimands) addressing treatment effects between week 0 and week 68 were also included. The tertiary estimand was identical to the primary treatment policy estimand, and the quaternary estimand was identical to the secondary trial product estimand, except values at week 20 (baseline) were replaced by those at week 0 (start of run-in) in the respective analyses.
Results are reported for the treatment policy estimand unless stated otherwise. Exploratory data and data from the run-in period (weeks 0-20) are summarized by descriptive statistics only.
Results
Study Participants
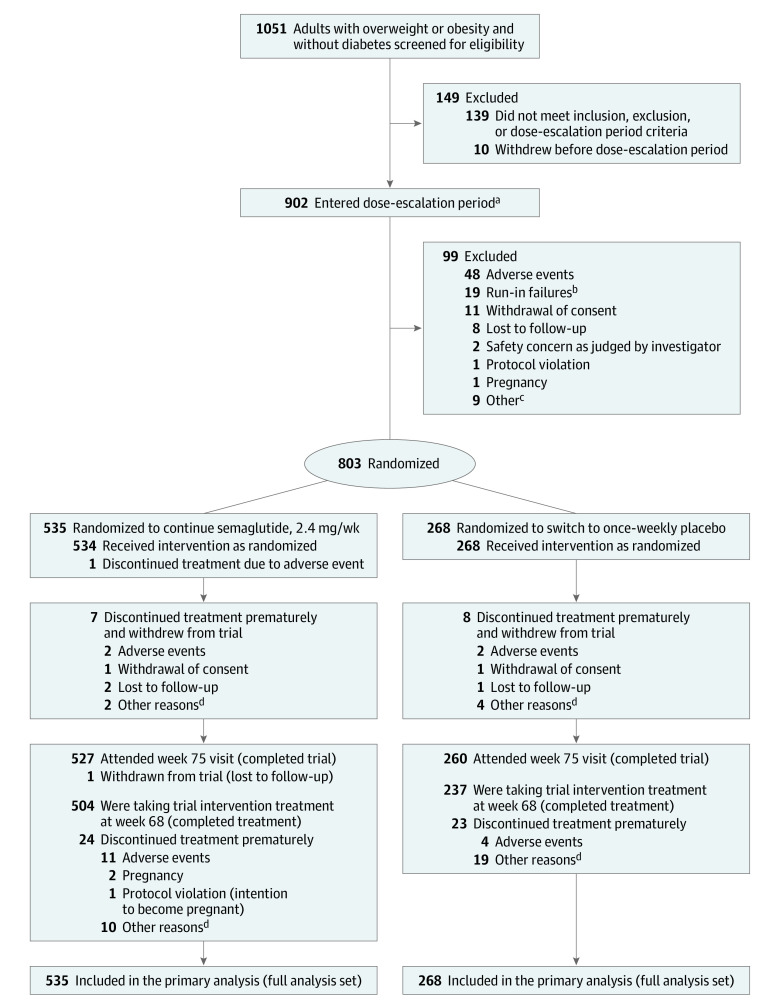
Overall, 1051 participants were screened and 902 entered the run-in. Of these, 803 (89.0%) were randomized at week 20 (continued semaglutide, n = 535; placebo, n = 268). Of the randomized participants, 787 (98.0%) completed the trial, 770 (95.9%) provided a body weight measurement at week 68, and 741 (92.3%) completed treatment (Figure 1). Of the 504 participants who continued semaglutide and completed treatment, 89.5% received 2.4 mg at week 68, 4.0% received 1.7 mg, and 2.8% received less than 1.7 mg; data were missing for 3.8%. One participant who switched to placebo received rescue intervention (liraglutide) during the randomized period. No participants had bariatric surgery.
Figure 1. Participant Flow in the Semaglutide Treatment Effect in People With Obesity (STEP) 4 Trial.
aThese participants received once-weekly semaglutide (safety analysis set).
bRun-in failures were defined as participants not meeting all 3 randomization criteria: attend the randomization visit, reach the semaglutide maintenance dose of 2.4 mg by week 16 (±3 days), and be receiving semaglutide, 2.4 mg, at week 20.
cOther reasons are listed in eTable 9 in Supplement 1.
dOther reasons are listed in eTable 10 in Supplement 1.
Of the 803 randomized participants, most were female (79.0%) and White (83.7%), with a mean age of 46.0 years, a mean body weight of 107.2 kg, a mean BMI of 38.4, and a mean waist circumference of 115.3 cm at week 0 (Table 1; eTable 2 in Supplement 1). Most (64.8%) had 1 to 3 comorbidities, with dyslipidemia and hypertension most prevalent (Table 1).
Table 1. Demographics and Clinical Characteristics at Week 0 and Week 20 (Full Analysis Set).
| Characteristics | Week 0 (start of run-in period with semaglutide treatment) (n = 803) | Change during run-in perioda | Week 20 (randomization) | |
|---|---|---|---|---|
| Continued semaglutide, 2.4 mg/wk (n = 535) | Switched to placebo (n = 268) | |||
| Age, mean (SD), y | 46 (12) | 47 (12) | 46 (12) | |
| Sex, No. (%) | ||||
| Female | 634 (79.0) | 429 (80.2) | 205 (76.5) | |
| Male | 169 (21.0) | 106 (19.8) | 63 (23.5) | |
| Race, No. (%)b | ||||
| White | 672 (83.7) | 446 (83.4) | 226 (84.3) | |
| Black or African American | 104 (13.0) | 69 (12.9) | 35 (13.1) | |
| Asian | 19 (2.4) | 15 (2.8) | 4 (1.5) | |
| Other | 8 (1.0) | 5 (0.9) | 3 (1.1) | |
| Hispanic or Latino ethnicity, No. (%) | 63 (7.8) | 42 (7.9) | 21 (7.8) | |
| Body weight, mean (SD), kg | 107.2 (22.7) | −11.1 (4.9) | 96.5 (22.5) | 95.4 (22.7) |
| Change, mean (SD), % | −10.6 (4.7) | |||
| Body mass indexc | ||||
| Mean (SD) | 38.4 (6.9) | −4.0 (1.7) | 34.5 (6.9) | 34.1 (7.1) |
| No. (%) | ||||
| <25 | 0 | 7 (1.3) | 9 (3.4) | |
| ≥25 to <30 | 22 (2.7) | 153 (28.6) | 69 (25.7) | |
| ≥30 to <35 | 274 (34.1) | 166 (31.0) | 97 (36.2) | |
| ≥35 to <40 | 249 (31.0) | 116 (21.7) | 52 (19.4) | |
| ≥40 | 258 (32.1) | 93 (17.4) | 41 (15.3) | |
| Waist circumference, mean (SD), cm | 115.3 (15.5) | −10.1 (6.2) | 105.5 (15.9) | 104.7 (16.9) |
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 127 (14) | −5.7 (13.6) | 121 (13) | 121 (13) |
| Diastolic | 81 (10) | −3.0 (8.8) | 78 (9) | 78 (9) |
| Hemoglobin A1c, mean (SD), % | 5.7 (0.3) | −0.4 (0.2)d | 5.4 (0.3) | 5.4 (0.3) |
| Fasting plasma glucose, mean (SD), mg/dL | 97.0 (10.7) | −9.5 (9.9) | 87.9 (7.7) | 86.9 (7.6) |
| Fasting lipids, median (IQR), mg/dLe,f | ||||
| Total cholesterol | 194.6 (170.3-218.1) [n = 798] | 0.9 (0.8-1.0)g | 177.2 (152.9-201.9) | 177.6 (156.0-198.8) |
| HDL-C | 50.2 (42.1-59.1) [n = 798] | 0.9 (0.8-1.0)g | 44.4 (37.8-51.7) | 44.0 (36.5-51.0) |
| LDL-C | 116.6 (97.3-138.6) [n = 798] | 1.0 (0.8-1.1)g | 110.4 (91.1-130.9) | 112.5 (93.6-130.9) |
| VLDL-C | 22.8 (17.4-32.0) [n = 798] | 0.8 (0.7-1.0)g | 18.5 (14.3-24.7) | 17.8 (13.5-24.7) |
| Free fatty acids | 13.0 (9.0-17.8) [n = 789] | 1.0 (0.7-1.4)g | 12.5 (9.0-18.0) [n = 534] | 12.5 (8.5-17.9) |
| Triglycerides | 117.5 (88.1-164.7) [n = 798] | 0.8 (0.7-1.0)g | 95.2 (73.9-125.5) | 90.8 (69.4-126.4) |
| SF-36 physical functioning score, mean (SD)e,h | 51.7 (6.4) [n = 801] | 2.2 (5.1) | 53.8 (5.7) [n = 534] | 54.1 (5.0) |
| Pulse, mean (SD), /mini | 71 (10) | 4.8 (9.3) | 76 (9) | 76 (9) |
| eGFR, median (IQR), mL/min/1.73 m2f,i,j | 100.5 (87.7-110.9) | 1.0 (0.9-1.0)g | 94.2 (81.3-106.6) | 95.9 (83.5-108.1) |
| Comorbidities at screening, No. (%) | ||||
| Dyslipidemia | 288 (35.9) | 189 (35.3) | 99 (36.9) | |
| Hypertension | 298 (37.1) | 199 (37.2) | 99 (36.9) | |
| Knee osteoarthritis | 99 (12.3) | 72 (13.5) | 27 (10.1) | |
| Obstructive sleep apnea | 94 (11.7) | 61 (11.4) | 33 (12.3) | |
| Asthma/COPD | 92 (11.5) | 57 (10.7) | 35 (13.1) | |
| Nonalcoholic fatty liver disease | 55 (6.8) | 37 (6.9) | 18 (6.7) | |
| Polycystic ovary syndrome | 25 (3.9) | 15 (3.5) | 10 (4.9) | |
| Coronary artery disease | 7 (0.9) | 4 (0.7) | 3 (1.1) | |
| Comorbidities at screening, No. (%)i,k | ||||
| 0 | 214 (26.7) | 144 (26.9) | 70 (26.1) | |
| 1 | 238 (29.6) | 160 (29.9) | 78 (29.1) | |
| 2 | 171 (21.3) | 103 (19.3) | 68 (25.4) | |
| 3 | 111 (13.8) | 77 (14.4) | 34 (12.7) | |
| 4 | 53 (6.6) | 38 (7.1) | 15 (5.6) | |
| ≥5 | 16 (2.0) | 13 (2.4) | 3 (1.1) | |
Abbreviations: COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
SI conversions: To convert HDL-C, LDL-C, and total cholesterol to millimoles per liter, multiply by 0.0259. To convert glucose to millimoles per liter, multiply by 0.055. To convert triglycerides to millimoles per liter, multiply by 0.0113.
Difference between values at week 0 and week 20 for individual participants in the total randomized population for selected parameters.
To meet regulatory requirements, race and ethnicity were recorded in this study and were determined by the participant according to fixed selection categories (with the option of answering “other,” “not applicable,” or “unknown”).
Calculated as weight in kilograms divided by height in meters squared.
Expressed as percentage points.
Participant numbers are provided where the number analyzed differed from the number in the full analysis set.
See eTable 3 in Supplement 1 for geometric mean (coefficient of variation) values for this parameter.
Observed median (IQR) ratio to week 0.
The Short Form 36 Version 2 Health Survey, Acute Version (SF-36) measures health-related quality of life and general health status. The SF-36 scores are norm-based scores, ie, scores transformed to a scale in which the 2009 US general population has a mean score of 50 and an SD of 10. The range of lowest to highest scores for the physical functioning domain is 19.03 to 57.60. An increase in score represents an improvement in health status. Further information on the SF-36 is provided in eAppendix 3 in Supplement 1.
Data are for the safety analysis set.
Assessed at screening (week −1).
Comorbidities were reported at screening and are presented for all randomized participants (week 0) and by randomized treatment group (week 20 continued semaglutide and placebo). Selected comorbidities are presented based on a history of any of the following, as reported at screening: dyslipidemia, hypertension, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, impaired glucose metabolism, reproductive system disorders, liver disease, kidney disease, osteoarthritis, gout, and asthma/COPD.
Among those not randomized at week 20 (n = 99 [11%]; Figure 1), mean body weight at week 0 (103.6 kg) was lower than in the randomized population; other characteristics were similar (Table 1; eTable 4 in Supplement 1).
Changes During the Run-in Period
During the 20-week run-in, mean body weight declined by 10.6% to 96.1 kg (Table 1). This was accompanied by reductions in waist circumference, BMI, systolic and diastolic blood pressure, hemoglobin A1c, and fasting plasma glucose and improvements in lipid profiles (Table 1; eTable 3 in Supplement 1). Participant characteristics at randomization (week 20) were comparable between treatment groups (Table 1; eTable 3).
Changes During the Randomized Period
Primary End Point
Following randomization, the estimated mean weight change from week 20 to week 68 was −7.9% with continued semaglutide vs +6.9% in participants switched to placebo (difference, −14.8 [95% CI, −16.0 to −13.5] percentage points; P < .001) (Table 2; eFigure 2A, eFigure 2B [cumulative distribution plot], and eFigure 3 in Supplement 1). For the trial product estimand, corresponding changes were −8.8% vs +6.5%, respectively (difference, −15.3 [95% CI, −16.5 to −14.1] percentage points; P < .001) (eTable 5 and eFigure 4 in Supplement 1). Results were similar when analyzed post hoc with study site as a random effect, with an estimated mean weight change from week 20 to week 68 of −7.9% with continued semaglutide vs +6.9% with placebo (difference, −14.7 [95% CI, −16.1 to −13.4] percentage points; P < .001).
Table 2. Changes in Efficacy End Points During the Randomized Period (Weeks 20-68; Treatment Policy Estimand; Full Analysis Set)a.
| End points | Estimated mean change (95% CI) | Difference (95% CI)b | P value | |
|---|---|---|---|---|
| Continued semaglutide, 2.4 mg/wk (n = 535) | Switched to placebo (n = 268) | |||
| Primary end point | ||||
| Body weight, % change | −7.9 (−8.6 to −7.2) | 6.9 (5.8 to 7.9) | −14.8 (−16.0 to −13.5) | <.001 |
| Confirmatory secondary end points | ||||
| Waist circumference, cm | −6.4 (−7.1 to −5.7) | 3.3 (2.3 to 4.3) | −9.7 (−10.9 to −8.5) | <.001 |
| Systolic blood pressure, mm Hg | 0.5 (−0.6 to 1.6) | 4.4 (2.9 to 6.0) | −3.9 (−5.8 to −2.0) | <.001 |
| SF-36 physical functioning scorec | 1.0 (0.6 to 1.4) | −1.5 (−2.2 to −0.7) | 2.5 (1.6 to 3.3) | <.001 |
| Supportive secondary end points | ||||
| Body weight, kg | −7.1 (−7.8 to −6.5) | 6.1 (5.1 to 7.0) | −13.2 (−14.3 to −12.0) | <.001 |
| Body mass indexd | −2.6 (−2.8 to −2.4) | 2.2 (1.8 to 2.5) | −4.7 (−5.2 to −4.3) | <.001 |
| Diastolic blood pressure, mm Hg | 0.3 (−0.4 to 1.1) | 0.9 (−0.4 to 2.1) | −0.6 (−2.0 to 0.9) | .46 |
| Hemoglobin A1c, % | −0.1 (−0.2 to −0.1) | 0.1 (0.1 to 0.1) | −0.2 (−0.3 to −0.2) | <.001 |
| Fasting plasma glucose, mg/dL | −0.8 (−1.7 to 0.1) | 6.7 (4.9 to 8.6) | −7.5 (−9.6 to −5.4) | <.001 |
| Fasting serum insulin, % changee | −18 (−22 to −14) | 0 (−10 to 11) | −18 (−27 to −8) | <.001 |
| Fasting lipid profile, % changee | ||||
| Total cholesterol | 5 (4 to 6) | 11 (10 to 13) | −6 (−8 to −4) | <.001 |
| HDL-C | 18 (17 to 20) | 18 (15 to 21) | 0 (−2 to 3) | .83 |
| LDL-C | 1 (−1 to 3) | 8 (5 to 10) | −6 (−9 to −3) | <.001 |
| VLDL-C | −6 (−9 to −2) | 15 (7 to 23) | −18 (−24 to −11) | <.001 |
| Free fatty acids | −18 (−27 to −7) | −14 (−24 to −4) | −5 (−20 to 13) | .59 |
| Triglycerides | −6 (−9 to −2) | 15 (7 to 23) | −18 (−24 to −11) | <.001 |
| SF-36 scoresc | ||||
| Physical component summary | 0.8 (0.3 to 1.3) | −0.9 (−1.8 to 0.0) | 1.7 (0.6 to 2.7) | .002 |
| Mental component summary | 0.1 (−0.5 to 0.7) | −3.4 (−4.3 to −2.4) | 3.4 (2.3 to 4.6) | <.001 |
| Participants who gained weight, No. (%)f | 79 (15.2) | 206 (82.4) | 0.0 (0.0 to 0.1) | <.001 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
SI conversions: To convert HDL-C, LDL-C, and total cholesterol to millimoles per liter, multiply by 0.0259. To convert glucose to millimoles per liter, multiply by 0.055. To convert triglycerides to millimoles per liter, multiply by 0.0113.
The treatment policy estimand assessed the treatment effect regardless of treatment discontinuation or rescue intervention using analysis of covariance, with randomized treatment as a factor and baseline end point value as a covariate, and a multiple imputation approach for missing data.10 Analyses were not controlled for multiple comparisons, except for changes in body weight (percent change), waist circumference, systolic blood pressure, and SF-36 physical functioning scores. eTable 5 in Supplement 1 shows corresponding data for the trial product estimand (which assessed the treatment effect assuming participants continued taking randomized treatment for the planned study duration without rescue intervention).
Data are absolute differences between estimated mean changes unless stated otherwise. The differences between mean percent changes in body weight, fasting serum insulin, and fasting lipid profile and mean changes in hemoglobin A1c are expressed in percentage points.
The Short Form 36 Version 2 Health Survey, Acute Version (SF-36) measures health-related quality of life and general health status. The SF-36 scores are norm-based scores, ie, scores transformed to a scale in which the 2009 US general population has a mean score of 50 and an SD of 10. The range of lowest to highest scores for the physical functioning domain is 19.03 to 57.60, for the physical component summary it is 6.11 to 79.67, and for the mental component summary it is −3.83 to 78.75. An increase in score represents an improvement in health status. Further information on the SF-36 is provided in eAppendix 3 in Supplement 1.
Calculated as weight in kilograms divided by height in meters squared.
These parameters were initially analyzed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups). For interpretation, these data are expressed as relative percent change and estimated relative percent difference between groups, respectively, and were calculated using the formula (estimated ratio − 1) × 100.
Data are observed proportions of participants who gained weight from week 20 to week 68, and estimated odds ratio (95% CI).
Confirmatory and Supportive Secondary End Points
Waist circumference (difference, −9.7 cm [95% CI, −10.9 to −8.5 cm]; P < .001) (Figure 2A; eFigure 3 in Supplement 1) and BMI (difference, −4.7 [95% CI, −5.2 to −4.3]) decreased from week 20 to week 68 with continued semaglutide and increased with placebo (Table 2; eTable 5 in Supplement 1).
Figure 2. Effect of Semaglutide, 2.4 mg Once Weekly, Compared With Placebo on Efficacy Outcomes During the Entire Trial (Full Analysis Set).
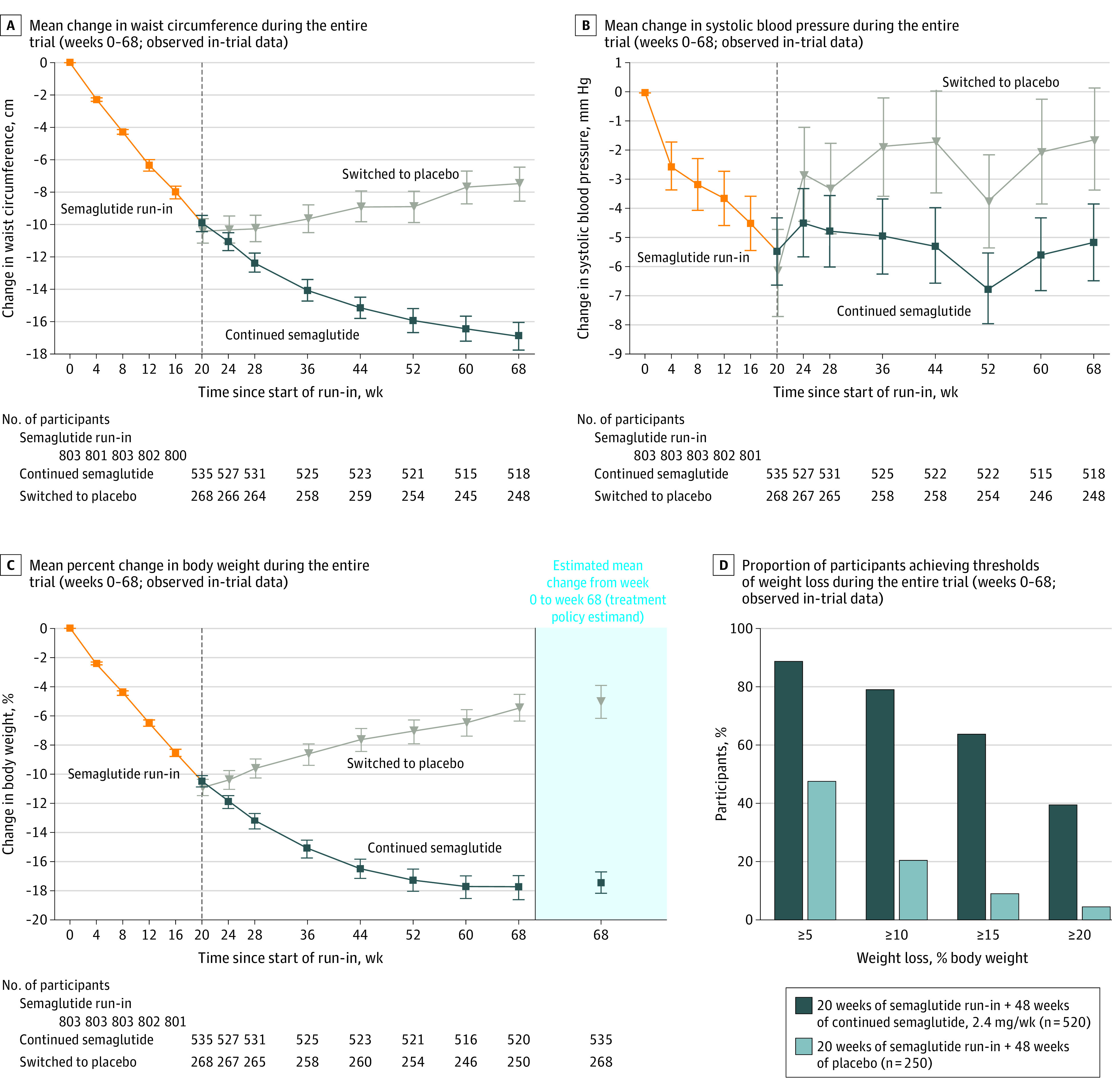
Data presented in panels A, B, and C are observed data for the full analysis set from the in-trial period (the time from week 0 to the date of last contact with trial site). Error bars represent 95% confidence intervals for the mean. Participant numbers shown denote those contributing to the mean. The dashed vertical line at week 20 represents the randomization time point. Data in the shaded area on the right in panel C are estimated mean changes from week 0 to week 68 for the treatment policy estimand, analyzed using the full analysis set. (The treatment policy estimand assessed the treatment effect regardless of treatment discontinuation or rescue intervention using analysis of covariance, with randomized treatment as a factor and baseline end point value as a covariate, and a multiple imputation approach for missing data.10) Data in panel D are observed data among all randomized participants with a week 68 assessment from the in-trial period (the time from week 0 to the date of last contact with trial site).
From week 20 to week 68, systolic blood pressure remained stable with continued semaglutide and significantly increased with placebo (difference, −3.9 mm Hg [95% CI, −5.8 to −2.0 mm Hg]; P < .001) (Figure 2B; eFigure 3 in Supplement 1), with no change in diastolic blood pressure in either treatment group (Table 2; eTable 5 and eFigure 5 in Supplement 1).
During week 20 to week 68, continued semaglutide led to additional reductions in hemoglobin A1c and fasting plasma glucose and improvements in lipid profile vs placebo (Table 2; eTable 5 in Supplement 1).
The SF-36 physical functioning scores significantly improved with continued semaglutide vs placebo from week 20 to week 68 (P < .001) (Table 2; eTable 5, eFigure 6, and eFigure 7 in Supplement 1). Improvements with continued semaglutide vs placebo were also seen in both the physical and mental component summary scores of the SF-36 (Table 2; eTable 5 and eFigure 7 in Supplement 1).
Further supportive secondary end point analyses, including absolute body weight changes, are reported in Table 2 and eTable 5 in Supplement 1.
Exploratory End Points
Among participants receiving antihypertensive medication at week 20 (continued semaglutide, n = 149; placebo, n = 67), greater proportions stopped or decreased use with continued semaglutide vs placebo (eTable 6 in Supplement 1). Corresponding data for lipid-lowering medications showed no noteworthy changes (eTable 7 in Supplement 1).
Changes Over the Entire Trial Period
For the treatment policy (tertiary) estimand, the estimated mean body weight change from week 0 to week 68 was −17.4% with continued semaglutide vs −5.0% with placebo (difference, −12.4 [95% CI, −13.7 to −11.0] percentage points) (Figure 2C; see eFigure 8 in Supplement 1 for cumulative distribution plot). For the trial product (quaternary) estimand, corresponding changes were −18.2% vs −5.2% (difference, −13.0 [95% CI, −14.3 to −11.7] percentage points).
The observed proportions of participants achieving 5% or more, 10% or more, 15% or more, and 20% or more body weight loss from week 0 to week 68 with continued semaglutide vs placebo were 88.7% vs 47.6%, 79.0% vs 20.4%, 63.7% vs 9.2%, and 39.6% vs 4.8%, respectively (Figure 2D; eFigure 9 in Supplement 1).
Adverse Events and Tolerability
During the run-in period, 84.3% of participants reported adverse events, with gastrointestinal tract disorders reported by 71.4% (eTable 8 in Supplement 1). In the randomized period, the proportions of those reporting new adverse events were 81.3% and 75.0% with continued semaglutide and placebo, respectively (Table 3). Gastrointestinal tract disorders were reported most frequently and in greater proportions with continued semaglutide than placebo (41.9% vs 26.1%, respectively) (Table 3). Most gastrointestinal events were mild to moderate in severity, and the majority of participants recovered without treatment discontinuation.
Table 3. Adverse Event and Tolerability Profile During the Randomized Period (Weeks 20-68; Safety Analysis Set).
| Adverse events | Continued semaglutide, 2.4 mg/wk (n = 535) | Switched to placebo (n = 268) | ||||
|---|---|---|---|---|---|---|
| No. (%) of participants | No. of events | Events per 100 patient-yearsa | No. (%) of participants | No. of events | Events per 100 patient-yearsa | |
| Any adverse event | 435 (81.3) | 1885 | 346.3 | 201 (75.0) | 779 | 292.8 |
| Serious adverse events | 41 (7.7) | 51 | 9.4 | 15 (5.6) | 19 | 7.1 |
| Discontinuation of trial product due to adverse eventsb | 13 (2.4) | 6 (2.2) | ||||
| Fatal eventsc,d | 1 (0.2) | 1 | 0.2 | 1 (0.4) | 2 | 0.7 |
| Adverse events reported in ≥5% of participantse | ||||||
| Diarrhea | 77 (14.4) | 114 | 20.9 | 19 (7.1) | 26 | 9.8 |
| Nausea | 75 (14.0) | 105 | 19.3 | 13 (4.9) | 13 | 4.9 |
| Constipation | 62 (11.6) | 75 | 13.8 | 17 (6.3) | 19 | 7.1 |
| Nasopharyngitis | 58 (10.8) | 77 | 14.1 | 39 (14.6) | 54 | 20.3 |
| Vomiting | 55 (10.3) | 88 | 16.2 | 8 (3.0) | 13 | 4.9 |
| Headache | 41 (7.7) | 48 | 8.8 | 10 (3.7) | 10 | 3.8 |
| Influenza | 39 (7.3) | 45 | 8.3 | 19 (7.1) | 23 | 8.6 |
| Abdominal pain | 35 (6.5) | 46 | 8.5 | 8 (3.0) | 10 | 3.8 |
| Back pain | 28 (5.2) | 32 | 5.9 | 18 (6.7) | 19 | 7.1 |
| Arthralgia | 25 (4.7) | 28 | 5.1 | 14 (5.2) | 16 | 6.0 |
| Safety areas of interest (MedDRA)f | ||||||
| Gastrointestinal disorders | 224 (41.9) | 607 | 111.5 | 70 (26.1) | 124 | 46.6 |
| Psychiatric disorders | 46 (8.6) | 55 | 10.1 | 35 (13.1) | 50 | 18.8 |
| Cardiovascular disordersc | 26 (4.9) | 32 | 5.7 | 30 (11.2) | 40 | 14.2 |
| Allergic reactions | 26 (4.9) | 29 | 5.3 | 11 (4.1) | 12 | 4.5 |
| Gallbladder-related disorders | 15 (2.8) | 17 | 3.1 | 10 (3.7) | 11 | 4.1 |
| Injection site reactions | 14 (2.6) | 15 | 2.8 | 6 (2.2) | 6 | 2.3 |
| Hepatic disorders | 11 (2.1) | 12 | 2.2 | 4 (1.5) | 4 | 1.5 |
| Malignant neoplasmsc | 6 (1.1) | 6 | 1.1 | 1 (0.4) | 2 | 0.7 |
| Hypoglycemia | 3 (0.6) | 3 | 0.6 | 3 (1.1) | 3 | 1.1 |
| Acute kidney failure | 1 (0.2) | 1 | 0.2 | 1 (0.4) | 1 | 0.4 |
| Acute pancreatitis | 0 | 0 | ||||
Events per 100 patient-years were calculated as (number of events/number of patient-years) × 100.
Based on the number of participants from the full analysis set who stated that an adverse event was the reason for permanent trial intervention discontinuation on the end-of-treatment form.
In-trial observation period data (time from randomization to last contact with trial site, irrespective of treatment discontinuation or rescue intervention).
In the continued semaglutide group, there was 1 death in a participant with a history of chronic obstructive pulmonary disease, hypertension, and lower leg edema; the cause of death per the death certificate was “natural causes with underlying chronic obstructive pulmonary disease”; event adjudication outcome: undetermined cause. In the placebo group, there was 1 death due to malignant lung cancer and malignant pericardial effusion in a participant who had discontinued placebo.
Most common adverse events by preferred term reported in 5% or more of participants in either treatment group.
Identified via Medical Dictionary for Regulatory Activities (MedDRA) searches.
Serious adverse events were reported in 2.3% of participants during the run-in period (eTable 8 in Supplement 1) and in 7.7% and 5.6% receiving continued semaglutide and placebo, respectively (Table 3). During the run-in, 5.3% of participants discontinued treatment because of adverse events, most of which were gastrointestinal tract disorders (eTable 8 in Supplement 1). In the randomized period, 2.4% and 2.2% of participants receiving continued semaglutide and placebo, respectively, discontinued treatment because of adverse events (Table 3). One death was reported in each treatment group during the randomized period, each considered unrelated to study treatment (Table 3). The death in the continued semaglutide group was attributed to natural causes with underlying chronic obstructive pulmonary disease on the death certificate; the death in the placebo group was due to metastatic lung cancer with pericardial effusion.
Gallbladder-related disorders (mostly cholelithiasis) were reported in 0.7% of participants during the run-in period and in 2.8% and 3.7% receiving continued semaglutide and placebo, respectively. Moderate acute pancreatitis was reported in 1 participant (during run-in), who recovered during the study. During the randomized period, malignant neoplasms occurred in 1.1% of participants taking continued semaglutide vs 0.4% taking placebo. Of the events in the continued semaglutide group, 3 were breast neoplasms (intraductal proliferative breast lesion, invasive breast cancer, and invasive ductal breast carcinoma), and the remaining 3 had no apparent clustering (endometrial adenocarcinoma, marginal zone lymphoma, and malignant melanoma). One event occurring in the placebo group was metastatic lung cancer. Other adverse events of special interest are described in Table 3 and in eTable 8 in Supplement 1.
Discussion
In this multicenter, randomized clinical trial, adults with obesity/overweight who continued once-weekly treatment with subcutaneous semaglutide, 2.4 mg, had ongoing and persistent weight loss vs participants who switched to placebo, who gained weight. Continued semaglutide also produced significantly better outcomes for waist circumference, systolic blood pressure, and SF-36 physical functioning scores vs placebo.
In those who continued semaglutide after randomization, weight loss achieved during the run-in period not only was sustained but continued, reaching a plateau at week 60 to week 68 and ultimately resulting in an estimated reduction of 17.4% over the entire trial. In contrast, participants who switched to placebo at week 20 gradually regained weight. The benefits of continuing semaglutide treatment for 68 weeks, rather than switching to placebo after 20 weeks, are consistent with findings from other withdrawal trials of antiobesity medications.23,24 These results emphasize the chronicity of obesity and the need for treatments that can maintain and maximize weight loss.
The significant and sustained weight loss demonstrated with continued semaglutide in this study was accompanied by sustained improvement in waist circumference, lipid profiles, and glucose metabolism, all of which are cardiometabolic risk factors. Sustained weight loss of a similar magnitude to that observed in this trial has been linked to improvements in obesity-related complications,25,26,27 such as type 2 diabetes,28 with treatment guidelines recommending sustained weight loss of 5% to 15% for people with these conditions.5,6 The sustained effects of semaglutide on body weight and cardiometabolic risk factors, as well as participants’ physical and mental functioning, indicate the potential for positive effects on such obesity-related complications.
The optimization and maintenance of weight loss are key goals of obesity management, but individuals can vary in their response to treatment29; it is therefore of clinical interest to examine the proportions of people achieving clinically relevant weight loss to support applicability in nonresearch clinical settings.20 In the present study, 40% of participants who continued semaglutide lost an additional 10% of body weight during the randomized period, as shown by eFigure 2B in Supplement 1, supporting its use for long-term treatment of obesity. Furthermore, 64% of those who took semaglutide for 68 weeks lost at least 15% of their week 0 body weight, targets not achieved by currently approved pharmacotherapy. For example, with the GLP-1 receptor agonist liraglutide, at a 3.0-mg/d dosage, which is approved for weight management, weight losses of 10% or more and 15% or more were achieved by 33% and 14%, respectively, among people treated for 56 weeks.30 Furthermore, weight loss with liraglutide in clinical trials was of lesser magnitude and appeared to plateau earlier (at 20 weeks31 or 40 weeks30) than with semaglutide in the present trial (at 60-68 weeks). Given the modest efficacy of currently approved pharmacotherapies,7,8,9 semaglutide may offer the potential to bridge the gap between behavioral and pharmacological options and bariatric surgery, which is currently considered the most effective and reliable intervention available for weight management.6,27,32 For example, 40% of participants in this trial who took semaglutide for 68 weeks lost 20% or more of their initial body weight, approaching the level of weight loss seen with sleeve gastrectomy.27,33
Although a higher maintenance dosage of semaglutide (2.4 mg/wk) was used in this trial, the adverse event profile and tolerability were consistent with data from a phase 2 study in people with obesity14 and semaglutide trials in patients with type 2 diabetes using lower maintenance dosages (up to 1.0 mg/wk),34 as well as with that reported for other GLP-1 receptor agonists,35 with no new concerns. Typical of this class, transient, mild to moderate gastrointestinal tract disorders were the most frequently reported adverse events. More of these events occurred during the run-in period, when semaglutide was escalated to the target dose, compared with the randomized period, despite the randomized period being twice as long. Over the entire trial, few participants in either group discontinued treatment because of adverse events, with the majority of participants who continued semaglutide and completed treatment receiving the 2.4-mg dose at week 68. These results indicate that most participants tolerated the strict up-titration schedule in the trial, and those who continued treatment at the 2.4-mg/wk dose beyond 20 weeks were unlikely to experience significant tolerability challenges thereafter.
The withdrawal design was a strength of the study, as participants receiving the experimental treatment continued to do so only if they achieved a desired target,36 while also enabling study of the withdrawal effect in participants who switched to placebo. Additional strengths of the study include the large sample size, blinded design, and high rates of treatment regimen and trial completion. The high treatment and trial completion rates were likely a result of multiple factors, including a focus on participant retention, selection of a population who could tolerate semaglutide during run-in, and inclusion of participants who had previously attempted to lose weight and so were likely motivated to continue in the trial following weight loss in the run-in period.
Limitations
This study has several limitations. First, there was inflexibility in the run-in period, which limited assessment to only participants tolerating the strict dose titration schedule, unlike how the medication might be used in clinical practice, with some patients not tolerating the medication after it is prescribed. The run-in period, as well as the trial setting, likely resulted in a study population who was both more tolerant of semaglutide and more adherent to medication use than would be the case in a typical setting. Because of this, the average effect size in clinical use is likely to be less than was seen in this trial. Second, there was no assessment of adherence to lifestyle interventions. Third, while the withdrawal design is a strength, it can also result in selection bias and favorable carryover effects to the randomized period, potentially resulting in overestimation of weight loss with continued semaglutide compared with what would be expected in a typical individual. To lessen any potential effect of the long half-life of semaglutide on adverse event reporting, adverse events were counted only during the randomized period if onset was after randomization.
Conclusions
Among adults with overweight or obesity who completed a 20-week run-in period with subcutaneous semaglutide, 2.4 mg once weekly, maintaining treatment with semaglutide compared with switching to placebo resulted in continued weight loss over the following 48 weeks.
eAppendix 1. Participant Enrollment and Exclusions by Study Site
eAppendix 2. Eligibility Criteria
eAppendix 3. Patient-Reported Outcome Assessments
eAppendix 4. Supportive Secondary Endpoints
eTable 1. Analysis and Imputation Methods to Address the Treatment Policy and Trial Product Estimands for the Primary and Confirmatory Secondary Endpoints in the Statistical Testing Hierarchy
eTable 2. Clinical Characteristics at Randomization (Week 20) for the Total Population (Full Analysis Set)
eTable 3. Geometric Mean Values for Fasting Lipid Parameters and Estimated Glomerular Filtration Rate at Weeks 0 and 20 (Full Analysis Set)
eTable 4. Demographics and Clinical Characteristics at Start of Run-In (Week 0) for Enrolled Participants and Non-Randomized Participants
eTable 5. Changes in Efficacy Endpoints During the Randomized Period (Weeks 20–68; Trial Product Estimand; Full Analysis Set)
eTable 6. Change in Antihypertensive Medication During the Randomized Period (Weeks 20–68; Observed In-Trial Data; Full Analysis Set)
eTable 7. Change in Lipid-Lowering Medication During the Randomized Period (Weeks 20–68; Observed In-Trial Data; Full Analysis Set)
eTable 8. Adverse Event and Tolerability Profile During the Run-In Period (Weeks 0–20; Safety Analysis Set)
eTable 9. Other Reasons for Withdrawal From Trial Prior to Randomization (Run-in Failure Participants)
eTable 10. Other Reasons for Premature Discontinuation of Trial Product After Randomization (Randomized Participants)
eFigure 1. STEP 4 Study Design
eFigure 2. Effect of Semaglutide 2.4 mg Once Weekly Compared With Placebo on Body Weight During the Randomized Period (Weeks 20–68; Full Analysis Set)
eFigure 3. Variability in Selected Efficacy Outcomes During the Entire Trial (Weeks 0–68; Observed In-Trial Data; Full Analysis Set)
eFigure 4. Percent Change in Body Weight During the Randomized Period (Weeks 20–68; Trial Product Estimand; Full Analysis Set)
eFigure 5. Mean Change in Diastolic Blood Pressure During the Entire Trial (Weeks 0–68; Observed In-Trial Data; Full Analysis Set)
eFigure 6. Mean SF-36 Physical Functioning Score During the Randomized Period (Weeks 20–68; Observed In-Trial Data; Full Analysis Set)
eFigure 7. Change in SF-36 Domain Scores During the Randomized Period (Weeks 20–68; Full Analysis Set)
eFigure 8. Cumulative Distribution Plot of Percent Change in Body Weight During the Entire Trial (Weeks 0–68; Observed In-Trial Data; Full Analysis Set)
eFigure 9. Proportion of Participants Achieving Thresholds of Weight Loss During the Entire Trial (Weeks 0–68; Observed On-Treatment Data; Full Analysis Set)
eReferences
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
Nonauthor Collaborators. STEP 4 Investigators
References
- 1.Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131-136. doi: 10.1159/000497124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibel RL, Seeley RJ, Darsow T, Berg EG, Smith SR, Ratner R. Biologic responses to weight loss and weight regain: report from an American Diabetes Association research symposium. Diabetes. 2015;64(7):2299-2309. doi: 10.2337/db15-0004 [DOI] [PubMed] [Google Scholar]
- 3.Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547-1559. doi: 10.2147/PPA.S103649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroeger CM, Hoddy KK, Varady KA. Impact of weight regain on metabolic disease risk: a review of human trials. J Obes. 2014;2014:614519. doi: 10.1155/2014/614519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garvey WT, Mechanick JI, Brett EM, et al. ; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 6.Yumuk V, Tsigos C, Fried M, et al. ; Obesity Management Task Force of the European Association for the Study of Obesity . European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402-424. doi: 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947-1956. doi: 10.1016/S0140-6736(16)00271-3 [DOI] [PubMed] [Google Scholar]
- 8.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237-248. doi: 10.1016/S2213-8587(17)30236-X [DOI] [PubMed] [Google Scholar]
- 9.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424-2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050-1061. doi: 10.1002/oby.22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration . Ozempic (semaglutide): prescribing information. Revised January 2020. Accessed July 22, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf
- 12.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754-762. doi: 10.1111/dom.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. doi: 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637-649. doi: 10.1016/S0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- 15.Lingvay I, Desouza CV, Lalic KS, et al. A 26-week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care. 2018;41(9):1926-1937. doi: 10.2337/dc17-2381 [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration . ICH E9 (R1): Statistical Principles for Clinical Trials: Addendum: Estimands and Sensitivity Analysis in Clinical Trials. Published October 2017. Accessed November 18, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9r1-statistical-principles-clinical-trials-addendum-estimands-and-sensitivity-analysis-clinical
- 18.Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203-2210. doi: 10.1111/dom.13804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Council for Harmonisation . Addendum on Estimands and Sensitivity Analysis in Clinical Trials To The Guideline on Statistical Principles for Clinical Trials E9I. Accessed October 27, 2020. https://database.ich.org/sites/default/files/E9-R1_Step4_Guideline_2019_1203.pdf
- 20.Wharton S, Astrup A, Endahl L, et al. Estimating and reporting treatment effects in clinical trials for weight management: using estimands to interpret effects of intercurrent events and missing data. Int J Obes (Lond). 2021. doi: 10.1038/s41366-020-00733-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEvoy BW. Missing data in clinical trials for weight management. J Biopharm Stat. 2016;26(1):30-36. doi: 10.1080/10543406.2015.1094814 [DOI] [PubMed] [Google Scholar]
- 22.Little RJA, Rubin DB. Statistical Analysis With Missing Data. John Wiley & Sons; 1987. [Google Scholar]
- 23.Sjöström L, Rissanen A, Andersen T, et al. ; European Multicentre Orlistat Study Group . Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352(9123):167-172. doi: 10.1016/S0140-6736(97)11509-4 [DOI] [PubMed] [Google Scholar]
- 24.Smith SR, Weissman NJ, Anderson CM, et al. ; Behavioral Modification and Lorcaserin for Overweight and Obesity Management Study Group . Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245-256. doi: 10.1056/NEJMoa0909809 [DOI] [PubMed] [Google Scholar]
- 25.Courcoulas AP, Christian NJ, Belle SH, et al. ; Longitudinal Assessment of Bariatric Surgery Consortium . Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416-2425. doi: 10.1001/jama.2013.280928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh M, Lee J, Gupta N, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity (Silver Spring). 2013;21(2):284-290. doi: 10.1002/oby.20279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879-887. doi: 10.1001/jama.2020.12567 [DOI] [PubMed] [Google Scholar]
- 28.Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541-551. doi: 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 29.Apovian CM, Garvey WT, Ryan DH. Challenging obesity: patient, provider, and expert perspectives on the roles of available and emerging nonsurgical therapies. Obesity (Silver Spring). 2015;23(suppl 2):S1-S26. doi: 10.1002/oby.21140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pi-Sunyer X, Astrup A, Fujioka K, et al. ; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 31.Wadden TA, Hollander P, Klein S, et al. ; NN8022-1923 Investigators . Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443-1451. doi: 10.1038/ijo.2013.120 [DOI] [PubMed] [Google Scholar]
- 32.Hellström PM. GLP-1 analogue liraglutide as adjunct treatment in diabetes type 2 after failed bariatric/metabolic surgery. Ann Transl Med. 2019;7(suppl 6):S240. doi: 10.21037/atm.2019.08.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567-1576. doi: 10.1056/NEJMoa1200225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi: 10.1016/j.diabet.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 35.Trujillo J. Safety and tolerability of once-weekly GLP-1 receptor agonists in type 2 diabetes. J Clin Pharm Ther. 2020;45(suppl 1):43-60. doi: 10.1111/jcpt.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration . Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products: Guidance for Industry. Published March 2019. Accessed July 22, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Participant Enrollment and Exclusions by Study Site
eAppendix 2. Eligibility Criteria
eAppendix 3. Patient-Reported Outcome Assessments
eAppendix 4. Supportive Secondary Endpoints
eTable 1. Analysis and Imputation Methods to Address the Treatment Policy and Trial Product Estimands for the Primary and Confirmatory Secondary Endpoints in the Statistical Testing Hierarchy
eTable 2. Clinical Characteristics at Randomization (Week 20) for the Total Population (Full Analysis Set)
eTable 3. Geometric Mean Values for Fasting Lipid Parameters and Estimated Glomerular Filtration Rate at Weeks 0 and 20 (Full Analysis Set)
eTable 4. Demographics and Clinical Characteristics at Start of Run-In (Week 0) for Enrolled Participants and Non-Randomized Participants
eTable 5. Changes in Efficacy Endpoints During the Randomized Period (Weeks 20–68; Trial Product Estimand; Full Analysis Set)
eTable 6. Change in Antihypertensive Medication During the Randomized Period (Weeks 20–68; Observed In-Trial Data; Full Analysis Set)
eTable 7. Change in Lipid-Lowering Medication During the Randomized Period (Weeks 20–68; Observed In-Trial Data; Full Analysis Set)
eTable 8. Adverse Event and Tolerability Profile During the Run-In Period (Weeks 0–20; Safety Analysis Set)
eTable 9. Other Reasons for Withdrawal From Trial Prior to Randomization (Run-in Failure Participants)
eTable 10. Other Reasons for Premature Discontinuation of Trial Product After Randomization (Randomized Participants)
eFigure 1. STEP 4 Study Design
eFigure 2. Effect of Semaglutide 2.4 mg Once Weekly Compared With Placebo on Body Weight During the Randomized Period (Weeks 20–68; Full Analysis Set)
eFigure 3. Variability in Selected Efficacy Outcomes During the Entire Trial (Weeks 0–68; Observed In-Trial Data; Full Analysis Set)
eFigure 4. Percent Change in Body Weight During the Randomized Period (Weeks 20–68; Trial Product Estimand; Full Analysis Set)
eFigure 5. Mean Change in Diastolic Blood Pressure During the Entire Trial (Weeks 0–68; Observed In-Trial Data; Full Analysis Set)
eFigure 6. Mean SF-36 Physical Functioning Score During the Randomized Period (Weeks 20–68; Observed In-Trial Data; Full Analysis Set)
eFigure 7. Change in SF-36 Domain Scores During the Randomized Period (Weeks 20–68; Full Analysis Set)
eFigure 8. Cumulative Distribution Plot of Percent Change in Body Weight During the Entire Trial (Weeks 0–68; Observed In-Trial Data; Full Analysis Set)
eFigure 9. Proportion of Participants Achieving Thresholds of Weight Loss During the Entire Trial (Weeks 0–68; Observed On-Treatment Data; Full Analysis Set)
eReferences
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
Nonauthor Collaborators. STEP 4 Investigators