Abstract
We describe a case of coronavirus disease 2019 (COVID-19) in a patient with mixed cellularity classical Hodgkin lymphoma (cHL) undergoing brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) therapy. A 43-year-old man presented to our hospital with a complaint of fever, for which he was diagnosed with COVID-19 after a positive polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and antiviral therapy with favipiravir and ciclesonide was started subsequently. The fever persisted for the first few days of treatment, but his respiratory status was stable, and he became asymptomatic and afebrile on day 9. Although the PCR tests remained positive, he met the updated discharge criteria of the World Health Organization (WHO) on day 12. However, his fever recurred, and his condition worsened on day 16. A chest X-ray showed a new opacity. It is likely that favipiravir and ciclesonide treatment probably did not completely eliminate the virus in the patient, and therefore the infection persisted. We added remdesivir from day 21, and the improvement was remarkable. He was discharged on day 29 after two consecutive PCR test results were negative. PCR tests are not mandatory for the updated WHO discharge criteria. However, even after antiviral therapy, COVID-19 patients with hematologic malignancies may have prolonged active infection with impaired viral excretion. Depending on the background disease and comorbidities, there may be some patient populations for whom it is not appropriate to simply comply with the current discharge criteria. Therefore, more emphasis may be needed on PCR examinations.
Keywords: COVID-19; SARS-CoV-2; Classical Hodgkin lymphoma; A+AVD therapy (brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine); Remdesivir, RT-PCR
Introduction
Since December 2019, the world has been confronting the emergence of the novel coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The incidence and mortality continue to rise unabated. Comorbidities such as chronic obstructive pulmonary disease, post-solid organ transplant, obesity, type 2 diabetes, cardiovascular disease, and chronic kidney disease have been shown to worsen disease severity and patient outcomes.1 , 2 Nonetheless, it remains unclear and controversial whether COVID-19 severity is associated with advanced cancer and chemotherapy3, 4, 5 In the realm of hematological malignancies, it is suggested that cancer and chemotherapy are associated with increased severity and a prolonged persistence of COVID-196 , 7 Herein, we describe a case of COVID-19 patient with mixed cellularity classical Hodgkin lymphoma (cHL) undergoing brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) therapy. We hope that our report will lead to reconsideration of the discharge criteria for COVID-19 patients with a background of hematologic malignancies.
Case and methods
A 43-year-old male presented to our hospital with a compliant of fever. Four months prior to the consultation, he was diagnosed with mixed cellularity cHL (Ann Arbor classification stage IV B), for which he had been on A+AVD therapy for 3 months. This therapy was favorable, and he was on the third cycle of A+AVD therapy. On day 17 of the third cycle, he complained of the aforementioned fever with a temperature of 38.4°C. His vital signs were: blood pressure 107/84 mm Hg, heart rate 106 bpm, respiratory rate 16 /min, and oxygen saturation 99% on ambient air. The rest of the physical examination findings were unremarkable. Laboratory tests revealed the following: white blood cell (WBC) count 5.2 × 109/L, neutrophils 4.9 × 109/L, lymphocytes 0.3 × 109/L, and C-reactive protein (CRP) 6.3 mg/dL. A chest computed tomography (CT) scan showed nonsegmental patchy ground-glass opacities (GGOs) in the lower lobe of the left lung (Fig 1 ). COVID-19 pneumonia was one of the differential diagnoses, and the patient was tested for SARS-CoV-2 infection. The polymerase chain reaction (PCR) test for SARS-CoV-2 using the patient's saliva specimen was positive; therefore, he was admitted to an isolation room. He was enrolled in a clinical trial wherein he received favipiravir and ciclesonide combination antiviral therapy. Although the fever persisted for the first few days of treatment, his respiratory status was stable, and he did not require oxygen support. The patient developed chemotherapy-induced pancytopenia for which he was administered filgrastim four times during the clinical course. On day 9, he became asymptomatic and afebrile. A chest radiograph showed no obvious worsening of the pneumonia; moreover, laboratory tests showed a steady decline in the inflammatory response, with CRP declining to 2.08 mg/dL on day 12. After day 9, the patient remained afebrile; therefore, he met the updated discharge criteria of the World Health Organization (WHO) on day 12.8 His discharge was anticipated after the maximum duration (14 days) of favipiravir and ciclesonide treatment was completed. Although PCR tests with nasal swab specimens remained positive on day 14, a negative PCR test result was not required for his discharge from the isolation ward as per the updated WHO discharge criteria.8 However, his fever recurred, and his condition worsened on day 16. A chest X-ray showed a new opacity in the right inferior pulmonary field on day 19; this was the first exacerbation since he was hospitalized. A CT scan performed on day 20 showed new GGOs in the right lung and an exacerbation of the previous left lung GGOs (Fig 2 ). Moreover, the inflammatory response increased, with a surge of CRP (5.01 mg/dL) on day 21. Although the patient completed 14 days of favipiravir and ciclesonide treatment, the SARS-CoV-2 pneumonia seemed to progress. Considering that the antiviral treatment might have been inadequate, we prolonged the hospitalization and added remdesivir from day 21. Remdesivir treatment was marked by an immediate resolution of the fever and a steady decline in the inflammatory response. Furthermore, on day 28, two consecutive PCR test results were negative for the first time since onset of COVID-19. Based on these results, remdesivir was administered for 8 days and he was discharged on day 29. A chest CT scan performed on discharge showed improvement in lung findings (Fig 3 ). The patient did not show recurrence of symptoms after his discharge.
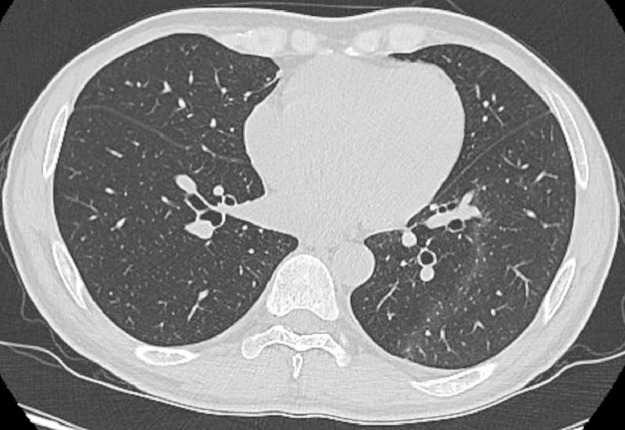
Fig. 1.
CT scan on admission. The initial CT scan revealed slight nonsegmental patchy GGOs in the left lower lobe.
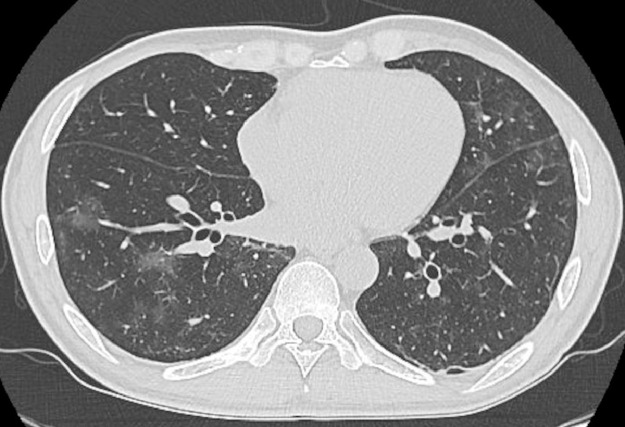
Fig. 2.
CT scan on day 20. The CT scan performed on day 20 (after fever recurrence) revealed a newly emerging GGO in the right lung and an exacerbation of the initial left lung GGO.
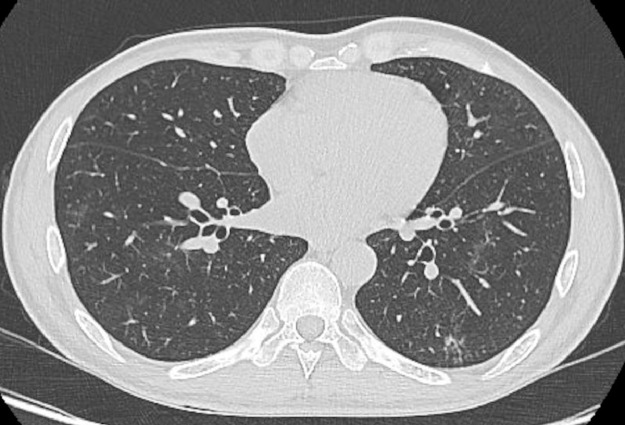
Fig. 3.
CT scan at discharge. The CT scan at discharge showed the disappearance of the bilateral GGOs.
Discussion
Our patient initially had complete relief from COVID-19 symptoms after the antiviral treatment with favipiravir and ciclesonide and met the discharge criteria; nevertheless, he experienced an exacerbation of fever and symptoms, as well as laboratory and radiological findings. Previous studies have reported secondary immune-related lung injury, which is induced by a cytokine storm (mainly by interleukin-6) in an excessive immune defense response to SARS-CoV-2.9 Secondary immune-related lung injuries have been reported to present as nonsegmental patchy consolidations and subpleural reticulations reminiscent of organizing pneumonia on chest CT.10 However, the CT scan on day 20 revealed the same peripheral-dominant GGOs as on admission, and the spread of GGOs widened and extended to bilateral lung fields, which is typical of exacerbation of SARS-CoV-2 pneumonia. Based on the CT findings and the remarkable effects of remdesivir on the clinical course, we concluded that the pathological condition was not secondary lung injury but an exacerbation of SARS-CoV-2 pneumonia itself. Given that the patient was isolated in a private room, the possibility of a second SARS-CoV-2 infection from the medical staffs was unlikely. Furthermore, the PCR test was consistently positive throughout favipiravir and ciclesonide treatment; however, it became negative during remdesivir treatment. Thus, favipiravir and ciclesonide treatment probably did not completely eliminate the virus from the patient, and the infection persisted. PCR test results can be positive even after the virus has been inactivated by appropriate antiviral treatments, and we believed that the PCR test after favipiravir and ciclesonide treatment showed a “non-pathogenic-positive” result by detecting a virus that had lost its pathogenicity.11 However, now we cannot help concluding that the PCR test result was “true-pathogenic-positive,” and the detected virus was pathogenic at the time the test was performed. In general, it is difficult to determine how long the infectivity and pathogenicity continue in patients with prolonged positive genetic tests. In order to distinguish between “non-pathogenic-positive” and “true-pathogenic-positive,” an attempt is currently being made to quantitatively estimate the viral load present in specimens based on the number of cycles (threshold cycle [Ct] value) required for amplification in the quantitative reverse transcription polymerase chain reaction (RT-PCR) assay.12 It has been reported that as the Ct value increases, the isolation rate of the virus that retained its pathogenicity decreases, even if the PCR test is positive.13 Likewise, a relatively inexpensive and rapid antigen examination for sensitive and quantitative detection of SARS-CoV-2 antigen using chemiluminescent enzyme immunoassay has been also developed. A method of inferring infectivity based on antibody for SARS-CoV-2 titers in the blood is also being considered, but no standardized method of measurement or criteria have been established. Given these backgrounds, perhaps by assessing the Ct values or the antigen quantification over time, it could be possible to distinguish whether the detected virus was one that had lost its pathogenicity after antiviral therapy or one that was still pathogenic. However, although we adopt an N-gene-specific quantitative RT-PCR assay in the clinical laboratory of our hospital, it is only treated as a qualitative test for the diagnosis of COVID-19 and the quantitative test results, including the Ct value, were not recorded. It is not possible to discuss this case from this perspective, and this point is a limitation of this report. Especially, in the realm of hematological malignancies, it is suggested that cancer and chemotherapy were related with prolonged persistence of COVID-196 , 7 Phil-Robin et al reported persisting SARS-CoV-2 viremia after rituximab therapy. They hypothesized that rituximab suppresses B cell function, thereby causing the patient to develop a severe combined cellular and humoral immunodeficiency, thus, rendering the viral infection severe and prolonged.14 Similarly, we considered that standard antiviral treatment alone could not eliminate the virus due to the underlying cHL and chemotherapy, resulting in prolonged disease duration. In the ECHELON-1 trial, which demonstrated that A+AVD regimen exhibited a superior modified progression-free survival for the frontline treatment of patients with stage III/IV cHL, the proportion of patients with severe viral infections as adverse events was not remarkably high.15 However, the United States Food and Drug Administration suggested progressive multifocal leukoencephalopathy (PML) as a possible adverse event of brentuximab vedotin.16 PML is a central nervous system demyelinating disease resulting from latent John Cunningham virus reactivation and is often fatal. The underlying mechanisms of reactivation of John Cunningham virus are not fully understood; however, it may be possible that A+AVD therapy reduces the immune response and viral excretion mechanisms more than the regular chemotherapy. The prolonged persistence of SARS-CoV-2 excretion in patients with hematological malignancies has significant implications for scheduling subsequent chemotherapy, shielding, and self-isolation. Considering the excessively long periods of isolation (which affect the society and hinder access to healthcare) for individuals with COVID-19, WHO updated its previous PCR test-based discharge criteria in May 2020. Previous discharge criteria, which required patients to be clinically recovered and to have two negative PCR test results on sequential samples taken at least 24 hours apart, were updated. Patients can now be discharged after 10 days from the onset of symptoms and at least 3 additional days without symptoms, regardless of the PCR test results.8 In this case, there was a potential risk of de-isolating our patient who had an active ongoing infection. Certainly, PCR retesting to determine discharge in all cases is unnecessary and may adversely affect the operation of the health care system. However, COVID-19 patients having an underlying hematologic malignancy (especially, those who received treatments resulting in a state of intense immunosuppression) should be discharged with caution, cognizant of the possibility of prolonged active or reactivated COVID-19.
Conclusion
Even after antiviral therapy, COVID-19 patients with hematologic malignancies may have prolonged active infection with impaired viral excretion. Depending on the background disease and comorbidities, compliance with the current discharge criteria may not be appropriate for some patient populations. Therefore, more emphasis is needed on PCR examinations after the COVID-19 symptoms are resolved. A better understanding of transmission risks among individuals with different clinical presentations or comorbidities will ameliorate the treatment safety of all patients. To refine the discharge criteria, future case evaluations and studies are warranted.
Declarations
Funding: None
Ethical approval and consent to participate: The patient in this report provided consent for participation in this study. The Institutional Review Board of the Japanese Red Cross Kyoto Daiichi Hospital approved this study.
Consent to publish: The patient in this report provided consent for the anonymous publication of her experiences. All authors of this report agree with and are greatly obliged to the Editorial Board for the publication of this report.
Disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. All authors declare that they have no conflicts of interest.
Author contributions
Hiroyuki Fujii and Taisuke Tsuji: Material preparation, data collection and analysis. Hiroyuki Fujii: Wrote the first draft of the manuscript. All authors: Study conception and design and manuscript revision. All authors read and approved the final manuscript.
Footnotes
Abbreviations: COVID-19, coronavirus disease 2019; cHL, classical Hodgkin lymphoma; A+AVD therapy, brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine therapy, PCR, polymerase chain reaction, SARS-CoV-2, acute respiratory syndrome coronavirus 2, WHO, World Health Organization, WBC, white blood cell, PCR, C-reactive protein, CT, computed tomography, GGOs, ground-glass opacities, Ct, threshold cycle, RT-PCR, reverse transcription polymerase chain reaction, PML, progressive multifocal leukoencephalopathy
References
- 1.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020:55. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennard Y.W., Jean-Baptiste C., Vasileios A., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer N., Choueiri T., Shah D., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robilotti E., Babady N., Mead P., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1101/2020.05.04.20086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah V., Ko T.Ko, Zuckerman M., et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King's College Hospital experience. Br J Haematol. 2020;190:e279–e282. doi: 10.1111/bjh.16935. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, Criteria for releasing COVID-19 patients from isolation. https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation, 2020 (accessed 17 June 2020).
- 9.Toshio H., Masaaki M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiroyuki F., Taisuke T., Tatsuya Y., et al. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review: high levels of anti-SSA/Ro antibodies in COVID-19. Clin Rheumatol. 2020;39:3171–3175. doi: 10.1007/s10067-020-05359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullard J., Dusk K., Funk D., et al. Predicting infectious SARS-CoV-2 from diagnostic Samples. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu D.K.W., Pan Y., Cheng S.M.S., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singanayagam A., Patel M., Charlett A., et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2001483. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phil-Robin T., Wali H., Mathias L., et al. Persisting SARS-CoV-2 viremia after rituximab therapy: two cases with fatal outcome and a review of literature. Br J Haematol. 2020;190:185–188. doi: 10.1056/1111/bjh.16896. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph M.C., Wojciech J., David J.S., et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA Drug Safety Communication, New Boxed Warning and Contraindication for Adcetris (brentuximab vedotin). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-new-boxed-warning-and-contraindication-adcetris-brentuximab-vedotin/, 2020 (accessed November 11, 2020).