Abstract
Rationale:
In order to understand mechanisms underlying tolerance and dependence following chronic benzodiazepine treatments, quantitative and reproducible behavioral models of these phenomena are required.
Objectives:
This research evaluated the ability of chronic treatment with a commonly-prescribed benzodiazepine, alprazolam, to induce tolerance to sedative effects and physical dependence using a novel set of behavioral measurements in rhesus monkeys.
Methods:
Four female rhesus monkeys (Macaca mulatta) were implanted with chronic intravenous catheters and administered i.v. alprazolam (1.0 mg/kg every 4 h, 38 days total). Quantitative observation measures were obtained during the 38 days of treatment. Acute administration of the benzodiazepine receptor antagonist flumazenil (0.1, 0.3 mg/kg, i.v.) was given to assess precipitated withdrawal. On day 39, saline was substituted for alprazolam and withdrawal signs were assessed for 7 days.
Results:
Maximal sedation (“deep sedation”) was evident on day 1 but was not significantly different from baseline levels by day 4 and was absent for the remainder of the 38 days of treatment. A milder form of sedation, “rest/sleep posture”, emerged by day 3 and did not decline over 38 days. Cessation of alprazolam treatment resulted in significant withdrawal signs (nose rub, vomit, procumbent posture, tremor/jerk, rigid posture) that dissipated by day 3. These signs also were observed with flumazenil (0.3 mg/kg).
Conclusions:
Chronic alprazolam treatment resulted in rapid tolerance to some behaviors (e.g., deep sedation) but no tolerance to others (e.g., rest/sleep posture). Physical dependence was observed via both spontaneous and precipitated withdrawal. Based on previous research, these phenomena may reflect differential plasticity at GABAA receptor subtypes.
Keywords: Benzodiazepine, Tolerance, Dependence, Alprazolam, Primate, Sedation
Introduction
Considerable preclinical and clinical research has demonstrated that chronic exposure to benzodiazepines (BZs) results in both tolerance to the behavioral effects of the drugs, as well as the development of physical dependence, as defined by the emergence of withdrawal signs following cessation of treatment or administration of an antagonist (for reviews, see Griffiths and Weerts 1997; Licata and Rowlett 2008). A particularly salient characteristic of tolerance to chronic BZ exposure is the observation that some behavioral effects (e.g. sedation) diminish at a more rapid rate than other effects (e.g., anxiolysis; Griffiths and Weerts 1997). Moreover, the withdrawal syndrome that defines BZ dependence symptomatically is not considered life threatening; however, it is a clinically significant phenomenon that likely contributes to maintenance of BZ use disorders (Griffiths and Weerts 1997; O’Brien 2005). The clinical efficacy of BZs and related drugs with long-term use remains controversial, with chronic treatment generally contraindicated, particularly among certain subpopulations (e.g., patients with alcohol use disorder, elderly patients; Griffiths and Weerts 1997; Airagnes et al. 2016). Although tolerance and dependence are significant impediments to BZ therapy, understanding the behavioral and neurobiological consequences of chronic BZ exposure remains a critical goal for pharmacology research (cf. Wafford 2005; Licata and Rowlett 2008; Gravielle 2016).
A key to understanding the behavioral consequences of chronic BZ exposure and its molecular/cellular underpinnings is to rigorously establish and validate behavioral measures, both in terms of reproducibility as well as translational value. Recently, we have developed procedures in rhesus monkeys to provide reliable metrics for sedation, based on scoring standards used by anesthesiologists for pediatric patients (Duke et al. 2018). The primary impetus for these new sedation measures was to improve “translational validity” between preclinical nonhuman animal models of sedation and human clinical trial results. The primary “disconnect” was that compounds lacking activity at the α1 subunit-containing receptor subtype (α1GABAA receptor) lacked sedative effects in animal models, yet were demonstrably sedative in the clinic (for review, see Skolnick 2012). In fact, we found that by parsing out sedation based on reaction to stimuli and arousal, a relatively mild form of sedation (rest/sleep posture) appeared to involve α2 and/or α3 subunit-containing receptors (α2GABAA, α3GABAA), whereas the most robust form of sedation (deep sedation) primarily involved α1GABAA receptors (Duke et al. 2018). The finding that subtypes other than α1GABAA receptors may play a key role in sedative-like effects of BZ ligands is consistent with findings from transgenic mouse approaches, providing construct validity (Behlke et al. 2016).
A primary goal of the present study was to determine the effects of chronic treatment with a non-selective BZ on our novel measures of sedation potentially associated with mediation by different GABAA receptor subtypes. We hypothesized that different rates of tolerance development might reflect distinct GABAA receptor mechanisms, with α1GABAA receptor-associated sedation dissipating at a more rapid rate than milder sedation associated with α2 and/or α3 subunit-containing receptors (α2/3GABAA subtypes). Another goal of these studies was to evaluate the extent to which spontaneous withdrawal (abrupt alprazolam cessation) and precipitated withdrawal (flumazenil injection) occurred, using a modified version of the approach pioneered by Griffiths, Ator, Weerts, and colleagues in baboons (e.g., Lukas and Griffiths 1982; Lamb and Griffiths 1984; Weerts et al. 1998; 2005; Ator et al. 2010). The relationship to these effects and plasma alprazolam levels also was determined.
Methods
Subjects and surgery
Four adult female rhesus monkeys (Macaca mulatta) weighing between 6 and 8 kg were housed in individual home cages under free-feeding conditions prior to the study, and limited to 2 h/day during the study (in AM). Water was available ad libitum. All monkeys participated in the experiments described in Duke et al (2018) with a minimum of 3 months of no treatments prior to beginning the present study. Rooms were maintained on a 12-h light/12-h dark schedule (lights on at 0630 h). Animals in this study were maintained in accordance with the Guide for Care and Use of Laboratory Animals (8th ed, 2011). Research protocols were approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
Monkeys were prepared with chronic indwelling venous catheters following the general surgical procedures described by Platt et al. (2011). The external end of the catheter was fed through a fitted jacket and tether system attached to a fluid swivel (Lomir Biomedical, Malone, NY) attached to the cages. The catheters were flushed daily with heparinized saline (100 IU/ml) and the exit site of the catheter was inspected routinely.
Drug preparation
The drugs used in this study were administered intravenously via syringe or syringe pump. The base form of alprazolam (Sigma-Aldrich, St. Louis, MO) and flumazenil (Tocris Bioscience, Bristol, UK) were dissolved in propylene glycol (50-80%), ethanol (5-10%), and sterile water. All drug solutions were filter-sterilized prior to infusion (0.2 μm pore syringe filters).
Observation procedures
The behavior of each monkey was scored using a focal animal approach as described in Platt and colleagues (2000; 2002) and modified for rhesus monkeys (see Rüedi-Bettschen et al. 2013; Duke et al. 2018). Briefly, a trained observer blind to the drug treatments observed a specific monkey for 5 minutes and recorded each instance that a particular behavior occurred during 15-second intervals. Scores, or “modified frequency scores”, for each behavior were calculated as the number of 15-second bins in which the behavior occurred (e.g., a maximum score would be 20). These behaviors are described as “species-typical behaviors” and are listed in Table 1, along with definitions (note that a summary of effects is also included in Table 1, see Results). In addition to rhesus monkey-specific behaviors, a motor function category was also scored, referred to as “observable ataxia” (any slip, trip, fall, or loss of balance).
Table 1.
Species-typical behaviors.
| Behavior | Brief Description | Summary of Effects | |
|---|---|---|---|
| Acute | Chronic | ||
| Passive Visual | Animal is standing or sitting motionless with eyes open | Yes | Yes |
| Locomotion | At least two directed steps in the horizontal and/or vertical plane | No | No |
| Self-Groom | Picking, scraping, spreading or licking of an animal’s own hair | Yes | No |
| Tactile/Oral Exploration | Any tactile or oral manipulation of the cage or environment | Yes | No |
| Scratch | Vigorous strokes of the hair with fingers or toenails | No | No |
| Stereotypy | Any repetitive, ritualized pattern of behavior that serves no obvious function | No | No |
| Forage | Sweeping and/or picking through wood chip substrate | No | No |
| Vocalization | Species-typical sounds emitted by monkey (not differentiated into different types) | No | No |
| Threat/Aggress | Multifaceted display involving one or more of the following: Open mouth stare with teeth partially exposed, eyebrows lifted, ears flattened or flapping, rigid body posture, piloerection, attack (e.g., biting, slapping) of inanimate object or other monkey | No | No |
| Yawn | To open mouth wide and expose teeth | No | No |
| Body Spasm | An involuntary twitch or shudder of the entire body; also “wet dog” shake | No | No |
| Present | Posture involving presentation of rump, belly, flank, and/or neck to observer or other monkey | No | No |
| Drink | Mouth contact to fluid delivery sippers | No | No |
| Fear Grimace | Grin-like facial expression involving the retraction of the lips exposing clenched teeth; may be accompanied by flattened ears, stiff, huddled body posture, screech/chattering vocalizations | No | No |
| Lip Smack | Pursing the lips and moving them together to produce a smacking sound, often accompanied by moaning | No | No |
| Cage Shake | Any vigorous shaking of the cage that may or may not make noise | No | No |
For sedation measures, structured exposure to stimuli were included in the observation sessions (Duke et al. 2018). When a monkey was observed to have closed eyes, an assessment of the animal’s responsiveness to the stimuli was determined. Specifically, the observer presented three stimuli: 1) walked at a normal pace towards the cage, 2) spoke the animal’s name, and 3) moved the lock used to secure the door of the cage. If the monkey responded immediately (i.e., opened eyes and oriented to the observer within 3 seconds of the initial sedation assessment), rest/sleep posture was scored. If the monkey attended more slowly (i.e., > 3 seconds following stimuli) and was observed to be assuming an atypical posture that differed from the characteristic rest/sleep posture (e.g., unable to keep an upright posture), the observer scored moderate sedation. If the monkey did not open eyes across the 15-s interval after all three stimuli, the observer noted the loss of ability to respond to external stimuli and made an assessment of deep sedation. As noted above, the assessment of sedation was initiated during the 5-min sampling period (divided into 60-sec epochs) if the animal presented with its eyes closed at any time during that period. The result of this assessment was recorded for each remaining 15-sec interval of the 60-sec epoch unless eyes opened. Afterwards, eyes closing again initiated the assessment. If eyes remained closed, then the assessment was repeated at the beginning of the next 60-sec epoch. The order in which animals were observed and the observer performing the scoring each day was randomized. Four observers participated in the scoring throughout the duration of the study; each observer underwent a minimum of 20 hours of training and met an inter-observer reliability criterion of ≥ 90% agreement with all other observers.
For chronic observation samplings, the five-minute sampling period occurred in the afternoon at ~1300 h. A preliminary study was conducted in order to determine if differences in observable behaviors occurred across the day, and only locomotion resulted in a significant change: Locomotion generally was absent in the morning but increased in the afternoon. The 1300 h sampling period was chosen to correspond to maximal levels of locomotion. For acute time-course determinations, the five-minute sampling periods were repeated at multiple times following i.v. injection of alprazolam or vehicle (minutes post injection): 0, 7.5, 15, 30, 60, 120, 240.
In addition to our standard measures of species-typical behavior and sedation, we included in this study an additional set of behaviors for scoring that were identified previously as associated with spontaneous and precipitated withdrawal following chronic BZ treatments in baboons (Lukas and Griffiths 1982; Lamb and Griffiths 1984; Weerts et al. 1998; 2005). We identified six behaviors common to these baboon studies (see Table 2, nose rub, lip droop, vomit/retch, rigid posture, tremor/jerk, seizure) and included an additional category of “other”, to allow for manifestations of withdrawal-like behavior that differed between rhesus monkeys and baboons. This category, a posteriori, resulted in scoring almost exclusively two behaviors that were combined into a new category called “prone/lean posture”, in which the monkey assumed postures associated with “rest”, i.e., leaning against the wall or laying down on the floor of the cage, but had eyes open and was apparently alert. These behaviors were incorporated into all samples, but we report withdrawal behaviors only in the context of flumazenil-precipitated withdrawal and spontaneous withdrawal (i.e., these behaviors did not occur following other treatment conditions).
Table 2.
Definitions of withdrawal-related behaviors.
| Behavior | Definition |
|---|---|
| Prone/Lean Posture | Laying chest facing down on bottom of cage and/or leaning against side of cage, eyes open. |
| Nose Rub | Repeated displacement of nose/muzzle from midline by either limb or cage bar. |
| Lip Droop | Bottom lip drooping, showing bottom teeth |
| Vomit/Retch | Disgorging stomach contents through the mouth or making an effort to do so (often accompanied by “gagging” sound); may not expel vomitus but its presence is assumed by extension of cheek pouch and/or chewing/swallowing. |
| Rigid Posture | Grasping cage with arms extended, appears to flex and/or stretch torso |
| Tremor/Jerk | Involuntary, rhythmic muscle contraction leading to shaking/jerking movements in one or more parts of the body |
| Seizure | Tonic, clonic, or both: Tonic is indicated by contraction of muscle groups, usually taking the form of extension of back and neck followed by limbs; Clonic is indicated by mild, repetitive tremor followed by brief, violent, rhythmic flexor spasms over entire body. |
Adapted from Lukas and Griffiths (1982); Lamb and Griffiths (1984); Weerts et al. (1998, 2005).
Alprazolam plasma concentrations
Blood samples for alprazolam exposure analyses were obtained via venipuncture at the same time (approximately 14:00 h) on days 2, 10, 17, and 38 of chronic alprazolam treatment, and day 40, which was 2 days following cessation of chronic treatment. Blood samples were obtained 30 min after an alprazolam or saline injection. Monkeys were sedated with 5-10 mg/kg ketamine (i.m.), and approximately 3 mL of blood were obtained for each sample. The samples were immediately centrifuged and the plasma was extracted. Plasma was placed in a separate plastic screw-top vial and frozen at −80° F. There was approximately 1.0-1.5 mL of plasma for each sample. Alprazolam in plasma was analyzed by gas chromatography as described by Greenblatt et al. (1990), using a commercial source (Enthalpy Analytical, Inc., Durham, NC).
Study design
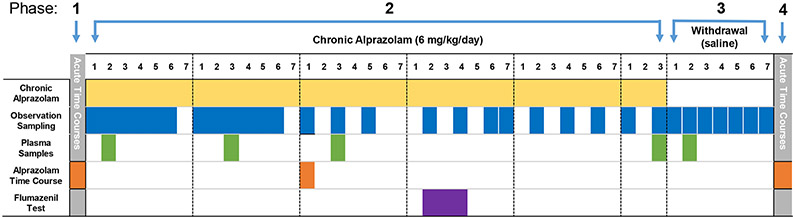
As illustrated in Fig. 1, the experiment was conducted in 4 phases. During phase 1, acute time-course studies were conducted with 1.0 mg/kg of alprazolam, 0.1 or 0.3 mg/kg of flumazenil, and drug vehicles, over approximately 2 weeks (i.e., single day tests in different monkeys on different days). Once completed and with at least 2 days drug-free, monkeys entered phase 2, chronic alprazolam treatment. Phase 3 consisted of replacing alprazolam with saline and assessing withdrawal (i.e., spontaneous withdrawal). Finally, phase 4 was conducted in the same manner as phase 1, in order to determine if any changes in baseline behavior occurred as a result of chronic treatments.
Figure 1.
Study design. Vertical columns represent time, in blocks of days (phase 1 and 4) and individual study days (phase 2 and 3). Rows represent treatments, indicated by colored blocks within each row. See Methods: Study design for procedural details.
For phase 1 and 4 acute studies, alprazolam was injected into the catheter followed by a 2-mL saline flush, and observation sampling occurred from 0 to 240 min as described above. For flumazenil alone tests, the same procedure was followed except time course studies ended after 30 min, based on pilot studies showing that antagonism of alprazolam’s behavioral effects dissipate before that time. For chronic administration (phase 2), alprazolam was administered 24 h per day, 7 days per week, as a single injection every 4 h via a syringe pump, which was programmed via Med Associates (St. Albans, VT) equipment in an adjacent room. During chronic treatment, observation sampling and blood sample collection occurred as shown in Fig. 1. An alprazolam time course was collected as described for phases 1 and 4 in all monkeys at the beginning of week 3. During this time-course, the syringe pump was inactivated, and re-activated as soon as the final observation sample was completed.
During week 4 of phase 2 (Fig. 1), flumazenil tests of precipitated withdrawal were conducted. These tests were conducted in the morning, immediately after a delivery of alprazolam via the syringe pump. Vehicle, 0.1 or 0.3 mg/kg, i.v., of flumazenil was administered via syringe, followed by observation sampling at 0, 7.5, 15, and 30 min after the injection, therefore the flumazenil test was completed prior to the next infusion of alprazolam.
Phase 3 consisted of tests for spontaneous withdrawal, i.e., cessation of drug exposure (Fig. 1). On day 1 of phase 3, alprazolam syringes were replaced with saline syringes in the AM at 0700 h, and saline injections (volume based on the amount of alprazolam infused) occurred 24 h per day for 7 days, once every 4 hours. Saline was used instead of the alprazolam vehicle, which tended to reduce catheter life. A pilot study with saline vs. vehicle revealed no changes in baseline behaviors (data not shown). Finally, all chronic injections were halted and after an additional week of no treatments, the acute time course studies (alprazolam and flumazenil) from phase 1 were repeated.
Data analysis
Our previous research demonstrated that modified frequency scores are normally distributed and similar analyses of the current data set revealed a similar finding (not shown), therefore parametric statistics were conducted (for discussion, see Duke et al. 2018). For time course studies, the modified frequency scores determined during phase 1 (acute time course studies), referred to as “Day 1” henceforth, were compared with the same tests conducted on Day 15 of chronic alprazolam administration, as well as with phase 4. A repeated measures ANOVA with Day and Time as independent variables was conducted, followed by Bonferroni t-tests compared each time point across Days. For analysis of effects during phase 2, the modified frequency scores were analyzed across chronic treatment and compared to similar samples that occurred prior to chronic treatment, using a repeated measures ANOVA with Bonferroni t-tests. Plasma samples of alprazolam on chronic treatment days 2, 10, 17, 38, and 40 were analyzed by repeated measures ANOVA with Bonferroni t-tests. For withdrawal tests, the withdrawal-associated behaviors recorded during the sampling period were summated into a single score, which was analyzed as a function of day of withdrawal and untreated (prior to chronic treatment), or as a function of flumazenil dose and time after flumazenil injection, by repeated measures ANOVA and Bonferroni t-tests. For all statistics, the family-wise error rate (alpha) was constrained to p ≤ 0.05.
Results
Overall behavior and acute effects of alprazolam
Under baseline conditions in which the monkeys did not receive injections and had adapted to the scorers (i.e., at least 2 weeks of daily observation sessions), the pattern of behavioral effects was similar to that reported previously (see Supplemental Fig. 1 in Duke et al. 2018). In this regard, the most commonly recorded behaviors were (in order): Passive/visual exploration (animal is standing or sitting motionless with eyes open), tactile/oral exploration (any tactile or oral manipulation of the cage or environment), self-groom (picking, scraping, spreading or licking of an animal’s own hair), and locomotion (at least two directed steps in the horizontal and/or vertical plane). All four behaviors accounted for ~65% of observed behaviors during baseline conditions, an overall pattern that also was observed following injections of alprazolam vehicle (data not shown).
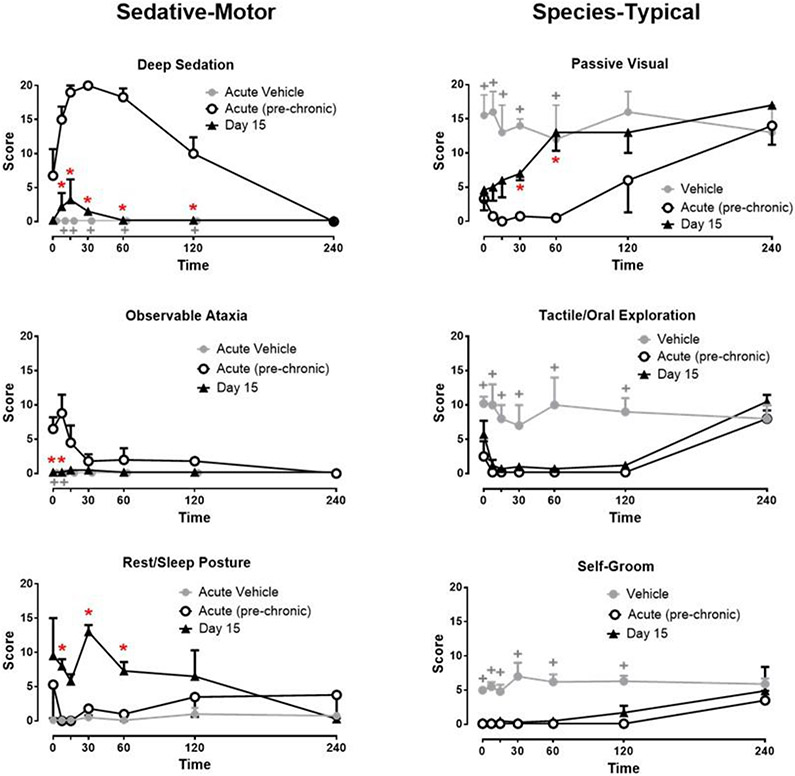
Following acute injections with 1.0 mg/kg, i.v. of alprazolam, the primary behavioral effect that was scored was deep sedation, which was reliably above vehicle levels at 7.5, 15, 30, 60, and 120 min post injection (see Fig. 2, upper left panel; Bonferroni t-tests, p<0.05). At 30 min post injection, all monkeys were scored as deep sedation for the entire 5 min sampling period. Notably, deep sedation was absent at 240 min post injection (Fig. 2). Observable ataxia also was increased significantly above vehicle levels for the 7.5 post-injection time point (Fig. 2, bottom left panel, Bonferroni t-tests, p<0.05). Behaviors that are non-independent of deep sedation, such as passive visual exploration, tactile/oral exploration, and self-groom were significantly decreased compared with acute vehicle treatment (compare lines with open circles to gray circles), presumably because they were incompatible with deep sedation (see “Species-Typical” column in Fig. 2, compare Acute (pre-chronic) to Vehicle; Bonferroni t-tests, p<0.05).
Figure 2.
Time-dependent effects of alprazolam on sedation-motor effects (deep sedation, observable ataxia, rest/sleep posture) and species-typical behaviors (passive visual, tactile/oral exploration, self-groom) in rhesus monkeys (N=4) during acute (pre-chronic treatment) tests and day 15 of chronic alprazolam treatment. Alprazolam was administered i.v. for all tests, and behavior was determined via focal sampling (5 min intervals) conducted 0, 7.5, 15, 30, 60, 120, and 240 min after the injection (see text for behavior definitions). Chronic alprazolam (1.0 mg/kg, i.v.) was administered via syringe pump 7 days per week, every 4 hours (6 mg/kg/day). Data are modified frequency scores, in which the occurrence of a behavior was scored during a 5-min sampling period divided into 15-sec intervals, with each behavior “score” calculated as the mean number of intervals in which the behavior occurred (± SEM). For species-typical behavior, gray lines and symbols are vehicle data (these results were zero for sedative-motor measures). *Note that red asterisks are acute (pre-chronic) test results vs. Day 15 results, Bonferroni t-tests, p< 0.05. +Note that grey plus symbols are tests for differences between acute vehicle and acute (pre-chronic) treatments, Bonferroni t-tests, p< 0.05.
In addition to definitions of behaviors, Table 1 summarizes the acute, as well as chronic effects of alprazolam on species-typical behaviors. As can be seen in Table 1, the majority of behaviors were not altered by acute (or chronic) alprazolam treatment; in most cases due to the very low levels of the scores. It is important to note, however, that alprazolam at this dose did not increase or induce any species-typical behaviors—in general it suppressed ongoing behaviors presumably by the induction of sedative-motor effects. The analyses of chronic alprazolam effects focuses on sedative-motor effects and species-typical effects that were suppressed by acute alprazolam treatment (passive visual, tactile/oral exploration, self-groom; Table 1).
Chronic alprazolam
In addition to the acute time course data for 1.0 mg/kg, i.v., of alprazolam, Fig. 2 displays the re-determination of the time course study following 15 days of 1.0 mg/kg alprazolam, administered i.v. every 4 hours. The asterisks in the Figure represent significant effects vs. the acute time course determination (i.e., pre-chronic alprazolam treatment). As seen in the upper left panel of the Figure, the robust, time-dependent increase in deep sedation was not observed after 15 days of chronic alprazolam treatment (Day 15 average scores vs. Acute average scores at 7.5, 15, 30, 60, and 120 min post injection, Bonferroni t-tests, p< 0.05). Similarly, observable ataxia (Fig. 2, middle left panel), which was apparent at the early time points, was absent after 15 days of treatment (Day 15 average scores vs. Acute average scores at 0 and 7.5 min post injection, Bonferroni t-tests, p< 0.05). These findings suggest tolerance to the sedative-motor effects of 1.0 mg/kg alprazolam as a result of chronic alprazolam treatments. In contrast to deep sedation and observable ataxia, the milder sedation measure, rest/sleep posture (Fig. 2, bottom left panel), was mostly absent during the acute time course study but was apparent at Day 15 (Day 15 average scores vs. Acute average scores at 7.5, 30, and 60 min post injection, Bonferroni t-tests, p< 0.05).
The three species-typical behaviors altered by acute alprazolam showed a somewhat more variable pattern of results (Fig. 2, right panels). For passive visual behavior, there was no difference between acute and day 15 results for the first 15 min; however, passive visual means returned to acute vehicle levels at 30 and 60 min post-injection (Fig. 2, top right panel, Bonferroni t-tests, p<0.05), likely due to the emergence of rest/sleep posture which is incompatible with scoring passive visual behavior. Regardless, these results suggest at least partial tolerance to the ability of 1.0 mg/kg of alprazolam to suppress passive visual behavior. Both tactile/oral exploration and self-groom, which were significantly attenuated during the acute time course studies, continued to be attenuated after 15 days of chronic alprazolam treatment (Fig. 2, middle and bottom right panels, Bonferroni t-tests, p> 0.05), although evidence of diminution of the effect occurred at the 240 min time point. In summary, these data show that while the effects of chronic alprazolam on one species-typical behavior displayed at least partial evidence of tolerance at later time points post-injection, two observable behaviors (tactile/oral exploration, self-grooming) did not show clear evidence of tolerance.
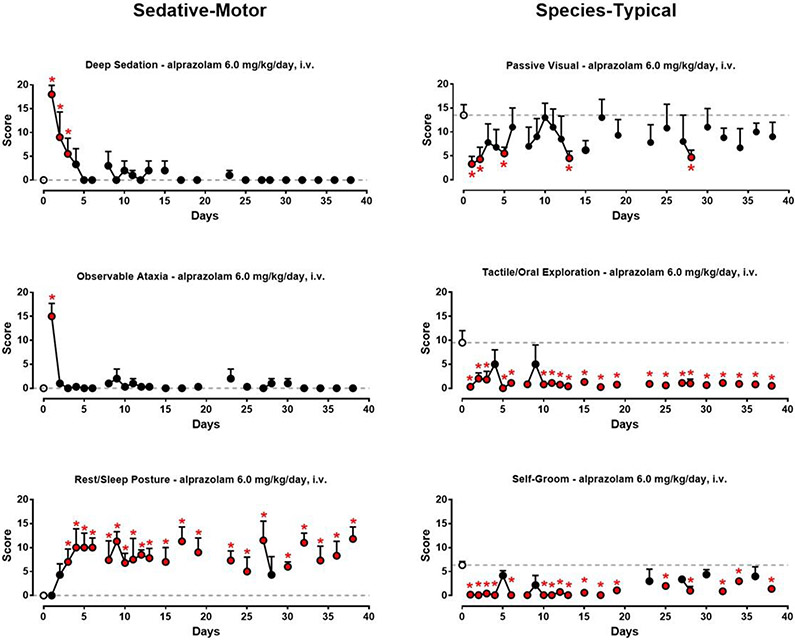
Fig. 3 shows the daily 5-min sampling data averaged across monkeys during chronic alprazolam treatment (Phase 2 in Fig. 1). As can be seen in the top left panel, deep sedation scores decreased across the first 5 days of chronic alprazolam treatment. By day 5, average deep sedation scores did not differ from baseline (untreated) scores across any of the treatment days (Bonferroni t-tests, p>0.05 vs. baseline). For observable ataxia (Fig. 3, middle left panel), significant levels of this behavior were evident only on day 1 of chronic treatment (Bonferroni t-tests vs. vehicle). In contrast, average rest/sleep posture scores (Fig. 3, bottom left panel) concomitantly increased from baseline, with the majority of daily scores significantly above baseline from day 3 to day 38 (Bonferroni t-tests, p< 0.05 vs. baseline).
Figure 3.
Sedation-motor effects (deep sedation, observable ataxia, and rest/sleep posture) and species-typical behavior (passive visual, tactile/oral exploration, self-groom) following chronic alprazolam treatment (1.0 mg/kg/4 h, or 6 mg/kg/day, i.v.) in rhesus monkeys (N=4). See text for behavior definitions. Data are mean modified frequency scores ± SEM (see Fig. 2 caption for details). Open circles and dashed lines represent baseline observations from 1 week prior to any drug treatment, averaged across 5 days for each monkey (the symbol and line are the averages of these individual values). *Note that red asterisks/symbols are differences from baseline observations, Bonferroni t-tests, p< 0.05.
The suppression of passive visual behavior by 1.0 mg/kg of alprazolam by- and-large showed a pattern consistent with tolerance, with the majority of means not differing significantly from baseline after day 5 (Fig. 3, top right panel, Bonferroni t-tests). However, it is worth noting that there was considerable variability in this measure, with returns to significant suppression on some days, and an overall tendency for the means to fall below the baseline data. Tactile/oral exploration scores (Fig. 3, middle right panel) were reduced to zero on day one of chronic alprazolam treatment, and the majority of scores for this behavior were significantly below baseline levels for the entire chronic treatment period (Bonferroni t-tests, p< 0.05). This same pattern of effects was evident for self-groom (Fig. 3, bottom right panel). In summary, these daily observation data suggest that tolerance occurred relatively rapidly for deep sedation and observable ataxia, which were largely replaced by a milder form of sedation (rest/sleep posture) that did not demonstrate tolerance over the chronic treatment period. Passive visual behavior also showed evidence of relatively rapid tolerance with chronic alprazolam treatment, but these results were more variable and as with the time course data, may be complicated by the emergence of rest/sleep posture. Finally, suppression of tactile/oral exploration and self-groom did not show clear evidence of tolerance over the 38-day chronic alprazolam treatment period.
Plasma levels
Table 3 shows the plasma alprazolam determinations from blood samples collected 30 min after an alprazolam injection during chronic treatment (phase 2) and the 2nd day of withdrawal (phase 3). No significant effects over the four alprazolam data points were observed (pairwise Bonferroni t-tests, p>0.05). On day 40 (day 2 of spontaneous withdrawal), plasma levels dropped to negligible levels (~1000-fold less than during chronic treatment).
Table 3.
Plasma alprazolam levels in rhesus monkeys (N=4) treated chronically with alprazolam or saline.
| Day | Treatment | Plasma alprazolam (mean ng/mL ± SEM) |
|---|---|---|
| 2 | Alprazolam (1.0 mg/kg/4 h, i.v.) | 722.8 ± 66.9 |
| 10 | Alprazolam (1.0 mg/kg/4 h, i.v.) | 669.8 ± 99.3 |
| 17 | Alprazolam (1.0 mg/kg/4 h, i.v.) | 781.5 ± 94.5 |
| 38 | Alprazolam (1.0 mg/kg/4 h, i.v.) | 718.3 ± 96.9 |
| 40 | Saline (0.2 mL/kg/4 hr) | 0.26 ± 0.16* |
N=3 (Below detection limit in one monkey).
Alprazolam levels in plasma were determined according to Greenblatt et al. (1990).
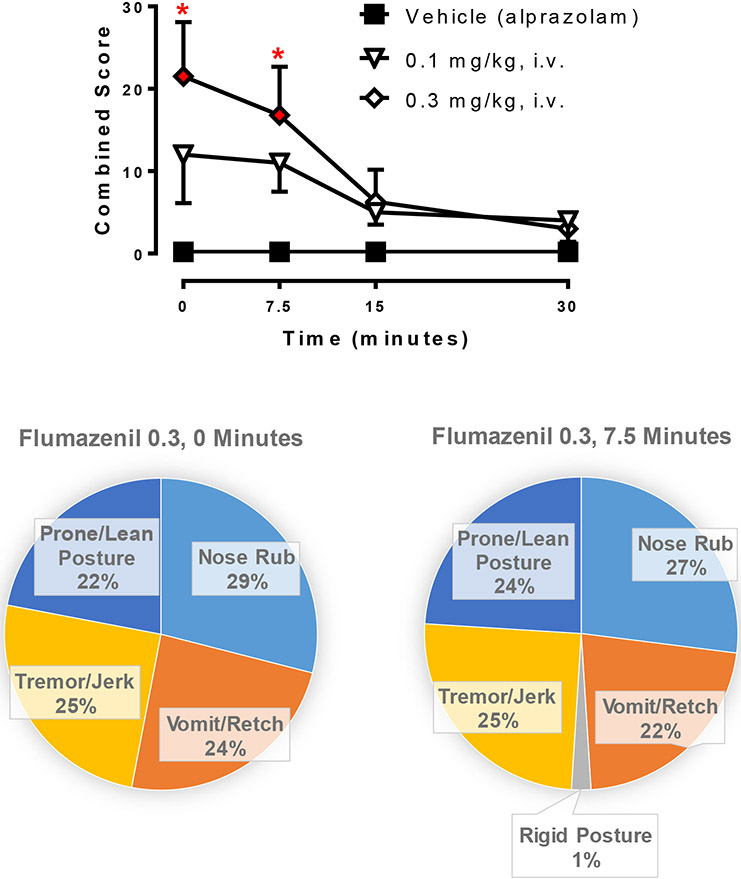
Precipitated and spontaneous withdrawal
During week 4 of chronic alprazolam treatment, i.v. injections of flumazenil or its vehicle were given in a session in the morning immediately after the programmed alprazolam injection. All withdrawal-related scores (Table 2) were summed to create a single “combined score” which was averaged across monkeys and shown in Fig. 4. The highest dose of flumazenil (0.3 mg/kg, i.v.) resulted in significantly higher combined withdrawal scores than observed after vehicle treatment (i.e., flumazenil vehicle injection, but alprazolam infusion occurred earlier). Withdrawal was evident at 0 and 7.5 min post flumazenil injection at this dose (Bonferroni t-tests, p< 0.05). The types of behavior observed with flumazenil withdrawal are shown in the bottom panel of Fig. 4, and consisted of prone/lean posture, nose rub, vomit/wretch, and tremor/jerk. Rigid posture was observed at the 7.5 min time point for 0.3 mg/kg, i.v., dose, albeit relatively infrequently (1% of the combined score).
Figure 4.
Effects of flumazenil treatment during the 4th week of chronic alprazolam treatment (1.0 mg/kg/4 h, or 6.0 mg/kg/day, i.v.) in rhesus monkeys (N=4). Top panel: Time-dependent effects of flumazenil and vehicle (note that chronic alprazolam injections continued during these tests). Data are mean ± SEM for a combined score of all withdrawal-related behaviors (see Table 1). *Note that p< 0.05 vs. Vehicle (alprazolam) treatment (Bonferroni t-tests). Bottom panels: Distribution of individual withdrawal-associated behaviors (see Table 1) that constituted the average combined score at the designated time points after flumazenil (0.3 mg/kg, i.v.) injection.
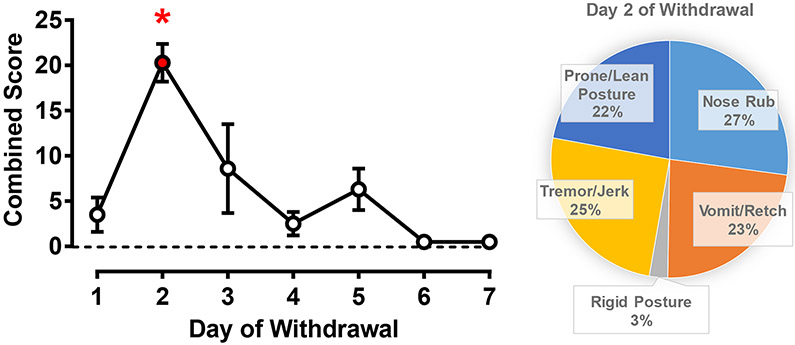
Fig. 5 shows results after substituting alprazolam with saline on day 1 of withdrawal (phase 3) for 7 days total. The average combined score for withdrawal was significantly different from pre-chronic treatment baseline on day 2 of withdrawal only (left panel; Bonferroni t-test, p< 0.05). On day 2, the withdrawal signs parsed almost evenly into prone/lean posture, nose rub, vomit/wretch, and tremor/jerk (right panel), with a small percentage (3%) of the combined score consisting of rigid posture. In summary, both flumazenil-precipitated and spontaneous withdrawal were observed in animals treated chronically with alprazolam, consistent with physical dependence development in our procedure. Finally, the re-determination of the acute alprazolam time course in phase 4 demonstrated time-dependent deep sedation that did not differ statistically from the data collected during phase 1 (data not shown), indicating there was no persistence of tolerance once the monkeys had been in spontaneous withdrawal for at least 7 days.
Figure 5.
Spontaneous withdrawal from chronic alprazolam (1.0 mg/kg/4 h or 6.0 mg/kg/day, i.v.) treatment in rhesus monkeys (N=4). On day 1 of withdrawal, saline was substituted for the first morning injection and administered i.v. (0.2 mL/kg) every 4 h for 7 days. Left panel: Data are mean ± SEM for a combined score of all withdrawal-related behaviors (see Table 1), observation sampling occurred in the morning and afternoon, with daily scores averaged. *Note that p< 0.05 vs. baseline (dashed horizontal line, pre-chronic baseline scores), Bonferroni t-tests. Right panel: Distribution of individual withdrawal-associated behaviors (see Table 1) that constituted the average combined score on day 2 of withdrawal.
Discussion
Chronic treatment with alprazolam and other conventional BZs (e.g., diazepam) consistently has been reported to induce tolerance to their behavioral effects as well as physical dependence (Griffiths and Weerts 1997; Licata and Rowlett 2008). Tolerance and dependence have been demonstrated following chronic BZ treatment in several species used in preclinical research, including non-human primates and rodents, since early studies by Yanagita and colleagues (e.g., Yanagita and Takahashi 1973). However, the GABAA receptor mechanisms underlying tolerance and dependence have remained somewhat elusive (cf., Wafford 2005; Gravielle 2016). Here, we describe a study in which both species-typical and drug-related behavior were recorded in a rigorous fashion in rhesus monkeys following acute and chronic alprazolam treatments, with the overall goal of establishing a robust approach to studying underlying mechanisms of behavioral phenomena that likely contribute to the abuse of BZs.
Tolerance as a result of chronic drug exposure may occur due to changes in absorption, distribution, metabolism, or excretion of the drug, thereby reducing the level of drug available at the binding site. Because of the general finding that rates of tolerance to BZ effects depends on the behavioral endpoint—as clearly demonstrated in the present study—it seems unlikely that decreasing drug exposure levels can account for tolerance development. Consistent with this observation, we found no evidence of declining plasma alprazolam levels in monkeys treated with 6.0 mg/kg/day of alprazolam, even after 38 days of treatment. Moreover, other investigators have demonstrated steady plasma and brain concentrations during chronic BZ treatment periods in which tolerance was observed (e.g. Cowley et al. 1995; Fernandes et al. 1996; Ferreri et al. 2015). As with this previous research, our findings suggest plasticity at the level of the receptor or receptor signaling/trafficking, rather than pharmacokinetic mechanisms.
A primary goal of this paper was to evaluate two previously-identified sedative effects, deep sedation and rest/sleep posture, as well as an observation-based ataxia measure, following chronic BZ treatment. Relatively rapid tolerance developed to both deep sedation (the more robust type of sedation representing a state in which the subject was resistant to arousal, Duke et al. 2018) and observable ataxia. Interestingly, the milder form of sedation, rest/sleep posture, was absent initially but emerged with chronic treatment. Further, species-typical behavior, which can be conceptualized as normative ongoing behavior of the monkey in an untreated state, was disrupted by both acute and chronic alprazolam treatment. In this regard, the passive visual measure was reduced but showed at least partial tolerance during chronic alprazolam treatment, perhaps indicative of the shift from deep sedation to the milder rest/sleep posture. Both tactile/oral exploration and self-groom (both of which are commonly occurring behaviors for monkeys) were disrupted acutely and did not return to baseline levels, even with the development of tolerance to deep sedation. Although it is unknown the extent to which these behaviors are completely suppressed by sedation, it is clear that even a shift to a milder type of sedative effect during chronic alprazolam treatment resulted in significant disruption of the animals’ ongoing “normal” behavior.
The quantitative observation approach used in the present study was chosen based on previous research by Duke et al. (2018), primarily because this approach has provided evidence for specific receptor subtype involvement in the acute behavioral effects of alprazolam. In this regard, deep sedation and observable ataxia engendered by alprazolam were sensitive to blockade by the α1GABAA receptor selective antagonist, βCCT, whereas rest/sleep posture and suppression of tactile/oral exploration were not blocked by βCCT (Duke et al. 2018). Moreover, compounds that had functional selectivity for α2GABAA and α3GABAA subtypes engendered rest/sleep posture but not deep sedation, while suppressing tactile/oral exploration and self-groom (Duke et al. 2018; Meng et al. 2019). To the extent to which GABAA subtypes differentially mediate these behavioral effects acutely, these data and those from the current study suggest that tolerance develops rapidly to α1GABAA subtype mediated effects (deep sedation, observable ataxia) whereas behaviors mediated by α2GABAA and/or α3GABAA receptors persist with chronic treatment.
The parameters established by our research will facilitate future studies with subtype-selective ligands to confirm the above hypothesis and to understand the potential role(s) of GABAA receptor subtypes in BZ-induced tolerance. At present, information on these subtypes and tolerance is relatively limited. Interestingly, a study using “knock-in” mouse technology suggested a requirement for the α5GABAA receptor subtype in tolerance to diazepam’s sedative effects (van Rijnsoever et al. 2004). More recently, Vinkers et al. (2012) used GABAA receptor subtype-selective ligands to show that the development of tolerance required concomitant activation of α1GABAA and α5GABAA receptors. We have yet to investigate the role of α5GABAA receptors in the chronic effects of BZ ligands, however, our acute studies do not point to a key role for this subtype in any of the behavioral effects induced by alprazolam in the present study (Duke et al. 2018).
In addition to tolerance to some behavioral effects of BZs, the development of physical dependence following chronic BZ treatment is a major impediment to use of these drugs as pharmacotherapies (Griffiths and Weerts 1997; Licata and Rowlett 2008). Moreover, physical dependence likely contributes to ongoing abuse of BZs, because drug taking may be maintained by the alleviation of withdrawal signs (i.e., negative reinforcement) and avoidance of detoxification may be a major reason for resistance to drug abstinence as well as high relapse rates (O’Brien 2005). The present study demonstrated that a dosing regimen based on duration of action of the drug (up to 4 h) and maintained 24 h per day, 7 days per week, resulted in both spontaneous withdrawal and withdrawal precipitated by the BZ site antagonist, flumazenil. For both types of withdrawal, the signs were essentially identical, consisting of abnormal postures, signs indicative of gastrointestinal distress, and tremors. Importantly, these signs are shared with human patients in BZ withdrawal (Griffiths and Weerts 1997; O’Brien 2005) as well as experimentally with baboons (Lukas and Griffiths 1982; Lamb and Griffiths 1984; Weerts et al. 1998; 2005; for review, see Griffiths and Weerts 1997). In the present study, the presence of spontaneous withdrawal was relatively short (statistically significant for one day only) which may reflect the use of the i.v. route and/or the dose of alprazolam used in this species. It is also noteworthy that we did not escalate the dose of alprazolam once tolerance was evident, as often practiced by patients addicted to BZs. Finally, when the time-dependent effects of the dose of alprazolam (1.0 mg/kg, i.v.) were re-evaluated following the brief withdrawal period, the deep sedation observed prior to chronic treatment re-emerged. Altogether, these data are consistent with a relatively mild physical dependence, consistent with previous research (for review, see Griffiths and Weerts 1997).
Our study design did not allow for conclusions on the amount of chronic exposure required to develop physical dependence with alprazolam, although a relatively simple design modification—insertion of multiple flumazenil tests—would address this issue. However, precipitated withdrawal in monkeys following a single injection of a BZ (i.e., acute dependence) has been demonstrated (Spealman 1986; Fischer et al. 2013). Moreover, Fischer et al. (2013) provided evidence that withdrawal appears to involve the α1GABAA receptor subtype, a conclusions which has received support using other pharmacological approaches and different species. For example, the α1GABAA subtype-preferring drugs zolpidem and zaleplon have been shown to result in physical dependence after chronic treatment in baboons (Weerts et al. 1998; Ator et al. 2000). Moreover, compounds lacking efficacy at α1GABAA subtypes had at least reduced propensities to result in physical dependence compared with conventional BZs (Mirza and Nielson 2006; Ator et al. 2010). It will be of considerable importance to determine the extent to which different GABAA receptor subtypes are involved in physical dependence following chronic BZ treatment.
Alprazolam (Xanax®) is a commonly prescribed anxiolytic, but unfortunately, is also consistently reported as one of the more frequently misused/abused BZs in the US (e.g., Substance Abuse and Mental Health Services Administration 2013). At present, the primary approach to treatment for addiction to BZs like alprazolam consists of detoxification, which is both difficult to achieve and associated with high rates of relapse. Our results are consistent with chronic alprazolam treatment resulting in physical dependence, as well as tolerance to some behavioral effects (e.g., deep sedation) associated with a specific GABAA receptor subtype (α1GABAA receptors). Knowledge of the underlying mechanisms of alprazolam-induced tolerance and dependence should help inform the development of improved anxiolytic medications, as well as potential treatments for BZ use disorder.
Acknowledgments of Funding and Grants:
Supported by NIH/NIDA grant DA043204, DA011792, and AA016179.
Footnotes
Conflict of Interest Statement: Dr. Duke, Dr. Platt, and Dr. Rowlett declare no conflicts of interest.
References
- Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F (2016) Benzodiazepine Misuse in the Elderly: Risk Factors, Consequences, and Management. Curr Psychiatry Rep, 89. doi: 10.1007/s11920-016-0727-9. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR (2010) Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at α1 and α2/3 subtypes. J Pharmacol Exp Ther 332: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson AJ, Yee BK, Zeilhofer HU, Engin E, Rudolph U (2016) A pharmacogenetic ‘restriction-of-function’ approach reveals evidence for anxiolytic-like actions mediated by α5-containing GABAA receptors in mice. Neuropsychopharmacology 41: 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley DS, Roy-Burne PP, Radant A, Ritchie JC, Greenblatt DJ, Nemeroff CB, Hommer DW (1995) Benzodiazepine sensitivity in panic disorder: effects of chronic alprazolam treatment. Neuropsychopharmacology 12: 147–157. [DOI] [PubMed] [Google Scholar]
- Duke AN, Meng Z, Platt DM, Atack JR, Dawson GR, Reynolds DS, Tiruveedhula VVNPB, Li G, Stephen MR, Sieghart W, Cook JM, Rowlett JK (2018) Evidence that sedative effects of benzodiazepines involve unexpected GABAA receptor subtypes: Quantitative observation studies in rhesus monkeys. J Pharmacol Exp Ther 366: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, File SE, Berry D (1996) Evidence against oppositional and pharmacokinetic mechanisms of tolerance to diazepam’s sedative effects. Brain Res 734: 236–242. [PubMed] [Google Scholar]
- Ferreri MC, Gutierrez ML, Gravielle MC (2015) Tolerance to the sedative and anxiolytic effects of diazepam is associated with different alterations of GABAA receptors in rat cerebral cortex. Neuroscience 310: 152–162. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Teixeira LP, van Linn ML, Namjoshi OA, Cook JM, Rowlett JK (2013) Role of gamma-aminobutyric acid type A (GABAA) receptor subtypes in acute benzodiazepine physical dependence-like effects: Evidence from squirrel monkeys responding under a schedule of food presentation. Psychopharmacology 227: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravielle MC (2016) Activation-induced regulation of GABAA receptors: Is there a link with the molecular basis of benzodiazepine tolerance? Pharmacol Res 109: 92–100. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Javaid JI, Lockniskar A, Harmatz JS, Shader RI (1990) Gas chromatographic analysis of alprazolam in plasma: replicability, stability and specificity. J Chromatogr 534: 202–207. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM (1997) Benzodiazepine self-administration in humans and laboratory animals – implications for problems of long-term use and abuse. Psychopharmacology 134: 1–37. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR (1984) Precipitated and spontaneous withdrawal in baboons after chronic dosing with lorazepam and CGS 9896. Drug Alcohol Depend 14: 11–17. [DOI] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK (2008) Abuse and dependence liability of benzodiazepine-type drugs: GABAA receptor modulation and beyond. Pharmacol Biochem Behav 90: 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR (1982) Precipitated withdrawal by a benzodiazepine receptor antagonist (Ro 15-1788) after 7 days of diazepam. Science 217: 1161–1163. [DOI] [PubMed] [Google Scholar]
- Meng Z, Berro LF, Sawyer EK, Rüedi-Bettschen D, Cook JE, Li G, Platt DM, Cook JM, Rowlett JK (2019) Evaluation of the anti-conflict, reinforcing, and sedative effects of YT-III-31, a ligand functionally selective for α3 subunit-containing GABAA receptors. J Psychopharmacol in press, available online, doi: 10.1177/0269881119882803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NO, Nielsen EØ (2006) Do subtype-selective γ-aminobutyric acidA receptor modulators have a reduced propensity to induce physical dependence in mice? J Pharmacol Exp Ther 316: 1378–1385. [DOI] [PubMed] [Google Scholar]
- O’Brien CP (2005) Benzodiazepine use, abuse, and dependence. J Clin Psychiatry 66(Suppl 2): 28–33. [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD (2011). Models of neurological disease (substance abuse): self-administration in monkeys. In: Current Protocols in Pharmacology 56:10.5.1–10.5.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P (2012) Anxioselective anxiolytics: on a quest for the holy grail. Trends in Pharmacological Sciences, 33: 611–620.22981367 [Google Scholar]
- Spealman RD (1986) Disruption of schedule-controlled behavior by Ro 15-1788 one day after acute treatment with benzodiazepines. Psychopharmacology 88: 398–400. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2013) Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- van Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Möhler H, Fritschy JM, Crestani F (2004) Requirement of α5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci 24: 6785–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers CH, van Oorschot R, Nielsen EO, Cook JM, Hansen HH, Groenink L, Olivier B, Mirza NR (2012) GABAA receptor a subunits differentially contribute to diazepam tolerance after chronic treatment. PLoS ONE 7: e43054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA (2005) GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opinion Pharmacol 5: 47–52 [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Kaminski BJ, Griffiths RR (2005) Comparison of the behavioral effects of bretazenil and flumazenil in triazolam-dependent and non-dependent baboons. Eur J Pharmacol 519: 103–113. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR (1998). Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther 285: 41–53. [PubMed] [Google Scholar]
- Yanagita T, Takahashi S (1973) Dependence liability of several sedative-hypnotic agents evaluated in monkeys. J Pharmacol Exp Ther 185: 307–316. [PubMed] [Google Scholar]