Abstract
Background:
This study sought to develop a clinical risk score (CRS) for resectable Colorectal Liver Metastasis (CRLM) by combining clinicopathologic and clinically available biologic indicators (i.e KRAS).
Methods:
A cohort of patients that underwent resection for CRLM at the Johns Hopkins Hospital (JHH) was analyzed to identify preoperatively assessable, independent predictors of overall survival (OS); these factors were combined into the GAME (Genetic And Morphological Evaluation) score. The score was compared to the current gold-standard (Fong score) and validated in an external cohort of patients from the Memorial Sloan Kettering Cancer Center (MSKCC).
Results:
In the JHH cohort (n = 502), 6 preoperatively available predictors of worse OS were identified on multivariable Cox regression. In turn, the GAME score was calculated by allocating points to each patient according to the presence of these predictive factors: KRAS mutated tumours: 1 point; carcinoembryonic antigen (CEA) ≥ 20 ng/mL: 1 point; primary tumour lymph node metastasis: 1 point; 3 ≤ Tumour Burden Score (TBS) < 9: 1 point or TBS ≥ 9: 2 points; extrahepatic disease: 2 points. The highest risk group (≥ 6 points) in the JHH cohort had a 5-year OS of 0% compared with 81.5% for low-risk patients (0 points). Importantly, in both the JHH and MSKCC (n = 747) cohorts the discriminatory capacity of the GAME score was superior to that of the Fong score, as demonstrated by the C-index and the Akaike Information Criterion (AIC).
Conclusion:
The GAME score is a preoperatively prognostic tool to inform treatment selection.
Introduction
Many studies have identified prognostic factors among patients undergoing hepatic resection for colorectal liver metastasis (CRLM). Although this effort has identified many factors associated with outcome, limited consensus exists regarding the relative importance of the reported prognostic factors and the implications of factor-specific cut-off values.1 In fact, it is now known that prognostic factors vary significantly between different study cohorts and may diverge over time in the same cohort.2, 3 In an attempt to address these limitations, previous studies have combined the power of the most consistently reported prognostic factors into clinical risk scores (CRS).4 CRS can still facilitate comparison of different populations, provide individualized prognostic information and improve stratification of patients in clinical trials.5 However, in contrast to biomarkers developed in other areas of medicine, most CRS for patients with CRLM have not undergone successful external validation.5
The most commonly used CRS was developed in 1999 based on 1001 patients who underwent resection of CRLM.6 Although the Fong score remains by far the most popular CRS due to the high quality and the large size of the cohort many additional risk scores have been developed, most notably the Iwatsuki, Rees and Nordlinger scores.7–9 However, concerns regarding their prognostic accuracy and applicability to external patient cohorts have limited their utility.9–11 In fact, Zakaria et al. recently demonstrated the limited prognostic accuracy and clinical value of 4 scores, after performing an external validation.1 These suboptimal results suggest that a prognostic model developed in one institution at a specific time period may not necessarily have wider applicability. For example, as most CRS were developed before modern chemotherapy, the applicability of currently available CRS to the contemporary clinical setting is limited, despite their original success. In turn, these limitations prevent CRS from exerting a meaningful impact on patient management preoperatively. This study was designed to develop a CRS including tumour morphology and biology.
Methods
JHH and MSKCC study populations
All adult patients that underwent complete resection of CRLM between 01/01/2000–12/31/2015 at either JHH or MSKCC and had available data on KRAS status were eligible for inclusion in the respective patient cohorts. Patients who only underwent ablation or palliative liver resection (R2 resection) were excluded. The Institutional Review Boards of JHH and MSKCC approved this study.
Detailed information was extracted from electronic patient records on the following clinicopathologic and demographic variables: patient sex, age, characteristics of the primary colorectal tumour [American Joint Committee on Cancer T stage, primary tumour location (colon vs rectum) and presence/absence of lymph node metastasis], preoperative factors [use of preoperative chemotherapy, chemotherapy regimens and/or biologic factors employed, preoperative carcinoembryonic antigen (CEA) levels and disease-free interval between the diagnosis of the primary tumour and CRLM], intraoperative factors [performance of major resection (resection of 3 or more liver segments) vs minor resection]. Metachronous disease was defined as the presentation of liver metastasis following a disease-free interval > 12 months after the diagnosis of primary CRC. CRLM characteristics [tumour size, number, presence of unilobar or bilobar disease in the liver, margin status (R0, defined as no tumour cells within 1 mm from the margin, vs R1)], KRAS mutation status and the receipt of postoperative therapy.12
Calculation of the Tumour Burden Score and determination of prognostic cut-off levels
Instead of analyzing tumour size and number as separate variables, tumour burden was assessed with the aid of a newly developed model, the “Tumour Burden Score” (TBS). TBS is a prognostic indicator that captures the cumulative impact of tumour size and tumour number on survival.13 TBS allows for discordant tumour size and number (patients with large tumour size, but with a limited number of lesions or patients with multiple but small tumours), as either may dominate the prognosis. It is calculated by assigning each patient a set of coordinates on a Cartesian plane, according to maximum tumour size (x-axis) and tumour number (y-axis)13 (Supplemental Figure 1). The Pythagorean theorem is then used to calculate the distance (an absolute value) of any given point (which corresponds to a patient with a specific tumour size and number) from the origin of the plane (0, 0) whereby (TBS2 = [Maximum tumour diameter]2 + [number of liver lesions]2). TBS values fall into three ‘zones’, associated with progressively worse overall survival (OS): 1) TBS < 3 (Zone 1), 2) 3 ≤ TBS < 9 (Zone 2) and 3) TBS ≥ 9 (Zone 3).13
Determination of KRAS mutation status and CEA cut-off
Genomic DNA was isolated from primary colorectal cancer (CRC) or CRLM tissue specimens using standard phenol-chloroform extraction and ethanol precipitation procedures. Subsequently, classical Sanger sequencing was performed in order to detect KRAS mutations (codons 12, 13 and 61).14 Receiver operating characteristic (ROC) curve analysis determined the most appropriate CEA cut-off value in the JHH cohort.
Primary outcome of the study, development of the GAME score and statistical analysis
The primary outcome of the study was OS calculated with the use of Kaplan Meier (KM) survival analysis; differences in OS were assessed with the log-rank test. A Cox stepwise regression analysis was performed to determine significant predictors of OS. Factors that can be assessed preoperatively were combined into a simple weighted scoring system while excluding those variables only assessed postoperatively (e.g. margin status).6,15 The Hazard Ratio (HR) of each prognostic factor identified through the Cox model was then used to determine the allocation of points in the scoring system (Supplemental Table 1). The sum of these points constituted a GAME score for each patient. After the GAME score was calculated, its discriminatory power compared with the Fong score was tested, in both the derivation (JHH) and validation (MSKCC) cohorts. ROC analysis with Harrell’s C-statistic and the Akaike Information Criterion (AIC) was used, as described.16 All statistical testing was two-sided, with significance defined as P < 0.05. All analyses were carried out with SPSS software ver. 23 (IBM SPSS, Chicago, IL, USA).
Results
A total of 502 adult patients were included in the JHH cohort with a median (interquartile range; IQR) follow up of 30.5 (15.7–55.1) months. The 1-, 3-, and 5-year OS rates were 94.5%, 67.4%, and 49.5%, respectively. With respect to the MSKCC validation cohort, a total of 747 adult patients were included with a median (IQR) follow up of 52.4 (29.7–76.6) months. The 1-, 3-, and 5-year OS rates were 95.8%, 77.9%, and 60.2%, respectively. Tables 1 and 2 summarize the clinicopathologic characteristics of the JHH and MSKCC cohorts. Of note, the CEA cut-off that displayed the maximum sensitivity and specificity in predicting inferior OS was found to be 20 ng/ml and was subsequently employed during the calculation of the GAME score. Table 3 illustrates the results of univariable and multivariable analysis of OS in the JHH dataset. Tumour size and number were not included in the univariable model, as they were collinear with TBS. Importantly, multivariable stepwise Cox regression identified 6 independent predictors of poor OS: tumour lymph node status. CEA levels ≥ 20 mg/mL, extrahepatic disease, presence of KRAS mutation, TBS 3–8 and TBS ≥ 9. Although margin status was a significant variable, it was not incorporated into the final score because it is not preoperatively available.
Table 1.
Patient Demographic and Clinicopathologic Characteristics in the JHH cohort
| All patients (n = 502) | |||
|---|---|---|---|
| Characteristics | No. | % | |
| Demographics | |||
| Age, years | Median (IQR) | 58.4 (49.8–66.4) | |
| Sex | Male : Female | 296 : 206 | 59.0 : 41.0 |
| Primary CRC characteristics | |||
| Tumour site | Colon : Rectum | 396 : 106 | 78.9 : 21.1 |
| T stage (n=454) | T1or T2 stage : T3 or T4 stage | 82 : 372 | 18.1 : 81.9 |
| Nodal metastases | Negative:Positive | 163 : 339 | 32.5 : 67.5 |
| Preoperative factors | |||
| Disease-free interval | < 12 months : > 12 months | 370 : 132 | 73.7 : 26.3 |
| Chemotherapy for liver disease | Total : Combined cytotoxic regimen : Combined cytotoxic regime and biologic agent | 335 : 306 : 201 | 66.7 : 91.3* : 60.0* |
| Preoperative CEA, ng/mL | Median (IQR) | 7.6 (3.5–21.3) | |
| Extrahepatic disease at the time of operation | 50 | 10.0 | |
| Tumour factors | |||
| No. of CRLM | Median (IQR) | 2.0 (1.0–4.0) | |
| Size of largest CRLM, cm | Median (IQR) | 2.6 (1.6–4.1) | |
| Tumour Burden Score | Median (IQR) | 4.1 (2.8–6.1) | |
| Bilobar disease | 214 | 42.6 | |
| KRAS mutation status | Wild-type : Mutated | 320 : 182 | 63.7 : 36.3 |
| Operative factors | |||
| Major resection | 163 | 32.5 | |
| Resection margin, mm | Median (IQR) | 5.0 (1.0–10.0) | |
| R1 | 52 | 8.6 | |
| Postoperative factors | |||
| Postoperative chemotherapy (n=451) | Yes : No | 321 : 130 | 71.2 : 28.8 |
CEA, carcinoembryonic antigen; CRC, colorectal cancer; CRLM, colorectal liver metastases; IQR, interquartile range; R1, resection margin exposure in pathology specimen
proportion in total preoperative chemotherapy for liver disease
Table 2.
Patient Characteristics in the two Cohorts
| Institute | No (%) | ||
|---|---|---|---|
| Characteristics | JHH (n=502) | MSKCC (n=747) | P |
| Patient characteristics | |||
| Age, years, Median (IQR) | 58.4 (49.8–66.4) | 58.1 (49.2–67.2) | 0.816 |
| CRC nodal metastases | 0.164 | ||
| Negative | 163 (32.5) | 272 (36.4) | |
| Positive | 339 (67.5) | 475 (63.6) | |
| Disease-free interval | 0.067 | ||
| < 12 months | 369 (73.5) | 583 (78.0) | |
| ≥ 12 months | 133 (26.5) | 164 (22.0) | |
| Preoperative CEA, ng/mL | 0.971 | ||
| Median, (IQR) | 7.6 (3.5–21.3) | 7.0 (3.4–22.1) | |
| Extrahepatic disease at the time of operation | 0.367 | ||
| Negative | 452 (90.0) | 684 (91.6) | |
| Positive | 50 (10.0) | 63 (8.4) | |
| No. of CRLM, Median (IQR) | 2 (1–4) | 2 (1–4) | 0.853 |
| Size of largest CRLM, cm, Median, (IQR) | 2.6 (1.6–4.1) | 2.5 (1.6–4.2) | 0.945 |
| KRAS mutation status | 0.551 | ||
| Wild-type | 320 (63.7) | 463 (62.0) | |
| Mutated | 182 (36.3) | 284 (38.0) | |
CEA, carcinoembryonic antigen; CRC, colorectal cancer; CRLM, colorectal liver metastases; IQR, interquartile range
Table 3.
Univariable and Multivariable Analysis of Overall Survival in the JHH cohort
| Factors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | ||||
| Age < 60 | Ref | |||
| Age ≥ 60 | 1.03 (0.79–1.34) | 0.86 | ||
| Gender | ||||
| Male | Ref | |||
| Female | 1.14 (0.86–1.50) | 0.36 | ||
| Tumour site | ||||
| Colon | Ref | |||
| Rectum | 0.81 (0.58–1.14) | 0.223 | ||
| CRC Nodal metastases | ||||
| Negative | Ref | Ref | ||
| Positive | 1.48 (1.10–1.99) | 0.01 | 1.55 (1.14–2.10) | <0.01 |
| Disease-free interval | ||||
| ≥ 12months | Ref | |||
| < 12 months | 1.18 (0.90–1.55) | 0.243 | ||
| Perioperative chemotherapy for CRLM | ||||
| No | Ref | |||
| Yes | 0.96 (0.63–1.44) | 0.831 | ||
| Preoperative CEA | ||||
| <20 | Ref | Ref | ||
| ≥20 | 1.86 (1.41–2.47) | < 0.01 | 1.90 (1.43–2.53) | < 0.01 |
| Extrahepatic disease | ||||
| Negative | Ref | Ref | ||
| Positive | 1.96 (1.28–3.00) | <0.01 | 2.10 (1.35–3.22) | <0.01 |
| Bilobar disease | ||||
| Negative | Ref | |||
| Positive | 1.24 (0.95–1.62) | 0.114 | ||
| KRAS mutation status | ||||
| Wild-type | Ref | Ref | ||
| Mutated | 1.35 (1.02–1.78) | 0.04 | 1.50 (1.13–2.00) | <0.01 |
| Resection margin width | ||||
| ≥ 1mm | Ref | Ref | ||
| < 1mm | 1.89 (1.39–2.56) | < 0.01 | 1.81 (1.32–2.48) | <0.01 |
| Tumour burden score | ||||
| < 3 | Ref | Ref | ||
| ≥ 3, < 9 | 1.70 (1.17–2.48) | <0.01 | 1.66 (1.14–2.44) | <0.01 |
| ≥ 9 | 3.00 (1.93–4.66) | < 0.01 | 3.23 (2.01–5.07) | < 0.01 |
CEA, carcinoembryonic antigen; CRC, colorectal cancer; CRLM, colorectal liver metastases; CI, confidence interval; HR, hazard ratio; Ref, reference
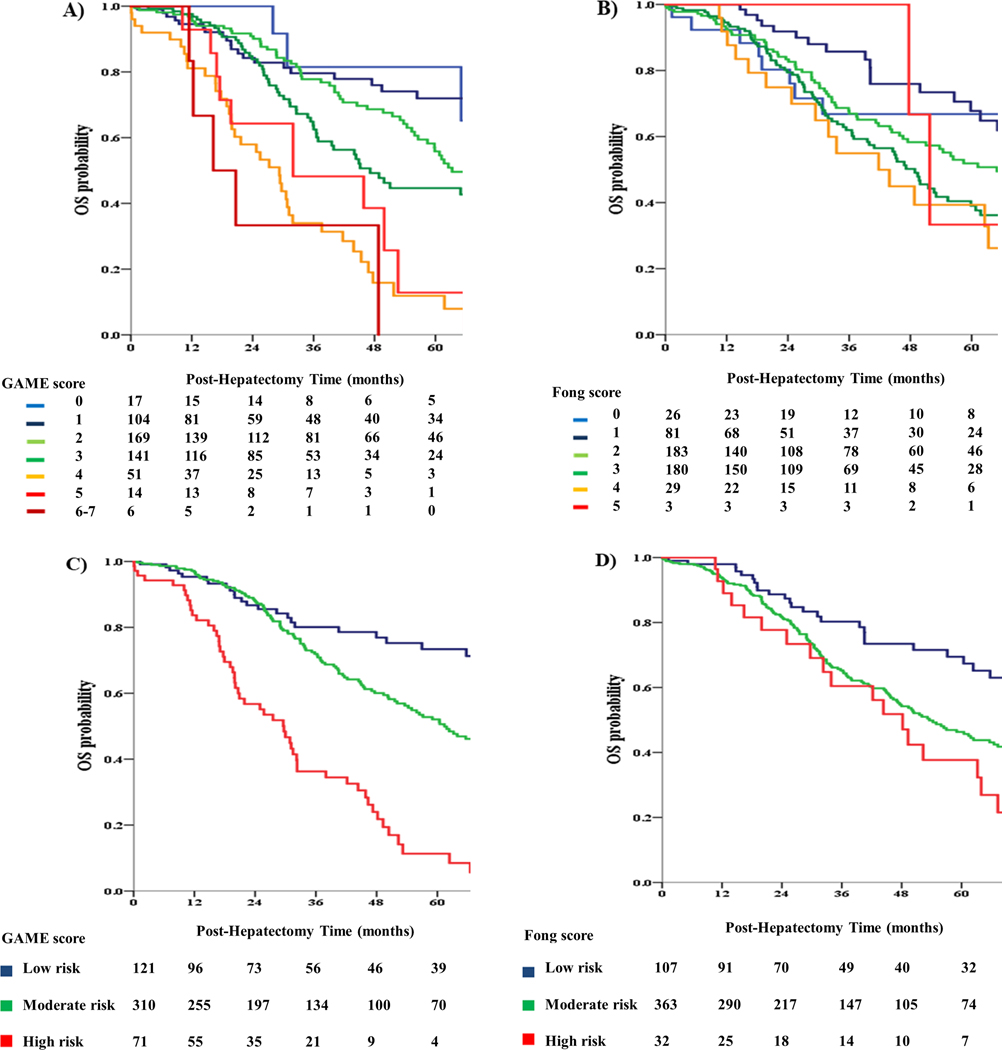
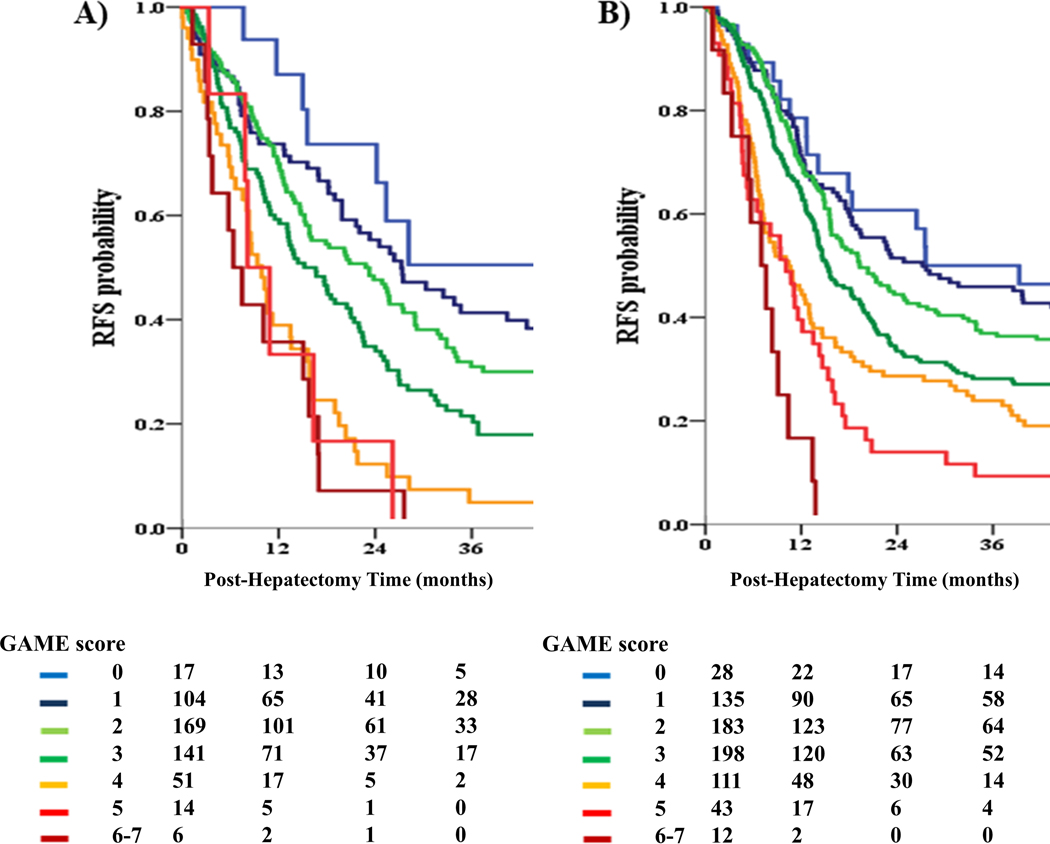
Patients were assigned to low-risk (GAME: 0–1), medium-risk (GAME: 2–3) and high-risk (GAME: ≥ 4) categories. To facilitate comparison, patients were also assigned to different risk categories according to Fong score, as previously described.17 In particular, each risk category was defined as follows: low risk (Fong: 0–1), medium risk (Fong: 2–3) and high risk (Fong: ≥ 4), respectively. OS between the 3 GAME score risk groups was compared using KM curves and log-rank testing, as demonstrated in Figure 1. The low-risk group demonstrated a better 5-year OS compared to the medium-risk group (73.4% vs 50.6%; P = 0.001). In turn, the medium risk group demonstrated a higher 5-year OS compared to the high-risk group (50.6% vs 11.3%; P < 0.001). It is also interesting to note, that although the GAME score was not developed to predict recurrence-free survival (RFS), patients who were assigned to higher GAME score risk categories demonstrated a higher risk of recurrence (Figure 3).
Figure 1.
A. Kaplan Meier analysis of overall survival (OS) for different values of the GAME score in the Johns Hopkins Hospital (JHH) cohort
B. Kaplan Meier analysis of OS for different values of the Fong score in the JHH cohort
C. Kaplan Meier analysis of OS for different GAME score risk subgroups in the JHH cohort
D. Kaplan Meier analysis of OS for different Fong score risk subgroups in the JHH cohort
Figure 3.
A. Kaplan Meier analysis of recurrence-free survival (RFS) for different values of the GAME score in the JHH cohort
B. Kaplan Meier analysis of recurrence-free survival (RFS) for different values of the GAME score in the MSKCC cohort
On the other hand, when the Fong score was used to stratify patients, the low-risk group demonstrated a higher 5-year OS of 67.4%, compared with 45.7% in the medium-risk group (P = 0.003). In contrast with the GAME score, however, no significant difference in 5-year OS was detected between the medium-risk and high-risk groups (45.7% vs 37.7%; P = 0.087). The GAME score significantly outperformed the Fong score with a Harrell’s C-index of 0.645 (0.598–0.692) vs 0.578 (0.530–0.625; P = 0.008), and an AIC of 2219 vs 2266 respectively.
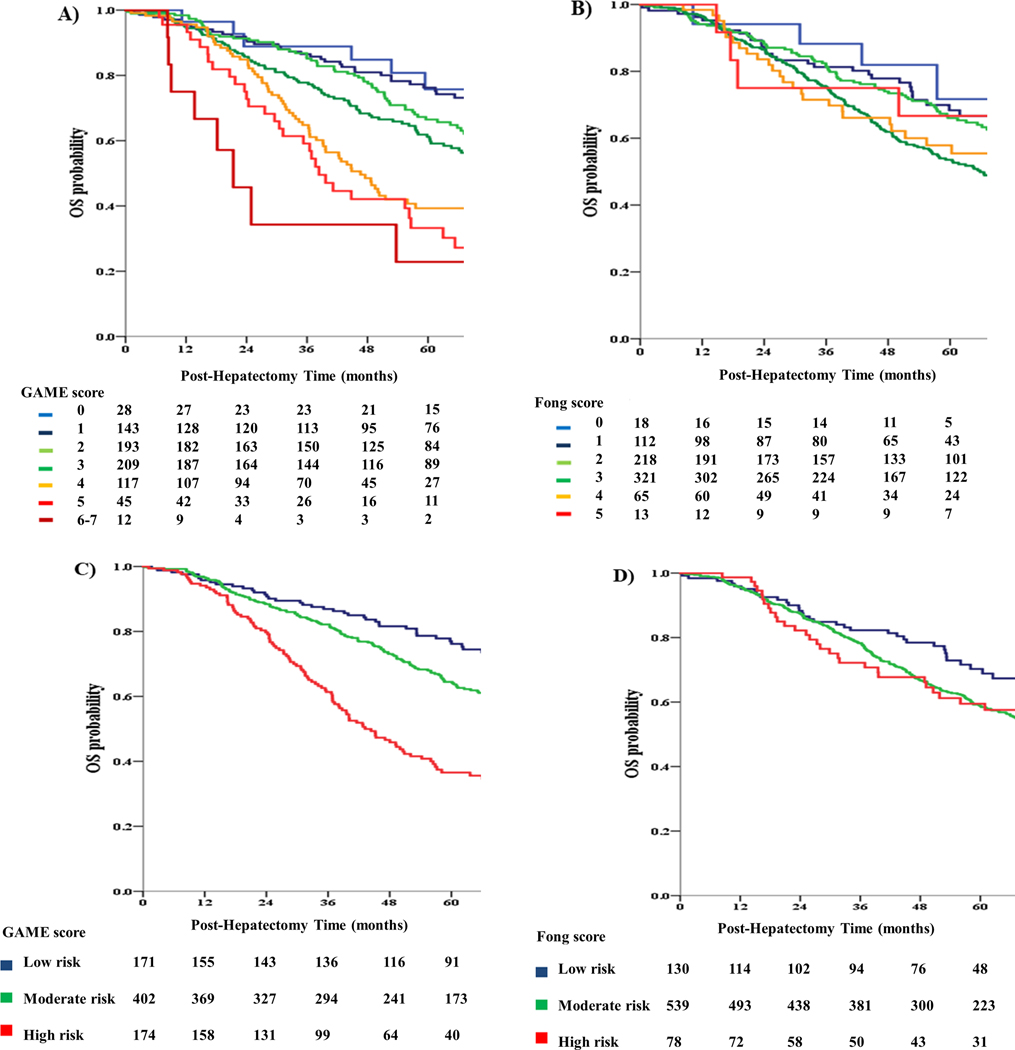
The OS between the 3 GAME score risk groups was compared using KM curves and log-rank testing (Figure 2). A significant difference in OS between the 3 risk groups was detected, with the low-risk group demonstrating a significantly higher 5-year OS compared to the medium-risk group (76.2% vs 63.7%; P = 0.024). In turn, the medium-risk group demonstrated a significantly higher 5-year OS compared to the high-risk group (63.7% vs 36.5%; P < 0.001). Interestingly, patients classified in different GAME score risk categories also demonstrated significant differences in RFS, as in the JHH cohort (Figure 3). On the other hand, when the Fong score was used to stratify patients, the low-risk group demonstrated a 5-year OS of 68.8%, which was higher than the 5-year OS of 58.4% that was observed in the medium-risk group (P = 0.008). In contrast with the GAME score, however, no difference in 5-year OS could be detected between the medium-risk and high-risk groups (58.4% vs 59.5%; P = 0.377). Importantly, the GAME score also outperformed the Fong score with respect to discriminatory ability. Specifically, Harrell’s C-index for the GAME score was 0.625 (0.584–0.662) vs. 0.584 (0.545–0.622) for the Fong score (P = 0.047). Consistent with these results, the AIC was lower for the GAME score compared to the Fong score, indicating better discriminatory ability (4169 vs 4197).
Figure 2.
A. Kaplan Meier analysis of OS for different values of the GAME score in the Memorial Sloan Kettering Cancer Center (MSKCC) cohort
B. Kaplan Meier analysis of OS for different values of the Fong score in the MSKCC cohort
C. Kaplan Meier analysis of OS for different GAME score risk subgroups in the MSKCC cohort
D. Kaplan Meier analysis of OS for different Fong score risk subgroups in the MSKCC cohort
Discussion
The current study reports on the development of the GAME score in a cohort from JHH and its external validation in a cohort from MSKCC. The study population included patients with CRLM and available genetic data, considerably exceeding previous studies. Importantly, the GAME score outperformed the Fong score in both cohorts. This is compatible with previous studies that have challenged the prognostic power of existing CRS and suggests that GAME may be a promising alternative.1, 4, 5, 18, 19 The excellent performance of the GAME might stem from its component variables, which are all powerful determinants of prognosis. Importantly, GAME is the first CRS to incorporate clinically available genetic information (KRAS status).20–23 Conversely, previous scores have been criticized for their reliance on traditional clinicopathologic variables, at a time when the importance of tumour biology is increasingly recognized.24, 25 Although the prognostic impact of many genetic markers has been evaluated, KRAS remains the most commonly utilized due to its wide availability and robust association with outcomes.20–23, 26 An additional advantage is that KRAS status can be safely inferred prior to CRLM resection, given the very high genetic concordance rate (95%) between primary and metastatic lesions.27–29 By contrast, other proposed markers require direct genetic or immunologic analysis of the metastatic lesions, a limitation that prevents their preoperative use.30 Furthermore, in contrast with KRAS mutational status, some of these markers are not commonly employed for clinical purposes thus limiting their potential applications.31, 32
Another advantage of the GAME score is that it incorporates a composite variable (TBS), reflecting both tumour size and number, as a proxy of tumour morphology. As such, TBS would be expected to capture more prognostic information than either tumour size or number alone, which have demonstrated limited predictive power in recent studies.33, 34 Building on this hypothesis, TBS was recently developed by our group and proved to be a more powerful prognostic indicator than the traditional morphologic criteria (e.g tumour size and number) used in previous CRS. Importantly, this result was confirmed in two external cohorts from Europe and Asia.13 Consequently, just as KRAS is a practical surrogate of tumour biology, TBS may serve as an accurate proxy of tumour morphology.
The primary tumour’s characteristics should also be considered during the development of any CRS, given the biologic continuity between primary and metastatic lesions.35 In turn, the presence of primary tumour lymph node metastasis remains the most consistent prognostic factor related to the primary tumour and was included in eight out of twelve previous CRS.5, 36, 37 Multivariable analysis in the JHH cohort confirmed these observations, resulting in the incorporation of primary lymph node status to the GAME score.
Preoperative CEA was also predictive of OS. Indeed, while pre-hepatectomy CEA levels are influenced by primary tumour characteristics and metastatic disease burden, CEA has long been recognized as an independent prognostic factor. However, CEA cut-off levels have been difficult to determine, a controversy reflected in previous CRS. For example, Schindl used CEA as a continuous variable, Lee used a cut-off level > 5 ng/ml, Rees > 60 ng/ml, and Fong and Konopke > 200 ng/ml.11, 38, 39 We determined a CEA cut off value of 20 ng/ml with the aid of ROC analysis. Interestingly, this value approximated the weighted average of the two cut-offs detected for the 2000–2007 and 2008–2015 periods, in a previous study of our group, thus maintaining continuity with our previous results.
Lastly, extrahepatic disease was also included in the GAME score. Interestingly, only 3 out of 12 previous CRC incorporated extrahepatic disease as a prognostic factor.5 This is hardly surprising, as most risk scores were developed during a time when the presence of extrahepatic disease precluded curative-intent hepatectomy. Even though this is no longer the case, few centers have accumulated significant experience in managing such patients. Nonetheless, as CRLM resection in the presence of extrahepatic disease will become increasingly common, a contemporary risk model should estimate its prognostic impact.40 To assess the applicability of the GAME score to centers that do not operate on patients with extrahepatic involvement, we repeated all analyses after excluding such patients; importantly, the results were essentially unchanged (results not shown).
Importantly, the GAME score was deliberately designed using only preoperative, rather than postoperative variables such as margin status. Consequently, the potential exists for the GAME score to be utilized preoperatively. This may have significant implications, as GAME could then help determine treatment selection. Indeed, while historical cohorts suggest that resection of CRLM benefits patients, no randomized trial has ever compared the efficacy of surgery vs chemotherapy. As such, it has been argued that patients with very aggressive disease may derive minimal benefit from surgery. Interestingly, previous risk scores were generally unsuccessful in identifying patients with extremely adverse prognosis. In fact, out of all available CRS, only the Grade C group of the Nagashima score reportedly had zero survival; however, this result was based on a single center study of only 81 patients.5 By contrast, the high-risk group of the Nordlinger score had an almost 50% OS rate, while the Iwatsuki score’s high-risk group had a 5-year OS of 30%. Furthermore, the high-risk group of the Fong score demonstrated an OS rate of 15%, while minimum survival rates were even higher in other CRS.1 Importantly, the very high-risk group (≥ 6) of the present model had an expected survival of 0% (Figure 1); in turn, it is possible that this group received no benefit from surgery. However, the number of patients in that group was too small to draw definite conclusions. As such, a case-control study, comparing the outcomes of medically and surgically treated patients according to GAME score may help to identify patients in whom medical management would be preferable to the costs and morbidity of major surgery.
The present prognostic model has several limitations. First of all, its development was based on data from a single institution. Although external validation in a cohort from MSKCC was conducted, this may not suffice given the considerable worldwide variation in patient characteristics and treatment strategies. To this end, external validation in cohorts from outside the US is currently underway. Of note, TBS was calculated using pathologic data, rather than preoperative radiographic imaging. However, we recently demonstrated that TBS values estimated through either imaging or pathology are equivalent in prognostic terms.41 As such, although we used pathologic data to estimate TBS and consequently the GAME score, it is probable that the GAME score can also be calculated with the aid of imaging thus supporting our decision to classify TBS as a preoperatively available value. Of note, no chemotherapy data were available in the MSKCC cohort. Lastly, although the aim of the study was to develop a novel CRS applicable to all patients who undergo curative intent resection for CRLM, future studies should separately assess the score’s performance among patients treated with either preoperative chemotherapy or immediate resection. Although the calculation of the GAME score is relatively straightforward compared to other risk models, clinical surgeons may still find the necessary mathematical calculations cumbersome in practice. The development of dedicated computer or mobile phone software capable of calculating GAME and similar risk models in real time could facilitate the effective utilization of these prognostic tools.
Collectively, the GAME score incorporated two novel (KRAS and TBS), one redefined (CEA levels) and two previously established (primary lymph node metastases and extrahepatic disease) prognostic factors and outperformed the most commonly used contemporary CRS, namely the Fong score in both the JHH cohort and the MSKCC cohort in which the latter originated. Importantly, GAME was not originally designed to guide patient selection for surgery but as it can be easily adapted to preoperative use, its role may eventually expand to encompass treatment selection. However, its prognostic value in medically treated patients will need to be assessed before this can occur. Ultimately, the inclusion of biomarkers of tumour biology (e.g KRAS status) in a clinical score for CRLM may have an impact in cases where the presence of extensive disease casts doubt on the role of aggressive surgical treatment.42
Supplementary Material
Supplemental Figure 1 Tumor burden score (TBS) was defined as the distance from the origin on a Cartesian plane that incorporated variables: maximum tumour size (x-axis) and number of liver lesions (y-axis). The Pythagorean theorem was then used to calculate the distance of any given point from the origin of the plane (0, 0).
Acknowledgments
Georgios Antonios Margonis was supported by the Bodossaki Foundation
Matthew J. Weiss was supported by the Drs. Keith and Valda Kaye Research Fund and the Carolyn Pastorini Research Fund
Footnotes
Conflicts of Interest / Financial Disclosures: None to report
References
- 1.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504; discussion 504–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505–510; discussion 511. [DOI] [PubMed] [Google Scholar]
- 4.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–764, 764–756. [DOI] [PubMed] [Google Scholar]
- 5.Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford). 2010;12:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. [DOI] [PubMed] [Google Scholar]
- 8.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 9.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueno H, Mochizuki H, Hatsuse K, Hase K, Yamamoto T. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–189. [DOI] [PubMed] [Google Scholar]
- 12.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 14.Margonis GA, Kim Y, Sasaki K, Samaha M, Amini N, Pawlik TM. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Cancer. 2016;122:2698–2707. [DOI] [PubMed] [Google Scholar]
- 15.Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, et al. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557–564. [DOI] [PubMed] [Google Scholar]
- 16.Akaike H. A New Look at the Statistical Model Identification. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected Papers of Hirotugu Akaike. New York, NY: Springer New York; 1998. p. 215–222. [Google Scholar]
- 17.Nakai T, Ishikawa H, Tokoro T, Okuno K. The clinical risk score predicts the effectiveness of adjuvant chemotherapy for colorectal liver metastasis. World J Surg. 2015;39:1527–1536. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Dennison AR, Robertson V, Jones MJ, Neal CP, Garcea G. Clinical risk scores in the current era of neoadjuvant chemotherapy for colorectal liver metastases. ANZ J Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, et al. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. Br J Surg. 2014;101:856–866. [DOI] [PubMed] [Google Scholar]
- 20.Margonis GA, Kim Y, Spolverato G, Ejaz A, Gupta R, Cosgrove D, et al. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015;150:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margonis GA, Spolverato G, Kim Y, Karagkounis G, Choti MA, Pawlik TM. Effect of KRAS Mutation on Long-Term Outcomes of Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. Ann Surg Oncol. 2015;22:4158–4165. [DOI] [PubMed] [Google Scholar]
- 22.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102:1175–1183. [DOI] [PubMed] [Google Scholar]
- 23.Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–626; discussion 626–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veen T, Soreide K. Can molecular biomarkers replace a clinical risk score for resectable colorectal liver metastasis? World J Gastrointest Oncol. 2017;9:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margonis GA, Sasaki K, Kim Y, Samaha M, Buettner S, Amini N, et al. Tumor Biology Rather Than Surgical Technique Dictates Prognosis in Colorectal Cancer Liver Metastases. J Gastrointest Surg. 2016;20:1821–1829. [DOI] [PubMed] [Google Scholar]
- 26.Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014;120:3965–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han CB, Li F, Ma JT, Zou HW. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: a meta-analysis and systematic review. Cancer Invest. 2012;30:741–747. [DOI] [PubMed] [Google Scholar]
- 28.Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:2622–2629. [DOI] [PubMed] [Google Scholar]
- 29.Paliogiannis P, Cossu A, Tanda F, Palmieri G, Palomba G. KRAS mutational concordance between primary and metastatic colorectal adenocarcinoma. Oncol Lett. 2014;8:1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunner SM, Kesselring R, Rubner C, Martin M, Jeiter T, Boerner T, et al. Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br J Surg. 2014;101:1681–1691. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Mo Q, Qin LX, Viale A, Maithel SK, Maker AV, et al. Gene expression profiles accurately predict outcome following liver resection in patients with metastatic colorectal cancer. PLoS One. 2013;8:e81680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balachandran VP, Arora A, Gonen M, Ito H, Turcotte S, Shia J, et al. A Validated Prognostic Multigene Expression Assay for Overall Survival in Resected Colorectal Cancer Liver Metastases. Clin Cancer Res. 2016;22:2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. [DOI] [PubMed] [Google Scholar]
- 34.Conrad C, You N, Vauthey JN. In patients with colorectal liver metastases, can we still rely on number to define treatment and outcome? Oncology (Williston Park). 2013;27:1078, 1083–1074, 1086. [PubMed] [Google Scholar]
- 35.Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F, et al. The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol. 2016;114:803–809. [DOI] [PubMed] [Google Scholar]
- 36.Tranchart H, Chirica M, Faron M, Balladur P, Lefevre LB, Svrcek M, et al. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg. 2013;37:2647–2654. [DOI] [PubMed] [Google Scholar]
- 37.Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: The evolution of determining prognosis. World J Gastrointest Oncol. 2013;5:207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WS, Kim MJ, Yun SH, Chun HK, Lee WY, Kim SJ, et al. Risk factor stratification after simultaneous liver and colorectal resection for synchronous colorectal metastasis. Langenbecks Arch Surg. 2008;393:13–19. [DOI] [PubMed] [Google Scholar]
- 39.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102. [DOI] [PubMed] [Google Scholar]
- 40.Leung U, Gonen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, et al. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki K MG, Andreatos N, et al. The prognostic utility of the “Tumor Burden Score” based on preoperative radiographic features of colorectal liver metastases. J Surg Oncol. 2017:9999:9991–9999. 10.1002/jso.24678. [DOI] [PubMed] [Google Scholar]
- 42.Soreide K, Sandvik OM, Soreide JA. KRAS mutation in patients undergoing hepatic resection for colorectal liver metastasis: a biomarker of cancer biology or a byproduct of patient selection? Cancer. 2014;120:3862–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Tumor burden score (TBS) was defined as the distance from the origin on a Cartesian plane that incorporated variables: maximum tumour size (x-axis) and number of liver lesions (y-axis). The Pythagorean theorem was then used to calculate the distance of any given point from the origin of the plane (0, 0).