Highlights
-
•
Low-income smokers preferred urine tests over breath tests to confirm cessation.
-
•
Rates of completing in-home urine tests were comparable to rates in prior studies.
-
•
Many digital photos of urine test results were inconclusive, limiting their value.
-
•
Urine tests are acceptable to low-income smokers, but need to yield clearer results.
Keywords: Smoking cessation, Biochemical verification, Cotinine, Low-income, Minority health
Abstract
Little is known about the acceptability and use of remote biochemical verification of self-reported cessation among low-income and racially diverse smokers. We compared responses to an in-person carbon monoxide breath test and in-home urine cotinine test among 270 adults who reported 7-day continuous abstinence at 6-month follow-up in a community-based randomized cessation trial. Half of participants (50%) reported annual household income below $10,000, one in four (28%) had not completed high school, and 69% were Black or African American. Regardless of whether the two tests were offered separately, sequentially, or as a head-to-head choice, participants were more likely to accept an offer to take the urine test than the breath test (89% vs. 32%), and complete it (46% vs. 13%). The proportion of participants completing the urine test and returning a digital photo of the test result is comparable to several studies completed with less disadvantaged samples. Self-report was confirmed by urine test for 74% of participants with a conclusive test result, although a high percentage (39%) of test results were inconclusive. In-home urine testing appears both acceptable and feasible for many low-income smokers, but challenges with testing technology and response rates currently limit its value to increase confidence in self-reports.
1. Introduction
In population-based, online and other minimal contact cessation studies, remote approaches to biochemical verification of self-reported quitting are often the only option. To date, this has mostly involved sending a test kit to participants and asking them to return it by mail or show the result with a digital photo or video call (Dahne, Tomko, McClure, Obeid, & Carpenter, 2020, Kim et al., 2018, Thrul et al., 2018).
In four US studies that have reported response rates to remote biochemical verification, rates ranged from 42 to 71%, with 64–88% confirming self-reported abstinence (Abroms et al., 2014, Cha et al., 2017, Stoops et al., 2019, Thrul et al., 2018). Most participants in these studies were white (73–84%) and college educated (47–80%), and only one study (Thrul et al., 2018) reported any income strata below $30,000 per year. All studies involved participants taking saliva samples for cotinine tests.
To expand upon findings from previous studies and increase their generalizability to a broader cross-section of smokers, we report new findings on biochemical verification in a community-based and racially diverse sample of low-income smokers. We compare two approaches – a carbon monoxide breath test and a urine cotinine test. We report the acceptance rate when participants are offered each test, their preference for one test or the other when offered a choice between the two, as well as completion rates and test results. To our knowledge, our study is the first to compare an in-person carbon monoxide breath test and an in-home urine cotinine test among low-income populations.
2. Methods
Data are from an ongoing community-based intervention trial testing effects of standard and specialized tobacco quitlines, with and without navigation to help address basic needs such as food, housing and utility bills (McQueen et al., 2019). Participants were adult daily smokers from across Missouri, identified when they called 2-1-1, a statewide community helpline for assistance with basic needs. Enrollment in the study began in June 2017, and analyses include data from participants who reported 7-day continuous abstinence from smoking on a 6-month follow-up survey between December 19, 2017 and April 2, 2020. Research procedures and materials were approved by the Human Research Protection Office at Washington University in St. Louis.
Participants who reported 7-day continuous abstinence at 6-month follow-up were offered biochemical verification in one of four ways: (1) breath test only; (2) urine test only; (3) urine test after being offered but not accepting or completing a breath test; or (4) choice of a breath or urine test. All offers were made by phone at the end of the 6-month follow-up survey. Participants who expressed interest in any test were re-contacted within 72 hours to confirm eligibility (not currently taking NRT, and for the urine test, have a mobile phone with the ability take and send photos) and obtain informed consent for biochemical verification. From August 1, 2019 to April 2, 2020, the eligibility/consent questions were added to the end of the 6-month follow-up survey to streamline the procedure.
For the breath test, a research team member called each participant, offered to meet them at a community location near their home to administer the test, and provided a $25 grocery store gift card for completing the test. Although smokers from across the state of Missouri were eligible for the study, the breath test was only offered to those in the St. Louis, MO metropolitan area, where the research team is physically located and could drive to meet participants. We used the coVita Micro + Smokerlyzer carbon monoxide (CO) breath test device developed by Bedfont Scientific (coVita, 2020). It measures the amount of CO exhaled and has been used to assess smoking status and validate abstinence (Bedfont Scientific Ltd, xxxx, Vasthare et al., 2018). We used the recommended cut point of 8 ppm CO to identify a participant as a smoker (SRNT Subcommittee on Biochemical Verification, 2002). Participants who completed the 6-month follow-up survey between December 19, 2017 and July 9, 2019 were offered this test; after July 9, 2019 we stopped offering breath tests due to low interest from participants.
For the urine test, we used NicAlert, an in-home, self-administered urine cotinine test. The test was offered to participants statewide (not just in St. Louis), and mailed to those who accepted. To use it, participants dipped a test strip into their urine sample at a depth of ½ inch for 20 seconds then laid it flat for 10–15 minutes. The result appeared as a red band within one of six “levels” on the test strip. Participants took a digital photo of the test strip and sent it to the study team by text or e-mail. Those returning the digital photo received a $35 grocery store gift card. NicAlert uses 100 ng/ml as the cut-point to identify someone as a smoker, corresponding to “Level 3” on the test strip. Digital photos were independently reviewed by two research team members and categorized as “unconfirmed abstinence” if the red band was at or above level 3, “confirmed abstinence” if it was below level 3, and “inconclusive” if the photo was unclear or a line could not be discerned; discrepancies between raters were resolved by a study investigator.
We began offering the urine test on July 18, 2018. In April 2020, NicAlert notified our team that its manufacturer had closed due to COVID-19 and test kits were no longer available. As a result, the last NicAlert tests in our inventory were mailed to participants who completed the 6-month follow-up survey by April 2, 2020.
A subset of participants who were offered the breath test and declined or did not complete it were later offered the urine test. These participants all completed the 6-month follow-up prior to July 18, 2018. Another group of participants were offered the choice of completing a breath test or a urine test. These participants all completed the 6-month follow-up between July 18, 2018 and July 9, 2019, and lived in the St. Louis, MO metropolitan area.
We report demographic characteristics and smoking history collected at baseline for all participants in this sample. Rates of test acceptance, completion and test results confirming self-reported abstinence are compared for each of the biochemical verification options. Descriptive statistics are reported for baseline variables in the full sample, comparing those who accepted a breath test or urine test when offered, and comparing those who completed versus did not complete either test. Differences are evaluated using chi-square tests for categorical variables and t-tests for continuous variables.
3. Results
There were 270 smokers who completed the 6-month follow-up survey between December 19, 2017 and April 2, 2020 and reported 7-day continuous abstinence from smoking. Most were Black or African American (69%) and half reported annual pre-tax household income below $10,000 (50%). One in four reported completing less than high school education (28%). Other sample characteristics are shown in Table 1.
Table 1.
Sample characteristics, by acceptance and completion of biochemical verification breath and urine tests (N = 270).
|
Frequency (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Total sample | Accepted breath test* | Accepted urine test* | p-value^ | Completed either test | Did not complete either test | p-value^ | |
| Sample characteristics | n = 270 | n = 35 | n = 190 | n = 58 | n = 212 | ||
| Age (years), mean (SD) | 49.1 (11.8) | 45.5 (12.7) | 49.0 (11.7) | 0.13 | 47.5 (10.1) | 49.6 (12.2) | 0.19 |
| Female | 203 (75.2) | 30 (85.7) | 137 (72.1) | 0.09 | 42 (72.4) | 161 (75.9) | 0.58 |
| Race | |||||||
| Black or African American | 186 (69.4) | 33 (94.3)† | 129 (68.6)† | 0.01† | 43 (75.4) | 143 (67.8) | 0.46 |
| White | 69 (25.7) | 2 (5.7)† | 49 (26.1)† | 11 (19.3) | 58 (27.5) | ||
| Other | 13 (4.9) | 0 (0.0)† | 10 (5.3)† | 3 (5.3) | 10 (4.7) | ||
| Hispanic | 8 (3.0) | 0 (0.0) | 6 (3.2) | 0.29 | 0 (0.0) | 8 (3.8) | 0.14 |
| Annual pre-tax household income | |||||||
| < $10,000 | 129 (50.2) | 19 (54.3) | 91 (50.8) | 0.56 | 32 (57.1) | 97 (48.3) | 0.29 |
| $10,000 - $19,999 | 78 (30.4) | 8 (22.9) | 56 (31.3) | 17 (30.4) | 61 (30.3) | ||
| ≥ $20,000 | 50 (19.5) | 8 (22.9) | 32 (17.9) | 7 (12.5) | 43 (21.4) | ||
| Education, | |||||||
| < High school | 76 (28.4) | 11 (31.4) | 57 (30.2) | 0.78 | 17 (29.8) | 59 (28.0) | 0.96 |
| High school/ GED | 78 (29.1) | 8 (22.9) | 54 (28.6) | 16 (28.1) | 62 (29.4) | ||
| > High school | 114 (42.5) | 16 (45.7) | 78 (41.3) | 24 (42.1) | 90 (42.7) | ||
| Insurance status | |||||||
| Medicaid | 75 (28.4) | 8 (23.5) | 56 (29.8) | 0.25 | 16 (27.6) | 59 (28.6) | 0.77 |
| Medicare | 30 (11.4) | 5 (14.7) | 21 (11.2) | 6 (10.3) | 24 (11.7) | ||
| Dual Medicaid and Medicare | 70 (26.5) | 6 (17.6) | 51 (27.1) | 14 (24.1) | 56 (27.2) | ||
| Uninsured | 59 (22.3) | 12 (35.3) | 37 (19.7) | 16 (27.6) | 43 (20.9) | ||
| Gateway to Better Health‡ | 21 (8.0) | 3 (8.8) | 14 (7.4) | 3 (5.2) | 18 (8.7) | ||
| Veterans Affairs | 9 (3.4) | 0 (0.0) | 9 (4.8) | 3 (5.2) | 6 (2.9) | ||
| Cigarettes per day, mean (SD) | 13.4 (9.2) | 12.8 (10.1) | 13.0 (8.3) | 0.88 | 12.2 (7.1) | 13.7 (9.7) | 0.20 |
| Age smoking initiation (years), mean (SD) | 16.5 (6.1) | 16.1 (5.8) | 16.6 (6.1) | 0.67 | 15.7 (4.7) | 16.8 (6.5) | 0.18 |
* “Accepted breath test” includes all those who indicated an interest in the breath test. “Accepted urine test” includes those who indicated an interest in the urine test when offered the choice between urine and breath tests or offered the urine test alone, it excludes those who were offered breath test then urine test.
^Chi-square tests for categorical variables and t-tests for continuous variables.
† The breath test was offered exclusively in the St. Louis, MO metropolitan area, which has a much higher Black or African American population than elsewhere in MO, thus the race difference.
‡ Gateway to Better Health is a health care program for uninsured adults in St. Louis City and County who are not eligible for Medicaid or Medicare.
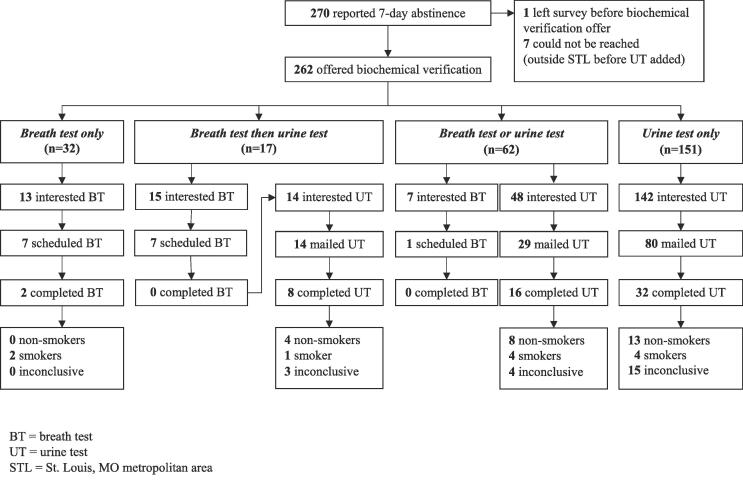
Fig. 1 shows results for each of the four biochemical verification options. Thirty-two participants were offered the breath test only, because they reported quitting before July 18, 2018, lived in the St. Louis, MO metropolitan area, and could not be reached once the urine test option was added. Of these, 13 participants (41%) expressed interest in completing the breath test, but only seven (22%) scheduled an appointment to take the test, and just two (6%) kept the appointment and took the test. In neither case did the test result confirm self-reported abstinence.
Fig. 1.
Flow diagram of interest, use and results of biochemical verification by breath test (BT) or urine test (UT) among low-income adults who reported 7-day abstinence from smoking at 6-month follow-up (N = 270).
When urine testing was added on July 18, 2018, we were able to reach 17 participants who had previously been offered a breath test, but declined or did not complete it. Each was offered the urine test (breath test then urine test in Fig. 1). Of the 17, 14 accepted the offer and were mailed a test (82%). Eight of these participants returned a digital photo of the test result (57%); four confirmed self-reported abstinence, one did not, and three of the photos were inconclusive.
Among 62 participants who were offered the choice between breath test or urine test, 7 (11%) expressed interest in the breath test and 48 (77%) expressed interest in the urine test. Of those interested the breath test, only one participant scheduled an appointment to take the test, but did not keep the appointment or take the test. Of those interested in the urine test, 17 could not be reached for eligibility and consent and 2 were no longer interested, so 29 were sent a test kit in the mail. Of these, 16 (55%) returned a digital photo of the test result; eight confirmed self-reported abstinence, four did not, and four of the photos were inconclusive.
One hundred fifty-one participants were offered the urine test only; 100 who completed the 6-month follow-up survey after July 9, 2019 and 51 who lived outside St. Louis when the breath test was offered. Of the 151, nearly all (n = 142; 94%) expressed interest in the urine test when offered. Of those interested, 29 could not be reached for eligibility and consent, 4 were no longer interested, and 29 did not meet eligibility criteria. The remaining 80 participants were mailed a test kit and 32 of them (40%) returned a digital photo of the test result. Of the 32 photos, 13 confirmed self-reported abstinence, four did not, and 15 were inconclusive.
Participants were more likely to accept an offer to take the urine test than the breath test and also more likely to complete it. Of 32 participants offered breath test alone, 13 were interested, while 142 of the 151 participants offered urine test alone were interested (41% vs. 94%; χ2(1) = 58.1, p < .001).
Of all breath test appointments that were scheduled (n = 15), 2 were completed, while 56 of the 123 urine testing kits that were mailed were completed (13% vs. 46%; χ2(1) = 5.7, p < .05). There were no significant differences in any demographic characteristics or tobacco use variables between those who accepted the urine versus breath test or between those who completed versus did not complete a test, except for a spurious association with race, explained by the breath test being offered only in St. Louis, where the proportion African American population greatly exceeds that of the rest of Missouri (Table 1).
4. Discussion
Participants were much more likely to accept and complete an in-home urine test than a breath test administered at a community location near them. This finding was consistent across separate sequential, and head-to-head comparisons of the two tests, and did not vary by participant characteristics measured in the study. The proportion of participants completing and returning a urine test – 46% of those who were mailed a test kit across all conditions in this study (56 completed urine tests out of 123 total mailed urine tests) – was comparable to several prior studies (Stoops et al., 2019; Thrul et al., 2018) but lower than others (Abroms et al., 2014, Cha et al., 2017) conducted in samples of less disadvantaged smokers. The relatively high rate of inconclusive test result photos – 39% of all photos across all conditions – means the functional return rate in our sample was lower, just 28%.
The general consensus among smoking cessation researchers is that biochemical verification is often neither feasible nor necessary in minimal-contact intervention trials conducted online, by phone or in community settings (Cha et al., 2017, Cheung et al., 2017, SRNT Subcommittee on Biochemical Verification, 2002), especially considering the unique challenges of executing it in underserved populations (Glasgow et al., 1993). Remote testing has been touted as a promising solution to both. The acceptability of in-home urine testing in our sample, and participants’ willingness and ability to share results using digital photos is encouraging, especially if the tests could be improved to yield a more easily discernable result. However, as tested in this study among a sample of very low-income smokers, the value of remote biochemical verification to increase confidence in self-report may be limited.
The comparisons made in this study were more exploratory than planned, a priori, and thus they have several limitations. Participants were not randomly assigned to different biochemical verification tests, the breath test was offered only to participants living in one city, and the amount of compensation provided for taking a test differed between the breath ($25) and urine ($35) tests due to when each was introduced. However, when participants were presented with the choice between breath and urine test, they were unaware of this difference, and their preference for the urine test was clear. Due to the design of our study, it is not possible to disentangle the effects of in-person versus in-home tests and breath versus urine tests.
Another limitation is the high rate of inconclusive results from the in-home urine tests. It is not clear whether a high rate of inconclusive results is inherent to the test or due to delays between completing the test and photographing the result. Future research should try to understand reasons for inconclusive test results and minimize their occurrence. It is also possible that participants’ test results could have been influenced by other tobacco use not assessed at follow-up.
Although our two biochemical verification methods were selected to address challenges experienced in low-income populations, the majority of participants who reported abstinence from smoking did not have their self-report biochemically verified. For the breath test in particular, we observed significant scheduling and transportation barriers, despite our team’s willingness to reschedule appointments and meet at familiar and convenient community locations. Although some participants declined to complete the urine test because they did not have a phone or camera to capture and send test results to research staff, these urine test-specific obstacles were expressed much less often than breath test obstacles. These finding suggest that in-home urine tests are acceptable and feasible for many low-income smokers in population-based studies.
CRediT authorship contribution statement
Rachel Garg: Conceptualization, Methodology, Formal analysis, Visualization, Writing - review & editing. Amy McQueen: Conceptualization, Methodology, Writing - review & editing. Jennifer Wolff: Project administration, Writing - review & editing. Taylor Butler: Project administration. Tess Thompson: Writing - review & editing. Charlene Caburnay: Writing - review & editing. Matthew W. Kreuter: Conceptualization, Methodology, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Role of funding source
Funding for this study was provided by the National Cancer Institute of the National Institutes of Health under award number R01CA201429. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributors
MK is PI of the study. He collaborated with Co-PI AM and RG, CC, and TT to develop the biochemical verification protocol. He collaborated with RG to draft the manuscript, collect and integrate feedback from all authors, and submit the final version of the manuscript. RG also managed and cleaned the data, conducted the analyses, and created the data table and flow diagram. TB and JW coordinated the distribution of biochemical verification tests, and collection and interpretation of test results. All authors read and contributed to writing this paper and reviewed and approved the final version.
Acknowledgements
The authors are grateful to our research partners at 2-1-1 in Missouri, our study participants for their time and trust, and the many research team members who helped carry out this study.
Contributor Information
Rachel Garg, Email: rminson@wustl.edu.
Amy McQueen, Email: amcqueen@wustl.edu.
Jennifer Wolff, Email: j.wolff@wustl.edu.
Taylor Butler, Email: butler.t@wustl.edu.
Tess Thompson, Email: tessthompson@wustl.edu.
Charlene Caburnay, Email: ccaburnay@wustl.edu.
Matthew W. Kreuter, Email: mkreuter@wustl.edu.
References
- Abroms L.C., Boal A.L., Simmens S.J., Mendel J.A., Windsor R.A. A randomized trial of Text2Quit: A text messaging program for smoking cessation. American Journal of Preventive Medicine. 2014;47(3):242–250. doi: 10.1016/j.amepre.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedfont Scientific Ltd. (n.d.). Smokerlyzer Range User Manual. Retrieved December 1, 2020, from https://www.bedfont.com/documents/2910-LAB679%20Smokerlyzer%20Manual%20-%20Issue%204.pdf.
- Cha S., Ganz O., Cohn A.M., Ehlke S.J., Graham A.L. Feasibility of biochemical verification in a web-based smoking cessation study. Addictive Behaviors. 2017;73:204–208. doi: 10.1016/j.addbeh.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.L., de Ruijter D., Hiligsmann M., Elfeddali I., Hoving C., Evers S.M., de Vries H. Exploring consensus on how to measure smoking cessation. A Delphi study. BMC Public Health. 2017;17(1):890. doi: 10.1186/s12889-017-4902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- coVita. Micro+ Smokerlyzer. (2020). Retrieve September 19, 2020, from https://www.covita.net/product/micropro-smokerlyzer/.
- Dahne J., Tomko R.L., McClure E.A., Obeid J.S., Carpenter M.J. Remote methods for conducting tobacco-focused clinical trials. Nicotine & Tobacco Research. 2020;22(12):2134–2140. doi: 10.1093/ntr/ntaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Mullooly J.P., Vogt T.M., Stevens V.J., Lichtenstein E., Hollis J.F.…Vogt M.R. Biochemical validation of smoking status: Pros, cons, and data from four low-intensity intervention trials. Addictive Behaviors. 1993;18(5):511–527. doi: 10.1016/0306-4603(93)90068-k. [DOI] [PubMed] [Google Scholar]
- Kim S.S., Bernstein K., Shim O., Fang H., McKee S., Ziedonis D. Remote Biochemical Verification of Smoking Abstinence via Mobile-Phone Video Call. Journal of Mobile Technology in Medicine. 2018;7(1):1–8. [Google Scholar]
- McQueen A., Roberts C., Garg R., Caburnay C., Fu Q., Gordon J.…Kreuter M. Specialized tobacco quitline and basic needs navigation interventions to increase cessation among low income smokers: Study protocol for a randomized controlled trial. Contemporary Clinical Trials. 2019;80:40–47. doi: 10.1016/j.cct.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stoops W.W., Johnson M.F., Strickland J.C., Knudsen H.K., Gilbert G.H., Massingale S.D.…Slade E. Feasibility of Collecting Saliva for Biological Verification of Tobacco Use Status in Dental Practices and Patients' Homes: Results from the National Dental PBRN. Community Dental Health. 2019;36(3):187–189. doi: 10.1922/CDH_4474Stoops03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrul J., Meacham M.C., Ramo D.E. A novel and remote biochemical verification method of smoking abstinence: Predictors of participant compliance. Tobacco Prevention & Cessation. 2018;4 doi: 10.18332/tpc/90649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasthare R., Kumar S., Arron L.Y. Carbon monoxide breath analyzers and its role in tobacco cessation: A narrative review of literature. Journal of International Oral Health. 2018;10(2):71–76. [Google Scholar]