Abstract
Diacylglycerol (DG) is a well-established lipid second messenger. Sphingomyelin synthase (SMS)-related protein (SMSr) produces DG and ceramide phosphoethanolamine (CPE) by the transfer of phosphoethanolamine from phosphatidylethanolamine (PE) to ceramide. We previously reported that human SMSr overexpressed in COS-7 cells significantly increased DG levels, particularly saturated and/or monounsaturated fatty acid–containing DG molecular species, and provided DG to DG kinase (DGK) δ, which regulates various pathophysiological events, including epidermal growth factor–dependent cell proliferation, type 2 diabetes, and obsessive–compulsive disorder. However, mammalian SMSr puzzlingly produces only trace amounts of CPE/DG. To clarify this discrepancy, we highly purified SMSr and examined its activities other than CPE synthase. Intriguingly, purified SMSr showed a DG-generating activity via hydrolysis of PE, phosphatidic acid (PA), phosphatidylinositol (PI), and phosphatidylcholine (PC) in the absence of ceramide. DG generation through the PA phosphatase (PAP) activity of SMSr was approximately 300-fold higher than that with PE and ceramide. SMSr hydrolyzed PI ten times stronger than PI(4,5)bisphosphate (PI(4,5)P2). The PAP and PC-phospholipase C (PLC) activities of SMSr were inhibited by propranolol, a PAP inhibitor, and by D609, an SMS/PC-PLC inhibitor. Moreover, SMSr showed substrate selectivity for saturated and/or monounsaturated fatty acid–containing PA molecular species, but not arachidonic-acid-containing PA, which is exclusively generated in the PI(4,5)P2 cycle. We confirmed that SMSr expressed in COS-7 cells showed PAP and PI-PLC activities. Taken together, our study indicated that SMSr possesses previously unrecognized enzyme activities, PAP and PI/PE/PC-PLC, and constitutes a novel DG/PA signaling pathway together with DGKδ, which is independent of the PI(4,5)P2 cycle.
Keywords: sphingomyelin synthase-related protein, phosphatidic acid phosphatase, phosphatidylinositol phospholipase C, phosphatidylethanolamine phospholipase C, diacylglycerol kinase
Abbreviations: Cer, ceramide; CHS, cholesteryl hemisuccinate; CPE, ceramide phosphoethanolamine; DDM, n-dodecyl-β-D-maltoside; DG, diacylglycerol; DGK, diacylglycerol kinase; ER, endoplasmic reticulum; I.S., internal standard; LPP, lipid phosphate phosphatase; MG-PLC, multi-glycerophospholipid PLC hydrolase; PA, phosphatidic acid; PAP, phospholipid phosphatase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PLC, phospholipase C; PMSF, phenylmethylsulfonyl fluoride; SM, sphingomyelin; SMS, sphingomyelin synthase; SMSr, sphingomyelin synthase-related protein
Diacylglycerol (DG) is a well-established lipid second messenger that activates protein kinase C (PKC) (1, 2). In addition to PKC, DG regulates a wide variety of signal transduction proteins, such as β2-chimaerin, protein kinase D, Ras guanyl nucleotide-releasing protein, and Unc-13 (3, 4, 5, 6). In eukaryotes, DG is generated from two major lipids: the glycerolipids, such as monoacylglycerol and triacylglycerol (7, 8, 9) via acyltransferase and lipase, respectively, and glycerophospholipids, such as phosphatidic acid (PA), phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), phosphatidylcholine (PC), and phosphatidylethanolamine (PE) via PA phosphatase (PAP)/lipid phosphate phosphatase (LPP) or phospholipase C (PLC) (10, 11, 12, 13). Although type II PAP/lipid phosphate phosphatase (PAP2/LPP), lipin (type I PAP) and PI(4,5)P2-specific phospholipase C (PLC), as DG-generating enzymes, have been cloned and extensively studied in mammals (10, 14, 15), the molecular entities (the genes and proteins) of PC- and PE-specific PLCs have not been identified until now (12, 16).
Sphingomyelin synthase (SMS) is a DG-generating enzyme (17, 18). There are three isoforms, SMS1, SMS2, and SMS-related protein (SMSr) (Table 1). Huitema et al. (19) identified SMS genes via a homology search for the sequences encoding the membrane proteins containing active site motifs common to PAP2/LPP. The SMS1 and SMS2 proteins generate DG and SM via the transfer of phosphocholine from PC to ceramide (Table 1). SMSr is an endoplasmic reticulum (ER)-resident, six-transmembrane protein and has no SMS activity but displays ceramide phosphoethanolamine (CPE) synthase (CPES) activity via the transfer of phosphoethanolamine from PE to ceramide (20) (Table 1). Puzzlingly, mammalian SMSr produces only trace amounts of CPE (approximately 300-fold lower than SM levels produced by SMS1) (20) (Table 1). Moreover, CPE levels in mammalian cells are extremely low (0.002%–0.005 mol% of total phospholipids) (21). Hence, it is supposed that SMSr has little or no ceramide-dependent DG-generating activity.
Table 1.
Comparison of SMSr with other SMSs
| Properties | SMSr (current study) | SMSr (previous reports) | SMS1 | SMS2 | |
|---|---|---|---|---|---|
| Subcellular localization | ER (active site: lumen) (20, 47) | Golgi (19, 73, 74) | Plasma membrane, Golgi (19, 73) | ||
| Reaction | Substrates | 1. PA 2. PI>PIP2 (selectivity 10:1) 3. PE 4. PC 5. PG |
Cer + PE (20) (approximately 300-fold lower than SM levels produced by SMS1) | 1. Cer + PC (19, 74) 2. Cer + PE (75) |
1. Cer + PC (19) 2. Cer + PE (47, 75) |
| Products (Lipids) | DG | CPE + DG | 1. SM, DG 2. CPE, DG |
1. SM, DG 2. CPE, DG |
|
| Inhibitor | Propranolol | PAP activity PC-PLC activity |
Unknown | Unknown | Unknown |
| D609 | PAP activity PC-PLC activity |
Unknown | SMS activity (40, 76, 77) | SMS activity (76, 77) | |
| Others (SM synthesis inhibitor) | Marabaricone C (78) SAPA (79) |
α-aminonitrile (compound D2) (80) 2-quinolone (77) 2-benzyloxybenzamides (Ly93) (81) Marabaricone C (78) |
|||
Recently, we demonstrated that SMSr interacted with the δ isozyme of DG kinase (DGKδ) (22, 23, 24), which is involved in the pathogenesis of type 2 diabetes (25, 26), obsessive–compulsive disorder (27, 28), and epidermal growth factor–dependent cell proliferation (29), via their sterile α motif domains (SAMDs) (30). Moreover, overexpression of both SMSr and DGKδ, but not of SMSr or DGKδ alone, enhanced PA production in COS-7 cells (30). In particular, the levels of saturated fatty acid (SFA)- and/or monounsaturated fatty acid (MUFA)-containing PA species, including 16:1/16:1-PA, 16:0/16:1-PA, 16:0/16:0-PA, 16:1/18:1-PA, and 16:0/18:1-PA, which were also produced by DGKδ in high-glucose-stimulated C2C12 myoblast cells (31), were significantly increased. Furthermore, SMSr overexpressed in COS-7 cells generated SFA- and/or MUFA-containing DG species. Therefore, it is possible that SMSr acts upstream of DGKδ and provides SFA- and/or MUFA-containing DG.
Taken together, SMSr can supply DG to DGKδ (31), whereas SMSr has little or no ceramide-dependent DG-generating activity (CPES activity) (20, 21). These contradictory results prompted us to postulate that SMSr possesses other DG-generating activity in addition to CPES activity. Here, we report that human SMSr, which was expressed in Sf9 cells and is highly purified, displayed DG-generating activity via hydrolysis of PA, PI, PE, and PC in the absence of ceramide. The PAP, PI-phospholipase C (PLC), PE-PLC, and PC-PLC activities were much stronger than the CPES activity (∼300-fold, ∼10-fold, ∼4-fold, and ∼3-fold, respectively). Intriguingly, SMSr exhibited a substrate specificity for SFA- and/or MUFA-containing PA and PC molecular species, but not polyunsaturated fatty acids (PUFA)-containing PA and PC. Taken together, we revealed the previously unrecognized enzyme activities of mammalian SMSr, novel PAP, PI-PLC, PE-PLC, and PC-PLC activities, to produce SFA and/or MUFA-containing DG molecular species independent of ceramide in the ER membrane.
Results
Purification of human SMSr
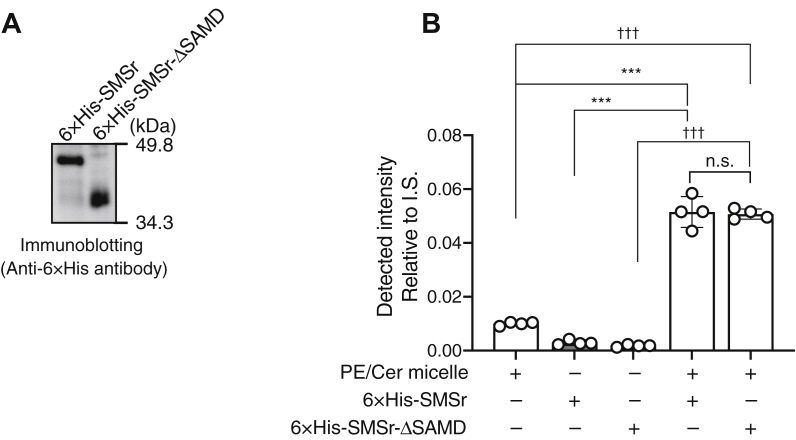
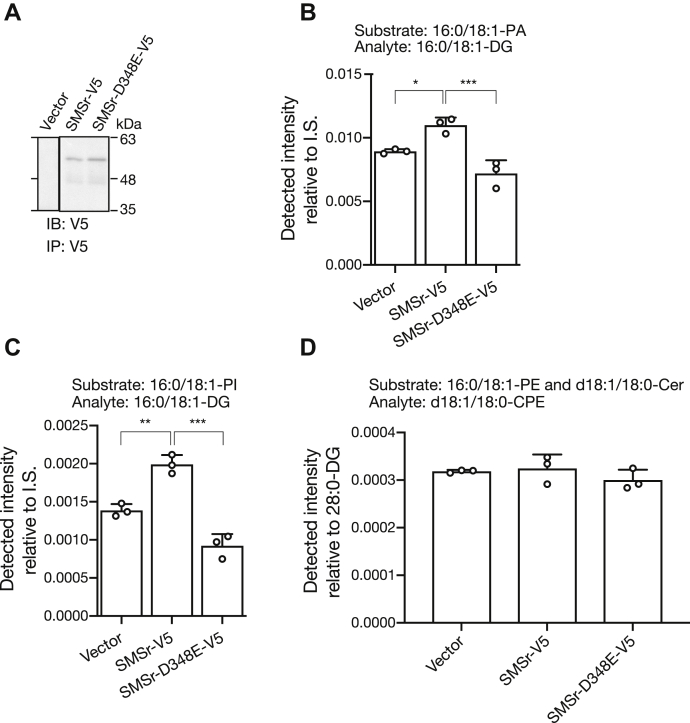
To determine whether SMSr possesses other DG-generating activity in addition to CPES activity, we first expressed N-terminal hexahistidine (6×His)-tagged human full-length (FL) SMSr and SMSr-ΔSAMD, which lacks an oligomer formation domain, SAMD (32, 33), in Sf9 cells and purified them from Sf9 cell membranes using Ni-affinity chromatography (Fig. 1A). SMSr-ΔSAMD showed no significant difference in DG production levels compared with FL-SMSr (Fig. 1B). However, the SMSr-ΔSAMD yield was approximately six-fold higher than that of FL-SMSr (data not shown). Therefore, we employed SMSr-ΔSAMD for further investigation.
Figure 1.
DG-generating activity of partially purified SMSr in the presence of PE and ceramide.A, partial purification of 6× His-SMSr and 6× His-SMSr-ΔSAMD expressed in Sf9 cells only via Ni-NTA agarose affinity chromatography. Partially purified 6× His-tagged proteins were detected via immunoblotting using anti-6× His antibody (PM032, Medical & Biological Laboratories, 1:1000 dilution). B, DG-generating activities of 6× His-tagged SMSr and SMSr-ΔSAMD were measured using LC-MS/MS. Partially purified 6× His-tagged proteins only via Ni-NTA agarose affinity chromatography (100 μl of sample) were used for the enzyme assay. The substrates (100 μl of 1 mM d18:1/18:0-ceramide, 1 mM 16:0/18:1-PE and 50 mM octyl-β-D-glucoside mixed-micelles) were used for the enzyme activity assay. The values are presented as the mean ±S.D. (n = 3). ∗∗∗p < 0.005 (versus 6× His-SMSr), †††p < 0.005 (versus 6× His-SMSr-ΔSAMD), n.s., not significant. PE and Cer mixed micelles contained detectable 16:0/18:1-DG (0.547 pmol/sample) that probably came from a PE stock solution. However, it was negligible because contamination rate was 0.0005%.
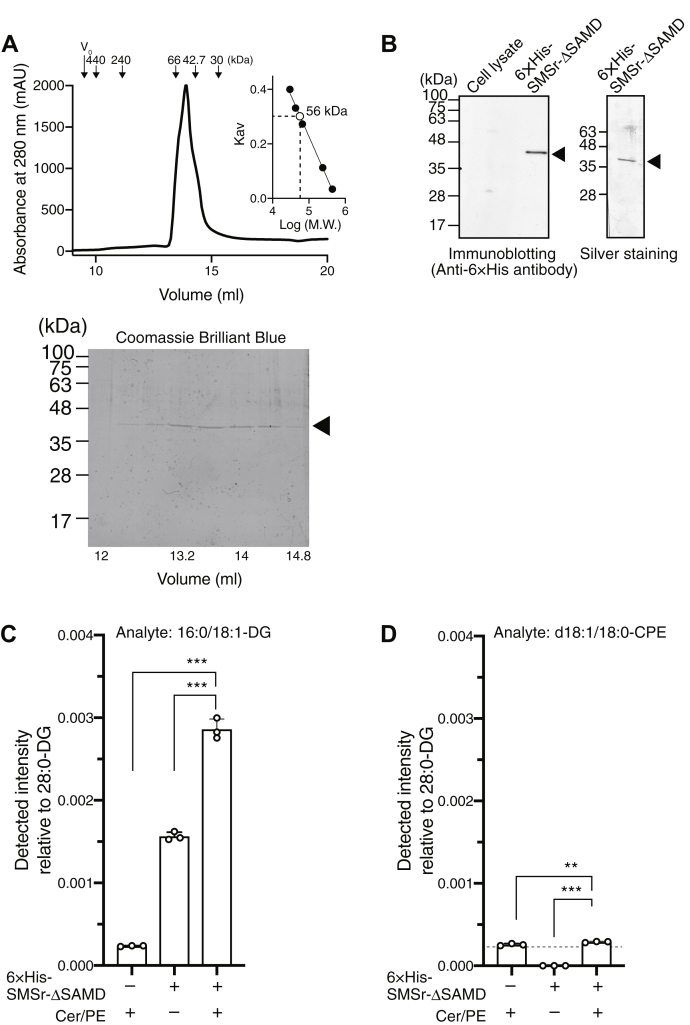
Next, to further purify 6× His-SMSr-ΔSAMD, size-exclusion chromatography was conducted after Ni-affinity chromatography. SMSr-ΔSAMD was eluted as a single peak in a volume of 13.9 ml (∼56.1 kDa) (Fig. 2A). Thus, the purified 6× His-SMSr-ΔSAMD is likely to be a monomer because its calculated molecular mass is 40.8 kDa (SMSr-ΔSAMD: 39.2 kDa +6×His: 1.6 kDa). Immunoblotting and silver staining showed that a single protein band at approximately 40 kDa was detected (Fig. 2B), indicating that 6× His-SMSr-ΔSAMD was obtained with a high purity. The yield was ∼6 μg per 1 L of an Sf9 cell culture. We confirmed that the purified SMSr-ΔSAMD slightly produced DG in the presence of PE and ceramide micelles (Fig. 2C). However, the DG-generating activity was exceedingly low (∼3 pmol/mg/min). Moreover, only trace amounts of CPE were generated by the purified SMSr-ΔSAMD (Fig. 2D).
Figure 2.
Purification of SMSr-ΔSAMD.A, elution profile of 6× His-SMSr-ΔSAMD from size-exclusion chromatography. The inset shows the calibration curve constructed using ferritin (440 kDa), catalase (240 kDa), bovine serum albumin (66 kDa), ovalbumin (42.7 kDa), and carbonic anhydrase (30 kDa). V0 is the void volume of the column (9.52 ml). The fractions corresponding to the peaks of SMSr-ΔSAMD were collected and concentrated. The separated proteins were detected by an SDS-PAGE (12%) analysis followed by Coomassie blue staining. B, purified 6× His-SMSr-ΔSAMD was analyzed via SDS–polyacrylamide gel electrophoresis (12% gel) followed by silver staining or immunoblotting using anti-6× His antibody (PM032, Medical & Biological Laboratories). C, the DG-generating activities of purified SMSr-ΔSAMD were measured using LC-MS/MS. d18:1/18:0-Cer and 16:0/18:1-PE micelles were used as substrates. The detected intensity of 16:0/18:1-DG in the sample was quantified using an internal standard (I.S.) (0.5 ng/μl of 14:0/14:0-DG). The values are presented as the mean ± S.D. (n = 3). Purified SMSr-ΔSAMD contained detectable 16:0/18:1-DG that probably came from Sf9 cell membranes. We calculated DG-generating activity of SMSr-ΔSAMD after subtracting these backgrounds. D, the CPES activity of SMSr-ΔSAMD was measured using LC-MS/MS. d18:1/18:0-Cer and 16:0/18:1-PE micelles were used as substrates. In positive ion mode, d18:1/18:0-CPE [M–H]+ (m/z 689.6) was selected as the precursor ion (Q1). The long-chain base (m/z 300.3) generated by CID of m/z 689.6 was selected as the product ion (Q3). The detected intensity of CPE in the sample was quantified using 0.5 ng/μl of 1,2-dimyristoyl-sn-glycerol (28:0-DG). The values are presented as the mean ±S.D. (n = 3).
Enzymological characterization of SMSr activity
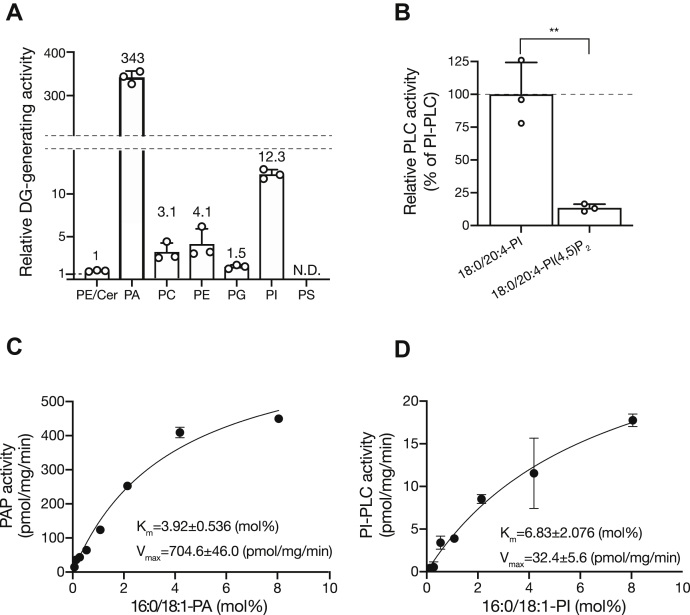
To test whether SMSr-ΔSAMD hydrolyzes other phospholipids, we compared the hydrolysis activity of 10 μl (0.03 μg/μl) of purified SMSr-ΔSAMD against various phospholipids (1-palmitoyl-2-oleoyl (16:0/18:1)-PA, 16:0/18:1-PC, 16:0/18:1-PE, 16:0/18:1-PG, 16:0/18:1-PI and 16:0/18:1-PS) in vitro by incubating the substrate micelle mixture followed by quantitation of the produced DG levels using liquid chromatography–tandem mass spectrometry (LC-MS/MS) (30, 34) (Fig. 3A). Compared with the DG-generating activity in the presence of ceramide and 16:0/18:1-PE (substrates for CPES), SMSr showed a much more intensive PAP activity (∼340-fold), even in the absence of ceramide. The enzyme also has stronger PI-PLC (∼12-fold), PE-PLC (∼4-fold), PC-PLC (∼3-fold), and PG-PLC (∼1.5-fold) activities. However, the PS hydrolysis activity of SMSr was not detectable. To further investigate the selectivity of SMSr for phosphoinositides, we compared the hydrolysis activities of purified SMSr-ΔSAMD against 18:0/20:4-PI and 18:0/20:4-PI(4,5)P2 (Fig. 3B). The enzyme showed a ten-fold stronger PI-PLC activity than the PI(4,5)P2-PLC activity. This result indicates that the catalytic selectivity for the phosphoinositides of SMSr is opposite to that of mammalian PI-PLC (PI(4,5)P2-PLC), which hydrolyzes PI(4,5)P2 much more strongly than PI (PI(4,5)P2:PI = 50:1) (Table 2) (35).
Figure 3.
Enzymological characterization of SMSr-ΔSAMD activity.A, purified 6× His-SMSr-ΔSAMD (10 μl (0.03 μg/μl) of sample) was used for the enzyme assay using N-Stearoyl-D-Sphingosine (d18:1/18:0-Cer) and 1-palmitoyl-2-oleoyl (16:0/18:1)-PE mixed micelles (Cer/PE), 16:0/18:1-PA, 16:0/18:1-PC, 16:0/18:1-PE, 16:0/18:1-PG, 16:0/18:1-PI, and 16:0/18:1-PS, as substrates. Relative DG-generating activities were compared with that in the presence of Cer/PE (0.8653 pmol/mg/min) (set to 1). The results are presented as the mean ± S.D. (n = 3). B, comparison of the enzyme activities for SMSr-ΔSAMD with 18:0/20:4-PI and 18:0/20:4-PI(4,5)P2 as substrates. The PLC activities of SMSr-ΔSAMD were measured using LC-MS/MS. The relative PIP2-PLC activity was standardized by comparison with the PI-PLC activity (7.97 pmol/mg/min) (set to 100%). The values are presented as the mean ± S.D. (n = 3). ∗∗p < 0.01. C and D, enzyme kinetic analyses of purified SMSr with (C) 16:0/18:1-PA and (D) 16:0/18:1-PI. The DG-generating activities were plotted as a function of (C) 16:0/18:1-PA concentration and (D) 16:0/18:1-PI concentration in mixed micelle (mol%). The values are the averages of triplicate determinations. The data are represented as the mean ± S.D.
Table 2.
Comparison of SMSr with other DG-generating enzymes
| Properties | SMSr (current study) | SMSr (previous reports) | LPP (PAP2) | Lipin-1 | PI(4,5)P2-PLC | PE-PLC | PC-PLC | |
|---|---|---|---|---|---|---|---|---|
| Subcellular localization | ER (active site: lumen) (20, 47) | PM>>ER, Golgi, endosome (14, 42, 51) | Cytosol or nucleus (82) | Cytosol (10, 35) | Unknown | Membrane bound? (83, 84, 85) | ||
| Reaction | Substrates | 1. PA 2. PI>PIP2 (selectivity 10:1) 3. PE 4. PC 5. PG |
Cer + PE | 1. PA 2. LPA 3. S1P 4. C1P |
PA | PI(4,5)P2>PI (selectivity 50:1) (54) | PE | PC |
| Products (Lipids) | DG | CPE + DG (20) | 1. DG 2. MG 3. Sphingosine 4. Cer |
DG | DG | DG | DG | |
| Inhibitor | Propranolol | PAP activity PC-PLC activity |
Unknown | PAP: inhibit (slightly) (36, 71) | inhibit (strongly) (37) | Unknown | Unknown | Unknown |
| D609 | PAP activity PC-PLC activity |
Unknown | Unknown | Unknown | Unknown | Inhibit (∼100 μM) (13) | Inhibit (∼100 μM) (40, 72) | |
| Kinetic parameter (Km) | 16:0/18:1-PA: 3.92 ± 0.536 mol% (97.4 ± 13.3 μM) 16:0/18:1-PI: 6.83 ± 2.08 mol% (169.7 ± 51.7 μM) |
Unknown | PA (36) LPP1: 3.4 ± 0.05 mol% LPP2: 0.44 ± 0.07 mol% LPP3: 0.61 ± 0.03 mol% |
PA (37) Lipin-1α: 4.2 ± 0.11 mol% Lipin-1β: 4.5 ± 0.07 mol% Lipin-1γ: 4.3 ± 0.14 mol% |
PLCδ1 (86) PI: 130 μM PI(4,5)P2: 170 μM |
Unknown | Unknown | |
These results suggested that SMSr not only acted as CPES but also a DG-generating enzyme through PAP, PI-PLC, PE-PLC, PC-PLC, PIP2-PLC, and PG-PLC activities. Next, a kinetic analysis of the enzyme was performed using n-dodecyl-β-D-maltoside (DDM)/Cholesteryl hemisuccinate (CHS)/phospholipid-mixed micelles. The Km value for 16:0/18:1-PA was 3.92 ± 0.536 mol% (Vmax: 704.6 ± 46 pmol/mg/min) (Fig. 3C), and the value for 16:0/18:1-PI was 6.83 ± 2.076 (mol%) (Vmax: 32.4 ± 5.6 pmol/mg/min) (Fig. 3D). The Km value and Vmax for 16:0/18:1-PA are comparable with those of LPPs (36) and lipin-1s (37) (Table 2).
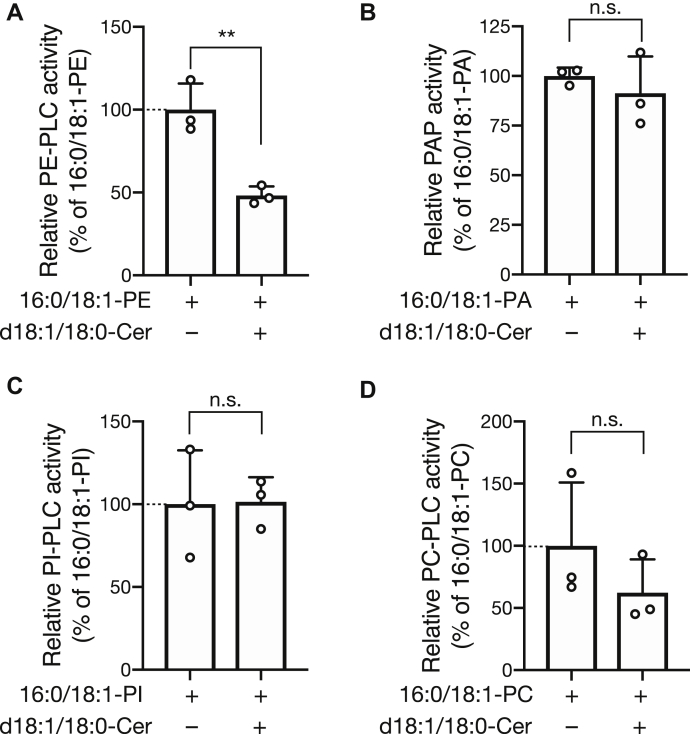
Because DG production activity by SMSr-ΔSAMD in the presence of d18:1/18:0-ceramide and PE was lower than that in presence of PE alone (Fig. 3A), we assumed that ceramide affects DG-generating activity of SMSr-ΔSAMD. Thus, the effects of d18:1/18:0-ceramide on hydrolysis activities of SMSr-ΔSAMD for other glycerophospholipids (PA, PI, and PC) were determined (Fig. 4). We confirmed that PE-PLC activity of SMSr-ΔSAMD was significantly decreased in presence of d18:1/18:0-ceramide (Fig. 4A). However, ceramide had no detectable effects on hydrolysis of PA, PI, and PC (Fig. 4, B–D), suggesting that the inhibitory effect of ceramide is PE selective.
Figure 4.
Effect of d18:1/18:0-ceramide on the DG-generating activity of SMSr-ΔSAMD. PE-PLC (A), PAP (B), PI-PLC (C), and PC-PLC (D) activities of the SMSr-ΔSAMD were analyzed in the presence or absence of 50 μM (2.1 mol%) d18:1/18:0-Cer, which is a substrate of SMSr as a CPE synthase (20). The values are presented as percentages of the activity of SMSr-ΔSAMD in the absence of d18:1/18:0-Cer (−) (16:0/18:1-PE-PLC, 7.42 pmol/mg/min;16:0/18:1-PAP, 194.7 pmol/mg/min; 16:0/18:1-PI-PLC 15.0 pmol/mg/min; 16:0/18:1-PC-PLC, 8.13 pmol/mg/min) (set to 100%). The values are presented as the mean ± S.D. (n = 3). ∗∗p < 0.01; n.s., not significant.
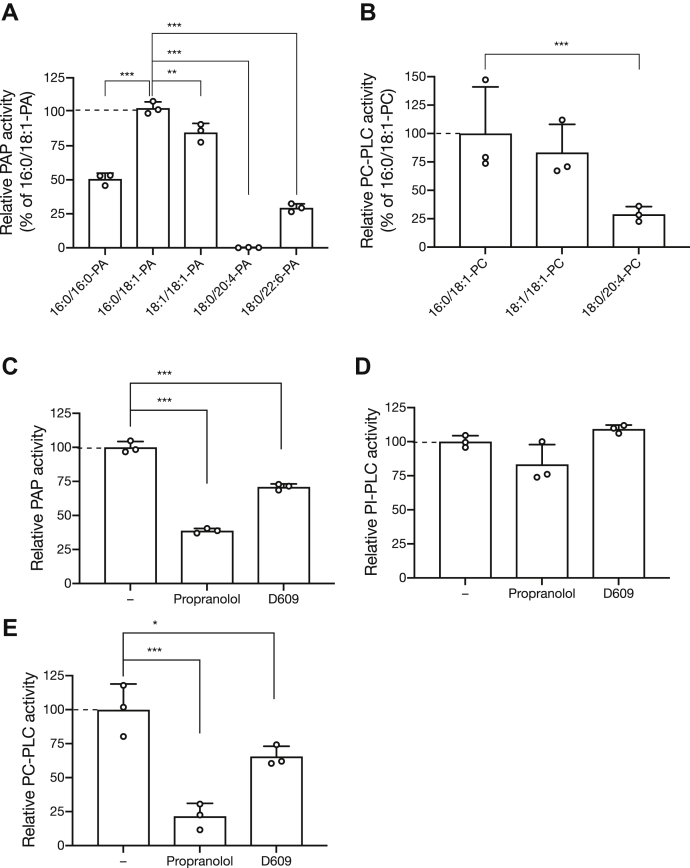
The substrate selectivity of SMSr for PA and PC having different acyl chains was examined (Fig. 5, A and B). SMSr was intensively active against SFA and/or MUFA-containing PA species, such as 16:0/18:1-, 16:0/16:0-, and 18:1/18:1-PA. However, SMSr exhibited relatively low activities against PUFA-containing PA, such as 18:0/20:4-PA, which is exclusively derived from the PI(4,5)P2 cycle (38), and 18:0/22:6-PA.
Figure 5.
Effects of acyl chains of phospholipids and inhibitors of PAP and SMS/PC-PLC on the SMSr-ΔSAMD activity.A, comparison of the enzyme activities for SMSr-ΔSAMD with 16:0/16:0-, 16:0/18:1-, 18:1/18:1-, 18:0/20:4-, and 18:0/22:6-PA as substrates. The detected intensity of the DG molecular species using LC-MS/MS was converted into the amount of DG produced (pmol) based on the calibration curves measured separately with a known concentration of each DG species. The relative PAP activity levels were standardized by comparison with the 16:0/18:1-PAP activity (505.2 pmol/mg/min) (set to 100%). B, comparison of the enzyme activities for SMSr-ΔSAMD with 16:0/18:1-, 18:1/18:1-, 18:0/20:4-PC as substrates. The relative PC-PLC activity was standardized by comparison with the 16:0/18:1-PC-PLC activity (2.75 pmol/mg/min) (set to 100%). C–E, PAP (C), PI-PLC (D) and PC-PLC (E) activities of the SMSr-ΔSAMD were analyzed in the presence of 1 mM propranolol, which is a PAP inhibitor (36, 71), or 500 μM D609, which is a PC-PLC and SMS inhibitor (12, 40, 72). The values are presented as percentages of the activity of SMSr-ΔSAMD in the absence of the inhibitor (−) (16:0/18:1-PAP, 817.1 pmol/mg/min; 16:0/18:1-PI-PLC, 29.3 pmol/mg/min; 16:0/18:1-PC-PLC, 2.66 pmol/mg/min) (set to 100%). The values are presented as the mean ± S.D. (n = 3). ∗p < 0.05; ∗∗∗p < 0.005 (versus control (−)).
We examined the effects of propranolol, which is a well-known PAP inhibitor (36, 39), and D609, which is a SMS and PC-PLC inhibitor (40,41) (Tables 1 and 2), on PAP, PI-PLC, and PC-PLC activities of SMSr (Fig. 5, C–E). The PAP and PC-PLC activities were significantly inhibited by 1 mM propranolol (approximately 60% and 80% decreases, respectively) and by 500 μM D609 (approximately 30% and 20% decreases, respectively) (Fig. 5, C and E). However, the inhibitors had no significant effects on the PI-PLC activity (Fig. 5D).
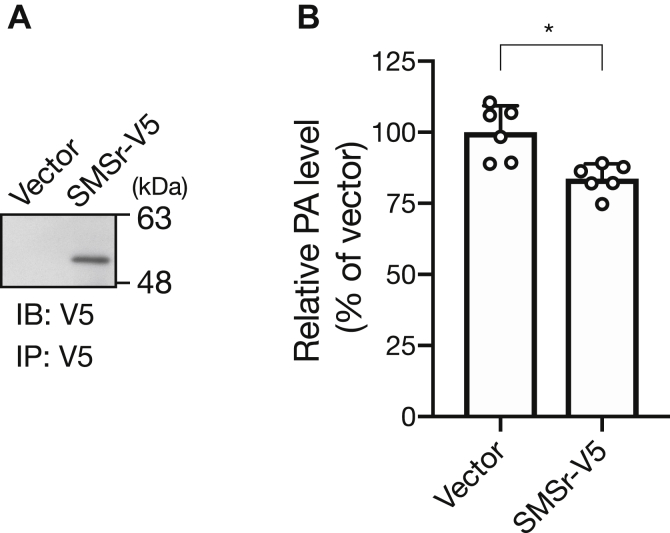
To test whether human SMSr expressed in mammalian cells, not insect cells, also displayed PAP and PI-PLC activities, C-terminal V5-tagged SMSr was expressed in COS-7 cells. The V5-tagged SMSr was immunoprecipitated using an anti-V5 tag antibody (Fig. 6A), and PAP, PI-PLC, and CPES activities in the immunoprecipitates were measured (Fig. 6, B–D). The SMSr expressed in the COS-7 cells exhibited PAP and PI-PLC activities (Fig. 6, B and C). However, a CPE synthase-inactive mutant of SMSr (D348E) (20) failed to show PAP and PI-PLC activities, indicating that Asp348 is also important for PAP and PI-PLC activities. On the other hand, the CPES activity was too low to detect in the precipitates (Fig. 6D), suggesting that human SMSr lacks the ability to synthesize considerable amounts of CPE, as Vacaru et al. reported (20).
Figure 6.
PAP activity of SMSr expressed in mammalian cells.A, SMSr-V5 and SMSr-D348E were overexpressed in COS-7 cells. After 24 h of transfection, the cells were lysed and immunoprecipitated with an anti-V5 antibody (MA5-15253, Invitrogen), followed by immunoblotting to confirm the expression of SMSr-V5 using the anti-V5 antibody. B, PAP, C, PI-PLC, and D, CPES activities in immunoprecipitates were measured by quantitation of 16:0/18:1-DG or d18:1/18:0-CPE levels using LC-MS/MS. The values are presented as the mean ± S.D. (n = 3). ∗p < 0.05 (versus SMSr-V5).
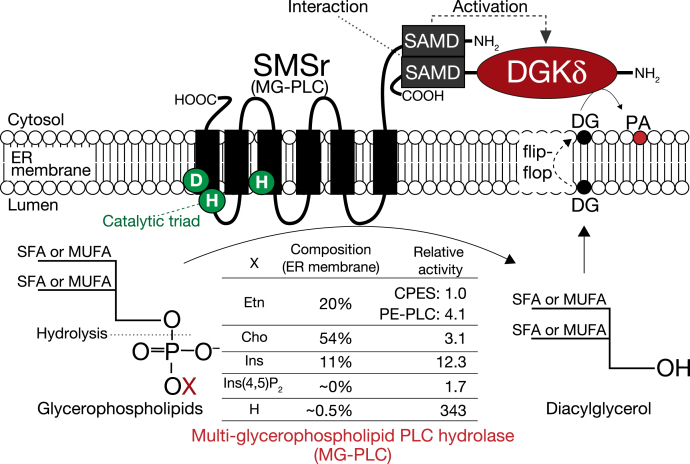
We previously reported that SMSr expressed in COS-7 cells generated DG (30). To determine whether SMSr indeed utilizes PA to produce DG in mammalian cells, we analyzed the changes in the amounts of PA in COS-7 cells overexpressing SMSr-V5 (Fig. 7A). The total PA levels in COS-7 cells overexpressing SMSr-V5 were significantly decreased (Fig. 7B). This result suggests that SMSr produced DG, at least in part, by hydrolyzing PA in mammalian cells.
Figure 7.
Changes in the amounts of total PA in COS-7 cells by overexpressing SMSr.A, human SMSr-V5 was overexpressed in COS-7 cells. After 24 h of transfection, the cells were lysed and immunoprecipitated with an anti-V5 antibody (MA5-15253, Invitrogen), followed by immunoblotting to confirm the expression of SMSr-V5 using the anti-V5 antibody. B, total PA levels in COS-7 cells transfected with vector alone or SMSr-V5 were measured using LC-MS/MS (30). The values are presented as the mean ± S.D. (n = 6). ∗p < 0.05.
Discussion
In the present study, we answered a major question: why does SMSr only exhibit negligible CPES activity? We found that SMSr is a new type of enzyme that is able to hydrolyze multiple glycerophospholipids, such as PA, PI, PE, and PC, to produce DG (Fig. 8). SMSr is a novel PAP that primarily acts in the ER lumen unlike the previously reported PAP/LPP (42, 43). Intriguingly, SMSr is true mammalian PI-PLC, while the previously found so-called PI-PLC is PIP2-PLC (10, 15, 35). Moreover, our data indicate that SMSr is a strong candidate for the elusive mammalian PE-PLC and PC-PLC activities, which were first described about 40 years ago (44, 45, 46). Furthermore, we answered another question: why does DGKδ, which does not show selectivity to DG species in vitro (31), apparently utilize limited DG species, 16:0 and/or 16:1-containing DG. The intrinsic selectivity of SMSr, which functions upstream of DGKδ, for 16:0 and/or 16:1-containing substrates would determine the limitation of DG species utilized by DGKδ.
Figure 8.
Model for a new DG-signaling pathway involving SMSr and DGKδ. In the present study, we demonstrated that SMSr displayed PAP, PI-PLC, PE-PLC, PC-PLC, and PG-PLC activities that were stronger than the CPE synthase activity (20). Moreover, the SMSr exhibited a substrate specificity for SFA- and/or MUFA-containing PA and PC molecular species, but not PUFA-containing PA and PC. It is possible that SMSr preferably hydrolyzes SFA- and/or MUFA-containing glycerophospholipids independent of the ceramide. Previously, we reported that SMSr and DGKδ functionally interact via their SAMDs (30). Taken together, it is possible that there is a new mammalian DG-signaling pathway consisting of SMSr and DGKδ independent of the PI(4,5)P2 cycle.
The purification of six-transmembrane proteins, SMS and SMSr, is generally difficult (18). However, in order to analyze enzymatic activities of SMSr beyond its previously reported CPES activity, purification was first needed. Thus, we expressed human SMSr using the baculovirus-insect cell system and, through trial and error, highly purified it via affinity and size-exclusion chromatography (Fig. 2, A and B). The purified enzyme gave almost a single band with a molecular mass of 43,000 via SDS gel electrophoresis. It is likely that size-exclusion chromatography with a buffer containing a nonionic, nondenaturing detergent, Nonidet P-40 (NP-40), at critical micelle concentration (0.018%) was critical for the successful purification.
Using the highly purified enzyme, we demonstrated for the first time that SMSr displayed not only CPES activity but also PAP, PE-PLC, PI-PLC, PC-PLC, and PG-PLC activities in vitro (Fig. 3, A and B). Interestingly, compared with the DG-generating activity in the presence of PE and ceramide (substrates of CPE synthase), purified human SMSr showed a ∼340-fold stronger PAP activity, even in the absence of ceramide. Considering the report that SMSr metabolized approximately 300-fold less ceramide than SMS1 (20) (Table 1), it is likely that the PAP activity of human SMSr is comparable with the SM synthase activity of SMS1. Moreover, we determined the Km values for 16:0/18:1-PA and 16:0/18:1-PI of human SMSr (3.92 and 6.83 mol%) (Fig. 3, C and D), which are almost the same as those of other PAPs, LPP1 (PAP2a) (36), and lipin-1 (37) (Table 2). We demonstrated that Asp348 of SMSr is part of the enzyme’s active site, which faces the ER lumen (19, 20, 47), was important for not only for CPES, but also for PAP and PI-PLC activities (Fig. 6, B and C). Therefore, SMSr probably shows CPES, PAP, PE-PLC, PI-PLC, PC-PLC, and PG-PLC activities through the same catalytic site.
In this study, we found that ceramide selectively affected PE-PLC activity of SMSr-ΔSAMD (Fig. 4A), but not its PAP, PI-PLC, or PC-PLC activity (Fig. 4, B–D). It is possible that PE-PLC activity of SMSr is competitive with CPE synthase activity of SMSr. SMSr has CPE synthase activity and, consequently, ceramide can occupy the substrate (ceramide/PE) binding site of SMSr (20). Therefore, it is likely that d18:1/18:0-ceramide interferes with PE-PLC activity by blocking access of PE to the active site. It was reported that mammalian SMSr acts as not only CPE synthase, but also ceramide sensor in the ER to protect cells against ceramide-induced mitochondrial apoptosis (20, 48). Taken together, SMSr modulates ceramide levels in ER via its SAMD, and vice versa, ceramide may selectively modulate the function (PE-PLC activity) of SMSr at least in part.
The PI-PLC, PE-PLC, and PC-PLC activities of purified SMSr were 28∼100-fold weaker than the PAP activity (Fig. 3A). However, two lines of evidence support that the PI-PLC activity of SMSr is comparable with its PAP activity in cells. First, the amount of PI, PC, and PE in the ER membrane was more than 20-fold, 100-fold, and 40-fold higher than that of PA, respectively (11%, 54%, and 20% versus less than 0.5% of total lipids in the ER membrane, respectively) (Fig. 8) (49, 50). As shown in Figure 3, C and D, SMSr showed a PAP activity of ∼60 pmol/mg/min at 0.5 mol% PA and ∼18 pmol/mg/min at 8 mol% PI. Second, the SMSr expressed in mammalian cells showed a PI-PLC activity as strong as (only 3.5-fold lower) the PAP activity (Fig. 5, B and C). These results suggest that the PI-PLC activity of SMSr expressed in mammalian cells is comparable with its PAP activity. Therefore, PAP and PI-PLC (and PE-PLC and PC-PLC) are likely to represent the substantial activity of SMSr.
What are the differences between SMSr as PAP and the already-identified PAPs (PAP2/LPP and lipin-1) (see Table 2)? LPPs have broad substrate specificity (see Table 2). However, they do not hydrolyze PC, PE, PI, or phosphoinositide (PI(4,5)P2) (11, 14, 51). Interestingly, SMSr hydrolyzed PA, PI, PI(4,5)P2, PE, PG, and PC (Fig. 3, A and B). LPPs contain six transmembrane domains and are primarily localized to the plasma membrane (14, 42, 51). It was reported that LPP2 and LPP3 were only partly localized to the ER in addition to the plasma membrane (52, 53). SMSr is an exclusive ER membrane protein (20, 33). Asp348 of SMSr is one of the catalytic triad on the ER luminal side (Fig. 8). It is possible that SMSr as PAP hydrolyzes PA and PI/PC/PE in the ER lumen, in contrast to other PAPs (LPPs and lipins, which are cytosolic proteins).
Lipin-1, the most characterized type I PAP, is specific for PA. Lipin-1 hydrolyzed 1,2-diunsaturated and 1-saturated-2-unsaturated PA molecular species but hydrolyzed little or no saturated PA (16:0/16:0- and 18:0/18:0-PA) (37). On the other hand, SMSr selectively hydrolyzed SFA and/or MUFA-containing PA and PC molecular species, but not 20:4-containing PA and PC (Fig. 4, A and B). This unique selectivity reinforces the hypothesis that SMSr and its downstream enzyme, DGKδ, are components of the alternative DG/PA-signaling pathway, which is independent of the PIP2 cycle (Fig. 8).
What are the differences between SMSr as PI-PLC and PI(4,5)P2-PLC? We found that SMSr also hydrolyzed 18:0/20:4-PI(4,5)P2 in vitro, and this activity was approximately tenfold weaker than the 18:0/20:4-PI-PLC activity (Fig. 3B). Notably, although mammalian PI(4,5)P2-PLC (so-called PI-PLC) also hydrolyzes both PI(4,5)P2 and PI, its PI(4,5)P2-PLC activity is 30∼50-fold stronger than the PI-PLC activity (35, 54). Therefore, the substrate specificity of SMSr as PI-PLC for phosphoinositide is different from PI(4,5)P2-PLC. The PI(4,5)P2 levels in the mammalian cells are ∼100-fold lower than the PI levels (55). Moreover, the majority of PI in mammalian cells is distributed in the ER membrane (56), whereas PI(4,5)P2 predominantly localizes at the plasma membrane (57, 58). Taken together, even though SMSr as PI-PLC hydrolyzed PI(4,5)P2 in vitro, it is possible that SMSr proteins hydrolyze PI more strongly than PI(4,5)P2 because little or no 18:0/20:4-PIP2 exists in the ER membrane.
It has been suggested that PC-specific phospholipase C (PC-PLC) plays pivotal roles in signal transduction pathways that are involved in atherogenesis, inflammation, and carcinogenesis (12, 40, 59). However, the molecular entity of mammalian PC-PLC has not yet been identified since the activity was reported 40 years ago (44). Moreover, we reported that coimmunoprecipitates using an anti-DGKδ antibody from C2C12 myoblast cells contained PC-PLC activity and that DGKδ interacted with SMSr (30, 31). Furthermore, D609, a PC-PLC inhibitor (40) (Table 2), attenuated the PC-PLC activity of SMSr (Fig. 5E). Therefore, it is likely that SMSr is PC-PLC, which has been awaited for molecular entity identification for quite some time (∼40 years).
PE-PLC activity in mammalian cells was discovered approximately 30 years ago (16, 45, 46, 60) and is inhibited by D609 (13). Although bacterial and plant PE-PLC has been cloned, mammalian PE-PLC molecules have not yet been identified. In the present study, we, for the first time, found the mammalian protein that displays PE-PLC activity. Therefore, it is possible that SMSr is the previously reported PE-PLC (46).
We previously reported that in C2C12 myoblasts, DGKδ apparently prefers to phosphorylate palmitic acid (16:0) and/or palmitoleic acid (16:1)-containing DG molecular species, but not 20:4-containing DG delivered from the PI(4,5)P2 cycle under high-glucose stimulation (31). However, DGKδ enigmatically fails to possess such DG species selectivity in vitro. Intriguingly, we recently found that SMSr interacted with DGKδ via their SAMDs and provided 16:0 and/or 16:1-containing DG species to the enzyme (30). However, it remains unclear whether SMSr itself determines DG species that are supplied to DGKδ. In the present study, SMSr preferentially produced 16:0 and/or 16:1-containing DG species in vitro. Therefore, SMSr likely limits DG species utilized by DGKδ. Thus, the molecular machinery of the novel DG supply pathway independent of the PI(4,5)P2 cycle becomes clearer.
In summary, the current study, for the first time, showed that human SMSr displayed PAP, PI-PLC, PE-PLC, PG-PLC, and PC-PLC activities in vitro, in addition to CPES activity. In particular, PAP (∼300-fold), PI-PLC (∼10-fold), PC-PLC (∼3-fold), and PE-PLC activity (∼4-fold) were stronger than the CPES activity. Thus, beyond the enzyme’s previously established CPES activity, SMSr can be categorized as a new type of PLC with a broad head group specificity, i.e., a multi-glycerophospholipid PLC hydrolase (MG-PLC). The current results allow us to propose the new DG/PA metabolic pathway “Luminal PA, PI, PC and PE → SMSr (MG-PLC) → DG → flip-flop → DGKδ → cytoplasmic PA” in the ER membrane, which is independent of the PI(4,5)P2 cycle (Fig. 8). Future studies exploring the enzymatic property of SMSr may contribute to understanding of the novel DG/PA metabolic pathway in the ER membrane and the pathogenetic mechanisms of various diseases related to DGKδ, type 2 diabetes (25, 26) and obsessive–compulsive disorder (27, 28) and, probably, to PC-PLC, atherogenesis, inflammation, and carcinogenesis (12, 40, 59).
Experimental procedures
Materials
Lipids: 1,2-dipalmitoyl-sn-glycero-3-phosphate (16:0/16:0-PA), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoinositol (16:0/18:1-PI), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (16:0/18:1-PS), 1,2-dioleoyl-sn-glycero-3-phosphate (18:1/18:1-PA), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphoinositol (18:0/20:4-PI), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phospho-(1'-myo-inositol-4',5'-bisphosphate) (18:0/20:4-PI(4,5)P2), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphate (18:0/20:4-PA), and 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphate (18:0/22:6-PA) were obtained from Avanti Polar Lipids. 1,2-dimyristoyl-sn-glycerol (14:0/14:0-DG), 1-palmitoyl-2-oleoyl-sn-3-phosphoglycerol (16:0/18:1-PG), and N-stearoyl-D-erythro-sphingosine (d18:1/18:0-Cer) were obtained from Cayman Chemical Company. 16:0/18:1-sn-glycero-3-phosphocholine (16:0/18:1-PC) was purchased from Sigma-Aldrich.
Detergents: n-dodecyl-β-D-maltoside (DDM) was obtained from Cayman Chemical Company. Cholesteryl hemisuccinate (CHS) was purchased from Sigma-Aldrich.
Plasmids
Human SMSr and SMSr-ΔSAMD (amino acid residues 79–413) tagged with N-terminal 6×His or C-terminal V5 were previously made (30). 6× His-tagged SMSr and SMSr-ΔSAMD were subcloned into the pOET3 vector (Oxford Expression Technologies) at XbaI/XhoI sites.
Antibodies
Rabbit polyclonal anti-His-tag antibody (PM032) was obtained from Medical and Biological Laboratories. Mouse monoclonal anti-V5 antibody (clone E10/V4RR, cat. no. MA5-15253) was obtained from Thermo Fisher Scientific. A peroxidase-conjugated goat anti-mouse IgG antibody was purchased from Bethyl Laboratories. A peroxidase-conjugated goat anti-rabbit IgG antibody (111-036-045) was obtained from Jackson Immuno Research.
Cell culture
COS-7 cells were maintained on 150-mm dishes (Thermo Fisher Scientific) in Dulbecco’s Modified Eagle’s Medium (DMEM) (Wako Pure Chemicals) containing 10% fetal bovine serum (Thermo Fisher Scientific), 100 units/ml penicillin G (Wako Pure Chemicals), and 100 μg/ml streptomycin (Wako Pure Chemicals) at 37 °C in an atmosphere containing 5% CO2 as described (30). For quantitation of PA and DG levels, 1 × 106 cells were plated on 100-mm dishes. After 24 h, plasmid cDNAs were transfected using PolyFect (Qiagen) according to the manufacturer’s instruction manual. After transfection, the cells were cultured for an additional 24 h and were used for the experiments.
Sf9 cells were maintained in Sf-900 II serum-free medium (Invitrogen) in sterile Erlenmeyer flask at 120 rpm and 28 °C without CO2 in the dark. Volume of the medium was kept at 20 to 30% of flask volume.
Expression of SMSr via baculovirus-insect cell system
To generate recombinant baculovirus, the flashBAC system (Oxford Expression Technologies) and the pOET3 vector were used as described in flashBAC System Transfection Protocol (https://www.mirusbio.com/assets/protocols/flashbac-baculovirus-expression-systems-protocol.pdf) (61, 62). For generation of baculovirus (P0), 1.0 × 106 cells were plated on 35-mm dishes. The DNAs (flashBAC and pOET3 vector) were transfected using TransIT-Insect Reagent (Mirus Bio) according to the manufacturer’s instruction manual. The baculoviral stocks (P0, ∼1 × 106 pfu/ml) were harvested at 5 days posttransfection supernatants and passed through a 0.22 μm filter.
Baculoviral stocks were amplified for seven rounds of amplification at 2 × 106 cells/ml, multiplicity of infection (MOI) of 0.1. Viral titer (plaque forming unit (pfu)/ml) was determined via plaque assay as described in flashBAC System Transfection Protocol. Viral stocks were stored at −80 °C until use.
To express recombinant proteins, Sf9 cells were seeded at 3 × 106 cells/ml and infected cells with recombinant baculovirus at MOI of 2. Infected cells were harvested after 24 h and the pellets were resuspended in 40% (v/v) glycerol diluted in phosphate-buffered saline. The cell samples were flash-frozen in liquid nitrogen and stored at −80 °C until use.
Preparation of Sf9 membranes containing overexpressed recombinant protein
The membranes of Sf9 cells containing overexpressed proteins were prepared via the protocol described by JCIMPT, an NIH Structural Biology RoadMap Initiative Project (https://commonfund.nih.gov/sites/default/files/JCIMPT_MembranePrep.pdf) (63, 64, 65).
Frozen Sf9 cells expressing recombinant proteins were thawed at 4 °C. After centrifugation at 10,000g for 30 min at 4 °C, cell pellets were resuspended in ice-cold hypotonic buffer (10 mM HEPES-NaOH (pH 7.5), 10 mM MgCl2, 20 mM KCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 μg/ml aprotinin, 20 μg/ml leupeptin, 20 μg/ml pepstatin and 20 μg/ml soybean trypsin inhibitor), and cells were homogenized on ice via homogenizer. After ultracentrifugation at 100,000g for 30 min at 4 °C, pellets were resuspended in ice-cold high-osmolarity buffer (10 mM HEPES-NaOH (pH 7.5), 1 M NaCl, 10 mM MgCl2, 20 mM KCl, 1 mM PMSF, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 20 μg/ml pepstatin, and 20 μg/ml soybean trypsin inhibitor) and homogenized on ice via homogenizer. This procedure using high-osmolarity buffer was repeated twice. After ultracentrifugation at 100,000g for 30 min at 4 °C, Sf9 membranes were resuspended in 40% (v/v) glycerol diluted in high-osmolarity buffer and homogenized. The Sf9 membranes were flash-frozen in liquid nitrogen and stored at −80 °C until use.
Purification of 6× His-tagged proteins
The 6× His-tagged proteins were partially purified via affinity chromatography. Frozen Sf9 membranes containing recombinant proteins were thawed at 4 °C. After ultracentrifugation at 100,000g for 30 min at 4 °C, the purified membranes were lysed via homogenization on ice with ice-cold lysis buffer (50 mM Tris-HCl, pH 8.0, containing 500 mM NaCl, 10% (v/v) glycerol, 1% (v/v) NP-40, 20 mM imidazole, 1 mM PMSF, 0.1 mM DTT, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 20 μg/ml pepstatin, and 20 μg/ml soybean trypsin inhibitor) and incubated at 4 °C for 1 h with gently stirring via magnetic stirrer. The supernatant (1% NP-40 soluble fraction) was isolated by ultracentrifugation at 200,000g for 30 min at 4 °C, and then the supernatant was incubated with nickel–nitrilotriacetic acid (Ni-NTA) agarose (Wako Pure Chemicals) at 4 °C for 2 h with gently rotation. After centrifugation at 200g for 10 min at 4 °C, the Ni-NTA agarose was resuspended in lysis buffer and transferred into an empty column. The beads were washed with 30 ml of lysis buffer, followed by washing with 10 ml of wash buffer (50 mM Tris-HCl, pH 8.0, containing 500 mM NaCl, 10% (v/v) glycerol, 0.5% (v/v) NP-40, 50 mM imidazole, 0.1 mM DTT). Subsequently, the bound proteins were eluted with an elution buffer (50 mM Tris-HCl, pH 8.0, containing 500 mM NaCl, 10% (v/v) glycerol, 0.1% (v/v) NP-40, 500 mM imidazole, 0.1 mM DTT). The partially purified proteins were further purified via size-exclusion chromatography. After concentration using Amicon Ultra-15 (Merk), the partially purified samples were subjected to size-exclusion chromatography on an ENrich SEC 650 column (Bio-Rad equipped with a Bio-Rad NGC chromatography system). The column was equilibrated with 20 mM Tris-HCl (pH 7.4), 200 mM NaCl, 5% glycerol, 0.5 mM dithiothreitol, and 0.018% NP-40.
Immunoblot analysis
The purified proteins were mixed with a 5×SDS sample buffer and incubated at 37 °C for 2 h instead of boiling. The samples were analyzed by SDS-PAGE and an immunoblot analysis, as described (30).
Preparation of DDM/CHS stock solutions
To prepare detergents stock solution (10% (w/v) n-dodecyl-β-D-maltoside (DDM) and 2% (w/v) cholesteryl hemisuccinate (CHS)), 5 g of DDM was added into 40 ml of 200 mM Tris-HCl (pH 8.0), followed by gently rotation until DDM goes into solution. The detergent (1 g of CHS) was added to DDM solution and sonicated until solution becomes translucent. After sonication, 10 ml of 200 mM Tris-HCl (pH 8.0) was added and incubated at 25 °C with gently rotation until the solution becomes transparent. The detergents solution was stored at −25 °C until use.
Preparation of micelles
The reaction solution for PLC and CPES activity assay was prepared as we reported (34). Phospholipids dissolved in chloroform/methanol (2:1 (v/v)) and d18:1/18:0-ceramide in chloroform were dried under N2 gas to yield a lipid film on the wall of the vial. The lipid film was resuspended in the 2×reaction buffer (40 mM Tris-HCl (pH 7.4), 30 mM KCl, 200 mM NaCl, 600 mM sucrose, 0.2% (w/v) DDM, 0.04% (w/v) CHS) to a final total lipid concentration of 0.1 mM each. The 2×reaction solutions containing lipids were vortexed for 3 min and sonicated in a bath sonicator (Sonifilter model 450) four times for 2 min at 65 °C.
Mixed micellar assay
The DG-generating activities (PLC, PAP, or CPES) of purified proteins were evaluated via a DDM mixed micellar phospholipase C assay previously published (66, 67) with a modification. Because DDM/CHS (5:1) micelles provide a more membrane-like environment, we adopted DDM/CHS (5:1) for a mixed micellar DG-generating enzyme activity assay.
SMSr-containing samples (10 μl) were diluted in 10 μl ice-cold 2×reaction buffer on ice (final concentration of 20 mM Tris-HCl (pH 7.4), 15 mM KCl, 200 mM NaCl, 300 mM sucrose, 0.1% (w/v) DDM, 0.02% CHS, and 50 μM phospholipids (16:0/18:1-PA, 16:0/18:1-PC, 16:0/18:1-PE, 16:0/18:1-PI, 16:0/18:1-PS or 16:0/18:1-PG)). For a CPE synthase activity assay, d18:1/18:0-ceramide was added into the reaction buffer (final concentration of 50 μM). Because SMSr is membrane-bound and lipid-dependent enzyme, the surface dilution kinetic scheme was used (68, 69). The surface concentration of phospholipids in the micelles (1.87 mM DDM and 0.41 mM CHS mixed micelle) is 2.14 mol% (68). The mol % of lipids in DDM/CHS/lipid-mixed micelle was calculated by the formula: mol% = 100 × [lipid (mol)]/([DDM (mol)] + [CHS (mol)] + [lipid (mol)]).
After incubation (the enzyme reaction) for 2 h at 37 °C, lipids were extracted by Bligh & Dyer method (70), followed by quantitation of 16:0/18:1-DG using LC-MS/MS (30, 34).
Statistical analysis
The data are represented as the means ± SD and were analyzed using Student’s t-test or one-way ANOVA using GraphPad Prism 8 (GraphPad).
Data availability
All data are contained within the article.
Conflict of interest
The authors declare no conflicts of interest associated with the contents of this article.
Acknowledgments
Author contributions
C. M. primarily designed and conducted the experiments, analyzed the data, and wrote the article. F. S. conceived the research and wrote the article.
Funding and additional information
This work was supported in part by grants from MEXT/JSPS KAKENHI (Grant Numbers: JP18J20003 (C. M.) and 25116704, 26291017, 20H03205, 17H03650 and 15K14470 (F. S.)); the Japan Science and Technology Agency (AS251Z01788Q and AS2621643Q) (F. S.); the Futaba Electronic Memorial Foundation (F. S.); the Ono Medical Research Foundation (F. S.); the Japan Foundation for Applied Enzymology (F. S.); the Food Science Institute Foundation (F. S.); the Skylark Food Science Institute (F. S.); the Hamaguchi Foundation for the Advancement of Biochemistry (F. S.); the Daiichi-Sankyo Foundation of Life Science (F. S.); the Terumo Life Science Foundation (F. S.); the Asahi Group Foundation (F. S.); the Japan Milk Academic Alliance (F. S.); the Japan Food Chemical Research Foundation (F. S.); and the SENSHIN Medical Research Foundation (F. S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Edited by Dennis Voelker
Footnotes
Present address for Chiaki Murakami: Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku 113-0033, Japan.
Contributor Information
Chiaki Murakami, Email: 12s3039@chiba-u.jp, cm55abcde@gmail.com.
Fumio Sakane, Email: sakane@faculty.chiba-u.jp.
References
- 1.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem. J. 1984;220:345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caloca M.J., Fernandez N., Lewin N.E., Ching D., Modali R., Blumberg P.M., Kazanietz M.G. Beta2-chimaerin is a high affinity receptor for the phorbol ester tumor promoters. J. Biol. Chem. 1997;272:26488–26496. doi: 10.1074/jbc.272.42.26488. [DOI] [PubMed] [Google Scholar]
- 4.Valverde A.M., Sinnett-Smith J., Van Lint J., Rozengurt E. Molecular cloning and characterization of protein kinase D: A target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8572. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebinu J.O., Bottorff D.A., Chan E.Y., Stang S.L., Dunn R.J., Stone J.C. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 6.Brose N., Rosenmund C. Move over protein kinase C, you've got company: Alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 7.Yen C.L., Stone S.J., Cases S., Zhou P., Farese R.V., Jr. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8512–8517. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen C.L., Farese R.V., Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 2003;278:18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 10.Kadamur G., Ross E.M. Mammalian phospholipase C. Annu. Rev. Physiol. 2013;75:127–154. doi: 10.1146/annurev-physiol-030212-183750. [DOI] [PubMed] [Google Scholar]
- 11.Brindley D.N., Waggoner D.W. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 1998;273:24281–24284. doi: 10.1074/jbc.273.38.24281. [DOI] [PubMed] [Google Scholar]
- 12.Podo F., Paris L., Cecchetti S., Spadaro F., Abalsamo L., Ramoni C., Ricci A., Pisanu M.E., Sardanelli F., Canese R., Iorio E. Activation of phosphatidylcholine-specific phospholipase C in breast and ovarian cancer: Impact on MRS-detected choline metabolic profile and perspectives for targeted therapy. Front. Oncol. 2016;6:171. doi: 10.3389/fonc.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiss Z., Tomono M. Compound D609 inhibits phorbol ester-stimulated phospholipase D activity and phospholipase C-mediated phosphatidylethanolamine hydrolysis. Biochim. Biophys. Acta. 1995;1259:105–108. doi: 10.1016/0005-2760(95)00148-6. [DOI] [PubMed] [Google Scholar]
- 14.Sigal Y.J., McDermott M.I., Morris A.J. Integral membrane lipid phosphatases/phosphotransferases: Common structure and diverse functions. Biochem. J. 2005;387:281–293. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y., Fukami K. Regulation and physiological functions of mammalian phospholipase C. J. Biochem. 2017;161:315–321. doi: 10.1093/jb/mvw094. [DOI] [PubMed] [Google Scholar]
- 16.Kiss Z., Anderson W.B. ATP stimulates the hydrolysis of phosphatidylethanolamine in NIH 3T3 cells. Potentiating effects of guanosine triphosphates and sphingosine. J. Biol. Chem. 1990;265:7345–7350. [PubMed] [Google Scholar]
- 17.Taniguchi M., Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta. 2014;1841:692–703. doi: 10.1016/j.bbalip.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi M., Okazaki T. Ceramide/Sphingomyelin rheostat regulated by sphingomyelin synthases and chronic diseases in murine models. J. Lipid Atheroscler. 2020;9:380–405. doi: 10.12997/jla.2020.9.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huitema K., van den Dikkenberg J., Brouwers J.F., Holthuis J.C. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vacaru A.M., Tafesse F.G., Ternes P., Kondylis V., Hermansson M., Brouwers J.F., Somerharju P., Rabouille C., Holthuis J.C. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J. Cell Biol. 2009;185:1013–1027. doi: 10.1083/jcb.200903152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bickert A., Ginkel C., Kol M., vom Dorp K., Jastrow H., Degen J., Jacobs R.L., Vance D.E., Winterhager E., Jiang X.C., Dormann P., Somerharju P., Holthuis J.C., Willecke K. Functional characterization of enzymes catalyzing ceramide phosphoethanolamine biosynthesis in mice. J. Lipid Res. 2015;56:821–835. doi: 10.1194/jlr.M055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakane F., Imai S., Kai M., Wada I., Kanoh H. Molecular cloning of a novel diacylglycerol kinase isozyme with a pleckstrin homology domain and a C-terminal tail similar to those of the EPH family of protein-tyrosine kinases. J. Biol. Chem. 1996;271:8394–8401. doi: 10.1074/jbc.271.14.8394. [DOI] [PubMed] [Google Scholar]
- 23.Sakane F., Imai S., Kai M., Yasuda S., Kanoh H. Diacylglycerol kinases: Why so many of them? Biochim. Biophys. Acta. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Sakane F., Hoshino F., Murakami C. New era of diacylglycerol kinase, phosphatidic acid and phosphatidic acid-binding protein. Int. J. Mol. Sci. 2020;21:6794. doi: 10.3390/ijms21186794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chibalin A.V., Leng Y., Vieira E., Krook A., Bjornholm M., Long Y.C., Kotova O., Zhong Z., Sakane F., Steiler T., Nylen C., Wang J., Laakso M., Topham M.K., Gilbert M. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Miele C., Paturzo F., Teperino R., Sakane F., Fiory F., Oriente F., Ungaro P., Valentino R., Beguinot F., Formisano P. Glucose regulates diacylglycerol intracellular levels and protein kinase C activity by modulating diacylglycerol kinase subcellular localization. J. Biol. Chem. 2007;282:31835–31843. doi: 10.1074/jbc.M702481200. [DOI] [PubMed] [Google Scholar]
- 27.Usuki T., Takato T., Lu Q., Sakai H., Bando K., Kiyonari H., Sakane F. Behavioral and pharmacological phenotypes of brain-specific diacylglycerol kinase delta-knockout mice. Brain Res. 2016;1648:193–201. doi: 10.1016/j.brainres.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q., Murakami C., Murakami Y., Hoshino F., Asami M., Usuki T., Sakai H., Sakane F. 1-Stearoyl-2-docosahexaenoyl-phosphatidic acid interacts with and activates Praja-1, the E3 ubiquitin ligase acting on the serotonin transporter in the brain. FEBS Lett. 2020;594:1787–1796. doi: 10.1002/1873-3468.13765. [DOI] [PubMed] [Google Scholar]
- 29.Crotty T., Cai J., Sakane F., Taketomi A., Prescott S.M., Topham M.K. Diacylglycerol kinase delta regulates protein kinase C and epidermal growth factor receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15485–15490. doi: 10.1073/pnas.0604104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami C., Hoshino F., Sakai H., Hayashi Y., Yamashita A., Sakane F. Diacylglycerol kinase delta and sphingomyelin synthase-related protein functionally interact via their sterile alpha motif domains. J. Biol. Chem. 2020;295:2932–2947. doi: 10.1074/jbc.RA119.012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai H., Kado S., Taketomi A., Sakane F. Diacylglycerol kinase δ phosphorylates phosphatidylcholine-specific phospholipase C-dependent, palmitic acid-containing diacylglycerol species in response to high glucose levels. J. Biol. Chem. 2014;289:26607–26617. doi: 10.1074/jbc.M114.590950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabukusta B., Köhlen J.A., Richter C.P., You C., Holthuis J.C. Monitoring changes in the oligomeric state of a candidate endoplasmic reticulum (ER) ceramide sensor by single-molecule photobleaching. J. Biol. Chem. 2016;291:24735–24746. doi: 10.1074/jbc.M116.749812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabukusta B., Kol M., Kneller L., Hilderink A., Bickert A., Mina J.G., Korneev S., Holthuis J.C. ER residency of the ceramide phosphoethanolamine synthase SMSr relies on homotypic oligomerization mediated by its SAM domain. Sci. Rep. 2017;7:41290. doi: 10.1038/srep41290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami C., Mizuno S., Kado S., Sakane F. Development of a liquid chromatography-mass spectrometry based enzyme activity assay for phosphatidylcholine-specific phospholipase C. Anal. Biochem. 2017;526:43–49. doi: 10.1016/j.ab.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Meldrum E., Parker P.J., Carozzi A. The PtdIns-PLC superfamily and signal transduction. Biochim. Biophys. Acta. 1991;1092:49–71. doi: 10.1016/0167-4889(91)90177-y. [DOI] [PubMed] [Google Scholar]
- 36.Roberts R., Sciorra V.A., Morris A.J. Human type 2 phosphatidic acid phosphohydrolases: Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 1998;273:22059–22067. doi: 10.1074/jbc.273.34.22059. [DOI] [PubMed] [Google Scholar]
- 37.Han G.S., Carman G.M. Characterization of the human LPIN1-encoded phosphatidate phosphatase isoforms. J. Biol. Chem. 2010;285:14628–14638. doi: 10.1074/jbc.M110.117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakane F., Mizuno S., Takahashi D., Sakai H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018;67:101–108. doi: 10.1016/j.jbior.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Kanoh H., Imai S., Yamada K., Sakane F. Purification and properties of phosphatidic acid phosphatase from porcine thymus membranes. J. Biol. Chem. 1992;267:25309–25314. [PubMed] [Google Scholar]
- 40.Adibhatla R.M., Hatcher J.F., Gusain A. Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: A mini-review of literature. Neurochem. Res. 2012;37:671–679. doi: 10.1007/s11064-011-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luberto C., Hannun Y.A. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J. Biol. Chem. 1998;273:14550–14559. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 42.Jia Y.-J., Kai M., Wada I., Sakane F., Kanoh H. Differential localization of lipid phosphate phosphatases 1 and 3 to cell surface subdomains in polarized MDCK cells. FEBS Lett. 2003;552:240–246. doi: 10.1016/s0014-5793(03)00931-1. [DOI] [PubMed] [Google Scholar]
- 43.Kai M., Sakane F., Jia Y.J., Imai S., Yasuda S., Kanoh H. Lipid phosphate phosphatases 1 and 3 are localized in distinct lipid rafts. J. Biochem. 2006;140:677–686. doi: 10.1093/jb/mvj195. [DOI] [PubMed] [Google Scholar]
- 44.Hostetler K.Y., Hall L.B. Phospholipase C activity of rat tissues. Biochem. Biophys. Res. Commun. 1980;96:388–393. doi: 10.1016/0006-291x(80)91227-9. [DOI] [PubMed] [Google Scholar]
- 45.Kiss Z., Crilly K., Chattopadhyay J. Ethanol potentiates the stimulatory effects of phorbol ester, sphingosine and 4-hydroxynonenal on the hydrolysis of phosphatidylethanolamine in NIH 3T3 cells. Eur. J. Biochem. 1991;197:785–790. doi: 10.1111/j.1432-1033.1991.tb15972.x. [DOI] [PubMed] [Google Scholar]
- 46.KISS Z. The long-term combined stimulatory effects of ethanol and phorbol ester on phosphatidylethanolamine hydrolysis are mediated by a phospholipase C and prevented by overexpressed α-protein kinase C in fibroblasts. Eur. J. Biochem. 1992;209:467–473. doi: 10.1111/j.1432-1033.1992.tb17311.x. [DOI] [PubMed] [Google Scholar]
- 47.Kol M., Panatala R., Nordmann M., Swart L., van Suijlekom L., Cabukusta B., Hilderink A., Grabietz T., Mina J.G.M., Somerharju P., Korneev S., Tafesse F.G., Holthuis J.C.M. Switching head group selectivity in mammalian sphingolipid biosynthesis by active-site-engineering of sphingomyelin synthases. J. Lipid Res. 2017;58:962–973. doi: 10.1194/jlr.M076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tafesse F.G., Vacaru A.M., Bosma E.F., Hermansson M., Jain A., Hilderink A., Somerharju P., Holthuis J.C. Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. J. Cell Sci. 2014;127:445–454. doi: 10.1242/jcs.138933. [DOI] [PubMed] [Google Scholar]
- 49.Casares D., Escriba P.V., Rossello C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019;20:2167. doi: 10.3390/ijms20092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zambrano F., Fleischer S., Fleischer B. Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim. Biophys. Acta. 1975;380:357–369. doi: 10.1016/0005-2760(75)90104-6. [DOI] [PubMed] [Google Scholar]
- 51.Waggoner D.W., Xu J., Singh I., Jasinska R., Zhang Q.X., Brindley D.N. Structural organization of mammalian lipid phosphate phosphatases: Implications for signal transduction. Biochim. Biophys. Acta. 1999;1439:299–316. doi: 10.1016/s1388-1981(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 52.Morris K.E., Schang L.M., Brindley D.N. Lipid phosphate phosphatase-2 activity regulates S-phase entry of the cell cycle in Rat2 fibroblasts. J. Biol. Chem. 2006;281:9297–9306. doi: 10.1074/jbc.M511710200. [DOI] [PubMed] [Google Scholar]
- 53.Gutiérrez-Martínez E., Fernández-Ulibarri I., Lázaro-Diéguez F., Johannes L., Pyne S., Sarri E., Egea G. Lipid phosphate phosphatase 3 participates in transport carrier formation and protein trafficking in the early secretory pathway. J. Cell Sci. 2013;126:2641–2655. doi: 10.1242/jcs.117705. [DOI] [PubMed] [Google Scholar]
- 54.Ellis M.V., James S.R., Perisic O., Downes C.P., Williams R.L., Katan M. Catalytic domain of phosphoinositide-specific phospholipase C (PLC): Mutational analysis of residues within the active site and hydrophobic ridge of PLCδ1. J. Biol. Chem. 1998;273:11650–11659. doi: 10.1074/jbc.273.19.11650. [DOI] [PubMed] [Google Scholar]
- 55.Balla T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarkes D., Rameh L.E. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem. J. 2010;428:375–384. doi: 10.1042/BJ20100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watt S.A., Kular G., Fleming I.N., Downes C.P., Lucocq J.M. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem. J. 2002;363:657–666. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma V.P., DesMarais V., Sumners C., Shaw G., Narang A. Immunostaining evidence for PI(4,5)P2 localization at the leading edge of chemoattractant-stimulated HL-60 cells. J. Leukoc. Biol. 2008;84:440–447. doi: 10.1189/jlb.0907636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B., Li H., Wang Z., Wang Y., Gao A., Cui Y., Liu Y., Chen G. Evidence for the role of phosphatidylcholine-specific phospholipase in experimental subarachnoid hemorrhage in rats. Exp. Neurol. 2015;272:145–151. doi: 10.1016/j.expneurol.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 60.Hafez M.M., Costlow M.E. Phosphatidylethanolamine turnover is an early event in the response of NB2 lymphoma cells to prolactin. Exp. Cell Res. 1989;184:37–43. doi: 10.1016/0014-4827(89)90361-3. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi D., Sakane F. Expression and purification of human diacylglycerol kinase alpha from baculovirus-infected insect cells for structural studies. PeerJ. 2018;6 doi: 10.7717/peerj.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saito T., Takahashi D., Sakane F. Expression, purification, and characterization of human diacylglycerol kinase ζ. ACS Omega. 2019;4:5540–5546. doi: 10.1021/acsomega.9b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morimoto K., Suno R., Hotta Y., Yamashita K., Hirata K., Yamamoto M., Narumiya S., Iwata S., Kobayashi T. Crystal structure of the endogenous agonist-bound prostanoid receptor EP3. Nat. Chem. Biol. 2019;15:8–10. doi: 10.1038/s41589-018-0171-8. [DOI] [PubMed] [Google Scholar]
- 64.Shimamura T., Shiroishi M., Weyand S., Tsujimoto H., Winter G., Katritch V., Abagyan R., Cherezov V., Liu W., Han G.W., Kobayashi T., Stevens R.C., Iwata S. Structure of the human histamine H1 receptor complex with doxepin. Nature. 2011;475:65–70. doi: 10.1038/nature10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson M.A., Cherezov V., Griffith M.T., Roth C.B., Jaakola V.P., Chien E.Y., Velasquez J., Kuhn P., Stevens R.C. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.James S.R., Paterson A., Harden T.K., Downes C.P. Kinetic analysis of phospholipase C beta isoforms using phospholipid-detergent mixed micelles. Evidence for interfacial catalysis involving distinct micelle binding and catalytic steps. J. Biol. Chem. 1995;270:11872–11881. doi: 10.1074/jbc.270.20.11872. [DOI] [PubMed] [Google Scholar]
- 67.Klein R.R., Bourdon D.M., Costales C.L., Wagner C.D., White W.L., Williams J.D., Hicks S.N., Sondek J., Thakker D.R. Direct activation of human phospholipase C by its well known inhibitor u73122. J. Biol. Chem. 2011;286:12407–12416. doi: 10.1074/jbc.M110.191783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carman G.M., Deems R.A., Dennis E.A. Lipid signaling enzymes and surface dilution kinetics. J. Biol. Chem. 1995;270:18711–18714. doi: 10.1074/jbc.270.32.18711. [DOI] [PubMed] [Google Scholar]
- 69.Deems R.A., Eaton B.R., Dennis E.A. Kinetic analysis of phospholipase A2 activity toward mixed micelles and its implications for the study of lipolytic enzymes. J. Biol. Chem. 1975;250:9013–9020. [PubMed] [Google Scholar]
- 70.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 71.Pascual F., Carman G.M. Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim. Biophys. Acta. 2013;1831:514–522. doi: 10.1016/j.bbalip.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Müller-Decker K. Interruption of TPA-induced signals by an antiviral and antitumoral xanthate compound: Inhibition of a phospholipase C-type reaction. Biochem. Biophys. Res. Commun. 1989;162:198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- 73.Yeang C., Ding T., Chirico W.J., Jiang X.C. Subcellular targeting domains of sphingomyelin synthase 1 and 2. Nutr. Metab. (Lond.) 2011;8:89. doi: 10.1186/1743-7075-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaoka S., Miyaji M., Kitano T., Umehara H., Okazaki T. Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J. Biol. Chem. 2004;279:18688–18693. doi: 10.1074/jbc.M401205200. [DOI] [PubMed] [Google Scholar]
- 75.Ding T., Kabir I., Li Y., Lou C., Yazdanyar A., Xu J., Dong J., Zhou H., Park T., Boutjdir M., Li Z., Jiang X.C. All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity. J. Lipid Res. 2015;56:537–545. doi: 10.1194/jlr.M054627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gusain A., Hatcher J.F., Adibhatla R.M., Wesley U.V., Dempsey R.J. Anti-proliferative effects of tricyclodecan-9-yl-xanthogenate (D609) involve ceramide and cell cycle inhibition. Mol. Neurobiol. 2012;45:455–464. doi: 10.1007/s12035-012-8254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adachi R., Ogawa K., Matsumoto S.I., Satou T., Tanaka Y., Sakamoto J., Nakahata T., Okamoto R., Kamaura M., Kawamoto T. Discovery and characterization of selective human sphingomyelin synthase 2 inhibitors. Eur. J. Med. Chem. 2017;136:283–293. doi: 10.1016/j.ejmech.2017.04.067. [DOI] [PubMed] [Google Scholar]
- 78.Othman M.A., Yuyama K., Murai Y., Igarashi Y., Mikami D., Sivasothy Y., Awang K., Monde K. Malabaricone C as natural sphingomyelin synthase inhibitor against diet-induced obesity and its lipid metabolism in mice. ACS Med. Chem. Lett. 2019;10:1154–1158. doi: 10.1021/acsmedchemlett.9b00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y.-L., Qi X.-Y., Jiang H., Deng X.-D., Dong Y.-P., Ding T.-B., Zhou L., Men P., Chu Y., Wang R.-X., Jiang X.-C., Ye D.-Y. Discovery, synthesis and biological evaluation of 2-(4-(N-phenethylsulfamoyl)phenoxy)acetamides (SAPAs) as novel sphingomyelin synthase 1 inhibitors. Bioorg. Med. Chem. 2015;23:6173–6184. doi: 10.1016/j.bmc.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 80.Deng X., Lin F., Zhang Y., Li Y., Zhou L., Lou B., Li Y., Dong J., Ding T., Jiang X., Wang R., Ye D. Identification of small molecule sphingomyelin synthase inhibitors. Eur. J. Med. Chem. 2014;73:1–7. doi: 10.1016/j.ejmech.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Li Y., Huang T., Lou B., Ye D., Qi X., Li X., Hu S., Ding T., Chen Y., Cao Y., Mo M., Dong J., Wei M., Chu Y., Li H. Discovery, synthesis and anti-atherosclerotic activities of a novel selective sphingomyelin synthase 2 inhibitor. Eur. J. Med. Chem. 2019;163:864–882. doi: 10.1016/j.ejmech.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 82.Reue K., Brindley D.N. Thematic review series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 2008;49:2493–2503. doi: 10.1194/jlr.R800019-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsuzawa Y., Hostetler K.Y. Properties of phospholipase C isolated from rat liver lysosomes. J. Biol. Chem. 1980;255:646–652. [PubMed] [Google Scholar]
- 84.Mateos M.V., Salvador G.A., Giusto N.M. Selective localization of phosphatidylcholine-derived signaling in detergent-resistant membranes from synaptic endings. Biochim. Biophys. Acta. 2010;1798:624–636. doi: 10.1016/j.bbamem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Mateos M.V., Uranga R.M., Salvador G.A., Giusto N.M. Coexistence of phosphatidylcholine-specific phospholipase C and phospholipase D activities in rat cerebral cortex synaptosomes. Lipids. 2006;41:273–280. doi: 10.1007/s11745-006-5097-3. [DOI] [PubMed] [Google Scholar]
- 86.Ginger R.S., Parker P.J. Expression, purification and characterisation of a functional phosphatidylinositol-specific phospholipase C-δ1 protein in Escherichia coli. Eur. J. Biochem. 1992;210:155–160. doi: 10.1111/j.1432-1033.1992.tb17403.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.