Abstract
Background:
Schizophrenia (SZ) is associated with devastating emotional, cognitive and language impairments. Understanding the deficits in each domain and their interactions is important for developing novel, targeted psychotherapies. This study tested whether negative-threat word processing is altered in individuals with SZ compared to healthy controls (HC), in relation to SZ symptom severity across domains.
Methods:
Thirty-one SZ and seventeen HC subjects were scanned with functional magnetic resonance imaging while silently reading negative-threat and neutral words. Post-scan, subjects rated the valence of each word. The effects of group (SZ, HC), word type (negative, neutral), task period (early, late), and severity of clinical symptoms (positive, negative, excitement/hostility, cognitive, depression/anxiety), on word valence ratings and brain activation, were analyzed.
Results:
SZ and HC subjects rated negative versus neutral words as more negative. The SZ subgroup with severe versus mild excitement/hostility symptoms rated the negative words as more negative. SZ versus HC subjects hyperactivated left language areas (angular gyrus, middle/inferior temporal gyrus (early period)) and the amygdala (early period) to negative words, and the amygdala (late period) to neutral words. In SZ, activation to negative versus neutral words in left dorsal temporal pole and dorsal anterior cingulate was positively correlated with excitement/hostility scores.
Conclusions:
A negatively-biased behavioral response to negative-threat words was seen in SZ with severe versus mild excitement/hostility symptoms. The biased behavioral response was mediated by hyperactivation of brain networks associated with semantic processing of emotion concepts. Thus, word-level semantic processing may be a relevant psychotherapeutic target in SZ.
Keywords: Schizophrenia, semantic processing, threat words, fMRI, fMRI dynamics, symptom factor analysis
1. INTRODUCTION
Schizophrenia (SZ) is a debilitating mental illness associated with a wide range of language, emotional, and cognitive deficits, and impaired social and occupational functioning (Tandon et al., 2009). Language dysfunction, presenting as disorganized language output, comprehension difficulties including of figurative language, and auditory verbal hallucinations, is suggested to reflect high-level semantic and contextual, and low-level perceptual, processing deficits (Kuperberg, 2010; Wible et al., 2009). Emotional dysfunction presents as difficulty understanding, expressing, and anticipating emotions (Kring and Elis, 2013; Phillips et al., 2003). Prior studies of emotional word processing, comparing SZ and healthy control (HC) groups on word valence rating or classification tasks, have reported either normal (Pinheiro et al., 2012), negatively-biased (Holt et al., 2006b; Jalenques et al., 2013), or blunted (Dowd and Barch, 2010), responses to negatively-valenced words. However, these seeming inconsistencies in emotional reactivity to words in SZ at the group level, could be due at least in part to individual differences in symptomatology. For example, negatively-biased word judgments have been associated with increased delusion symptoms (Holt et al., 2006b), whereas blunted responses have been associated with increased anhedonia (Dowd and Barch, 2010).
The Positive and Negative Syndrome Scale (PANSS) classifies SZ symptoms into three categories, positive, negative, and general (Kay et al., 1987). However, factor analyses suggest that a five-category classification to positive (e.g., delusions), negative (e.g., apathy), cognitive (e.g., conceptual disorganization), excitement/hostility (positive and cognitive symptoms related to excitement and hostility), and anxiety/depression (e.g., guilt feelings) symptoms is more adequate for this multidimensional disorder (Anderson et al., 2015; Lancon et al., 1998; Lindenmayer et al., 1994; Van den Oord et al., 2006). Language symptoms do not fall neatly into one category, and can also be assigned to different categories in different factor analyses, perhaps reflecting complex interactions of language difficulties with other symptoms in SZ. For example, difficulty in abstract thinking (N5) is usually assigned to the cognitive factor but may also load significantly on the positive factor, and unusual thought content (G9) is usually assigned to the positive factor but may also load on the cognitive factor (van der Gaag et al., 2006).
This study tested whether negative word processing is altered in individuals with SZ compared to HC, in relation to SZ symptom severity across domains. A previous within-group study (Perez et al., 2015) in part of the same SZ cohort analyzed here found that the severity of suspiciousness/persecution symptoms was positively correlated with the level of activation in visual, semantic, and limbic/paralimbic brain areas during the reading of threat versus neutral words. The present study compared behavioral and brain responses in SZ and HC subjects tested with the same word reading paradigm designed to probe emotional linguistic processing (Perez et al., 2015; Protopopescu et al., 2005). The time course of brain responses was assessed in addition to the magnitude, because abnormal neural dynamics have been associated with clinical symptoms in other mental disorders, and may have relevance for psychotherapeutic interventions (Protopopescu et al., 2005). In a subset of SZ subjects, the effects of symptom severity on threat word processing were assessed across five PANSS factors (Citrome et al., 2011).
2. MATERIALS AND METHODS:
2.1. Subjects:
Participants were 31 SZ subjects (one with schizoaffective disorder), and 17 HC subjects with no psychiatric disorder, as determined based on the Structured Clinical Interview for the DSM-IV Axis 1 Disorders (First, 1997) (Table 1). Inclusion criteria included: only minimal negative symptoms and cognitive impairment, no electroconvulsive therapy in the past year, English reading proficiency at or above 8th grade level ascertained by a reading score of at least 42 (Wide Range Achievement Test III (Wilkinson and Robertson, 2006)), and capacity to provide informed consent. Exclusion criteria included: diagnosis of schizoaffective disorder with mainly affective symptoms, prior manic episode, substance abuse within the past six months, and history of major neurological or medical illness. The SZ and HC groups were matched on all demographic variables, except that the mean age was higher by 7.8 years in the SZ group (p=0.008), and three SZ but no HC subjects were left handed. SZ subjects were receiving anti-psychotic medications, and psychotropic medications such as mood stabilizers, anti-depressants, benzodiazepines and anti-cholinergic medications. The anti-psychotic medication doses were converted to a mean daily dose equivalence of Chlorpromazine (CPZ) (Andreasen et al., 2010). Subject variables, consisting of age, sex, and paradigm order, were modeled as nuisance covariates in all the between-group analyses. For analyses within the SZ group, CPZ-equivalent medication dose was entered as an additional nuisance regressor.
Table 1:
Demographic characteristics of SZ and HC, and clinical characteristics of SZ. The scores in the three traditional PANSS subscales (10) are presented here as the arithmetic mean across items in each subscale. The PANSS factor analysis scores correspond to the weighted mean for each factor, as computed in (18). Additional subject characteristics are given in the Supplement. SD - standard deviation; Min - minimum; Max - maximum.
| SZ | HC | |
|---|---|---|
| Demographic Variables | N=31 | N=17 |
| Age, years, Mean (SD) | 34.7 (10.7) | 26.9 (5.4) |
| Gender, male, N (%) | 25 (80.6) | 12 (70.6) |
| WRAT reading score, mean (SD) | 54.9 (7.5) | 61.1 (5.1) |
| CPZ equivalent, mean (SD) | 559.7 (290.1) | |
| PANSS Scores, mean (SD, Min, Max)(10) | N=31 | |
| Positive score | 2.2 (0.8, 1, 4) | |
| Negative score | 2.1 (0.7, 1, 3.3) | |
| General score | 1.8 (0.6, 1, 3) | |
| Total score | 2.0 (0.7, 1, 3.5) | |
| PANSS Factor scores, mean (SD, Min, Max)(18) | N=20 | |
| Excitement/Hostility score | 3.4 (1.4, 2.45, 7.35) | |
| Cognition score | 7.3 (2.4, 4.45, 11.7) | |
| Positive score | 5.7 (2.5, 2.7, 11.8) | |
| Negative score | 10.3 (4.1, 4.7, 16.1) | |
| Depression/Anxiety score | 5.2 (2.0, 2.7, 10.3) |
For 20 SZ subjects for whom all 30 PANSS items were administered, five PANSS subscores representing Positive, Negative, Cognitive, Excitement/Hostility, and Depression/Anxiety symptoms, as defined in a PANSS factor analysis (Citrome et al., 2011), were computed. This PANSS factor analysis (Citrome et al., 2011) was selected because it was conducted on a large sample of SZ subjects and based on all 30 PANSS items. For analyses of the Excitement/Hostility factor, two subjects with outlying (more than two standard deviations above the mean) subscores were excluded, such that the sample size was 18 in that analysis.
All subjects provided informed consent as part of a protocol that included independent assessment of capacity for informed consent, approved by the New-York Presbyterian Hospital/Weill Cornell Medical College institutional review board. Data analyses were approved by Partners Human Research Committee.
2.2. Emotional word paradigm:
Participants underwent two fMRI paradigms, instructed-fear/safety (reported in (Perez et al., 2015)) and emotional word, with order counterbalanced. In the emotional word paradigm, 24 negative (Neg) and 24 neutral (Neu) written words were presented that were matched on mean word length and mean frequency within the lexicon (Carroll et al., 1971). The Neg words were selected for their negative valence and threatening content relevant to paranoid delusions. The Neu words were selected for their neutral valence and low relevance to schizophrenia. The full list of words is given in Supplementary Table 3. For the Neg versus Neu words, normative valence ratings on a 9-point scale ranging from negative to positive were lower (mean rating ± standard deviation: Neg=3.1±1.0, Neu=5.5±0.6, p<10−5), and normative arousal ratings on a 9-point scale ranging from unarousing to arousing were higher (mean rating ± standard deviation: Neg=5.35±0.84, Neu=3.74±0.71, p<10−5)(Warriner et al., 2013), confirming that the selected word lists differed as designed. In each trial, a word was presented for 2s, followed by a jittered inter-stimulus interval ranging 1.8–3.8s (uniformly distributed), for a total of 28.8s per block. Participants were instructed to read each word silently and then press a button with the right index finger. The words were presented in 4 blocks of 6 per valence type, in pseudorandom order. Blocks were separated by a rest period (24s) during which subjects were instructed to look at a dash centered on the screen. The paradigm started and ended with 12s rest periods, for a total duration of 7.44min. Stimulus presentation and response collection was controlled by the Integrated Functional Imaging System (MRI Devices Corporation, Waukesha, WI) and E-Prime (Psychology Software Tools, Pittsburgh, PA). Immediately following the scan, an unannounced memory test was conducted: 48 words seen during the scan intermixed with 24 novel words (12 negative, 12 neutral, matched on length, frequency) were presented, and subjects were instructed to indicate the words previously seen in the scanner. Subsequently, subjects rated the emotional valence of all the words used in the scanner and memory tasks on a seven-point Likert scale ranging from −3 (strongly negative) to +3 (strongly positive), with 0 indicating neutral. For analyses, the range of valence scores was shifted to 1 to 7.
2.3. Behavioral data analysis:
The behavioral data analysis tested the hypotheses that 1) There are Group (SZ, HC) differences in valence ratings of Neg versus Neu words. 2) There are SZ Subgroup (severe, mild, on each of 5 PANSS symptom subscales) differences in valence ratings of Neg versus Neu words. For each subject, the mean valence ratings were calculated for each word type. An ANCOVA was used to test for effects of Group (HC, SZ) and Word Valence Type (Neg, Neu) on the subjective word valence ratings. Group and Valence were entered as within-subjects factors, and age, sex, and order of paradigm were entered as nuisance regressors. Within the SZ group, mild and severe subgroups were defined for each of five PANSS symptom factors (Positive, Negative, Cognitive, Excitement/Hostility, Depression/Anxiety) (Citrome et al., 2011), corresponding to the patients with scores in the lower and upper half (median split), respectively. Separate ANCOVAs were used to test for effects of SZ Subgroup (mild, severe) in each of the five symptom subscales, and Valence (Neg, Neu), on the subjective word valence ratings. For analyses within the SZ group, CPZ-equivalent medication dose was entered as an additional nuisance regressor.
2.4. Image acquisition:
Scanning was conducted on two General Electric-Signa 3 Tesla scanners (max gradient strength 40 mT/m; max slew rate 150T/m/s), using identical scan parameters. An ANCOVA revealed no effects of scanner on blood-oxygen-level-dependent (BOLD) signal, confirming that the datasets could be combined across scanners, with scanner modeled as a nuisance covariate in group-level analyses. The scan protocol consisted of a localizer scan, high-resolution T1-weighted anatomical scan, reference 2D T1-weighted anatomical scan matching the slice placement and thickness of the subsequent echo planar images (EPI), and EPI for fMRI. The high-resolution T1 was acquired with a spoiled gradient recalled acquisition sequence (TR/TE=30/8ms, flip angle=45, field of view=220mm, 140 coronal slices with thickness=contiguous 1.5mm, number of averages=1, matrix=256×256, voxel resolution=0.8594×1.5×0.8594mm3). After shimming to maximize homogeneity, functional scans were collected using gradient echo EPI (TR=1200ms,TE=30ms, 15 or 21 5mm slices, 1mm gap, FoV=240mm, matrix=64×64), with a z-shimming algorithm (Gu et al., 2002) to reduce signal loss due to susceptibility artifact in ventral brain regions.
2.5. Image processing and analyses:
Image processing was performed with a customized Statistical Parametric Mapping (SPM) software package (www.fil.ion.ucl.ac.uk/spm), as detailed in the Supplement.
The neuroimaging data analysis tested the hypotheses that 1) There are Group (SZ, HC) differences in brain activation to Neg versus Neu words, in early versus late Task Periods. 2) In SZ, the brain activation to Neg versus Neu words, in the early versus late Periods, varies as a function of the severity of Excitement/Hostility and Cognition symptoms. These two PANSS factors were selected for the neuroimaging data analysis because they showed an effect on the behavioral word valence ratings. Processed fMRI data were submitted to a two-level voxel-wise linear mixed-effects model, using a customized version of fmristat software within an ANCOVA framework (Worsley et al., 2002). First-level (subject) models consisted of whole-brain voxel-wise multiple linear regressions. Block-wise stimulus onset times convolved with a canonical hemodynamic response function (HRF) were entered as regressors of interest. Nuisance regressors consisted of the global signal, realignment parameters, physiological fluctuations, and the first order temporal derivative of the regressors of interest, to account for individual variation from the canonical HRF. The first-level models included temporal filtering to reduce effects of baseline drifts and higher frequency noise, and a first order autoregressive model to account for autocorrelation in the residuals. The regression model was fit to each brain voxel using the Expectation Maximization algorithm. Linear contrasts were applied to test for condition-specific effects of Word Valence (Neg, Neu) and Task Period (Early, Late). The early and late periods corresponded to the first and second half of the scan, respectively. The second-level analyses with Subject as the random-effect summarized brain responses within-Group and between-Group as a function of Valence and Period, and their interaction, using a multiple regression model with nuisance regressors of age, sex, scanner, and order of paradigm. Additionally, the effects within the SZ Group of the hypothesis-driven contrasts of Valence (Neg vs Neu) and Period (Early vs Late) were examined for their association with two PANSS factors (Excitement/Hostility, Cognition) via separate multiple regression models, each using the PANSS factor as the main regressor and the same nuisance regressors plus CPZ-equivalent medication dose. These group-level correlation effect estimates generated statistical maps of the t-statistic, and the statistical significance of the t-maps was evaluated in the final step of inference. Statistical significance of the t-maps was determined using random field theory, and the voxel-wise p<0.01 was corrected based on family-wise error rate over the whole brain at pcorrected<0.05. A small volume correction was applied to two regions of interest (ROI): the amygdala as defined in the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002), and posterior middle temporal gyrus (TE1p) as defined in the Human Connectome Project atlas (Glasser et al., 2016) (Fig. 2B, Table 2B). These ROIs were selected for their relevance to emotional word processing, and implication in abnormal processing in SZ, indicated by prior neuroimaging work using a similar emotional word paradigm (Goldstein et al., 2007; Perez et al., 2015; Protopopescu et al., 2005).
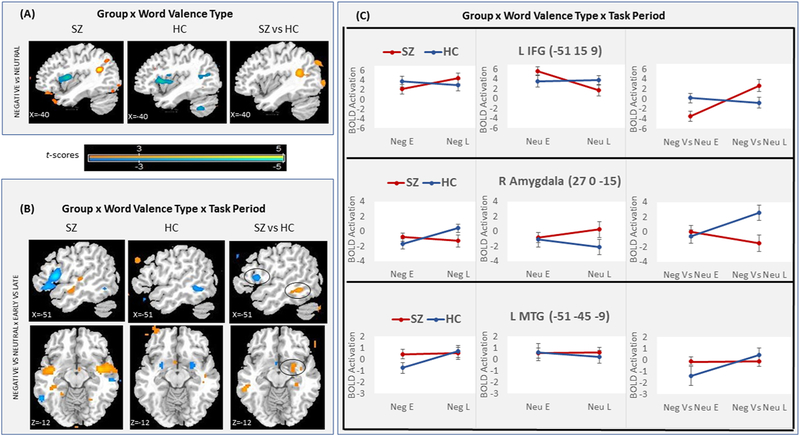
Figure 2:
Brain activation in SZ and HC. (A) fMRI t-maps of the Neg versus Neu contrast for within-SZ, within-HC, and SZ versus HC. (B) fMRI t- maps of the Neg versus Neu × Early versus Late interaction for within-SZ, within-HC, and SZ versus HC. (C) BOLD response effect sizes (in %) at the left IFG, right amygdala, and left MTG/ITG peaks (indicated by black circles in B), for Neg, Neu, and Neg versus Neu words, in early (E) and late (L) task periods, in SZ and HC. For visualization, fMRI maps shown at voxel-wise p<0.01.
Table 2:
Brain regions showing SZ and HC Group differences in (A) the Word Valence Type contrast over the whole brain at pcorrected<0.05, and (B) the Word Valence Type X Task Period interaction over the whole-brain at pcorrected <0.06 and in two ROIs at small-volume pcorrected <0.05 (marked *). The activation peak coordinates in Montreal Neurological Institute (MNI) space are listed along with the corresponding Brodmann area, peak z-score, corrected p-value, and cluster size. L - left; R - right.
| (A) SZ vs HC: Negative vs Neutral words | |||||||
| Brain region | Brodmann area | Peak coordinate (MNI space) | Peak Z | p-corrected (whole-brain) | Cluster size (mm3) | ||
| x | y | z | |||||
| Increase in activation | |||||||
| R middle frontal gyrus | 46/9 | 30 | 21 | 30 | 4.171 | 0.010 | 1998 |
| L posterior middle temporal gyrus / angular gyrus | 39 | −39 | −48 | 18 | 4.148 | 0.011 | 1782 |
| L middle occipital gyrus | 18 | −24 | −96 | −3 | 3.801 | 0.035 | 3078 |
| (B) SZ vs HC: Negative vs Neutral words X Early vs Late periods | |||||||
| Brain region | Brodmann area | Peak coordinate (MNI space) | Peak Z | p-corrected (whole-brain, small-volume*) | Cluster size (mm3) | ||
| x | y | z | |||||
| Increase in activation | |||||||
| R Amygdala | 34 | 27 | 0 | −15 | 3.168 | 0.026 * | 999 |
| L middle/inferior temporal gyrus | 20 | −51 | −45 | −9 | 3.239 | 0.037 * | 1296 |
| Decrease in activation | |||||||
| L inferior frontal gyrus | 44 | −54 | 15 | 9 | 3.368 | 0.063 | 1674 |
3. RESULTS:
3.1. Behavioral:
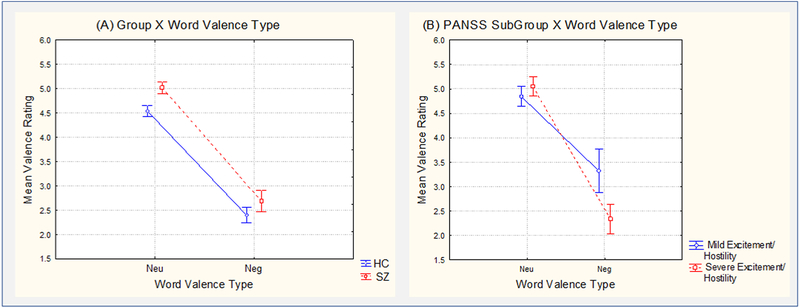
In the SZ versus HC Group analysis, there was a significant main effect of Valence on the subjective word valence ratings (mean (M)± standard error (SE): Neu =4.8±0.7, Neg =2.6±1.0; (F1,89)=152.73, p<10−4), and a marginal effect of Group (M±SE: SZ=3.9±1.5, HC=3.5±1.2; (F1,89)=3.71, p=0.057), with no significant interaction between Valence and Group (M±SE, SZ: Neu =5.0±0.1, Neg =2.7±0.2; HC: Neu =4.5±0.1, Neg =2.4±0.2; F(1,89)=, F(1,89)=0.26, p=0.61) (Figure 1A).
Figure 1:
Behavioral word valence ratings in SZ and HC Groups (A) and in mild and severe Excitement/Hostility SZ Subgroups (B). Vertical bars denote standard errors.
However, in the severe versus mild SZ Subgroup analysis, there was a significant Excitement/Hostility Subgroup by Valence interaction (M±SE, severe SZ: Neu=5.1±0.2, Neg=2.3±0.3; mild SZ: Neu=4.8±0.2, Neg=3.3±0.4; F(1,32)=5.49, p=0.025), which was due to the Neg words being rated as more negative (Fisher’s LSD, p=0.01) and the Neu words being rated the same (Fisher’s LSD, p=0.56) in severe versus mild SZ (Figure 1B). There was a similar trend for the Cognitive Subgroup by Valence interaction (M±SE, severe SZ: Neu=4.8±0.2, Neg=2.2±0.2; mild SZ: Neu=5.2±0.2, Neg=3.4±0.4; F(1,32)=2.34, p=0.136), which was also due to the Neg words being rated as more negative (Fisher’s LSD, p=0.01) and the Neu words being rated the same (Fisher’s LSD, p=0.3) in severe versus mild SZ. There were no effects of the other PANSS factors on word valence ratings.
3.2. Neuroimaging:
In SZ versus HC, the 2-way interaction of Group by Valence showed positive activations in the left posterior middle temporal/angular gyri (pMTG/LAG, Brodmann area (BA)39), left middle occipital gyrus (MOG), and right middle frontal gyrus (MFG, BA46/9), that were due to increased response to Neg words and decreased response to Neu words in SZ versus HC (Figure 2A, Table 2A, Suppl. Table 1A). The 3-way interaction of Group by Valence by Period revealed a negative activation trend in the left inferior frontal gyrus (IFG, BA44) that was due to decreased early and increased late response to Neg words, and the opposite pattern for Neu words, in SZ versus HC. Positive activation trends were seen in the right amygdala and left middle/inferior temporal gyrus (MTG/ITG, BA20) that were due to increased early response to Neg words, and in the amygdala also decreased late response to Neg words and increased late response to Neu words, in SZ versus HC (Figure 2B–C, Table 2B, Suppl. Table 1B).
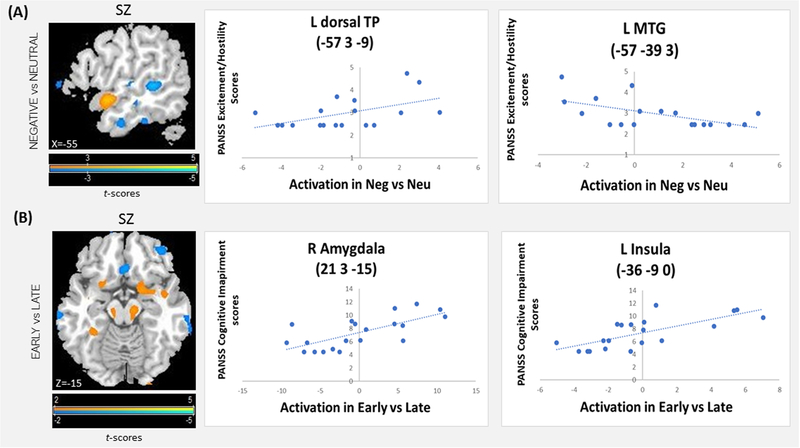
Within the SZ group, there was a positive correlation between PANSS Excitement/Hostility scores and activation in the Valence contrast in the left dorsal temporal pole (dTP, BA21/38), left middle frontal gyrus (MFG, BA10/11/46), and dorsal anterior cingulate (dAC, BA32), that was due to a positive correlation with the response to Neg words and negative correlation with the response to Neu words, and in the middle and superior occipital gyri (MOG, SOG) that was due to a more positive correlation with the response to Neg words than Neu words. There was a negative correlation between Excitement/Hostility scores and activation in the left middle temporal gyrus (MTG, BA22), left inferior frontal gyrus (IFG, BA44), and ventromedial prefrontal cortex (vmPFC, BA11), that was due to a positive correlation between the PANSS Excitement/Hostility scores and the response to Neu words and a negative correlation with the response to Neg words (Figure 3A, Table 3A, Suppl. Table 2A). The PANSS Cognitive scores were positively correlated with activation in the right amygdala and parahippocampus, and bilateral insula, in the Period contrast. Greater activation in the early, and weaker in the late, period was seen in these areas (across word types) in SZ with more severe Cognitive symptoms (Figure 3B, Table 3B, Suppl. Table 2B). Correlations of the Excitement/Hostility scores with the period contrast activation, and of the Cognitive scores with the Valence contrast activation, did not reveal significant findings.
Figure 3:
Correlations in the SZ Group between activation amplitude in the Word Valence Type contrast and PANSS Excitement/Hostility scores (A), and between activation amplitude in the Task Period contrast and PANSS Cognitive scores (B). For visualization, fMRI maps are shown at voxelwise p<0.01. Scatter plots show individual PANSS scores and t-values at the activation peaks.
Table 3:
Brain regions showing a correlation in the SZ Group (A) between activation amplitude in the Word Valence Type contrast and PANSS Excitement/Hostility scores, and (B) between activation amplitude in the Task period contrast and PANSS Cognitive scores. Activation clusters exceeding whole-brain pcorrected<0.05 are listed, along with the corresponding Brodmann area, MNI peak coordinates, peak z-score, corrected p-value, and cluster size. L - left; R - right.
| (A) Correlation of Excitement/Hostility scores and activation in Negative vs Neutral words contrast | |||||||
| Brain region | Brodmann area | Peak coordinate (MNI space) | Peak Z | p-corrected (whole brain) | Cluster size (mm3) | ||
| x | y | z | |||||
| Positive correlation | |||||||
| L middle frontal gyrus | 10/11/46 | −30 | 57 | 9 | 3.758 | 0.04 | 4293 |
| R anterior cingulate cortex | 32 | 15 | 45 | 15 | 3.723 | 0.045 | 4266 |
| L temporal pole/ superior temporal gyrus | 21/38 | −57 | 3 | −9 | 3.86 | 0.029 | 1269 |
| L superior occipital gyrus | 19 | −21 | −84 | 30 | 3.686 | 0.05 | 8532 |
| L middle occipital gyrus | 19 | −36 | −78 | 12 | 4.065 | 0.014 | 2241 |
| R middle occipital gyrus | 37 | 39 | −72 | 9 | 4.119 | 0.012 | 1998 |
| Negative correlation | |||||||
| L inferior frontal gyrus, pars opercularis | 44 | −63 | 18 | 24 | 4.24 | 0.012 | 945 |
| L middle temporal gyrus | 22 | −57 | −39 | 3 | 3.9 | 0.025 | 2862 |
| R ventromedial prefrontal cortex | 11 | 15 | 21 | −33 | 4.23 | 0.013 | 4266 |
| (B) Correlation of Cognitive Impairment scores and activation in Early vs Late period contrast | |||||||
| Brain region | Brodmann area | Peak coordinate (MNI space) | Peak Z | p-corrected (whole brain) | Cluster size (mm3) | ||
| x | y | z | |||||
| Positive correlation | |||||||
| R frontal superior orbital gyrus | 11 | 18 | 30 | −24 | 3.857 | 0.029 | 1053 |
| R amygdala | 34 | 21 | 3 | −15 | 3.956 | 0.021 | 4941 |
| L insula | 13 | −36 | −9 | 0 | 4.221 | 0.008 | 5805 |
| R insula | 13 | 36 | 12 | 3 | 4.095 | 0.013 | 8262 |
| Negative correlation | |||||||
| L cerebellum | −6 | −51 | −42 | 3.667 | 0.053 | 4050 | |
4. DISCUSSION:
Behaviorally, there were marginal differences between the SZ and HC groups in subjective word valence ratings, and no significant interaction as a function of word valence type. However, within the SZ group, there were significant differences between the subgroups with mild versus severe excitement/hostility symptoms, and marginal differences between the subgroups with mild versus severe cognitive symptoms, whereby the subgroup with severe symptoms showed a negative bias for negative words and no difference for neutral words relative to the subgroup with mild symptoms. These results are consistent with the hypothesis that negatively-biased processing of threat words is associated with the positive (excitement, hostility) and cognitive (uncooperativeness, poor impulse control) symptoms of SZ, captured here in the excitement/hostility PANSS factor (Citrome et al., 2011). It is interesting that the word ratings varied as a function of the excitement/hostility (and marginally the cognition), but not the other, PANSS factors. This finding may be driven by the threatening theme of the negative words. That is, words with a sad or anxious theme may be better probes for the symptoms of the depression/anxiety factor. Single words may also be suboptimal for probing concrete, stereotypic, or unusual thinking (captured in the cognitive and positive factors), because they reflect difficulties in establishing causal relationships and context which are better probed by sentences or narrative. Overall, the present findings converge with those of prior studies (Cohen and Minor, 2010; Dowd and Barch, 2010; Holt et al., 2006b; Potvin et al., 2016) in highlighting that emotional biases in stimulus processing may be a significant marker of SZ. However, the specific biases that are observed likely vary as a function of individual symptomatology, and the experimental stimuli and testing paradigms used to probe the symptoms.
The fMRI maps revealed overall increased activation in semantic, visual, and emotion expression and appraisal areas in SZ versus HC. Activation was elevated in SZ for negative versus neutral words in left language (pMTG/AG, MTG/ITG in the early period), limbic (amygdala in the early period), prefrontal (MFG), and visual (MOG), areas, and for neutral versus negative words in the amygdala (late period). In SZ, the PANSS excitement/hostility scores were positively correlated with the amplitude of response to negative versus neutral words in left language (dTP), dorsomedial (dAC) and dorsolateral (MFG) prefrontal, and visual (MOG, SOG), areas, and to neutral versus negative words in a left language (L MTG) area.
The left-lateralized temporoparietal network that was hyperactivated in SZ comprised areas considered to be amodal, highly interconnected, and part of a distributed system specialized for language comprehension (Turken and Dronkers, 2011; Mesulam et al., 2014). The left AG (BA39) may be important for conceptual integration and narrative comprehension (Binder et al., 2009; Seghier, 2013; Wilson et al., 2007). The left dTP (BA38) may be important for conceptual knowledge of social behaviors including the representation of emotions (Ross and Olson, 2010; Zahn et al., 2007), and it is strongly connected with ipsilateral perisylvian language areas, and limbic and dorsomedial prefrontal areas associated with the expression and appraisal of emotions (Bludau et al., 2014; Etkin et al., 2011; Pascual et al., 2013). The results are consistent with the idea that the excitement and hostility symptoms of SZ may be mediated by hyperactivation of a higher-order language network comprising left anterior temporal language and dorsomedial prefrontal areas, associated with knowledge of emotion and social concepts, and assignment of emotional value to stimuli.
Verbal hallucinations have been linked to hyperactivation of the posterior superior temporal gyrus (pSTG) and AG (Silbersweig and Stern, 1996; Wible et al., 2009). Structural abnormalities in the left pSTG/AG, and the middle longitudinal fasciculus connecting the temporal pole (TP) with AG, have been associated with thought disorder (Asami et al., 2013; Shenton et al., 1992). Abnormal left TP connectivity with the frontal cortex via the uncinate fasciculus (Price et al., 2008; van den Heuvel et al., 2010), and abnormal left TP functional connectivity (Pu et al., 2014), have also been shown in SZ. The findings of hyperactivation of a temporoparietal language network in thought disorder and verbal hallucinations (Wible et al., 2009), as well as in emotionally-biased word processing (this study), are consistent with the possibility of a shared neuropathological basis for different aspects of language dysfunction in schizophrenia, and the possibility that therapeutic intervention targeted at single word semantic processing will address multiple language-related symptoms. Further supporting the possibility of shared neuropathological basis for different language deficits in SZ is the common finding of MFG (BA46) hyperactivation in this and prior studies of verbal hallucination (Lennox et al., 2000; Wible et al., 2009).
Hyperactivation of visual areas in SZ was previously reported during viewing of affectively-valenced faces (Wolf et al., 2011), and a positive correlation with PANSS persecutory delusion scores was found in the visual word form area to negative and neutral words (Perez et al., 2015). The present findings further suggest that hyperactivation of high-order visual areas may be related to perceptual biases, and severe excitement/hostility symptoms, in schizophrenia.
In contrast to the positive correlation in reactive areas, a negative correlation with excitement/hostility scores was seen in left IFG (BA44), an area associated with cognitive control of language (Binder et al., 2009; Gabrieli et al., 1998; Roskies et al., 2001), and in vmPFC (BA11), an area implicated in the regulation of emotion (36). This may indicate decreased top-down regulation of emotional function in the presence of increasing excitement/hostility symptoms, and is consistent with studies demonstrating deficits in these regions across a range of neuropsychiatric disorders (Protopopescu et al., 2008; Silbersweig et al., 2007).
Finally, the dynamics of fMRI activation (early vs late period) were abnormal in SZ. In the amygdala and left MTG/ITG, the response to negative words was elevated early and remained stable late in SZ, whereas it built-up over time in HC. The response to neutral words in the amygdala built-up over time in SZ, whereas it declined over time in HC. In contrast in the left IFG, the activation built-up for negative words and declined for neutral words in SZ, whereas it remained stable in HC. The PANSS cognitive scores were positively correlated with the amplitude of activation in early versus late periods (across word types) in the right amygdala and bilateral insula. The amygdala and insula are important for emotional salience detection, and abnormally elevated activation in these areas has been associated with hypersensitivity to emotional (or otherwise salient) stimuli (Adolphs et al., 1998; Morris et al., 1998; Phelps and LeDoux, 2005; Sander et al., 2003), including neutral stimuli in SZ (Hall et al., 2008; Holt et al., 2006a; Mier et al., 2014; Potvin et al., 2016). The insula may play a key role in emotional awareness (Craig, 2009; Mesulam and Mufson, 1982). Medial temporal lobe structures have been implicated in the pathophysiology of psychotic syndromes and mood regulation (Butler et al., 2012; Ellison-Wright and Bullmore, 2010; Epstein et al., 1999; Lee et al., 2016). Hypoactivity of frontal regulation systems and abnormal frontal-temporal connectivity are considered central to the neuropathology of SZ, especially within systems responsible for emotion processing (Carter et al., 1998; Friston, 1998; Goldman-Rakic, 1991; McTeague et al., 2017). The abnormal activation dynamics of frontal and temporal networks observed here, and association with cognitive symptoms, may indicate weaker cognitive control of emotional reactivity in SZ. The abnormal amygdala time-course to threat and neutral words in SZ is similar to that seen in post-traumatic stress disorder to trauma and neutral words, respectively (Protopopescu et al., 2005), consistent with the idea that abnormal emotional reactivity related to fronto-limbic pathology may be central to cognitive impairment across these disorders. The findings are also relevant to interventions using repeated exposure to symptom-related stimuli, in which the time course of habituation and sensitization to the stimuli is key, as they suggest that brain reactivity to threat words varies within a period comparable to that of a single-exposure therapy session (Protopopescu et al., 2005). Methodologically, the results demonstrate the importance of considering the time course of emotional reactivity in studies of SZ.
5. CONCLUSIONS:
The behavioral and neuroimaging results suggest that negatively-biased reactivity to threat words, in individuals with schizophrenia and severe excitement and hostility symptoms, may be mediated by hyperactivation of semantic, visual, and emotion, neural networks. The results raise the possibility of a shared neuropathological basis for different aspects of language dysfunction in schizophrenia, and the possibility that psychotherapy effectively targeting word-level semantics could alleviate symptoms of thought disorder and verbal hallucinations.
Future research should examine biases in negative word processing associated with other symptoms such as depression and anxiety, and biases in positive word processing that may be particularly relevant to individuals with excitement/hostility symptoms. It would also be interesting to examine the contribution to abnormal word processing of attributes that interact with emotional valence, such as word concreteness (Vigliocco et al., 2014).
The study has several limitations. The SZ subjects were on psychotropic medications, which we attempted to control for by including the medication dose equivalent as a nuisance covariate. Differences in language ability may also have affected the present results. In our sample, the SZ group was found to have lower reading scores than the SC group (raw-score mean ± standard deviation: SZ= 54.9±7.6, HC= 61.1±5.1, t=2.37, p=0.02). Future studies should collect more detailed measurements of language ability (including reading comprehension) in larger samples, to incorporate in neurobiological models of language processing in SZ, and to improve our understanding of this disease.
Supplementary Material
Acknowledgments:
The authors would like to thank Monica Bennett for her assistance in organizing the data for analysis and publication.
Role of the Funding Source
This work was supported by National Institute of Mental Health R01 grant MH074808 (Silbersweig, PI), and Brain and Behavior Research Foundation Independent Investigator grant 22249 (Liebenthal, PI). The sponsors had no direct involvement in study design, data collection and analysis, writing the report, and the decision to submit for publication.
Declaration of Interests:
Emily Stern, MD is a founder and CEO of Ceretype Neuromedicine, Inc., a spin-out from Brigham and Women’s Hospital, and has received founders shares (unrelated). She has four patents submitted: (1) System and Method for z-Shim Compensated Echo-Planar Magnetic Resonance Imaging, and Systems (issued), (2) System and Methods for Generating Biomarkers Based on Multivariate Classification of Functional Imaging and Associated Data (issued), (3) System and Method for a Multivariate, Automated, Systematic and/or Hierarchical Searching System for Biosignature Extraction and Biomarker Discovery via task-based fMRI Imaging spacetime data. (4) PhyNFI: System and Method for Physiological-Noise-Free Functional Magnetic Resonance Imaging (pending). David Silbersweig, MD is a founder and Chair of Ceretype Neuromedicine, Inc., a spin-out from Brigham and Women’s Hospital, and has received founders shares (unrelated). He has submitted the same four patents mentioned above. Adam Savitz is a full-time employee of Janssen Research & Development and owns stock in Johnson & Johnson, the parent company of Janssen R&D. Janssen R&D did not support the work involved in this publication. The other authors, Sara Dar, Einat Liebenthal, Hong Pan, Thomas Smith, and Yulia Landa, reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adolphs R, Tranel D, Damasio AR, 1998. The human amygdala in social judgment. Nature 393(6684), 470–474. [DOI] [PubMed] [Google Scholar]
- Anderson A, Wilcox M, Savitz A, Chung H, Li Q, Salvadore G, Wang D, Nuamah I, Riese SP, Bilder RM, 2015. Sparse factors for the positive and negative syndrome scale: which symptoms and stage of illness? Psychiatry Res 225(3), 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC, 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 67(3), 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Saito Y, Whitford TJ, Makris N, Niznikiewicz M, McCarley RW, Shenton ME, Kubicki M, 2013. Abnormalities of middle longitudinal fascicle and disorganization in patients with schizophrenia. Schizophr Res 143(2), 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL, 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19(12), 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, Schleicher A, Zilles K, Amunts K, 2014. Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage 93, 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Weisholtz D, Isenberg N, Harding E, Epstein J, Stern E, Silbersweig D, 2012. Neuroimaging of frontal-limbic dysfunction in schizophrenia and epilepsy-related psychosis: toward a convergent neurobiology. Epilepsy Behav 23(2), 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B, Davies P, 1971. The American Heritage word frequency book. Houghton Mifflin Boston. [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD, 1998. Functional hypofrontality and working memory dysfunction in schizophrenia. Am Journal of Psychiatry 155(9), 1285–1287. [DOI] [PubMed] [Google Scholar]
- Citrome L, Meng X, Hochfeld M, 2011. Efficacy of iloperidone in schizophrenia: a PANSS five-factor analysis. Schizophr Res 131(1), 75–81. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS, 2010. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull 36(1), 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel--now? The anterior insula and human awareness. Nature Rev Neurosci 10(1):59–70. [DOI] [PubMed] [Google Scholar]
- Dowd EC, Barch DM, 2010. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry 67(10), 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E, 2010. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res 117(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Epstein J, Stern E, Silbersweig D, 1999. Mesolimbic activity associated with psychosis in schizophrenia. Symptom-specific PET studies. Ann N Y Acad Sci 877, 562–574. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognit Sci 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MG,M; Williams JBW, 1997. Structured Clinical Interview for DSM-IV (SCID).,, Washington, D.C. [Google Scholar]
- Friston KJ, 1998. The disconnection hypothesis. Schizophr Res 30(2), 115–125. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE, 1998. The role of left prefrontal cortex in language and memory. Proc of the Natl Acad of Sci 95(3), 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536(7615), 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P, 1991. Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. Psychopathol and the Brain, 1–23. [Google Scholar]
- Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, Yang Y, Thomas K, Levy K, Silverman M, Clarkin J, Posner M, Kernberg O, Stern E, Silbersweig D, 2007. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage 36(3), 1026–1040. [DOI] [PubMed] [Google Scholar]
- Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E, Yang Y, 2002. Single-shot interleaved z-shim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage 17, 1358–1364. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, Baig BJ, Gountouna V-E, Job DE, Donaldson DI, 2008. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry 64(1), 70–73. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S, 2006a. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res 82(2–3), 153–162. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, Judge A, Kuperberg GR, 2006b. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res 83(2), 247–256. [DOI] [PubMed] [Google Scholar]
- Jalenques I, Enjolras J, Izaute M, 2013. Emotional valence of words in schizophrenia. L’Encephale 39(3), 189–197. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opfer LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2), 261. [DOI] [PubMed] [Google Scholar]
- Kring AM, Elis O, 2013. Emotion deficits in people with schizophrenia. Annual Rev of Clin Psychology 9, 409–433. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, 2010. Language in schizophrenia part 1: an introduction. Language and Linguistics Compass 4(8), 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancon C, Aghababian V, Llorca P, Auquier P, 1998. Factorial structure of the Positive and Negative Syndrome Scale (PANSS): a forced five-dimensional factor analysis. Acta Psychiatrica Scandinavica 98(5), 369–376. [DOI] [PubMed] [Google Scholar]
- Lee SH, Niznikiewicz M, Asami T, Otsuka T, Salisbury DF, Shenton ME, McCarley RW, 2016. Initial and Progressive Gray Matter Abnormalities in Insular Gyrus and Temporal Pole in First-Episode Schizophrenia Contrasted With First-Episode Affective Psychosis. Schizophr Bull 42(3), 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Park SB, Medley I, Morris PG, Jones PB, 2000. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res 100(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Bernstein-Hyman R, Grochowski S, 1994. A new five factor model of schizophrenia. Psychiatric Quarterly 65(4), 299–322. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A, 2017. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am Journal of Psychiatry 174(7), 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Rogalski EJ, Wieneke C, Hurley RS, Geula C, Bigio EH, Thompson CK, Weintraub S, 2014. Primary progressive aphasia and the evolving neurology of the language network. Nature Reviews Neurology 10(10), 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Mufson EJ, 1982. Insula of the old world monkey. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. Journal of Comp Neurology 212(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Mier D, Lis S, Zygrodnik K, Sauer C, Ulferts J, Gallhofer B, Kirsch P, 2014. Evidence for altered amygdala activation in schizophrenia in an adaptive emotion recognition task. Psychiatry Res 221(3), 195–203. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ, 1998. Conscious and unconscious emotional learning in the human amygdala. Nature 393(6684), 467–470. [DOI] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding S-L, Dickerson BC, 2013. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex 25(3), 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Pan H, Weisholtz DS, Root JC, Tuescher O, Fischer DB, Butler T, Vago DR, Isenberg N, Epstein J, 2015. Altered threat and safety neural processing linked to persecutory delusions in schizophrenia: a two-task fMRI study. Psychiatry Research: Neuroimaging 233(3), 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE, 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry 54(5), 515–528. [DOI] [PubMed] [Google Scholar]
- Pinheiro AP, McCarley RW, Thompson E, Gonçalves OF, Niznikiewicz M, 2012. From semantics to feelings: how do individuals with schizophrenia rate the emotional valence of words? Schizoph Res and Treat 2012:431823. doi: 10.1155/2012/431823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Tikasz A, Mendrek A, 2016. Emotionally Neutral Stimuli Are Not Neutral in Schizophrenia: A Mini Review of Functional Neuroimaging Studies. Front Psychiatry 7, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA, 2008. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. Neuroimage 39(3), 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, 2005. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry 57(5), 464–473. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, Altemus M, Polanecsky M, McEwen B, Stern E, Silbersweig D, 2008. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 108(1–2), 87–94. [DOI] [PubMed] [Google Scholar]
- Pu W, Rolls ET, Guo S, Liu H, Yu Y, Xue Z, Feng J, Liu Z, 2014. Altered functional connectivity links in neuroleptic-naïve and neuroleptic-treated patients with schizophrenia, and their relation to symptoms including volition. NeuroImage: Clin 6, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskies A, Fiez J, Balota D, Raichle M, Petersen S, 2001. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognit Neurosci 13(6), 829–843. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR, 2010. Social cognition and the anterior temporal lobes. Neuroimage 49(4), 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T, 2003. The human amygdala: an evolved system for relevance detection. Rev Neurosci 14(4), 303–316. [DOI] [PubMed] [Google Scholar]
- Seghier ML, 2013. The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist 19(1), 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, 1992. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. New England Journal of Med 327(9), 604–612. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E, 2007. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry 164(12), 1832–1841. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Stern E, 1996. Functional neuroimaging of hallucinations in schizophrenia: toward an integration of bottom-up and top-down approaches. Mol Psychiatry 1(5), 367–375. [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS, 2009. Schizophrenia,“just the facts” 4. Clinical features and conceptualization. Schizophr Res 110(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF, 2011. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Syst Neurosci 10;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Pol HEH, 2010. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. Journal of Neurosci 30(47), 15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oord EJ, Rujescu D, Robles JR, Giegling I, Birrell C, Bukszár J, Murrelle L, Möller H-J, Middleton L, Muglia P, 2006. Factor structure and external validity of the PANSS revisited. Schizophr Res 82(2), 213–223. [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, van Harten PN, Valmaggia L, de Hert M, Cuijpers A, Wiersma D, 2006. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res 85(1–3), 280–287. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Kousta ST, Della Rosa PA, Vinson DP, Tettamanti M, Devlin JT, Cappa SF, 2014. The neural representation of abstract words: the role of emotion. Cereb Cortex 24(7), 1767–1777. [DOI] [PubMed] [Google Scholar]
- Warriner AB, Kuperman V, Brysbaert M, 2013. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behav Res Methods 45(4), 1191–1207. [DOI] [PubMed] [Google Scholar]
- Wible CG, Preus AP, Hashimoto R, 2009. A cognitive neuroscience view of schizophrenic symptoms: abnormal activation of a system for social perception and communication. Brain Imaging and Behav 3(1), 85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G, Robertson G, 2006. Wide Range Achievement Test 4 professional manual: Psychological Assessment Resources. Inc. [Google Scholar]
- Wilson SM, Molnar-Szakacs I, Iacoboni M, 2007. Beyond superior temporal cortex: intersubject correlations in narrative speech comprehension. Cereb Cortex 18(1), 230–242. [DOI] [PubMed] [Google Scholar]
- Wolf C, Linden S, Jackson MC, Healy D, Baird A, Linden DE, Thome J, 2011. Brain activity supporting working memory accuracy in patients with paranoid schizophrenia: a functional magnetic resonance imaging study. Neuropsychobiol 64(2), 93–101. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao C, Aston J, Petre V, Duncan G, Morales F, Evans A, 2002. A general statistical analysis for fMRI data. Neuroimage 15(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J, 2007. Social concepts are represented in the superior anterior temporal cortex. Proc of the Natl Acad of Sci 104(15), 6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.