Abstract
Background and Aims
Global plant trait datasets commonly identify trait relationships that are interpreted to reflect fundamental trade-offs associated with plant strategies, but often these trait relationships are not identified when evaluating them at smaller taxonomic and spatial scales. In this study we evaluate trait relationships measured on individual plants for five widespread Protea species in South Africa to determine whether broad-scale patterns of structural trait (e.g. leaf area) and physiological trait (e.g. photosynthetic rates) relationships can be detected within natural populations, and if these traits are themselves related to plant fitness.
Methods
We evaluated the variance structure (i.e. the proportional intraspecific trait variation relative to among-species variation) for nine structural traits and six physiological traits measured in wild populations. We used a multivariate path model to evaluate the relationships between structural traits and physiological traits, and the relationship between these traits and plant size and reproductive effort.
Key Results
While intraspecific trait variation is relatively low for structural traits, it accounts for between 50 and 100 % of the variation in physiological traits. Furthermore, we identified few trait associations between any one structural trait and physiological trait, but multivariate regressions revealed clear associations between combinations of structural traits and physiological performance (R2 = 0.37–0.64), and almost all traits had detectable associations with plant fitness.
Conclusions
Intraspecific variation in structural traits leads to predictable differences in individual-level physiological performance in a multivariate framework, even though the relationship of any particular structural trait to physiological performance may be weak or undetectable. Furthermore, intraspecific variation in both structural and physiological traits leads to differences in plant size and fitness. These results demonstrate the importance of considering measurements of multivariate phenotypes on individual plants when evaluating trait relationships and how trait variation influences predictions of ecological and evolutionary outcomes.
Keywords: Protea, Proteaceae, South Africa, Cape Floristic Region, functional traits, intraspecific trait variation, trait combinations, ecophysiology, trait–fitness relationship
INTRODUCTION
Plant ecologists often study readily measured traits under the assumption that observed traits reflect the outcome of selective pressures on growth and demographic rates, and are thus mechanistically linked to performance and fitness (Ackerly et al., 2000; McGill et al., 2006). Understanding patterns of variation in these traits would thus provide insight into processes that influence species distributions (Cavender-Bares et al., 2004; Wright et al., 2004; Chave et al., 2009; Mitchell et al., 2015), the composition of communities (Keddy, 1992; Fukami et al., 2005; Kraft and Ackerly, 2010) and even ecosystem function (Díaz and Cabido, 2001; Lavorel and Garnier, 2002; Zirbel et al., 2017; Cadotte, 2017).
The Leaf Economics Spectrum (LES) is a useful framework when evaluating traits and trait trade-offs measured at the leaf level, which reflects the carbon costs and gains associated with photosynthesis (Wright et al., 2004; Shipley et al., 2006; Blonder et al., 2015). The Wood Economics Spectrum (WES) provides a similar framework, but at the level of xylem traits and whole-plant architecture, structure and function (Chave et al., 2009). The trait models and trait trade-offs associated with the LES and WES have been empirically supported when evaluating relationships between traits across phylogenetic and spatial scales (Wright et al., 2004, 2010; Chave et al., 2009; Díaz et al., 2016). However, these relationships have been most extensively studied at broad geographical and phylogenetic scales using species means and ignoring individual trait variation (Funk and Cornwell, 2013; Anderegg et al., 2018). At lower taxonomic and spatial scales, trait relationships are often weak and uncertain, or even in a direction that opposes predictions based on the LES and WES (Messier et al., 2017b, 2018; Anderegg et al., 2018). Given that explanations for trait relationships and key trait trade-offs observed in the LES and WES are often considered in terms of biophysical relationships at the level of individual plants or individual organs (e.g. leaves and stems), advances could be made by focusing on how individual trait variation influences individual variation in performance and fitness.
Furthermore, traits that may be described as structural or morphological characteristics of individuals (e.g. leaf area and stem density) may serve as indicators both of plant strategies (e.g. resource conservation vs. resource spending) and of physiological performance through univariate (Wright et al., 2004; Chave et al., 2009) and multivariate trait associations (Messier et al., 2018). Structural traits that reflect plant strategies and measures of physiological performance are themselves likely to be related to size and fitness. This suggests that studies should simultaneously account for how structural traits influence physiological performance and how both structural traits and physiological performance influence fitness at the level of individual plants in a multivariate framework. In this study we examine the following questions:
Does the proportion of variance attributable to individual differences within species depend on whether the traits are structural (i.e. organ-level, morphological traits) or physiological (i.e. dynamic traits that reflect rates of carbon assimilation and water conductance)?
Are individual differences in structural and physiological traits associated with differences in indicators of fitness, such as size and reproductive effort?
To the extent that individual differences in size or reproductive effort are associated with structural and physiological traits, are they primarily the result of univariate associations with individual traits or of multivariate associations with combinations of traits?
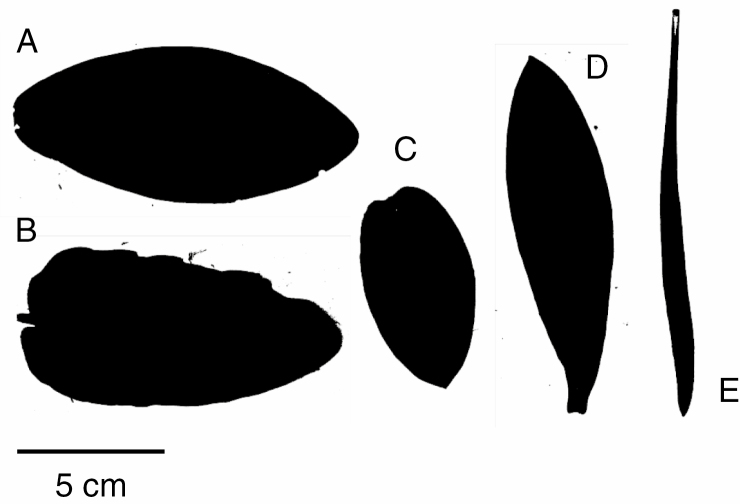
To answer these questions we measured a suite of leaf, stem and whole-plant traits on individuals of five widespread Protea species that are common to and abundant in the Cape Floristic Region (CFR), South Africa. We also measured aspects of physiological performance and fitness on the same individuals. The shape and size of leaves differs markedly among the species (Fig. 1), and it has been argued that these differences could promote coexistence of congeners within local communities (Cody, 1986; but see Potts et al., 2011). Substantial intraspecific trait variation in many commonly measured leaf and stem structural traits has been observed in Protea species as well, and may reflect adaptation of ecotypes to the local environment (Carlson et al., 2011, 2016; Prunier et al., 2012). Steep precipitation and temperature gradients in the CFR (Linder, 2005) may be strong selective factors on physiological traits related to the trade-off between carbon uptake and water conservation.
Fig. 1.
Outlines of leaves from the five Protea species: (A) P. nitida, (B) P. eximia, (C) P. punctata, (D) P. laurifolia and (E) P. repens.
MATERIALS AND METHODS
Study sites
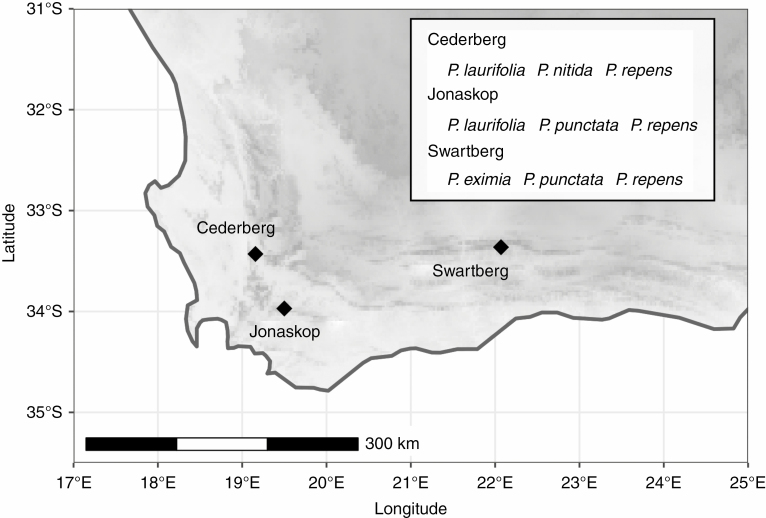
We measured structural traits, physiological performance and components of fitness on individual plants from each of the five Protea (L.) (Proteaceae) species described below at three sites in the Western Cape Province (Fig. 2) from July to September 2016 (summarized in Table 1). Jonaskop is located in the central part of the province in the Riviersonderend Mountain Range. It is relatively hot and wet with rainfall largely restricted to the winter months (May–August). Cederberg is located in the northern part of the province. It is relatively cool and wet, and also lies within the winter rainfall region. Swartberg Pass is located in the eastern part of the province. It is relatively hot and dry, with no seasonal trend in rainfall. The sampled populations occurred within the fynbos ecosystem, which is dominated by plant species in three families (Restionaceae, Ericaceae and Proteaceae), and is characterized as semi-arid with nutrient-poor soils (Bergh et al., 2014). It is a fire-dominated ecosystem, with fires recurring approximately every 10–13 years (VanWilgen et al., 2010).
Fig. 2.
Distribution of the three study sites where individuals were sampled (three populations per site) between July and September 2016. See Table 1 for more information about the sampled populations and climatic characteristics of each site.
Table 1.
Sites where Protea populations were sampled. The site is characterized by climate data from (Schulze 1997): MAT (mean annual temperature), MAP (mean annual precipitation), and Seasonality (either a winter rainfall site, or an aseasonal site)
| Site | Species sampled at site | n | Approximate stand age (years) | Population elevation (m) | Population GPS location |
|---|---|---|---|---|---|
| Cederberg | |||||
| MAT: 11.2 °C | P. repens | 19 | 8 | 916 | −32.43125, 19.14387 |
| MAP: 509 cm | P. laurifolia | 13 | 18 | 916 | −32.43027, 19.15748 |
| Seasonality: winter rainfall | P. nitida | 8 | 20+ | 916 | −32.43027, 19.15748 |
| Jonaskop | |||||
| MAT: 16.3 °C | P. repens | 20 | 9 | 983 | −33.94628, 19.51844 |
| MAP: 568 cm | P. laurifolia | 20 | 15 | 857 | −33.92868, 19.52423 |
| Seasonality: winter rainfall | P. punctata | 20 | 10 | 1503 | −33.97053, 19.49953 |
| Swartberg | |||||
| MAT: 16.5 °C | P. repens | 20 | 10 | 1110 | −33.36245, 22.09049 |
| MAP: 170 cm | P. punctata | 16 | 10 | 1188 | −33.36281, 22.06939 |
| Seasonality: aseasonal | P. eximia | 15 | 13 | 1188 | −33.36281, 22.06939 |
Study species
We focused our sampling on five common Protea species: P. eximia, P. laurifolia, P. nitida, P. punctata and P. repens. These species are among the most geographically widespread in the genus, presumably reflecting broad environmental tolerances at the species level (Rebelo, 2001). All of them have relatively thick, sclerophyllous, long-lived leaves that are typical of shrubs in Mediterranean climates, and they are long-lived, woody plants with an erect shrub-like or tree-like growth form. Large individuals may be up to 5 m tall (Rebelo, 2001). The genus Protea is monophyletic and originated in the CFR (Valente et al., 2010; Schnitzler et al., 2011), but within this clade, the species we sampled are not closely related (Mitchell et al., 2017).
Like all other members of the genus, the large inflorescences of these species develop into seedheads that are retained until fire. The seeds are released from the seedheads during fire, and the seeds germinate and the seedlings emerge in dense stands in the months following fire with the onset of rain. In P. eximia, P. laurifolia, P. punctata and P. repens, post-fire re-establishment occurs through recruitment of new seedlings. In P. nitida, post-fire recruitment occurs predominantly through resprouting of surviving individuals (Rebelo, 2001).
Sampling design
At each of the three sites (Fig. 2), we sampled co-occurring populations of our focal Protea species during the Austral winter 2016, from July to September (Table 1). Only the most widespread species, P. repens, could be sampled at every site. We sampled P. laurifolia at Jonaskop and Cederberg, P. punctata at Jonaskop and Swartberg, P. nitida at Cederberg, and P. eximia at Swartberg. Sampled populations were not always adjacent. At Jonaskop, the P. punctata population was located near the peak of Jonaskop Mountain on a south-facing slope, while the P. laurifolia population was located at mid-elevation near the elevational transition into true fynbos habitat. Protea repens was sampled at an elevation ~100 m higher than P. laurifolia. At Swartberg all three populations were sampled on the south-facing side of Swartberg Pass. Protea eximia and P. punctata populations were located adjacent to one another. Protea repens was located ~2 km west of them and further downslope. At Cederberg, P. laurifolia and P. nitida were adjacent to one another, but P. nitida occurred along a south-facing rocky hillside directly above the P. laurifolia. Protea repens at Cederberg was ~1.2 km west of the P. nitida and P. laurifolia populations but at a similar elevation.
Between ten and 20 individuals were sampled per population for structural trait and physiological trait measurements (see methods below). We define structural traits here as those traits associated with leaf and stem organ morphology (e.g. leaf length/width ratio, stomatal pore size and bark thickness) and/or those that reflect the allocation of biomass to different tissues within an organ (e.g. leaf lamina density and stem specific density). These structural traits are themselves probably associated with aspects of plant fitness (Lechowicz and Blais, 1988), in addition to their effects on variation in whole-plant rates of carbon assimilation and water use (Niinemets et al., 2015), which we describe below and refer to as physiological traits.
On each plant we also measured the height of the main stem, the basal diameter of the main stem and canopy area (calculated as an ellipsoid, using the distance of two perpendicular measurements of the canopy). The height and canopy area measures were included as size traits in our analysis described below. We also estimated the approximate age of each measured individual by counting the number of branching events (i.e. the number of internodes) on the main stem, which has been shown to provide a good estimate of age (Carlson et al., 2011). In reseeding species, all seedlings emerge at roughly the same time shortly after a fire. As a result, all individuals in these populations were about the same age (±2 years). It is common for reseeding species to reach reproductive maturity within 2–3 years of seedling establishment following fire (Protea Atlas Project; Rebelo, 2001, 2006). All of our populations of reseeding populations consisted of adult plant stands that were greater than 7 years old (Table 1) and thus well past the age of first flowering and nearing the end of their expected lifespan of ~10–13 years, which is determined by the fire return interval. For the one resprouting species, P. nitida, we sampled individuals in the population that were approximately the same age, although individuals of different ages can occur within a population. In general, even though there were differences in age between populations and sites, all sampled individuals were adults and all individuals sampled within a population were roughly the same age (Table 1).
All Protea species retain seeds in their seedheads until fire. Thus, the number of inflorescences and seedheads (hereafter ‘seedheads’) is a direct proxy for reproductive output, a key component of fitness. We counted the total number of inflorescences and seedheads on each individual as an index of fitness. Previous work in other Protea species has shown a strong positive association between the total estimated number of seeds per plant and the number of inflorescences (Carlson and Holsinger, 2013).
Structural trait measurements
On each sampled individual we collected a branch, cut at the second internode, from which we obtained leaf and wood traits. We selected the first fully expanded, sun-exposed leaf, which was always from the previous year’s growth, because the current year’s leaves were not fully matured. Fresh leaves were scanned and the images were later analysed using the software ImageJ (Schneider et al., 2012) to determine the single-sided leaf lamina area (cm2) and lamina length/width ratio. We calculated length/width ratio (LWR, unitless) as the length of the longest part of the leaf blade excluding the petiole divided by the width of the widest part of the leaf blade. We used a digital micrometer (Mitutoyu IP65) to measure the thickness of fresh leaves. We took three measurements per leaf, avoiding major veins, and we took the average of these measurements as a measure of lamina thickness. We dried the leaves at 60 °C for at least 3 d in a drying oven before measuring dry mass. We estimated leaf mass per area (LMA, g cm−2) as total dry mass divided by the total fresh area, and we estimated lamina density (LD, g cm−3) as LMA divided by lamina thickness (Kitajima and Poorter, 2010).
Before drying the leaves we made a stomatal peel on both the adaxial and the abaxial sides of each fresh leaf by applying clear nail polish to the bottom, right portion of the leaf, letting it dry, and lifting the peel up with transparent tape. Another piece of tape was placed on top of the peel to preserve it. We subsequently analysed these peels under a light microscope to estimate stomatal density, stomatal length and stomatal pore index. Protea species have stomata that are sunken within an epistomatal cavity (J. E. Carlson, National Parks Service, USA and C. S. Jones, University of Connecticut, USA, unpubl. res.). Thus, our peels do not directly measure guard cell length. Instead, we measured the size of the stomatal pore by measuring the longest length from the edges of the epistomatal cavities. We measured the stomatal pore length (mm) of three stomata from three different views of the peel on both the abaxial and the adaxial surfaces of the leaf. Stomatal pore densities (mm−2) were quantified as the number of stomata per mm2 and were also measured from three different views of the peel on both surfaces of the leaf. As the densities and lengths did not differ between the sides, we hence used the average of the abaxial measures in our analyses. Stomatal pore index (SPI, unitless) was calculated as the density multiplied by the square of stomatal pore length. It is proportional to total stomatal area across the surface of the leaf (Carlson et al., 2016).
We also measured wood density (g cm−3) and bark thickness (mm) for each sampled individual. To measure wood density we removed the bark from a section of 2-year-old sapwood and hydrated it under a vacuum until it was completely saturated. We determined wet volume of the stem as the mass of the water displaced. The sample was then oven-dried at ~75 °C for 5 d, after which we measured its mass. Wood density was calculated as the oven-dry mass of the stem divided by its wet volume (following Martinez-Cabrera et al., 2009). We measured bark thickness (phloem plus cortex and epidermis) on hydrated stems using a digital micrometer. We measured the thickness of bark at the top and bottom of each stem section (the section on which wood density was calculated) using the average of the two measurements as our estimate of bark thickness.
Physiological trait measurements
Photosynthesis, stomatal conductance and instantaneous water use efficiency.
We measured the light-saturated photosynthetic rate under ambient conditions on all individuals sampled for morphological traits. All measurements were taken on clear days from July to August 2016 between 0900 and 1400 h using a LiCor 6400XT with a CO2 mixing system and a red/blue LED light source (Lincoln, NE, USA). We measured photosynthesis on the same leaves that we used for structural trait analysis. We maintained the conditions inside the LiCor chamber as close as possible to ambient conditions on a bright and clear day. We set photosynthetically active radiation to 1500 μmol m−2 s−1, CO2 to 400 μmol mol−1, temperature to 25 °C (±2 °C) and relative humidity to 35–45 %. When taking gas exchange measurements, we first allowed the leaf to acclimate to chamber conditions for 3–5 min, or until fluctuations in internal carbon concentrations were minimal. We then took three measurements for each leaf, waiting 1–2 min between each measurement. We used the average of these three measurements as our estimate of area-based light-saturated photosynthetic rate (Aarea) for that individual. In instances where the leaf did not fill the entire 3 × 2-cm2 chamber, we corrected the raw estimate using our measurement of leaf area for that leaf. In some cases with P. repens the total leaf area in the chamber using a single leaf was very small. To provide stable estimates of photosynthetic rate, we placed two, non-overlapping leaves side-by-side in the chamber to increase total area. We also recorded transpiration and stomatal conductance and used them in the analysis as indicators of water conservation and carbon assimilation. Finally, we calculated instantaneous water use efficiency (WUE) as assimilation rate divided by transpiration (Lambers et al., 2008).
Sapwood-specific hydraulic conductivity
After sampling all individuals in the population, we collected the branches on which we measured photosynthetic traits and structural traits. We cut the stems below the second most recent node, and the portion of the stem between the second and first nodes (i.e. the portion of the branch that was 2 years old) was used to measure maximum hydraulic conductance (Kmax). Stems were immediately placed in a bucket of deionized water and kept under water until they were hydrated. Kmax was measured between 8 h and 4 d later. Before measuring Kmax we cut back a portion of 2-year-old stem, keeping track of the bottom (root-oriented) and top (leaf-oriented) ends.
We removed all leaves from the stem segments before hydrating them for 12–24 h under a vacuum using deionized and degassed water following the protocol from Espino and Schenk (2011). After hydration, no bubbles emerged from the top of the stem indicating that all air had been removed from the vascular column. The water used for the measurements was deionized and had been degassed by pumping it through a 0.2-μm filter at an absolute pressure of ~30 kPa. We immediately removed the bark from the top portion of the stem, submerged the stem in a tray of deionized water, cut ~1 cm of stem from the base of the stem to remove any embolisms introduced during stem transfer, and attached the stem to tubing that fed into a XY’LEM embolism meter (Bronkhorst, Montigny les Cormeilles, France) so that there was a continuous water column from the XY’LEM water source to the stem. To calculate Kmax it is necessary to quantify the flow rate across the stem relative to the change in pressure. We report sapwood-specific hydraulic conductivity (Ks) as it takes into account the length of the stem segment as well as the cross-sectional area of the stem (Pérez-Harguindeguy et al., 2013):
where F is the flow rate (kg s −1), L is the length of the stem (m), ΔP is the pressure differential (MPa−1) and Asw is the cross-sectional area of the stem (m−2). Note that the XY’LEM reports flow rate, but this rate is corrected for flow under zero pressure (F0). This correction was measured for each run and incorporated into the calculated flow rate. Additionally, the recorded conductivities were adjusted assuming the water was at 20 °C to account for change in water viscosity, following Espino and Schenk (2011). We recorded flow rates after they stabilized (~2–3 min). In the absence of flow, we cut back the stem at the top and base under water, and re-measured. If flow was still absent, we rehydrated the stem and repeated the measurements. After the flow rates were measured we recorded the length of the stem and its cross-sectional area (average of the areas from the base and top of the segment).
Leaf-specific conductivity and leaf-specific photosynthetic rate
The Huber value is the sapwood cross-sectional area relative to the total leaf area and it is an indication of the total photosynthetic biomass produced for a given investment in wood (Menuccini and Bonosi, 2001). To measure the Huber value of each sample, we removed all of the leaves above the point of the cut branch. We measured the cross-sectional area of the sapwood at the base of the stem. We then scanned all of the leaves on a digital scanner and determined their total area using ImageJ. The sampled branches had minimal leaf damage, removing the effect of leaf loss and herbivory, which can bias the total leaf area measurements.
We used Huber values to determine leaf-specific conductance (LSC; sapwood-specific conductivity divided by total leaf area of the stem, Tyree and Zimmerman, 2002) and leaf-specific photosynthetic rate (LSP; light-saturated photosynthetic rate per area divided by Huber value). LSC measures the investment in photosynthetic area given the rate at which the leaves receive water, and is a proxy for the balance between water loss through transpiration relative to water supply (e.g. Sterck et al., 2008). Similarly, leaf-specific photosynthetic rate is a proxy for the total amount of carbon assimilation relative to investment in sapwood.
Statistical analyses
Comparison of variance structure between structural and physiological traits.
To evaluate the variance structure (i.e. the proportion of variance in traits within species relative to among species) for each structural and physiological trait we used a random intercept model with a species random effect. As we used a Bayesian modelling framework, we were able to estimate the mean proportion of within-species variance for each structural and physiological trait, in addition to the degree of uncertainty associated with this estimate by evaluating the full posterior distribution for each variance component. Specifically, we implemented the following model in R (v.3.6.1) using the rstanarm package (Stan Development Team, 2018):
From this, the within-species variance is the square of the sigma parameter estimate, the among-species variance is the variance among the five species random intercepts, and the proportional within-species variance percentage is the within-species variance divided by the sum of the within-species variance and the among-species variance, multiplied by 100:
Analysis of trait, size and fitness relationships
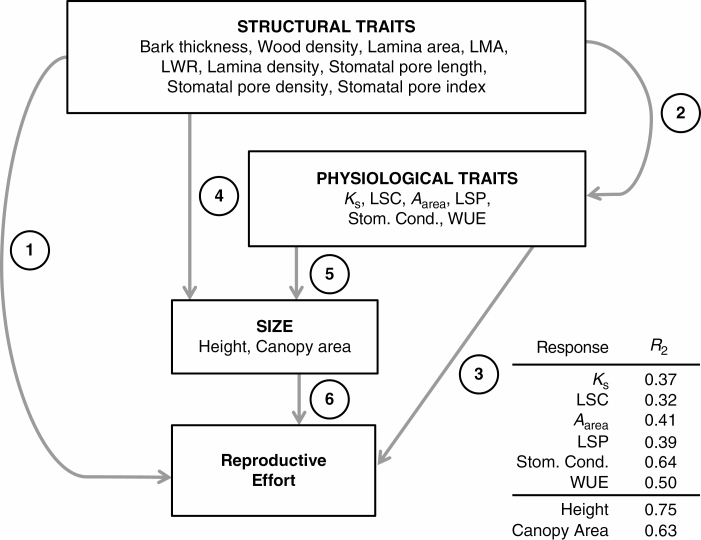
To allow us to simultaneously evaluate associations among structural traits, physiological traits, plant size and reproductive effort, we constructed a Bayesian path analysis model in Stan (Carpenter et al., 2017), and implemented the analysis using the rstan package in R (Stan Development Team, 2018). Figure 3 provides a schematic of the relationships used in the model, and the complete diagram of the relationships among variables is provided in Supplementary Data Fig. S3.
Fig. 3.
Diagram representing the path model simultaneously analysing relationships between structural traits, physiological traits, size and reproductive effort. See Methods for description of analysis. Presented are the Bayesian R2 values associated with physiological and size trait responses.
Each path in the model was expressed as a linear model of the form
where is the observation of the kth individual, of species i in the jth site. β0 is the intercept, βp is the path coefficient for covariate p, xp is the value of covariate p, is the random effect associated with species i, and is the random effect of site j (nested within species i). For example, the path leading to LSP is a multiple regression of LSP on wood density (wd), leaf lamina area (lf_area), LMA, leaf density (ld), leaf length/width ratio (lw), bark width (bw), stomatal length (s_length), stomatal density (s_dens) and stomatal pore index (spi). Other physiological traits are regressed on the same set of structural traits, and the residual variance associated with the regression in each physiological trait is treated as independent of the residual variance in regressions involving other physiological traits. The regressions of size traits (e.g. height and canopy area) are treated analogously with the covariates including all structural and physiological traits. Finally, the number of seedheads is regressed on all structural, physiological and size traits. Furthermore, because the number of seedheads is positive and discrete, we modelled the number of seedheads using a Poisson regression with a log link, so that
where has the same linear structure as above. To facilitate the choice of priors and comparison of the influence of different covariates, all variables were standardized to a mean of zero and a standard deviation of one before the analysis. Source code and data files for these analyses are available at https://kholsinger.github.io/Protea-traits-physiology-fitness/. We used independent N(0,1) priors on β0 and βp, and a half Cauchy(0,5) on the variance parameters. We used four chains in the analysis with a burn-in of 1250 iterations and a sample of 1250 iterations for a total of 5000 samples from the posterior. Because Stan uses a Hamiltonian Monte Carlo method, posterior draws are very efficient. Thinning was unnecessary, estimates of Rhat were all less than 1.05, and no divergent transitions were reported. We report posterior means for path coefficients, and we identify coefficients of whose sign we are confident based on whether 80 or 95 % symmetric credible intervals (CIs) overlap zero. To assess the extent to which covariates account for variation in the dependent variable, we calculated a Bayesian version of R2 (Gelman et al., 2017).
RESULTS
Intraspecific trait variation of structural and physiological traits
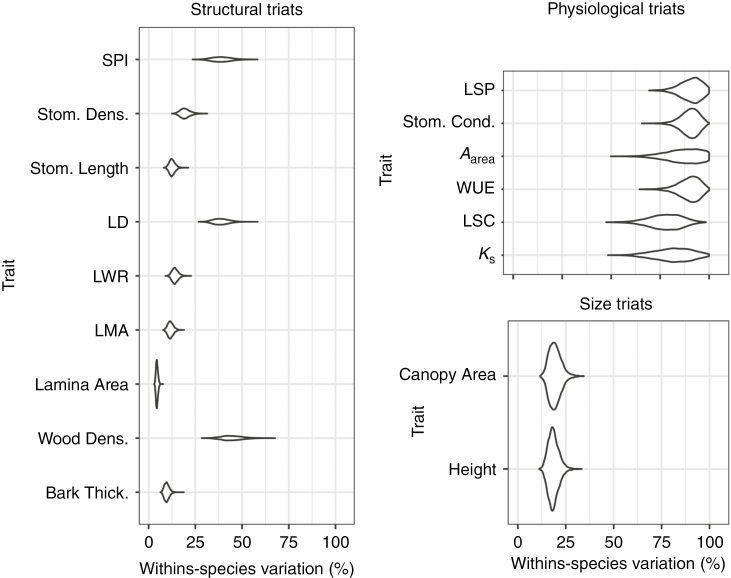
We evaluated the variance structure (i.e. the proportional intraspecific trait variation relative to among-species variation) for the nine structural traits and the six physiological traits measured. The average within-species variation observed in structural traits ranged between 13 and 45 %, while within-species variance accounted for between 75 and 90 % of the variation observed in physiological traits (Fig. 4; see Supplementary Data Table S1 for the mean raw trait values for each species, and Figs S1 and S2 for the distribution of raw trait values for each species at each site). Only ~22 % of the variation in our measures of size was attributed to within-species variance, which is unsurprising given that individuals within a population were all approximately the same age. Our approach also allowed us to evaluate the uncertainty associated with the mean estimates for the variance partitioning by summarizing the posterior distribution of each within-species variance estimate (i.e. evaluating the 95 % credible intervals for each mean estimate). We observed much greater uncertainty in our estimates of within-species variance for the physiological traits than for the structural traits. Despite these broad credible intervals it is clear that for the traits we measured, within-species variance was substantially greater for physiological traits, while the variation in structural traits was largely attributed to among-species differences.
Fig. 4.
Posterior distributions of the within-species variance for all structural, physiological and size traits estimated using a random intercept model. The entire posterior distribution is plotted for each trait to demonstrate the mean estimate of the within-species proportional variance in addition to the uncertainty associated with variance partitioning estimates.
Evidence of pairwise relationships: structural trait and physiological trait associations
To assess the significance of the pairwise associations in the path model, we evaluated the magnitude of an estimate and its uncertainty (given as either the 80 % or the 95 % credible interval) for each regression coefficient in each path of the model (see Fig. 3 for a summary of the path model and Supplementary Data Fig. S3 for the full path model). For example, to evaluate the effect of LMA on photosynthetic rates we looked at the multiple regression in the path model indicated by arrow 2 in Fig. 3, in which photosynthetic rate is a function of all nine structural traits (see Methods for a full description of the model and the inclusion of random effects). From this multiple regression, we obtained mean estimates and associated uncertainties for all nine predictor covariates. Given that we scaled all of the trait data to have a mean of zero and a standard deviation of one, the estimates will be similarly scaled and estimates with a magnitude of zero indicate no association between the two traits of interest. The mean effect of LMA on photosynthetic rate is −0.172 (Table 2) indicating a negative association between LMA and photosynthetic rate. However, the 95 % credible interval for this estimate overlaps zero (95 % CI: −0.505, 0.164), which means that we do not have strong evidence to support this association in the context of the full path model. Moving forward, we conclude we have strong evidence of an association between two variables if the 95 % CIs for the estimate do not overlap zero. We conclude there is a weak association, albeit uncertain, if the 80 % CIs do not overlap zero.
Table 2.
Mean estimates from the hierarchical path model. Bold indicates the 95 % credible intervals do not overlap zero. Italics indicate the 80 % credible intervals do not overlap zero
| Physiological traits | Size | Fitness | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K s | LSC | A area | LSP | Stomatal conductance | WUE | Height | Canopy area | Reproductive effort | ||
| Structural traits | Bark thickness | 0.268 | 0.302 | 0.018 | 0.256 | −0.043 | −0.002 | −0.134 | −0.149 | −0.234 |
| Wood density | −0.108 | −0.129 | −0.011 | −0.080 | −0.067 | 0.154 | −0.062 | −0.057 | −0.069 | |
| Lamina area | −0.048 | 0.062 | 0.181 | 0.477 | 0.015 | −0.144 | 0.228 | 0.045 | 0.102 | |
| LMA | −0.008 | −0.033 | −0.172 | −0.038 | 0.009 | −0.014 | 0.135 | 0.075 | 0.015 | |
| Lamina density | 0.153 | 0.078 | 0.130 | 0.030 | 0.116 | −0.032 | 0.010 | −0.023 | 0.032 | |
| LWR | 0.454 | 0.527 | −0.154 | 0.198 | 0.215 | −0.317 | 0.015 | −0.041 | −0.040 | |
| Stomatal pore length | 0.659 | 0.515 | −0.094 | 0.638 | −0.670 | 0.325 | 0.266 | 0.444 | 0.222 | |
| Stomatal pore density | 0.566 | 0.381 | −0.016 | 0.283 | −0.171 | 0.281 | 0.126 | 0.287 | 0.202 | |
| SPI | −0.293 | −0.239 | 0.230 | −0.189 | 0.304 | −0.337 | −0.175 | −0.301 | −0.184 | |
| Physiological traits | K s | −0.194 | −0.197 | 0.122 | ||||||
| LSC | 0.242 | 0.296 | −0.148 | |||||||
| A area | 0.032 | 0.073 | −0.036 | |||||||
| LSP | −0.171 | −0.179 | 0.059 | |||||||
| Stomatal conductance | −0.070 | −0.009 | 0.093 | |||||||
| WUE | 0.030 | −0.011 | −0.035 | |||||||
| Size | Height | 0.170 | ||||||||
| Canopy area | 0.689 | |||||||||
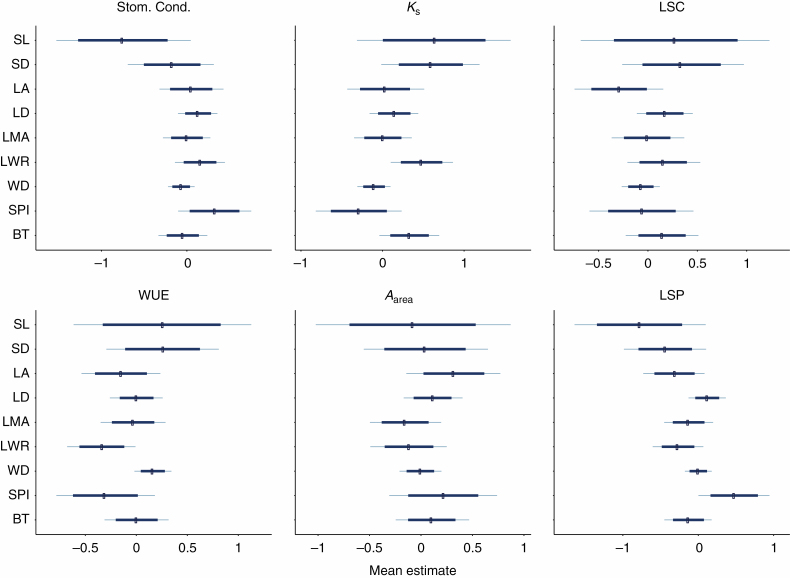
In total, we evaluated 54 bivariate associations between structural traits and physiological traits in the context of the path model (Fig. 3: arrow 2; with nine structural traits included as covariates in six multiple regressions reflecting the six physiological response traits). Of these 54 relationships, we find strong evidence for only four associations between structural traits and physiological traits and weak evidence for an additional nine associations (Table 2; Fig. 5). There is a strong positive association between leaf length/width ratio and both hydraulic conductance and leaf specific conductance. Given these positive associations, it is not surprising that we also found a negative association between leaf length/width ratio and water use efficiency. Leaf area is positively associated with leaf-specific photosynthetic rate. Similarly, stomatal pore length and stomatal pore density are positively associated with hydraulic conductance, albeit weakly. The remaining associations between structural traits and physiological traits were weaker and the sign of the associations was uncertain.
Fig. 5.
Coefficient estimates for each of the structural traits on each of the physiological traits from the path model (also summarized in Table 2). The dashed vertical line indicate the mean of the posterior distribution for the coefficient, the bold bar indicates the 80 % credible interval of the posterior distribution, and the outer line indicates the 95 % credible interval. The dashed vertical line in each plot is zero. The structural traits represented on the y-axis in each panel are ranked according to their association with fitness in the path model, as follows: stomatal length (SL), stomatal density (SD), leaf lamina area (LA), lamina density (LD), leaf mass per area (LMA), leaf length/width ratio (LWR), wood density (WD), stomatal pore index (SPI) and bark thickness (BT).
Evidence of pairwise relationships: trait and size associations
We evaluated a total of 25 pairwise associations between structural traits and size (Fig. 3: arrow 4) and physiological traits and size (Fig. 3: arrow 5). The only strongly supported associations between traits and either size measure (e.g. height or canopy area) were the positive association between leaf-specific conductivity and canopy area and the negative association between leaf-specific photosynthetic rates and canopy area (Table 2). Other associations between physiological traits and size were weaker and the sign of the association was uncertain, and none of the structural traits show convincing associations with either height or canopy area (Fig. 3: arrow 4; Table 2).
Evidence of pairwise relationships: trait and size associations with reproductive effort
Our model estimates a total of 17 pairwise relationships among structural traits and reproductive effort (Fig. 3: arrow 1), physiological traits and reproductive effort (Fig. 3: arrow 3), and plant size and reproductive effort (Fig. 3: arrow 6). First, we find strong evidence for associations between six of the nine structural traits and reproductive effort (mean estimates summarized in Table 2). Individual plants with thicker bark, more dense wood and higher stomatal pore index have lower reproductive effort. Individuals with larger leaves, larger stomatal pores and higher stomatal density have higher reproductive effort. Second, individuals also had higher reproductive effort if they have higher hydraulic conductance, leaf-specific photosynthetic rate and stomatal conductance. They have lower reproductive effort if they have higher values of leaf-specific conductivity and water use efficiency. Lastly, both plant height and canopy area are positively associated with reproductive effort, indicating that our measures of plant size are reasonable proxies for plant reproductive output, congruent with earlier studies of South African Proteaceae (e.g. Mustart and Cowling, 1992).
Strength of multiple regression models
In addition to evaluating the pairwise relationships in the context of the path model, we also evaluated the support for the individual multiple regressions for each of the physiological traits, and for each of the size traits. The multivariate regression of individual physiological traits on structural traits accounted for 35–70 % of the variation in physiological traits (Fig. 3). Similarly, the multivariate regression accounted for 78 and 64 % of the variation in plant height and canopy area, respectively (Fig. 3). For comparison, we analysed all univariate models relating structural traits to physiological traits (54 models in total), all univariate models relating structural traits to size traits (18 models in total), and all univariate models relating physiological traits to size traits (12 models in total) and calculated Bayesian R2 values for each of these models (summarized in Supplementary Data Fig. S4). Associations revealed in univariate regressions of physiological traits on structural traits showed that most individual structural traits were poor predictors of physiological performance (mean R2 = 0.27, range = 0.18–0.46). In contrast, univariate regressions of size on physiological or structural traits revealed that many structural traits (mean R2 = 0.65, range = 0.59–0.71) and physiological traits (mean R2 = 0.65, range = 0.60–0.72) were good predictors of plant size. In our multivariate path model, we see that the individual estimates for structural and physiological traits on plant size measures are large, but they have broad CIs, indicating the uncertainty in assigning confidence to these estimates. The strong associations between structural and physiological traits with plant size in the univariate models are probably a result of the strong covariance among these traits, and their correlated effects on plant size. For the relationships between structural traits and physiological traits, however, we see that while individual structural trait associations may be weak, variable or uncertain, the combinations of structural traits have a predictable relationship with plant physiological performance.
DISCUSSION
Although much work in plant ecology over the past decade has focused on analysis of functional traits, relatively few have examined the association between those traits and indicators of physiological performance or fitness (Caruso et al., 2020), and even fewer have examined those associations at the level of individuals (see Swenson et al., 2020 for a recent review). Here we demonstrate in five species of Protea from the western Cape region of South Africa that individual differences in structural traits are associated with individual differences in physiological performance, and that both are associated with individual differences in overall plant size and reproductive effort. In addition, we show that in spite of substantial structural trait differences among species, differences in physiological traits among species are relatively modest. Finally, we show that a substantial fraction of the differences in physiological traits among individuals can be accounted for by multivariate differences in structural traits even when associations of individual traits with physiological trait measures are weak or uncertain.
Structural traits differ greatly but physiological traits differ little between species
While studies of trait-based ecology have often focused on differences among species (Albert et al., 2010, 2011), recent work demonstrates that intraspecific trait variation observed both within and across populations is often substantial (Albert et al., 2010; Hulshoff and Swenson, 2010; Siefert et al., 2015). As expected, our results show that most of the variation in structural traits is a result of differences among species. In contrast, we found that most of the variation in physiological traits is a result of differences among individuals within species. The substantial intraspecific trait variation observed in physiological traits is probably not due to developmental differences among individuals within a population, as individual plants within a population were all approximately the same age and demonstrated little variation in plant size (e.g. height and canopy area) relative to differences observed among species.
To our knowledge only a few studies have compared the intra- and interspecific partitioning of structural and physiological trait variation, but Marks’ (2007) simulation study of traits and trait covariances in seedlings may be informative. Physiological traits represent more integrated aspects of individual plants, because rates of photosynthesis or stem water conductance are determined by a variety of structural traits, only some of which are commonly measured. Interestingly, Marks (2007) found that traits exhibiting more integration also exhibited more variation within than among species, consistent with the results presented here. Similarly, Siefert et al. (2015) found that ‘whole-plant traits’ were much more variable within populations than among species in a global-scale meta-analysis of trait variation within plant communities.
Trait differences may suggest functional differentiation along a resource conservation axis
Our results reveal identifiable relationships between individual structural traits and reproductive effort even after accounting for their association with physiological traits and plant size. Some of these associations suggest that differences in reproductive effort are associated with differences among individuals in the degree to which they invest in resource conservation. For example, individuals with thicker bark and higher wood density also had lower reproductive effort. Investment in thicker bark and denser wood provides protection against fire (Larjavaara and Muller-Landau, 2010; Lawes et al., 2013; Hempson et al., 2014; Charles-Dominique et al., 2017), but it may also represent a structural cost that is associated with reduced investment in seed production (Chave et al., 2009). In contrast, individuals with larger leaves also had higher reproductive effort, consistent with higher rates of carbon acquisition leading to faster growth and greater reproductive investment.
Similarly, we found a strong, positive association between leaf-specific photosynthetic rates and reproductive effort. Leaf-specific photosynthetic rate is an index of the rate of instantaneous carbon gain as integrated across the entire canopy. As a result, it is likely to be a better indicator of total carbon acquisition than area- or mass-based photosynthetic rates. Indeed, Aarea showed only a weak (and possibly negative) association with reproductive effort.
While we did not detect a strong association between leaf-level photosynthesis and reproductive effort, we did find convincing evidence that individuals with higher rates of hydraulic conductance and lower water use efficiency also had greater reproductive effort, which is consistent with a water-spending strategy that maximizes transpiration and carbon gain during photosynthetically active periods. In contrast, individuals with higher levels of water conductance per unit leaf area produced fewer seedheads, which suggests that there is selection in this system against a phenotype that does not efficiently balance carbon acquisition with water loss through transpiration.
In other instances, the association between reproductive effort and resource conservation is less apparent. For example, individuals with larger stomata at a given stomatal density or higher stomatal density for a given stomatal size also had higher reproductive effort. While we did not detect a convincing association between stomatal size and stomatal conductance in the species we studied, other studies have identified such associations (Aasamaa et al., 2001; Taylor et al., 2012). Similarly, previous work in Protea repens (Carlson et al., 2016) showed that in hot and dry conditions individuals with higher stomatal density also had both higher stomatal conductance and higher reproductive effort. Given these observations, the negative association we detected between stomatal pore index and reproductive effort might reflect the commonly observed trade-off between stomatal size and stomatal density (Franks et al., 2009).
Trait combinations best predict plant performance
Our path model allowed us to evaluate 101 associations among structural traits, physiological traits, plant size and reproductive effort. Many of the trait–trait associations expected based on biophysical models that underlie the LES and the WES are weak and poorly supported. For example, we detected a negative association between wood density and stem hydraulic conductance, as expected from the WES (Chave et al., 2009), but it is only weakly supported (i.e. the 80 % CI overlaps 0). Of the 54 associations between structural traits and physiological traits included in our model, we found strong evidence (i.e. the 95 % CI does not overlap zero) for just four associations. Nonetheless, multivariate regressions using these traits reveal clear associations between individual-level traits and physiological traits (Fig. 3, R2 = 0.37–0.64), demonstrating the importance of considering trait combinations and multivariate phenotypes when evaluating plant strategies and performance (Laughlin and Messier, 2015; Messier et al., 2017a).
While the paucity of strong associations between single structural traits and physiological traits appears to conflict with studies demonstrating clear trade-offs in pairwise trait analyses (Wright et al., 2004; Chave et al., 2009), the signs of the individual regression coefficients in the multivariate analysis are consistent with differentiation along a whole plant axis representing differences in investment for growth and reproduction vs. safety and storage. Differentiation along such an axis seems reasonable given that these plants are adapted to a fire-dominated ecosystem in which they have a limited timespan to maximize lifetime fitness before the next fire cycle returns.
CONCLUSIONS
Recent research demonstrates that trait–trait associations observed at broad geographical and phylogenetic scales may not exist when considering smaller phylogenetic (Mitchell et al., 2015; Anderegg et al., 2018; Messier et al., 2018) and spatial scales (Funk and Cornwell, 2013; Messier et al., 2017b), leading some to question whether the LES and WES provide useful insights for trait-based ecology. If trade-offs in LES and WES arise from biophysical constraints, we should find them at the level of individual leaves or individual plants (Swenson et al., 2020). At other hierarchical levels the relationships, if they exist, are likely to reflect macro-ecological and macro-evolutionary processes (Messier et al., 2018).
Here we explicitly test the strength of trait–performance relationships at the level of individual plants. Although we reliably detect only a few associations when evaluating individual structural traits, multivariate combinations of structural traits show strong associations with physiological traits. Furthermore, multivariate combinations of both structural and physiological traits show strong associations with size. In spite of these strong associations, co-occurring species differ substantially more in structural traits than in physiological traits. This may seem surprising, as ecologists commonly assume that coexistence requires trait differences (‘limiting similarity’ sensuMacarthur and Levins, 1967), but also recognize that co-occurring species may be similar in traits related to competitive hierarchies (Herben and Goldberg, 2014). If species differ substantially in performance, after all, the one with higher performance will exclude the other.
The dynamics of natural selection may provide an additional explanation for the relatively small among-species differences in physiological traits that we observed. As Fisher (1930) pointed out nearly a century ago, natural selection erodes additive genetic variance over time. Thus, traits strongly associated with fitness are likely to harbour less additive genetic variance than those that are weakly related to fitness (summarized in Mousseau and Roff, 1987). Physiological trait measures associated with carbon and water balances seem likely to be more closely associated with whole-plant performance and fitness than other structural elements of the LES or WES. Previous work in Protea (Carlson et al., 2011; Carlson and Holsinger, 2012; Prunier et al., 2012) has demonstrated that among-population variation in structural traits includes a significant genetic component. Similarly, we detect substantial among-species differences in these traits, consistent with the hypothesis that structural traits associated with the LES and WES are less tightly tied to individual fitness within populations than measures of physiological performance.
While it is generally understood that trait differences are required for evolutionary responses, ecologists often focus on differences in mean trait values between species and neglect the role of intraspecific trait variation (Bolnick et al., 2011). Our results show that differences in structural traits measured at the individual plant level lead to differences in physiological traits, and the variation in these physiological performance measures are largely the result of differences between individual plants rather than between species. Taken together, these results suggest that ecological investigations of species coexistence are incomplete without attention to performance differences among individuals. Lastly, our results suggest that understanding the mechanistic basis of trade-offs in the LES, WES or other axes of life history variation requires a focus on trait relationships measured on individual organisms so that we can properly scale the resulting associations to higher taxonomic and spatial scales.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: boxplots of all nine structural traits for all populations. Figure S2: boxplots of all six physiological traits for all populations. Figure S3: diagram of the complete path model. Figure S4: histogram showing the distribution of the Bayesian R2 values calculated from analysis of univariate models. Table S1: mean trait values for all species and all traits.
ACKNOWLEDGEMENTS
We are grateful to A. G. Rebelo, M. Treurnicht, J. A. Slingsby, K. J. Esler and J. E. Carlson for their advice, generosity and logistical support during project development and data collection. We thank Tanisha Williams for her assistance in the field, Sarah Hossain and Colleen Walsh for assistance processing leaf and wood samples, and C. S. Jones, M. Urban, R. Bagchi and two anonymous reviewers for substantial editorial comments on an earlier version of the manuscript. We thank Cape Nature for their assistance in obtaining collecting permits and additional field support.
FUNDING
This work was supported in part by grants awarded to K.M.N. from The Explorer’s Club and the Ronald H. Bamford Award from the EEB Department at the University of Connecticut, and in part by the National Science Foundation (DEB-1046328).
LITERATURE CITED
- Aasamaa K, Sõber A, Rahi M. 2001. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Functional Plant Biology 28: 765–774. [Google Scholar]
- Ackerly DD, Dudley SA, Sultan SE, et al. 2000. The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50: 979. [Google Scholar]
- Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. 2011. When and how should intraspecific variability be considered in trait-based plant ecology? Perspectives in Plant Ecology, Evolution and Systematics 13: 217–225. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S. 2010. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Functional Ecology 24: 1192–1201. [Google Scholar]
- Anderegg LDL, Berner LT, Badgley G, Sethi ML, Law BE, HilleRisLambers J. 2018. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecology Letters 21: 734–744. [DOI] [PubMed] [Google Scholar]
- Bergh NG, Verboom GA, Rouget M, Cowling RM. 2014. Vegetation types of the Greater Cape Floristic Region. In: Allsopp N, Colville JF, Verboom GA, eds. Fynbos: ecology, evolution, and conservation of a megadiverse region. Oxford: Oxford University Press, 15–60. [Google Scholar]
- Blonder B, Vasseur F, Violle C, Shipley B, Enquist BJ, Vile D. 2015. Testing models for the leaf economics spectrum with leaf and whole-plant traits in Arabidopsis thaliana. AoB Plants 7: plv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends in Ecology & Evolution 26: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadotte MW. 2017. Functional traits explain ecosystem function through opposing mechanisms. Ecology Letters 20: 989–996. [DOI] [PubMed] [Google Scholar]
- Carlson JE, Adams CA, Holsinger KE. 2016. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Annals of Botany 117: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE. 2012. Developmental plasticity in Protea as an evolutionary response to environmental clines in the Cape Floristic Region. PLoS One 7: e52035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE. 2013. Direct and indirect selection on floral pigmentation by pollinators and seed predators in a color polymorphic South African shrub. Oecologia 171: 905–919. [DOI] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE, Prunier R. 2011. Plant responses to climate in the Cape Floristic Region of South Africa: evidence for adaptive differentiation in the Proteaceae. Evolution 65: 108–124. [DOI] [PubMed] [Google Scholar]
- Carpenter B, Gelman A, Hoffman M, et al. 2017. Stan: a probabilistic programming language. Journal of Statistical Software, Articles 76: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso CM, Maherali H, Martin RA. 2020. A meta-analysis of natural selection on plant functional traits. International Journal of Plant Sciences 181: 44–55. [Google Scholar]
- Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. 2004. Phylogenetic overdispersion in Floridian oak communities. The American Naturalist 163: 823–843. [DOI] [PubMed] [Google Scholar]
- Charles-Dominique T, Midgley GF, Bond WJ. 2017. Fire frequency filters species by bark traits in a savanna–forest mosaic. Journal of Vegetation Science 28: 728–735. [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Cody ML. 1986. Structural niches in plant communities. In: Diamond J, Case TJ, eds. Community ecology. New York: Harper and Row, 381–405 [Google Scholar]
- Díaz S, Cabido M. 2001. Vive la différence: plant functional diversity matters to ecosystem processes. Trends in Ecology & Evolution 16: 646–655. [DOI] [PubMed] [Google Scholar]
- Díaz S, Kattge J, Cornelissen JH, et al. 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Espino S, Schenk HJ. 2011. Mind the bubbles: achieving stable measurements of maximum hydraulic conductivity through woody plant samples. Journal of Experimental Botany 62: 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. 1930. The genetical theory of natural selection. Oxford: Clarendon Press. [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences of the United States of America 106: 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami T, Martijn Bezemer T, Mortimer SR, Putten WH. 2005. Species divergence and trait convergence in experimental plant community assembly. Ecology Letters 8: 1283–1290. [Google Scholar]
- Funk JL, Cornwell WK. 2013. Leaf traits within communities: context may affect the mapping of traits to function. Ecology 94: 1893–1897. [DOI] [PubMed] [Google Scholar]
- Gelman A, Goodrich B, Gabry J, Ali I. 2017. R-squared for Bayesian regression models. http://www.stat.columbia.edu/~gelman/research/unpublished/bayes_R2.pdf (4 November 2018).
- Hempson GP, Midgley JJ, Lawes MJ, Vickers KJ, Kruger LM. 2014. Comparing bark thickness: testing methods with bark-stem data from two South African fire-prone biomes. Journal of Vegetation Science 25: 1247–1256. [Google Scholar]
- Herben T, Goldberg DE. 2014. Community assembly by limiting similarity vs. competitive hierarchies: testing the consequences of dispersion of individual traits. The Journal of Ecology 102: 156–166. [Google Scholar]
- Hulshof CM, Swenson NG. 2010. Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. Functional Ecology 24: 217–223. [Google Scholar]
- Keddy PA. 1992. Assembly and response rules: two goals for predictive community ecology. Journal of Vegetation Science 3: 157–164. [Google Scholar]
- Kitajima K, Poorter L. 2010. Tissue-level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. The New Phytologist 186: 708–721. [DOI] [PubMed] [Google Scholar]
- Kraft NJB, Ackerly DD. 2010. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecological Monographs 80: 401–422. [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. 2008. Plant physiological ecology, 2nd edn. New York: Springer. [Google Scholar]
- Larjavaara M, Muller-Landau HC. 2010. Rethinking the value of high wood density. Functional Ecology 24: 701–705. [DOI] [PubMed] [Google Scholar]
- Laughlin DC, Messier J. 2015. Fitness of multidimensional phenotypes in dynamic adaptive landscapes. Trends in Ecology & Evolution 30: 487–496. [DOI] [PubMed] [Google Scholar]
- Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology 16: 545–556. [Google Scholar]
- Lawes MJ, Midgley JJ, Clarke PJ. 2013. Costs and benefits of relative bark thickness in relation to fire damage: a savanna/forest contrast. The Journal of Ecology 101: 517–524. [Google Scholar]
- Lechowicz MJ, Blais PA. 1988. Assessing the contributions of multiple interacting traits to plant reproductive success: environmental dependence. Journal of Evolutionary Biology 1: 255–273. [Google Scholar]
- Linder PH. 2005. Evolution of diversity: the Cape flora. Trends in Plant Science 10: 536–541. [DOI] [PubMed] [Google Scholar]
- Macarthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101: 377–385. [Google Scholar]
- Marks CO. 2007. The causes of variation in tree seedling traits: the roles of environmental selection versus chance. Evolution 61: 455–469. [DOI] [PubMed] [Google Scholar]
- Martínez-Cabrera HI, Jones CS, Espino S, Schenk HJ. 2009. Wood anatomy and wood density in shrubs: responses to varying aridity along transcontinental transects. American Journal of Botany 96: 1388–1398. [DOI] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21: 178–185. [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Bonosi L. 2001. Leaf/sapwood area ratios in Scots pine show acclimation across Europe. Canadian Journal of Forest Research 31: 442–456. [Google Scholar]
- Messier J, Lechowicz MJ, McGill BJ, Violle C, Enquist BJ. 2017a. Interspecific integration of trait dimensions at local scales: the plant phenotype as an integrated network. The Journal of Ecology 105: 1775–1790. [Google Scholar]
- Messier J, McGill BJ, Enquist BJ, Lechowicz MJ. 2017b. Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography 40: 685–697. [Google Scholar]
- Messier J, Violle C, Enquist BJ, Lechowicz MJ, McGill BJ. 2018. Similarities and differences in intrapopulation trait correlations of co-occurring tree species: consistent water-use relationships amid widely different correlation patterns. American Journal of Botany 105: 1477–1490. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Lewis PO, Lemmon EM, Lemmon AR, Holsinger KE. 2017. Anchored phylogenomics improves the resolution of evolutionary relationships in the rapid radiation of Protea L. American Journal of Botany 104: 102–115. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Moore TE, Mollmann HK, et al. 2015. Functional traits in parallel evolutionary radiations and trait-environment associations in the Cape Floristic Region of South Africa. The American Naturalist 185: 525–537. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Roff DA. 1987. Natural selection and the heritability of fitness components. Heredity 59: 181–197. [DOI] [PubMed] [Google Scholar]
- Mustart PJ, Cowling RM. 1992. Impact of flower and cone harvesting on seed banks and seed set of serotinous Agulhas Proteaceae. South African Journal of Botany 58: 337–342. [Google Scholar]
- Niinemets Ü, Keenan TF, Hallik L. 2015. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. The New Phytologist 205: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Potts AJ, Midgley JJ, Child MF, Larsen C, Hempson T. 2011. Coexistence theory in the Cape Floristic Region: revisiting an example of leaf niches in the Proteaceae. Austral Ecology 36: 212–219. [Google Scholar]
- Prunier R, Holsinger KE, Carlson JE. 2012. The effect of historical legacy on adaptation: do closely related species respond to the environment in the same way? Journal of Evolutionary Biology 25: 1636–1649. [DOI] [PubMed] [Google Scholar]
- Rebelo AG. 2001. Proteas: a field guide to the proteas of southern Africa. Vlaeberg: Fernwood Press. [Google Scholar]
- Rebelo AG. 2006. Protea atlas project.https://www.proteaatlas.org.za/package.htm.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler J, Barraclough TG, Boatwright JS, et al. 2011. Causes of plant diversification in the Cape biodiversity hotspot of South Africa. Systematic Biology 60: 343–357. [DOI] [PubMed] [Google Scholar]
- Schulze RE. 1997. South African atlas of agrohydrology and climatology. Technical Report. Report TT82/96. Pretoria: Water Resource Commission. [Google Scholar]
- Shipley B, Lechowicz MJ, Wright I, Reich PB. 2006. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87: 535–541. [DOI] [PubMed] [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, et al. 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18: 1406–1419. [DOI] [PubMed] [Google Scholar]
- Stan Development Team. 2018. RStan: the R interface to Stan. R package version 2.18.1. http://mc-stan.org/ (20 November 2019).
- Sterck FJ, Zweifel R, Sass-Klaassen U, Chowdhury Q. 2008. Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescens). Tree Physiology 28: 529–536. [DOI] [PubMed] [Google Scholar]
- Swenson NG, Worthy SJ, Eubanks D, et al. 2020. A reframing of trait–demographic rate analyses for ecology and evolutionary biology. International Journal of Plant Sciences 181: 33–43. [Google Scholar]
- Taylor SH, Franks PJ, Hulme SP, et al. 2012. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. The New Phytologist 193: 387–396. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmerman MH. 2002. Springer series in wood science. Berlin: Springer. [Google Scholar]
- Valente LM, Reeves G, Schnitzler J, et al. 2010. Diversification of the African genus Protea (Proteaceae) in the Cape biodiversity hotspot and beyond: equal rates in different biomes. Evolution 64: 745–760. [DOI] [PubMed] [Google Scholar]
- Van Wilgen BW, Forsyth GG, De Klerk H, Das S, Khuluse S, Schmitz P. 2010. Fire management in Mediterranean-climate shrublands: a case study from the Cape fynbos, South Africa: fire regimes in Cape fynbos. The Journal of Applied Ecology 47: 631–638. [Google Scholar]
- Wright SJ, Kitajima K, Kraft NJ, et al. 2010. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 91: 3664–3674. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Zirbel CR, Bassett T, Grman E, Brudvig LA. 2017. Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. The Journal of Applied Ecology 54: 1070–1079. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.