Abstract
Background
As a result of the constant increase in carbapenem resistance amongst gram‐negative bacteria in several countries, the inappropriate use of carbapenems must be reduced. Antimicrobial stewardship programmes (ASPs) aim to improve carbapenem usage by implementing interventions, including the promotion of the de‐escalation (DE) strategy. Thus, this study aimed to evaluate the impact of this strategy on carbapenem use based on a clear definition of DE.
Methods
The post‐prescription review and feedback (PPRF) strategy, which is used to optimise carbapenem use, was implemented by the antimicrobial stewardship team (AST). We compared the DE rate during the pre‐AST intervention period (from April 2017 to March 2018) and post‐AST intervention period (from April 2018 to March 2019).
Result
A total of 1500 patients (n = 771 in the pre‐AST intervention period and n = 729 in the intervention post‐AST period) were admitted to the hospital. The average duration of antibiotic therapy decreased from 9.9 to 7.7 days. The DE rate significantly increased in the post‐AST intervention period compared with the pre‐AST intervention period (51.4% vs 40.3%; P < .001).
Conclusion
The PPRF strategy implemented by the AST could improve the carbapenem usage by increasing the DE rate of carbapenem.
What is known
Antimicrobial stewardship (AS) improves the optimisation of antibiotic prescription using the post‐prescription review and feedback (PPRF) strategy.
Carbapenem is a wide‐spectrum antibiotic, and its use must be limited to maintain biological sensitivity.
Because de‐escalation is not well defined, the prescription after de‐escalation is not evaluated.
What is new
The PPRF strategy improves the optimal utilisation of antimicrobials and reduces carbapenem use by enhancing de‐escalation therapy.
Based on the definition of de‐escalation, the prescription trend of carbapenem was assessed.
The rate of switching to narrow‐spectrum β‐lactams significantly increased in the post‐AST intervention period.
The trend of carbapenem use significantly decreased by switching to narrow‐spectrum antibiotics and by shortening the duration of carbapenem treatment.
1. INTRODUCTION
Considering the continuous increase in carbapenem resistance amongst gram‐negative bacteria in several countries, the appropriate use of carbapenems and other types of antibiotics must be implemented to solve this problem. 1 , 2 Antimicrobial stewardship programmes (ASPs) aim to address carbapenem resistance by implementing interventions that improve carbapenem use, which include promoting the de‐escalation (DE) strategy. 3 , 4 Limiting the unnecessary use of carbapenem in hospitals can reduce resistance rates amongst problematic gram‐negative bacteria, including Pseudomonas aeruginosa. 5
When carbapenem is inappropriately used in some patients, including those who received prolonged therapy, the DE strategy is recommended for cost‐saving and preventing antimicrobial resistance (AMR) when susceptible bacteria are identified. Although the strategy is considered beneficial in terms of patient safety and prevention of AMR, it has not been utilised in diverse clinical settings. 6 , 7 , 8 Furthermore, although this strategy is widely recommended, it does not have a clear definition and is difficult to sufficiently evaluate. 9 , 10 , 11
Thus, a multidisciplinary antimicrobial stewardship team (AST) comprising trained pharmacists who focus on carbapenem stewardship with the use of the post‐prescription review and feedback (PPRF) strategy was established. 12 , 13 Thus, this study aimed to evaluate the impact of this strategy on the DE, duration, and use of carbapenem based on a clear definition of DE.
2. MATERIALS AND METHODS
2.1. Study design and setting
This study was conducted at Showa University Fujigaoka Hospital, a 584‐bed tertiary care centre with a 14‐bed intensive care unit, in Japan. A quasi‐experimental uncontrolled intervention was provided to determine the impact of the ASP on carbapenem treatment. The study was approved by the institutional review board (no. F2018C25) of the institution, and the need for informed consent was waived because of the retrospective design of the study.
From April 2018 to March 2019, the AST implemented an intervention that promotes DE strategies for patients treated with carbapenem. This analysis included consecutive patients who were admitted to any department of the hospital and received carbapenems. The control group only received carbapenem therapy from April 2017 to March 2018. Carbapenem therapy included both definitive therapy for documented infection and empirical therapy for suspected infection.
2.2. Data collection
Data, including demographic information of patients (eg, age, serum creatinine, granulocyte count, and weight), antimicrobial regimen, and days of therapy (DOT) per 100 patient‐days in the hospital, were collected retrospectively from electronic charts. The clinical data of mortality, antibiotic prescription (category, dose, and duration), blood culture results of individuals who received carbapenem treatments, recommendations made by the AST, and acceptance rate were obtained. The susceptibility rates of gram‐negative bacteria to meropenem were calculated as the proportion of susceptible isolates to total isolates of each bacteria detected during the period. The isolated bacteria were counted per patient and specimen in hospital in‐patients during the period. Drug susceptibility was interpreted according to Clinical and Laboratory Standards Institute document M100‐S22.
2.3. Intervention
The AST had a conference on patients receiving carbapenems once a week. The PPRF strategy aims to optimise antimicrobial use by encouraging a prescriber to comprehensively engage in blood culture, microbial examination, imaging or blood examination, and antibiotic selection and to follow the appropriate dose or frequency as scheduled, discontinuation of treatment, and duration of therapy. The AST then provided recommendations to the attending physicians regarding the aforementioned interventions according to the data obtained from the medical charts. If the prescriber does not follow the recommendation after 3‐5 days, the physician in the AST discussed the situation with the prescriber and made recommendations via telephone. More than one recommendation could be made per patient.
The members of the AST in this study included physicians (respiratory and emergency physicians and paediatricians) and a full‐time pharmacist, infection control nurse, and microbiology technologist. The carbapenem PPRF strategy implemented by the AST was used by physicians during conference, decision‐making, and recommendations (with a total full‐time equivalent [FTE] of 0.05); by the pharmacist when selecting patients and checking the prescription and patient's condition every weekday, attending conferences, and performing follow‐ups after recommendations (FTE of 0.5); by the microbiology laboratory technician when assessing biological data (FTE of 0.1); and by the infection control nurse when evaluating primary disease and medical device (FTE of 0.05). The FTEs of each health care worker did not include other AST activities, such as infectious disease consultation, infection control implementation, and provision of education to hospital staff.
The AST collaborated with a ward‐based clinical pharmacist (CP) if needed. The CP confirmed the dosage to the physician based on renal function and blood culture results. The AST follows and supported the recommendation of the CP. 8 Moreover, the team focused on educating all medical staff, new staff, nurses, pharmacists, and medical residents. The leader physician of the AST had a discussion with the head of the medical department about treatment policy and importance of blood culture. In the pre‐AST intervention period, we had no intervention strategy of antimicrobial stewardship against any antibiotics prescription including carbapenems.
2.4. Definitions
We performed an evaluation of carbapenem therapy, which was classified into the following groups based on prescription status within 7 days: (a) discontinuation of treatment: inhibition of carbapenem therapy because of the absence of proven bacterial infection 11 , 14 , 15 , 16 , 17 ; (b) switch to a narrow‐spectrum antibiotic: changing to narrower‐spectrum β‐lactams, such as the penicillin, piperacillin/tazobactam, and cephem groups or a combination of these groups 14 , 15 , 16 , 18 ; (c) continuation of treatment: continuous treatment with carbapenem over 7 days; (d) Change to other classes: switching to non‐β‐lactam antibiotics: switching to non‐β‐lactam antibiotics 16 ; and (e) oral route: switch from parenteral to any oral antibiotics. DE was defined as either discontinuation, narrowing of therapy, or switch to oral route. 11 , 14 , 19 , 20
2.5. Outcome
The primary outcome was the DE rate between the pre‐ and post‐AST intervention periods. The secondary outcomes included DOT per 100 bed‐days for carbapenems, length of carbapenem therapy, 30‐day mortality, and in‐hospital mortality.
2.6. Statistical analysis
Categorical and continuous variables were compared using Pearson's chi‐squared test or Fisher's exact test and Student's t‐test, respectively. All statistical analyses were two‐tailed, and P < .05 were considered statistically significant.
An interrupted time series analysis was performed to identify the effect of the intervention on the DOT of carbapenem. A total of 24 monthly data points in the pre‐ and post‐AST intervention periods were available for analysis. The model included an intercept (β0), baseline trend (β1), level change after the start of the intervention (β2), and trend change after initiating the intervention (β3).
Statistical analyses were performed using the Statistical Package for the Social Sciences software version 23.0 (IBM Japan).
3. RESULTS
3.1. Characteristics of the participants
In total, 1500 in‐patients were treated with carbapenem during the 12‐month pre‐AST intervention period (n = 771) and the 12‐month post‐AST intervention period (n = 729). The characteristics of the patients at baseline were similar between the pre‐ and post‐AST periods, except in those who were on renal replacement therapy and who presented with neutropenia (Table 1).
TABLE 1.
Characteristics of the patients (n = 1500)
| Variables | All patients | Pre‐AST intervention period | Post‐AST intervention period | P value |
|---|---|---|---|---|
| (n = 1500) | (n = 771) | (n = 729) | ||
| Age, y | 67.1 ± 20.2 | 67.5 ± 19.3 | 66.7 ± 21.1 | .444 |
| Body weight, kg | 52.7 ± 14.7 | 52.5 ± 15.0 | 52.9 ± 14.5 | .367 |
| Renal replacement therapy | 130 (8.7) | 81 (10.5) | 49 (6.7) | .009 |
| Creatinine clearance, mL/min a | 73.7 ± 47.6 | 72.3 ± 47.8 | 75.1 ± 47.4 | .275 |
| Neutropenia, granulocytes <500/µL | 195 (13.0) | 78 (10.1) | 117 (16.0) | .001 |
| Carbapenems | ||||
| Meropenem | 1298 (86.5) | 642 (83.3) | 656 (90.0) | <.001 |
| Doripenem | 52 (3.5) | 35 (4.5) | 17 (2.3) | .019 |
| Imipenem/cilastatin | 50 (3.3) | 40 (5.2) | 10 (1.4) | <.001 |
| Panipenem/betamipron | 37 (2.5) | 20 (2.6) | 17 (2.3) | .744 |
| Biapenem | 63 (4.2) | 34 (4.4) | 29 (4.0) | .677 |
| Combination therapy | 256 (17.1) | 131 (17.0) | 125 (17.1) | .936 |
| β‐lactam agent | ||||
| Penicillin G | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1.000 |
| Third‐generation cephalosporins | 9 (0.6) | 6 (0.8) | 3 (0.4) | .508 |
| Aminoglycosides | 52 (3.4) | 22 (2.9) | 30 (4.1) | .041 |
| Macrolides | 8 (0.5) | 7 (0.9) | 1 (0.1) | .070 |
| Quinolones | 24 (1.6) | 18 (2.3) | 6 (0.8) | .020 |
| Anti MRSA agents | 164 (10.9) | 78 (10.1) | 86 (11.8) | .297 |
| Others | 14 (0.9) | 9 (1.2) | 5 (0.7) | .332 |
| Intensive care unit | 133 (8.9) | 72 (9.3) | 61 (8.4) | .509 |
| Mortality | ||||
| 30‐day | 219 (14.6) | 110 (14.3) | 109 (15.0) | .707 |
| In‐hospital | 298 (19.9) | 150 (19.5) | 148 (20.3) | .681 |
Data were presented as mean ± standard deviation or n (%).
Abbreviations: AST, antimicrobial stewardship team; MRSA, methicillin‐resistant Staphylococcus aureus.
The creatinine clearance values of patients who had received renal replacement therapy were not included.
3.2. Process measures
During the intervention, the AST gave 447 recommendations for 218 patients. Figure 1 shows the type of interventions provided by the AST and the proportion of patients who accepted the treatment. The total acceptance rate was 68.7% (307/447). The proportion of patients receiving carbapenem treatment who underwent blood culture was higher in the post‐AST intervention period than in the pre‐AST intervention period (74.9% vs 54.5%; P < .05).
FIGURE 1.
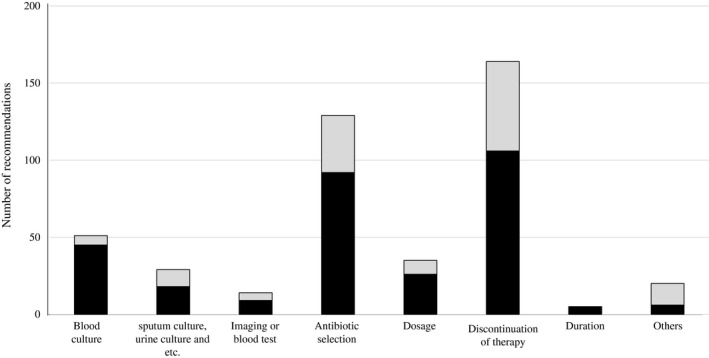
Number of recommendations made by the antimicrobial stewardship team. Black bars, recommendations that were accepted; gray bars, recommendations that were not accepted
3.3. Primary outcome
The DE rate was significantly higher in the post‐AST intervention period than in the pre‐AST intervention period (51.4% vs 40.3%; P < .001) (Table 2). Notably, the rate of switching to narrower‐spectrum β‐lactams increased; the occurrence of prolonged carbapenem therapy decreased.
TABLE 2.
Trends of carbapenem prescription during the pre‐ and post‐AST intervention periods
| Variables | Pre‐AST intervention period | Post‐AST intervention period | P value |
|---|---|---|---|
| (n = 771) | (n = 729) | ||
| Duration of carbapenem therapy, days | 9.9 ± 8.5 | 7.7 ± 4.8 | <.001 |
| De‐escalation | 311 (40.3) | 375 (51.4) | <.001 |
| Discontinuation of therapy (within 7 days) | 236 (30.6) | 234 (32.1) | .534 |
| Switch to narrow‐spectrum antibiotics | 63 (8.2) | 120 (16.5) | <.001 |
| Penicillin group | 8 (1.0) | 17 (2.3) | .050 |
| Ampicillin | 3 (0.3) | 3 (0.4) | 1.000 |
| Ampicillin and sulbactam | 4 (0.5) | 12 (1.6) | .043 |
| Piperacillin and tazobactam | 5 (0.6) | 14 (1.9) | .028 |
| Cephem group | 46 (6.0) | 83 (11.4) | <.001 |
| First generation cephem | 10 (1.3) | 24 (3.3) | .009 |
| Second Generation cephem | 14 (1.8) | 19 (2.6) | .297 |
| Third Generation cephem | 22 (2.9) | 37 (0.4) | .027 |
| Fouth Generation cephem | 0 (0.0) | 3 (1.0) | .115 |
| Combination | 4 (0.5) | 5 (0.7) | .465 |
| Switch from parenteral to oral route | 12 (1.6) | 22 (3.0) | .057 |
| Continuous treatment (more than 8 days) | 446 (57.8) | 341 (46.8) | <.001 |
| Change to other classes a | 14 (1.8) | 12 (1.6) | .801 |
| Anti MRSA drugs | 6 (0.8) | 4 (0.5) | .754 |
| Others | 7 (0.9) | 5 (0.7) | .629 |
| Combination | 1 (0.1) | 4 (0.5) | .170 |
Data were presented as mean ± standard deviation or n (%).
Abbreviations: AST, antimicrobial stewardship team; MRSA, methicillin‐resistant Staphylococcus aureus.
Change to other classes was defined as a switch to non‐β‐lactam intravenous agents.
3.4. Secondary outcomes
No significant differences were observed in in‐hospital mortality and 30‐day mortality between the pre‐ and post‐AST periods (19.5% vs 20.3%, P = .681 and 14.3% vs 15.0%; P = .707, respectively).
Table 3 shows the susceptibility rates of gram‐negative bacteria during the periods. No significant differences were observed in the susceptibility between the pre‐ and post‐ AST intervention periods.
TABLE 3.
Susceptibility rates of gram‐negative bacteria to meropenem during the pre‐ and post‐AST intervention periods
| Pre‐AST intervention period | Post‐AST intervention period | P value | |||
|---|---|---|---|---|---|
| Escherichia coli | 100% | (1001/1001) | 100% | (1008/1008) | >.99 |
| Klebsiella pneumoniae | 99.1% | (583/588) | 99.4% | (652/656) | .617 |
| Enterobacter cloacae | 89.7% | (131/146) | 94.1% | (143/152) | .167 |
| Pseudomonas aeruginosa | 87.4% | (456/522) | 88.3% | (505/572) | .638 |
Data were presented as susceptibility rates (No. of isolates).
Abbreviation: AST, antimicrobial stewardship team.
3.5. Trend analysis of carbapenem use
The trend of carbapenem use increased during the pre‐AST intervention period (β1 = 0.101, P = .007). After the AST intervention, the level (β2 = −0.686, P = .049) and the slope decreased (β3 = −0.211, P < .001) (Figure 2). DOT per 100 patient‐days was reduced in the post‐AST intervention period compared with the pre‐AST intervention period (2.95 vs 3.59). The mean (± standard deviation) days of carbapenem therapy was significantly shorter during the post‐AST intervention period than in the pre‐AST intervention period (7.7 ± 4.8 vs 9.9 ± 8.5; P < .001).
FIGURE 2.
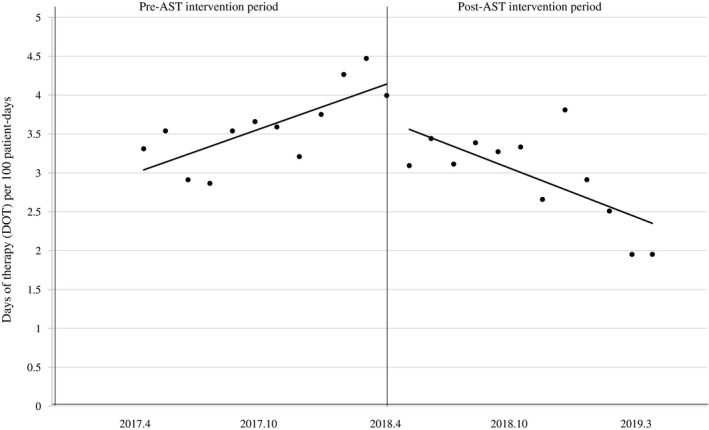
The line graph shows a segmented linear regression of therapy duration (days of therapy per 100 patient‐days) during the pre‐ and post‐AST intervention periods. The trend of carbapenem use increased during the pre‐AST intervention period (β1 = 0.101, P = .007). After the AST intervention, the level (β2 = −0.686, P = .049) and the slope decreased (β3 = −0.211, P < .001)
4. DISCUSSION
This study revealed that the PPRF strategy for carbapenem use that was implemented by the multidisciplinary AST promoted the DE strategy without compromising patient safety. Notably, the trend of carbapenem use was reduced by switching to narrow‐spectrum antibiotics and by shortening the length of carbapenem use.
In this study, we focused on carbapenem stewardship by promoting DE using the intervention implemented by the AST. DE is considered an important strategy for ASPs and is a default practice whenever broad‐spectrum antimicrobials are prescribed. Several studies have attempted to define DE. However, its definition remains unclear because of the complexity of clinical settings and limited data available. 10 , 11 Moreover, we attempted to evaluate the AST intervention focusing on appropriate carbapenem use based on a clear definition of DE. Because carbapenem has the widest spectrum of antimicrobial activity, switching to other narrower‐spectrum β‐lactams is considered. 6 , 11 Piperacillin‐tazobactam is recognised broad‐spectrum antibiotic. Piperacillin‐tazobactam has been tried for use as an alternative therapy to carbapenems, from the standpoint of cost and efficacy. 1 , 6 Because carbapenem has various high stabile β‐lactamases and high therapeutic cost, compared with piperacillin‐tazobactam, we defined the switch from carbapenem to piperacillin‐tazobactam as DE, according to previous studies. 6 , 11
Previous studies have defined DE as early discontinuation of antimicrobial treatment. 17 , 21 , 22 However, the initiation of DE from the start of antimicrobial use has been controversial. 10 , 11 Ideally, DE should be performed as soon as possible after obtaining culture results. Because the final blood culture results can be obtained after a week in our hospital, DE was performed within 7 days of carbapenem use. In the present study, the PPRF strategy that was evaluated and classified into five was implemented for carbapenem stewardship. Previous studies have shown that the AST intervention is effective in decreasing the use of targeted broad‐spectrum antimicrobials, including carbapenems. 12 , 13 However, these studies have not assessed the outcomes based on a clear definition of DE. The implementation of interventions by promoting DE based on a clear definition is useful for the carbapenem stewardship programme in various health care facilities.
The AST without an infectious disease (ID) physician and with small FTEs for each member other than the pharmacist only had one activity per week. The AST collaborated with ward‐based CPs in providing appropriate carbapenem dosage and obtaining blood culture results, which indicated a high acceptance rate of these recommendations.
In contrast, our recommendations (such as antibiotic selection and discontinuation) had low acceptance rates, which was caused by the shortage of manpower in the AST. The attending physician often believes that carbapenem is highly effective against severe infections. Importantly, patient safety should be considered in DE. 6 Data obtained from the MERINO trial, which supports the efficacy of carbapenem, showed that patients with bloodstream infections caused by third‐generation cephalosporin‐resistant gram‐negative bacteria who were treated with meropenem had lower mortality than those who received piperacillin‐tazobactam. 23 These therapeutic recommendations require a broad knowledge of infectious diseases, and a thorough discussion between ID specialists and prescribers is necessary to achieve successful interventions. In a Japanese nationwide survey, ID physicians and health care workers in the AST had low FTEs. 24 To enhance comprehensive antimicrobial stewardship that includes DE strategy, more FTE support of ID physician and ID‐trained pharmacist will be needed. 25
The present study had several limitations. First, the effect of the strategy on AMR could not be evaluated. Preventing the unnecessary use of carbapenem along with the utilisation of infection control measures may decrease selection pressure and prevent the occurrence of AMR. 26 A well‐designed quasi‐experimental study that evaluates the effect of what on AMR must be conducted. Another limitation is the limited term of this study. ITSA requires a sufficient number of observations, which is ideally 100 points for each data in the time series. 27 In 2019, there was a significant shortage of various antimicrobials other than carbapenems in Japan. 28 The AST intervention against promoting DE in this period was challenging to evaluate.
In conclusion, the PPRF strategy implemented by the AST enhanced the carbapenem stewardship programme based on a clear definition and application of DE.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors meet the ICMJE authorship criteria.
Suzuki A, Maeda M, Yokoe T, Hashiguchi M, Togashi M, Ishino K. Impact of the multidisciplinary antimicrobial stewardship team intervention focusing on carbapenem de‐escalation: A single‐centre and interrupted time series analysis. Int J Clin Pract. 2021;75:e13693. 10.1111/ijcp.13693
REFERENCES
- 1. Wilson A, Livermore DM, Otter JA, et al. Prevention and control of multi‐drug‐resistant Gram‐negative bacteria: recommendations from a Joint Working Party. J Hosp Infect. 2015;2016(92):S1‐S44. 10.1016/j.jhin.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 2. del Arco A, Tortajada B, de la Torre J, et al. The impact of an antimicrobial stewardship programme on the use of antimicrobials and the evolution of drug resistance. Eur J Clin Microbiol Infect Dis. 2014;34(2):247‐251. 10.1007/s10096-014-2225-5 [DOI] [PubMed] [Google Scholar]
- 3. Dellit TH. Summary of the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Infect Dis Clin Pract. 2007;15(4):263‐264. 10.1097/IPC.0b013e318068b1c0 [DOI] [PubMed] [Google Scholar]
- 4. Zhang DI, Cui K, Lu W, et al. Evaluation of carbapenem use in a tertiary hospital: Antimicrobial stewardship urgently needed. Antimicrob Resist Infect Control. 2019;8(1):1‐7. 10.1186/s13756-018-0449-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pakyz AL, Oinonen M, Polk RE. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem‐resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53(5):1983‐1986. 10.1128/AAC.01535-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leone M, Bechis C, Baumstarck K, et al. De‐escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non‐blinded randomized noninferiority trial. Intensive Care Med. 2014;40(10):1399‐1408. 10.1007/s00134-014-3411-8 [DOI] [PubMed] [Google Scholar]
- 7. la Martire G, Robin C, Oubaya N, et al. De‐escalation and discontinuation strategies in high‐risk neutropenic patients: an interrupted time series analyses of antimicrobial consumption and impact on outcome. Eur J Clin Microbiol Infect Dis. 2018;37(10):1931‐1940. 10.1007/s10096-018-3328-1 [DOI] [PubMed] [Google Scholar]
- 8. Katsios CM, Burry L, Nelson S, et al. An antimicrobial stewardship program improves antimicrobial treatment by culture site and the quality of antimicrobial prescribing in critically ill patients. Crit Care. 2012;16(6):R216. 10.1186/cc11854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujibayashi A, Niwa T, Takeichi S, et al. Clinical impact of a prospective audit with intervention and feedback without carbapenem restriction in patients receiving carbapenem injection. Int J Clin Pract. 2019;73(1):1‐8. 10.1111/ijcp.13262 [DOI] [PubMed] [Google Scholar]
- 10. Tabah A, Bassetti M, Kollef MH, et al. Antimicrobial de‐escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patient. Intensive Care Med. 2020;46(2):245‐265. 10.1007/s00134-019-05866-w [DOI] [PubMed] [Google Scholar]
- 11. Weiss E, Zahar J‐R, Lesprit P, et al. Elaboration of a consensual definition of de‐escalation allowing a ranking of β‐lactams. Clin Microbiol Infect. 2015;21(7):649.e1‐649.e10. 10.1016/j.cmi.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 12. Honda H, Murakami S, Tagashira Y, et al. Efficacy of a postprescription review of broad‐spectrum antimicrobial agents with feedback: a 4‐year experience of antimicrobial stewardship at a tertiary care center. Open Forum Infect Dis. 2018;5(12):ofy314. 10.1093/ofid/ofy314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akazawa T, Kusama Y, Fukuda H, et al. Eight‐year experience of antimicrobial stewardship program and the trend of carbapenem use at a tertiary acute‐care hospital in Japan—the impact of postprescription review and feedback. Open Forum Infect Dis. 2019;6(10):1‐6. 10.1093/ofid/ofz389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kollef MH. Optimizing antibiotic therapy in the intensive care unit setting. Crit Care. 2001;5(4):189‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morel J, Casoetto J, Jospé R, et al. De‐escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico‐surgical intensive care unit. Crit Care. 2010;14(6):R225. 10.1186/cc9373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joung MK, Lee J‐A, Moon S‐Y, et al. Impact of de‐escalation therapy on clinical outcomes for intensive care unit‐acquired pneumonia. Crit Care. 2011;15(2):10‐17. 10.1186/cc10072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohji G, Doi A, Yamamoto S, Iwata K. Is de‐escalation of antimicrobials effective? A systematic review and meta‐analysis. Int J Infect Dis. 2016;49:71–79. 10.1016/j.ijid.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de‐escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. 10.1186/cc12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garnacho‐Montero J, Gutiérrez‐Pizarraya A, Escoresca‐Ortega A, et al. De‐escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32‐40. 10.1007/s00134-013-3077-7 [DOI] [PubMed] [Google Scholar]
- 20. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De‐escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533‐1540. 10.1007/s00134-007-0619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madaras‐Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de‐escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103‐1113. 10.1086/677633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paul M, Dickstein Y, Raz‐Pasteur A. Antibiotic de‐escalation for bloodstream infections and pneumonia: systematic review and meta‐analysis. Clin Microbiol Infect. 2016;22(12):960‐967. 10.1016/j.cmi.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 23. Harris PNA, Tambyah PA, Lye DC, et al. Effect of piperacillin‐tazobactam vs meropenem on 30‐day mortality for patients with e coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance. JAMA. 2018;320(10):984‐994. 10.1001/jama.2018.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maeda M, Muraki Y, Kosaka T, et al. The first nationwide survey of antimicrobial stewardship programs conducted by the Japanese Society of Chemotherapy. J Infect Chemother. 2019;25(2):83‐88. 10.1016/j.jiac.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 25. Maeda M, Muraki Y, Kosaka T, et al. Essential human resources for antimicrobial stewardship teams in Japan: estimates from a nationwide survey conducted by the Japanese Society of Chemotherapy. J Infect Chemother. 2019;25(9):653‐656. 10.1016/j.jiac.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 26. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic‐resistant bacteria and Clostridium difficile infection: a systematic review and meta‐analysis. Lancet Infect Dis. 2017;17(9):990–1001. 10.1016/S1473-3099(17)30325-0 [DOI] [PubMed] [Google Scholar]
- 27. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299‐309. 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 28. Komagamine J, Yabuki T, Hiraiwa T. A trend in prevalence of antimicrobial use and appropriateness of antimicrobial therapy in an acute care hospital from 2018 to 2019: repeated prevalence surveys in Japan. BMC Res Notes. 2019;12(1):811. 10.1186/s13104-019-4849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


