FIGURE 1.
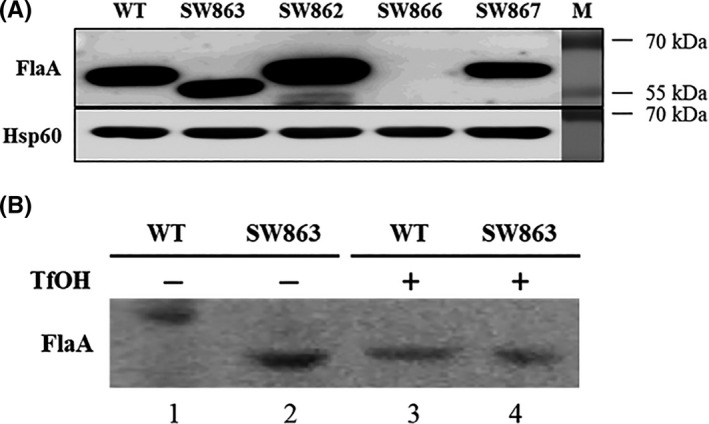
Western blot analysis of FlaA in H.pylori. (A) Detection of FlaA in the wild‐type and mutant strains. The whole‐cell proteins of the wild type (WT), the ∆jhp0106 (SW863), ∆jhp0106 revertant (SW862), ∆flaA mutant (SW866), and ∆flaA revertant (SW867) were probed with anti‐FlaA mouse polyclonal antibody. Anti‐Hsp60 mouse monoclonal antibody was used as internal control. (B) Glycosylation of FlaA verified by trifluoromethanesulphonic acid (TfOH) treatment. The total proteins extracted from the WT and SW863 strains were treated with or without TfOH and analyzed by Western blotting. TfOH chemically removed conjugated carbohydrates that were glycosylated on FlaAs (represented in the form of higher molecular mass ≈ 57 kDa) (lane 1), resulting in non‐glycosylated FlaAs (represented in the form of lower molecular mass ≈ 53 kDa) (lanes 2, 3, and 4)
