Abstract
Chronic inflammation pain is a debilitating disease, and its mechanism still remains poorly understood. This study attempted to illuminate the metabolic mechanism of chronic inflammation pain induced by complete Freund’s adjuvant (CFA) injection, especially at spinal level. The chronic inflammation pain model was established by CFA administration. Behavioral testing including mechanical allodynia and thermal hyperalgesia was performed. Meanwhile, a liquid chromatography–mass spectrometry‐based metabolomics approach was applied to analyze potential metabolic biomarkers. The orthogonal partial least squares discrimination analysis mode was employed for determining metabolic changes, and a western blot was performed to detect the protein expression change. The results showed that 27 metabolites showed obviously abnormal expression and seven metabolic pathways were significantly enriched, comprising aminoacyl‐tRNA biosynthesis, arginine and proline metabolism, histidine metabolism, purine metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glutathione metabolism, and phenylalanine metabolism. Meanwhile, the results showed that the expression of arginase I and nitric oxide levels were elevated in the CFA group compared with the control group, while the argininosuccinate synthetase and argininosuccinatelyase proteins were not significantly different between the groups. These findings demonstrate that metabolic changes of the spinal cord may be implicated in neurotransmitter release and pain conductivity following CFA administration.
Keywords: chronic inflammation pain, complete Freund’s adjuvant, metabolomics
1. INTRODUCTION
Chronic pain results in dramatic decline in life quality, substantial medical expenses and a massive economic burden (Henderson & Keay, 2018). Survey data demonstrate that the prevalence of chronic pain ranges from 13.5 to 47% globally, and afflicts at least 50 million American adults (Dahlhamer et al., 2018; Tsuji et al., 2019). Generally, patients with chronic pain have symptoms of anxiety and depression, poor concentration and irritability (Gureje, Von Korff, Simon, & Gater, 1998). Some research shows that patients with chronic pain have multiple inflammatory and neuropathic conditions (Finnerup, 2013). Additionally, chronic inflammation pain, as one type of chronic pain, is attracting growing interest from clinicians and scientists. A previous study documented that chronic inflammation pain was derived from chemical stimuli, tissue damage or autoimmune processes. These stimuli directly caused the release of inflammatory mediators comprising prostaglandins, histamine and neurogenic factors, and elicited a series of chain reactions, thereby contributing to pain sensation by stimulating the peripheral afferent fibers (Kidd, Photiou, & Inglis, 2004). The potential mechanism regarding the chronic inflammation pain has been extensively investigated. Yet, it is of great importance in the clinical practice, while its pathogenesis has not been clarified comprehensively.
More recently, systems biology strategies such as metabolomics have been widely applied in medical fields to investigate the pathogenic mechanism, which facilitated the development of novel biomarkers for disease diagnosis and therapy (Hocher & Adamski, 2017; Yang et al., 2018; Zhang et al., 2018). Metabolism is a complex dynamic process including generating energy and producing macromolecules for sustaining cell growth and survival (Patti et al., 2012). Metabolites are downstream molecules of gene transcription and translation processes, which are closely correlated to the disease phenotype (Lains et al., 2019). Metabolic shift is identified as a hallmark of disease, and provides a noninvasive method to monitor the disease progress (Ohman & Forsgren, 2015). Furthermore, emerging evidence has revealed the relationship between inflammatory and metabolic dysregulation (Jha et al., 2015; Jiang et al., 2016; Palomer, Salvado, Barroso, & Vazquez‐Carrera, 2013). A previous study revealed that aberrant metabolism may be involved in triggering inflammatory cascade reactions (O'Neill & Hardie, 2013). Notwithstanding, there are no adequate data to uncover the role of metabolism alteration in chronic inflammation pain. Therefore, the complete Freund’s adjuvant (CFA) model was established to investigate the potential mechanism for chronic inflammation pain in this study. Moreover, a metabolomics method was employed to analyze the changes in spinal metabolites. Interestingly, our results indicated that certain metabolic pathways were obviously enriched in the chronic inflammatory process, and these findings may provide new perspectives for comprehending the underlying mechanism of chronic inflammation pain.
2. MATERIALS AND METHODS
2.1. Animals
All experiments were performed on 8–12‐week‐old male C57BL/6 mice purchased from Shanghai SLAC Laboratory Animal Co. Ltd. For experiments, mice (20–35 g) were housed four or five per cage at constant room temperature (25 ± 1°C) and relative humidity (50 ± 5%) under a 12 h light/dark schedule (lights on 07:00–19:00); food and water were available ad libitum. For behavioral tests, the mice were allowed to adapt to laboratory conditions for about one week and to habituate to the testing situation for at least 15 min before experiments. The Animal Care and Use Committee of Zhejiang University approved all of the mouse protocols (approval no. 11978).
2.2. CFA‐induced chronic inflammation pain
Animals were randomly divided into two groups as follows: (a) a control group, injected with 10 μl saline (n = 10); and (b) a CFA group, injected with 10 μl 50% CFA in saline (n = 10). Chronic inflammatory pain was induced by administration of CFA as described previously (Pan et al., 2014). Briefly, an emulsion containing 10 μl of CFA with saline (proportion 1:1) was injected into the left posterior plantar of mice (n = 10). The control group received the same procedure with saline (Y. Liu et al., 2017b). Mice were allowed to acclimatize to the home cage and environment.
2.3. Behavioral testing
2.3.1. Mechanical allodynia
Mice were placed in individual black wood boxes without a bottom and allowed to acclimatize for at least 30 min to quantify the mechanical sensitivity of the hindpaw according to the previous literature (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994). Mechanical paw withdrawal threshold in response to the stimulation of von Frey filaments was measured using the “up–down” method (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994). Filaments were applied to the plantar surface of left hindpaw until they bent. A quick withdrawal or shaking of the stimulated paw or biting or licking of the paw was regarded as a positive withdrawal response, while other responses were regarded as a negative withdrawal response. A positive withdrawal response was followed by the application of a lower force filament and vice versa for a negative response until a change in behavior occurred (Zhao, Hiraoka, Ogawa, & Tanaka, 2018). The test started with the application of a 0.16 g filament. Every trial was repeated three times at ~2 min intervals. According to the method described by Dixon, the 50% paw withdrawal threshold was calculated based on this assessment.
2.3.2. Thermal hyperalgesia
To assess thermal hyperalgesia, mouse paw withdrawal latency (PWL) was measured using radiant heat (Bao et al., 2014; Bao et al., 2015). Mice were placed individually in plastic cages and allowed to acclimatize at least for 30 min. Each left hind paw received at least three stimuli with a 10 min interval between, and the average of the three values was defined as the PWL. The heat was maintained at a constant intensity and the cut‐off time was set to 21 s to prevent paw damage.
2.3.3. Sample preparation
Animals were anesthetized with 3% isoflurane on day 7 after CFA administration. Then these mice were sacrificed through decapitation. A laminectomy of L4–6 was carried out, and the spinal cord tissues were exposed. Complete incision of L4–6 was performed and the intervening tissue was removed. Thereafter, the spinal cord was removed and stored in a liquid nitrogen box immediately for future use.
2.3.4. Metabolite extraction
In brief, the spinal cord tissue was homogenized in 1,500 μl methanol with water (1:1) in a 2 ml glass tissue homogenizer, and centrifuged at 15,000g for 10 min (Tube 1). The supernatant was transferred to a 2 ml centrifuge tube, tube 2, then concentrated at room temperature in vacuum. A 120 μl methanol–water (1:1) solution was applied to dissolve the concentrated product. The precipitate of tube 1 was homogenized with 1,600 μl cold dichloromethane–methanol (3:1), then centrifuged at 15,000g for 10 min. The culture liquid was transferred to 2 ml centrifuge tube, tube 3, which was concentrated at room temperature in vacuum, and redissolved with 120 μl methanol–water (1:1). The solutions of centrifuge tubes 2 and 3 were mixed and centrifuged once again (15,000g for 10 min). The supernatant was determined by HPLC–MS. During the study, 10 QC samples were pooled from all spinal cord samples to equilibrate the HPLC–MS system (Zhou et al., 2018).
2.3.5. Liquid chromatography–mass spectrometry analysis
The metabolomics data were determined using a Nexera UHPLC LC‐30A system (Shimadzu, Japan), while the chromatographic separation was processed on a Waters HSS T3 (150 × 3 mm, 1.8 μm) column at 25°C, with a flow rate of 0.3 ml/min. The analysis was completed with mobile phases A (acetonitrile) and B (0.1% CH3COOH–H2O). The gradient program was 100% B at 0–10 min; 50% A and 50% B at 10–13 min; 95% A and 5% B at 13–14 min; 100% B at 14–15 min. All samples were kept at 4°C during the procedures.
The high‐resolution MS system was performed using a TripleTOF5600 + mass spectrometer (AB SCIEX™, USA). Both positive and negative modes was used to acquire the data. Source parameters are defined as follows: scanning range, m/z 100–1,500; scanning mode,data‐independent acquisition (DIA); capillary voltage, 5,000 V (positive) and 4,500 V (negative); capillary temperature, 500°C; declustering potential (DP), 60 V; collision energy (CE), 35 V; collision energy spared (CES), 15 V.
2.3.6. Data processing
The raw LC–MS data was imported into MS‐DIAL3.96 software for preprocessing, then peak extraction, de‐noise, deconvolution and peak alignment, and a 3D data matrix in CSV format was exported. The peak information was compared with metabolites from online databases including MassBank, Respect and GNPS. The three‐dimensional matrix comprising sample information, retention time, mass nuclear ratio and mass spectrometry response intensity (peak area) was analyzed. Principal components analysis, partial least squares discriminate analysis and orthogonal partial least squares discrimination analysis were carried out to make multivariate statistical analysis using SIMCA‐P (version 11.0, Umetrics, Umea, Sweden) software (Rezig et al., 2018).
2.3.7. Western blot analysis
The mouse spinal cord tissues (L4–6) were harvested and homogenized using RIPA buffer (Beyotime, P0013B) supplemented with 1× protease inhibitor cocktail (Sigma‐Aldrich; P8304), phosphatase inhibitor cocktail II and III (Sigma‐Aldrich; P5726). The supernatant was collected by centrifugation at 12,000g for 10 min, and the protein concentration was detected using a bicinchoninic acid protein assay kit (Beyotime, P0012S). An aliquot of 50 μg protein from each sample was separated using SDS‐PAGE and transferred to a PVDF membrane, then blocked with 5% nonfat milk in TBST (pH 7.4). Thereafter, the membranes were incubated with primary antibodies including arginase I (1:1000; CST; #93668), argininosuccinate synthetase (1:1000; abcam; ab17095), argininosuccinatelyase (1:1000; abcam; ab97370) and actin (1:1000; ABclonal; AC026). After incubation with the appropriate horseradish peroxidase (HRP) conjugated secondary antibodies (IgG, against rabbit, 1:1000; ABclonal; AS014), the immune complexes were visualized using the SuperSignal West Pico Substrate (34,077, Pierce). The digital images were quantified using densitometric measurements by Quantity‐One software (Bio‐Rad).
2.3.8. NO level detection
The spinal cord tissues (L4–6) were acquired and the level of nitric oxide (NO) was determined. Briefly, the NO detection kit (A012‐1‐2; Nanjing Jiancheng Biotechnology Co. Ltd; China) was purchased and the experiment protocol was performed according to the operating manual.
2.3.9. Statistical analysis
Data are presented as the mean ±standard deviation. An unpaired Student’s t‐test was conducted using GraphPad Prism 8.0 (Graphpad, CA, USA). A value of P < 0.05 was considered statistically significant.
3. RESULTS
3.1. CFA‐induced mechanical and thermal hypersensitivities
The mechanical and thermal hypersensitivities were examined on the fifth day after CFA injection. The results showed that the PWL and paw withdrawal threshold values were remarkable decreased in the CFA group compared with the control group (Figure 1a,b; P < 0.05).
FIGURE 1.
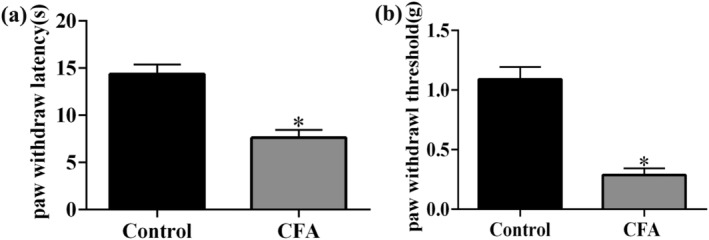
Mechanical and thermal allodynia in mice induced by complete Freund's adjuvant (CFA) injection. Effect of CFA injection on the paw withdrawal responses to thermal (a) and mechanical (b) stimuli at 5 days. *P < 0.05, compared with control group
3.2. Metabolic profiling analysis
To confirm whether chronic inflammation pain induced dramatic shifts in the metabolites in the spinal cord, an LC–MS method was applied to analyze the differences between the control and CFA groups. Principal components analysis (Figure 2a) and partial least squares discrimination analysis methods (Figure 2b) were used to detect the differences. The results showed that the two methods did not isolate differentially expressed metabolites (Figure 2a,b). Therefore, orthogonal partial least squares discrimination analysis mode was employed, and the metabolites were separated into two categories (Figure 2c). Meanwhile, the model was subjected to a parametric test, and the results indicated that the prediction rate of metabolites was 14.4%, the prediction rate of the grouping was 75.4% and the accuracy of model prediction was 58.6% (Figure 2d). In order to prevent a false positive of the model, it was detected by response arrangement tests (100 runs), and the results showed that the prediction rate of grouping was 99.0%, and the accuracy of model prediction was 72.3% (Figure 2e). To obtain different metabolite candidates, P‐value < 0.05 and fold change > 2 were set as threshold values. The heat map and volcano plot of metabolites are separately shown in Figure 2f and g, and the details of the different metabolites are attached to Table 1.
FIGURE 2.
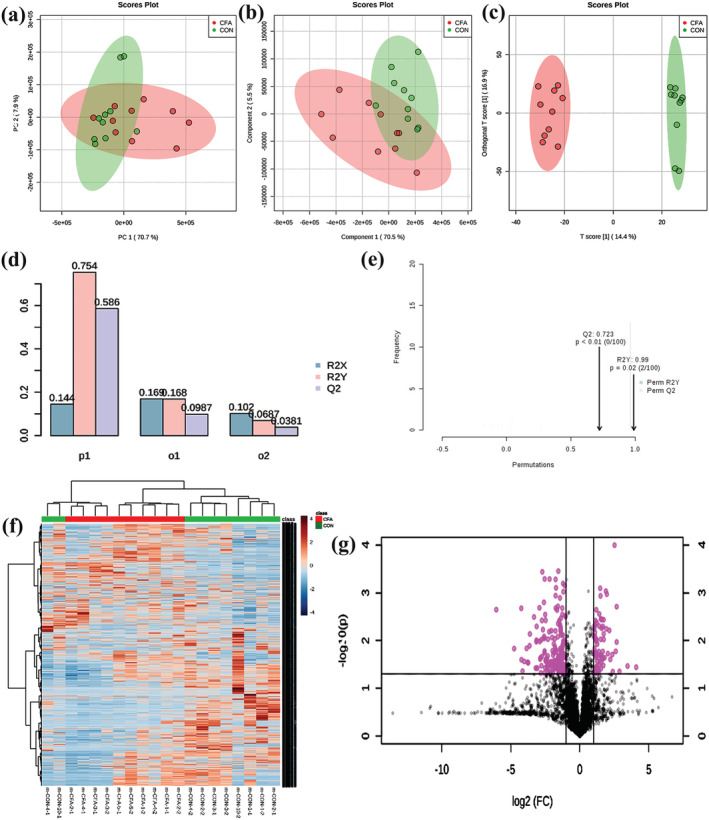
Metabolic profiling analysis: (a) principal components analysis; (b) partial least squares discrimination analysis; (c) orthogonal partial least squares discrimination analysis; (d, e) parametric test. (f) Heat map analysis of metabolites between control group and CFA group (the color scale shows the relative metabolites expression in certain slide: blue indicates low relative expression levels; red indicates high relative expression levels; yellow indicates no change); (g) volcano plot of metabolites between control group and CFA group (red indicates the metabolites expression was significantly down/up‐regulated in CFA group compared with control group; P < 0.05). R 2 X represents the prediction rate of metabolites, R 2 Y represents the prediction rate of grouping, and Q 2 represents the accuracy of model prediction
TABLE 1.
The details of metabolites in different groups
| Alignment ID | Average retention time (min) | Average Mz | Metabolite name | Adduct type | MS/MS assigned | Reference m/z | Formula | Ontology | INCHIKEY | SMILES | MS1 isotopic spectrum | MS/MS spectrum | m‐CON‐1‐1 | m‐CON‐1‐2 | m‐CON‐2‐1 | m‐CON‐2‐2 | m‐CON‐3‐1 | m‐CON‐3‐2 | m‐CON‐4‐1 | m‐CON‐4‐2 | m‐CON‐5‐1 | m‐CON‐5‐2 | m‐CFA‐1‐1 | m‐CFA‐1‐2 | m‐CFA‐2‐1 | m‐CFA‐2‐2 | m‐CFA‐3‐1 | m‐CFA‐3‐2 | m‐CFA‐4‐1 | m‐CFA‐4‐2 | m‐CFA‐5‐1 | m‐CFA‐5‐2 | QC‐1 | QC‐2 | QC‐3 | QC‐4 | QC‐5 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | CON | CON | CON | CON | CON | CON | CON | CON | CON | CFA | CFA | CFA | CFA | CFA | CFA | CFA | CFA | CFA | CFA | QC | QC | QC | QC | QC | VIP (CFA vs. CON) | FC (CFA vs. CON) | TTEST (CFA vs. CON) | |||||||||||||
| 44 | 3.798 | 104.05289 | N‐Methylalanine | [M + H]+ | True | 104.0706 | C4H9NO2 | Alanine and derivatives | GDFAOVXKHJXLEI‐VKHMYHEASA‐N | CN[C@@H](C)C(O)=O | 104.05318:10556 105.05653:4518 106.05989:904 | 58.07356:42104.11706:42 | 2,194 | 813 | 6,113 | 23,888 | 45,403 | 29,632 | 46,466 | 46,437 | 50,186 | 6,318 | 52,019 | 38,489 | 45,164 | 35,816 | 72,491 | 40,667 | 48,518 | 38,682 | 43,410 | 72,211 | 3,369 | 2,412 | 35,398 | 1967 | 37,982 | 0.060775 | 1.8934433871 | 0.0041166709 |
| 47 | 2.967 | 104.07158 | a‐Aminoisobutyrate | [M + H]+ | True | 104.0706 | C4H9NO2 | Alpha amino acids | FUOOLUPWFVMBKG‐UHFFFAOYSA‐N | CC(C)(N)C(O)=O | 104.06766:5184 105.07101:4230 106.07437:2256 | 56.05714:83 58.07238:2343 58.11862:142 58.14444:98 58.21546:42 58.37488:42 59.05377:43 59.08196:319 59.1004:63 59.91335:48 60.08711:1660 60.11774:104 60.19324:42 60.48803:42 61.00925:63 69.03474:42 71.08587:42 87.05281:63104.10562:1224 104.28419:42 | 62,703 | 54,945 | 78,732 | 42,530 | 87,202 | 80,753 | 98,610 | 104,309 | 96,175 | 82,126 | 91,306 | 110,792 | 92,627 | 107,516 | 125,306 | 114,844 | 154,416 | 108,947 | 156,350 | 139,787 | 20,239 | 3,645 | 41,971 | 67,113 | 61,782 | 0.10933 | 1.5250778787 | 0.0002315302 |
| 634 | 3.507 | 132.101 | Isoleucine | [M + H]+ | True | 132.1028 | C6H13NO2 | Isoleucine and derivatives | AGPKZVBTJJNPAG‐UHFFFAOYNA‐N | O=C(O)C(N)C(C)cc | 132.101:275158 133.10435:40078 134.10771:5289 | 53.00695:63 53.02544:42 55.02148:42 55.06335:83 56.06026:171 57.06399:150 57.07038:149 58.05942:83 58.0691:63 58.07985:63 62.94052:21 69.04636:63 69.07684:478 69.10146:102 69.21879:42 71.07623:42 72.06087:146 72.08722:42 72.94139:146 73.06555:63 74.0699:42 85.8249:42 86.09304:87 86.1022:2372 86.20303:179 86.27247:83 86.34325:83 86.53212:42 87.06313:210 87.08025:133 87.09867:104 89.60625:44 90.05688:982 90.90903:42114.07255:63115.05105:63117.0801:42119.07763:21127.86925:43132.07666:840132.11559:42 | 14,260 | 715 | 869 | 106,976 | 120,636 | 816 | 154,014 | 34,887 | 3,356 | 859 | 15,242 | 1,267 | 1,462,686 | 8,721 | 1,404,377 | 1,314,712 | 1,672,418 | 3,525 | 1,578 | 6,252 | 49,284 | 40,378 | 42,667 | 43,929 | 38,667 | 1.4409 | 13.4680832579 | 0.017904852 |
| 1,006 | 4.964 | 146.16362 | Spermidine | [M + H]+ | True | 146.16518 | C7H19N3 | Dialkylamines | ATHGHQPFGPMSJY‐UHFFFAOYSA‐N | NCCCCNCCCN | 146.16512:4739 147.16847:688148.17183:0 | 56.96902:21 58.07324:42 72.08226:146 72.09304:104 84.08359:63112.11149:42 | 53 | 69 | 1,045 | 3,522 | 8,376 | 7,891 | 12,487 | 8,258 | 28,946 | 582 | 12,503 | 25,642 | 29,045 | 21,045 | 21,998 | 26,846 | 70,867 | 113,281 | 56,294 | 62,733 | 741 | 71 | 346 | 63 | 856 | 0.097503 | 6.1808252257 | 0.0010790023 |
| 1,093 | 3.779 | 150.05882 | Methionine | [M + H]+ | True | 150.05832 | C5H11NO2S | Methionine and derivatives | FFEARJCKVFRZRR‐UHFFFAOYNA‐N | CSCCC(N)C(O)=O | 150.056:51970 151.05935:5422 152.06271:8131 | 53.04919:42 53.06153:42 55.87885:31 56.05933:1175 56.10476:77 56.12907:45 58.99855:42 60.82431:42 60.85072:48 61.01922:1374 61.07874:47 61.54713:21 66.04822:31 70.9967:21 73.64085:21 74.02877:191 74.05183:31 74.06519:73 75.03236:31 77.00658:31 84.04641:83 84.05804:63 85.03094:83 87.0238:53 87.02644:234 90.04098:31 93.06465:21102.05724:190104.05369:350105.00134:31120.0837:21129.12486:10133.03197:514134.11136:42135.12294:31150.05905:83150.07806:42150.12646:52 | 991 | 2,193 | 3,376 | 7,331 | 5,339 | 1925 | 248,496 | 8,429 | 224,333 | 1867 | 12,403 | 9,580 | 203,402 | 208,821 | 281,816 | 185,274 | 234,987 | 262,779 | 342,045 | 404,916 | 410 | 4,593 | 2,204 | 824 | 219,231 | 0.43378 | 4.2556179107 | 0.0022510742 |
| 1,149 | 4.075 | 156.0755 | Histidine | [M + H]+ | True | 156.07675 | C6H9N3O2 | Histidine and derivatives | HNDVDQJCIGZPNO‐UHFFFAOYNA‐N | O=C(O)C(N)CC1=CN=CN1 | 156.07379:27948 157.07714:3718 158.0805:3718 | 50.02816:21 54.04945:63 56.06555:147 66.04694:83 68.05582:83 71.95312:42 81.04317:42 81.04952:169 82.05473:167 82.07135:63 83.05714:44 83.06357:331 83.10729:42 86.06815:21 93.04813:366 95.05525:22 95.06213:63109.721:65110.07299:883110.1026:69111.05206:42115.50999:21156.07678:83 | 2,559 | 3,836 | 10,967 | 5,033 | 9,758 | 57,934 | 130,906 | 8,141 | 45,796 | 2,921 | 167,330 | 124,687 | 40,716 | 143,928 | 67,014 | 47,851 | 26,308 | 44,872 | 318,432 | 290,404 | 3,874 | 2,741 | 3,303 | 6,566 | 4,009 | 0.26255 | 4.5763448755 | 0.0061254643 |
| 1,254 | 3.967 | 161.12683 | l‐β‐Homolysine | [M + H]+ | True | 161.12845 | C7H16N2O2 | β Amino acids and derivatives | PJDINCOFOROBQW‐LURJTMIESA‐N | NCCCC[C@H](N)CC(O)=O | 161.12852:5683 162.13187:1610 163.13523:1610 | 70.07452:21 72.08102:125 84.08356:83 84.10168:63139.03064:21144.10316:63146.07782:42161.1118:42 | 4,751 | 1,240 | 3,089 | 56,286 | 40,507 | 44,075 | 15,866 | 60,653 | 13,569 | 372 | 77,838 | 74,484 | 15,263 | 81,451 | 31,709 | 26,618 | 22,144 | 20,078 | 22,976 | 94,105 | 3,417 | 867 | 2,832 | 2,743 | 7,909 | 0.059781 | 1.9411417257 | 0.0421885982 |
| 1,285 | 3.391 | 162.11143 | l‐carnitine | [M + H]+ | True | 162.11247 | C7H15NO3 | Carnitines | PHIQHXFUZVPYII‐ZCFIWIBFSA‐N | C[N+](C)(C)C[C@H](O)CC([O‐])=O | 162.11374:29728 163.11709:5364 164.12045:1490 | 57.03634:42 58.07023:104 59.07545:63 60.09476:104 85.03355:104102.09293:104103.03878:104103.04737:146146.09703:63162.11127:503 | 137,818 | 126,043 | 189,429 | 120,297 | 182,996 | 226,630 | 171,583 | 230,633 | 230,463 | 1,617 | 354,025 | 440,909 | 1,259,888 | 305,598 | 292,446 | 335,317 | 1,768,962 | 338,206 | 430,799 | 398,995 | 1,008 | 1,020 | 117,255 | 123,861 | 125,978 | 1.1382 | 3.6631295405 | 0.007597821 |
| 1,291 | 3.753 | 162.112 | Carnitine | [M + H]+ | True | 162.11247 | C7H15NO3 | Carnitines | PHIQHXFUZVPYII‐ZCFIWIBFSA‐N | C[N+](C)(C)C[C@H](O)CC([O‐])=O | 162.11185:524328 163.1152:89133 164.11856:11269 | 54.93892:42 55.95581:43 57.04489:618 57.06727:96 57.09925:54 58.07563:878 59.07764:378 59.35122:63 60.01388:48 60.08713:1754 60.11119:177 60.12213:95 60.13635:111 61.0302:125 61.06437:42 84.08521:42 84.76316:63 85.03355:2327 85.0882:99 86.05917:42 97.97429:42 98.96687:42102.09148:1763 103.04305:2444 103.08173:102103.22644:83104.41814:42114.96052:42146.10208:63161.58708:83162.10399:264162.11296:4560 162.25131:66162.34656:125162.44545:104 | 114,299 | 2,126,919 | 2,391,938 | 55,919 | 39,264 | 15,737 | 7,897 | 45,777 | 2049 | 2,570,282 | 27,836 | 16,605 | 9,793 | 17,451 | 1,601 | 9,851 | 14,734 | 10,385 | 11,670 | 23,891 | 2,141,254 | 2,090,007 | 104,083 | 166,766 | 41,578 | 1.9093 | 0.0195136254 | 0.0288782904 |
| 1,572 | 3.702 | 182.08066 | Tyrosine | [M + H]+ | True | 182.08118 | C9H11NO3 | Tyrosine and derivatives | OUYCCCASQSFEME‐UHFFFAOYNA‐N | O=C(O)C(N)CC1=CC=C(O)C=C1 | 182.08034:80922 183.08369:11783 184.08705:2561 | 51.03576:63 53.04057:148 53.06729:42 55.02642:31 64.8437:32 65.04722:246 67.05714:42 74.79868:21 77.0445:333 77.07794:36 79.05739:73 79.08247:21 81.03526:21 81.06955:52 88.02511:21 90.77292:42 91.05811:2809 91.10388:61 91.16583:43 93.05737:31 94.04394:42 94.75534:52 95.05221:776 95.08797:26 95.10586:26 99.93855:21101.04308:31103.05828:94106.06608:22107.05055:501108.08651:21109.06851:52117.05843:83118.06648:167118.66663:42119.05116:1528 119.09734:34120.05537:31121.0638:83122.64606:21123.04481:1296 123.37523:21135.06503:31135.66908:32136.07527:2217 137.07275:42145.26024:21147.04427:415153.06671:32165.05525:458182.07918:52 | 8,060 | 623 | 884 | 226,568 | 388,549 | 282,080 | 350,601 | 507,047 | 346,685 | 2 | 433,140 | 282,783 | 263,639 | 318,859 | 309,281 | 265,574 | 326,698 | 310,226 | 493,607 | 582,405 | 230 | 1,033 | 343,879 | 8,427 | 382,659 | 0.38975 | 1.6987417454 | 0.0247046305 |
| 2,218 | 1.391 | 220.11549 | d‐(+)‐pantothenic acid | [M + H]+ | True | 220.11795 | C9H17NO5 | Secondary alcohols | GHOKWGTUZJEAQD‐ZETCQYMHSA‐N | CC(C)(CO)[C@@H](O)C(O)=NCCC(O)=O | 220.11549:34997 221.11884:3891 222.1222:1196 | 56.01493:42 57.07793:63 59.06138:42 67.06334:83 69.08517:42 70.03354:63 72.04784:63 77.04002:42 79.05535:42 83.05476:42 85.06741:63 87.08044:42 90.05307:85 90.05977:337 94.07184:42 95.05688:83 98.01903:42 98.02183:83115.06196:42118.09472:63122.10828:21124.07578:146129.08815:42131.08987:42142.08527:104145.0995:42156.11259:42177.12654:104184.09784:125202.10573:104202.14784:21205.12944:63205.1638:167205.17592:104220.11571:125220.14291:42 | 210,166 | 167,154 | 220,827 | 109,474 | 147,563 | 108,886 | 56,756 | 58,130 | 89,778 | 218,550 | 133,007 | 121,659 | 44,553 | 87,255 | 76,023 | 81,978 | 30,429 | 92,765 | 114,592 | 121,395 | 95,250 | 291,906 | 285,777 | 247,110 | 218,772 | 0.12778 | 0.6513850084 | 0.0240294256 |
| 2,298 | 4.286 | 227.11293 | l‐Carnosine | [M + H]+ | True | 227.11386 | C9H14N4O3 | Hybrid peptides | CQOVPNPJLQNMDC‐ZETCQYMHSA‐N | NCCC(O)=N[C@@H](CC1=CN=CN1)C(O)=O | 227.1124:74781 228.11575:11134 229.11911:1465 | 55.04132:42 68.05227:42 82.05465:63 83.06734:215 83.08278:64 84.96175:42 93.0494:146 93.84448:43 95.0634:235109.72236:47110.07436:1878 110.10692:69110.16467:57119.07593:63122.07659:361122.0875:147136.08853:63141.10925:21146.08275:63151.03488:21152.08218:83155.33369:43156.07483:712161.68329:21164.07979:230172.06091:21180.08009:63181.10577:125192.07181:42210.0862:188210.1046:104224.81111:21227.10995:104227.12697:83 | 18,611 | 8,659 | 3,375 | 691,174 | 7,229 | 672,318 | 676,762 | 765,788 | 534,700 | 11,967 | 994,556 | 706,764 | 307,627 | 838,688 | 664,714 | 681,011 | 67,650 | 941,312 | 717,540 | 803,866 | 459 | 802 | 451,869 | 13,045 | 501,460 | 0.88068 | 1.9830595505 | 0.0156686685 |
| 2,657 | 1.416 | 245.07281 | Uridine | [M + H]+ | True | 245.07681 | C9H12N2O6 | Pyrimidine nucleosides | DRTQHJPVMGBUCF‐UHFFFAOYNA‐N | O=C1N=C(O)C=CN1C2OC(co)C(O)C2O | 245.07242:3838 246.07577:452247.07913:0 | 70.02979:42 71.02264:21 96.01913:63 97.0379:42113.02923:65113.03673:537113.06073:42245.22159:63 | 69,991 | 42,498 | 24,696 | 39,146 | 37,751 | 48,829 | 31,960 | 31,420 | 14,001 | 31,382 | 9,996 | 37,357 | 14,007 | 17,556 | 2,538 | 28,768 | 2,617 | 11,325 | 1824 | 1,048 | 30,913 | 36,493 | 47,935 | 42,544 | 40,265 | 0.064638 | 0.3417941529 | 0.0004340064 |
| 2,948 | 6.998 | 261.03534 | d‐Mannose‐6‐phosphate | [M + H]+ | True | 261.03699 | C6H13O9P | Hexose phosphates | NBSCHQHZLSJFNQ‐QTVWNMPRSA‐N | OC1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H](O)[C@@H]1O | 261.03534:50962 262.03869:4518 263.04205:1061 | 53.04903:63 57.04477:63 63.03564:21 67.02851:21 69.03809:106 71.04882:42 80.98725:85 81.03551:281 85.03593:230 93.06441:21 97.04065:43 98.98625:426 99.02415:71 99.04521:149100.90723:42103.3968:44109.03044:725109.09969:23118.94981:21127.04094:383127.05207:127145.04797:63160.98993:21207.00189:42225.00177:42225.01447:104243.02344:146 | 62,030 | 31,393 | 42,363 | 123,232 | 220,245 | 70,522 | 88,688 | 424,738 | 442,372 | 825,961 | 540,513 | 331,605 | 354,822 | 396,896 | 424,848 | 373,235 | 413,106 | 497,578 | 948,371 | 689,780 | 426 | 6,442 | 11,014 | 48,095 | 52,117 | 0.69733 | 2.1319580501 | 0.0090084967 |
| 3,133 | 2.362 | 269.08701 | Inosine | [M + H]+ | True | 269.08804 | C10H12N4O5 | Purine nucleosides | UGQMRVRMYYASKQ‐KQYNXXCUSA‐N | OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2O | 269.08591:112501 270.08926:17870 271.09262:3310 | 55.02217:63 55.03473:42 57.04338:83 57.06256:63 67.03049:42 69.03893:63 71.02114:63 73.03501:83 82.05434:83 85.02518:42 85.03168:63 85.04599:63 92.02988:42 94.03973:213 95.21723:21 97.02918:42 99.83833:42103.04815:43110.03708:674115.04003:85118.6619:43119.03718:769120.0197:104133.04759:83136.60722:209137.04619:23885 137.17505:1682 137.26263:667137.3519:250137.39655:42137.48917:42137.55205:167137.64471:125137.81856:125138.30925:63138.40051:42140.32079:42215.12384:42219.2112:21 | 1,744,192 | 2,301,859 | 1,189,128 | 1,606,597 | 1,083,286 | 1,455,366 | 1,113,897 | 1,444,050 | 899,913 | 1,494,445 | 1,074,040 | 1,458,989 | 656,759 | 1,296,131 | 533,352 | 1,233,329 | 494,316 | 966,957 | 696,056 | 1,027,346 | 1,380,913 | 1,675,873 | 1,554,619 | 1,649,054 | 1,345,126 | 1.2935 | 0.6584421129 | 0.0042157925 |
| 4,947 | 6.973 | 364.06473 | Guanosine 5′‐monophosphate | [M + H]+ | True | 364.06528 | C10H14N5O8P | Purine ribonucleoside monophosphates | RQFCJASXJCIDSX‐UUOKFMHZSA‐N | O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)N1C=NC2=C1NC(=N)N=C2O | 364.05899:1155 365.06234:434366.0657:87 | 110.05524:10135.02759:21149.02536:21152.05627:125152.08411:63169.94139:10264.26987:10 | 683 | 2,643 | 263 | 6,031 | 1,126 | 1,516 | 323 | 5,970 | 5,029 | 4,968 | 1877 | 7,575 | 1,051 | 9,004 | 11,751 | 1790 | 17,554 | 11,665 | 7,104 | 8,026 | 49 | 129 | 273 | 28 | 443 | #N/A | 2.710738302 | 0.007436557 |
| 55 | 1.398 | 115.00401 | Maleic acid | [M − H]− | True | 115.00368 | C4H4O4 | Dicarboxylic acids and derivatives | VZCYOOQTPOCHFL‐UHFFFAOYSA‐N | O=C(O)C=cc(=O)O | 115.00503:9050 116.00838:577117.01174:576 | 71.05444:21 71.05563:21 | 55,633 | 77,348 | 48,858 | 62,670 | 32,151 | 49,493 | 32,421 | 69,355 | 21,203 | 39,650 | 26,928 | 50,276 | 11,637 | 51,514 | 32,987 | 25,449 | 20,385 | 39,037 | 39,291 | 29,654 | 45,173 | 36,089 | 41,432 | 43,091 | 38,137 | 0.042704 | 0.6693331587 | 0.0155992248 |
| 217 | 3.937 | 154.06157 | His | [M − H]− | True | 154.06219 | C6H9N3O2 | Histidine and derivatives | HNDVDQJCIGZPNO‐YFKPBYRVSA‐N | N[C@@H](CC1=CN=CN1)C(O)=O | 154.06317:5394 155.06652:675156.06988:630 | 80.04959:42 91.03069:21 93.0517:83137.04097:42154.07217:63 | 2,130 | 584 | 2,731 | 2,805 | 1,596 | 25,392 | 32,088 | 2,772 | 46,927 | 1924 | 37,518 | 31,430 | 44,829 | 28,002 | 50,461 | 38,582 | 49,224 | 31,520 | 50,570 | 2,223 | 1,664 | 3,377 | 1898 | 4,881 | 3,229 | 0.064842 | 3.063153116 | 0.0012762611 |
| 263 | 3.67 | 164.07458 | l‐(−)‐Phenylalanine | [M − H]− | True | 164.0717 | C9H11NO2 | Phenylalanine and derivatives | COLNVLDHVKWLRT‐QMMMGPOBSA‐N | N[C@@H](CC1=CC=CC=C1)C(O)=O | 164.07387:12947 165.07722:2343 166.08058:1340 | 72.01447:42 91.05745:21103.06355:42134.04286:21147.05013:104164.08469:63 | 59,723 | 1,231 | 4,593 | 37,941 | 77,746 | 57,819 | 75,580 | 88,057 | 81,639 | 137 | 100,927 | 61,536 | 6,396 | 65,599 | 92,867 | 78,271 | 85,666 | 57,850 | 103,083 | 116,152 | 59,012 | 58,157 | 61,426 | 63,599 | 64,054 | 0.075006 | 1.5859668171 | 0.0361989234 |
| 301 | 6.907 | 171.00775 | Glycerophosphate(2) | [M − H]− | True | 171.00639 | C3H9O6P | Glycerophosphates | AWUCVROLDVIAJX‐GSVOUGTGSA‐N | OC[C@@H](O)COP(O)(O)=O | 171.01015:13672 172.0135:624173.01686:286 | 77.99744:43 78.96368:579 81.30538:21 | 193,915 | 455,002 | 195,998 | 313,002 | 258,938 | 165,165 | 293,164 | 184,300 | 291,288 | 304,236 | 128,549 | 153,126 | 10,999 | 162,820 | 9,070 | 9,053 | 9,921 | 189,087 | 256,611 | 127,301 | 125,714 | 155,695 | 163,806 | 208,021 | 204,086 | 0.42234 | 0.3979411738 | 0.0003830492 |
| 312 | 1.694 | 173.00899 | cis‐Aconitate | [M − H]− | True | 173.00916 | C6H6O6 | Tricarboxylic acids and derivatives | GTZCVFVGUGFEME‐IWQZZHSRSA‐N | OC(=O)C\C(=C\C(O)=O)C(O)=O | 173.01173:4774 174.01508:988175.01844:188 | 85.03636:63 | 26,885 | 37,094 | 29,617 | 1902 | 1,439 | 1,126 | 1,095 | 1,447 | 1725 | 52,483 | 1791 | 2,320 | 682 | 1,536 | 1,215 | 1,062 | 1,274 | 1,506 | 2057 | 2,623 | 24,108 | 19,080 | 22,670 | 20,288 | 20,534 | #N/A | 0.1037768146 | 0.0177163028 |
| 317 | 4.026 | 173.10483 | l‐(+)‐Arginine | [M − H]− | True | 173.1044 | C6H14N4O2 | l‐α‐Amino acids | ODKSFYDXXFIFQN‐BYPYZUCNSA‐N | N[C@@H](CCCNC(N)=N)C(O)=O | 173.10464:16345 174.10799:2098 175.11135:74 | 105.03072:21131.0858:378173.11749:42 | 9,483 | 93,266 | 131,938 | 8,595 | 20,461 | 7,508 | 6,163 | 15,823 | 9,093 | 60,177 | 10,077 | 6,734 | 1,693 | 9,333 | 6,493 | 6,422 | 4,270 | 7,258 | 14,847 | 34,069 | 17,284 | 17,708 | 8,559 | 20,185 | 9,741 | 0.069043 | 0.2791559887 | 0.0419059935 |
| 803 | 6.769 | 229.0134 | d‐Ribulose 5‐phosphate | [M − H]− | True | 229.01187 | C5H11O8P | Pentose phosphates | FNZLKVNUWIIPSJ‐UHNVWZDZSA‐N | OCC(=O)[C@H](O)[C@H](O)COP(O)(O)=O | 229.00995:5537 230.0133:849231.01666:282 | 78.95767:63 78.96269:294 91.06715:21 96.97551:125 97.03944:42 | 59,792 | 54,366 | 146,915 | 51,521 | 86,127 | 4,758 | 31,327 | 69,766 | 35,181 | 109,452 | 14,016 | 7,827 | 28,820 | 11,103 | 33,714 | 42,339 | 35,598 | 68,688 | 61,508 | 9,504 | 38,938 | 49,583 | 50,449 | 52,324 | 56,902 | 0.0888 | 0.4823083618 | 0.0170326847 |
| 1718 | 5.74 | 322.0506 | Cytidine‐3′‐monophosphate | [M − H]− | True | 322.04459 | C9H14N3O8P | Ribonucleoside 3′‐phosphates | UOOOPKANIPLQPU‐XVFCMESISA‐N | OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=CC(=N)N=C1O | 322.0498:4108 323.05315:759324.05651:333 | 78.96099:125 96.97363:42211.01308:42322.06519:63 | 33,820 | 128,567 | 23,557 | 160,602 | 21,139 | 14,234 | 34,175 | 26,041 | 13,700 | 33,224 | 10,321 | 34,112 | 10,600 | 21,033 | 19,820 | 12,858 | 16,402 | 12,454 | 13,615 | 9,089 | 29,710 | 32,516 | 39,322 | 35,315 | 39,940 | 0.086863 | 0.3277804927 | 0.0305979778 |
| 1731 | 6.877 | 323.02869 | Uridine 5′‐monophosphate | [M − H]− | True | 323.02859 | C9H13N2O9P | Pyrimidine ribonucleoside monophosphates | DJJCXFVJDGTHFX‐XVFCMESISA‐N | O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)N1C=CC(O)=NC1=O | 323.03195:4429 324.0353:820325.03866:412 | 78.96327:167 80.51807:21 96.97334:83111.02354:42211.00215:63323.03467:63 | 29,233 | 149,656 | 17,963 | 202,163 | 18,277 | 21,042 | 29,042 | 20,533 | 14,499 | 28,719 | 13,747 | 23,658 | 1,557 | 17,368 | 1978 | 2031 | 2,303 | 20,274 | 14,242 | 5,319 | 34,845 | 38,533 | 36,869 | 43,415 | 47,390 | 0.11326 | 0.1929425542 | 0.0284777076 |
| 3,024 | 7.264 | 476.09399 | 8‐Methylthiooctyl glucosinolate | [M − H]− | True | 476.10883 | C16H31NO9S3 | Alkylglucosinolates | CWOJBEDMJKZKAB‐STPBKMPXSA‐N | CSCCCCCCCC\C(S[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)=N/OS(O)(=O)=O | 476.0961:3018 477.09945:479478.10281:695 | 96.98495:42357.10968:42389.07697:104458.12701:21476.10638:167476.12793:188 | 33,371 | 34,322 | 19,035 | 24,637 | 14,148 | 28,477 | 7,809 | 21,548 | 27,341 | 22,910 | 13,362 | 10,208 | 10,621 | 9,090 | 6,317 | 19,145 | 6,381 | 14,649 | 9,622 | 6,535 | 9,484 | 13,047 | 20,190 | 11,853 | 19,247 | 0.033732 | 0.4534713482 | 0.0001821421 |
3.3. Protein expression and pathway analysis
The decrease in arginine levels may be involved in the alteration of key enzymes of the arginine–NO cycle including argininosuccinate synthetase and argininosuccinatelyase, and NO level and arginase I expression. To validate the hypothesis, the Western Blot (WB) assay was performed, and the results showed that the expression of arginase I was elevated in the CFA group compared with the control group, while the proteins of argininosuccinate synthetase and argininosuccinatelyase were not significantly different between the CFA group and the control group (Figure 3a). The NO level was obviously increased in the CFA group compared with the control group (Figure 3b, P < 0.05). In order to screen significantly enriched pathways, the different metabolites were analyzed based on the KEGG and HMDB databases. In Table 2, metabolic pathways with raw P and impact values are listed. In addition, the impact of metabolic pathway is delineated in Figure 3c, and the pathways marked with letters were severely affected by chronic inflammation pain, with the details as follows (A–G): (A) aminoacyl‐tRNA biosynthesis; (B) arginine and proline metabolism; (C) histidine metabolism; (D) purine metabolism; (E) phenylalanine; (F) tyrosine and tryptophan biosynthesis; and (G) glutathione metabolism and phenylalanine metabolism. Moreover, to provide insight into the pathobiological mechanism of chronic inflammation pain, the interaction networks among these seven metabolic pathways were generated and are presented in Figure 3d.
FIGURE 3.
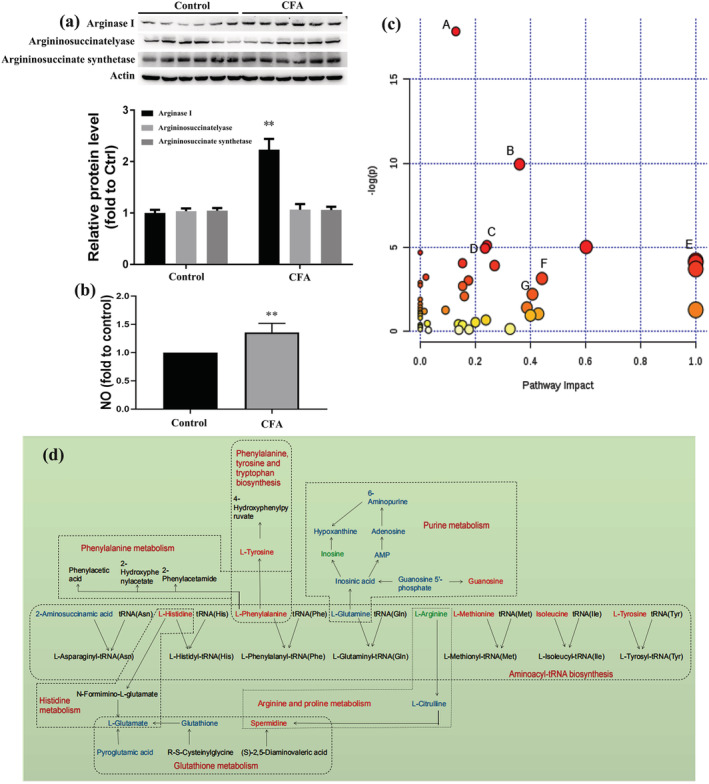
Pathway analysis of the different metabolites: (a) Western Blot (WB) assay for key protein expression; (b) NO level detection; and (c) bubble plot for pathway analysis of the different metabolites. The x‐axis represents the pathway impact and the y‐axis describes the impact value. The circular area is proportional to the number of metabolites assigned to the term and the color accords with the P‐value; (d) the regulatory relationship of metabolic pathways in response to CFA‐induced inflammatory pain. Potential biomarkers are marked as red (up‐regulated), green (down‐regulated) and blue (without significant changes). Other undetected metabolites in the metabolic pathway are labeled in black. The names of related metabolic pathways are marked in red in the corresponding dashed box
TABLE 2.
The potential metabolic pathways
| Total | Expected | Hits | Raw P | #name? | Holm adjust | FDR | Impact | |
|---|---|---|---|---|---|---|---|---|
| Aminoacyl‐tRNA biosynthesis | 69 | 3.6034 | 17 | 1.81 × 10−8 | 17.827 | 1.48E‐06 | 1.48E‐06 | 0.12903 |
| Arginine and proline metabolism | 44 | 2.2978 | 10 | 4.84 × 10−5 | 9.9365 | 0.0039184 | 0.0019834 | 0.36034 |
| Histidine metabolism | 15 | 0.78335 | 4 | 0.0060344 | 5.1103 | 0.48275 | 0.11698 | 0.24194 |
| Alanine, aspartate and glutamate metabolism | 24 | 1.2534 | 5 | 0.0066071 | 5.0196 | 0.52196 | 0.11698 | 0.60232 |
| Purine metabolism | 68 | 3.5512 | 9 | 0.0071327 | 4.9431 | 0.55635 | 0.11698 | 0.23524 |
| Nitrogen metabolism | 9 | 0.47001 | 3 | 0.0091513 | 4.6939 | 0.70465 | 0.12507 | 0 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 4 | 0.20889 | 2 | 0.015078 | 4.1945 | 1 | 0.15742 | 1 |
| Valine, leucine and isoleucine biosynthesis | 11 | 0.57445 | 3 | 0.016665 | 4.0944 | 1 | 0.15742 | 0.99999 |
| Pyrimidine metabolism | 41 | 2.1411 | 6 | 0.017278 | 4.0583 | 1 | 0.15742 | 0.1534 |
| Glycine, serine and threonine metabolism | 31 | 1.6189 | 5 | 0.019841 | 3.92 | 1 | 0.1627 | 0.26989 |
| d‐Glutamine and d‐glutamate metabolism | 5 | 0.26112 | 2 | 0.024285 | 3.7179 | 1 | 0.18103 | 1 |
| Pantothenate and CoA biosynthesis | 15 | 0.78335 | 3 | 0.039543 | 3.2304 | 1 | 0.26972 | 0.02041 |
| Glutathione metabolism | 26 | 1.3578 | 4 | 0.04276 | 3.1522 | 1 | 0.26972 | 0.44179 |
| Cysteine and methionine metabolism | 27 | 1.41 | 4 | 0.048266 | 3.031 | 1 | 0.2827 | 0.17491 |
| β‐Alanine metabolism | 17 | 0.88779 | 3 | 0.054848 | 2.9032 | 1 | 0.29984 | 0 |
| Biosynthesis of unsaturated fatty acids | 42 | 2.1934 | 5 | 0.063642 | 2.7545 | 1 | 0.32333 | 0 |
| Glycerophospholipid metabolism | 30 | 1.5667 | 4 | 0.067032 | 2.7026 | 1 | 0.32333 | 0.15371 |
| Phenylalanine metabolism | 11 | 0.57445 | 2 | 0.10909 | 2.2156 | 1 | 0.49694 | 0.40741 |
| Amino sugar and nucleotide sugar metabolism | 37 | 1.9323 | 4 | 0.12318 | 2.0941 | 1 | 0.53164 | 0.16012 |
| Ubiquinone and other terpenoid–quinone biosynthesis | 3 | 0.15667 | 1 | 0.14873 | 1.9056 | 1 | 0.60979 | 0 |
| Lysine biosynthesis | 4 | 0.20889 | 1 | 0.19328 | 1.6436 | 1 | 0.75471 | 0 |
| Biotin metabolism | 5 | 0.26112 | 1 | 0.23553 | 1.4459 | 1 | 0.86 | 0 |
| Glyoxylate and dicarboxylate metabolism | 18 | 0.94001 | 2 | 0.24122 | 1.4221 | 1 | 0.86 | 0.38709 |
| Cyanoamino acid metabolism | 6 | 0.31334 | 1 | 0.27559 | 1.2888 | 1 | 0.886 | 0 |
| Linoleic acid metabolism | 6 | 0.31334 | 1 | 0.27559 | 1.2888 | 1 | 0.886 | 1 |
| Citrate cycle (TCA cycle) | 20 | 1.0445 | 2 | 0.28093 | 1.2697 | 1 | 0.886 | 0.09164 |
| Sphingolipid metabolism | 21 | 1.0967 | 2 | 0.30076 | 1.2014 | 1 | 0.91341 | 0.01504 |
| Valine, leucine and isoleucine degradation | 38 | 1.9845 | 3 | 0.31867 | 1.1436 | 1 | 0.93324 | 0 |
| Lysine degradation | 23 | 1.2011 | 2 | 0.34013 | 1.0784 | 1 | 0.9556 | 0 |
| Taurine and hypotaurine metabolism | 8 | 0.41778 | 1 | 0.34961 | 1.0509 | 1 | 0.9556 | 0.42857 |
| Ascorbate and aldarate metabolism | 9 | 0.47001 | 1 | 0.38377 | 0.95772 | 1 | 0.9834 | 0 |
| Methane metabolism | 9 | 0.47001 | 1 | 0.38377 | 0.95772 | 1 | 0.9834 | 0.4 |
| Riboflavin metabolism | 11 | 0.57445 | 1 | 0.44686 | 0.80551 | 1 | 1 | 0 |
| Nicotinate and nicotinamide metabolism | 13 | 0.6789 | 1 | 0.50357 | 0.68603 | 1 | 1 | 0.2381 |
| Pentose and glucuronate interconversions | 16 | 0.83557 | 1 | 0.57805 | 0.54809 | 1 | 1 | 0.2 |
| Glycerolipid metabolism | 18 | 0.94001 | 1 | 0.62147 | 0.47567 | 1 | 1 | 0.0256 |
| Starch and sucrose metabolism | 19 | 0.99224 | 1 | 0.64149 | 0.44396 | 1 | 1 | 0.13815 |
| Fatty acid biosynthesis | 43 | 2.2456 | 2 | 0.6688 | 0.40227 | 1 | 1 | 0 |
| Fructose and mannose metabolism | 21 | 1.0967 | 1 | 0.67845 | 0.38794 | 1 | 1 | 0.15342 |
| Butanoate metabolism | 22 | 1.1489 | 1 | 0.6955 | 0.36313 | 1 | 1 | 0 |
| Galactose metabolism | 26 | 1.3578 | 1 | 0.7552 | 0.28077 | 1 | 1 | 0 |
| Fatty acid elongation in mitochondria | 27 | 1.41 | 1 | 0.76823 | 0.26367 | 1 | 1 | 0 |
| Porphyrin and chlorophyll metabolism | 27 | 1.41 | 1 | 0.76823 | 0.26367 | 1 | 1 | 0 |
| Arachidonic acid metabolism | 36 | 1.88 | 1 | 0.85855 | 0.15251 | 1 | 1 | 0.32601 |
| Fatty acid metabolism | 39 | 2.0367 | 1 | 0.88011 | 0.12771 | 1 | 1 | 0 |
| Tryptophan metabolism | 40 | 2.0889 | 1 | 0.88655 | 0.12042 | 1 | 1 | 0.17715 |
| Tyrosine metabolism | 44 | 2.2978 | 1 | 0.90906 | 0.095343 | 1 | 1 | 0.14045 |
| Primary bile acid biosynthesis | 46 | 2.4023 | 1 | 0.9186 | 0.084902 | 1 | 1 | 0.02976 |
4. DISCUSSION
Chronic inflammatory pain is universally regarded as a difficult medical problem worldwide and only partial therapy options are available. Various methods have been employed to investigate the potential mechanisma. However, the complex biochemical processes of chronic inflammatory pain remain poorly understood and little relief has been achieved in spite of the enormous efforts that have been made in basic medical and clinical research. Therefore, illuminating the underlying mechanism may provide novel strategies to alleviate pain with fewer side effects. Recently, systems biology strategies including metabolomics analysis have been widely applied to explore the pathogenic mechanism. In this study, the metabolites of CFA‐induced chronic inflammation pain were analyzed based on a metabolomics method. The analysis showed that 27 metabolites were significantly altered in response to CFA injection and seven metabolic pathways were obviously enriched.
4.1. The association between chronic inflammatory pain and metabolites
Inflammatory pain is a complex symptom involving multiple modulators consisting of neurotransmitters, receptors, ion channels and signaling pathways (Jiao et al., 2020). Previous studies documented that NF‐κB, as a ubiquitously expressed transcription factor, could effectively initiate the inflammatory response to mediate cell proliferation, apoptosis and metastasis (Sethi, Sung, & Aggarwal, 2008). Insulin resistance was enhanced by the NF‐κB pathway to accelerate the progress of inflammatory reactions (Wang, Zhang, Wang, Wang, & Liu, 2019). Inflammatory and oxidative stress were closely correlated with the development of metabolic complications, and NF‐κB signaling may promote the deterioration of nonalcoholic fatty liver disease by inducing the accumulation of triacylglycerol in the liver (Kang et al., 2017; Valenzuela & Videla, 2020). Moreover, emerging evidence has shown that metabolic disturbance may participate in regulating excitable membranes, synaptic transmission and synaptic plasticity. Surveys suggested several metabolites as biological markers that are sensitive to pain pathology induced by CFA injection. Similarly, the differentially expressed metabolites were screened, and the results showed that the expression of 26 metabolites was significantly changed in response to CFA injection. Hence, the potential regulatory network was analyzed, and a hub metabolite was sought out for developing a therapeutic method of chronic inflammation pain.
4.2. The metabolic alterations elicited by chronic inflammatory pain
Several metabolites induced by chronic inflammatory pain were identified, which may be implicated in nervous impulse transmission. To clarify the metabolic process, spinal cord tissues were acquired and the regulatory process of metabolites was analyzed. Generally, arginine is susceptible to the level of guanidine compounds, and thereby results in citrullination (Wang et al., 2019). In addition, a previous study showed that arginine downregulation exacerbated the inflammatory reactions, and thereby resulted in the degradation of amino acids (Schroecksnadel et al., 2006). In this study, our results showed that the level of arginine was significantly decreased in the CFA group compared with the control group, which may directly mediate the inflammatory response and cause inflammatory pain. In addition, related documents revealed that arginine participated in the synthesis of NO neurotransmitter, which could produce anti‐nociceptive natural opioids and N‐methyl‐d‐aspartate receptor‐mediated pain‐promoting effect (Chen et al., 2016; Rondon et al., 2018), whereas neurotransmitter depletion derived from arginine decrease may contribute to inflammatory pain. Moreover, histidine is closely related to the inflammation processes by regulating the synthesis of histamine neurotransmitters (Shell et al., 2016). The metabolomics data showed that histidine expression was enhanced following CFA injection and ultimately led to chronic inflammation pain.
4.3. Phenylalanine and tyrosine metabolism
Tyrosine is an essential amino acid, which is partially synthetized from phenylalanine. The accumulation of phenylpyruvate is toxic to the central nervous system (Rausell et al., 2019). Previous research found that the levels of phenylalanine and tyrosine were remarkably increased in cerebrospinal fluid of patients with regional pain syndrome (Meissner et al., 2014). Dopamine, norepinephrine and epinephrine produced by the phenylalanine and tyrosine metabolic reactions play a critical role in the brain. Norepinephrine released from the sympathetic nerves can activate β2ARs receptors, and result in production and secretion of the proinflammatory cytokine, subsequently causing hyperalgesia of sensory neurons and increasing chronic inflammatory pain (Li et al., 2013). Interestingly, our findings indicated that the pronounced increase of phenylalanine and tyrosine may accelerate pain signal transduction by increasing the concentration of neurotransmitters in spinal cord.
Currently, data suggest that metabolic changes are relevant to many diseases, and metabolites obtained from accessible samples such as urine or plasma may serve as potential biomarkers for diagnosis of chronic inflammatory pain in the clinic (Liu et al., 2017a; Liu et al., 2017b). The spinal cord is the primary center of transmission signals. The signals of nociceptive stimuli are transmitted to the posterior horn of the spinal cord by fine fibers, and eventually pass to the cerebral cortex after processing in the spinal cord (Descalzi et al., 2015; Meacham, Shepherd, Mohapatra, & Haroutounian, 2017).
Therefore, investigating the alteration of metabolites in spinal cord may help to illuminate the neuronal communication mechanism regarding CFA injection‐induced chronic inflammation pain. Collectively, this study provides a new perspective for comprehending the pathological process of CFA‐induced chronic inflammation pain, and enhancing efforts to develop new therapeutic strategies.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (nos 81371214 and 81671063), the Natural Science Foundation of Zhejiang Province, China (no. LY16H090008), and the Key Program of the Natural Science Foundation of Zhejiang Province, China (no. LZ19H090003).
Zhang W, Lyu J, Xu J, et al. The related mechanism of complete Freund's adjuvant‐induced chronic inflammation pain based on metabolomics analysis. Biomedical Chromatography. 2021;35:e5020. 10.1002/bmc.5020
REFERENCES
- Bao, Y. , Gao, Y. , Hou, W. , Yang, L. , Kong, X. , Zheng, H. , … Hua, B. (2015). Engagement of signaling pathways of protease‐activated receptor 2 and mu‐opioid receptor in bone cancer pain and morphine tolerance. International Journal of Cancer, 137(6), 1475–1483. 10.1002/ijc.29497 [DOI] [PubMed] [Google Scholar]
- Bao, Y. , Hou, W. , Liu, R. , Gao, Y. , Kong, X. , Yang, L. , … Hua, B. (2014). PAR2‐mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Molecular Pain, 5(10), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan, S. R. , Bach, F. W. , Pogrel, J. W. , Chung, J. M. , & Yaksh, T. L. (1994). Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods, 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Chen, G. , Xie, R. G. , Gao, Y. J. , Xu, Z. Z. , Zhao, L. X. , Bang, S. , … Ji, R. R. (2016). Beta‐arrestin‐2 regulates NMDA receptor function in spinal lamina II neurons and duration of persistent pain. Nature Communications, 7, 12531. 10.1038/ncomms12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer, J. , Lucas, J. , Zelaya, C. , Nahin, R. , Mackey, S. , DeBar, L. , … Helmick, C. (2018). Prevalence of chronic pain and high‐impact chronic pain among adults—United States, 2016. MMWR. Morbidity and Mortality Weekly Report, 67(36), 1001–1006. 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descalzi, G. , Ikegami, D. , Ushijima, T. , Nestler, E. J. , Zachariou, V. , & Narita, M. (2015). Epigenetic mechanisms of chronic pain. Trends in Neurosciences, 38(4), 237–246. 10.1016/j.tins.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup, N. B. (2013). Pain in patients with spinal cord injury. Pain, 154(Suppl 1), S71–S76. 10.1016/j.pain.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Gureje, O. , Von Korff, M. , Simon, G. E. , & Gater, R. (1998). Persistent pain and well‐being: A World Health Organization study in primary care. JAMA, 280(2), 147–151. 10.1001/jama.280.2.147 [DOI] [PubMed] [Google Scholar]
- Henderson, L. A. , & Keay, K. A. (2018). Imaging acute and chronic pain in the human brainstem and spinal cord. The Neuroscientist, 24(1), 84–96. 10.1177/1073858417703911 [DOI] [PubMed] [Google Scholar]
- Hocher, B. , & Adamski, J. (2017). Metabolomics for clinical use and research in chronic kidney disease. Nature Reviews. Nephrology, 13(5), 269–284. 10.1038/nrneph.2017.30 [DOI] [PubMed] [Google Scholar]
- Jha, M. K. , Song, G. J. , Lee, M. G. , Jeoung, N. H. , Go, Y. , Harris, R. A. , … Suk, K. (2015). Metabolic connection of inflammatory pain: Pivotal role of a pyruvate dehydrogenase kinase–pyruvate dehydrogenase–lactic acid axis. The Journal of Neuroscience, 35(42), 14353–14369. 10.1523/JNEUROSCI.1910-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Liu, J. , Wang, T. , Gao, J. R. , Sun, Y. , Huang, C. B. , … Qin, X. J. (2016). Urinary metabolite profiling provides potential differentiation to explore the mechanisms of adjuvant‐induced arthritis in rats. Biomedical Chromatography, 30(9), 1397–1405. 10.1002/bmc.3697 [DOI] [PubMed] [Google Scholar]
- Jiao, Q. , Ren, Y. , Ariston Gabrie, A. N. , Wang, Q. , Wang, Y. , Du, L. , … Wang, Y. S. (2020). Advances of immune checkpoints in colorectal cancer treatment. Biomedicine & Pharmacotherapy, 123, 109745. 10.1016/j.biopha.2019.109745 [DOI] [PubMed] [Google Scholar]
- Kang, H. H. , Kim, I. K. , Lee, H. I. , Joo, H. , Lim, J. U. , Lee, J. , … Moon, H. S. (2017). Chronic intermittent hypoxia induces liver fibrosis in mice with diet‐induced obesity via TLR4/MyD88/MAPK/NF‐κB signaling pathways. Biochemical and Biophysical Research Communications, 490(2), 349–355. 10.1016/j.bbrc.2017.06.047 [DOI] [PubMed] [Google Scholar]
- Kidd, B. L. , Photiou, A. , & Inglis, J. J. (2004). The role of inflammatory mediators on nociception and pain in arthritis. Novartis Foundation Symposium, 260, 122–133.discussion 133–128, 277–129 [PubMed] [Google Scholar]
- Lains, I. , Gantner, M. , Murinello, S. , Lasky‐Su, J. A. , Miller, J. W. , Friedlander, M. , & Husain, D. (2019). Metabolomics in the study of retinal health and disease. Progress in Retinal and Eye Research, 69, 57–79. 10.1016/j.preteyeres.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Li, W. , Shi, X. , Wang, L. , Guo, T. , Wei, T. , Cheng, K. , … Clark, J. D. (2013). Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain, 154(8), 1224–1236. 10.1016/j.pain.2013.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R. , Xu, H. , Zhang, X. , Wang, X. , Yuan, Z. , Sui, Z. , … Li, Q. (2017a). Metabolomics strategy using high resolution mass spectrometry reveals novel biomarkers and pain‐relief effect of traditional Chinese medicine prescription Wu‐Zhu‐Yu decoction acting on headache modelling rats. Molecules, 22(12). 10.3390/molecules22122110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Chen, H. , Lu, J. , Jiang, Y. , Yang, R. , Gao, S. , … Chen, W. (2017b). Urinary metabolomics of complete Freund's adjuvant‐induced hyperalgesia in rats. Biomedical Chromatography, 31(6). 10.1002/bmc.3886 [DOI] [PubMed] [Google Scholar]
- Meacham, K. , Shepherd, A. , Mohapatra, D. P. , & Haroutounian, S. (2017). Neuropathic pain: Central vs. peripheral mechanisms. Current Pain and Headache Reports, 21(6), 28. 10.1007/s11916-017-0629-5 [DOI] [PubMed] [Google Scholar]
- Meissner, A. , van der Plas, A. A. , van Dasselaar, N. T. , Deelder, A. M. , van Hilten, J. J. , & Mayboroda, O. A. (2014). 1H‐NMR metabolic profiling of cerebrospinal fluid in patients with complex regional pain syndrome‐related dystonia. Pain, 155(1), 190–196. 10.1016/j.pain.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Ohman, A. , & Forsgren, L. (2015). NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson's disease and controls. Neuroscience Letters, 594, 36–39. 10.1016/j.neulet.2015.03.051 [DOI] [PubMed] [Google Scholar]
- O'Neill, L. A. , & Hardie, D. G. (2013). Metabolism of inflammation limited by AMPK and pseudo‐starvation. Nature, 493(7432), 346–355. 10.1038/nature11862 [DOI] [PubMed] [Google Scholar]
- Palomer, X. , Salvado, L. , Barroso, E. , & Vazquez‐Carrera, M. (2013). An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. International Journal of Cardiology, 168(4), 3160–3172. 10.1016/j.ijcard.2013.07.150 [DOI] [PubMed] [Google Scholar]
- Pan, Z. , Zhu, L. J. , Li, Y. Q. , Hao, L. Y. , Yin, C. , Yang, J. X. , … Cao, J. L. (2014). Epigenetic modification of spinal miR‐219 expression regulates chronic inflammation pain by targeting CaMKIIgamma. The Journal of Neuroscience, 34(29), 9476–9483. 10.1523/JNEUROSCI.5346-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti, G. J. , Yanes, O. , Shriver, L. P. , Courade, J. P. , Tautenhahn, R. , Manchester, M. , & Siuzdak, G. (2012). Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nature Chemical Biology, 8(3), 232–234. 10.1038/nchembio.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell, D. , Garcia‐Blanco, A. , Correcher, P. , Vitoria, I. , Vento, M. , & Chafer‐Pericas, C. (2019). Newly validated biomarkers of brain damage may shed light into the role of oxidative stress in the pathophysiology of neurocognitive impairment in dietary restricted phenylketonuria patients. Pediatric Research, 85(2), 242–250. 10.1038/s41390-018-0202-x [DOI] [PubMed] [Google Scholar]
- Rezig, L. , Servadio, A. , Torregrossa, L. , Miccoli, P. , Basolo, F. , Shintu, L. , & Caldarelli, S. (2018). Diagnosis of post‐surgical fine‐needle aspiration biopsies of thyroid lesions with indeterminate cytology using HRMAS NMR‐based metabolomics. Metabolomics, 14(10), 141. 10.1007/s11306-018-1437-6 [DOI] [PubMed] [Google Scholar]
- Rondon, L. J. , Farges, M. C. , Davin, N. , Sion, B. , Privat, A. M. , Vasson, M. P. , … Courteix, C. (2018). l‐Arginine supplementation prevents allodynia and hyperalgesia in painful diabetic neuropathic rats by normalizing plasma nitric oxide concentration and increasing plasma agmatine concentration. European Journal of Nutrition, 57(7), 2353–2363. 10.1007/s00394-017-1508-x [DOI] [PubMed] [Google Scholar]
- Schroecksnadel, K. , Winkler, C. , Duftner, C. , Wirleitner, B. , Schirmer, M. , & Fuchs, D. (2006). Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clinical Rheumatology, 25(3), 334–337. 10.1007/s10067-005-0056-6 [DOI] [PubMed] [Google Scholar]
- Sethi, G. , Sung, B. , & Aggarwal, B. B. (2008). Nuclear factor‐kappaB activation: From bench to bedside. Experimental Biology and Medicine (Maywood, N.J.), 233(1), 21–31. [DOI] [PubMed] [Google Scholar]
- Shell, W. E. , Pavlik, S. , Roth, B. , Silver, M. , Breitstein, M. L. , May, L. , & Silver, D. (2016). Reduction in pain and inflammation associated with chronic low back pain with the use of the medical food Theramine. American Journal of Therapeutics, 23, e1353–e1362. 10.1097/MJT.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji, H. , Tetsunaga, T. , Tetsunaga, T. , Nishida, K. , Misawa, H. , & Ozaki, T. (2019). The factors driving self‐efficacy in intractable chronic pain patients: A retrospective study. Journal of Orthopaedic Surgery and Research, 14(1), 473. 10.1186/s13018-019-1535-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela, R. , & Videla, L. A. (2020). Impact of the co‐administration of N‐3 fatty acids and olive oil components in preclinical nonalcoholic fatty liver disease models: A mechanistic view. Nutrients, 12(2). 10.3390/nu12020499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Zhang, J. , Wang, H. , Wang, X. , & Liu, S. (2019). Vitamin D deficiency enhances insulin resistance by promoting inflammation in type 2 diabetes. International Journal of Clinical and Experimental Pathology, 12(5), 1859–1867. [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Bi, C. , Pang, W. , Liu, Y. , Yuan, Y. , Zhao, H. , … Li, Y. (2019). Plasma metabolic profiling analysis of gout party on acute gout arthritis rats based on UHPLC–Q‐TOF/MS combined with multivariate statistical analysis. International Journal of Molecular Sciences, 20(22). 10.3390/ijms20225753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q. J. , Zhao, J. R. , Hao, J. , Li, B. , Huo, Y. , Han, Y. L. , … Guo, C. (2018). Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. Journal of Cachexia, Sarcopenia and Muscle, 9(1), 71–85. 10.1002/jcsm.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Wei, T. T. , Li, Y. , Li, J. , Fan, Y. , Huang, F. Q. , … Qi, L. W. (2018). Functional metabolomics characterizes a key role for N‐acetylneuraminic acid in coronary artery diseases. Circulation, 137(13), 1374–1390. 10.1161/CIRCULATIONAHA.117.031139 [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Hiraoka, Y. , Ogawa, H. , & Tanaka, K. (2018). Region‐specific deletions of the glutamate transporter GLT1 differentially affect nerve injury‐induced neuropathic pain in mice. Glia, 66, 1988–1998. [DOI] [PubMed] [Google Scholar]
- Zhou, P. , Zhou, N. , Shao, L. , Li, J. , Liu, S. , Meng, X. , … Wu, A. (2018). Diagnosis of Clostridium difficile infection using an UPLC–MS based metabolomics method. Metabolomics, 14(8), 102. 10.1007/s11306-018-1397-x [DOI] [PubMed] [Google Scholar]


