Abstract
Background:
Human infection with Strongyloides stercoralis and hookworm parasites is usually under reported due to less sensitive diagnostic methods. Agar plate culture (APC) is the most sensitive technique for parasites having larval stage. However, using APC in routine diagnosis is uncommon. This study aimed to determine the detection rate and sensitivity of APC in comparison with formal ether concentration technique (FECT) and spontaneous tube sedimentation techniques (STSTs) for S. stercoralis and hookworm larvae.
Methods:
Stool samples collected from 844 schoolchildren in Amhara Regional State, northwestern Ethiopia in 2019, transported to nearby health institutions and processed by APC, FECT and STSTs. The prevalence of S. stercoralis and hookworm was computed by descriptive statistics and Chi-square. The diagnostic agreement among the three techniques was evaluated using Kappa value.
Results:
The overall prevalence of S. stercoralis and hookworm infections by combining the three methods was 13.2% (111/844) and 33.8% (277/844), respectively. Using APC alone, the prevalence of S. stercoralis and hookworm were found to be 10.9% (92/844) and 24.5% (207/844), respectively. Agar plate culture was 5.4 and 2.7 times respectively more sensitive than FECT and STST, with slight and fair agreement in the detection of S. stercoralis. Hookworm diagnostic agreement was moderate between APC and FECT, and APC and STST. The Kappa value between STST and FECT diagnostic methods was substantial.
Conclusion:
APC has a better detection rate of S stercoralis and hookworm larvae. Therefore, APC can be used as an alternative routine diagnostic method to S. stercoralis and hookworm co-endemic countries.
Keywords: Strongyloides stercoralis, Hookworm, Agar plate culture, Detection rate
Introduction
Strongyloides stercoralis and hookworm are intestinal parasites that belong to the soil-transmitted helminths (STHs). They are found in soil polluted with feces in the wet and warm climates especially in rural communities where poor sanitation and hygiene are being practiced (1). Globally, an estimated 370 and 500 million people are affected by S. stercoralis and hookworm, respectively (2,3). To date, the exact burden of S. stercoralis is not yet known. However, in the case of hookworm infection disability-adjusted life year (DALYs) has been estimated to vary between 60,000 and 22.1 million DALYs (4).
Strongyloides stercoralis and hookworm are highly neglected intestinal nematodes (5). Human infections occur when larvae living in faecally contaminated soil penetrate the intact skin. The disease outcomes of these parasite infections are manifested by gastrointestinal complications, which lead to malnutrition, unhealthy condition, and low productivity of infected individuals (6).
Among the diagnostic methods of S. stercoralis and hookworm species, FECT is a simple rapid diagnostic test for the detection of S. stercoralis and hookworm from stool samples. However, it is less sensitive to detect the diagnostic stages of these two parasites (7) as compared to APC. The STST has better sensitivity in the detection of S. stercoralis and hookworm larval infections, but it is not yet adopted as a routine diagnostic technique (8). Although APC is also highly sensitive in the detection of S. stercoralis and hookworm larvae (9), it has not been used as a routine diagnostic method in poor countries like Ethiopia where S. stercoralis and hookworm are highly prevalent (10,11). Currently, a novel approach for immunodiagnosis of S. stercoralis infections using rSsFAR with reliable sensitivity and specificity was developed (12).
The above facts indicated a need for having improved diagnostic parasitological techniques with high degree of sensitivity and specificity in geographical locations where S. stercoralis and hookworm infections are co-endemic and where limited access to more sophisticated techniques.
Co-endemic areas of hookworm and S. stercoralis parasites should be known using highly sensitive diagnostic techniques. Moreover, S. stercoralis should be included in the current STHs prevention and control program. This of course ultimately helps to minimize the burden of S. stercoralis and hookworm infections among human populations in co-endemic areas.
Therefore, we aimed to compare and evaluate the detection rate and sensitivity of APC, FECT and STST in co-endemic areas of the two parasites in Amhara Regional State.
Materials and Methods
Study design, area and period
A cross-sectional study was conducted among schoolchildren in Amhara Regional State, northwestern Ethiopia in 2019. Primary schoolchildren aged from 6–14 yr, volunteer to give stool and consent were included in the study. Children who had taken anthelmintic drugs for the last three months before and during the data collection time were excluded.
The sample size was calculated using simple population proportion formula by taking P= 50%, 95% confidence interval (CI), 5% (d=0.05) margin of error, and 2 design effects (13).
By adding 10% for none response (384 x1/10=38+384=422), and 2 designs effect (2x 422=844). Overall, 844 schoolchildren were randomly selected in each class and screened for S. stercoralis and hookworm infections using APC, FECT and STSTs.
Laboratory Data Collection
Approximately, seven-gram of fresh stool sample was collected from each study participant using a stool cup and was transported to the nearby health institution to confirm S. stercoralis and hookworm infection using FECT, STST, and APC tests. Approximately, half a gram, three-gram and three-gram of fresh stool samples were used in FECT, STST, and APC techniques, respectively. Detection and identification of the larval stages of S. stercoralis and hookworm species were done by light microscope. In S. stercoralis larvae, the buccal cavity is short and the tail is short and notched. However, in hookworm species, the buccal cavity is long and the tail region is long and pointed (14). Those schoolchildren who were found to be positive for either S. stercoralis or hookworm using any one of the above diagnostic tests were considered as positive.
In the FECT, about half a gram stool was processed based on a modified Ritchie’s method (15) and two and half milliliters of 10% formalin and one milliliter of ethyl acetate was added in the collection tube, mixed with approximately half a gram of stool and let stood for a minute. The cover of the sample collection tube was discarded and the filtration concentration unit with conical tube was introduced. Both pieces of the device were screwed until well closed. The sample was mixed up carefully, turned over and centrifuged at 1000-rpm, for three minutes. The supernatant was removed and a small amount of the sediment was put on a glass slide, well mixed, covered with cover slide and looked for the presence of ova of hookworm and rhabditiform larvae of S. stercoralis using a microscope first with 10x and them with 40x (15).
In the STST, approximately three-gram of unrefrigerated stool sample was weighed and homogenized in 10 ml of normal saline solution (0.85%). The mixture was filtered through surgical gauze into a 50 ml plastic tube and then the tube was filled with more normal saline solution, plugged, and shaken vigorously. The tube was left to stand for 45 minutes. The supernatant was discarded and a sample was taken from the bottom and put on a microscope slide. The slide was observed using a microscope to check the presence of ova of hookworm and rhabditiform larvae of S. stercoralis (8).
In the APC, about three-gram of stool was placed on the center of a nutrient agar-plate in a 100 × 15 mm petri dish. The petri dish was sealed with adhesive tape and incubated at 26 °C for 48 hours. The surface of the agar-plate was analyzed daily with dissection microscope or visually with naked eye for the presence of furrows/tracks of moving larvae (16). The adhesive tape was removed and then after five milliliters of a 10% formalin solution was added to the agar surface. Five minutes later, the formalin suspension was transferred from the agar plate to a conical test tube and centrifuged at 1500 rpm for 5 minutes. Finally, the sediment was looked on a microscope with 10x and then with 40x for classification of the S. stercoralis and hookworm larvae by identifying the buccal cavity and the tail region (17).
Comparison of the detection rate of the diagnostic methods
The detection rate of FECT, STST and APC for S. stercoralis and hookworm was checked. The diagnostic agreements of methods were evaluated by Kappa value, number of observed agreement, number of agreements expected by chance and standard error of Kappa. Kappa results were interpreted as follows: values ≤ 0 as no agreement; 0.01–0.20 as none to slight; 0.21–0.40 as fair; 0.41– 0.60 as moderate; 0.61–0.80 as substantial and 0.81–1.00 as almost perfect agreement (18).
Data quality assurance
Training of the laboratory staffs on sample collection and diagnosis as well as an explanation about the study were given before stool sample collection. Proper labeling of the stool cup with serial numbers was done. The amount of stool sample was checked during collection and transported immediately to the nearby health institution laboratory. Each test was performed by following standard operating procedure (SOP). To eliminate observer bias, smeared stool slides were examined independently by two laboratory technologists and the results of their observations were recorded for later comparison on separate sheets. The discordant results were re-checked by the principal investigator. Generally, the quality assurance was checked during pre-analytical, analytical and post-analytical stages.
Data Analyses
Data were analyzed using SPSS (ver. 23, Chicago, IL, USA) statistical software. The overall prevalence of S. stercoralis and hookworm infections were calculated using descriptive statistics and Chi-square at 95% CI. Kappa value, number of observed agreement, number of agreements expected by chance and standard error of Kappa were also computed at 95% CI.
Ethical clearance was obtained from the Ethical Review Committee of Science College, Bahir Dar University. Permission letters were also secured from the Amhara Regional Health Bureau, Amhara Regional Education Bureau, Zonal and Woreda Education Offices. Written informed consent was obtained from the parents/guardians after explaining the purpose and objective of the study. Enrollment in the study was purely on voluntary basis and the study participants’ laboratory results were kept confidential. Study participants who were positive for any intestinal parasite were referred to medical doctors for treatment.
Results
Socio-demographic characteristics of study participants
Overall, 844 primary school children with a mean age of 10.3 yr (range: 6–14 yr) and a standard deviation of 1.77, were included. The majority of the study participants (43.1%) were in the age category of 10–11 years. Most of the participants (88.3%) were rural dwellers and male students accounted for 51.7% (Table 1).
Table 1:
Strongyloides stercoralis and hookworm infections across socio-demographic variables of primary schools students using combined diagnosis results of FECT, STST, and APC
| Variables | Total examined N (%) | S. stercoralis | Hookworm spp. | |||
|---|---|---|---|---|---|---|
| Positive (N,%) | Negative (N,%) | Positive (N,%) | Negative (N,%) | |||
| Age (yr) | 6–9 | 254 (30.1) | 23 (9.1) | 231 (90.9) | 74 (29.1) | 180 (70.9) |
| 10–11 | 364 (43.1) | 53 (14.6) | 311 (85.4) | 118 (32.4) | 246 (67.6) | |
| 12–14 | 226 (26.8) | 35 (15.5) | 191 (84.5) | 88 (38.9) | 138 (61.1) | |
| Sex | M | 436 (51.7) | 63 (14.4) | 373 (85.6) | 154 (35.7) | 282 (64.3) |
| F | 408 (48.3) | 48 (11.8) | 360 (88.2) | 123 (30.1) | 285 (69.9) | |
| Residence | Urban | 99 (11.7) | 12 (12.1) | 87 (87.9) | 32 (32.3) | 67 (67.7) |
| Rural | 745 (88.3) | 99 (13.3) | 646 (86.7) | 245 (32.9) | 500 (67.1) | |
| Total | 844 (100) | 111 (13.2) | 733 (86.8) | 277 (32.8) | 567 (67.2) | |
M=male; F= female
Detection and identification of S. stercoralis and hookworm in agar plate culture media
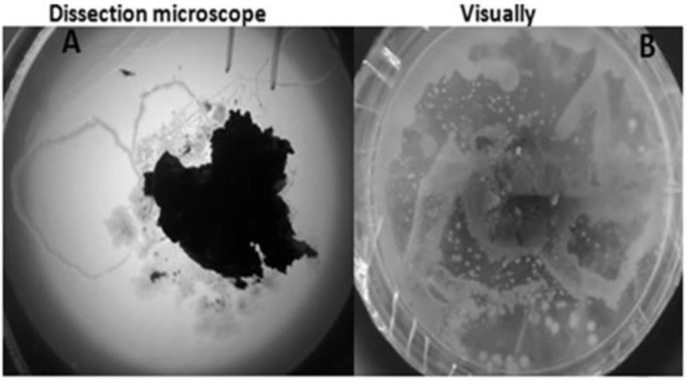
Furrows left on agar plates by larvae and free-living adults of S. stercoralis and larvae of hookworm were seen using dissection microscope or naked eye (Fig. 1). The tracks left by S. stercoralis larvae were characteristically much thinner (Fig. 1A) than those left by hookworm larvae on the agar plate culture (Fig. 1B).
Fig. 1:
The growth and crawling of S. stercoralis and hookworm on agar plate culture media; A= furrow left by S. stercoralis larvae, B= Furrow left by hookworm larvae
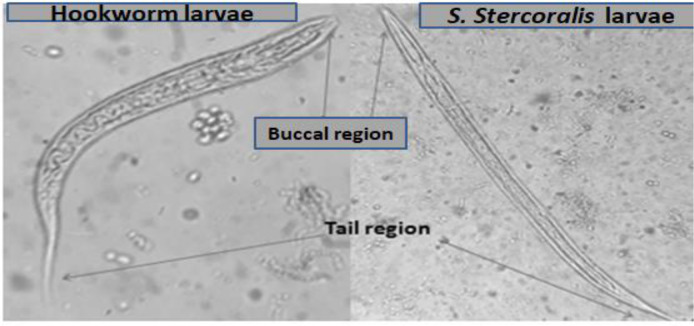
Identification of S. stercoralis and hookworm larvae was done through microscope by considering the buccal cavity and tail regions of the larvae. The buccal cavity is short in S. stercoralis larva whereas in the hookworm larva, it is long. The tail region of S. stercoralis is short and notched whereas the tail of hookworm larva is long and pointed (Fig. 2).
Fig. 2:
The difference between S. stercoralis and hookworm larva
Prevalence of S. stercoralis and hookworm
The overall prevalence rates of S. stercoralis and hookworm infections using a combination of FECT, STST and APC methods were 13.2% (95 % CI:11.0–15.6%) and 32.8% (95 % CI: 29.74–36.06%), respectively (Table 1). The prevalence of co-infection of S. stercoralis and hookworm was 5.1% (43/844). High prevalence rates of S. stercoralis (10.9%) and hookworm (24.5%) were recorded by APC. Agar plate culture was respectively 5.4 and 2.7 times more sensitive than FECT and STST in the detection of S. stercoralis. However, APC was almost equally sensitive as the other two techniques in the detection of hookworms. Similarly, STST was almost equally sensitive to FECT in the detection of both S. stercoralis and hookworms (Table 2). Among the combination of two methods employed for diagnosis, a combination of STST and APC had the highest detection rates for both S. stercoralis (12.7%) and hookworm (32.5%) parasites (Table 2).
Table 2:
The prevalence rates of S. stercoralis and hookworm parasites as diagnosed using FECT, STST, APC techniques individually and their combinations
| Types of methods | Total examined (N) | S. stercoralis | Hookworm spp. | ||
|---|---|---|---|---|---|
| Pos (N) | % at 95%CI | Pos (N) | % at 95%CI | ||
| FECT | 844 | 17 | 2.0 (1.26–3.20) | 181 | 21.5 (18.81–24.35) |
| STST | 844 | 34 | 4.0 (2.90–5.58) | 199 | 23.6 (20.84–26.56) |
| APC | 844 | 92 | 10.9 (8.92–13.18) | 207 | 24.5 (21.75–27.54) |
| FECT+STST | 844 | 42 | 5.0 (3.71–6.66) | 246 | 29.2 (26.18–32.30) |
| FECT+APC | 844 | 98 | 11.6 (9.62–13.95) | 255 | 30.2 (27.21–33.39) |
| STST+APC | 844 | 107 | 12.7 (10.60–15.10) | 274 | 32.5 (29.39–35.69) |
| FECT+STST+APC | 844 | 111 | 13.2 (11.04–15.60) | 277 | 32.8 (29.74–36.06) |
Pos = Positive
Diagnostic agreement of methods
The diagnostic agreement between APC and FECT (kappa value=0.174), and APC and STST (kappa value=0.258) to detect S. stercoralis was slight and fair, respectively. The agreement between STST and FECT in the diagnosis of S. stercoralis was fair (Kappa valve=0.335). The number of observed agreements between APC and FECT and that of APC and STST was comparable in the detection of S. stercoralis. However, the agreement was high between STST and FECT (Table 3).
Table 3:
The diagnostic agreement of FECT, STST, and APC techniques in S. stercoralis detection
| Methods | APC | Kappa value (95%CI | NOA (N,%) | NAEC (N, %) | SEK | χ2, P-value | ||
|---|---|---|---|---|---|---|---|---|
| Pos | Neg | |||||||
| FECT | Pos | 11 | 6 | 0.174 (0.077–0.270) | 757 (89.69) | 738.7 (87.52) | 0.049 | 51.7, 0.000 |
| Neg | 81 | 746 | ||||||
| STST | Pos | 19 | 15 | 0.258 (0.153–0.363) | 756 (89.57) | 725.4 (85.95) | 0.054 | 73.8, 0.000 |
| Neg | 73 | 737 | ||||||
| STST | ||||||||
| FECT | Pos | 9 | 8 | 0.335 (0.165–0.505) | 811 (96.09) | 794.4 (94.12) | 0.087 | 107.4; 0.000 |
| Neg | 25 | 802 | ||||||
NOA=Number of observed agreement, NAEC=Number of agreements expected by chance, SEK=Standard error of Kappa, Pos=Positive, Neg=Negative
The diagnostic agreement between APC and FECT (kappa value=0.592), and that of APC and STST (kappa value=0.540) to detect hookworm was moderate. The Kappa value between STST and FECT in the diagnosis of hookworm was substantial (kappa value=0.620). The number of observed agreements between APC and FECT, APC and that of STST and STST and FECT to detect hookworm was comparable (Table 4).
Table 4:
The diagnostic agreement of FECT, STST, and APC techniques in hookworm detection
| Methods | APC | Kappa value (95%CI | NOA (N,%) | NAEC (N, %) | SEK | χ2, P-value | ||
|---|---|---|---|---|---|---|---|---|
| Pos | Neg | |||||||
| FECT | Pos | 133 | 48 | 0.592 (0.527–0.657) | 722 (85.55) | 544.8 (64.55) | 0.033 | 298.3, 0.000 |
| Neg | 74 | 589 | ||||||
| STST | Pos | 132 | 67 | 0.540 (0.473–0.606) | 702 (83.18) | 535.6 (63.47) | 0.034 | 245.9, 0.000 |
| Neg | 75 | 570 | ||||||
| FECT | Pos | 134 | 47 | 0.620 (0.556–0.684) | 732 (86.73) | 549.4 (65.09) | 0.033 | 325.5, 0.000 |
| Neg | 65 | 598 | ||||||
NOA=Number of observed agreement, NAEC=Number of agreements expected by chance, SEK=Standard error of Kappa, Pos=Positive, Neg=Negative
Discussion
Strongyloides stercoralis and hookworm are public health important helminths, which are co-endemic in tropics and sub-tropics including Ethiopia (11). The prevalence of S. stercoralis in the current study was 13.2%. This result is in agreement with 12.7% in south-central Côte d’Ivoire (19), but it is lower than 48.6% reported in Cambodia (20). This difference could be due to the difference in the sanitation and shoes wearing habit, working with bare hands, and the variation in the combination of methods used for the diagnosis of S. stercoralis.
On the other hand, in the present study, the prevalence of hookworm (34.9%) is lower than 41.3% in northwest Ethiopia (21), 51.0% in south-central Côte d’Ivoire (19) and 49.0% in Cambodia (20). The low prevalence in the present study might be due to differences in sanitation, shoes wearing habit, working with bare hands local endemicity of the parasite and employment of different laboratory diagnosis.
The detection rate of FECT to S. stercoralis in the current study was low 2.0%. This result is comparable with previous findings from rural Bahir Dar, Ethiopia (3.5%) (11), but it is lower than previously reported (12.3%) in Addis Ababa, Ethiopia (22). The low detection rate of S. stercoralis by FECT might be attributed to several factors. For instance, the amount of stool sample used in the procedure, the sampling technique, filtration of the stool suspension through the gauze that might remove the larvae in the final sediment and also the immune status of the study participants.
In the present study, APC was superior to the FECT in the detection of S. stercoralis. This current result was consistent with previously conducted researches (9, 22–24). This high detection rate by APC might be ascribed to the use of large amount of stool sample (three-gram), the longer 48 h incubation time which might have helped the S. stercoralis larvae to emerge from stool, as well as the absence of debris coming from fungus growing on the APC medium.
Hookworm can be diagnosed by different parasitological diagnostic methods with different sensitivity. In the present study, APC showed the highest detection rate for hookworm larvae. This result is consistent with former study conducted in East Sikkim, India (25). Similarly, in the present study, APC had better sensitivity than FECT in detection of hookworm larvae. This finding is consistent with previous reports (26,27).
In the present study, the detection of hookworm by STST was comparable with APC in a 1:1 ratio, but it was superior in the detection of S. stercoralis (2:1) ratio compared with FECT. This high detection rate of S. stercoralis and hookworm by STST in the present study is supported by the previous study conducted in Peru (8). Although STST has better performance in the detection of S. stercoralis, application of this test in routine diagnostic activity is limited.
Conclusion
From the three techniques, APC is superior to the other two techniques in detection of S. stercoralis and hookworms. The STST is also better in sensitivity than FECT. Therefore, APC can be recommended for epidemiological and routine clinical diagnosis in places where S. stercoralis and hookworm are endemic.
Acknowledgements
We would like to acknowledge Bahir Dar University and Mundo Sano Foundation, Spain that provided financial support to conduct this research and the study participants who provided stool samples to evaluate the diagnostic techniques.
Footnotes
Conflicts of interest
Authors declare that they have no competing interests.
Funding source
Bahir Dar University, Science College and Mundo Sano Foundations, Institute of Health Carlos III, Spain.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, et al. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008; 118(4):1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: A plea for action. PLoS Negl Trop Dis. 2013; 7(5):e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utzinger J, Raso G, Brooker S, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009; 136(13):1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krolewiecki AJ, Lammie P, Jacobson J, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013; 7(5):e2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7(7):e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meurs L, Polderman AM, Vinkeles Melchers NVS, et al. Diagnosing Polyparasitism in a High-Prevalence Setting in Beira, Mozambique: Detection of Intestinal Parasites in Fecal Samples by Microscopy and Real-Time PCR. PLoS Negl Trop Dis. 2017; 11(1):e0005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tello R, Terashima A, Marcos LA, et al. Highly effective and inexpensive parasitological technique for diagnosis of intestinal parasites in developing countries: spontaneous sedimentation technique in tube. Int J Infect Dis. 2012; 16(6):e414–416. [DOI] [PubMed] [Google Scholar]
- 9.Jongwutiwes S, Charoenkorn M, Sitthichareonchai P, et al. Increased sensitivity of routine laboratory detection of Strongyloides stercoralis and hookworm by agar-plate culture. Trans R Soc Trop Med Hyg. 1999; 93(4):398–400. [DOI] [PubMed] [Google Scholar]
- 10.Teklemariam Z, Abate D, Mitiku H, et al. Prevalence of Intestinal Parasitic Infection among HIV Positive Persons Who Are Naive and on Antiretroviral Treatment in Hiwot Fana Specialized University Hospital, Eastern Ethiopia. ISRN AIDS. 2013; 2013:324329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amor A, Rodriguez E, Saugar JM, et al. High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up. Parasit Vectors. 2016; 9(1): 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masoori L, Meamar AR, Bandehpour M, et al. Fatty acid and retinol-binding protein: A novel antigen for immunodiagnosis of human strongyloidiasis. PLoS One. 2019; 14(7): e0218895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel WW. A foundation for analysis in the health sciences. Biostatistics, 1995; pp 591–598. [Google Scholar]
- 14.Inês SJ, Souza JN, Santos RC, et al. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimen. Acta Trop. 2011; 120(3): 206–210. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie L. An ether sedimentation technique for routine stool examinations. Bull U S Army Med Dep. 1948; 8(4):326. [PubMed] [Google Scholar]
- 16.Arakaki T, Iwanaga M, Kinjo F, et al. Efficacy of agar-plate culture in detection of Strongyloides stercoralis infection. J Parasitol.1990; 76(3):425–428. [PubMed] [Google Scholar]
- 17.Blatt JM, Cantos GA. Evaluation of Techniques for the Diagnosis of Strongyloides stercoralis in Human Immunodeficiency Virus (HIV) Positive and HIV Negative Individuals in the City of Itajaí, Brazil. Braz J Infect Dis. 2003; 7(6):402–408. [DOI] [PubMed] [Google Scholar]
- 18.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012; 22(3): 276–282. [PMC free article] [PubMed] [Google Scholar]
- 19.Becker SL, Sieto B, Silué KD, et al. Diagnosis, Clinical Features, and Self-Reported Morbidity of Strongyloides stercoralis and Hookworm Infection in a Co-Endemic Setting. PLoS Negl Trop Dis. 2011; 5(8):e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrer A, Khieu V, Schär F, et al. Strongyloides stercoralis and hookworm co-infection: spatial distribution and determinants in Preah Vihear Province, Cambodia. Parasit Vectors. 2018; 11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tadesse H, Bayeh A, Wondemagegn M, et al. Efficacy of single dose albendazole and praziquantel drugs among helminths infected school children at Rural Bahir Dar, northwest Ethiopia. Trop Doct. 2018; 48(4):270–272. [DOI] [PubMed] [Google Scholar]
- 22.Tamirat H, Beyene P, Tekola E. Evaluation of Parasitological Methods for the Detection of Strongyloides Stercoralis among Individuals in Selected Health Institutions In Addis Ababa, Ethiopia. Ethiop J Health Sci. 2017; 27(5):515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Intapan PM, Maleewong W, Wongsaroj IT, et al. Comparison of the Quantitative Formalin Ethyl Acetate Concentration Technique and Agar Plate Culture for Diagnosis of Human Strongyloidiasis. J Clin Microbiol. 2005; 43(4):1932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh TS, Chanu NO, Dutta S. Comparative evaluation of Harada–Mori and agar plate culture for the identification of hookworm species under limited resources. J Nat Sci Biol Med. 2018; 9:127–31. [Google Scholar]
- 25.Anamnart W, Intapan PM, Maleewong W. Modified formalin-ether concentration technique for diagnosis of human strongyloidiasis. Korean J Parasitol. 2013; 51(6):743–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glinz D, Silué KD, Knopp S, et al. Comparing Diagnostic Accuracy of Kato-Katz, Koga Agar Plate, Ether-Concentration, and FLOTAC for Schistosoma mansoni and Soil-Transmitted Helminths. PLoS Negl Trop Dis. 2010; 4(7):e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buonfrate D, Formenti F, Perandin F, et al. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015; 21(6): 543–552. [DOI] [PubMed] [Google Scholar]