Abstract
Background:
In this study, we assessed the in vitro antischistosomal activity of the active ingredients of Allium sativum (allicin) and Curcuma longa (curcumin) on Schistosoma mansoni.
Methods:
This study was conducted in Faculty of Science, Port said University, Egypt (2018). Adult worms were exposed to a range of concentrations of AL or CU, and worm survival was assessed 24 h post-exposure to calculate the lethal concentration of the compounds. Scanning electron microscopy was used to assess ultrastructural changes in the surface of AL- or CU- treated worms. The genotoxicities of AL and CU on S. mansoni were determined by DNA fragmentation analysis.
Results:
We determined the concentrations of AL and CU required to kill 50% of S. mansoni (LC50 ). The LC50 of AL was 8.66 μL/mL, whereas 100% mortality of S. mansoni was achieved by AL at concentrations of 50 μL/mL. The LC50 of CU was 87.25 μL/mL, with the highest mortality of 91.3% seen after 24 h exposure to 100 μg/mL CU. Ultrastructural studies revealed that exposure to either AL or CU led to mild or severe surface damage to S. mansion, respectively. The degree of damage in the worms was sex-dependent. Interestingly, while CU exposure resulted in DNA fragmentation in S. mansoni worms, we observed no genotoxic effects of AL.
Conclusion:
Both AL and CU exhibit antischistosomal activity; the study provided evidence suggesting that these compounds act through distinct mechanisms. These promising results encourage further investigation into these compounds as potential antischistosomal agents, either alone or as complementary treatments to praziquantel.
Keywords: Schistosoma mansoni, Allicin, Curcumin, Ultrastructural, DNA
Introduction
Schistosomiasis is a disease caused by parasitic worms and is considered a high-priority, neglected tropical disease by WHO (1). It can result in acute and long-term pathological disorders (2,3). There are limited drugs available for the treatment of Schistosoma spp. in humans, and while the current drug of choice is praziquantel (PZQ) (4), PZQ efficacy has been reduced (5–7). Such drugs may be found in natural resources, for example in medicinal plants recommended for the treatment of schistosomiasis (8–11).
Allicin (AL) is the main active ingredient of Allium sativum (commonly known as garlic) and is considered responsible for many of the beneficial effects associated with this plant (12–16). While many in vitro studies have examined the efficacy of garlic extract as an antischistosomiasis drug (17,18), few have studied the effects of AL in isolation (19). Curcumin (CU), the active ingredient of Curcuma longa (commonly known as turmeric) is a phenolic compound and a yellow pigment often used as a spice and food colorant (20). Curcumin has multiple therapeutic properties (21–24). Most of the studies dealt with turmeric crude as antischistosomal agent (25–27). In vitro studies on the antischistosomal activity of curcumin have focused on its genotoxic effects on the adult stages (28,29).
It is important to assess the effects of drugs against S. mansoni in a variety of ways, rather than focusing solely on mortality. We herein examined ultrastructural changes in the worm tegument by scanning electron microscopy; these analyses enable us to better understand the mechanism of action of each drug (30,31). The molecular applications were more recently used in the evaluation of the therapies (29, 32). Furthermore, PZQ is less effective at treating immature developmental stages of S. mansoni than it is at treating adult worms (33). Thus, it is important to find new drugs that are also effective against earlier stages of S. mansoni (34–36).
Little is known of the antischistosomal activity of allicin or curcumin. In this study, we evaluate the in vitro antischistosomal activity of these compounds on different developmental stages of S. mansoni. In response to allicin and curcumin, we assayed worm mortality, ultrastructural surface changes, and genotoxicity in adult worms.
Methods
This study was conducted in the Faculty of Science, Port said University, Egypt in 2018.
Active ingredients
Allicin (AL; C6H10OS2 ) was obtained in liquid form from Sciencemed (Egypt). Curcumin (CU; C21H20O6 ) was obtained as a powder from Sigma Aldrich (St. Louis, MO, USA) and dissolved in phosphate buffer saline (PBS).
Miracidiacidal and cercaricidal activity of AL and CU
Biomphalaria alexandrina snails were maintained at a temperature of 25 ± 2 °C (37). Infected snails were kept in glass jars (up to 10 animals per jar) containing: sand, 100 mL deionized and dechlorinated water, and snail food. Snails were incubated with a range of concentrations of AL or CU to determine the LC50 of these compounds. The calculated LC50 values of these compounds were then used as a stock concentration for ensuing miracidiacidal and cercaricidial assays.
For miracidiacidal assays, 20 freshly hatched miracidia were maintained in each well of a tissue culture plate with 1 mL dechlorinated water. A series of dilutions of the calculated LC50 values of AL and CU were prepared, and a different concentration was added to each well. Each drug concentration was tested in triplicate wells. Untreated control miracidia were incubated in 1 mL dechlorinated water alone. Viability of miracidia was assessed visually by dissecting microscope, and the time of miracidia death was recorded (38).
For cercaricidal assays, 1 mL water containing immediately emitted cercariae were placed in each well of a tissue culture plate. A series of dilutions of the calculated LC50 values of AL and CU were prepared, and a different concentration was added to each well. Each drug concentration was tested in triplicate wells. Untreated control freshly shed cercariae were incubated in 1 mL dechlorinated water alone (39). Viability of cercariae was assessed visually by stereomicroscope. The viability of cercariae was determined by exposing the worms to AL or CU for 5 min and replacing it with fresh water and monitoring them within 60 minutes.
In vitro preparation of worms
Ten male hamsters (Mesocricetus auratus) of similar age and mass (100-140 g) were obtained from the Schistosome Biological Supply Center (Theodor Bilharz Research Institute, Giza, Egypt). Hamsters were incubated with 80-100 S. mansoni cercariae for 1 h using a partial immersion technique, as previously described (40). At 8 wk post-infection, adult worms were recovered from the animals by hepatic portal vein perfusion (41). Adult S. mansoni worms were then incubated at 37 °C in 24-well plates, with 24-30 worms per well. Worms were maintained in 1 mL RPMI-1640 media supplemented with penicillin (100 U/mL), streptomycin (100 mg/mL), 10 % fetal calf serum (Gibco), 2 g/L glucose, 0.39 g/L glutamate, and 20 g/L NaHCo3 (39).
Assessing mortality of adult S. mansoni worms for three successive days
A range of concentrations of either AL or CU was added to each well of a tissue culture plate containing adult S. mansoni worms, maintained as described above. Worm mortality was assessed visually by stereomicroscope for up to 3 days (42,43).
Determination of the LC50 values of AL and CU against adult S. mansoni
Adult worms were incubated in wells of a tissue culture plate with a range of concentrations of either AL or CU. Worms were monitored for 24 h after exposure to a given compound, and mortality assessed visually by stereomicroscope. The LC50 values of AL and CU were determined using SPSS statistical program (ver.20, Chicago, IL, USA).
Scanning electron microscopy of adult S. mansoni
S. mansoni worms were exposed to the determined LC50 of AL or CU for 24 h. Worms were fixed in equal volumes of 4% glutaraldehyde and cacodylate 0.2 M for 2 hours. Worms were then washed in equal volumes of sucrose 0.4M and cacodylate 0.2 M for 2 h and post-fixed in osmium 2% and cacodylate 0.3M for 1 h. Fixing was carried out at 4 °C. Samples were washed with distilled water and gradually dehydrated in increasing concentrations of ethyl alcohol for 5 min each (30%, 50%, 70%, and 90%) and finally, in absolute alcohol for 10 minutes. Samples were then air-dried and mounted on copper stubs using double-sided adhesive tape, coated with gold using an S150A sputter coater (Edwards, UK). Images were captured and were analyzed using a Philips XL30 scanning electron microscope (Philips, Eindhoven, Netherlands) operated at 10-30 kV, at the Electron Microscopy Unit of the Theodor Bilharz Research Institute.
Assessing DNA fragmentation by agarose gel analysis
DNA was extracted from either untreated control S. mansoni adult worms or worms exposed to the determined adult schistosomal LC50 of AL or CU for 24 hours (44). DNA was separated by gel electrophoresis on a 2% agarose gel containing 1% GelRed (1:500). DNA fragmentation was visualized using a BIO-RAD Gel DOC TM XR+.
Results
The molluscicidal LC50 values for AL and CU here were 315 μL/L and 5690 μg/L, respectively. We then assayed the miracidiacidal and cercaricidial activity of AL and CU using a series dilution of the molluscicidal LC50 (Table 1).
Table 1:
Miracidiacidal and cercaricidal activity of AL and CU
| Drug | Dose | Miracidia (N=20) | Cercariae (N=20) | ||
|---|---|---|---|---|---|
| Mortality rate (%) | Death time (min) | Mortality rate (%) | Death time (min) | ||
| AL | LC50 | 100 | 8 | 100 | 5 |
| 1/2th LC50 | 100 | 20 | 100 | 9 | |
| 1/4th LC50 | No effect after 60 min | 100 | 60 | ||
| 1/8th LC50 | No effect after 60 min | No effect after 60 min | |||
| CU | LC50 | 100 | 20 | 100 | 50 |
| 1/2th LC50 | 100 | 40 | 100 | 60 | |
| 1/4th LC50 | No effect after 60 min | No effect after 60 min | |||
N: number of miracidia or cercariae. LC50: lethal concentration 50
At the determined LC50 of AL, we observed 100% killing of both miracidia and cercariae within 8 and 5 min, respectively. No effects on miracidia viability were observed after 1 h incubation with 1/4th LC50. No antischistosomal activity of AL was seen against miracidia or cercariae when applied at a concentration of 1/8th LC50. At the determined LC50 of CU, we observed 100% killing of both miracidia and cercariae within 20 and 50 min, respectively. No effects of CU on either miracidia or cercariae viability were observed after 1 h incubation with 1/4th LC50.
Next assessed the mortality of S. mansoni adults after exposure to a range of concentrations of AL or CU for three successive days (Table 2). Exposure of adult worms to concentrations of 30 μL/mL AL resulted in 100 % mortality after 24 hours. Exposure to concentrations of 100 μg/mL CU resulted in 100 % mortality of S. mansoni adults by day two. Viability assays were used to determine the LC50 of AL and CU following 24 h exposure of adult S. mansoni worms. In this case, the LC50 of AL was found to be 8.66 μL/mL for AL and 87.25 μg/mL for CU (Table 2).
Table 2:
Mortality rate (%) of S. mansoni worms exposed to different concentrations of AL and CU for three consecutive days in vitro
| Group | Mortality rate (%) | Group | Mortality rate (%) | ||||
|---|---|---|---|---|---|---|---|
| AL (μL/mL) | First day | Second day | Third day | CU (μg/mL) | First day | Second day | Third day |
| 5 | 4.33 | 73.33 | 86.67 | 5 | NU | NU | NU |
| 10 | 54.55 | 81.82 | 100 | 10 | 0 | 33.3 | 66.67 |
| 20 | 71.43 | 100 | 100 | 20 | 0 | 37.5 | 75 |
| 30 | 100 | 100 | 100 | 30 | NU | NU | NU |
| 40 | 100 | 100 | 100 | 40 | NU | NU | NU |
| 50 | 100 | 100 | 100 | 50 | 4 | 40 | 80 |
| 60 | NU | NU | NU | 60 | 4.17 | 45.83 | 95.83 |
| 80 | NU | NU | NU | 80 | 12 | 64 | 100 |
| 100 | NU | NU | NU | 100 | 91.3 | 100 | 100 |
| LC50 | 8.66 | LC50 | 87.25 | ||||
NU: not used. LC50: lethal concentration 50
We examined the surface ultrastructure of drug-treated worms using scanning electron microscopy. Here, we observed mild to severe changes in the surface ultrastructure of adult S. mansoni worms exposed to an LC50 of AL or CU in comparison with control worms (Fig. 1A, 1B, 1G, and 1J). The damage was specifically seen in the tegument of S. mansoni worms exposed to AL or CU. Male worms exposed to AL displayed significant atrophy of the tubercles, with loss of spines and intertubular ridges, and swelling between tubercles (Fig. 1C and 1D). Exposure of male worms to CU was associated with severe erosion of the tubercles (Fig. 1E) and the peeling of the tegument (Fig. 1F). While few protuberances were seen in the gynecophoral canal of male worms exposed to AL (Fig. 1H), damage to the gynecophoral canal edge (Fig. 1I) was apparent in males exposed to CU. Furthermore, though female worms exposed to AL showed wrinkling of the tegument and small tegument dimples (Fig. 1K), wrinkling was more pronounced following exposure to CU (Fig. 1L).
Fig. 1:
Scanning electron micrographs of untreated control S. mansoni and S. mansoni exposed to an LC50 of AL or CU. Images are shown of the male tegument (A-F) and gynecophoral canal (G-I), and the female tegument (J-L). tu - tubercles, black arrows - intertubular ridges, sp - spines, white arrow - swelling, gc - gynecophoral canal, black arrow head - protuberances, white arrow head - dimples. Higher magnifications are shown as inset images (7988X, scale bar, 10μm) and (6000X, scale bar 20 μm).
To assess the genotoxic effects of AL and CU on S. mansoni, we used DNA fragmentation analysis on separated genomic DNA (Fig. 2). Lane profile analysis of DNA from either untreated control worms or worms exposed to an LC50 of AL or CU is shown in Fig. 3. DNA fragmented bands were not detected in control worms and those exposed to AL (Fig. 3B and 3C). Here, we observed DNA fragmentation of S. mansoni upon exposure to CU, where separation yielded 4 distinct fragments (Fig. 3D). The analysis of these bands is illustrated in Table 3 in comparison with the reference ladder. The sizes of these bands were 800 bp, 625.8 bp, 454.4 bp, and 284 bp with a relative front of 0.64, 0.68, 0.73, and 0.80, respectively.
Fig. 2:
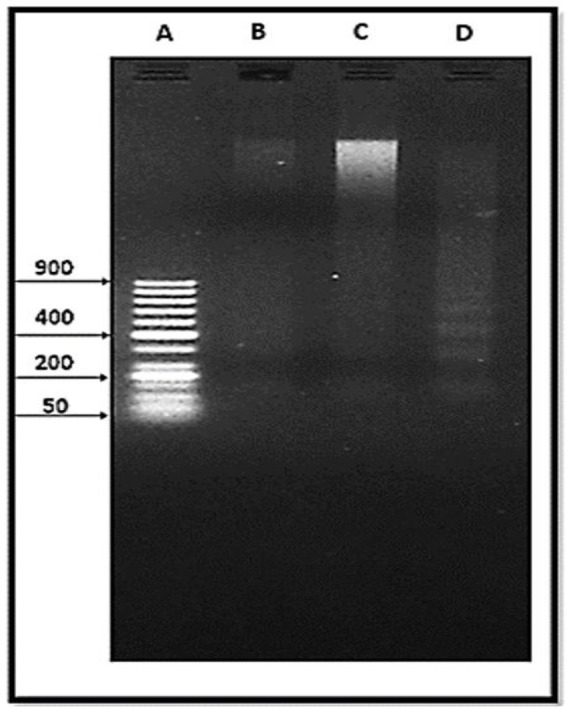
Electrophoretic separated genomic DNA from S. mansoni. 50 bp DNA ladder (A). Untreated control S. mansoni (B). S. mansoni worms exposed to an LC50 of AL (C). S. mansoni worms exposed to an LC50 of CU (D).
Fig. 3:
Lane profile analysis of S. mansoni DNA fragments. Samples were run alongside a 50 bp DNA ladder (A). Untreated control S. mansoni (B). S. mansoni worms exposed to an LC50 of AL (C). S. mansoni worms exposed to an LC50 of CU (D).
Table 3:
Band profile analysis of DNA fragments
| Lane | Band number | Base pair (bp) | Relative front (Rf) | Volume (Int) | Band (%) | Lane (%) |
|---|---|---|---|---|---|---|
| A | 1 | 900 | 0.61 | 55.14 | 6.6 | 5.9 |
| 2 | 800 | 0.64 | 55.68 | 6.7 | 6 | |
| 3 | 700 | 0.66 | 53.92 | 6.5 | 5.8 | |
| 4 | 600 | 0.69 | 46.62 | 5.6 | 5 | |
| 5 | 500 | 0.72 | 49.63 | 6 | 5.3 | |
| 6 | 400 | 0.75 | 153.12 | 18.5 | 16.5 | |
| 7 | 300 | 0.78 | 57.09 | 6.9 | 6.1 | |
| 8 | 250 | 0.83 | 48.93 | 5.9 | 5.3 | |
| 9 | 200 | 0.85 | 134.62 | 16.2 | 14.5 | |
| 10 | 150 | 0.88 | 19.30 | 2.3 | 2.1 | |
| 11 | 100 | 0.91 | 43.04 | 5.2 | 4.6 | |
| 12 | 50 | 0.94 | 112.80 | 13.6 | 12.2 | |
| D | 1 | 800 | 0.64 | 5.31 | 15.5 | 3.5 |
| 2 | 625.8 | 0.68 | 6.59 | 19.2 | 4.3 | |
| 3 | 454.4 | 0.73 | 9.25 | 27 | 6 | |
| 4 | 284 | 0.80 | 13.12 | 38.3 | 8.5 |
A: ladder. D: CU
Discussion
This is one of the first studies to examine the antischistosomal activity of AL and CU on the different developmental stages of S. mansoni (miracidia, cercariae, and adult). In this study, we also assay the antischistosomal effect of AL and CU on adult worms in a variety of ways, by assessing: animal mortality, ultrastructural morphology, and genotoxicity upon exposure.
We determined the molluscicidal LC50 values of AL and CU to be 315 μL/L and 5690 μg/L, respectively. Both the molluscicidal LC50 and 1/2 LC50 of AL and CU were efficient in the elimination of 100% of miracidia and cercariae, while 1/4 LC50 of AL was only effective against cercariae. Together these results indicate that AL exerts a higher biocidal effect against miracidia, cercariae, and adult worms than CU (45). CU showed a marked effect against cercariae in vitro and resulted in ultrastructural damage, which resulted in deficiencies of cercariae ability to infect mice (46).
Continuous exposure of adult S. mansoni worms to either AL or CU for three days revealed higher mortality rates in response to AL than CU. Here, exposure to 30 μL/mL of AL resulted in 100% mortality from the first day, while the highest recorded mortality rate for CU exposure was 91.3% at the higher concentration of 100 μg/mL. The impressive antischistosomal activity of AL is in agreement with previous results obtained using A. sativum extract. A. sativum displayed the highest antischistosomal activity when compared with four other plant extracts (Dryopteris filixmas, Tanacetum vulgare, Juglans nigra, Syzygium aromaticum) (17). In contrast with the present study, exposure of S. mansoni to turmeric extract at a concentration of 50 μM resulted in 100% mortality after 24 h (47). The mortality rates affected consequently on LC50 of both tested drugs, as it was 8.66 μL/mL for AL and 87.25 μg/mL for CU. Of note, the LC50 values of both AL and CU were higher than those of mefloquine and praziquantel, which were 3.96 and 6.67 μg/mL, respectively (48).
The tegument is the primary interface between the parasite and the host environment, thus analyzing this structure upon exposure to a drug allows us to better understand the therapeutic mechanism (49,50). In contrast to observations from our mortality assays, ultrastructural changes in the worms were more apparent upon exposure to an LC50 of CU than to an LC50 of AL. Here, while S. mansoni exposed to AL exhibited moderate surface damage, CU induced severe tegumental deformations in the worms. AL exposure resulted in moderate perturbations to the suckers, though these changes were more pronounced in CU-exposed worms. In agreement with a previous study, we found that male worms exposed to AL displayed atrophied tubercles and loss of spines (19). We also observed erosion and peeling of the tubercles in CU-exposed males. The peeling of the tegument in S. mansoni worms was subjected to polyvalent vaccine (30). Female worms exhibited either slight or moderate wrinkling of the tegument in response to AL or CU, respectively. Interestingly, both compounds showed a less pronounced effect on the tegument of females than males. Male S. mansoni worms are more susceptible to the venom of Cerastes cerastes snake than female worms (31). This may serve to explain why the tegument of male worms showed more damage than those of female worms.
The effects of an LC50 of CU were also observable at the molecular level. Lane profile and band analysis revealed that the genotoxicity of CU resulted in DNA fragmentation of S. mansoni. These findings are similar to previous observations in which DNA fragmentation of S. mansoni was detected in response to in vitro exposure to CU (29). In contrast, S. mansoni showed no evidence of DNA fragmentation upon exposure to an LC50 of AL.
Conclusion
Both AL and CU exhibit mild antischistosomal activity. Our findings encourage further investigation into the use of these compounds as antischistosomal agents, either alone or as complementary treatments to PZQ. In future studies, we hope to elucidate further the links between AL- and CU-induced ultrastructural changes, genotoxicity, and worm mortality in S. mansoni. We also wish to investigate the effects of these compounds on RNA, and better understand their efficacy in vivo.
Acknowledgements
The study was self-funded.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Quansah E, Sarpong E, Karikari TK. Disregard of neurological impairments associated with neglected tropical diseases in Africa. eNeurologicalSci. 2015;3:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deribew K, Tekeste Z, Petros B, et al. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed. 2013;3(4):307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coltart C, Whitty CJ. Schistosomiasis in non-endemic countries. Clin Med (lond). 2015;15(1):67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) . Report of the WHO informal consultation on schistosomiasis control. WHO/CDS/CPC/SIP/99, 2. Geneva: WHO; 1999. [Google Scholar]
- 5.Melman SD, Steinauer ML, Cunningham C, et al. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis. 2009;3(8):e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Dai JR, Li HJ, et al. Is there reduced susceptibility to praziquantel in Schistosoma japonicum? Evidence from Chin. Parasitology. 2010;137(13):1905–12. [DOI] [PubMed] [Google Scholar]
- 7.Crellen T, Walker M, Lamberton PH, et al. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin Infect Dis. 2016;63(9):1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EL Shenawy NS, Soliman MFM, Reyad SI. The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in mice. Rev Inst Med Trop Sao Paulo. 2008;50(1):29–36. [DOI] [PubMed] [Google Scholar]
- 9.Soliman FM. Evaluation of avocado/soybean unsaponifiable alone or concurrently with praziquantel in murine schistosomiasis. Acta Trop. 2012;122(3):261–6. [DOI] [PubMed] [Google Scholar]
- 10.Ali SA, El-Regal NS, Saeed SM. The antischistosomal activity of two active constituents isolated from the leaves of Egyptian medicinal plants. Infect Dis (Auckl). 2015;8:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selem R, Rashed S, Younis M, et al. A novel green approach for treatment of immature Schistosomiasis mansoni infection in mice; Arabic gum (Acacia Senegal) antischistosomal properties. Afr J Pharm Pharmacol. 2018;12(29):436–45. [Google Scholar]
- 12.Abdullah TH, Kandil O, Elkadi A, et al. Garlic revisited: therapeutic for the major diseases of our times. J Natl Med Assoc. 1988;80(4):439–45. [PMC free article] [PubMed] [Google Scholar]
- 13.Patya M, Zahalka MA, Vanichkin A, et al. Allicin stimulates lymphocytes and elicits an antitumor effect: a possible role of p21ras. Int Immunol. 2004;16(2):275–81. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Song Y, Lian J, et al. Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol Lett. 2017;14(1):468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metwally DM, Al-Olayan EM, Alanazi M, et al. Antischistosomal and anti-inflammatory activity of garlic and allicin compared with that of praziquantel in vivo. BMC Complement Altern Med. 2018;18(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunita K, Singh DK. Fascioliasis control: in vivo and in vitro phytotherapy of vector snail to kill Fasciola larva. J Parasitol Res. 2011;2011:240807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metwalley KM. Assessment of the antischistosomal activity of some plant extracts against Schistosoma mansoni infection. World J Med Sci. 2015;12(2):162–9. [Google Scholar]
- 18.Sadrefozalayi S, Aslanipour B, Alan M, et al. Determination and comparison of in vitro radical scavenging activity of both garlic oil and aqueous garlic extracts and their in vivo antioxidant effect on schistosomiasis disease in mice. Tur J Agric Food Sci Technol (TURJAF). 2018;6(7):820–7. [Google Scholar]
- 19.Lima CMBL, Freitas FIS, de Morais LCSL, et al. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev Soc Bras Med Trop. 2011;44(3):327–30. [DOI] [PubMed] [Google Scholar]
- 20.Buescher R, Yang L. Turmeric. In: Lauro GJ, Francis FJ, editors. Natural food colorants. New York: Marcel Dekker; 2000. p. 205–26. [Google Scholar]
- 21.Wang X, Hang Y, Liu J, et al. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol Lett. 2017;13(6):4825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol. 1998;124(3):425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu C-T, Han B, Liu HC, et al. Curcumin protects mice against concanavalin A-induced hepatitis by inhibiting intrahepatic intercellular adhesion molecule-1(ICAM-1) and CXCL10 expression. Mol Cell Biochem. 2011;358(1–2):53–60. [DOI] [PubMed] [Google Scholar]
- 25.Allam G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology. 2009;214(8):712–27. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoud EA, Elbessoumy AA. Hematological and Biochemical Effects of Curcumin in Schistosoma Mansoni Infested Mice. Assiut Vet Med J. 2014;60(142):184–95. [Google Scholar]
- 27.Hussein A, Rashed S, El hayawan I, et al. Evaluation of the anti-schistosomal effects of turmeric (Curcuma longa) versus praziquantel in Schistosoma mansoni infected mice. Iran J Parasitol. 2017;12(4):587–96. [PMC free article] [PubMed] [Google Scholar]
- 28.Morais ER, Oliveira KC, Magalhães LG, et al. Effects of curcumin on the parasite Schistosoma mansoni: A transcriptomic approach. Mol Biochem Parasitol. 2013;187:91–7. [DOI] [PubMed] [Google Scholar]
- 29.De Aguiar DP, Moscardini MBM, Morais ER, et al. Curcumin generates oxidative stress and induces apoptosis in adult Schistosoma mansoni worms. PLoS One. 2016;11(11):e0167135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel-Zaher M, Abed GH, Abdel-Hakeem SS. Ultrastructural changes of Schistosoma mansoni worms associated with the administration of its polyvalent vaccine. JZOS. 2016;3(6):9–20. [Google Scholar]
- 31.Hassan EA, Abdel-Rahman MA, Ibrahim MM, et al. In vitro antischistosomal activity of venom from the Egyptian snake Cerastes cerastes. Rev Soc Bras Med Trop. 2016;49(6):752–7. [DOI] [PubMed] [Google Scholar]
- 32.De Oliveira RN, Ferreira PM, Calado M, et al. Sesquiterpenes effects on DNA of Schistosoma mansoni after in vivo treatment. Gene Rep. 2018;11:205–12. [Google Scholar]
- 33.Sabah AA, Fletcher C, Webbe G, et al. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61(3):294–303. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed AM, Metwally NM, Mahmoud SS. Sativa seeds against Schistosoma mansoni different stages. Mem Inst Oswaldo Cruz. 2005;100(2):205–11. [DOI] [PubMed] [Google Scholar]
- 35.de Moraes J, Nascimento C, Yamaguchi LF, et al. Schistosoma mansoni: In vitro schistosomicidal activity and tegumental alterations induced by piplartine on schistosomula. Exp Parasitol. 2012;132(2):222–7. [DOI] [PubMed] [Google Scholar]
- 36.El-Faham MH, Eissa MM, Igetei JE, et al. Treatment of Schistosoma mansoni with miltefosine in vitro enhances serological recognition of defined worm surface antigens. PLoS Negl Trop Dis. 2017;11(8):e0005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO) . Molluscicide screening and evaluation. Bull World Health Organ. 1965;33(4):567–81. [PMC free article] [PubMed] [Google Scholar]
- 38.Tchounwou PB, Englande AJ, Malek EA, et al. The effects of Bayluscide and Malathion on miracidial survival in schistosomiasis control. J Environ Sci Health B. 1991;26(1):69–82. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie LS, Lopez VA, Cola JM. Prolonged application of an organation against Biomphalaria glabrata and Schistosoma mansoni. In: Tomas C, editor. Molluscicides in Schistosomiasis Control. New York, London: Academic Press; 1974. p. 77–88. [Google Scholar]
- 40.Olivier L, Stirewalt MA. An efficient method for exposure of mice to cercariae of Schistosoma mansoni. J Parasitol. 1952;38(1):19–23. [PubMed] [Google Scholar]
- 41.Smithers SE, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55(4):695–700. [DOI] [PubMed] [Google Scholar]
- 42.Holtfreter MC, Loebermann MCM, Klammt S, et al. Schistosoma mansoni: schistosomicidal effect of mefloquine and primaquine in vitro. Exp Parasitol. 2011;127(1):270–6. [DOI] [PubMed] [Google Scholar]
- 43.El Bardicy S, El Sayed I, Yousif F, et al. Schistosomicidal and molluscicidal activities of amino alkylamino substituted neo- and norneocryptolepine derivatives. Pharm Biol. 2012;50(2):134–40. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring: Harbour Laboratory Press; 2001. [Google Scholar]
- 45.Mantawy MM, Aly HF, Zayed N, et al. Antioxidant and schistosomicidal effect of Allium sativum and Allium cepa against Schistosoma mansoni different stages. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 3:69–80. [PubMed] [Google Scholar]
- 46.Shoheib ZS, El-Nouby KA, Deyab FA, et al. Potential effect of Curcuma longa extract on infectivity and pathogenicity of Schistosoma mansoni cercariae. J Egypt Soc Parasitol. 2008;38(1):141–59. [PubMed] [Google Scholar]
- 47.Magalhães LG, Machado CB, Morais ER, et al. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol Res. 2009;104(5):1197–201. [DOI] [PubMed] [Google Scholar]
- 48.Abou-Shady OM, Mohammed SS, Attia SS, et al. In vitro effect of mefloquine on adult Schistosoma mansoni. Res J Parasitol. 2015;10(3):111–9. [Google Scholar]
- 49.Hoffmann KF, Strand M. Molecular identification of a Schistosoma mansoni tegumental protein with similarity to cytoplasmic dynein light chains. J Biol Chem. 1996;271(42):26117–23. [DOI] [PubMed] [Google Scholar]
- 50.Shuhua X, Binggui S, Utzinger J, et al. Ultrastructural alterations in adult Schistosoma mansoni caused by artemether. Mem Inst Oswaldo Cruz. 2002;97(5):717–24. [DOI] [PubMed] [Google Scholar]




