Abstract
Background:
Due to numerous side effects of common drugs in treatment of leishmaniasis, new therapeutic approaches focus on herbal compounds. Therefore, we aimed to determine the effect of crocin and stigmasterol on in-vitro growth of promastigotes and amastigotes of Leishmania major in the Department of Parasitology, Pasteur Institute, Tehran, Iran in 2018.
Methods:
The effect of different concentrations of crocin and stigmasterol were evaluated by determining their in-vitro inhibitory effects on promastigotes and amastigotes of the L. major using MTT assay.
Results:
The fatality rate was 65.27% and 71.96% for crocin and stigmasterol respectively at 24 h post-culture in concentration of 50 μg/mL. The mean inhibitory effect of crocin and stigmasterol on L major amastigotes after 72 h were 52.22% and 38.96%.
Conclusion:
The crocin and stigmasterol had efficient adverse effects on promastigote and amastigotes of L. major, hence, further studies on the anti-leishmanial effects of these herbal compounds in human and animal models are recommended.
Keywords: Crocin, Tigmasterol, Leishmania major, Promastigote, Amastigote, In vitro
Introduction
Leishmaniasis is a group of protozoan diseases commonly found in both human and animal (Zoonoses) (1). Cutaneous lleishmaniasis (also known as oriental sore, tropical sore, chiclero ulcer, Aleppo boil) is the most common form of leishmaniasis in both dry (urban) and wet (rural) forms worldwide including Iran (2). It has been considered as one of the six major diseases in the World Health Organization’s Tropical Diseases Survey (3), about 19000 cases of reported annually (4, 5).
Unfortunately, despite the high prevalence of cutaneous leishmaniasis in Iran, no efficient prevention and treatment method has been proposed. Therefore, efforts to discover new drugs with the least side effects need to be continued more decisively (6). Systemic therapies are the first line of treatment against leishmaniasis (7). These compounds are toxic to parasites. Long duration of treatment, parasites resistance, high expense, and severe toxicity on the heart, liver and kidney could be referred as the most notable disadvantages of these drugs. Keeping in view these facts, researchers are trying to use plant compounds to treat leishmaniasis (8). Commonly, useful botanical compounds are identified through study of plant extracts. In a study, it was collected the Iranian traditional herbal remedies against leishmaniasis. Many native plants constituents such as Zajuria multiflora Boiss, Lawsonia inermis, Calendula officinalis, Nerium oleander, etc. have been effective in controlling the disease in different studies (9).
Crocin is one of the most consequential alkaloids ingredients in plants such as saffron, and many of its helpful effects on health, such as antioxidant, anti-cancer, learning and memory invigoration effects, have been proven in many studies (10). On the other hand, stigmasterol is also an herbal sterol with beneficial therapeutic functions, including anti-inflammatory, anti-cancer effects, as well as protective influence on the immune system (11).
Considering the importance of using plant compounds, the main objective of the present study was the in-vitro investigation of beneficial effects of the two herbal compounds, crocin and stigmasterol, on L. major standard strain (MHOM/IR/75/ER).
Materials and Methods
Our study has the control group included L. major promastigotes, uninfected macrophage and infected macrophage by L. major without crocin and stigmasterol, and case group including promastigotes of L. major, uninfected macrophages and macrophage infected with L. major treated with different doses of crocin and stigmasterol. This project was performed in the Department of Parasitology, Pasteur Institute, Tehran, Iran in 2018.
Parasite culture
L. major standard strains (MHOM/IR/75/ER) were prepared from Parasitology Department of Pasteur Institute of Iran and cultured in flasks containing RPMI-1640 medium with 7%–10% inactivated FCS.
Preparation of different concentrations of crocin and stigmasterol
About 1 mg of crocin and stigmasterol powders purchased from Sigma Company, dissolved in 200 μl of methanol separately and various concentrations including 1.61, 3.12, 6.24, 12.5, 25, 50 μg / mL were prepared from both compounds dissolving in the RPMI-1640 medium and stored in the 4 °C (12). The concentrations of crocin and stigmasterol were calculated according to Table 1.
Table 1:
Calculation of different concentrations of crocin and stigmometol compounds
| Ccompounds (Concentration (μg/ml)) | ||||||
|---|---|---|---|---|---|---|
| Crocin | ||||||
| Mass (Microgram) | 1.61 | 3.12 | 6.24 | 12.5 | 25 | 50 |
| Concentration (micromolar) | 1.6377 | 3.1936 | 6.3872 | 12.7948 | 25.5896 | 51.179 |
| Volume (Milliliter) | 1 | |||||
| m. weight (g/mol) | 976.96 | |||||
| Stigmasterol | ||||||
| Mass (Microgram) | 1.61 | 3.12 | 6.24 | 12.5 | 25 | 50 |
| Concentration (Micromolar) | 3.877 | 7.754 | 15.1203 | 30.2891 | 60.5782 | 121.1563 |
| Volume (Milliliter) | 1 | |||||
| m. weight (g/mol) | 412.69 | |||||
Promastigotes viability Determination
107 parasites were cultured in 96-well plates in RPMI-1640 medium with 10% bovine serum. Crocin and Stigmasterol, with final concentrations of 1.61, 3.12, 6.24, 12.5, 25, 50 μg/mL were added to the wells in three repetitions (triplicate) (13). The wells were examined 24 h post-culture by MTT method in which 20μl of MTT solution was added into wells and incubated at 37 °C in the dark for 2 to 5 hours. After centrifuging at 2500 rpm, the supernatant was discarded and 20μl of DMSO added and light absorption at 650 nm was examined (14).
Amastigotes viability Determination
To evaluate the EC50 of crocin and stigmasterol on L. major amastigotes, mouse macrophage J774A.1 cells were used. Primarily, the macrophage cells cultured in RPMI-1640 and incubated at 37 °C with CO2 5% for allowing them to adhere and were then exposed to L. major promastigotes. To infect macrophages, 106 promastigotes of the parasite added to each well-containing macrophage and kept at 37 °C with CO2 5%. After 6 h, the supernatant discarded to remove the unshielded macrophages, fresh culture medium were replaced, and solution light absorption was measured by MTT assay.
Statistical Analysis
The experiment was arranged in Completely Randomized Design (CRD) with three replications per treatment. All data were assessed by using SPSS version 20.0. (Chicago, IL, USA). The homogeneity of variance was checked using One-Sample Kolmogorov-Smirnov Test. Differences between treatment means in all experiments were analyzed based on Scheffea test using the probability of five percent.
Results
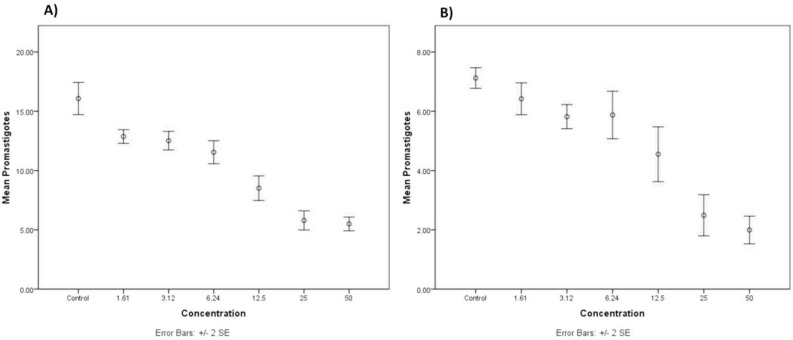
The mean of promastigotes counted in wells containing various concentrations of crocin and stigmasterol (0, 1.61, 3.12, 6.24, 12.5, 25, 50 μg/mL) were 15.77, 12.87, 12.61, 11.54, 8.51, 5.79, 5.49 (Fig. 1.A) and 7.12, 6.41, 5.85, 5.87, 4.55, 2.49, 1.99, respectively (Fig. 1.B). The results of One-Sample Kolmogorov-Smirnov Test clearly confirmed the homogeneity of variance in both of crocin (P=0.79) and stigmasterol (P=0.52) groups. According to the findings, the mean number of promastigotes showed highly significant differences in different concentrations of crocin (P≤0.0001). The mean number of promastigotes counted stigmasterol was also significantly different (P≤0.0001). In different concentrations ranging from 0 to 50 μg/mL).
Fig. 1:
The means and standard errors of promastigotes counted in different concentrations of A) crocin, and B) stigmasterol
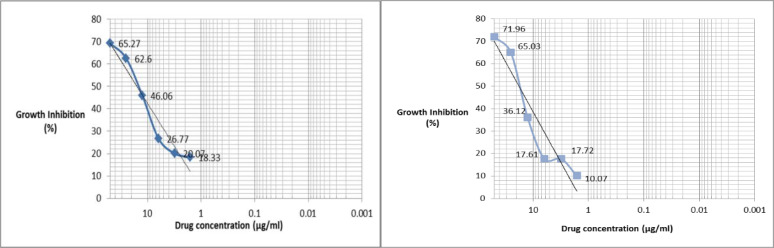
According to Fig. 2, the graphs determined that all examined concentrations of crocin and stigmasterol had a significant effect on the number of parasites compared to control group. In another word, by increasing the concentration, there was a significant decrease in the number of parasites. After 24 h, the concentration of crocin and 19 of stigmasterol causes 50% of the parasite population to be dead. The lowest concentration of stigmasterol showed 10.07% growth inhibitory effect after 24 h and higher concentrations were more efficient so that at 50 and 25 μg/ml, the percentage of fatality was reported to be 71.96% and 65.03%, respectively.
Fig. 2:
The growth inhibitory effect of different concentrations of crocin and stigmasterol on L. major promastigotes Standard strain in RPMI 1640 after 24 h
The light absorption indicated less parasites exist in higher concentrations
As shown in Table 1, the optical density (15) decreased when the concentration of extractions increased from 0 to 50 μg/mL. The OD was 0.95 in 1.61 μg/mL of Crocin; meanwhile, the OD reached the lowest number (0.49) in the highest concentration (50 μg/mL). The same pattern could observe in various concentrations of stigmasterol showing that more the concentrations increased, more the OD decreased (Table 2).
Table 2:
The measured OD of crocin and stigmasterol in various concentrations
| Concentration (μM) | OD* | ||
|---|---|---|---|
| Crocin | Stigmasterol | Crocin | Stigmasterol |
| 0 ** | 0 ** | 1.40 ±0.003 | 9.80 ±0.08 |
| 1.6 | 3.8 | 0.95 ±0.07 | 0.68 ±0.11 |
| 3.1 | 7.7 | 0.81 ±0.01 | 0.63 ±0.08 |
| 6.3 | 15.1 | 0.85 ±0.02 | 0.61 ±0.44 |
| 12.7 | 30.2 | 0.77 ±0.02 | 0.49 ±0.13 |
| 25.5 | 60.5 | 0.71 ±0.04 | 0.32 ±0.02 |
| 51.1 | 121.1 | 0.49 ±0.02 | 0.22 ±0.15 |
Data are presented in Mean ± SD
Control group
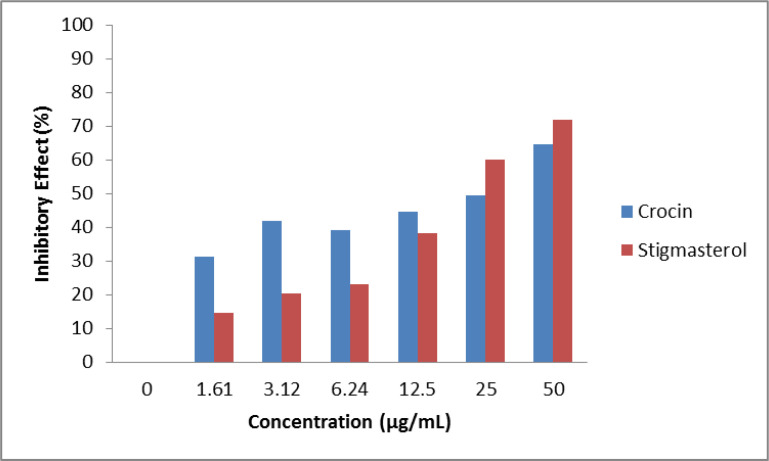
L. major promastigotes exposing to high concentrations has been led to death more in comparison to counterparts exposed to low concentrations in both crocin and stigmasterol extractions (Fig. 2). The crocin had better inhibitory effect in lower concentrations. However, in higher concentrations including 25 μg/mL and 50 μg/mL, the stigmasterol extraction had efficacy compared to crocin (Fig. 3).
Fig. 3:
The crocin and stigmasterol inhibitory effect on L. major promastigote standard strain in different concentrations ranging from 0 to 50 μg/mL
The mean of amastigotes counted in each macrophage decreased after 72 h in control, Crocin-treated and Stigmasterol-treated groups in three treatments. Table 3 shows the mean number of amastigotes counted in 100 macrophages in control, Crocin-treated and Stigmasterol-treated groups in the three treatments (Table 2). The mean inhibitory effect of these compounds on L. major amastigotes has been measured 52.2% and 38.96% for crocin and Stigmasterol, respectively. The results indicate the higher efficiency of crocin anti-leishmaniasis effect compared to stigmasterol (Table 2).
Table 3:
The crocin and stigmasterol EC50 inhibition activity on L. major promastigote standard strain
| Groups | Mean (100 macrophages ×106) | Growth inhibitory (%) | ||
|---|---|---|---|---|
| Crocin | Stigmasterol | Crocin | Stigmasterol | |
| Treated Control 1 | 2.25 | 2.79 | 0 | 0 |
| Crocin/ stigmasterol treated 1 | 1.06 | 1.61 | 52.48 | 42.2 |
| Treated Control 2 | 2.17 | 3.04 | 0 | 0 |
| Crocin/ stigmasterol treated 2 | 0.99 | 1.88 | 57.64 | 38.18 |
| Treated Control 3 | 2.21 | 2.89 | 0 | 0 |
| Crocin/ stigmasterol treated 3 | 1.18 | 1.83 | 46.48 | 36.51 |
Discussion
Leishmaniasis is a group of parasitic diseases caused by the protozoan Leishmania species (16). Annually, between 1.5 and 2 million people suffer from cutaneous leishmaniasis globally, 90% of whom live in 9 countries, including Iran (17). Since the efforts for designing vaccine has failed in clinical trials so far, there is a dire need for new treatments. Herbal bioactive compounds extracted from a variety of plants exhibit anti-leishmanial properties (18).
Traditional medicine, particularly those are based on the use of plants, has been regarded as an ancient root stone to prevent and treat many infections from centuries ago to now (19, 20). Natural products and their derivatives that are originated from plants have potential activities against microbial agents including parasites (21, 22).
Stigmasterol has been shown to stimulate cytokines release by which the differentiation of CD4+ T cells into Th1 cells occurs (23). Macrophages, are known as parasite host cells and presents parasitic antigens to immune cells (24). Stigmasterol can stimulate cellular immunity and activates macrophage cells and natural killers cells (NKs) (25).
In a study, anticoagulant activity and the effects of immune modulation of tannins were investigated. The effects of polyphenol on intracellular leishmaniasis parasites are due to activation of macrophages instead of direct antiparasitic activity (26).
Stigmasterol induce the caspase-3 activation and anti-apoptotic protein Bcl-2 decrement and thus results in apoptosis (27). L. major contributes to its survival in the host body by preventing the release of cytochrome C from mitochondria and as a result, harnesses Caspase-3 activation and apoptosis in infected macrophage (28). Phytosterols such as stigmasterol trigger the activation of Caspase-3 and induce internal pathways apoptosis (mitochondrial) (29). In addition to activating caspase, stigmasterol increases the production and activation of certain enzymes involved in the L. delimitation, such as calmodulin and cellular kinases (30).
The investigation of Inhibitory effect of saffron and its major components, Safranal and Crocin, revealed that Helicobacter pylori was vulnerable to saffron aquatic and alcoholic extracts (31). Safranal and crocin had more antibacterial effects in comparison to alcoholic extracts (15). In this regard, the Iranian native plants had potential therapeutic effects on cutaneous leishmaniasis. They pointed to medicinal plants of different parts in Iran, although the saffron compounds were not named due to expensive, the high prevalence of leishmaniasis in Khorasan Province and the abundance of this product in the city shows the importance of the effective compounds in this Area (32).
Two herbal compounds of saffron, stigmasterol and crocin had concentration-dependent inhibitory effect on the growth of L. major amastigotes and promastigotes in macrophage cells. As expected, we deduced that the higher concentration of stigmasterol and crocin accompany the lower growth rate of the parasite. Therefore, the survival rate of the parasite promastigote and amastigote is also dependent on the concentration of these compounds.
Conclusion
Since stigmasterol and crocin have anti-leishmaniasis influence in-vitro, the in-vivo studies in animal models are recommended in achieving the appropriate medicine combination in the treatment of cutaneous leishmaniasis.
Acknowledgements
This study was financially supported in part by “Clinical Research Development Center of Baqiyatallah Hospital.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Reithinger R, Dujardin J-C, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–96. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz E, Hatz C, Blum J. New world cutaneous leishmaniasis in travellers. Lancet Infect Dis. 2006;6(6):342–9. [DOI] [PubMed] [Google Scholar]
- 3.Alvar J, Vélez InD, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One.2012;7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, Uzonna JE. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol.2012;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehravaran A, Jaafari MR, Jalali SA, et al. The role of ISCOMATRIX bilayer composition to induce a cell mediated immunity and protection against leishmaniasis in BALB/c mice. Iran J Basic Med Sci.2016;19(2):178–86. [PMC free article] [PubMed] [Google Scholar]
- 6.Farahmand M, Nahrevanian H, Shirazi HA, et al. An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran. Braz J Infect Dis.2011;15(1):17–21. [PubMed] [Google Scholar]
- 7.Pourmohammadi B, Motazedian M, Handjani F, et al. Glucantime efficacy in the treatment of zoonotic cutaneous leishmaniasis. Southeast Asian J Trop Med Public Health.2011;42(3):502–8. [PubMed] [Google Scholar]
- 8.Sadeghian G, Ziaei H, Bidabadi LS, et al. Decreased effect of glucantime in cutaneous leishmaniasis complicated with secondary bacterial infection. Indian J Dermatol.2011;56(1):37–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahmani M, Saki K, Ezatpour B, et al. Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis. Asian Pac J Trop Biomed. 2015;5(9):695–701. [Google Scholar]
- 10.Rezaee R, Mahmoudi M, Abnous K, et al. Cytotoxic effects of crocin on MOLT-4 human leukemia cells. J Complement Integr Med. 2013;10(1). doi: 10.1515/jcim-2013-0011. [DOI] [PubMed] [Google Scholar]
- 11.Kaur N, Chaudhary J, Jain A, et al. Stigmasterol: a comprehensive review. Int J Pharm Sci Res.2011;2(9):2259–2265. [Google Scholar]
- 12.Ghaffarifar F. Leishmania major: in vitro and in vivo anti-leishmanial effect of cantharidin. Exp Parasitol.2010;126(2):126–9. [DOI] [PubMed] [Google Scholar]
- 13.Khademvatan S, Gharavi MJ, Rahim F, et al. Miltefosine-induced apoptotic cell death on Leishmania major and L. tropica strains. Korean J Parasitol. 2011;49(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroufi Y, Ghaffarifar F, Dalimi A, et al. A study on the cytotoxic effect of cantharidin on Leishmania major promastigote and amastigote survival in vitro. KAUMS Journal(FEYZ). 2012;16(5):406–413. [Google Scholar]
- 15.Nakhaei M, Khaje-Karamoddin M, Ramezani M. Inhibition of Helicobacter pylori growth in vitro by saffron (Crocus sativus L.). Iran J Basic Med Sci. 2008;11:91–6. [Google Scholar]
- 16.Kaye P, Scott P. Leishmaniasis: complexity at the host–pathogen interface. Nat Rev Microbiol.2011;9(8):604–15. [DOI] [PubMed] [Google Scholar]
- 17.Monge-Maillo Ba, López-Vélez R. Therapeutic options for old world cutaneous leishmaniasis and new world cutaneous and mucocutaneous leishmaniasis. Drugs.2013;73(17):1889–920. [DOI] [PubMed] [Google Scholar]
- 18.De Monte C, Bizzarri B, Gidaro MC, et al. Bioactive compounds of Crocus sativus L. and their semi-synthetic derivatives as promising anti-Helicobacter pylori, anti-malarial and anti-leishmanial agents. J Enzyme Inhib Med Chem. 2015;30(6):1027–33. [DOI] [PubMed] [Google Scholar]
- 19.Khomarlou N, Aberoomand-Azar P, Lashgari AP, et al. Essential oil composition and in vitro antibacterial activity of Chenopodium album subsp. striatum. Acta Biol Hung. 2018; 69(2): 144–55. [DOI] [PubMed] [Google Scholar]
- 20.Mirzaie A, Halaji M, Dehkordi FS, et al. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complement Ther Clin Pract. 2020;40:101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonaidi Jafari N, Kargozari M, Ranjbar R, et al. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. Journal of Food Processing and Preservation. 2018; 42(1): e13336. [Google Scholar]
- 22.Aminnezhad S, Kermanshahi RK, Ranjbar R. Evaluation of synergistic interactions between cell-free supernatant of Lactobacillus strains and Amikacin and genetamicin against Pseudomonas aeruginosa. Jundishapur J Microbiol. 2015;8(4):e16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nylén S, Gautam S. Immunological perspectives of leishmaniasis. J Glob Infect Dis.2010;2(2):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C-H, Cheng J-Y, Deng M-C, et al. Prebiotic effect of diosgenin, an immunoactive steroidal sapogenin of the Chinese yam. Food Chem.2012; 132(1):428–32. [DOI] [PubMed] [Google Scholar]
- 25.Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci.2015;72(23):4445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolodziej H, Kiderlen AF. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry. 2005;66(17):2056–71. [DOI] [PubMed] [Google Scholar]
- 27.Kabeer FA, Sreedevi GB, Nair MS, et al. Isodeoxyelephantopin from Elephantopus scaber (Didancao) induces cell cycle arrest and caspase-3-mediated apoptosis in breast carcinoma T47D cells and lung carcinoma A549 cells. Chin Med.2014;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looi CY. Pharmacological Activities and Chemical Constituents of. J Med Sci.13(4):236–43. [Google Scholar]
- 29.Sirisha N, Sreenivasulu M, Sangeeta K, et al. Antioxidant properties of Ficus species–a review. Int J Pharmtech Res. 2010;2. [Google Scholar]
- 30.Brasili E, Praticò G, Marini F, et al. A non-targeted metabolomics approach to evaluate the effects of biomass growth and chitosan elicitation on primary and secondary metabolism of Hypericum perforatum in vitro roots. Metabolomics.2014;10:1186–1196. [Google Scholar]
- 31.Rosenthal E, Marty P. Recent understanding in the treatment of visceral leishmaniasis. J. Postgrad Med. 2003;49(1):61–8. [DOI] [PubMed] [Google Scholar]
- 32.Moghaddas E, Khamesipour A, Mohebali M, et al. Iranian Native Plants on Treatment of Cutaneous Leishmaniosis: A Narrative Review. Iran J Parasitol. 2017;12(3):312–322. [PMC free article] [PubMed] [Google Scholar]