Abstract
Background:
Schistosomiasis has been identified as a major public health problem in tropical countries. The present study aimed to investigate the schistosomicidal effects of the methanolic extract of Argemone mexicana L. and its active component, berberine against Schistosoma mansoni on in-vitro experiments.
Methods:
S. mansoni adults were used. Various concentrations of the methanolic extract (10 – 200 μg/ml) and berberine (2.5 – 50 μM) were tested from 24 to 72 h. The viability of S. mansoni was confirmed with an invertoscope-microscope. Furthermore, cytotoxic (Hemolysis test), and antioxidant (DPPH radical scavenging assay) capacities were determined.
Results:
The viability tests on S. mansoni showed that A. mexicana at 50 μg/mL is lethal at 48 h and berberine at 10 μM is lethal at 24 h. The hemolytic activity at 1,000 μg/mL was 2.9% for A. mexicana and 90.2% for berberine. The antioxidant capacities shown by A. mexicana and berberine, were EC50 156.3 and 84.1 μg/mL, respectively.
Conclusion:
The extract of A. mexicana and berberine demonstrated high antischistosomal activities in low concentration and short exposure time on the in-vitro model.
Keywords: Anthelmintics, Medicinal plants, Papaveraceae, Schistosoma mansoni
Introduction
Several clinical significant parasites can affect the human intestine, schistosomiasis is a neglected tropical disease caused by blood flukes of the Schistosoma genus, being Schistosoma mansoni, S. haematobium, and S. japonicum responsible for most human infections (1). More than 250 million people have been infected in more than 75 countries where the disease is endemic (2). In the absence of a vaccine, the first-line treatment for the control of schistosomiasis is praziquantel (PZQ) used since the 1970s in mass administration programs (3). That situation facilitated the development of resistant strains to PZQ, so there is a need to investigate new antischistosomal agents (4).
The use of plant-derived natural compounds is currently considered a promising approach in schistosomiasis treatment (5). The challenge is to obtain highly effective compounds, able to stimulate host-defense mechanisms and with no side effects. In this sense, natural products have the advantage of offering access to the development of new schistosomicidal compounds due to their well-documented transition to synthetic drugs in many other diseases (6).
Argemone mexicana L. (Papaveraceae) is a plant widely distributed in tropical countries. In traditional medicine, A. mexicana shows activity against Plasmodium falciparum, Leishmania donovani and Trypanosoma brucei rhodesiense (7,8). The presence of several alkaloids has been reported in this plant, being berberine its main component (9). Berberine is perhaps the constituent responsible for the biological activity of A. mexicana since its effectiveness has been attributed against L. tropica and L. infantum (10). Berberine causes morphological changes on Entamoeba histolytica and Giardia lamblia, inducing chromatin agglutination in the nucleus, formation of autophagic vacuoles and aggregates in the cytoplasm (11). Berberine has also been reported to possess activity against a variety of bacteria, fungi, viruses (12).
The aim of this work was focused on evaluating the antischistosomal activity of the methanolic extract of A. mexicana (Am), as well as the main component berberine.
Materials and Methods
Ethical statement
The procedures with animals comply with the European Union guidelines (European Directive 2010/63/CE) for the experimentation and use of laboratory animals. Accredited facilities at the USAL were used (Reg.No.PAE/SA/001). The Ethics Committee of the UANL approved the procedures (Reg.No.335) for the use of human blood.
Chemicals
Overall, 2,2-diphenyl-1-picrylhydrazyl (DPPH), penicillin, streptomycin sulfate, berberine, dimethylsulfoxide (DMSO), ethylenediaminetetraacetic acid (EDTA), L-glutamine, absolute methanol (MeOH), praziquantel (PZQ), and quercetin were purchased from Sigma-Aldrich ® (Merck, USA). RPMI-1640 culture medium and Fetal Bovine Serum (FBS) were purchased from Invitrogen (Invitrogen, USA).
Plant material
The aerial part of A. mexicana was collected in Feb 2018 in the city of Monterrey (25°46′24.38″N & 100°20′59.28″W), México. The taxonomic identification of the plant was carried out at the Botany Laboratory of the School of Biological Science (Reg.029128).
Parasites
S. mansoni cercariae (strain-LE) were routinely maintained in Biomphalaria glabrata snails as an intermediate host, and in CD1 female mice (Charles River-Criffa. Spain), as the definitive host. Forty-day old female mice, weighing 25 g, were infected with 150 cercariae by abdominal skin exposure (13). The mice were kept with food and water ad-libitum in an environment under an alternant cycle of 12 h light/12 h dark at 20 °C. After 8 wk, mice were sacrificed with a lethal dose of 60 mg/kg pentobarbital plus heparin (2 IU/mL) and then perfused with PBS and heparin (500 IU/L) to obtain couples of adult S. mansoni from the portal and mesenteric veins. The worms were washed in RPMI-1640 culture medium (pH 7.5), supplemented with 10% heat-inactivated (56 °C) FBS, L-glutamine (2mM), penicillin (100 IU/mL), and streptomycin (100 mg/mL) (14).
Preparation of the methanolic extract and phytochemical analysis
The dried plant (200 g) was ground and extracted by the maceration method with 500 mL of absolute-MeOH for 96 h, in-room temperature. The solvent was removed into a rotary evaporator and the extract was concentrated to dryness and stored at −20 °C, until testing. The functional groups present in the methanolic extract and the active component berberine had been previously determined in a publication previously published by our group in 2020 (15).
Activity against S. mansoni
The activity against S. mansoni in the adult stage was carried out using the technique previously described to obtain the worms (16). Stock solutions of Am or berberine (1 mg/mL) in DMSO (1% w/v) were prepared. The solutions were sterilized with membrane filters (0.22 μm). Serial dilutions were made from the stock solutions, with RPMI-1640 + 10% FSB. The concentrations of the solutions were 10 to 200 μg/mL for Am, and 2.5 to 50 μM for berberine in culture plates with a final volume of 3 mL (considering the constant culture media volume in each well) and applied to 24-well plates filled with a final volume of 3 mL culture medium. Nine worms were cultured per cavity at 37 °C, 5% CO2 and 95% humidity immediately after perfusion of animals to ensure vitality (17). Negative control (C-) wells contained adults incubated with 1% DMSO plus culture media while positive control (C+) wells contained worms incubated with 10 μM PZQ plus the culture media. All experiments were performed in triplicate and microplates were checked at 0, 24, 48, and 72 h using an invertoscope-microscope.
Cytotoxicity assay
Cytotoxicity was determined in a suspension of human erythrocytes by the hemolysis test. Serum was separated and EDTA (1.5 mg/mL blood) was added to wash out the erythrocytes, which mix and separate at 1,000 rpm (5 min/37 °C). The erythrocytes were centrifuged three times in pH 7.4 (10 mM PBS) phosphate buffer solution removing the supernatants. The erythrocytes obtained were used to prepare the 5% v/v red blood cell suspension in PBS). For the evaluation of Am and berberine cytotoxicity in erythrocytes, the prepared suspension was incubated with different concentrations of the extract, berberine and controls (50 to 1,000 μg/mL) for 30 min (37 °C), these were classified as treatments (Tr). The negative control was untreated erythrocytes (C-), positive control was distilled water to produce osmotic hemolysis (C+) (18). After incubation, treatments were centrifuged for 4 min at 13,000 rpm (4 °C), 200 μL were taken and placed in a 96-well microplate. Hemolysis was determined spectrophotometrically at 540 nm. The readings were recorded as absorbance (Abs) for each treatment minus the absorbance presented by the vehicle (AbsTr). The percentage of hemolysis was calculated with the formula:
Antioxidant activity
It was evaluated by the DPPH radical scavenging method (19). Treatments (Tr) were evaluated at concentrations of 20 to 2,500 μg/mL. DPPH was prepared at 125 μM in MeOH, 100 μL of each respective concentration was taken and 100 μL of DPPH was added. Samples remained protected from light for 30 minutes. A quercetin solution was used as C+ and as C-MeOH. Absorbance at 517 nm was measured and the percentage reduction was calculated as:
Statistical analysis
Statistical differences were calculated using analysis of variance (ANOVA) and the Tukey test to establish differences between groups. Mean inhibitory concentration (CI50) and mean effective concentration (EC50) were determined by the Probit test, with a 95% confidence interval. The analyses were performed with SPSS software, version 22.0 (Chicago, IL, USA). Differences were considered significant at P<0.05. All tests were performed in triplicate in at least three different tests.
Results
Phytochemical analysis
An extraction yield of 10.22% was obtained. Conventional phytochemical tests indicated the presence of unsaturations, quinones, triterpenes -sterols, phenols, saponins, flavonoids, carbohydrates, and alkaloids mainly. Finally, by HPLC-DAD and mass-spectrometry, the berberine alkaloid was determined as the most abundant component in the extract.
Activity against S. mansoni
Am and berberine can inhibit the viability of the worms and even separate couples. Both treatments produced an alteration of the integument, with a clear dose-response relationship since the percentage of viability decreases as the concentration of the extract or alkaloid increases (Table 1). In all treatments, at 0 h, all couples were viable and without integument release. The positive control showed 100% mortality after 4 h of incubation, while the negative control did not reveal a decrease in viability (Fig. 1A).
Table 1:
In-vitro effect of Argemone mexicana methanolic extract and berberine on adult S. mansoni couples
| Variable | Initial | 24 h | 48 h | 72 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Concentration | Couples | S.C. | M% | T.D. | V% | S.C. | M% | T.D. | V% | S.C. | M% | T.D. | V% |
| Untreated (C-) | 9 | 0 | 100 | - | 100 | 0 | 100 | - | 100 | 0 | 100 | - | 70.5 | |
| PZQ (C+) | 9 | 9 | 0 | ++ | 6 | 9 | 0 | +++ | 0.9 | 9 | 0 | +++ | 0 | |
| Am (μg/ml) | 10 | 9 | 0 | 100 | - | 100 | 0 | 100 | - | 100 | 0 | 100 | - | 77 |
| 25 | 9 | 0 | 100 | - | 100 | 1 | 75 | + | 85 | 3 | 75 | ++ | 54* | |
| 50 | 9 | 5* | 75 | ++ | 69* | 8** | 50** | ++ | 62* | 8** | 25** | +++ | 23** | |
| 100 | 9 | 9** | 25** | +++ | 15** | 9** | 0** | +++ | 0** | 9** | 0** | +++ | 0** | |
| 200 | 9 | 9** | 0** | +++ | 0** | 9** | 0** | +++ | 0** | 9** | 0** | +++ | 0** | |
| ANOVA p | 0.002 | 0.004 | <0.001 | 0.004 | 0.005 | 0.001 | 0.004 | 0.001 | <0.001 | |||||
| F | 23.04 | 16.44 | 8.79 | 16.30 | 16.71 | 3.86 | 17.44 | 23.61 | 4.38 | |||||
| IC50 (μg/mL) | 65.35 | 43.20 | 23.75 | |||||||||||
| Berbrine (μM) | 2,5 | 9 | 0 | 100 | - | 86 | 1 | 100 | - | 77 | 3 | 75 | - | 41** |
| 5 | 9 | 6** | 75 | + | 65* | 8** | 50** | + | 46** | 8** | 25** | + | 16** | |
| 10 | 9 | 9** | 0** | ++ | 27** | 9** | 0** | ++ | 11** | 9** | 0** | +++ | 2.6** | |
| 25 | 9 | 9** | 0** | +++ | 5.1** | 9** | 0** | +++ | 0** | 9** | 0** | +++ | 0** | |
| 50 | 9 | 9** | 0** | +++ | 0** | 9** | 0** | +++ | 0** | 9** | 0** | +++ | 0** | |
| ANOVA p | 0.018 | <0.001 | 0.006 | 0.020 | <0.001 | 0.002 | 0.109 | 0.001 | <0.001 | |||||
| F | 8.71 | 43.49 | 5.89 | 6.64 | 78.53 | 6.13 | 3.35 | 36.48 | 6.54 | |||||
| IC50 (μM) | 8.76 | 4.82 | 1.68 | |||||||||||
S.C.: Separated couples. M%: Mean percentage of worm motility. T.D.: Tegument damage. V%: Viability percent. The tegument damage was classified as follows: Absent -, Present +, Abundant ++, and Very abundant +++. Statistical differences compared to untreated worm culture HDS Tukey post-hoc test *P 0.05, **P 0.001
Fig. 1:
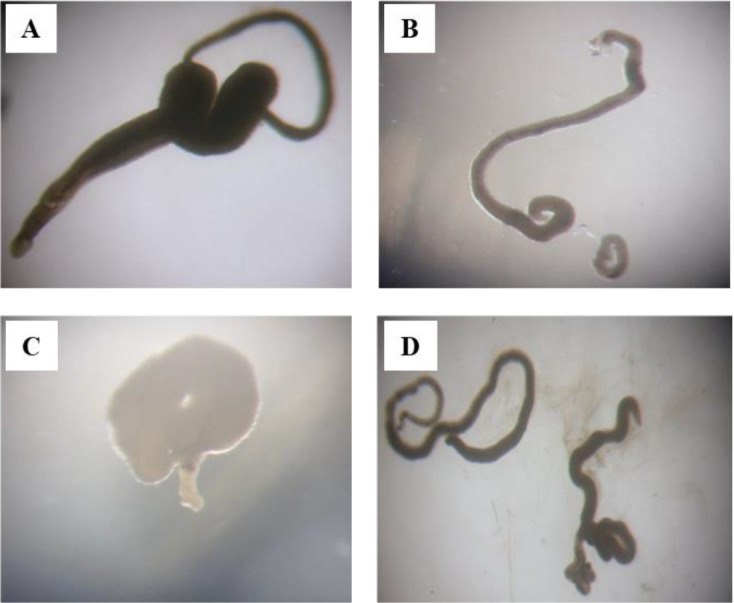
In-vitro investigation of adult S. mansoni after 72 h of incubation. A: Negative control. B: Positive control (10 μM PZQ). C: A worm treated with 25 μg/mL Am. D: A worm treated with 10 μM berberine
Am presented IC50 of 65.35, 43.20 and 23.75 μg/mL at 24, 48 and 72 h respectively. It was observed that at a 10 μg/mL concentration there were no significant changes, however, at 48 h and 72 h in concentrations of 50 μg/mL and 25 μg/mL (Fig. 1C) respectively the effect is remarkable since at that time it was noted the detachment of all the pairs and an increase in the liberation of the integument. In concentrations of 100 and 200 μg/mL Am was effective after 24 h of incubation. Berberine presented IC50 of 8.76, 4.82 and 1.68 μM at 24, 48 and 72 h respectively, it also showed high effectiveness in 5 μM, with the pairs detaching at 48 h and being lethal at 48 h in the case of males and 72 h in the case of females (Fig. 1D), in both cases, there is a detachment of the integument, which increases as the concentration of berberine increases.
Cytotoxicity of the methanolic extract and berberine
The percentage of cytotoxicity are shown in Table 2. At 1,000 μg/mL, Am had a hemolytic activity of 2.9%, which decreases as the concentration of the extract decreases (Table 2). For the lowest concentration of 50 μg/mL, the hemolysis presented in the erythrocytes was 0.1%. In the case of berberine, the hemolysis determined at 1,000 μg/mL was 90.2% and at 50 μg/mL it was 1.3%. Compared to C- which did not present hemolysis and C+ had 100% hemolysis, berberine presented a highly significant difference (P<0.001).
Table 2:
Evaluation of the cytotoxicity of Am and berberine. Data are expressed as the mean ± SD (P<0.05) of the % of hemolysis plus standard error (SE). Different letters within the same column are significantly different analyzed via the Tukey test
| Treatments | Cytotoxicity | |
|---|---|---|
| μg/mL | Am | Berberine |
| Distilled water (C+) | 100 ± 0.0 | 100 ± 0.0 |
| Untreated (C-) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 50 | 0.1 ± 0.0a** | 1.3 ± 0.1a** |
| 100 | 0.3 ± 0.0a** | 2.8 ± 0.4b** |
| 200 | 0.9 ± 0.1b** | 11.8 ± 1.4c* |
| 400 | 1.2 ± 0.4b** | 23.8 ± 3.2d* |
| 600 | 1.6 ± 0.3b** | 55.9 ± 4.1e |
| 800 | 2.2 ± 0.4c** | 72.9 ± 2.3f |
| 1,000 | 2.9 ± 0.4d** | 90.2 ± 9.3g |
| SE | 0.4 | 13.6 |
| ANOVA p | <0.001 | <0.001 |
| F | 3.87 | 3.87 |
Statistical differences compared to positive control HDS Tukey post-hoc test *P 0.05, **P 0.001
Antioxidant capacity
The median effective concentration (EC50) in which a treatment induces a median response after an exposure time showed, that the amount of sample necessary to decrease the DPPH concentration by 50%, for Am was 156.3 ± 6.5 μg/mL and for berberine 84.1 ± 7.4 μg/mL, however, the quercetin standard was more effective 1.03 ± 0.2 μg/mL.
Discussion
Interest in natural products for the treatment of different diseases, including schistosomiasis, is increasing, due to the scarce resources for their treatment, (20). The development of new drugs against this disease is required until an effective vaccine can be administered (21). The control of schistosomiasis is based on praziquantel which has many deficiencies (22). Many investigations have evaluated several substances from plant origin, such as berberine, abundant in A. mexicana, shown promising properties against L. tropica, L. infantum, Trichomonas vaginalis, and E. histolytica (15,23,24). However, no studies have been conducted against S. mansoni.
This research demonstrated that A.mexicana and its main component berberine, have a great capacity to inhibit the viability of S. mansoni in the adult stage. The effects of both were dependent on time and dose. The highest efficacy was observed in berberine at a concentration of 5 μM during an incubation period of 72 h, and from 10 μM at 24 h with effect in both sexes, combined to the separation of the couples. However, the effect was much more pronounced against the male. Additionally, schistosomes exposed to both treatments showed mobility changes with atypical muscle contractions that became slower over time when treatment concentration increased and even parasite death was observed. Other researchers reported similar effects through in vitro tests, using compounds isolated from other plant species such as piplartine isolated from Piper tuberculatum, 8-hydroxyquinoline, and berberine chloride extracted from Coptis chinensis (13) evaluated against schistosomules, and adult worms. However, the mechanisms of action have not been clarified to date.
Previous studies have demonstrated the biological activity of berberine. Berberine from B. vulgaris significantly decreased the viability of Echinococcus granulosus, L. tropica, and L. infantum in in-vitro experiments (24,25). This may be because berberine inhibits the synthesis of nucleic acids and proteins, as well as the activity of telomerase (26). Plants containing alkaloids such as berberine are mainly related to the presence of quaternary nitrogen and the functions of oxygen necessary for the strong anti-plasmodic activity (27). The relationship between oxygenation and anti-plasmodic activity provides clues for possible molecular frameworks of synthesis and structure-activity relationship studies that could lead to the identification of new drugs (8). In-vitro studies indicate that berberine binds to tubulin inducing depolymerization of interphase and mitotic microtubules (28).
Concerning the tests with PZQ, males, and females, contracted without any movement at 4 h in addition to the detachment of the pairs and the detachment of the tegument. Previous studies indicated the effects of PZQ on nematodes, causing contractions when exposed to concentrations of 0.1 and 1 μg/mL, this is because PZQ causes a rapid influx of calcium followed by contraction, paralysis, and tegumental damage (29). PZQ caused severe muscle contractions and partial bend of the worms, as did berberine which caused paralysis and muscle contraction; however, the exact mechanisms underlying this effect are still unclear. In Am’s case, it caused paralysis of the worms, but no contraction or curving. As the incubation time increased at higher doses of Am, the release of the tegument by the worms increased. In the case of berberine, it was observed from 5 μM, this is important because the tegument is involved in many functions that are of vital importance to S. mansoni, such as the absorption of nutrients (30). The tegument is the interface between the parasite and the host environment; therefore, it represents an important target for many drug actions. Different investigations evaluated natural compounds to evaluate the in-vitro and in-vivo activity and found alterations of the tegument surface as in our study, they were able to observe, induction of tegument damage in the immature and mature stages after in-vitro incubation, which depended on the dose and time, i.e. it intensified progressively as the incubation period and the concentration of treatments increased, and it was also possible to observe shrinkage, corrugation, unfolding and widening of the gynecophoral canal (31).
To evaluate the safety of the extracts, hemolysis tests were performed. As can be seen in Table 2, Am did not show high toxicity, being 2.9% at its highest concentration tested and presented almost no toxicity at the lowest concentration tested, this following Karimi et al. criteria (32), in which Am could be classified as a low toxicity, the probable hemolysis presented is related to mechanical stress at the time of agitation during the incubation time. In the case of berberine, hemolysis was proportional to the concentration, since as the dose was increased, hemolysis also increased. Berberine at 5 μM is effective against S. mansoni at 48 h, this dose is far below the lowest concentration evaluated in the cytotoxicity test, berberine stands out since in minimal doses it′s biological activity is very effective. These data are consistent with those previously reported (15). Therefore, at concentrations of interest, below 200 μg/mL (Table 2), Am and berberine were not found to be significantly cytotoxic (P<0.001). Berberine is not toxic, cytotoxic, or mutagenic at doses based on clinical situations, however, its side effects may result from high doses (33).
DPPH radical assays are considered representative among the various methods for evaluating the ability to capture free radicals, the antioxidant activity of plants varies according to the part of the plant used, its state of maturation, this is related to the phenolic content of the plant and the radical scavenging efficiency of DPPH will depend on this (34). The EC50 determined for Am and berberine was 156.3 and 84.1 μg/mL respectively, in the case of quercetin, it was 1.03 μg/mL. Based on the results Am and berberine, have a moderate capacity to capture free radicals compared to quercetin. These results are relevant because recent studies have shown that the combinations of antioxidant and anthelmintic activities could show antiparasitic synergistic effects (35). The ability to capture free radicals in plants of the Berberidaceae family has been reported due to some flavonoids present such as anthocyanins, which have multiple functions in plants such as protection under ultraviolet radiation (36).
Conclusion
The methanolic extract of A. mexicana and the principal compound berberina, showed high activity against S. mansoni in low concentration in the in-vitro model. However, in-vivo efficacy requires to be evaluated in an animal model.
Acknowledgements
The authors thank the USAL Foundation, the Santander Bank of Spain, the Carlos III Health Institute of Spain, and the National Council of Science and Technology (CONACYT) of Mexico, for the support given to J.H. Elizondo-Luevano (Grant 418935) project: CB176853.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Financial support
This work was financed by the National Council of Science and Technology (CONACYT) of México (Project CB176853) Scholarship 418935. This study was supported by the Carlos III Health Institute, Spain, scholarships: RICET: RD16/0027/0018, PI16/01784, and USAL foundation - Santander Bank of Spain.
References
- 1.Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol. 2014;36(8):347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enk MJ, Lima ACL, Drummond SC, et al. The effect of the number of stool samples on the observed prevalence and the infection intensity with Schistosoma mansoni among a population in an area of low transmission. Acta Trop. 2008;108(2–3):222–28. [DOI] [PubMed] [Google Scholar]
- 3.Cederberg S, Sikasunge CS, Andersson A, et al. Short communication: In vitro efficacy testing of praziquantel, ivermectin, and oxfendazole against Taenia solium cysts. J Parasitol Res. 2012;2012:363276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magalhães LG, Machado CB, Morais ER, et al. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol Res. 2009;104(5):1197–201. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira RN, Rehder VLG, Santos Oliveira AS, et al. Schistosoma mansoni: In vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp Parasitol. 2012;132(2):135–43. [DOI] [PubMed] [Google Scholar]
- 6.Harvey A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov Today. 2000;5(7):294–300. [DOI] [PubMed] [Google Scholar]
- 7.Brahmachari G, Gorai D, Roy R. Argemone mexicana: chemical and pharmacological aspects. Rev Bras Farmacogn. 2013;23(3):559–75. [Google Scholar]
- 8.Malebo HM, Wenzler T, Cal M, et al. Anti-protozoal activity of aporphine and protoberberine alkaloids from Annickia kummeriae (Engl. & Diels) Setten & Maas (Annonaceae). BMC Complement Altern Med. 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang F-R, Khalil AT, Wu Y-C, et al. Cytotoxic Benzophenanthridine and Benzylisoquinoline Alkaloids from Argemone mexicana. Z Naturforsch C J Biosci. 2003;58(7–8):521–6. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoudv H, Sharififar F, Sharifi I, et al. In vitro inhibitory effect of Berberis vulgaris (Berberidaceae) and its main component, Berberine against different leishmania species. Iran J Parasitol. 2014;9(1):28–36. [PMC free article] [PubMed] [Google Scholar]
- 11.Birdsall T, GS K. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Alt Med Rev. 1997;2(2):94–103. [Google Scholar]
- 12.Singh B, Srivastava JS, Khosa RL, et al. Individual and combined effects of berberine and santonin on spore germination of some fungi. Folia Microbiol (Praha). 2001;46(2):137–42. [DOI] [PubMed] [Google Scholar]
- 13.Dkhil MA, Moneim AEA, Al-Quraishy S. Berberine protects against Schistosoma mansoni-induced oxidative damage in renal and testicular tissues of mice. Pak J Zool. 2014;46(3):763–71. [Google Scholar]
- 14.Yepes E, Varela-M RE, López-Abán J, et al. Inhibition of granulomatous inflammation and prophylactic treatment of schistosomiasis with a combination of edelfosine and Praziquantel. PLoS Negl Trop Dis. 2015;9(7):e0003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elizondo-Luevano JH, Verde-Star J, González-Horta A, et al. In Vitro Effect of Methanolic Extract of Argemone mexicana against Trichomonas vaginalis. Korean J Parasitol. 2020;58(2):135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yepes E, Varela-M RE, López-Abán J, et al. In vitro and in vivo anti-schistosomal activity of the alkylphospholipid analog edelfosine. PLoS One. 2014;9(10):e109431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eraky MA, Aly NS, Selem RF, et al. In vitro schistosomicidal activity of phytol and tegumental alterations induced in juvenile and adult stages of Schistosoma haematobium. Korean J Parasitol. 2016;54(4):477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakshmi G, Smitha N, Ammu SV, et al. Phytochemical profile, in vitro antioxidant and hemolytic activities of various leaf extract of Nymphaea Nouchali Linn: An in vitro study. Int J Pharm Pharm Sci. 2014;6(6):548–52. [Google Scholar]
- 19.Ledy G, Catalina L, Azucena O. Free radical scavenging and cytotoxic activity of five commercial standardized extracts (red wine, green tea, pine bark, polygonum and pomegranate). Afr J Biotechnol. 2012;11(102):16725–30 [Google Scholar]
- 20.Panic G, Duthaler U, Speich B, et al. Repurposing drugs for the treatment and control of helminth infections. Int J Parasitol Drugs Drug Resist. 2014;4(3):185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mølgaard P, Nielsen SB, Rasmussen DE, et al. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol. 2001;74(3):257–64. [DOI] [PubMed] [Google Scholar]
- 22.Keiser J, Chollet J, Xiao SH, et al. Mefloquine - An aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3(1):e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elizondo-Luévano JH, Castro-Ríos R, Sánchez-García E, et al. In Vitro Study of Antiamoebic Activity of Methanol Extracts of Argemone mexicana on Trophozoites of Entamoeba histolytica HM1-IMSS. Can J Infect Dis Med Microbiol. 2018;2018:7453787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoudvand H, Shakibaie M, Tavakoli R, et al. In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against Iranian isolate of sensitive and glucantime-resistant Leishmania tropica. Iran J Parasitol. 2014;9(4):452–60. [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoudvand H, Saedi Dezaki E, Sharififar F, et al. Protoscolecidal effect of Berberis vulgaris root extract and its main compound, berberine in cystic echinococcosis. Iran J Parasitol. 2014;9(4):503–10. [PMC free article] [PubMed] [Google Scholar]
- 26.Wright CW, Marshall SJ, Russell PF, et al. In vitro antiplasmodial, antiamoebic, and cytotoxic activities of some monomeric isoquinoline alkaloids. J Nat Prod. 2000;63(12):1638–40. [DOI] [PubMed] [Google Scholar]
- 27.Grycová L, Dostál J, Marek R. Quaternary protoberberine alkaloids. Phytochemistry. 2007;68(2):150–75. [DOI] [PubMed] [Google Scholar]
- 28.Raghav D, Ashraf SM, Mohan L, et al. Berberine Induces Toxicity in HeLa Cells through Perturbation of Microtubule Polymerization by Binding to Tubulin at a Unique Site. Biochemistry. 2017;56(20):2594–611. [DOI] [PubMed] [Google Scholar]
- 29.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21(6):659–67. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann KF, Strand M. Molecular identification of a Schistosoma mansoni tegumental protein with similarity to cytoplasmic dynein light chains. J Biol Chem. 1996;271(42):26117–23. [DOI] [PubMed] [Google Scholar]
- 31.Shuhua X, Binggui S, Utzinger J, et al. Ultrastructural alterations in adult Schistosoma mansoni caused by artemether. Mem Inst Oswaldo Cruz. 2002;97(5):717–24. [DOI] [PubMed] [Google Scholar]
- 32.Karimi G, Aghasizadeh M, Razavi M, et al. Protective effects of aqueous and ethanolic extracts of Nigella sativa L. and Portulaca oleracea L. on free radical induced hemolysis of RBCs. Daru. 2011;19(4):295–300. [PMC free article] [PubMed] [Google Scholar]
- 33.Fatehi M, Saleh TM, Fatehi-Hassanabad Z, et al. A pharmacological study on Berberis vulgaris fruit extract. J Ethnopharmacol. 2005; 102(1):46–52. [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Rodríguez P, Pabón Baquero LC, Rodríguez Álvarez MF. Propiedades químicas y biológicas de Arbutus unedo: una planta con potencial medicinal. Rev Cuba de Farm. 2015;49(1):144–55. [Google Scholar]
- 35.Gouveia MJ, Brindley PJ, Rinaldi G, et al. Combination Anthelmintic/Antioxidant Activity against Schistosoma mansoni. Biomolecules. 2019;9(2):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun LL, Gao W, Zhang MM, et al. Composition and Antioxidant Activity of the Anthocyanins of the Fruit of Berberis heteropoda Schrenk. Molecules. 2014;19(11):19078–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


