Abstract
Excited-state catalysis, a process that involves one or more excited catalytic species, has emerged as a powerful tool in organic synthesis because it allows access to the excited-state reaction landscape for the discovery of novel chemical reactivity. Herein, we report the first excited-state palladium-catalyzed 1,2-spin-center shift reaction that enables site-selective functionalization of carbohydrates. The strategy features mild reaction conditions with high levels of regio- and stereoselectivity that tolerate a wide range of functional groups and complex molecular architectures. Mechanistic studies suggest a radical mechanism involving the formation of hybrid palladium species that undergoes a 1,2-spin-center shift followed by the reduction, deuteration, and iodination to afford functionalized 2-deoxy sugars. The new reactivity will provide a general approach for the rapid generation of natural and unnatural carbohydrates.
Graphical Abstract
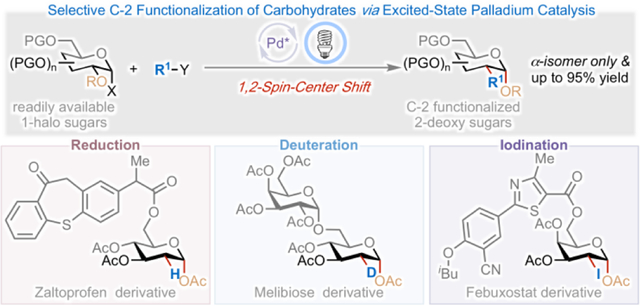
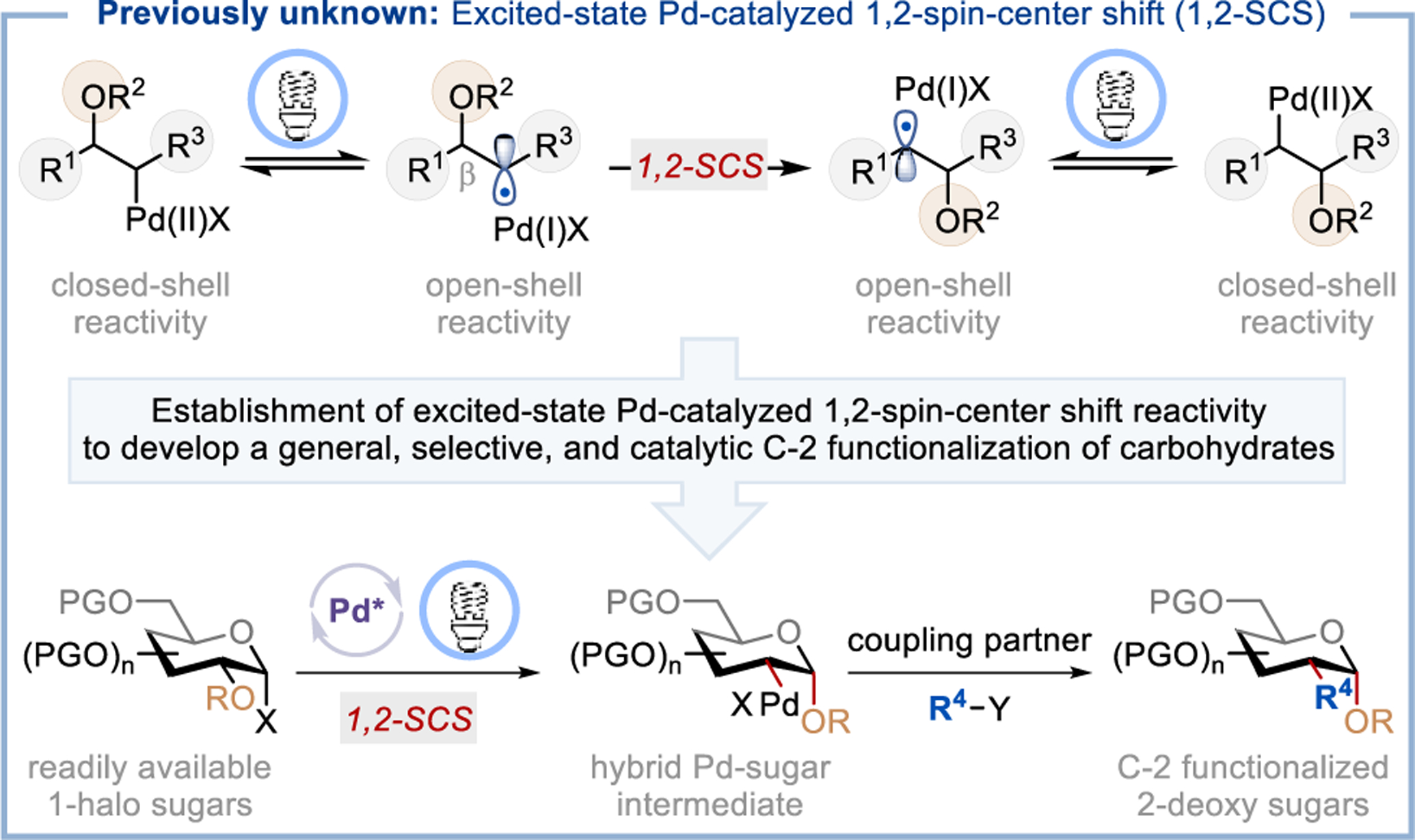
Visible-light-induced excited-state palladium catalysis has emerged as a promising strategy for developing valuable reactions.1 Seminal work by Gevorgyan,2 Fu,3 and Yu4 showed that photoexcited Pd-complexes undergo rapid, radical oxidative addition into aryl/alkyl-halide bonds, forming aryl/alkyl-Pd species with hybrid reactivity in which the closed-shell Pd(II) complex is in equilibrium with an open-shell alkyl radical/Pd(I) intermediate through a reversible photoexcitation/recombination process (Figure 1).5 This hybrid reactivity has been exploited in a range of transformations, such as desaturation reactions,2a, 6 Mizoroki-Heck reactions,2b, 3, 7 difunctionalization of conjugated dienes,8 and others.4, 9 Despite these recent advances, the application of either ground-state or excited-state Pd-catalysis to mediate 1,2-spin-center shift (SCS)10 remains elusive. We envisioned that with a functional group such as acyloxy at the β-position, the alkyl radical/Pd(I) species could undergo a 1,2-SCS, accessing a new reaction site for further functionalization (Figure 1).11 The establishment of such reactivity is significant because it will (i) enable unique reactions capable of the rapid generation of molecular complexity and late-stage functionalization of complex molecules; (ii) provide new strategic bond formation that leads to otherwise difficult or unobtainable molecular architecture; and (iii) guide the design and development of new chemical reactions.
Figure 1.
Development and exploitation of excited-state palladium-catalyzed 1,2-spin-center shift for selective C-2 functionalization of carbohydrates.
Carbohydrates, the most abundant biomolecules, have indispensable roles in a wide range of biological processes, including cell-cell recognition, protein folding, neurobiology, inflammation, and infection.12 The possibility of modifying sugar structure(s) to enhance or otherwise alter the physiological properties of the parent molecule is therefore highly attractive.13 Selective C-2 functionalization of carbohydrates has attracted significant interest because the resulting 2-deoxy sugar derivatives, in which the C-2 hydroxyl group of sugar has been replaced by other functional groups, are ubiquitous in nature and are found in medicine, molecular imaging, cell engineering, and catalysis.14 Conventional methods to access C-2 functionalized 2-deoxy sugars rely on the derivatization of advanced intermediates such as glycals and 1,2-epoxy- or 1,2-cyclopropyl-sugars.15 These protocols often involve multi-step precursor syntheses and harsh reaction conditions, and have limited reaction scope. We envisaged that the establishment of excited-state Pd-catalyzed 1,2-SCS reactivity would enable a general, controllable, and selective catalytic strategy for C-2 functionalization of carbohydrates using readily available 1-halosugars.16
The mechanistic hypothesis of the proposed transformation is outlined in Figure 2. We envisioned that photoexcited palladium catalyst [Pd(0)]* undergoes radical oxidative addition with 1-halo sugar 1, generating the hybrid 1-glycosyl-Pd-X complexes IIa and IIb.2b, 3 The glycosyl radical IIa favors the B2,5 boat conformation (IIIa) because of the hyperconjugation between the singly occupied molecular orbital (SOMO) and σ*C-O orbital of the C-2–OAc group.17 Such an interaction is more pronounced in glycosyl radicals because the lone pair electron of the endocyclic-O (ηO, anomeric interaction) raises the SOMO energy level. Such an extended anomeric interaction weakens the C-2–OAc bond and promotes the 1,2-SCS through a concerted [2,3]-acyloxy rearrangement with a cyclic five-membered ring transition state IIIb,11b, 17a forming the deoxypyranosan-2-yl radical IVa that prefers the 4C-1 chair conformation.18 Although the anomeric radical is more stable than the secondary alkyl radical, the molecular stability gained from the formation of an anomeric C–O bond in IVa drives the desired 1,2-SCS.19 Under visible-light irradiation, the intermediate IVa is in equilibrium with alkyl-Pd(II)X complex IVb, which allows access to both open- and closed-shell reactivities. We anticipate that these hybrid Pd species can engage in a wide range of cross-coupling reactions through processes such as (i) transmetalation followed by reductive elimination, or (ii) radical coupling or atom/group transfer followed by reduction of Pd(I)X to furnish the desired C-2 functionalized carbohydrate 2 and regenerate Pd(0) catalyst, completing the catalytic cycle.
Figure 2.
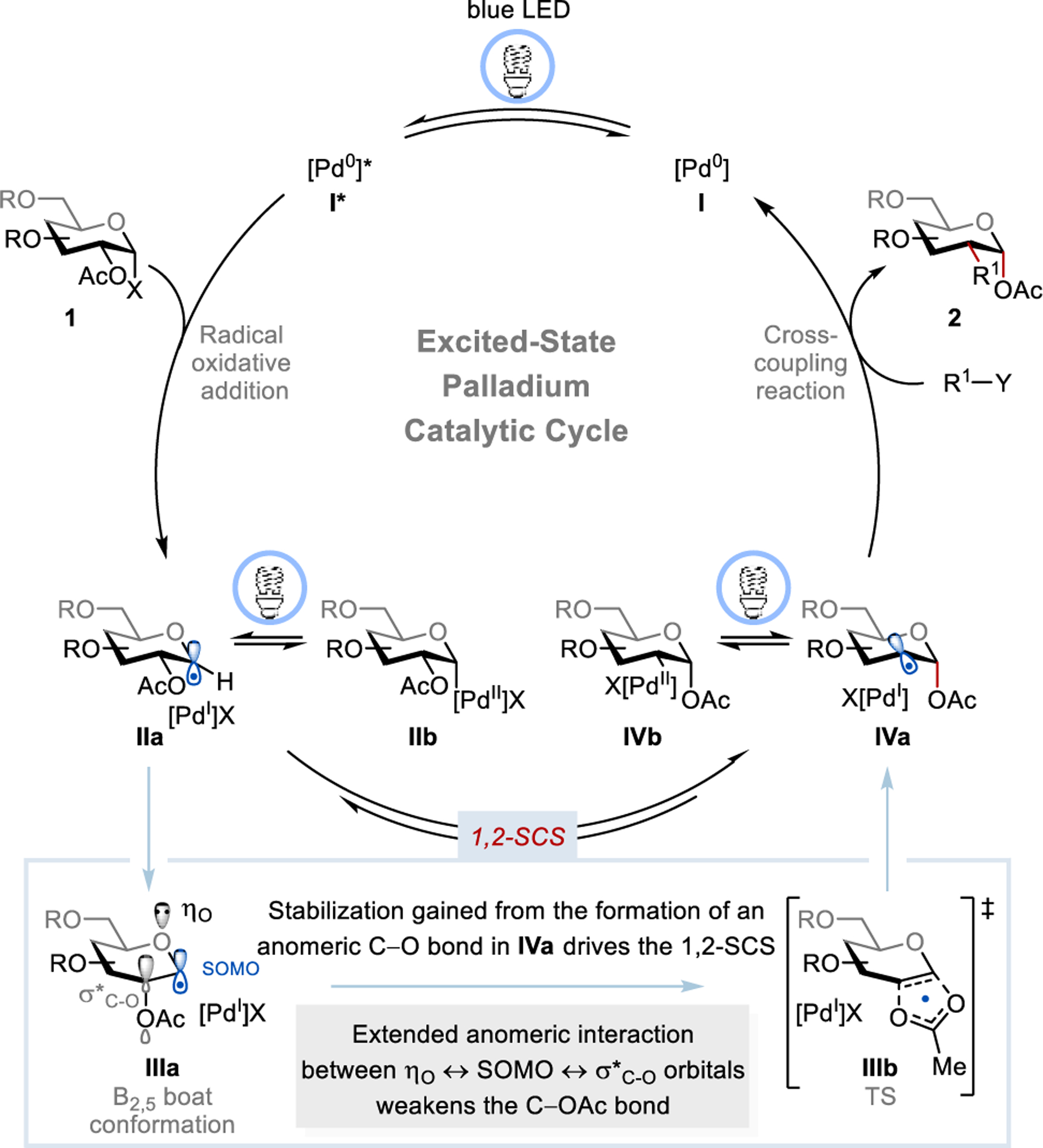
Proposed catalytic cycle for the excited-state Pd-catalyzed C-2 functionalization of carbohydrates.
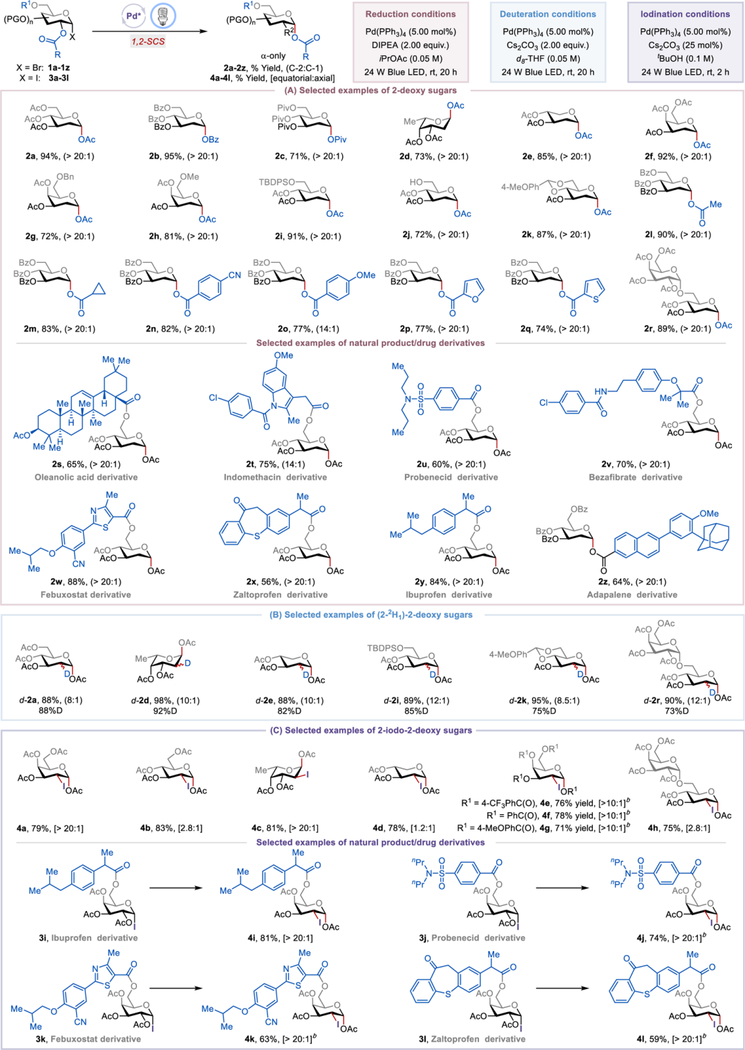
With this hypothesis in mind, we started our investigations using readily available α-glucosyl bromide (1a) as a model substrate. Initial experiments showed that upon exposing 1a to 24 W blue light-emitting diodes (LED) in the presence of Pd(PPh3)4 (5.00 mol%), N, N-diisopropylethylamine (DIPEA, 2.00 equiv) in isopropyl acetate (i-PrOAc, 0.05 M) at room temperature for 20 h, we observed 94% yield of α-only product 2a with >20:1 C-2 selectivity (Table S1, entry 1). The Pd(PPh3)4 catalyst was shown to be critical for the desired reactivity because replacing it with PPh3, Pd(PPh3)Cl2, led to no reaction or significantly lower yield and selectivity (entries 2 & 3). We recognized that the relative rates of the intramolecular 1,2-SCS and the intermolecular hydrogen atom transfer must be controlled to achieve high levels of regioselectivity. It was envisioned that the unique inner-sphere coordination interaction between the Pd catalyst and alkyl radical could stabilize and modulate the reactivity of radical intermediates, thus minimizing the premature C-1 reduction.1e, 20 Indeed, the use of other common Ru-, Ir-, and organic-based photoredox catalysts, where inner-sphere coordination is not feasible, proved to be ineffective and afforded the product with low yields and selectivity (entries 4–6). Reactions in acetonitrile were sluggish and were accompanied by the erosion of the regioselectivity (entry 7). Control experiments showed that DIPEA, an oxygen-free environment, and light were all essential for the desired reactivity (entries 8–10).
With the optimized conditions in hand, we next examined the scope of the reaction. In general, a wide range of α-bromosugars afforded the desired 2-deoxy sugars with up to 95% yield and >20:1 regioselectivity (Table 1A).21 α-Glucosyl bromides with different ester protecting groups such as acetyl, benzoyl, or pivaloyl worked well (2a-2c). Other α-bromosugars, including those derived from acetylated L-fucose, D-xylose, and D-galactose, were also viable substrates (2d-2f).22 Substrates with benzyl-, methyl-, and the acid-sensitive tert-butyldimethylsilyl-protected C-6 hydroxyl groups were well tolerated, affording the desired products 2g-2i in 72–91% yields with >20:1 C-2 selectivity. A free C-6 hydroxyl group, which is useful for further functionalization and often serves as a glycosyl acceptor, reacted smoothly and gave product 2j in 72% yield. A fused ring structure also proved to be compatible with the reaction conditions (2k). The structure of the migratory ester group has little effect on the reaction efficiency because C-2 esters substituted with alkyl, aryl, or heteroaryl groups underwent excited-state Pd-catalyzed 1,2-SCS smoothly, forming the corresponding products 2l-2q in 74–90% yields. A melibiose derivative gave the desired 2-deoxy-disaccharide 2r in 89% yield. Notably, the reaction affords the α−2-deoxyglycosides exclusively, and the corresponding β-isomers were not observed.
Table 1.
Scope of C-2 reduction, deuteration, and iodination of α-glycosyl halides via excited-state Pd-catalysis.a
|
See Supporting Information for experimental details.
% of deuterium incorporation, C-2:C-1 ratio, and equatorial:axial (eq:ax) ratio were determined by 1H-NMR.
Benzene instead of tBuOH was used as a solvent.
The synthetic utility of this process is further highlighted by its amenability to (i) a late-stage modification of functionally dense natural product- and drug-conjugated sugar derivatives and (ii) the synthesis of deuterated 2-deoxy sugars (Table 1). For example, α-Bromoglucose derivatives of oleanolic acid, Indomethacin, Probenecid, Bezafibrate, Febuxostat, Zaltoprofen, Ibuprofen, and Adapalene reacted and afforded the desired products 2s-2z in good yields and excellent levels of regioselectivity, demonstrating that the method can be used in the preparation of pharmaceutically relevant compounds. Furthermore, deuterium-labeled sugars are versatile probes for the study of biological processes such as metabolic and biosynthetic pathways,23 and useful chiral building blocks for the synthesis of chiral deuterated precursors of bioactive molecules.24 Using d8-THF as the solvent and Cs2CO3 as the base under otherwise identical reaction conditions, we successfully obtained a series of (2-2H1)-2-deoxy sugars d-2a, d-2d, d-2e, d-2i, d-2k, and d-2r in yields of 88–98% and with high levels of regioselectivity and deuterium incorporation (Table 1B).
The reaction can be further extended to the synthesis of 2-iodo-2-deoxy sugars using α-iodosugars as starting materials (Table 1C). For example, α-iodosugar derivatives of D-galactose, D-glucose, L-fucose, and D-xylose were converted to the corresponding 2-iodo-2-deoxy sugars 4a-4d with good yields and up to >20:1 equatorial/axial selectivity. The electronic nature of the migrating group had a negligible impact on reaction efficiency and stereoselectivity, as demonstrated by substrates 3e-3f that afforded the desired products 4e-4f in similar yields and diastereoselectivity. Disaccharide and D-galactose derivatives of pharmaceuticals such as Ibuprofen, Probenecid, Febuxostat, and Zaltoprofen could be used to generate the corresponding products 4h-4l with >20:1 equatorial/axial ratios in good yield. Notably, other photocatalysts such as Ru(bpy)32+, Ir(ppy)3, and Eosin Y failed to catalyze this iodination reaction. Given that 2-iodo-2-deoxy sugars are (i) excellent glycosyl donors that control the stereochemistry of the newly formed glycosidic bond25 and (ii) versatile intermediates for further sugar derivatizations,26 our protocol will find a useful application in the synthesis of complex glycans for the discovery and development of new bioactive compounds.
Our mechanistic hypothesis of the excited-state Pd-catalyzed C-2 functionalization of carbohydrates depicted in Figure 2 is supported by UV-Vis measurements, Stern-Volmer quenching studies, radical trapping experiments, deuterium labeling studies, quantum yield measurements, radical clock and cross-over experiments, and kinetic studies (Figure 3). UV-Vis measurements showed the absence of any reaction between the acetylated α-glucosyl bromide (1a) and the ground-state Pd(PPh3)4 (Figure 3A). Irradiation of the reaction mixture with blue LED light for 5 min, however, led to a significant bathochromic shift (Δλabs = 43 nm) with a λabs at 362 nm. The UV-Vis data suggested that an excited Pd(PPh3)4 catalyst readily undergoes a radical oxidative addition with 1a, generating a putative Pd(II)-species.4 Stern-Volmer quenching studies demonstrated that only 1-halosugar quenches the excited Pd(PPh3)4 (Fig. S2). The radical nature of the reaction is further supported by a radical trapping experiment (Figure 3B). Deuterium labeling studies where Cs2CO3 was replaced by DIPEA under deuteration reaction conditions shifted the 2a:d-2a ratio from 1.0:7.3 to 1.9:1.0, showing that DIPEA serves as a hydrogen atom donor (Figure 3C). Because the quantum yields of the C-2 reduction and iodination reactions were 0.09 and 0.24, respectively, an extended radical chain propagation is unlikely under our reaction conditions (Fig. S9 and Fig. S10).
Figure 3.
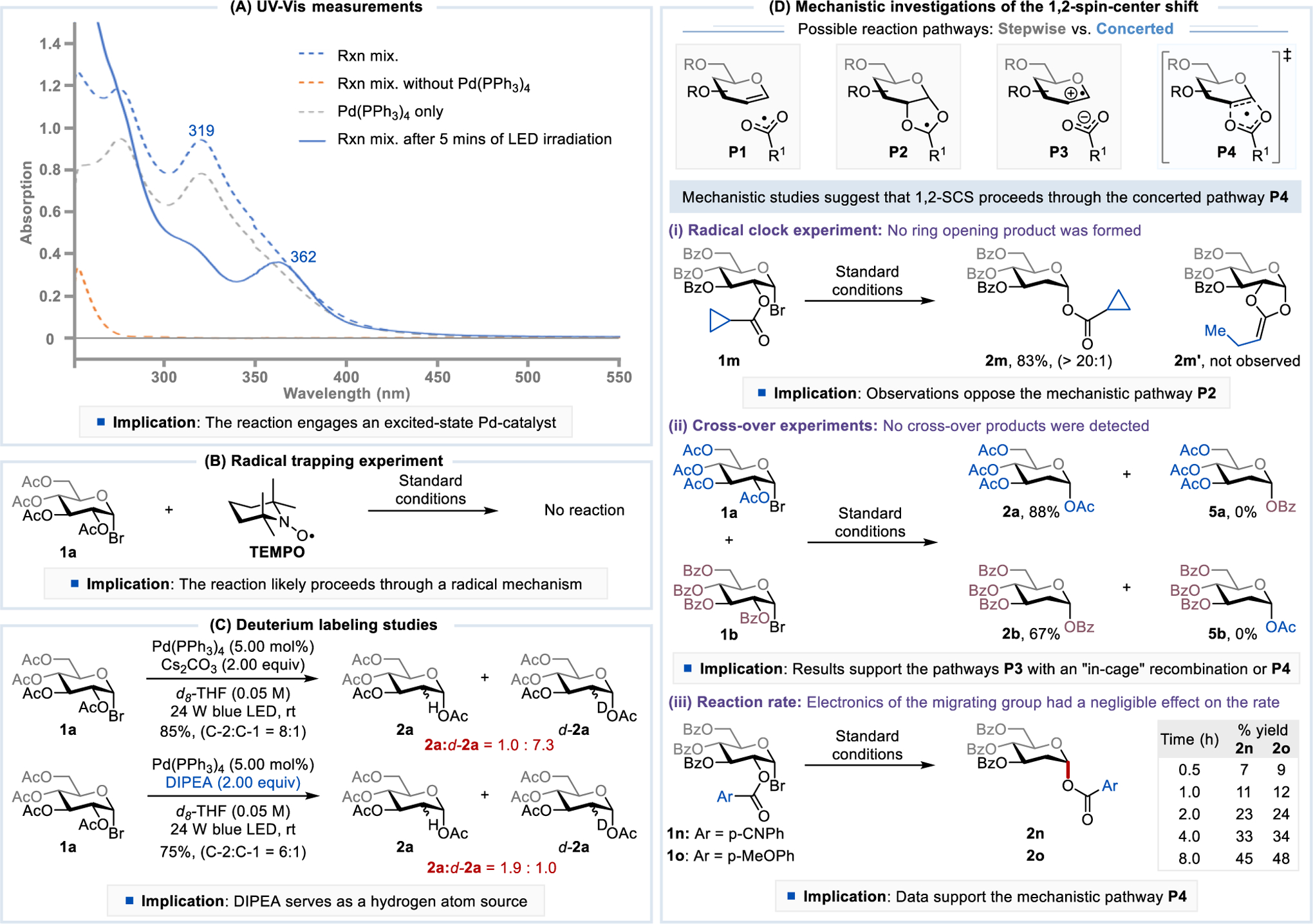
Mechanistic studies of excited-state Pd-catalyzed C-2 functionalization of carbohydrates. See Supporting Information for experimental details. % of deuterium incorporation, C-2:C-1 ratio, and reaction yields were determined by 1H-NMR using CH2Br2 as an internal standard.
The key 1,2-spin-center shift involving the acyloxy migration may proceed through one of the following reaction pathways: (P1) fragmentation to an acyloxy radical and an alkene with subsequent recombination; (P2) formation of a cyclic 1,3-dioxolanyl radical followed by ring-opening; (P3) fragmentation to an alkene radical cation and an acyloxy anion followed by recombination; or (P4) a concerted process involving a cyclic five-membered ring transition state (Figure 3D).11b The P1 pathway can be eliminated because the decarboxylation of an acyloxy radical (k = 109 s−1)27 is much faster than the migration (k = 102 s−1),19 and no decarboxylation was observed in the reaction. Pathway P2 is also unlikely because a radical clock experiment using a substrate bearing the cyclopropyl acetate group (1m) afforded the desired product 2m without the formation of a ring-opening side product 2m’. No cross-over products 5a and 5b were formed in cross-over experiments using substrates 1a and 1b, indicating that the reaction could proceed through either pathway P3 with an “in-cage” recombination or pathway P4. Since the electronics of the migrating group has a negilible effect on the reaction rate, the acyloxy migration most likely proceeds through the natural, concerted pathway P4, and this agrees with the DFT calculations.28
In summary, we established and exploited the first excited-state Pd-catalyzed 1,2-SCS reaction for the synthesis of C-2 functionalized carbohydrates from readily available 1-halosugars. The reaction features high levels of regio- and stereoselectivity, broad substrate scope, and mild reaction conditions that tolerate a wide range of functional groups and complex molecular structures. Detailed mechanistic studies suggest a non-chain radical reaction mechanism involving an excited Pd catalytic species and a 1,2-SCS via a concerted [2,3]-acyloxy rearrangement. Given the versatile reactivity of Pd catalysts in carbon-carbon and carbon-heteroatom bond forming reactions, we anticipate that our strategy will (i) offer a general, catalytic approach for the C-2 selective functionalization of carbohydrates to access a wide array of unexplored carbohydrate mimics, establish tools that tackle fundamental questions in glycobiology, and aid the discovery and development of new therapeutics; and (ii) guide the design and development of new synthetic strategies beyond carbohydrate chemistry.
Supplementary Material
ACKNOWLEDGMENT
The research reported in this publication was supported by the National Institutes of Health (NIH) under award number R35 GM119652 and National Science Foundation (NSF) CAREER Award under the award number CHE1848463. The Shimadzu UPLC/MS used for portions of this work were purchased with funds from NIGMS equipment administrative supplement, Shimadzu Scientific Instruments grant, and Office of the Vice Presi-dent for Research at Stony Brook University. We thank Mr. Chaudhary Harris for editing the manuscript.
Footnotes
Supporting Information
Experimental details and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This paper is dedicated to Prof. Barry M. Trost on the occasion of his 80th birthday. The authors declare no competing financial interests.
REFERENCES
- (1).For selected reviews, see:; (a) Chuentragool P; Kurandina D; Gevorgyan V, Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem. Int. Ed 2019, 58, 11586–11598; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Chuentragool P; Gevorgyan V, Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis 2019, 51, 985; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kancherla R; Muralirajan K; Arunachalam S; Rueping M, Visible Light-Induced Excited-State Transition-Metal Catalysis. Trends in Chemistry 2019, 1, 510–523; [Google Scholar]; (d) Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G, Visible Light-Induced Palladium-Catalysis in Organic Synthesis. Chem. Lett 2019, 48, 181–191; [Google Scholar]; (e) Cheng W-M; Shang R, Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS Catal 2020, 10, 9170–9196. [Google Scholar]
- (2).(a) Parasram M; Chuentragool P; Sarkar D; Gevorgyan V, Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers. J. Am. Chem. Soc 2016, 138, 6340–6343; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Parasram M; Gevorgyan V, Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem. Int. Ed 2017, 56, 14212–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wang G-Z; Shang R; Cheng W-M; Fu Y, Irradiation-Induced Heck Reaction of Unactivated Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2017, 139, 18307–18312. [DOI] [PubMed] [Google Scholar]
- (4).Zhou WJ; Cao GM; Shen G; Zhu XY; Gui YY; Ye JH; Sun L; Liao LL; Li J; Yu DG, Visible-Light-Driven Palladium-Catalyzed Radical Alkylation of C–H Bonds with Unactivated Alkyl Bromides. Angew. Chem. Int. Ed 2017, 56, 15683–15687. [DOI] [PubMed] [Google Scholar]
- (5).Pd(I)/radical intermediates were proposed in some ground-state Pd-catalyzed reactions. For selected reviews, see:; (a) Liu Q; Dong X; Li J; Xiao J; Dong Y; Liu H, Recent Advances on Palladium Radical Involved Reactions. ACS Catal 2015, 5, 6111–6137; [Google Scholar]; for selected examples, see:; (b) Bloome KS; McMahen RL; Alexanian EJ, Palladium-Catalyzed Heck-Type Reactions of Alkyl Iodides. J. Am. Chem. Soc 2011, 133, 20146–20148; [DOI] [PubMed] [Google Scholar]; (c) Xiao B; Liu Z-J; Liu L; Fu Y, Palladium-Catalyzed C–H Activation/Cross-Coupling of Pyridine N-Oxides with Nonactivated Secondary Alkyl Bromides. J. Am. Chem. Soc 2013, 135, 616–619; [DOI] [PubMed] [Google Scholar]; (d) Feng Z; Min QQ; Xiao YL; Zhang B; Zhang X, Palladium-Catalyzed Difluoroalkylation of Aryl Boronic Acids: A New Method for the Synthesis of Aryldifluoromethylated Phosphonates and Carboxylic Acid Derivatives. Angew. Chem. Int. Ed 2014, 53, 1669–1673; [DOI] [PubMed] [Google Scholar]; (e) Peacock DM; Roos CB; Hartwig JF, Palladium-Catalyzed Cross Coupling of Secondary and Tertiary Alkyl Bromides with a Nitrogen Nucleophile. ACS Cent. Sci 2016, 2, 647–652; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang C; Dong G, Direct B-Alkylation of Ketones and Aldehydes Via Pd-Catalyzed Redox Cascade. J. Am. Chem. Soc 2018, 140, 6057–6061. [DOI] [PubMed] [Google Scholar]
- (6).(a) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V, General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chuentragool P; Parasram M; Shi Y; Gevorgyan V, General, Mild, and Selective Method for Desaturation of Aliphatic Amines. J. Am. Chem. Soc 2018, 140, 2465–2468; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cheng WM; Shang R; Fu Y, Irradiation-Induced Palladium-Catalyzed Decarboxylative Desaturation Enabled by a Dual Ligand System. Nat. Commun 2018, 9, 5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Kurandina D; Rivas M; Radzhabov M; Gevorgyan V, Heck Reaction of Electronically Diverse Tertiary Alkyl Halides. Org. Lett 2018, 20, 357–360; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang G-Z; Shang R; Fu Y, Irradiation-Induced Palladium-Catalyzed Decarboxylative Heck Reaction of Aliphatic N-(Acyloxy) Phthalimides at Room Temperature. Org. Lett 2018, 20, 888–891; [DOI] [PubMed] [Google Scholar]; (c) Koy M; Sandfort F; Tlahuext-Aca A; Quach L; Daniliuc CG; Glorius F, Palladium-Catalyzed Decarboxylative Heck-8Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light. Chem. - Eur. J 2018, 24, 4552–4555; [DOI] [PubMed] [Google Scholar]; (d) Kancherla R; Muralirajan K; Maity B; Zhu C; Krach PE; Cavallo L; Rueping M, Oxidative Addition to Palladium(0) Made Easy through Photoexcited-State Metal Catalysis: Experiment and Computation. Angew. Chem. Int. Ed 2019, 58, 3412–3416; [DOI] [PubMed] [Google Scholar]; (e) Chuentragool P; Yadagiri D; Morita T; Sarkar S; Parasram M; Wang Y; Gevorgyan V, Aliphatic Radical Relay Heck Reaction at Unactivated C (Sp3)–H Sites of Alcohols. Angew. Chem. Int. Ed 2019, 58, 1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Huang H-M; Koy M; Serrano E; Pflüger PM; Schwarz JL; Glorius F, Catalytic Radical Generation of Π-Allylpalladium Complexes. Nat. Catal 2020, 3, 393–400; [Google Scholar]; (b) Huang H-M; Bellotti P; Pflueger PM; Schwarz JL; Heidrich B; Glorius F, A Three-Component, Interrupted Radical Heck/Allylic Substitution Cascade Involving Unactivated Alkyl Bromides. J. Am. Chem. Soc 2020, 142, 10173–10183; [DOI] [PubMed] [Google Scholar]; (c) Shing Cheung KP; Kurandina D; Yata T; Gevorgyan V, Photoinduced Palladium-Catalyzed Carbofunctionalization of Conjugated Dienes Proceeding Via Radical-Polar Crossover Scenario: 1,2-Aminoalkylation and Beyond. J. Am. Chem. Soc 2020, 142, 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Ratushnyy M; Parasram M; Wang Y; Gevorgyan V, Palladium-Catalyzed Atom-Transfer Radical Cyclization at Remote Unactivated C(Sp3)−H Sites: Hydrogen-Atom Transfer of Hybrid Vinyl Palladium Radical Intermediates. Angew. Chem. Int. Ed 2018, 57, 2712–2715; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheng W-M; Shang R; Fu Y, Irradiation-Induced Palladium-Catalyzed Decarboxylative Desaturation Enabled by a Dual Ligand System. Nat. Commun 2018, 9, 1–9; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Koy M; Bellotti P; Katzenburg F; Daniliuc CG; Glorius F, Synthesis of All-Carbon Quaternary Centers by Palladium-Catalyzed Olefin Dicarbofunctionalization. Angew. Chem. Int. Ed 2020, 59, 2375–2379; [DOI] [PubMed] [Google Scholar]; (d) Ratushnyy M; Kvasovs N; Sarkar S; Gevorgyan V, Visible-Light-Induced Palladium-Catalyzed Generation of Aryl Radicals from Aryl Triflates. Angew. Chem. Int. Ed 2020, 59, 10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Spin-center shift is broadly defined as shifting the position of the radical center to another atom in the course of the reaction. For selected review, see:; (a) Wessig P; Muehling O, Spin-Center Shift (SCS)–A Versatile Concept in Biological and Synthetic Chemistry. Eur. J. Org. Chem 2007, 2007, 2219–2232; [Google Scholar]; for selected recent examples, see:; (b) Jin J; MacMillan DW, Alcohols as Alkylating Agents in Heteroarene C–H Functionalization. Nature 2015, 525, 87–90; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nacsa ED; MacMillan DW, Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols. J. Am. Chem. Soc 2018, 140, 3322–3330; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dimakos V; Gorelik D; Su HY; Garrett GE; Hughes G; Shibayama H; Taylor MS, Site-Selective Redox Isomerizations of Furanosides Using a Combined Arylboronic Acid/Photoredox Catalyst System. Chem. Sci 2020, 11, 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).(a) Tanner DD; Law FC, Free-Radical Acetoxy Group Migration. J. Am. Chem. Soc 1969, 91, 7535–7537; [Google Scholar]; (b) Beckwith ALJ; Crich D; Duggan PJ; Yao Q, Chemistry of β-(Acyloxy)Alkyl and β-(Phosphatoxy)Alkyl Radicals and Related Species: Radical and Radical Ionic Migrations and Fragmentations of Carbon−Oxygen Bonds. Chem. Rev 1997, 97, 3273–3312. [DOI] [PubMed] [Google Scholar]
- (12).Varki A, Essentials of Glycobiology /Edited by Varki A; Cummings RD; Esko JD; Stanley P; Hart GW; Aebi M; Darvill AG; Kinoshita T; Packer NH; Prestegard JH; Schnaar RL; Seeberger PH Cold Spring Harbor Press: 2017. [PubMed] [Google Scholar]
- (13).(a) Ernst B; Magnani JL, From Carbohydrate Leads to Glycomimetic Drugs. Nat. Rev. Drug Discovery 2009, 8, 661–677; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fernández-Tejada A; Cañada FJ; Jiménez-Barbero J, Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. - Eur. J 2015, 21, 10616–10628. [DOI] [PubMed] [Google Scholar]
- (14).(a) Lu S; Li X; Wang A, A New Chiral Diphosphine Ligand and Its Asymmetric Induction in Catalytic Hydroformylation of Olefins. Catal. Today 2000, 63, 531–536; [Google Scholar]; (b) Hang HC; Bertozzi CR, Ketone Isosteres of 2-N-Acetamidosugars as Substrates for Metabolic Cell Surface Engineering. J. Am. Chem. Soc 2001, 123, 1242–1243; [DOI] [PubMed] [Google Scholar]; (c) De Lederkremer RM; Marino C, Deoxy Sugars: Occurrence and Synthesis. Adv. Carbohydr. Chem. Biochem 2007, 61, 143–216; [DOI] [PubMed] [Google Scholar]; (d) Pajak B; Siwiak E; Sołtyka M; Priebe A; Zieliński R; Fokt I; Ziemniak M; Jaśkiewicz A; Borowski R; Domoradzki T, 2-Deoxy-D-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci 2020, 21, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) Inghardt T; Frejd T, Oxirane Ring Opening of Anhydrosugars with Alkynyl- and Alkenylaluminates. Synthesis 1990, 1990, 285–291; [Google Scholar]; (b) Harvey JE; Hewitt RJ; Moore PW; Somarathne KK, Reactions of 1,2-Cyclopropyl Carbohydrates. Pure Appl. Chem 2014, 86, 1377–1399; [Google Scholar]; (c) Bennett CS; Galan MC, Methods for 2-Deoxyglycoside Synthesis. Chem. Rev 2018, 118, 7931–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Although C-2 reduction of 1-bromosugars using a stoichiometric amount of tin hydride/bulky tris(trimethylsilyl)silane and a radical initiator are known, a selective, catalytic process remains elusive.; (a) Giese B; Gröninger KS; Witzel T; Korth HG; Sustmann R, Synthesis of 2-Deoxy Sugars. Angew. Chem. Int. Ed 1987, 26, 233–234; [Google Scholar]; (b) Giese B; Kopping B; Chatgilialoglu C, Tris(Trimethylsilyl)Silane as Mediator in Organic Synthesis Via Radicals. Tetrahedron Lett 1989, 30, 681–684; [Google Scholar]; (c) Guiard J; Rahali Y; Praly JP, NaBH3CN: A Janus Substitute for Tin-Free Radical-Based Reactions. Eur. J. Org. Chem 2014, 2014, 4461–4466. [Google Scholar]
- (17).(a) Dupuis J; Giese B; Rüegge D; Fischer H; Korth HG; Sustmann R, Conformation of Glycosyl Radicals: Radical Stabilization by β-CO Bonds. Angew. Chem. Int. Ed 1984, 23, 896–898; [Google Scholar]; (b) Abe H; Shuto S; Matsuda A, Highly A-and B-Selective Radical C-Glycosylation Reactions Using a Controlling Anomeric Effect Based on the Conformational Restriction Strategy. A Study on the Conformation− Anomeric Effect− Stereoselectivity Relationship in Anomeric Radical Reactions. J. Am. Chem. Soc 2001, 123, 11870–11882. [DOI] [PubMed] [Google Scholar]
- (18).Korth H-G; Sustmann R; Gröninger KS; Witzel T; Giese B, Electron Spin Resonance Spectroscopic Investigation of Carbohydrate Radicals. Part 3. Conformation in Deoxypyranosan-2-, −3-, and −4-yl Radicals. J. Chem. Soc., Perkin Trans 2 1986, 1461–1464. [Google Scholar]
- (19).Korth HG; Sustmann R; Groeninger KS; Leisung M; Giese B, Electron Spin Resonance Spectroscopic Investigation of Carbohydrate Radicals. 4. 1, 2-Acyloxyl Migration in Pyranosyl Radicals. J. Org. Chem 1988, 53, 4364–4369. [Google Scholar]
- (20).Engl S; Reiser O, Copper Makes the Difference: Visible-Light-Mediated Atom Transfer Radical Addition (ATRA) Reactions of Iodoform with Olefins. ACS Catal 2020, 10, 9899–9906. [Google Scholar]
- (21). In some cases, we observed a trace amount of elimination and/or C-1 reduction side products.
- (22). We also tested mannose derivative where the B2,5 boat confirmation is less favorable, we only observed trace amount of C-2 reduction product.
- (23).(a) He X; Agnihotri G; Liu H. w., Novel Enzymatic Mechanisms in Carbohydrate Metabolism. Chem. Rev 2000, 100, 4615–4662; [DOI] [PubMed] [Google Scholar]; (b) Zhang L; Shi L; Shen Y; Miao Y; Wei M; Qian N; Liu Y; Min W, Spectral Tracing of Deuterium for Imaging Glucose Metabolism. Nat. Biomed. Eng 2019, 3, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Levy DE; Fügedi P, The Organic Chemistry of Sugars CRC Press: 2005. [Google Scholar]
- (25).Roush WR; Bennett CE, A Highly Stereoselective Synthesis of 2-Deoxy-β-Glycosides Using 2-Deoxy-2-Iodo-Glucopyranosyl Acetate Donors. J. Am. Chem. Soc 1999, 121, 3541–3542. [Google Scholar]
- (26).(a) Yang X; Fu B; Yu B, Total Synthesis of Landomycin a, a Potent Antitumor Angucycline Antibiotic. J. Am. Chem. Soc 2011, 133, 12433–12435; [DOI] [PubMed] [Google Scholar]; (b) Peng P; Liu H; Gong J; Nicholls JM; Li X, A Facile Synthesis of Sialylated Oligolactosamine Glycans from Lactose via the Lafont Intermediate. Chem. Sci 2014, 5, 3634–3639. [Google Scholar]
- (27).Hilborn JW; Pincock JA, Rates of Decarboxylation of Acyloxy Radicals Formed in the Photocleavage of Substituted 1-Naphthylmethyl Alkanoates. J. Am. Chem. Soc 1991, 113, 2683–2686. [Google Scholar]
- (28).Zipse H, [1, 2]-Acyloxy Shifts in Radicals. A Computational Investigation of Substituent and Solvent Effects. J. Am. Chem. Soc 1997, 119, 1087–1093 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


