Abstract
Transient Receptor Potential (TRP) channels expressed in specific subsets of airway sensory nerves function as transducers and integrators of a diverse range of sensory inputs including chemical, mechanical and thermal signals. These TRP sensors can detect inhaled irritants as well as endogenously released chemical substances. They play an important role in generating the afferent activity carried by these sensory nerves and regulating the centrally mediated pulmonary defense reflexes. Increasing evidence reported in recent investigations has revealed important involvements of several TRP channels (TRPA1, TRPV1, TRPV4 and TRPM8) in the manifestation of various symptoms and pathogenesis of certain acute and chronic airway diseases. This mini-review focuses primarily on these recent findings of the responses of these TRP sensors to the biological stresses emerging under the pathophysiological conditions of the lung and airways.
Keywords: transient receptor potential channel, airway, lung, respiratory disease, vagus, C-fiber
1. Introduction
1.1. Airway sensory nerves
Activities of sensory nerves arising from the lung and airways are conducted in two separate neural pathways: 1) vagal nerves and their branches innervate the entire respiratory tract, from larynx, conducting airways to lung parenchyma, and project to the nucleus tractus solitarius in the medulla, and 2) sympathetic afferents innervate lung parenchyma, pulmonary vessels and pleural region, and travel via the white rami communicants to the dorsal root ganglia (DRG) of spinal cord cervical and thoracic segments. An important role of vagal bronchopulmonary afferents in regulating the functions of the respiratory system under both healthy and disease conditions has been extensively studied and well documented [1–3]. These afferents are grossly classified into three major subgroups: slowly and rapidly adapting receptors conducted by myelinated fibers, and bronchopulmonary C-fibers [1,4]. The primary function of these afferents is to maintain homeostasis by regulating breathing pattern, bronchomotor tone and other essential cardiopulmonary functions. In comparison, transduction properties and regulatory functions of the sympathetic afferents are less clearly defined [2], and further investigations are warranted.
Another important function of vagal bronchopulmonary C-fibers and a subset of rapidly adapting receptors is to detect inhaled irritants and certain endogenous chemical substances; and elicit centrally mediated defense reflexes such as cough, mucous secretion and reflex bronchoconstriction [2,5]. In addition, intense or sustained stimulation of bronchopulmonary C-fibers by chemical irritants is known to evoke the release of neuropeptides including tachykinins and calcitonin-gene related peptide (CGRP) from their sensory terminals, which can in turn interact or act on a number of effector cells in the airways, trigger local “axon reflexes” and neurogenic inflammation resulting bronchoconstriction, protein extravasation, inflammatory cell chemotaxis [6]. These actions of tachykinins on airway functions are extensively documented in various airway diseases, but a marked difference in their potencies between different species has also been well recognized [6].
1.2. TRP channels in respiratory tract
The mammalian transient receptor potential (TRP) superfamily consists of 28 non-selective cation channels that can be divided into six subgroups on the basis of protein and nucleotide sequence homology: canonical (TRPC, 7 channels), vanilloid (TRPV, 6 channels), ankyrin (TRPA, 1 channel), melastatin (TRPM, 8 channels), polycystin (TRPP, 3 channels) and mucolipin (PRTML, 3 channels) families. Each of them contains six transmembrane spanning regions (S1–S6) with a pore-forming loop between S5 and S6 regions [7,8]. They are expressed in various cell types of the mammalian organ systems and also located in the plasma membrane of a number of cell organelles (e.g., endoplasmic reticulum, etc.) [9]. In the respiratory tract, they are found in sensory nerves (e.g., TRPV1, TRPA1, TRPM8), airway and vascular smooth muscles (TRPV4, TRPC3 & C6), airway epithelial cells and capillary endothelial cells (TRPV1 & V4, TRPC6), fibroblasts (TRPV4, TRPA1), alveolar macrophages (TRPV2 & V4), and neutrophils (TRPV4) [8,10,11]. It is extensively documented that these TRP channels play an important role in detecting a diverse range of biological signals and regulating the function of these cells.
Upon activation of TRP channels in the airway sensory nerves, the influx of cations can generate depolarization of the neuronal membrane leading to activation or inactivation of voltage-gated ion channels, and modulate the cell excitability. Increasing evidence continues to reveal the important biological properties and functions of these TRP channels, their involvements in regulating the physiological responses to various physicochemical stresses, and the up-regulation of their sensitivity and expression during airway inflammation.
Several comprehensive reviews of the roles of TRP channels as therapeutic targets in respiratory diseases have been published in the last decade [8,11–14]. In view of increasing new findings on the following TRP channels: TRPV1, TRPV4, TRPA1 and TRPM8, this brief review will focus specifically on their respective roles in the neural control of airway functions. Particular emphases have been given to their potential involvements in the development of pathophysiological conditions in the lung and airways.
2. TRP channels expressed in airway sensory nerves
The cell bodies of vagal sensory neurons innervating the respiratory tract are located in two separate but adjacent vagal sensory ganglia: nodose and jugular ganglia. It has been clearly documented that the neurons within these two ganglia are of different embryological origins and distinct phenotypes, including the differences in their distributions in the lung and airway structures and expression of TRP channels [3]. An in-depth review of this important and complex subject is presented in another article by Taylor-Clark et al. in this special issue and therefore not included in this mini-review.
2.1. TRPV1
TRPV1 is recognized as a “molecular gateway” to nociceptive sensation in somatic and visceral tissues and can be activated by a wide range of nociceptive chemical (e.g., acid), physical (e.g., heat) and biological stimuli (e.g., capsaicin) [8,15].
In the respiratory tract, although the expression of TRPV1 is also found in other cell types such as airway epithelial cells [16] and airway smooth muscles [17], TRPV1 is predominantly expressed in the sensory nerves, particularly in the C-fibers that represent a majority (>70%) of the vagal bronchopulmonary afferents [18]. In fact, TRPV1 is known as a reliable biomarker for identifying the C-fiber sensory neurons [2,19]. Indeed, one of the most distinct characteristics of these C-fiber afferents is their exquisite sensitivity to capsaicin, the pungent extract of hot peppers and a selective and potent activator of TRPV1 [15,19]. In contrast, their myelinated counterpart of the vagal pulmonary afferents, slowly and rapidly adapting receptors, exhibit either no or negligible sensitivity to capsaicin when the reflex contraction of airway smooth muscles is prevented in intact animals under normal condition [2,19].
TRPV1 is a polymodal transducer that can be activated directly or indirectly by a number of endogenous chemical substances such as hydrogen ion [20], anandamide [21,22], bradykinin [23], lysophosphatidic acid [24] and lipoxygenase metabolites [25]. TRPV1 can also detect physicochemical perturbations in the airway tissues; for example, TRPV1 expressed in the respiratory tract are extremely sensitive to a slight increase (Δ = ~3 °C) in tissue temperature within the normal physiological range [26,27]. More recent studies further revealed that TRPV1 and TRPA1 can be activated by air borne particulate matters [28,29] and volatile anesthetics such as isoflurane [30]. The superficial and strategic locations of these TRPV1-expressing sensory terminals in the airway mucosa [31] render them an important sensor to detect inhaled chemical irritants and elicit the protective airway reflexes.
Bronchial hypersensitivity, characterized by exaggerated sensory (e.g., airway irritation) and reflex mediated responses (e.g., cough, bronchoconstriction) to inhaled irritants and certain endogenous inflammatory mediators, is a prominent pathophysiological feature in patients with airway inflammatory diseases such as asthma. Increasing evidence reported in recent studies suggested a pivotal role of TRPV1 in the manifestation of various symptoms of bronchial hypersensitivity in these patients [32–34]. For example, certain endogenous TRPV1 activators such as H+, lipoxygenase metabolites (e.g., 12S- and 15S-hydroperoxyeicosatetraenoic acid), bradykinin and sphingosine-1-phosphate are consistently detected in the bronchoalveolar lavage fluid, sputum and/or exhaled breath condensate of patients with asthma [9,25,35–38]. Cough sensitivity to TRPV1 activators was markedly elevated in patients with asthma or other airway inflammatory diseases [39,40]. Furthermore, certain endogenous inflammatory mediators and cytokines (e.g., prostaglandin E2, tumor necrosis factor α [TNFα], etc.), though not a TRPV1 activator themselves, can enhance the sensitivity of TRPV1 [34,41]; TRPV1 has several consensus phosphorylation sites that can be phosphorylated by protein kinases A (PKA), PKC and PKG, tyrosine kinase, etc. [41–44]. It is evident that TRPV1 functions not only as a transducer but also an integrator of biological actions generated by multiple endogenous activators and modulatory molecules.
Recent studies have further revealed that the allergic inflammation-induced airway hyperresponsiveness was associated with an enhanced bronchopulmonary C-fiber sensitivity to TRPV1 activators [45,46], accompanied by an upregulation of TRPV1 expression in Aδ vagal afferents of nodose ganglionic origin [46–48], mainly rapidly adapting receptors. As such, a near-threshold level of stimulation is expected to evoke a greater afferent discharge of bronchopulmonary C-fibers and consequently more intense sensory and reflex responses, resulting in bronchial hypersensitivity [34]. For example, a recent report showed that a slight increase in airway temperature triggered vigorous coughs and a transient bronchoconstriction mediated through cholinergic reflex pathway in patients with mild asthma, but not in heathy individuals [49].
2.2. TRPV4
TRPV4, originally identified as an osmosensor on mammalian cells [50], is now recognized as a molecule integrator of diverse stimuli [51]. It can be activated by physical stimuli including hypotonicity, moderate heat and shear stress, as well as a number of endogenous and exogenous chemical stimuli, such as acidic pH, anandamide, arachidonic acid metabolites, 4α-PDD and GSK1016790A [52]. TRPV4 is widely expressed in respiratory track, including vascular smooth muscle, endothelial cells, epithelial cells of alveoli, trachea and bronchi, and inflammatory cells such as macrophages and neutrophils [13,51]. Although TRPV4 mRNA has been detected in pulmonary sensory neurons [26], the functional expression of this TRP channel in vagal bronchopulmonary afferents and its role as a peripheral nociceptor in the lungs and airways are yet to be unequivocally defined.
In anesthetized rats, right atrial injection of selective TRPV4 agonist GSK1016790A evoked a slowly-developing rapid shallow breathing and an increased pulmonary chemosensitivity to capsaicin. The stimulating and sensitizing effects were abolished by sectioning and perineural capsaicin treatment of both cervical vagi, TRPV4 antagonist GSK2193874, or by systemic infusion of the COX inhibitor indomethacin. Surprisingly, patch clamp recordings showed that GSK1016790A or 4α-PDD failed to activate or sensitize isolated bronchopulmonary sensory neurons [53]. These results suggested that activation of TRPV4 regulated the respiration in rats through an indirect stimulation of vagal bronchopulmonary afferents, likely via the effect on other TRPV4-expressing cells, e.g., macrophages, epithelial or endothelial cells in the lungs and airways. Similarly, in a study of airway sensory nerves, Bonvini et al. [54] showed that TRPV4 agonists induced Ca2+ flux in guinea pig nodose ganglion neurons, depolarization of guinea pig, mouse and human vagus nerves, discharge of Aδ fibers (not C-fibers), and coughing in conscious guinea pigs. However, the single-cell RT-PCR study showed that TRPV4 was expressed on 1 out of 32 and 0 out of 32 guinea pig nodose and jugular ganglia neurons, respectively, suggesting that TRPV4 exerted its effects on accessory cells and acted on sensory neurons via an indirect mechanism. The study further proposed the TRPV4-ATP-P2X3 interaction as a key osmotic sensing pathway involved in airway sensory nerve reflexes [54].
2.3. TRPA1
TRPA1 is expressed in a sub-population of TRPV1-expressing airway neurons [55,56] (e.g., Fig. 1A), and is considered as the major airway irritant sensing receptor [57]. TRPA1 can be activated by cold temperature (< 17°), a wide range of pungent compounds and environmental irritants such as allicin, allyl isothiocyanate (AITC), cannabinoids, acrolein, ozone, formaldehyde, cinnamaldehyde, crotonaldehyde, commonly used tear gases, heavy metals zinc, cadmium and copper, and anesthetics isoflurane, lidocaine and nicotine [13,57,58]. It can also be activated by a variety of endogenous mediators such as hydrogen sulfide [59], reactive and electrophilic byproducts of oxidative (ROS), nitrative (RNS), and carbonilyc (RCS) stress [14,60,61]. A recent study demonstrated a direct interaction between diesel exhaust particles and airway C-fiber afferents in guinea pig and human vagus nerves that was mediated through an oxidative stress pathway and activation of TRPA1 channels expressed on these airway afferents [62]. In addition, TRPA1 has been suggested to play a crucial role in detection of lipopolysaccharides (LPS), a microbial signature molecule, by sensory neurons in flies and mammals [63].
Fig. 1. Co-expression of TRPV1 and TRPA1 in airway sensory neurons and a comparison of neural and cough responses to their respective agonists.
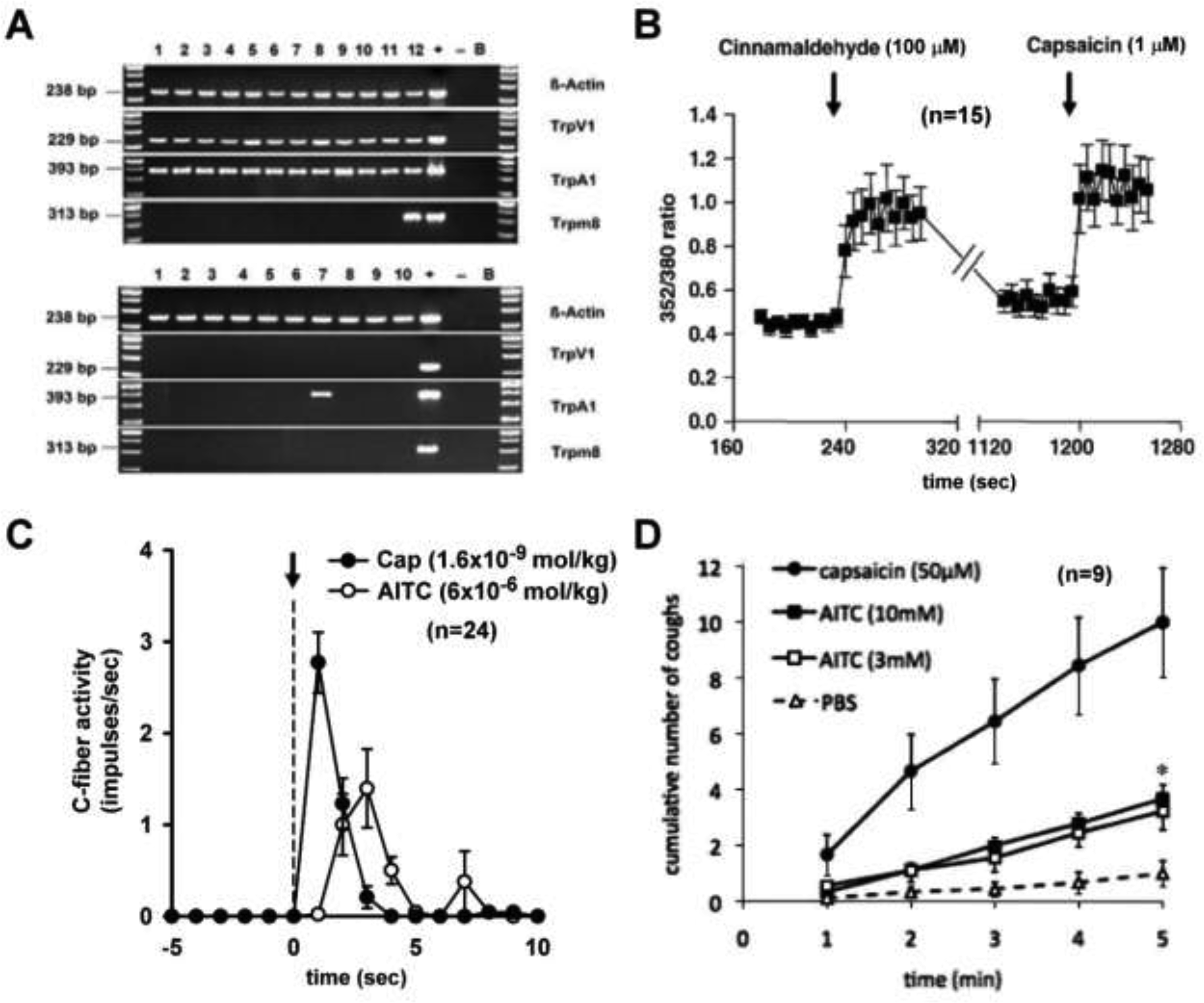
A: Single cell RT-PCR of TRPV1+ (top) and TRPV1− (bottom) mouse jugular/nodose cells retrogradely labelled from the lung and airways. Samples in which the reverse transcriptase was omitted (‘−’) served as negative control. cDNA obtained from a whole jugular/nodose ganglion served as positive control (‘+’); B: bath solution was used as a template. B: Effect of cinnamaldehyde and capsaicin, selective agonists of TRPA1 and TRPV1, respectively, on intracellular [Ca2+]free (expressed as 352/380 ratio) in dissociated DiI-labelled jugular/nodose neurons from mouse lung. C: Responses of pulmonary C-fibers activity to intravenous injections (dashed line) of allyl isothiocyanate (AITC; ave 6×10−6 mol/kg), a selective agonist of TRPA1, and capsaicin (Cap; ave 1.6×10−9 mol/kg) in anesthetized rats. Note that the potency of the stimulatory effect of Cap on bronchopulmonary C-fibers is >1000-fold higher than that of AITC. D: Time course of the cough induced by inhalation of AITC and capsaicin (paired study, n=9) in awake guinea pigs. Note that increasing the concentration of AITC from 3 mM to 10 mM did not further increase cough frequency, indicating that the maximally effective concentration of AITC was attained; AITC (10 mM) was significantly less effective in inducing cough than capsaicin (50 μM); *, P<0.05, Cap vs AITC. Data are means ± SEM; n is shown in each panel. (A&B, C and D modified from references 56, 70 and 86, respectively)
2.4. TRPM8
TRPM8 is gated by cool and noxious cold temperatures, and can also be activated by the cooling compounds menthol, icilin, and eucalyptol [64]. It is predominantly expressed in a subset of primary afferent neurons within the DRG and trigeminal ganglia that are largely distinct from neurons expressing TRPV1 and TRPA1 [65,66]. The expression of TRPM8 in vagal bronchopulmonary sensory neurons seems relatively sparse. In a study of vagal afferent nerves innervating the mouse lungs, there was little evidence of TRPM8 expression [56]. In acutely dissociated vagal ganglion neurons innervating rat airways and lungs, 16% neurons expressed TRPM8 as demonstrated by both cold and menthol sensitivity [67]. Using an ex vivo rat lung preparation, another study showed that a similar small fraction (15.3%) of afferent nerves were activated by both cold temperature and menthol, but not a TRPA1 agonist, cinnamaldehyde [68]; this is an interesting distinction since TRPA1 can also be activated by either cold or menthol in vitro (57).
3. Interaction between TRP channels and its implications
As described above, a majority of these TRP channels are polymodal transducers; for example, TRPV1 can be activated by low pH, high temperature, anandamide, bradykinin and other endogenous inflammatory mediators; TRPA1 by various environmental chemical irritants, ROS, bradykinin and other inflammatory mediators; TRPV4 by hypotonicity, shear stress, anandamide, etc. As such, more than one TRP channels can be activated in the same cell by the same chemical mediator. Furthermore, because both TRPA1 and TRPV1 can be activated by a number of endogenous inflammatory mediators, it is highly probable that they can be activated simultaneously during airway inflammatory reaction. In addition, both TRPV1 and TRPA1 are abundantly and selectively expressed in vagal pulmonary C-fiber sensory nerves [56] (Fig. 1), and interestingly, their co-localization in the same cells increased from 55% to 80% in trigeminal ganglion neurons after a treatment of nerve growth factor [69]. More importantly, recent studies showed that simultaneous activations of TRPA1 and TRPV1 with their respective selective agonists at near-threshold concentrations evoked an abrupt and striking potentiating effect; this synergistic effect was clearly illustrated in both studies of vagal bronchopulmonary C-fiber recording in intact animals [70] and in isolated bronchopulmonary sensory neurons [71]. The synergism was completely abrogated in isolated neurons when Ca2+ was removed from the extracellular solution [71], suggesting an involvement of activation of certain intracellular signaling pathway(s) and molecule(s) initiated by the Ca2+ influx conducted through these channels (Fig. 2). Such an important role of intracellular Ca2+ as a mediator regulating the interaction between the TRPA1 and TRPV1 channels has been reported in the nociceptor neurons during acute inflammatory hyperalgesia [72,73], as illustrated in Fig. 2 [74].
Fig. 2. Synergistic effect of simultaneous activations of TRPA1 and TRPV1 channels in pulmonary sensory neurons.
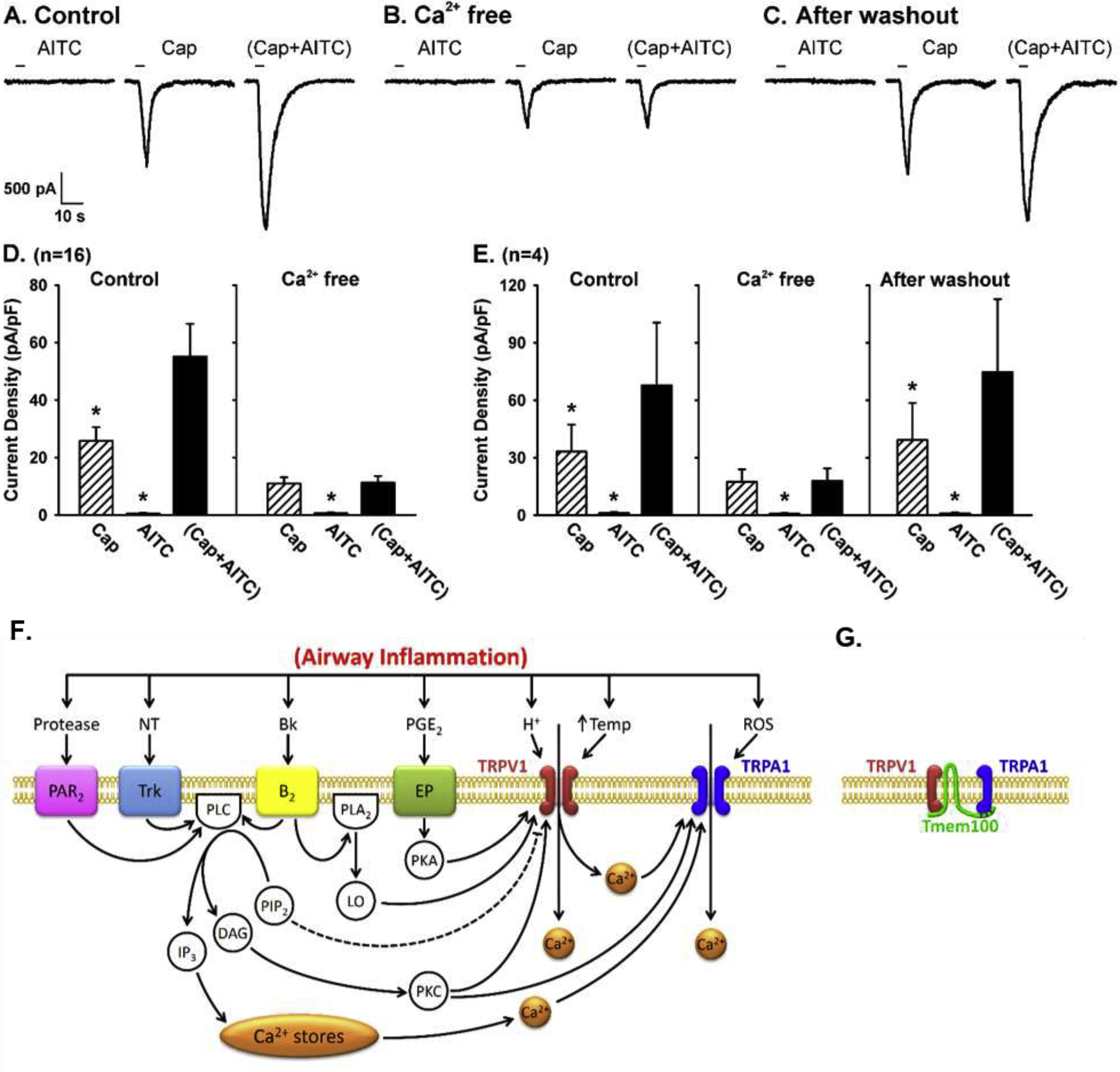
A: experimental records illustrating the positive interaction of TRPA1 and TRPV1 channels during control in a jugular neuron (27.4 pF). B: the positive interaction was completely abolished after the same neuron was perfused by Ca2+-free ECS for 10 min. C: the positive interaction returned after the same neuron was perfused by regular ECS again for 20 min. D: group data of the responses (n=16) when neurons were perfused with regular ECS (Control) and Ca2+-free ECS, respectively. E: in 4 of these 16 neurons, group data were also obtained after the Ca2+-free ECS was washed out. Data are means ± SEM. *, significantly (P < 0.05) different from the response to (Cap+AITC). F: hypothesized mechanisms involved in the TRPA1-TRPV1 interaction in pulmonary sensory neurons during airway inflammation. Dashed line depicts an inhibitory pathway. Bk, bradykinin; B2, bradykinin B2 receptor; DAG, diacyglycerol; EP, prostanoid EP receptors; IP3, inositol 1,4,5-triphosphate; LO, lipoxygenase products; NT, neurotrophins; PAR2, protease-activated receptor-2; PGE2, prostaglandin E2; PLC, phospholipase C; PLA2, phospholipase A2; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; ROS, reactive oxygen species; Temp, temperature; Trk receptors, tryosine receptor kinase receptors A and B. G: potential physical interaction between TRPA1 and TRPV1 subunits in a heteromeric A1/V1 channel complex; Tmem100, a two-transmembrane adaptor protein. (A-E and F-G are modified from references 71 and 74, respectively)
A recent study further revealed that Tmem100, a two-transmembrane adaptor protein with a putative TRPA1 binding site at its C-terminus, can alter the physical association between TRPA1 and TRPV1, and thereby regulate the excitability of the TRPA1-TRPV1 heteromeric channel complex [75]. In the presence of Tmem100, the physical association between TRPA1 and TRPV1 was weakened and the sensitivity to TRPA1 agonist was enhanced in a TRPV1-dependent manner (Fig. 2C). Taken together, these findings suggest a potentially important role of the TRPA1-TRPV1 interaction in regulating the excitability and function of bronchopulmonary C-fiber sensory nerves during airway inflammatory reaction [70,71,74].
Both TRPA1 and TRPM8 can be activated by cold temperature, but at different temperature thresholds (25–28 °C in TRPM8 and 17 °C in TRPA1), in addition to their respective specific chemosensitivities. However, they are expressed in two distinct populations of sensory neurons in DRG [65]: TRPA1 is expressed in nociceptive neurons containing tachykinins, whereas TRPM8 does not co-express with tachykinins [76]. In addition, in rat DRG neurons all TRPM8-expressing cells also express TrkA, whereas the expression of TRPA1 is independent of TrkA expression [55]. Despite a lack of evidence of their co-expression in the same neurons, a selective activation of TRPM8 by menthol or eucalyptol, was able to inhibit or attenuate the irritant effect evoked by activating TRPA1 or TRPV1 in mice and in isolated murine DRG neurons, but the underlying mechanism was not entire clear [77].
4. Roles of TRP channels in pathophysiological conditions
4.1. Cough hypersensitivity
TRPV1.
The reproducible dose-dependent cough responses to inhalation of aerosolized capsaicin has been extensively reported in both animal and human studies, without serious short- or long- term side effects [78,79]; as such, capsaicin inhalation challenge has been commonly used for testing the cough reflex sensitivity in humans [80]. There is a significant correlation between cough sensitivity and the density of TRPV1-expressing nerves in the mucosa of chronic cough patients [17,81]. These observations led to the assumption of TRPV1 expressed in the airway sensory nerves is likely the cough sensor mediating the pathophysiological condition of cough hypersensitivity [82]. However, in clinical trial studies, despite the profound inhibitory effects the TRPV1 antagonists, SB-705498 and XEN-D0501, on capsaicin-evoked cough, neither of them has proven to be effective in the treatment of patients with refractory chronic cough [83,84]. These results raised the questions about the precise role of TRPV1 in the pathogenesis of refractory chronic cough and/or the possible involvements of multiple cough sensors in this disease.
TRPA1.
Activation of TRPA1 in airway sensory nerves can evoke cough in both animals and humans. Acrolein, cinnamaldehyde, crotonaldehyde and AITC have been shown to cause cough in guinea pigs [85,86]. Cinnamaldehyde induces a concentration-dependent cough response in human volunteers [85]. TRPA1 blockade diminishes these responses as well as the coughing induced by endogenous mediators PGE2 and bradykinin [87]. Citric acid–induced cough response is similarly alleviated by selective TRPA1 blocker [88]. Based upon these observations, TRPA1 was suggested as a promising target for developing therapeutic treatment for chronic cough [10,89]. However, collective data indicated a distinctly lower efficacy of its selective agonists (e.g., AITC or cinnamaldehyde) as a tussive agent in awake guinea pigs [86] and also a markedly lower potency as a stimulant of isolated airway sensory neurons [56] or bronchopulmonary C-fibers [70] than capsaicin, a selective agonist of TRPV1 (Fig. 1). Furthermore, the clinical trial of the selective TRPA1 antagonist, GRC 17536, has not yielded successful outcome in patients with chronic cough [90], once again illustrating the unpredictability of success in translating animal experimental data to human clinical trial outcome.
TRPM8.
Breathing cold air can provoke cough, airway constriction, mucosal secretion and trigger an asthma attack [91], such responses are thought to be mediated by vagal afferent nerves [92,93], and through the activation of TRPM8 channels [67,94]. However, TRPM8 agonist menthol has been widely used as antitussive treatment. It can inhibit citric acid induced cough [95], decreases capsaicin cough reflex sensitivity [96], and is often used for suppressing respiratory irritancy [8]. It is worth noting that menthol can also affect a number of other ion channel targets besides TRPM8 that could in part account for its inhibition of irritating sensations or cough (97). It has been proposed that the antitussive effect of menthol may result from TRPM8-dependent activation of nasal trigeminal, rather than vagal bronchopulmonary sensory nerves [66]. In a recent pilot clinical study, a TRPM8 agonist, AX-8, given as a lozenge significantly reduced cough frequency during waking hours in refractory chronic cough patients compared with baseline (no therapy), accompanied by reductions in the patient-reported outcomes of cough severity, throat irritation and urge-to-cough [98].
4.2. Asthma
TRPV1.
Cumulative evidence have suggested potential involvement of TRPV1 in the pathogenesis of asthma. A general hypothesis has been as follows: inhaled irritants (e.g., acid) or endogenous TRPV1 activators (e.g., lipoxygenase metabolites) activate TRPV1 that are overexpressed and sensitized through intracellular pathways driven by inflammatory mediators, which leads to exaggerated TRPV1 activation and bronchopulmonary C- and Aδ- fiber discharges. The resulting various aspects of tachykinin-mediated “neurogenic inflammation” contribute to the manifestation of clinical features of asthma, including excessive cough [12,33]. Despite the extensive supportive results obtained from pre-clinical studies, the precise role of locally released tachykinins play in the pathogenesis of asthma and cough hypersensitivity remains uncertain as results obtained from clinical trials in testing the efficacy of tachykinin receptor antagonists have been largely either non-conclusive or disappointing [99,100].
In allergic animal models, pretreatment with capsaicin to degenerate airway TRPV1-expressing afferents inhibited allergen-induced bronchoconstriction in sensitized guinea pigs [101] and airway hyperresponsiveness in allergic rabbits [102]. Capsaicin pretreatment and depletion of sensory neuropeptides also inhibited inflammatory cells accumulation and airway hyperresponsiveness in a mouse model of non-atopic asthma [103]. An issue with the above mentioned studies is that they could not differentiate whether the effects are due to degeneration of TRPV1 channels specifically, or degeneration of all TRPV1-expressing sensory nerves [104], the subpopulation of which are known to also include TRPA1-expressing neurons [13,57]. Studies with TRPV1 antagonism supported an important role for TRPV1 in asthma. Two TRPV1 antagonists SB-705498 and PF-04065463 significantly inhibited airway hyperresponsiveness to histamine in ovalbumin (OVA)-sensitized guinea pigs [105]. In a mouse model of OVA-induced asthma, blocking the TRPV1 pathway by capsazepine or TRPV1 siRNA inhalation significantly alleviated airway hyperresponsiveness, allergic inflammation and airway remodeling [106].
TRPV4.
Activation of TRPV4 is known to cause bronchoconstriction, a key symptom of asthma [107], but the underlying mechanism is not yet clear. McAlexander et al. [108] demonstrated that TRPV4 agonist GSK1016790A produced a slow-onset, long-lasting contraction of guinea pig and human isolated airways. This contraction could be blocked by TRPV4 antagonist, but also by cysteinyl leukotriene (cystLT) antagonists and a 5 lipoxygenase inhibitor, indicating that the activation of TRPV4 did not induce airway smooth muscle contraction directly, and instead reliant upon the production of cystLT. A recent study by Bonvini et al. [109] further suggested that activation of TRPV4 induced release of ATP from airway smooth muscle cells which triggered P2X4-dependent release of cysteinyl leukotrienes from nearby mast cells and resulted in airway smooth muscle contraction. It would be interesting to examine whether this novel TRPV4-ATP-cystLT axis is also involved in the indirect stimulation of bronchopulmonary sensory nerves upon TRPV4 activation.
TRPA1.
Exposure to certain chemical irritants such as ozone [110] and toluene diisocyanate [111], which are now known to activate TRPA1, has been shown to produce asthma-like symptoms such as cough, dyspnea, wheezing and subsequent hypersensitivity to chemical, physical and/or electrical stimuli [112–114]. Indeed, TRPA1-activating stimuli such as chlorine, aldehydes, ROS, and scents are among the most prevalent triggers of asthma [115]. Acetaminophen, via its reactive metabolite N-acetyl-pbenzo-quinoneimine, was reported to induce airway neurogenic inflammation through TRPA1 activation and subsequent release of sensory neuropeptides substance P and CGRP. The responses were abated by TRPA1 antagonism and absent in TRPA1-deficient mice, indicating that TRPA1-dependent neuronal inflammation may contribute to the increased risk of asthma and other atopic and inflammatory conditions that are associated with acetaminophen consumption [116].
In a non-allergic mouse model of asthma, the induction of airway hyperresponsiveness without bronchial inflammatory cells by exposure to hypochlorite-OVA was shown to result from a neuroimmune interaction between sensory neurons and mast cell activation which was significantly reduced in TRPA1-deficient mice [117]. In an OVA-induced mouse model of allergic asthma, the allergen-induced leukocyte infiltration, cytokine and mucus production as well as airway hyperreactivity were significantly attenuated in TRPA1-deficient mice or wild-type mice treated with TRPA1 inhibitor HC-030031 [115]. In both cases, the neuronal expression of TRPA1 channels was most probably responsible for disease symptoms [11]. The TRPA1-dependent airway inflammation shown in mouse OVA model of asthma has been reproduced in recently developed TRPA1-deficient rats [118].
TRPM8.
A TRPM8 gene polymorphism is positively associated with cold-evoked airway hyperresponsiveness, a characteristic feature of bronchial asthma [119]. TRPM8 is also responsible for cold-induced mucus hypersecretion through Ca2+- PLC-PIP2-MARCKS signaling pathway [120]. TRPM8 seems also contribute to airway inflammatory responses due to the activation of this channel in non-neuronal cells in the respiratory tract. The expression of TRPM8 in human bronchial epithelial cells is well established [120,121], activation of which by cold or menthol increases the expression of many pro-inflammatory cytokines, an effect inhibited by nonselective TRPM8 antagonist BCTC, and siRNA knockdown [122]. A recent in vitro study [123] demonstrated that the extracts of menthol cigarette smoke induced greater reactive oxygen species-sensitive, TRPM8-mediated, mitogen-activated protein kinase (MAPK) - dependent inflammatory responses in human lung epithelial cells, than those of non-menthol cigarette smoke. A follow up study [124] further showed that sub-chronic exposure in mice to menthol cigarette smoke induced greater TRPM8-mediated activation of MAPKs and lung inflammation than that to non-menthol cigarette smoke. The augmented inflammatory effects likely resulted from menthol-evoked additional stimulation of TRPM8 channels on lung epithelial cells. Furthermore, TRPM8 is expressed in mast cells, where it has been implicated in cold- and menthol-elicited histamine release [125], which offers an alternative explanation for the involvement of TRPM8 in the menthol- and cold-induced airway allergic and inflammatory responses.
4.3. COPD
The involvement of TRP channels in the development of COPD has been suggested [11,14]. TRPV1 and TRPV4 mRNA levels were increased in lung tissue samples from patients with COPD, and cigarette smoke exposure–induced ATP release from primary bronchial epithelial cells was attenuated by blockers of both TRP channels [126]. Polymorphisms in the TRPV4 gene have been associated with COPD [127]. Tiotropium, a drug widely prescribed for its bronchodilator activity in patents with COPD and asthma, inhibited neuronal TRPV1-mediated effects in guinea pigs through a mechanism unrelated to its anticholinergic activity [128].
Recent evidence indicates that TRPA1 could be the primary TRP channel implicated in the pathogenesis of COPD [129]. TRPA1 is a major neuronal sensor of oxidative stress, which is known to be substantially increased in COPD [130]. The vast majority of COPD patients are smokers. Crotonaldehyde, α,β-unsaturated aldehydes and acrolein contained in cigarette smoke, the major causative factor of COPD, cause irritation and inflammation in the respiratory tract, via activation of TRPA1 [73,131]. Cigarette smoke extract increases TRPA1 expression and induces IL8 release in human bronchial epithelial cells, the latter can be attenuated by TRPA1 inhibitor HC-030031 or TRPA1 siRNA [132]. Nicotine, the major chemical constituent of cigarette smoke, has also been reported to activate TRPA1 directly; however, this action required a concentration of nicotine much higher (>100 folds) than that delivered by cigarette smoke [133,134].
Exposure to biomass fuels and biomass smoke is also a risk factor for COPD development [11,135]. Wood smoke particulate matters has been shown to cause calcium influx in trigeminal sensory neurons through TRPA1 activation [136]. Diesel exhaust particles activate TRPA1 channels in airway C-fiber afferents in guinea pig and human vagus through an oxidative stress pathway [62]. Ample evidence suggests that TRPA1 antagonism might be a promising option in clinical management of COPD [89].
4.4. Airway viral infection
In addition to the injury of alveolar walls and lung parenchyma cells, respiratory viral infection induces inflammatory reaction in the airway mucosa that is densely innervated by TRPV1-expressing sensory nerves [31], which can lead to the development of airway hypersensitivity. Indeed, cough sensitivity to inhalation of TRPV1 activators, capsaicin or citric acid aerosol, was markedly elevated in patients during and recovering from upper respiratory infection [39,40]. The evidence of a pronounced upregulation of TRPV1 expression was found in the airway mucosa of patients with chronic cough during recovery from respiratory tract infections caused by rhinovirus, respiratory syncytial virus and measles virus [81,137,138]. In addition, the viral infection increased the tachykinin synthesis in C- and Aδ-fiber airway afferents and upregulated the neurokinin receptor expression on the target cells in the lung [139–141]; it also inhibited the preganglionic muscarinic M2 autoreceptors in the airways [142]. Together, they augmented the reflex bronchoconstriction mediated through both cholinergic and tachykininergic mechanisms as well as the cough responsiveness to TRPV1 activation.
In the current pandemic of coronavirus (COVID-19) infectious diseases, one of the major causes of mortality is respiratory failure [143]; possible involvements of abnormal function of TRPV1 and TRPV4 in the development of lung dysfunction have been suggested [144,145]. The “cytokine storm”, a primary pathogenic factor of acute respiratory distress syndrome (ARDS) and multiple organ failures in COVID-19 patients, is triggered by a dysregulated and severe immune reaction as indicated by excessively high levels of inflammatory markers and cytokines in the plasma [146,147]. Some of these endogenous mediators (e.g., prostaglandins, leukotrienes) and cytokines (e.g., TNFα, interleukin-1β) are known to activate pulmonary C-fibers [148–151] via sensitization or direct stimulation of TRPV1 receptors [149–150], which in turn leads to airway constriction, increased alveolar capillary permeability and inflammatory cell chemotaxis through both centrally-mediated and local axon reflexes [2].
TRPV4 is also abundantly expressed on alveolar epithelial and capillary endothelial cells (Section 2.2). A recent clinical trial phase-1 study has demonstrated effective protective actions of the TRPV4 inhibitor in the treatment of cardiogenic pulmonary edema [152], suggesting a possible role of TRPV4 in regulating the integrity of the alveolus/capillary barrier [153,154]. In view of the fact that a disruption of the alveolus/capillary barrier integrity can lead to alveolar edema and other serious detrimental consequences of the ARDS, it seems plausible that a selective TRPV4 antagonist may provide a potentially effective therapeutic action on the alveolar edema caused by COVID-19 infection [144].
5. Conclusion
The new information obtained from these recent studies has provided valuable insights and broadened our knowledge about the potential involvements of these TRP channels in the development of certain pathophysiological conditions in the lung and airways, but many important and challenging questions still remain. First and foremost, it is imperative that these new findings be tested in clinical trial studies to further evaluate their significance and impact on developing the new therapeutic strategies for treating patients with these respiratory diseases.
It is well documented that TRP sensors are also expressed in a wide spectrum of non-neuronal cells in the respiratory tract as described earlier (Section 1.2.). Although their actions and interactions with sensory neurons are beyond the scope of this brief review, existing evidence clearly indicates their important roles in regulating the overall function of airway nervous system under both normal and pathophysiological conditions, which cannot be overlooked. For example, it is well recognized that both nervous and immune systems are the cornerstones of the important pulmonary defense function. Despite the clear evidence of expression of various TRP channels (e.g., TRPC1, TRPC3, TRPA1, TRPV1, TRPV4) in a number of immune and inflammatory cells (T-cell, B-cell, mast cell, neutrophil, macrophage, etc.) [155,156], the role of these TRP channels in modulating the interaction between neural and immune cells and thereby regulating the function of pulmonary defense mechanisms is certainly very important and requires further investigations.
Highlights.
Transient Receptor Potential (TRP) channels expressed in airway sensory nerves function as transducers and integrators of a diverse range of sensory inputs including chemical, mechanical and thermal signals.
These TRP sensors are extremely sensitive to inhaled irritants as well as endogenously released chemical substances.
They play an important role in generating the afferent activity carried by these sensory nerves and regulating the centrally mediated pulmonary defense reflexes.
This review focuses primarily on the recent findings of the responses of these TRP sensors to the biological stresses emerging under the pathophysiological conditions of the lung and airways.
Acknowledgements:
Authors thank Dr. An-Hsuan Lin for the assistance in the preparation of this manuscript.
Funding:
The publication was supported in part by National Institutes of Health grants AI123832, UL1TR001998 and P30 ES026529–04S1. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- [1].Coleridge JC, Coleridge HM, Afferent vagal C fibre innervation of the lungs and airways and its functional significance, Rev. Physiol. Biochem. Pharmacol. 99 (1984) 1–110. 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- [2].Lee LY, Yu J, Sensory nerves in lung and airways, Compr. Physiol. 4 (2014) 287–324. 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- [3].Mazzone SB, Undem BJ, Vagal afferent innervation of airways in health and disease, Physiol. Rev. 96 (2016) 975–1024. 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sant’Ambrogio G, Information arising from the tracheobronchial tree of mammals, Physiol. Rev. 62 (1982) 531–569. 10.1152/physrev.1982.62.2.531. [DOI] [PubMed] [Google Scholar]
- [5].Coleridge HM, Coleridge JC, Reflexes evoked from tracheobronchial tree and lungs in Handbook of Physiology, in: Cherniack NS, Widdicombe JG (Eds.), Bethsda: American Physiological Society, 1986, pp. 395–430. [Google Scholar]
- [6].Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW, Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease, Physiol. Rev. 94 (2014) 265–301. 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clapham DE, TRP channels as cellular sensors, Nature. 426 (2003) 517–524. 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- [8].Nilius B, Szallasi A, Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine, Pharmacol. Rev. 66 (2014) 676–814. 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- [9].Gees M, Colsoul B, Nilius B, 2010. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2, a003962. 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moran MM, McAlexander MA, Bíró T, Szallasi A, Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug. Discov. 10 (2011) 601–620. 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- [11].Dietrich A, Modulators of transient receptor potential (TRP) channels as therapeutic options in lung disease, Pharmaceuticals (Basel). 12 (2019). 10.3390/ph12010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Banner KH, Igney F, Poll C, TRP channels: emerging targets for respiratory disease, Pharmacol. Ther. 130 (2011) 371–384. 10.1016/j.pharmthera.2011.03.005. [DOI] [PubMed] [Google Scholar]
- [13].Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG, Transient receptor potential (TRP) channels in the airway: role in airway disease, Br. J. Pharmacol. 171 (2014) 2593–2607. 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].De Logu F, Patacchini R, Fontana G, Geppetti P, TRP functions in the broncho-pulmonary system, Semin. Immunopathol. 38 (2016) 321–329. 10.1007/s00281-016-0557-1. [DOI] [PubMed] [Google Scholar]
- [15].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D, The capsaicin receptor: a heat-activated ion channel in the pain pathway, Nature. 389 (1997) 816–824. 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [16].McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, Ashraf S, McGahon MK, Curtis TM, Arron J, Choy D, Warke TJ, Bradding P, Ennis M, Zholos A, Costello RW, Heaney LG, Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma, J. Allergy. Clin. Immunol. 133 (2014) 704–712. 10.1016/j.jaci.2013.09.016. [DOI] [PubMed] [Google Scholar]
- [17].Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH, Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough, Exp. Lung Res. 31 (2005) 295–306. 10.1080/01902140590918803. [DOI] [PubMed] [Google Scholar]
- [18].Jammes Y, Fornaris E, Mei N, Barrat E, Afferent and efferent components of the bronchial vagal branches in cats, J. Auton. Nerv. Syst. 5 (1982) 165–176. 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- [19].Ho CY, Gu Q, Lin YS, Lee LY, Sensitivity of vagal afferent endings to chemical irritants in the rat lung, Respir. Physiol. 127 (2001) 113–124. 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- [20].Gu Q, Lee LY, Characterization of acid signaling in rat vagal pulmonary sensory neurons, Am. J. Physiol. Lung. Cell. Mol. Physiol. 291 (2006) 58–65. 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin YS, Lee LY, Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors, J. Physiol. 539 (2002) 947–955. 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ross RA, Anandamide and vanilloid TRPV1 receptors, Br. J. Pharmacol. 140 (2003) 790–801. 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carr MJ, Kollarik M, Meeker SN, Undem BJ, A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals, J. Pharmacol. Exp. Ther. 304 (2003) 1275–1279. 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- [24].Nieto-Posadas A, Picazo-Juárez G, Llorente I, Jara-Oseguera A, Morales-Lázaro S, Escalante-Alcalde D, Islas LD, Rosenbaum T, Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site, Nat. Chem. Biol. 8 (2011) 78–85. 10.1038/nchembio.712. [DOI] [PubMed] [Google Scholar]
- [25].Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U, Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances, Proc. Natl. Acad. Sci. USA. 97 (2000) 6155–6160. 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ni D, Gu Q, Hu H-Z, Gao N, Zhu MX, Lee L-Y, Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors, Am. J. Physiol. Regul. Integr. Comp. Physiol. 291 (2006) R541–R550. 10.1152/ajpregu.00016.2006. [DOI] [PubMed] [Google Scholar]
- [27].Ni D, Lee LY, Effect of increasing temperature on TRPV1-mediated responses in isolated rat pulmonary sensory neurons, Am. J. Physiol. Lung Cell. Mol. Physiol. 294 (2008) 563–571. 10.1152/ajplung.00336.2007. [DOI] [PubMed] [Google Scholar]
- [28].Akopian AN, Fanick ER, Brooks EG, TRP channels and traffic-related environmental pollution-induced pulmonary disease, Semin. Immunopathol. 38 (2016) 331–338. 10.1007/s00281-016-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Deering-Rice CE, Stockmann C, Romero EG, Lu Z, Shapiro D, Stone BL, Fassl B, Nkoy F, Uchida DA, Ward RM, Veranth JM, Reilly CA, Characterization of transient receptor potential vanilloid-1 (TRPV1) variant activation by coal fly ash particles and associations with altered transient receptor potential ankyrin-1 (TRPA1) expression and asthma, J. Biol. Chem. 291 (2016) 24866–24879. 10.1074/jbc.M116.746156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kichko TI, Niedermirtl F, Leffler A, Reeh PW, Irritant volatile anesthetics induce neurogenic inflammation through TRPA1 and TRPV1 channels in the isolated mouse trachea, Anesth. Analg. 120 (2015) 467–471. 10.1213/ANE.0000000000000568. [DOI] [PubMed] [Google Scholar]
- [31].Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV, Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system, Neuroscience. 141 (2006) 1533–1543, 2006. 10.1016/j.neuroscience.2006.04.073. [DOI] [PubMed] [Google Scholar]
- [32].Geppetti P, Materazzi S, Nicoletti P, The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur. J. Pharmacol. 533 (2006) 207–214. 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- [33].Jia Y, Lee L-Y, Role of TRPV receptors in respiratory diseases, Biochim. Biophys. Acta. 1772 (2007) 915–927. 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [34].Lee LY, Gu Q, Role of TRPV1 in inflammation-induced airway hypersensitivity, Curr. Opin. Pharmacol. 9 (2009) 243–249. 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B, Endogenous airway acidification. Implications for asthma pathophysiology, Am. J. Respir. Crit. Care. Med. 161 (2000) 694–699. 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- [36].Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA, Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma, FASEB. J. 15 (2001) 1212–1214. 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- [37].Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, Wenzel SE, Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition, Clin. Exp. Allergy 32 (2002) 1558–1565. 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- [38].Patil MJ, Meeker S, Bautista D, Dong X, Undem BJ, Sphingosine-1-phosphate activates mouse vagal airway afferent C-fibres via S1PR3 receptors, J. Physiol. 597 (2019) 2007–2019. 10.1113/JP277521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Connell F, Thomas VE, Studham JM, Pride NB, Fuller RW, Capsaicin cough sensitivity increases during upper respiratory infection. Respir. Med. 90 (1996) 279–286. 10.1016/s0954-6111(96)90099-2. [DOI] [PubMed] [Google Scholar]
- [40].Doherty MJ, Mister R, Pearson MG, Calverley PM, Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease, Thorax. 55 (2000) 643–649. 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kwong K, Lee LY, PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J. Appl. Physiol. 93 (2002) 1419–1428. 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- [42].Premkumar LS, Ahern GP, Induction of vanilloid receptor channel activity by protein kinase C, Nature. 408 (2000) 985–990. 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- [43].Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA, Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide, J. Physiol. 534 (2001) 813–825. 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gu Q, Kwong K, Lee LY, Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in rat vagal sensory neurons: role of cAMP/PKA signaling pathway, J. Neurophysiol. 89 (2003) 1985–1993. 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- [45].Zhang G, Lee LY, Sensitizing effects of chronic exposure and acute inhalation of ovalbumin aerosol on pulmonary C fibers in rats, J. Appl. Physiol. (1985) 105 (2008) 128–138. 10.1152/japplphysiol.01367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY, Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat, J. Physiol. 586 (2008) 5771–5786. 10.1113/jphysiol.2008.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Watanabe N, Horie S, Spina D, Michael GJ, Page CP, Priestley JV, Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized Guinea pigs, Int. Arch. Allergy. Immunol. 146 (2008) 28–32. 10.1159/000126057. [DOI] [PubMed] [Google Scholar]
- [48].Lieu TM, Myers AC, Meeker S, Undem BJ, TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors, Am. J. Physiol. Lung. Cell. Mol. Physiol. 302 (2012) 941–948. 10.1152/ajplung.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hayes D Jr., Collins PB, Khosravi M, Lin RL, Lee LY, Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex, Am. J. Respir. Crit. Care. Med. 185 (2012) 1190–1196. 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S, Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor, Cell. 103 (2000) 525–535. 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].White JPM, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I, TRPV4: Molecular conductor of a diverse orchestra, Physiol. Rev. 96 (2016) 911–973. 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- [52].Heller S, O’Neil RG, Molecular mechanisms of TRPV4 gating, in: Liedtke WB, Heller S (Eds.), Boca Raton (FL), 2007. [PubMed] [Google Scholar]
- [53].Gu QD, Moss CR 2nd, Kettelhut KL, Gilbert CA, Hu H, Activation of TRPV4 regulates respiration through indirect activation of bronchopulmonary sensory neurons, Front. Physiol. 7 (2016) 65. 10.3389/fphys.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bonvini SJ, Birrell MA, Grace MS, Maher SA, Adcock JJ, Wortley MA, Dubuis E, Ching Y-M, Ford AP, Shala F, Miralpeix M, Tarrason G, Smith JA, Belvisi MG, Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: Role of adenosine triphosphate, J. Allergy Clin. Immunol. 138 (2016) 249–261.e12. 10.1016/j.jaci.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K, Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors, J. Comp. Neurol. 493 (2005) 596–606. 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- [56].Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ, Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs, J. Physiol. 586 (2008) 1595–1604. 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zygmunt PM, Högestätt ED, TRPA1, Handb. Exp. Pharmacol. 222 (2014) 583–630. 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]
- [58].Taylor-Clark TE, Undem BJ, Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels, J. Physiol. 588 (2010) 423–433. 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hsu CC, Lin RL, Lee LY, Lin YS Hydrogen sulfide induces hypersensitivity of rat capsaicin-sensitive lung vagal neurons: role of TRPA1 receptors, Am. J. Physiol. Regul. Integr. Comp. Physiol. 305 (2013) 769–779. 10.1152/ajpregu.00202.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt S-E, TRPA1 is a major oxidant sensor in murine airway sensory neurons, J. Clin. Invest. 118 (2008) 1899–1910. 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y, TRPA1 underlies a sensing mechanism for O2, Nat. Chem. Biol. 7 (2011) 701–711. 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- [62].Robinson RK, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Chen S, McGilvery CM, Hu S, Shaffer MSP, Bonvini SJ, Maher SA, Mudway IS, Porter AE, Carlsten C, Tetley TD, Belvisi MG, Mechanistic link between diesel exhaust particles and respiratory reflexes, J. Allergy Clin. Immunol. 141 (2018) 1074–1084.e9. 10.1016/j.jaci.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn J-A, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F, TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins, Nat. Commun. 5 (2014) 3125. 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Almaraz L, Manenschijn J-A, de la Peña E, Viana F, TRPM8, Handb. Exp. Pharmacol. 222 (2014) 547–579. 10.1007/978-3-642-54215-2_22. [DOI] [PubMed] [Google Scholar]
- [65].Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A, ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures, Cell. 112 (2003) 819–829. 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- [66].Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, Mori N, Canning BJ, The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol, J. Appl. Physiol. 115 (2013) 268–274. 10.1152/japplphysiol.01144.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Xing H, Ling JX, Chen M, Johnson RD, Tominaga M, Wang C-Y, Gu J, TRPM8 mechanism of autonomic nerve response to cold in respiratory airway, Mol. Pain. 4 (2008) 22. 10.1186/1744-8069-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhou Y, Sun B, Li Q, Luo P, Dong L, Rong W, Sensitivity of bronchopulmonary receptors to cold and heat mediated by transient receptor potential cation channel subtypes in an ex vivo rat lung preparation, Respir. Physiol. Neurobiol. 177 (2011) 327–332. 10.1016/j.resp.2011.05.011. [DOI] [PubMed] [Google Scholar]
- [69].Diogenes A, Akopian AN, Hargreaves KM, NGF up-regulates TRPA1: Implications for orofacial pain, J. Dent. Res. 86 (2007) 550–555. 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- [70].Lin YJ, Lin RL, Ruan T, Khosravi M, Lee LY, A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents, J. Appl. Physiol. 118 (2015) 273–281. 10.1152/japplphysiol.00805.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hsu CC, Lee LY, Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons, J. Appl. Physiol. 118 (2015) 1533–1543. 10.1152/japplphysiol.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR SR, Petrus MJ, Earley TJ, Patapoutian A, Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin, Neuron. 41 (2004) 849–857. 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- [73].Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D, TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents, Cell. 124 (2006) 1269–1282. 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- [74].Lee LY, Hsu CC, Lin YJ, Lin RL, Khosravi M, Interaction between TRPV1 and TRPA1: synergy on pulmonary sensory nerves, Pulm. Pharmacol. Therap. 35 (2015) 87–93. 10.1016/j.pupt.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo CH, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X, Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain, Neuron. 85 (2015) 833–846. 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McKemy DD, Neuhausser WM, Julius D, Identification of a cold receptor reveals a general role for TRP channels in thermosensation, Nature. 416 (2002) 52–58. 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- [77].Willis DN, B Liu MA Ha, S-E. Jordt, J.B. Morris, Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants, FASEB. J. 25 (2011) 4434–4444. 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bolser DC, Aziz SM, Chapman RW, Ruthenium red decreases capsaicin and citric acid-induced cough in guinea pigs, Neurosci. Lett. 126 (1991) 131–133. 10.1016/0304-3940(91)90536-3. [DOI] [PubMed] [Google Scholar]
- [79].Dicpinigaitis PV, Alva RV, Safety of capsaicin cough challenge testing, Chest. 128 (2005) 196–202. 10.1378/chest.128.1.196. [DOI] [PubMed] [Google Scholar]
- [80].Morice AH, Fontana GA, Belvisi MG, Birring SS, Chung KF, Dicpinigaitis PV, Kastelik JA, McGarvey LP, Smith JA, Tatar M, Widdicombe J, ERS guidelines on the assessment of cough, Eur. Respir. J. 29 (2007) 1256–1276. 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- [81].Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF, Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough, Am. J. Respir. Crit. Care Med. 170 (2004) 1276–1280. 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- [82].Materazzi S, Nassini R, Gatti R, Trevisani M, Geppetti P Cough sensors. II. Transient receptor potential membrane receptors on cough sensors, Handb. Exp. Pharmacol. 187 (2009) 49–61. 10.1007/978-3-540-79842-2_3. [DOI] [PubMed] [Google Scholar]
- [83].Khalid S, Murdoch R, Newlands A, Smart K, Kelsall A, Holt K, Dockry R, Woodcock A, Smith JA, Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial, J. Allergy Clin. Immunol. 134 (2014) 56–62. 10.1016/j.jaci.2014.01.038. [DOI] [PubMed] [Google Scholar]
- [84].Belvisi MG, Birrell MA, Wortley MA, Maher SA, Satia I, Badri H, Holt K, Round P, McGarvey L, Ford J, Smith JA, XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough, Am. J. Respir. Crit. Care Med. 196 (2017) 1255–1263. 10.1164/rccm.201704-0769OC. [DOI] [PubMed] [Google Scholar]
- [85].Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH, TRPA1 agonists evoke coughing in guinea pig and human volunteers, Am. J. Respir. Crit. Care Med. 180 (2009) 1042–1047. 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Brozmanova M, Mazurova L, Ru F, Tatar M, Kollarik M, Comparison of TRPA1-versus TRPV1-mediated cough in guinea pigs, Eur. J. Pharmacol. 689 (2012) 211–218. 10.1016/j.ejphar.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG, Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin, Thorax. 67 (2012) 891–900. 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mukhopadhyay I, Kulkarni A, Aranake S, Karnik P, Shetty M, Thorat S, Ghosh I, Wale D, Bhosale V, Khairatkar-Joshi N, 2014. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One. 9, e97005. 10.1371/journal.pone.0097005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mukhopadhyay I, Kulkarni A, Khairatkar-Joshi N, Blocking TRPA1 in respiratory disorders: Does it hold a promise?, Pharmaceuticals (Basel). 9 (2016). 10.3390/ph9040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chung KF, Advances in mechanisms and management of chronic cough: The Ninth London International Cough Symposium 2016, Pulm. Pharmacol. Ther. 47 (2017) 2–8. 10.1016/j.pupt.2017.02.003. [DOI] [PubMed] [Google Scholar]
- [91].Carlsen K-H, Carlsen KCL, Exercise-induced asthma, Paediatr. Respir. Rev. 3 (2002) 154–160. 10.1097/MCP.0b013e32831da8ab. [DOI] [PubMed] [Google Scholar]
- [92].Suzuki S, Ishii M, Sasaki J, Takishima T, Bronchial responsiveness to methacholine during airway cooling in normal subjects, Clin. Allergy. 16 (1986) 33–40. 10.1111/j.1365-2222.1986.tb01951.x. [DOI] [PubMed] [Google Scholar]
- [93].Giesbrecht GG, Pisarri TE, Coleridge JC, Coleridge HM, Cooling the pulmonary blood in dogs alters activity of pulmonary vagal afferents, J. Appl. Physiol. 74 (1993) 24–30. 10.1152/jappl.1993.74.1.24. [DOI] [PubMed] [Google Scholar]
- [94].Fisher JT, TRPM8 and dyspnea: from the frigid and fascinating past to the cool future?, Curr. Opin. Pharmacol. 11 (2011) 218–223. 10.1016/j.coph.2011.06.004. [DOI] [PubMed] [Google Scholar]
- [95].Morice AH, Marshall AE, Higgins KS, Grattan TJ, Effect of inhaled menthol on citric acid induced cough in normal subjects, Thorax. 49 (1994) 1024–1026. 10.1136/thx.49.10.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wise PM, Breslin PAS, Dalton P, Sweet taste and menthol increase cough reflex thresholds, Pulm. Pharmacol. Ther. 25 (2012) 236–241. 10.1016/j.pupt.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Oz M, El Nebrisi EG, Yang K-HS, Howarth FC, Al Kury LT, Cellular and molecular targets of menthol actions, Front. Pharmacol. 8 (2017) 472. 10.3389/fphar.2017.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].EU Clinical Trials Register [Internet]: Amsterdam (Netherlands): European Medicines Agency; 1995- Identifier 2017–003108-27. Clinical Trial Results: A Pilot Study of the Efficacy, Safety, and Tolerability of AX-8 for the Treatment of Refractory Chronic Cough; 2017. October 19 [cited 2021 Jan 30]; [20 pages]. Available from https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-003108-27/results. [Google Scholar]
- [99].Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, Cohen J, Sterk PJ, Miller B, Paccaly A, Burggraaf J, Cohen AF, Diamant Z, Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma, Am. J. Respir. Crit. Care Med. 175 (2007) 450–457. 10.1164/rccm.200608-1186OC. [DOI] [PubMed] [Google Scholar]
- [100].Badri H, Smith JA, Emerging targets for cough therapies; NK1 receptor antagonists, Pulm. Pharmacol. Ther. 59 (2019) 101853. 10.1016/j.pupt.2019.101853. [DOI] [PubMed] [Google Scholar]
- [101].Manzini S, Maggi CA, Geppetti P, Bacciarelli C, Capsaicin desensitization protects from antigen-induced bronchospasm in conscious guinea-pigs, Eur. J. Pharmacol. 138 (1987) 307–308. 10.1016/0014-2999(87)90451-1. [DOI] [PubMed] [Google Scholar]
- [102].Herd CM, Gozzard N, Page CP, Capsaicin pre-treatment prevents the development of antigen-induced airway hyperresponsiveness in neonatally immunised rabbits, Eur. J. Pharmacol. 282 (1995) 111–119. 10.1016/0014-2999(95)00291-r. [DOI] [PubMed] [Google Scholar]
- [103].Buckley TL, Nijkamp FP, Airways hyperreactivity and cellular accumulation in a delayed-type hypersensitivity reaction in the mouse. Modulation by capsaicin-sensitive nerves, Am. J. Respir. Crit. Care Med. 149 (1994) 400–407. 10.1164/ajrccm.149.2.8306037. [DOI] [PubMed] [Google Scholar]
- [104].Rogerio AP, Andrade EL, Calixto JB, C-fibers, but not the transient potential receptor vanilloid 1 (TRPV1), play a role in experimental allergic airway inflammation, Eur. J. Pharmacol. 662 (2011) 55–62. 10.1016/j.ejphar.2011.04.027. [DOI] [PubMed] [Google Scholar]
- [105].Delescluse I, Mace H, Adcock JJ, Inhibition of airway hyper-responsiveness by TRPV1 antagonists (SB-705498 and PF-04065463) in the unanaesthetized, ovalbumin-sensitized guinea pig, Br. J. Pharmacol. 166 (2012) 1822–1832. 10.1111/j.1476-5381.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Choi JY, Lee HY, Hur J, Kim KH, Kang JY, Rhee CK, Lee SY, TRPV1 blocking alleviates airway inflammation and remodeling in a chronic asthma murine model, Allergy Asthma Immunol. Res. 10 (2018) 216–224. 10.4168/aair.2018.10.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA, Functional TRPV4 channels are expressed in human airway smooth muscle cells, Am. J. Physiol. Lung Cell. Mol. Physiol. 287 (2004) L272–L278. 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- [108].McAlexander MA, Luttmann MA, Hunsberger GE, Undem BJ, Transient receptor potential vanilloid 4 activation constricts the human bronchus via the release of cysteinyl leukotrienes, J. Pharmacol. Exp. Ther. 349 (2014) 118–125. 10.1124/jpet.113.210203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bonvini SJ, Birrell MA, Dubuis E, Adcock JJ, Wortley MA, Flajolet P, Bradding P, Belvisi MG, Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma, Eur. Respir. J. (2020). 10.1183/13993003.01458-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Taylor-Clark TE, Undem BJ, Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels, J. Physiol. 588 (2010) 423–433. 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA, Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation, Am. J. Respir. Cell Mol. Biol. 40 (2009) 756–762. 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Moller DR, McKay RT, Bernstein IL, Brooks SM, Persistent airways disease caused by toluene diisocyanate, Am. Rev. Respir. Dis. 134 (1986) 175–176. 10.1164/arrd.1986.134.1.175. [DOI] [PubMed] [Google Scholar]
- [113].Coffey MJ, Wheeler CS, Gross KB, Eschenbacher WL, Sporn PH, Peters-Golden M, Increased 5-lipoxygenase metabolism in the lungs of human subjects exposed to ozone, Toxicology. 114 (1996) 187–197. 10.1016/s0300-483x(96)03487-7. [DOI] [PubMed] [Google Scholar]
- [114].Schelegle ES, Walby WF, Vagal afferents contribute to exacerbated airway responses following ozone and allergen challenge, Respir. Physiol. Neurobiol. 181 (2012) 277–285. 10.1016/j.resp.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt S-E, A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma, Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 9099–9104. 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nassini R, Materazzi S, Andrè E, Sartiani L, Aldini G, Trevisani M, Carnini C, Massi D, Pedretti P, Carini M, Cerbai E, Preti D, Villetti G, Civelli M, Trevisan G, Azzari C, Stokesberry S, Sadofsky L, McGarvey L, Patacchini R, Geppetti P, Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 24 (2010) 4904–4916. 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- [117].Hox V, Vanoirbeek JA, Alpizar YA, Voedisch S, Callebaut I, Bobic S, Sharify A, De Vooght V, Van Gerven L, Devos F, Liston A, Voets T, Vennekens R, Bullens DMA, De Vries A, Hoet P, Braun A, Ceuppens JL, Talavera K, Nemery B, Hellings PW, Crucial role of transient receptor potential ankyrin 1 and mast cells in induction of nonallergic airway hyperreactivity in mice, Am. J. Respir. Crit. Care Med. 187 (2013) 486–493. 10.1164/rccm.201208-1358OC. [DOI] [PubMed] [Google Scholar]
- [118].Reese RM, Dourado M, Anderson K, Warming S, Stark KL, Balestrini A, Suto E, Lee W, Riol-Blanco L, Shields SD, Hackos DH, Behavioral characterization of a CRISPR-generated TRPA1 knockout rat in models of pain, itch, and asthma, Sci. Rep. 10 (2020) 979. 10.1038/s41598-020-57936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Naumov DE, Perelman JM, Kolosov VP, Potapova TA, Maksimov VN, Zhou X, Transient receptor potential melastatin 8 gene polymorphism is associated with cold-induced airway hyperresponsiveness in bronchial asthma, Respirology. 20 (2015) 1192–1197. 10.1111/resp.12605. [DOI] [PubMed] [Google Scholar]
- [120].Li M, Li Q, Yang G, Kolosov VP, Perelman JM, Zhou XD, Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism, J. Allergy Clin. Immunol. 128 (2011) 625–626. 10.1016/j.jaci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- [121].Sabnis AS, Shadid M, Yost GS, Reilly CA, Human lung epithelial cells express a functional cold-sensing TRPM8 variant, Am. J. Respir. Cell Mol. Biol. 39 (2008) 466–474. 10.1165/rcmb.2007-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sabnis AS, Reilly CA, Veranth JM, Yost GS, Increased transcription of cytokine genes in human lung epithelial cells through activation of a TRPM8 variant by cold temperatures, Am. J. Physiol. Lung Cell. Mol. Physiol. 295 (2008) L194–L200. 10.1152/ajplung.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lin A-H, Liu M-H, Ko H-KB, Perng D-W, Lee T-S, Kou YR, Inflammatory effects of menthol vs. non-menthol cigarette smoke extract on human lung epithelial cells: a double-hit on TRPM8 by reactive oxygen species and menthol, Front. Physiol. 8 (2017) 263. 10.3389/fphys.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lin A-H, Liu M-H, Ko H-K, Perng D-W, Lee T-S, Kou YR, Menthol cigarette smoke induces more severe lung inflammation than non-menthol cigarette smoke does in mice with subchronic exposure - role of TRPM8, Front. Physiol. 9 (2018) 1817. 10.3389/fphys.2018.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Cho Y, Jang Y, Yang YD, Lee C-H, Lee Y, Oh U, TRPM8 mediates cold and menthol allergies associated with mast cell activation, Cell Calcium. 48 (2010) 202–208. 10.1016/j.ceca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- [126].Baxter M, Eltom S, Dekkak B, Yew-Booth L, Dubuis ED, Maher SA, Belvisi MG, Birrell MA, Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung, Thorax. 69 (2014) 1080–1089. 10.1136/thoraxjnl-2014-205467. [DOI] [PubMed] [Google Scholar]
- [127].Zhu G, Gulsvik A, Bakke P, Ghatta S, Anderson W, Lomas DA, Silverman EK, Pillai SG, Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease, Hum. Mol. Genet. 18 (2009) 2053–2062. 10.1093/hmg/ddp111. [DOI] [PubMed] [Google Scholar]
- [128].Birrell MA, Bonvini SJ, Dubuis E, Maher SA, Wortley MA, Grace MS, Raemdonck K, Adcock JJ, Belvisi MG, Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: A beneficial off-target effect?, J. Allergy Clin. Immunol. 133 (2014) 679–687.e9. 10.1016/j.jaci.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Taylor-Clark TE, Role of reactive oxygen species and TRP channels in the cough reflex, Cell Calcium, 60 (2016) 155–162. 10.1016/j.ceca.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wallace H, Airway pathogenesis is linked to TRP channels, in: Emir TLR (Ed.), Boca Raton (FL), 2017: pp. 251–264. 10.4324/9781315152837-13. [DOI] [PubMed] [Google Scholar]
- [131].Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R, Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents, J. Clin. Invest. 118 (2008) 2574–2582. 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lin A-H, Liu M-H, Ko H-K, Perng D-W, Lee T-S, Kou YR, Lung epithelial TRPA1 transduces the extracellular ROS into transcriptional regulation of lung inflammation induced by cigarette smoke: the role of influxed Ca2+, Mediators Inflamm. 2015 (2015) 148367. 10.1155/2015/148367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JAJ, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T, Nicotine activates the chemosensory cation channel TRPA1, Nat. Neurosci. 12 (2009) 1293–1299. 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- [134].Lee LY, Lin RL, Khosravi M, Xu F, Reflex bronchoconstriction evoked by inhaled nicotine aerosol in guinea pigs: role of the nicotinic acetylcholine receptor, J. Appl. Physiol. 125 (2018) 17–123. 10.1152/japplphysiol.01039.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Salvi SS, Barnes PJ, Chronic obstructive pulmonary disease in non-smokers, Lancet (London, England). 374 (2009) 733–743. 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- [136].Shapiro D, Deering-Rice CE, Romero EG, Hughen RW, Light AR, Veranth JM, Reilly CA, Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material, Chem. Res. Toxicol. 26 (2013) 750–758. 10.1021/tx400024h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zaccone EJ, Undem BJ, Airway vagal neuroplasticity associated with respiratory viral infections, Lung. 194 (2016) 25–29. 10.1007/s00408-015-9832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Omar S, Clarke R, Abdullah H, Brady C, Corry J, Winter H, Touzelet O, Power UF, Lundy F, McGarvey LP, Cosby SL, 2017. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS One. 12, e0171681. 10.1371/journal.pone.0171681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X, Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs, Am. J. Physiol. 277 (1999) 831–840. 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- [140].Carr MJ, Hunter DD, Jacoby DB, Undem BJ, Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs, Am. J. Respir. Crit. Care. Med. 165 (2002) 1071–1075. 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- [141].Dakhama A, Park JW, Taube C, El Gazzar M, Kodama T, Miyahara N, Takeda K, Kanehiro A, Balhorn A, Joetham A, Loader JE, Larsen GL, Gelfand EW, Alteration of airway neuropeptide expression and development of airway hyperresponsiveness following respiratory syncytial virus infection, Am. J. Physiol. Lung. Cell. Mol. Physiol. 288 (2005) 761–770. 10.1152/ajplung.00143.2004. [DOI] [PubMed] [Google Scholar]
- [142].Lee AM, Fryer AD, van Rooijen N, Jacoby DB, Role of macrophages in virus-induced airway hyperresponsiveness and neuronal M2 muscarinic receptor dysfunction, Am. J. Physiol. Lung. Cell. Mol. Physiol. 286 (2004) 1255–1259. 10.1152/ajplung.00451.2003. [DOI] [PubMed] [Google Scholar]
- [143].Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K, Pathophysiology of COVID-19: mechanisms underlying disease severity and progression, Physiology (Bethesda). 35 (2020) 288–301. 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kuebler WM, Jordt SE, Liedtke WB, Urgent reconsideration of lung edema as a preventable outcome in COVID-19: inhibition of TRPV4 represents a promising and feasible approach, Am. J. Physiol. Lung. Cell. Mol. Physiol. 318 (2020) 1239–1243. 10.1152/ajplung.00161.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Nahama A, Ramachandran R, Cisternas AF, Ji H, The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: A review, Med. Drug. Discov. 5 (2020) 100033. 10.1016/j.medidd.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].De Virgiliis F, Di Giovanni S, Lung innervation in the eye of a cytokine storm: neuroimmune interactions and COVID-19, Nat. Rev. Neurol. 25 (2020) 1–8. 10.1038/s41582-020-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hammock BD, Wang W, Gilligan MM, Panigrahy D, Eicosanoids: The overlooked storm in coronavirus disease 2019 (COVID-19)?, Am. J. Pathol. 190 (2020) 1782–1788. 10.1016/j.ajpath.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yu J, Lin S, Zhang J, Otmishi P P, Guardiola JJ, Airway nociceptors activated by pro-inflammatory cytokines, Respir. Physiol. Neurobiol. 156 (2007) 116–119. 10.1016/j.resp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [149].Hu YM, Gu QH, Lin RL, Kryscio R, Lee LY, Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-alpha in rat pulmonary sensory neurons, Am. J. Physiol. Lung. Cell. Mol. Physiol. 299 (2010) 483–492. 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lin RL, Gu Q, Lee LY, Hypersensitivity of vagal pulmonary afferents induced by tumor necrosis factor alpha in mice, Front. Physiol. 8 (2017) 411. 10.3389/fphys.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hsu CC, Lin YS, Lin RL, Lee LY, Immediate and delayed potentiating effects of tumor necrosis factor-α on TRPV1 sensitivity of rat vagal pulmonary sensory neurons, Am. J. Physiol. Lung. Cell. Mol. Physiol. 313 (2017) 293–304. 10.1152/ajplung.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Goyal N, Skrdla P, Schroyer R, Kumar S, Fernando D, Oughton A, Norton N, Sprecher DL, Cheriyan J, Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects, Am. J. Cardiovasc. Drugs. 19 (2019) 335–342. 10.1007/s40256-018-00320-6. [DOI] [PubMed] [Google Scholar]
- [153].Morty RE, Kuebler WM, TRPV4: an exciting new target to promote alveolocapillary barrier function, Am. J. Physiol. Lung. Cell. Mol. Physiol. 307 (2014) L817–L821. 10.1152/ajplung.00254.2014. [DOI] [PubMed] [Google Scholar]
- [154].Pairet N, Mang S, Fois G, Keck M, Kühnbach M, Gindele J, Frick M, Dietl P, Lamb DJ, 2018. TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS One. 13, e0196055. 10.1371/journal.pone.0196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Parenti A, De Logu F, Geppetti P, Benemei S, What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 173 (2016) 953–969. 10.1111/bph.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Khalil L, Alliger K, Weidinger C, Yerinde C, Wirtz S, Becker C, Engel MA, Functional role of transient receptor potential channels in immune cells and epithelia, Front. Immunol. 9 (2018) 174. 10.3389/fimmu.2018.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]


