Abstract
Long non-coding RNA metastasis-associated urothelial carcinoma associated 1 (UCA1) plays a pivotal role in various human diseases. Its gene expression is regulated by several factors, including transcription factors, chromatin remodeling and epigenetic modification. UCA1 is involved in the regulation of the PI3K/AKT, Wnt/β-catenin, MAPK, NF-κB and JAK/STAT signaling pathways, affecting a series of cellular biological functions, such as cell proliferation, apoptosis, migration, invasion and tumor drug resistance. Furthermore, UCA1 is used as a novel potential biomarker for disease diagnosis and prognosis, as well as a target for clinical gene therapy. The present review systematically summarizes and elucidates the mechanisms of upstream transcriptional regulation of UCA1, the regulatory role of UCA1 in multiple signaling pathways in the occurrence and development of several diseases, and its potential applications in clinical treatment.
Keywords: lncRNA, UCA1, signaling pathway, diagnosis, prognosis
1. Introduction
The whole genome sequencing of eukaryotes has confirmed that ~93% of the DNA in the human genome can be transcribed into RNA (1). Notably, only 2% of mRNAs encode proteins and the remaining 98% are non-coding RNAs (1). Among the different types of non-coding transcripts, long non-coding RNAs (lncRNAs) have attracted great interest. lncRNAs do not contain any extended open reading frames (ORFs) and are >200 nucleotides in length (2). They have been identified as indispensable regulators in a variety of physiological and pathological processes, including epigenetic inheritance, cell cycle, post-transcriptional regulation, translation and chromatin modification (3). In addition, lncRNAs participate in the regulation of cell proliferation, differentiation, apoptosis, invasion, migration and tumor drug resistance (4). lncRNAs play important roles in the occurrence and development of various diseases, particularly malignant tumors (5,6). Previous studies have demonstrated that lncRNAs function as either oncogenes or tumor suppressors, and are involved in the regulation of tumorigenesis and progression of various tumors (5,6).
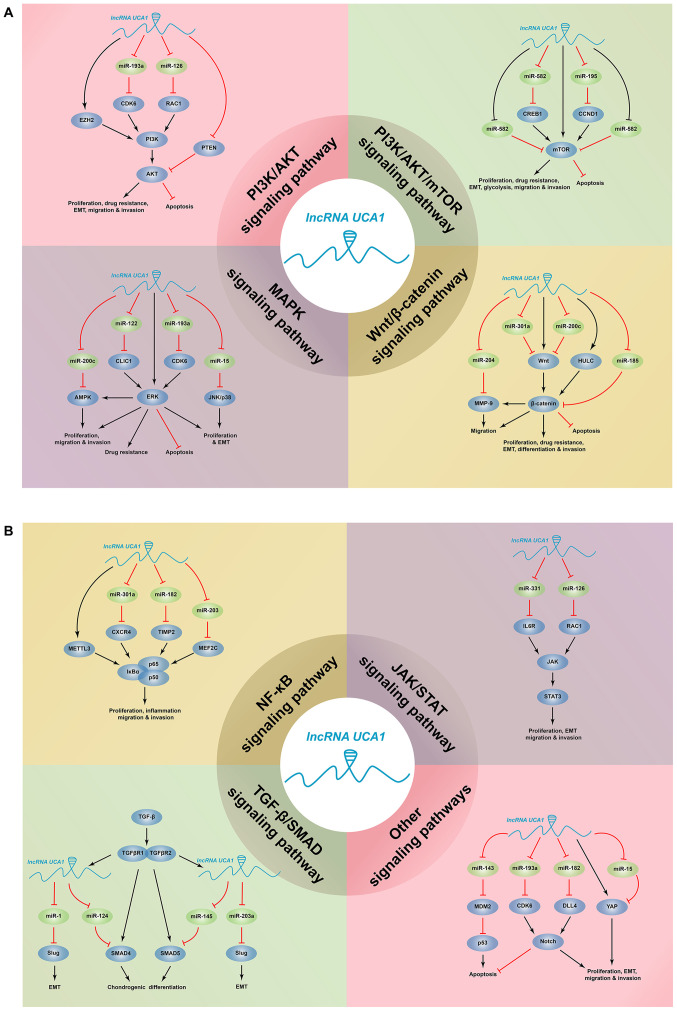
Urothelial carcinoma associated 1 (UCA1) was initially identified in bladder cancer by high-throughput RNA sequencing, and is associated with the progression of bladder cancer (7). UCA1 has been demonstrated to be highly expressed in several human tumors, such as gastric cancer (GC) and cholangiocarcinoma, which is closely associated with tumor-node-metastasis (TNM) stage, depth of invasion, vascular invasion, lymph node metastasis, overall survival (OS) and relapse-free survival (RFS) (8–10). In addition, UCA1 is involved in regulating cell proliferation, apoptosis, migration, invasion and drug resistance (11). Several studies have reported that UCA1 participates in the regulation of multiple cellular signaling pathways at transcriptional, post-transcriptional and epigenetic levels (Fig. 1) (12,13). The present review discusses the molecular mechanisms and clinical potential of UCA1 in the regulation of signaling pathways and transcription in human diseases.
Figure 1.
Signaling pathways in diseases mediated by UCA1. UCA1 regulates key signaling pathways by acting as a competitive endogenous RNA, thus affecting biological activities, such as cell proliferation, apoptosis, migration, invasion and drug resistance in cancers and non-cancerous diseases. UCA1, urothelial carcinoma associated 1; CCND1, cyclin D1; CDK6, cyclin-dependent kinase 6; CLIC1, chloride intracellular channel 1; CREB1, cAMP response element-binding protein 1; CXCR4, C-X-C chemokine receptor type 4; DLL4, Delta-like 4; EZH2, enhancer of zeste homolog 2; HULC, highly upregulated in liver cancer; MEF2C, myocyte enhancer factor 2C; METTL3, methyltransferase-like 3; MMP-9, matrix metalloproteinase-9; mTOR, mammalian target of rapamycin; RAC1, RAS-related C3 botulinus toxin substrate 1; TGF-β, transforming growth factor-β; TIMP2, tissue inhibitor of metalloproteinase-2; YAP, yes-associated protein; miR, microRNA; EMT, epithelial-to-mesenchymal transition; lncRNA, long non-coding RNA.
2. Overview of UCA1
In 2006, UCA1 was demonstrated to be highly specific and sensitive in the diagnosis of bladder cancer, particularly in patients with superficial G2-G3 (7). Furthermore, UCA1 is considered to be the most specific gene for bladder transitional cell carcinoma (TCC) (7). UCAI exhibits a notable differential diagnostic function in several urinary tract diseases without TCC (7).
The UCA1 gene contains three exons and two introns, and is located on the positive strand of human chromosome 19p13.12 (7). Notably, the UCA1 sequence contains multiple termination sequences, without any conservative long ORFs (14,15). Initially, the full-length cDNA of UCA1 was identified as 1.4 kb (7). However, recent studies have demonstrated that UCA1 has three different isoforms (1.4, 2.2 and 2.7 kb) (16,17). Currently, the 1.4 kb isoform has attracted great interest (14). In addition to its associations with non-cancerous diseases, including systemic lupus erythematosus (SLE), diabetic nephropathy (DN) and Parkinson's disease (PD) (18–20), UCA1 is also involved in the progression of different types of cancer, including bladder cancer, colorectal cancer (CRC) and hepatocellular carcinoma (HCC) (21–23). Furthermore, overexpression of UCA1 is closely associated with clinicopathological characteristics, including poor prognostic factors, such as TNM stage, vascular invasion and lymph node metastasis (8,24,25).
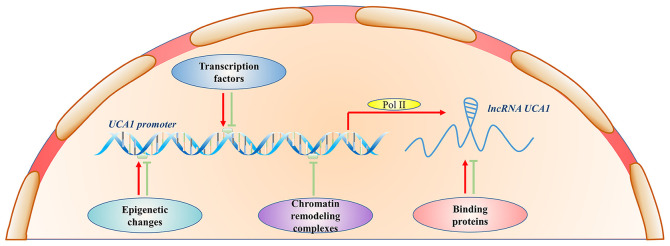
Increasing evidence suggest that UCA1 functions as an oncogene, which plays an important role in the tumorigenesis and development of different types of cancer, including papillary thyroid carcinoma (26), pancreatic cancer (PC) (27) and lung adenocarcinoma (28). UCA1 expression is regulated by several factors, such as transcription factors, chromatin remodeling and epigenetic modification (Fig. 2) (29–31). Furthermore, UCA1 contains microRNA (miRNA/miR) binding sites that regulate the expression of target genes through the sponging of miRNAs (Table I) (8,11,24).
Figure 2.
Regulatory mechanisms of UCA1. Transcription factors, epigenetic changes, chromatin remodeling complexes and binding proteins positively or negatively regulate UCA1 expression. UCA1, urothelial carcinoma associated 1; lncRNA, long non-coding RNA; Pol II, RNA polymerase II.
Table I.
Regulation of signaling pathways mediated by UCA1 as a competitive endogenous RNA of miRNAs.
| miRNA | Target gene | Signaling pathways | Biological functions | Diseases | (Refs.) |
|---|---|---|---|---|---|
| miR-193a | CDK6 | PI3K/AKT, ERK/MAPK and Notch | Migration, invasion and apoptosis | Glioma | (61) |
| miR-126 | RAC1 | PI3K/AKT and JAK/STAT | Proliferation, migration and invasion | AML | (62) |
| miR-582 | CREB1 | PI3K/AKT/mTOR | EMT | Osteosarcoma | (72) |
| miR-143 | HK2 | PI3K/AKT/mTOR | Glycolysis | Bladder cancer | (79) |
| miR-200c | – | PI3K/AKT/mTOR, AMPK and Wnt/β-catenin | Proliferation, migration and invasion | Hemangioma | (78) |
| miR-185-5p | MMP-9 | Wnt/β-catenin | EMT | Melanoma | (84) |
| miR-122 | – | PI3K/AKT/mTOR and JNK/p38 MAPK | Apoptosis | AMI | (76) |
| miR-122 | CLIC1 | ERK/MAPK | Metastasis | CCA | (89) |
| miR-203 | MEF2C | NF-κB | Inflammation | Epilepsy | (93) |
| miR-331-3p | IL6R | JAK2/STAT3 | Proliferation and apoptosis | MM | (98) |
| miR-15a | – | Hippo and JNK/p38 MAPK | Proliferation and EMT | Thyroid cancer | (90) |
| miR-124 | JAG1 | Notch | Invasion and EMT | Tongue cancer | (46) |
| miR-145-5p | SMAD5 | TGF-β/SMAD | Chondrogenic differentiation | Osteoarthritis | (103) |
| miR-124-3p | SMAD4 | TGF-β/SMAD | Chondrogenic differentiation | Osteoarthritis | (103) |
| miR-1 | Slug | TGF-β/SMAD | EMT | BC and glioma | (33,104) |
| miR-203a | |||||
| miR-182-5p | DLL4 | Notch | Proliferation, migration and apoptosis | Renal cancer | (11) |
| miR-143 | MDM2 | PI3K/AKT/mTOR and p53 | Apoptosis | AMI | (77) |
UCA1, urothelial carcinoma associated 1; miR, microRNA; CDK6, cyclin-dependent kinase 6; AMI, acute myocardial infarction; AML, acute myeloid leukemia; BC, breast cancer; CCA, cholangiocarcinoma; CLIC1, chloride intracellular channel 1; CREB1, cAMP response element-binding protein 1; DLL4, Delta-like 4; EMT, epithelial-to-mesenchymal transition; ERK, extracellular signal-regulated kinase; HK2, hexokinase 2; JAG1, jagged 1; MAPK, mitogen-activated protein kinases; MM, multiple myeloma; MMP-9, matrix metalloproteinase-9; mTOR, mammalian target of rapamycin; PE, pre-eclampsia; RAC1, RAS-related C3 botulinus toxin substrate 1; TGF-β, transforming growth factor-β; -, not available.
3. Upstream regulation of UCA1 expression
As presented in Table II, upstream regulators of UCA1 include transcription factors, chromatin remodeling complexes, epigenetic changes and binding proteins (29,31–33). The core promoter of the UCA1 gene can bind with several transcription factors, such as CCAAT/enhancer binding protein α (C/EBPα) (32), C/EBPβ (34), E26 transformation-specific transcription factor 2 (35), specificity protein 1 (36), MYB (37) and E1A binding protein p300 (EP300) (38). These transcription factors interact with the promoter to upregulate UCA1 expression, and SND1 can upregulate UCA1 expression through transcriptional activator MYB and promote 5-fluorouracil (5-FU)-induced apoptosis of HCC cells (37). Similarly, macrophage-derived chemokine CCL18 upregulates UCA1 expression through transcription factor EP300 in osteosarcoma (38). In addition, the combination of hyaluronic acid and CD44 stimulates the signal transduction of PI3K and AKT, resulting in phosphorylation of C/EBPα, which binds to the promoter of the UCA1 gene to induce transcriptional activation of UCA1, resulting in migration and invasion of HSC-3 cells in human head and neck squamous cell cancer (39).
Table II.
Upstream regulation of UCA1 expression.
| Type of factor | Regulation | Regulatory factor | Diseases | (Refs.) |
|---|---|---|---|---|
| Transcription factors | Promotion | C/EBPα | Bladder cancer | (32) |
| Promotion | C/EBPβ | Bladder cancer | (34) | |
| Promotion | Ets-2 | Bladder cancer | (35) | |
| Promotion | SP1 | GC | (36) | |
| Promotion | MYB | HCC | (37) | |
| Promotion | EP300 | Osteosarcoma | (38) | |
| Promotion | C/EBPα | HNSCC | (39) | |
| Chromatin remodeling complexes | Inhibition | SATB1 | BC | (29) |
| Inhibition | ARID1A | BC | (30) | |
| Inhibition | CAPERα/TBX3 repressor complex | UMS | (43) | |
| Epigenetic changes | Inhibition | SAM | HCC | (31) |
| Binding proteins | Promotion | HnRNPI | BC | (44) |
| Inhibition | IMP1 | BC | (45) | |
| Others | Promotion | TGF-β | Tongue cancer | (46) |
| Promotion | BMP9 | BC | (33) | |
| Promotion | TAZ/YAP and TEAD complexes | BC | (47) | |
| Inhibition | Metformin | EH | (50) | |
| Inhibition | Metformin | PE | (51) | |
| Inhibition | lncRNA GAS8-AS1 | Osteosarcoma | (52) | |
| Inhibition | UPF1 | HCC | (53) | |
| Promotion | PA | GC | (54) | |
| Promotion | CAFs | CRC | (55) | |
| Promotion | HBx | HCC | (56) |
UCA1, urothelial carcinoma associated 1; ARID1A, AT-rich interaction domain 1A; BC, breast cancer; BMP9, bone morphogenetic protein 9; C/EBP, CCAAT/enhancer binding protein; CAFs, cancer-associated fibroblasts; CRC, colorectal cancer; EH, endometrial hyperplasia; EP300, E1A binding protein p300; ETS-2, E26 transformation-specific transcription factor 2; GC, gastric cancer; HBx, hepatitis B virus X protein; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cancer; IMP1, insulin-like growth factor 2 mRNA-binding protein 1; PA, palmitic acid; PE, pre-eclampsia; SAM, S-adenosylmethionine; SATB1, special AT-rich sequence binding protein 1; SP1, specificity protein 1; TAZ, transcriptional co-activator with PDZ binding motif; TEAD, transcriptional enhancer TEA domain; TGF-β, transforming growth factor-β; UMS, ulnar-mammary syndrome; UPF1, up-frameshift protein 1; YAP, yes-associated protein.
AT-rich interaction domain 1A (ARID1A) is one of the major members of SWItch/Sucrose non-fermentable chromatin remodeling complexes, which is often found to be loss-of-function mutations in different types of cancer, such as non-small cell lung cancer (NSCLC) (40), breast cancer (BC) (41) and pancreatic ductal adenocarcinoma (PDAC) (42). ARID1A inhibits UCA1 expression by regulating chromatin access of transcription factor C/EBPα (30). It has been confirmed that the CAPERα/TBX3 complex directly inhibits UCA1 transcription and promotes cancer cell senescence by regulating chromatin structure (43). Furthermore, the AT-rich sequence binding protein 1 suppresses UCA1 expression by closing the chromatin structure of the UCA1 promoter region in BC (29). Notably, epigenetic modification participates in regulating UCA1 expression. S-adenosylmethionine inhibits UCA1 transcription by increasing DNA methyltransferase or decreasing DNA demethylase (31). The stability of UCA1 is notably improved due to the formation of functional ribonucleoprotein complexes between hnRNPI and UCA1 (44). Conversely, UCA1 interacts with insulin-like growth factor 2 mRNA-binding protein 1 and is downregulated due to its reduced stability (45).
Bone morphogenetic protein 9 (BMP9), a member of the BMP family, belongs to the transforming growth factor-β (TGF-β) superfamily. TGF-β is a well-known inducer of epithelial-to-mesenchymal transition (EMT) (33,46). BMP9 or TGF-β can upregulate UCA1 expression to promote invasion and metastasis of cancer cells, including BC and GC cells (33,46). In addition, TGF-β inactivates the Hippo pathway by regulating the complex of transcriptional co-activator with PDZ binding motif/yes-associated protein (YAP) and transcriptional enhancer TEA domain (TEAD), which subsequently upregulates UCA1 expression and promotes the migration and invasion of BC cells (47). Some clinical trials have reported that metformin exhibits anticancer potential and can be used as an adjuvant for cancer prevention or an AMPK activator for cancer treatment (48,49). Metformin can decrease endometrial hyperplasia via the UCA1/miR-144/TGF-β1/AKT signaling pathway (50). In addition, metformin suppresses the migratory ability of trophoblast cells by regulating the signal transduction pathway of UCA1/miR-204/MMP-9 (51). Notably, lncRNA GAS8-AS1 inhibits migration and invasion of osteosarcoma cells by downregulating UCA1 expression (52). Furthermore, up-frameshift protein 1, palmitic acid, cancer-associated fibroblasts and hepatitis B virus X protein can also regulate UCA1 expression and affect the proliferation of cancer cells, including HCC, GC and CRC cells (53–56).
4. Signaling pathways regulated by UCA1
PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway is involved in important physiological activities, including cell proliferation, invasion and metastasis, and is closely associated with cancer (16,35), DN (19), SLE (18), myocardial fibrosis (57) and PD (20). Recently, the regulatory mechanisms of lncRNAs in the PI3K/AKT pathway and their effects on diseases have attracted great interest (58–60).
It has been reported that UCA1 can promote malignant phenotypes of PDAC by activating the AKT signaling pathway (16). This process includes the promotion of cell proliferation, invasion, EMT, inhibition of apoptosis and enhancement of 5-FU resistance (16). In bladder cancer (35) and SLE (18), UCA1 is highly expressed and promotes cell proliferation by mediating the PI3K/AKT signaling pathway. UCA1 knockdown inhibits proliferation and migration of glioma cells through miR-193a-mediated downregulation of cyclin-dependent kinase 6 (CDK6), and inactivates the PI3K/AKT pathway by decreasing the expression levels of phosphorylated PI3K and AKT proteins (61). In acute myeloid leukemia (AML), UCA1 can be used as an endogenous sponge to compete with miR-126, which in turn suppresses activation of the PI3K/AKT signaling pathway by inhibiting the expression of RAS-related C3 botulinus toxin substrate 1 (RAC1) (62).
Recent studies have demonstrated that enhancer of zeste homolog 2 (EZH2) can promote cell cycle progression by affecting the PI3K/AKT pathway or the expression levels of cyclins (63–65). EZH2 can also be post-translationally modified by phosphorylation of AKT (66). UCA1 directly interacts with EZH2 in GC and enhances EZH2 expression, which in turn activates the AKT/GSK3β/cyclin D1 (CCND1) axis to increase cell proliferation (36). However, UCA1 suppresses p27Kip1/CDK2 signal transduction and promotes cell proliferation and tumorigenesis by recruiting EZH2 (56). In PC (67) and BC (44), UCA1 promotes the proliferation of cancer cells by inhibiting p27 expression. UCA1 can also inhibit the level of cAMP response element-binding (CREB) protein, and regulate the cell cycle of bladder cancer cells via the PI3K-dependent pathway (68). A study has reported that UCA1 impairs the binding of brahma-related gene 1 (BRG1) to the p21 promoter and the chromatin remodeling activity of BRG1 to accelerate the proliferation of bladder cancer cells (69). Cisplatin and gemcitabine resistance in bladder cancer cells is mediated by UCA1-CREB-miR-196a-5p paradigm (70).
UCA1 can inhibit PTEN and promote p-AKT expression to induce the proliferation of osteosarcoma cells (71). Notably, p-PTEN is a phosphatase, and PTEN is a negative regulator of PI3K. Dephosphorylation of PTEN can decrease activation of AKT and block its downstream signal transduction (71). In vivo studies have demonstrated that UCA1 functions via the PI3K/AKT signaling pathway (19,48,72). In the Sprague-Dawley rat model of DN, inhibition of UCA1 decreases renal pathological damage, improved renal function and decreased inflammation in DN rats by suppressing the PI3K/AKT signaling pathway (19). In addition, downregulation of UCA1 expression can ameliorate the damage of dopaminergic neurons by inhibiting the PI3K/AKT signaling pathway to decrease oxidative stress and inflammation in PD (20). Thus, UCA1 participates in the regulation of the PI3K/AKT signaling pathway by affecting EZH2, CDK6, RAC1 and PTEN, which promote cell proliferation, invasion, metastasis and drug resistance, and inhibit apoptosis in diseases (36,61,62,71).
Several studies have demonstrated that mammalian target of rapamycin (mTOR) is the core component downstream of the PI3K/AKT pathway (73–75). lncRNAs participate in cell proliferation, apoptosis, invasion and energy metabolism by regulating the mTOR signaling pathway (73). It has been reported that upregulation of UCA1 expression confers tamoxifen resistance in BC cells, partly by activating the mTOR signaling pathway (74). In addition, UCA1 interacts with mTOR to inhibit p27 and miR-143 expression; however, the expression levels of Kirsten rat sarcoma viral oncogene homolog and CCND1 significantly increase, which results in the proliferation, EMT and metastasis of CRC cells (55). Notably, it has been demonstrated that UCA1 increases CREB1 expression by acting as a competitive endogenous RNA (ceRNA) of miR-582, thus promoting EMT via the CREB1-mediated mTOR pathway, which results in osteosarcoma metastasis (72). In cardiomyocytes, UCA1 decreases miR-122 and miR-143 expression, and regulates their downstream mTOR signaling pathway to inhibit oxygen-glucose deprivation (OGD) or hypoxia/reoxygenation (H/R)-induced apoptosis and injury (76,77). UCA1 knockdown can also regulate signal transduction of mTOR via miR-200c and miR-195, and inhibit the proliferation and invasion of hemangioma cells and microvascular endothelial cells (78). Furthermore, bladder cancer cells preferentially metabolize glucose by aerobic glycolysis, known as the Warburg effect (79). UCA1 promotes glycolysis and upregulates hexokinase 2 expression via the mTOR-STAT3/miR-143 pathway in bladder cancer cells (79). Taken together, these findings suggest that UCA1 can directly or indirectly activate the mTOR signaling pathway by serving as a regulator of miR-582 and miR-195, and affect the expression of proteins, such as CREB1 and CCND1, to promote cell proliferation, drug resistance and inhibit apoptosis.
Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is a developmental signal-transduction pathway. Activation of this pathway can result in the proliferation, invasion, metastasis and differentiation of cancer cells, ultimately inducing tumorigenesis (80). The canonical Wnt pathway activates gene transcription through β-catenin accumulation in the cytoplasm and translocation to the nucleus (80).
Several studies have demonstrated that UCA1 promotes cell proliferation and EMT in osteosarcoma (38), glioma (81) and papillary thyroid carcinoma (82) by activating the Wnt/β-catenin signaling pathway. In addition, the Wnt/β-catenin signaling pathway is closely associated with chemoresistance (12,81,83). UCA1 induces chemoresistance to cisplatin and temozolomide in glioma and decreases the sensitivity of BC cells to tamoxifen via the Wnt/β-catenin signaling pathway (81,83). Notably, UCA1 can also increase cisplatin resistance of bladder cancer cells by activating Wnt signaling in a Wnt6-dependent manner (12).
Previous studies have reported that UCA1 regulates the Wnt/β-catenin signaling pathway via miR-200c and miR-185-5p, and affects the proliferation and EMT in hemangioma (78) and melanoma cells (84), respectively. In osteosarcoma, UCA1 knockdown inhibits miR-301a expression and induces the silencing of C-X-C chemokine receptor type 4 (CXCR4), thus decreasing cell proliferation and metastasis (85). In addition, downregulation of miR-301a expression decreases the levels of Wnt3a, Wnt5a and β-catenin proteins, which in turn inhibits the Wnt/β-catenin signaling pathway (85). UCA1 regulates matrix metalloproteinase-9 expression, which is a downstream gene of Wnt/β-catenin (86), via miR-204 and increases trophoblast cells migration (51). Notably, enhanced UCA1 expression upregulates the expression of highly upregulated in liver cancer (HULC) by inhibiting HULC promoter methylation, and upregulates β-catenin by promoting β-catenin promoter-enhancer chromatin DNA looping formation, which in turn promotes the malignant transformation of hepatocyte-like cells (87). Collectively, these findings suggest that UCA1 is involved in tumorigenesis via the Wnt/β-catenin signaling pathway by regulating miRNAs, such as miR-204 and miR-301a, and lncRNAs, such as HULC.
MAPK signaling pathway
The MAPK pathway participates in the regulation of cell proliferation, invasion, metastasis, apoptosis and differentiation, and is closely associated with the occurrence of various diseases such as PDAC (16), melanocytes (13) and acute myocardial infarction (76). Its functional pattern predominantly involves phosphorylating nuclear transcription factors, cytoskeletal proteins and enzymes (88). The MAPK signaling pathway includes four pathways: The ERK, P38, SAPK/JNK and ERK5/BMK1 pathways. As an important regulatory gene, UCA1 can participate in the regulation of the MAPK signaling pathway (88).
A previous study demonstrated that UCA1 promotes the proliferation, migration and invasion of PDAC cells, and decreases apoptosis and increases 5-FU resistance by activating the ERK signaling pathway (16). In addition, UCA1 negatively regulates the CREB/MITF/melanogenesis axis by suppressing the ERK and JNK signaling pathways in melanocytes, thus inhibiting the expression of melanogenesis-related genes in melanocytes (13).
UCA1 knockdown activates the AMPK signaling pathway by upregulating a series of miRNAs (61,76,78,89). In addition, UCA1 knockdown regulates AMPK signal transduction via miR-200c and suppresses the proliferation, migration and invasion of hemangioma cells (78). Furthermore, in acute myocardial infarction, UCA1 decreases OGD aroused cell apoptosis and injury by downregulating miR-122 expression and suppressing its downstream JNK/p38 MAPK signaling pathway (76). Knockdown of UCA1 inactivates the MAPK signaling pathway by downregulating CDK6 expression mediated by miR-193a, which decreases the proliferation and promotes the apoptosis of glioma cells (61). In cholangiocarcinoma, UCA1 upregulates chloride intracellular channel 1 (CLIC1) expression by inhibiting miR-122 expression and activating the ERK/MAPK signaling pathway to promote the metastasis of malignant cells (89). In addition, UCA1 promotes the viability and EMT of TPC-1 cells by competing with miR-15a and activating the Hippo and JNK signaling pathways in human thyroid cancer (90). Taken together, these findings suggest that UCA1 activates the MAPK signaling pathways by regulating CLIC1 and CDK6 expression, inducing cell proliferation, migration and drug resistance, and inhibiting apoptosis.
Nuclear factor-kappa B (NF-κB) signaling pathway
NF-κB is an extensively expressed multi effect transcription factor. It is a heterotrimer composed of p50, p65 and IκB (91). The NF-κB signaling pathway is activated by several factors, including extracellular stimulation and transcription factors (91). Activated NF-κB signaling pathway mediates several biological processes, such as cell proliferation, tumor metastasis and inflammation (91).
UCA1 can activate the NF-κB signaling pathway through inflammatory cytokines like IL6 (92) and transcription factors like MEF2C (93). Several studies have demonstrated that the NF-κB signaling pathway can be regulated by UCA1 (85,94). In osteosarcoma, inhibition of UCA1 downregulates miR-301a expression and decreases CXCR4 expression, thus suppressing cell proliferation and metastasis (85). Furthermore, miR-301a positively regulates the phosphorylation of IκB and p65 proteins and activates the NF-κB signaling pathway (85). UCA1 knockdown exerts an anticancer effect on GC cells by rescuing miR-182 expression to activate the NF-κB and PI3K/AKT/GSK3β signaling pathways (94). In HCC, the synergism between UCA1 and inflammatory cytokine IL6 promotes SUV39H1 expression. Notably, methyltransferase-like 3, which binds to SUV39H1 mRNA, is also upregulated. Excessive SUV39H1 expression can increase the expression of tri-methylation of histone 3 lysine 9 trimethylation (H3K9me3) (92). Notably, under inflammatory conditions, H3K9me3 induces phosphorylation of NF-κB, resulting in the malignant transformation of hepatocyte-like stem cells (92). This synergism between UCA1 and IL6 can aggravate the malignant transformation of hepatocyte-like stem cells via the NF-κB signaling pathway (92). In addition, UCA1 regulates the inflammatory responses in epilepsy by regulating the miR-203-mediated myocyte enhancer factor 2C/NF-κB signaling pathway (93).
JAK/STAT signaling pathway
The JAK/STAT signaling pathway is a common pathway for various cytokines and growth factors to transmit signals within cells (95). It mediates several biological responses, including cell proliferation, differentiation, migration, apoptosis and immune regulation (95). Previous studies have demonstrated that lncRNAs play a regulatory role in the JAK/STAT signaling pathway (96,97).
UCA1 promotes the development of cancer cells by activating the JAK/STAT signaling pathway, including multiple myeloma (MM) (98), AML (62) and pre-eclampsia (99). UCA1 activates the JAK2/STAT3 pathway via the miR-331-3p/IL6R axis in MM, and regulates cell proliferation and apoptosis (98). In AML, UCA1 competes with miR-126 as an endogenous sponge. miR-126 suppresses activation of the JAK/STAT signaling pathway by decreasing RAC1 expression, thus inhibiting cell proliferation, migration and invasion (62). Notably, UCA1 exerts opposite effects on the regulation of the JAK/STAT signaling pathway in some non-cancerous cells (99,100). A study has reported that UCA1 expression is upregulated in trophoblast cells of pre-eclampsia pregnancy, and it can inhibit the invasion and proliferation of trophoblast cells by downregulating JAK2 expression (99). In addition, overexpression of UCA1 inhibits activation of the JAK/STAT signaling pathway, which in turn inhibits activation of astrocytes in temporal lobe epilepsy (100). It is suggested that UCA1 promotes IL6R and RAC1 expression to stimulate the JAK/STAT signaling pathway, which increases cell proliferation and invasion in several tumors, while UCA1 exerts opposing effects in some non-cancerous cells (62,98).
TGF-β/SMAD signaling pathway
The TGF-β superfamily is composed of secretory polypeptide molecule TGF-β, activins, inhibins and BMPs, and participates in various biological activities, such as cell invasion and migration (101). The TGF-β superfamily is involved in cartilage and bone formation, inflammation, regulation of endocrine functions, and formation and development of tumors (101). SMADs are important molecules for intracellular TGF-β signal transduction (101).
Previous studies have demonstrated that EMT induced by TGF-β1 is an important reason for the invasion and migration of cancer cells (46,102). The UCA1/miR-124 axis regulates TGF-β1-induced EMT and invasion of tongue cancer cells (46). In addition, UCA1 promotes the proliferation of MM cells by targeting TGF-β (102). SMAD4 and SMAD5, two members of SMADs, are important elements involved in the TGF-β pathway (103). It has been confirmed that UCA1 regulates the TGF-β signaling pathway via the miR-145-5p/SMAD5 and miR-124-3p/SMAD4 axes and promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells (103). Notably, UCA1 competitively binds with miR-1 and miR-203a to upregulate the expression of Slug, a downstream effector of TGF-β (104), and subsequently regulates EMT in glioma cells (104) and BC cells (33).
Other signaling pathways
UCA1 also participates in the regulation of other signaling pathways, including the Notch, Hippo and p53 signaling pathways. In human glioma, UCA1 knockdown inhibits the proliferation and migration of cells through miR-193a-mediated downregulation of CDK6, and blocks the Notch signaling pathway by decreasing the expression levels of phosphorylated Notch1, Notch2 and Notch3 proteins (61). In renal cell carcinoma, UCA1 upregulates Delta-like 4 (DLL4) expression by acting as a ceRNA of miR-182-5p, and induces the EMT process via the DLL4-mediated Notch pathway, which in turn promotes cell proliferation and migration, and inhibits apoptosis (11). Furthermore, UCA1 can form a ribonucleoprotein complex with Mps one binder kinase activator-1, large tumor suppressor 1 and YAP, which decreases the phosphorylation of YAP (105). Dephosphorylated YAP translocates into the nucleus and combines with TEAD protein, inhibiting the key proteins of the Hippo signaling pathway and promoting proliferation, migration and invasion of PC cells (105). Conversely, UCA1 activates the Hippo signaling pathway by downregulating miR-15a expression and increasing cell viability and EMT in human thyroid cancer (90). In cardiomyocytes, UCA1 inhibits H/R-induced apoptosis by decreasing miR-143 expression and suppressing its downstream p53 signaling pathway (77). Furthermore, UCA1 can regulate the p53 signaling pathway via miR-143 and MDM2; the Notch signaling pathway via CDK6 and DLL4 or regulate YAP and other signaling pathways, which promotes cell proliferation, EMT, migration and invasion, and inhibits cell apoptosis (11,61,105).
5. Clinical applications of UCA1
Several studies have reported that UCA1 has potential clinical applications (106–109). Currently, there are three common clinical applications of UCA1: Acts as a diagnostic biomarker (110,111), prognostic biomarker (112,113) and therapeutic target (87). The use of UCA1 as a therapeutic target for reversing drug resistance has been extensively investigated (22,83).
UCA1 as a diagnostic biomarker
Early diagnosis is beneficial to the prognosis of diseases. Poor prognosis is mainly attributed to the metastasis of cancer cells, drug resistance and tumor recurrence, which are closely associated with late diagnosis (114). Thus, it is important to improve the early diagnostic rate of patients. Recently, several studies have demonstrated that lncRNAs have great potential to be used as biomarkers for disease diagnosis, including UCA1 (115–117).
UCA1 is highly specific and easy to be detected in serum, plasma and urine. Liquid biopsy is becoming a novel method for disease detection (72,118). In laryngeal squamous cell carcinoma (110), NSCLC (111), osteosarcoma (72) and HCC (118), serum UCA1 levels of patients are higher than that of the healthy control population. Notably, UCA1 secreted by exosomes into the serum of patients can promote the development of prostate cancer (PCa) (114). In addition, serum UCA1 expression is significantly higher in patients with HCC than those with benign liver disease, which helps to distinguish the two groups (119). In non-cancerous diseases, UCA1 can limit the inflammatory responses in epilepsy (93), and UCA1 expression is higher in patients with non-refractory epilepsy than those with refractory epilepsy (120). Notably, combined measurement of UCA1 levels in serum and plasma can increase the sensitivity and specificity for the diagnosis of some malignancies (119). In addition, UCA1 expression is higher in the urine of patients with PCa compared with healthy controls (114). Taken together, these findings suggest that UCA1 has the potential to be used as a biomarker for diagnosis and screening of diseases.
Increasing evidence suggest that the combination of UCA1 and other lncRNAs may exhibit better diagnostic performance. As a predictor of CRC, the combination of UCA1, HOXA transcript at the distal tip and plasmacytoma variant translocation 1 has better diagnostic performance compared with UCA1 alone, and can be used to screen patients with advanced CRC (115). Another study demonstrated that the combination of UCA1, gastric cancer high expressed transcript 1, taurine upregulated gene 1 and p21-associated ncRNA DNA damage activated can improve the diagnostic ability and significance of GC (116). Furthermore, when comparing patients with bladder cancer with healthy controls, the combination of UCA1, circFARSA and circSHKBP1 has better diagnostic performance compared with UCA1 alone (117).
Early differential diagnosis of cancer and precancerous lesions is an important method to decrease the risk of recurrence and improve prognosis (121). A study has demonstrated that the combination of UCA1, HOX transcript antisense intergenic RNA, hydatidiform mole associated and imprinted and metastasis-associated lung adenocarcinoma transcript 1 can distinguish between patients with bladder cancer and patients with urocystitis, with a sensitivity and specificity of 95.7% and 94.3%, respectively (121). Collectively, these findings suggest that it is feasible to use UCA1 as a diagnostic biomarker. However, further studies are required to verify the clinical applications of UCA1.
UCA1 as a prognostic biomarker
With the rapid growth of disease morbidity and mortality, overall prognosis will become the principal determinant of global public health (122). Surgery is one of the routine therapies for cancer; however, its complications and recurrence affect the prognosis of patients (122). Recent studies have demonstrated that UCA1 is closely associated with clinicopathological characteristics, including vascular invasion, lymph node metastasis and TNM stage, and can be used as a prognostic biomarker of diseases (24,25,123). However, there is no doubt whether further investigation on the role of UCA1 as a prognostic marker will contribute to the treatment of cancer.
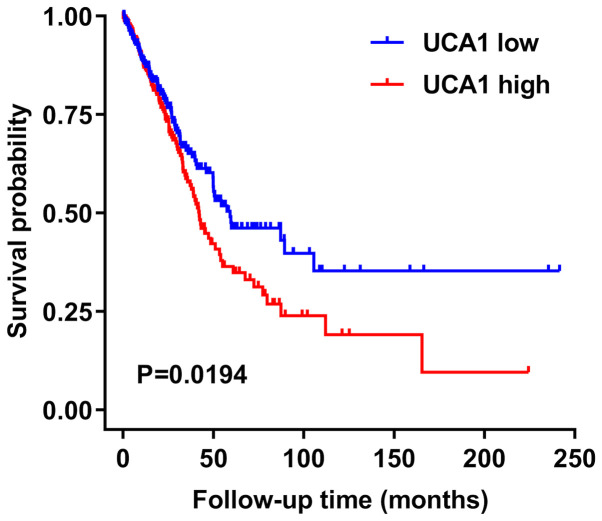
UCA1 is closely associated with tumor size, histological differentiation, stage of lymph node metastasis, depth of invasion, vascular invasion, OS, RFS and prognostic biomarkers (Table III). It has been reported that in some cancers, such as CRC (24), HCC (23), GC (8), adrenal cortical carcinoma (124) and esophageal cancer (125), survival probability of patients is associated with UCA1 expression. The Cancer Genome Atlas (https://cancergenome.nih.gov) data for lung adenocarcinoma revealed that the survival probability of patients with NSCLC, with high UCA1 expression, is unfavorable than those with low UCA1 expression (Fig. 3). Previous studies have suggested that overexpression of UCA1 in CRC indicates a large tumor, advanced TNM stage, deeply infiltrated lymph node, positive lymph node metastasis and poor OS (22,24). Other studies have reported that UCA1 expression in GC tissues and cells is positively associated with TNM stage, lymph node infiltration, lymph node metastasis and OS (8,9). Notably, UCA1 expression in the plasma of patients with GC significantly decreases following surgery (126). Thus, UCA1 can be used as an index of postoperative prognosis. In addition, UCA1 expression is significantly upregulated in SLE, which is positively associated with SLE disease activity index (18). Collectively, these findings suggest that UCA1 can be used as an independent prognostic factor to monitor the occurrence and development of diseases.
Table III.
Role of UCA1 in clinical correlation and prognosis of diseases.
| Disease | Upregulation/downregulation | Clinical correlation | (Refs.) |
|---|---|---|---|
| Renal cancer | Upregulation | Degree of differentiation, TNM stage and lymph node metastasis | (11,107) |
| SLE | Upregulation | SLEDAI | (18) |
| CRC | Upregulation | Tumor size, tumor stage, lymph node infiltration, lymph node metastasis and OS | (22,24) |
| HCC | Upregulation | Tumor stage, TNM stage, Microvascular infiltration and OS | (23) |
| GC | Upregulation | TNM stage, tumor stage, lymph node infiltration, lymph node metastasis and OS | (8,9) |
| Tongue cancer | Upregulation | TNM staging, lymph node metastasis, lymph node infiltration and OS | (46) |
| Osteosarcoma | Upregulation | Tumor stage, tumor size and OS | (72) |
| OSCC | Upregulation | TNM stage, lymph node metastasis and OS | (108) |
| PTC | Upregulation | Tumor size, tumor stage, lymph node metastasis and OS | (26,82) |
| MM | Upregulation | OS | (98,102) |
| PC | Upregulation | Tumor stage and OS | (27,105) |
| LSCC | Upregulation | Lymph node metastasis and OS | (110) |
| NSCLC | Upregulation | TNM stage, tumor size and OS | (111) |
| PCa | Upregulation | Edmondson-Steiner grade, TNM stage and lymph node metastasis | (112,114) |
| Epilepsy | Upregulation | Classification of epilepsy | (120) |
| Glioma | Upregulation | TNM stage, tumor size, lymph node metastasis and OS | (109) |
| Cholangiocarcinoma | Upregulation | TNM stage, tumor stage, lymph node infiltration, lymph node metastasis, RFS and OS | (10) |
| OC | Upregulation | TNM stage, lymph node metastasis and OS | (128) |
| NPC | Upregulation | Tumor stage, lymph node metastasis and OS | (113) |
| ACC | Upregulation | TNM stage and lymph node metastasis | (124) |
| LUAD | Upregulation | TNM stage, lymph node metastasis, RFS and OS | (28) |
| Melanoma | Upregulation | Tumor stage and lymph node metastasis | (106) |
| EC | Upregulation | Degree of differentiation, TNM stage, lymph node metastasis and OS | (125) |
UCA1, urothelial carcinoma associated 1; ACC, adrenal cortical carcinoma; CRC, colorectal cancer; EC, esophageal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; LSCC, laryngeal squamous cell carcinoma; LUAD, lung adenocarcinoma; MM, multiple myeloma; NPC, nasopharyngeal carcinoma; NSCLC, non-small cell lung cancer; OC, ovarian cancer; OS, overall survival; OSCC, oral squamous cell carcinoma; PC, pancreatic cancer; PCa, prostate cancer; PTC, papillary thyroid carcinoma; RFS, relapse-free survival; SLEDAI, systemic lupus erythematosus disease activity index; TNM, tumor-node-metastasis.
Figure 3.
Association between UCA1 expression and survival time of patients with non-small cell lung cancer, based on The Cancer Genome Atlas data. UCA1, urothelial carcinoma associated 1.
UCA1 as a therapeutic target
With a better understanding of the pathogenesis of diseases, several molecules and signal transduction pathways are likely to be suitable for targeted therapy (122). Given that UCA1 exerts carcinogenic effects associated with several molecules and cascade reactions, it has been confirmed as an ideal therapeutic target (22,70,81). Targeted knockout of UCA1 can be used to improve radiosensitivity, inhibit cancer metastasis, prevent cancer growth in vivo, promote apoptosis and reverse drug resistance of cancer cells (21,37,72). Clinical applications of UCA1 as a therapeutic target for reversing drug resistance has attracted great interest (21,127). UCA1 is also known as a cancer upregulated drug resistant gene (87). Previous studies have reported that downregulating UCA1 expression can reverse drug resistance in cancers, and some drugs for chemotherapy exert an anticancer role by mediating UCA1 (Table IV), including cisplatin (70,81), tamoxifen (83), paclitaxel (128), 5-FU (22), Adriamycin (129), temozolomide (81), cetuximab (130), trastuzumab (131), imatinib (132), docetaxel (133) and gemcitabine (70). These drugs provide the possibility of chemotherapy-related clinical applications, and studies have demonstrated that UCA1 knockdown can reverse multidrug resistance in retinoblastoma (127) and bladder cancer (21). In addition, the combination of UCA1-targeted therapy and programmed cell death 1 (PD-1) immune checkpoint blockade has a better synergistic effect following CRISPR-Cas9-mediated UCA1 knockdown (134). However, further studies are required to confirm the therapeutic value of UCA1 for diseases.
Table IV.
Role of UCA1 in tumor drug resistance.
| Cancer type | Drug resistance | Mechanisms | (Refs.) |
|---|---|---|---|
| PDAC | 5-FU | UCA1 promotes EMT by activating the AKT and ERK signaling pathways in PDAC cells | (16) |
| Bladder cancer | Cisplatin/gemcitabine | UCA1 inhibits cisplatin/gemcitabine-induced apoptosis via targeting p27Kip1 | (70) |
| Bladder cancer | Cisplatin | UCA1 upregulates Wnt6 expression | (12) |
| Bladder cancer | Multidrug resistance | UCA1 inhibits autophagy by upregulating ATG7 expression | (21) |
| OC | Paclitaxel | UCA1 upregulates ABCB1 expression | (128) |
| CRC | 5-FU | UCA1 upregulates CREB1 expression | (22) |
| AML | Adriamycin | UCA1 upregulates the HK2 expression | (129) |
| BC | Tamoxifen | UCA1 activates the mTOR signaling pathway | (74) |
| BC | Tamoxifen | UCA1 activates the Wnt/β-catenin signaling pathway | (83) |
| BC | Trastuzumab | UCA1 upregulates YAP1 expression | (131) |
| Glioma | Cisplatin/temozolomide | UCA1 activates the Wnt/β-catenin signaling pathway | (81) |
| CRC | Cetuximab | UCA1 in exosomes transmits cetuximab resistance | (130) |
| CML | Imatinib | UCA1 upregulates MDR1 expression | (132) |
| PCa | Docetaxel | UCA1 upregulates Sirt1 expression | (133) |
| Retinoblastoma | Multidrug resistance | UCA1 upregulates STMN1 expression | (127) |
UCA1, urothelial carcinoma associated 1; 5-FU, 5-fluorouracil; AML, acute myeloid leukemia; BC, breast cancer; CML, chronic myeloid leukemia; CRC, colorectal cancer; CREB1, cAMP response element-binding protein 1; HK2, hexokinase 2; MDR1, multidrug resistance protein 1; mTOR, mammalian target of rapamycin; OC, ovarian cancer; PCa, prostate cancer; PDAC, pancreatic ductal adenocarcinoma; STMN1, stathmin 1; YAP1, yes-associated protein 1.
6. Conclusions and perspective
UCA1 can regulate a series of signaling pathways by acting as a ceRNA of several miRNAs, and affect epigenetic, transcriptional and post-transcriptional regulation. It plays a regulatory role in several biological functions, including cell proliferation, apoptosis, migration, invasion and drug resistance. In addition, UCA1 can be used as a potential biomarker for disease diagnosis and treatment. For some diseases, the combination of UCA1 and other lncRNAs has demonstrated better diagnostic and screening performance. Strategies, such as CRISPR-Cas9 system or siRNA-mediated knockout, and the combination of UCA1-targeted therapy and PD-1 immune checkpoint blockade may be used to transform UCA1 from basic research into clinical practice.
Recently, although great progress has been made in understanding the biology of UCA1, even in its therapeutic applications, several unknown areas in UCA1 research remain. First, the lack of effective methods to investigate RNA secondary and tertiary structures and nuclear ultrastructure of UCA1 impedes the study on the composition of its isoforms and tissue-specific regulation (4). Secondly, several studies have confirmed that lncRNAs can promote the drug resistance of cancer cells by regulating their stemness feature (27,135,136,104); however, the mechanism by which UCA1 affects the drug resistance of cancer stem cells remains unclear. Some lncRNAs may play important regulatory roles in cellular biological processes via multiple pathways (5,6). However, whether binding miRNAs affects the expression or function of UCA1 remains unclear. In conclusion, UCA1, as a well-known lncRNA with great clinical potential, requires increasing comprehension and in-depth study.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- 5-FU
5-fluorouracil
- AML
acute myeloid leukemia
- ARID1A
AT-rich interaction domain 1A
- BC
breast cancer
- BMP9
bone morphogenetic protein 9
- BRG1
brahma-related gene 1
- C/EBPα
CCAAT/enhancer binding protein α
- CCND1
cyclin D1
- CDK6
cyclin-dependent kinase 6
- ceRNA
competitive endogenous RNA
- CLIC1
chloride intracellular channel 1
- CRC
colorectal cancer
- CREB
cAMP response element-binding
- CXCR4
C-X-C chemokine receptor type 4
- DLL4
Delta-like 4
- DN
diabetic nephropathy
- EMT
epithelial-to-mesenchymal transition
- EP300
E1A binding protein p300
- EZH2
enhancer of zeste homolog 2
- GC
gastric cancer
- H/R
hypoxia/reoxygenation
- H3K9me3
histone 3 lysine 9 trimethylation
- HCC
hepatocellular carcinoma
- HULC
highly upregulated in liver cancer
- lncRNA
long non-coding RNA
- miRNA/miR
microRNA
- MM
multiple myeloma
- mTOR
mammalian target of rapamycin
- NSCLC
non-small cell lung cancer
- OGD
oxygen-glucose deprivation
- ORFs
open reading frames
- OS
overall survival
- PC
pancreatic cancer
- PD
Parkinson's disease
- PD-1
programmed cell death 1
- PDAC
pancreatic ductal adenocarcinoma
- RAC1
RAS-related C3 botulinus toxin substrate 1
- RFS
relapse-free survival
- SLE
systemic lupus erythematosus
- TCC
transitional cell carcinoma
- TEAD
TEA domain
- TGF-β
transforming growth factor-β
- TNM
tumor-node-metastasis
- UCA1
urothelial carcinoma associated 1
- YAP
yes-associated protein
Funding Statement
The present review was funded by the National Natural Science Foundation of China (grant nos. 81870105 and 81770107), the Scientific Research Fund Project of Hunan Provincial Health Commission (grant no. 20201921), the National Key Research and Development Program of China (grant no. 2018YFA0107800) and the Scientific Research and Innovation Project of postgraduates in Hunan Province (grant no. CX20200970).
Funding
The present review was funded by the National Natural Science Foundation of China (grant nos. 81870105 and 81770107), the Scientific Research Fund Project of Hunan Provincial Health Commission (grant no. 20201921), the National Key Research and Development Program of China (grant no. 2018YFA0107800) and the Scientific Research and Innovation Project of postgraduates in Hunan Province (grant no. CX20200970).
Availability of data and materials
Not applicable.
Authors' contributions
ZL and YW contributed to drafting the review and figures. SY, FW, and JL revised the manuscript for important intellectual content. JZ and LZ conceived the project and revised the manuscript for important intellectual content. ZL and YW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao H, Wahlestedt C, Kapranov P. Strategies to annotate and characterize long noncoding RNAs: Advantages and pitfalls. Trends Genet. 2018;34:704–721. doi: 10.1016/j.tig.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Kaikkonen MU, Adelman K. Emerging roles of non-coding RNA transcription. Trends Biochem Sci. 2018;43:654–667. doi: 10.1016/j.tibs.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci. 2019;44:33–52. doi: 10.1016/j.tibs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Xiao G, Yao J, Kong D, Ye C, Chen R, Li L, Zeng T, Wang L, Zhang W, Shi X, et al. The Long Noncoding RNA TTTY15, Which is located on the Y chromosome, promotes prostate cancer progression by sponging let-7. Eur Urol. 2019;76:315–326. doi: 10.1016/j.eururo.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XS, Zhang Z, Wang HC, Cai JL, Xu QW, Li MQ, Chen YC, Qian XP, Lu TJ, Yu LZ, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–4858. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Xiong JB, Zhang GY, Liu Y, Jie ZG, Li ZR. Long noncoding RNA UCA1 regulates PRL-3 expression by sponging MicroRNA-495 to promote the progression of gastric cancer. Mol Ther Nucleic Acids. 2020;19:853–864. doi: 10.1016/j.omtn.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY, Liu Q, Zhao G, Zhang ZZ. The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol Cancer. 2019;18:115. doi: 10.1186/s12943-019-1032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li O, Yi W, Yang P, Guo C, Peng C. Long non-coding RNA UCA1 promotes proliferation and invasion of intrahepatic cholangiocarcinoma cells through targeting microRNA-122. Exp Ther Med. 2019;18:25–32. doi: 10.3892/etm.2019.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Hu W, Wang Y, An Y, Song L, Shang P, Yue Z. Long non-coding RNA UCA1 promotes malignant phenotypes of renal cancer cells by modulating the miR-182-5p/DLL4 axis as a ceRNA. Mol Cancer. 2020;19:18. doi: 10.1186/s12943-020-1132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. Febs J. 2014;281:1750–1758. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 13.Pei S, Chen J, Lu J, Hu S, Jiang L, Lei L, Ouyang Y, Fu C, Ding Y, Li S, et al. The long noncoding RNA UCA1 negatively regulates melanogenesis in melanocytes. J Invest Dermatol. 2020;140:152–163.e5. doi: 10.1016/j.jid.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Neve B, Jonckheere N, Vincent A, Van Seuningen I. Epigenetic Regulation by lncRNAs: An Overview Focused on UCA1 in Colorectal Cancer. Cancers (Basel) 2018;10:440. doi: 10.3390/cancers10110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao K, Xu J, Yang W, You X, Zhong Q, Wang X. The research progress of LncRNA involved in the regulation of inflammatory diseases. Mol Immunol. 2018;101:182–188. doi: 10.1016/j.molimm.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Qi M, Wu R, Liu A, Chen D, Tang L, Chen J, Hu X, Li W, Zhan L, et al. Long non-coding RNA CUDR promotes malignant phenotypes in pancreatic ductal adenocarcinoma via activating AKT and ERK signaling pathways. Int J Oncol. 2018;53:2671–2682. doi: 10.3892/ijo.2018.4574. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Li M, Song Y, Xu J, Qi F. Overexpression of long noncoding RNA CUDR promotes hepatic differentiation of human umbilical cord mesenchymal stem cells. Mol Med Rep. 2020;21:1051–1058. doi: 10.3892/mmr.2019.10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang CR, Li TH. Circulating UCA1 is highly expressed in patients with systemic lupus erythematosus and promotes the progression through the AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:2364–2371. doi: 10.26355/eurrev_201804_14828. [DOI] [PubMed] [Google Scholar]
- 19.Shi CH, Huang Y, Li WQ, Chen RG. Influence of LncRNA UCA1 on glucose metabolism in rats with diabetic nephropathy through PI3K-Akt signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:10058–10064. doi: 10.26355/eurrev_201911_19573. [DOI] [PubMed] [Google Scholar]
- 20.Cai L, Tu L, Li T, Yang X, Ren Y, Gu R, Zhang Q, Yao H, Qu X, Wang Q, Tian J. Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson's disease through the inhibition of the PI3K/Akt signaling pathway. Int Immunopharmacol. 2019;75:105734. doi: 10.1016/j.intimp.2019.105734. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Li W, Ning J, Yu W, Rao T, Cheng F. Long noncoding RNA UCA1 targets miR-582-5p and contributes to the progression and drug resistance of bladder cancer cells through ATG7-mediated autophagy inhibition. Onco Targets Ther. 2019;12:495–508. doi: 10.2147/OTT.S183940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu Y, Feng Y, Liu H, Fei B, Mao Y, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B, Lu Y, Cao X, Zhu W, Kong L, Ji H, Zhang F, Lin X, Guan Q, Ou K, et al. MiRNA-124 inhibits the proliferation, migration and invasion of cancer cell in hepatocellular carcinoma by downregulating lncRNA-UCA1. Onco Targets Ther. 2019;12:4509–4516. doi: 10.2147/OTT.S205169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luan Y, Li X, Luan Y, Zhao R, Li Y, Liu L, Hao Y, Oleg Vladimir B, Jia L. Circulating lncRNA UCA1 promotes malignancy of colorectal cancer via the miR-143/MYO6 Axis. Mol Ther Nucleic Acids. 2020;19:790–803. doi: 10.1016/j.omtn.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Hu CP. Long Non-Coding RNA urothelial carcinoma associated 1 (UCA1): Insight into its role in human diseases. Crit Rev Eukaryot Gene Expr. 2015;25:191–197. doi: 10.1615/CritRevEukaryotGeneExpr.2015013770. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Cui M, Yu P, Li Q. Correlations of lncRNAs with cervical lymph node metastasis and prognosis of papillary thyroid carcinoma. Onco Targets Ther. 2019;12:1269–1278. doi: 10.2147/OTT.S191700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Feng W, Gu S, Wang H, Zhang Y, Chen W, Xu W, Lin C, Gong A, Xu M. The UCA1/KRAS axis promotes human pancreatic ductal adenocarcinoma stem cell properties and tumor growth. Am J Cancer Res. 2019;9:496–510. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Cao P, Wu Q, Guo Y, Yang Y, Chen F. Overexpression of LncRNA-UCA1 correlates with lung adenocarcinoma progression and poor Prognosis. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2018.180739. [DOI] [PubMed] [Google Scholar]
- 29.Lee JJ, Kim M, Kim HP. Epigenetic regulation of long noncoding RNA UCA1 by SATB1 in breast cancer. BMB Rep. 2016;49:578–583. doi: 10.5483/BMBRep.2016.49.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X, Zhang Y, Mayakonda A, Madan V, Ding LW, Lin LH, Zia S, Gery S, Tyner JW, Zhou W, et al. ARID1A and CEBPα cooperatively inhibit UCA1 transcription in breast cancer. Oncogene. 2018;37:5939–5951. doi: 10.1038/s41388-018-0371-4. [DOI] [PubMed] [Google Scholar]
- 31.Sadek KM, Lebda MA, Nasr NE, Nasr SM, El-Sayed Y. Role of lncRNAs as prognostic markers of hepatic cancer and potential therapeutic targeting by S-adenosylmethionine via inhibiting PI3K/Akt signaling pathways. Environ Sci Pollut Res Int. 2018;25:20057–20070. doi: 10.1007/s11356-018-2179-8. [DOI] [PubMed] [Google Scholar]
- 32.Xue M, Li X, Wu W, Zhang S, Wu S, Li Z, Chen W. Upregulation of long non-coding RNA urothelial carcinoma associated 1 by CCAAT/enhancer binding protein alpha contributes to bladder cancer cell growth and reduced apoptosis. Oncol Rep. 2014;31:1993–2000. doi: 10.3892/or.2014.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li GY, Wang W, Sun JY, Xin B, Zhang X, Wang T, Zhang QF, Yao LB, Han H, Fan DM, et al. Long non-coding RNAs AC026904.1 and UCA1: A ‘one-two punch’ for TGF-β-induced SNAI2 activation and epithelial-mesenchymal transition in breast cancer. Theranostics. 2018;8:2846–2861. doi: 10.7150/thno.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin B, Gong Y, Li H, Jiao L, Xin D, Gong Y, He Z, Zhou L, Jin Y, Wang X, et al. C/EBPβ promotes the viability of human bladder cancer cell by contributing to the transcription of bladder cancer specific lncRNA UCA1. Biochem Biophys Res Commun. 2018;506:674–679. doi: 10.1016/j.bbrc.2018.10.152. [DOI] [PubMed] [Google Scholar]
- 35.Wu W, Zhang S, Li X, Xue M, Cao S, Chen W. Ets-2 regulates cell apoptosis via the Akt pathway, through the regulation of urothelial cancer associated 1, a long non-coding RNA, in bladder cancer cells. PLoS One. 2013;8:e73920. doi: 10.1371/journal.pone.0073920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ZQ, Cai Q, Hu L, He CY, Li JF, Quan ZW, Liu BY, Li C, Zhu ZG. Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis. 2017;8:e2839. doi: 10.1038/cddis.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui X, Zhao C, Yao X, Qian B, Su C, Ren Y, Yao Z, Gao X, Yang J. SND1 acts as an anti-apoptotic factor via regulating the expression of lncRNA UCA1 in hepatocellular carcinoma. RNA Biol. 2018;15:1364–1375. doi: 10.1080/15476286.2018.1534525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su Y, Zhou Y, Sun YJ, Wang YL, Yin JY, Huang YJ, Zhang JJ, He AN, Han K, Zhang HZ, et al. Macrophage-derived CCL18 promotes osteosarcoma proliferation and migration by upregulating the expression of UCA1. J Mol Med (Berl) 2019;97:49–61. doi: 10.1007/s00109-018-1711-0. [DOI] [PubMed] [Google Scholar]
- 39.Bourguignon LYW. Matrix hyaluronan-CD44 interaction activates MicroRNA and LncRNA Signaling Associated With chemoresistance, invasion, and tumor progression. Front Oncol. 2019;9:492. doi: 10.3389/fonc.2019.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung YP, Redig A, Hornick JL, Sholl LM. ARID1A mutations and expression loss in non-small cell lung carcinomas: Clinicopathologic and molecular analysis. Mod Pathol. 2020;33:2256–2268. doi: 10.1038/s41379-020-0592-2. [DOI] [PubMed] [Google Scholar]
- 41.Wang T, Gao X, Zhou K, Jiang T, Gao S, Liu P, Zuo X, Shi X. Role of ARID1A in epithelial-mesenchymal transition in breast cancer and its effect on cell sensitivity to 5-FU. Int J Mol Med. 2020;46:1683–1694. doi: 10.3892/ijmm.2020.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferri-Borgogno S, Barui S, McGee AM, Griffiths T, Singh PK, Piett CG, Ghosh B, Bhattacharyya S, Singhi A, Pradhan K, et al. Paradoxical Role of AT-rich interactive domain 1a in restraining pancreatic carcinogenesis. Cancers (Basel) 2020;12:2695. doi: 10.3390/cancers12092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar PP, Emechebe U, Smith R, Franklin S, Moore B, Yandell M, Lessnick SL, Moon AM. Coordinated control of senescence by lncRNA and a novel T-box3 co-repressor complex. Elife. 2014;3:e02805. doi: 10.7554/eLife.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Zhou N, Watabe K, Lu Z, Wu F, Xu M, Mo YY. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1) Cell Death Dis. 2014;5:e1008. doi: 10.1038/cddis.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Meng X, Chen S, Li W, Li D, Singer R, Gu W. IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Res. 2018;20:32. doi: 10.1186/s13058-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang TH, Liang LZ, Liu XL, Wu JN, Su K, Chen JY, Zheng QY. LncRNA UCA1/miR-124 axis modulates TGFβ1-induced epithelial-mesenchymal transition and invasion of tongue cancer cells through JAG1/Notch signaling. J Cell Biochem. 2019;120:10495–10504. doi: 10.1002/jcb.28334. [DOI] [PubMed] [Google Scholar]
- 47.Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo J, Li Y, Duan H, Yuan L. Metformin suppresses the proliferation and promotes the apoptosis of colon cancer cells through inhibiting the expression of long noncoding RNA-UCA1. Onco Targets Ther. 2020;13:4169–4181. doi: 10.2147/OTT.S245091. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Li T, Sun X, Jiang X. UCA1 involved in the metformin-regulated bladder cancer cell proliferation and glycolysis. Tumour Biol. 2017;39:1010428317710823. doi: 10.1177/1010428317710823. [DOI] [PubMed] [Google Scholar]
- 50.Guo M, Zhou JJ, Huang W. Metformin alleviates endometrial hyperplasia through the UCA1/miR144/TGFbeta1/AKT signaling pathway. Int J Mol Med. 2020;45:623–633. doi: 10.3892/ijmm.2019.4438. [DOI] [PubMed] [Google Scholar]
- 51.Ding Y, Yuan X, Gu W, Lu L. Treatment with metformin prevents pre-eclampsia by suppressing migration of trophoblast cells via modulating the signaling pathway of UCA1/miR-204/MMP-9. Biochem Biophys Res Commun. 2019;520:115–121. doi: 10.1016/j.bbrc.2019.09.099. [DOI] [PubMed] [Google Scholar]
- 52.Zha Z, Han Q, Liu W, Huo S. lncRNA GAS8-AS1 downregulates lncRNA UCA1 to inhibit osteosarcoma cell migration and invasion. J Orthop Surg Res. 2020;15:38. doi: 10.1186/s13018-020-1550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Li Y, Wang N, Li X, Zheng J, Ge L. UPF1 inhibits the hepatocellular carcinoma progression by targeting long non-coding RNA UCA1. Sci Rep. 2019;9:6652. doi: 10.1038/s41598-019-43148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan J, Dai Q, Zhang T, Li C. Palmitate acid promotes gastric cancer metastasis via FABP5/SP1/UCA1 pathway. Cancer Cell Int. 2019;19:69. doi: 10.1186/s12935-019-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jahangiri B, Khalaj-Kondori M, Asadollahi E, Sadeghizadeh M. Cancer-associated fibroblasts enhance cell proliferation and metastasis of colorectal cancer SW480 cells by provoking long noncoding RNA UCA1. J Cell Commun Signal. 2019;13:53–64. doi: 10.1007/s12079-018-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu JJ, Song W, Zhang SD, Shen XH, Qiu XM, Wu HZ, Gong PH, Lu S, Zhao ZJ, He ML, Fan H. HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci Rep. 2016;6:23521. doi: 10.1038/srep23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lv S, Yuan P, Lu C, Dong J, Li M, Qu F, Zhu Y, Zhang J. QiShenYiQi pill activates autophagy to attenuate reactive myocardial fibrosis via the PI3K/AKT/mTOR pathway. Aging (Albany NY) 2021 Feb 11; doi: 10.18632/aging.202482. (Epub ahead of print). doi: 10.18632/aging.202482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou S, Zhu Y, Li Z, Zhu Y, He Z, Zhang C. Exosome-derived long non-coding RNA ADAMTS9-AS2 suppresses progression of oral submucous fibrosis via AKT signalling pathway. J Cell Mol Med. 2021;25:2262–2273. doi: 10.1111/jcmm.16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goyal B, Yadav SRM, Awasthee N, Gupta S, Kunnumakkara AB, Gupta SC. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188502. doi: 10.1016/j.bbcan.2021.188502. [DOI] [PubMed] [Google Scholar]
- 60.Ghafouri-Fard S, Abak A, Tondro Anamag F, Shoorei H, Majidpoor J, Taheri M. The emerging role of non-coding RNAs in the regulation of PI3K/AKT pathway in the carcinogenesis process. Biomed Pharmacother. 2021;137:111279. doi: 10.1016/j.biopha.2021.111279. [DOI] [PubMed] [Google Scholar]
- 61.Xin H, Liu N, Xu X, Zhang J, Li Y, Ma Y, Li G, Liang J. Knockdown of lncRNA-UCA1 inhibits cell viability and migration of human glioma cells by miR-193a-mediated downregulation of CDK6. J Cell Biochem. 2019;120:15157–15169. doi: 10.1002/jcb.28777. [DOI] [PubMed] [Google Scholar]
- 62.Sun MD, Zheng YQ, Wang LP, Zhao HT, Yang S. Long noncoding RNA UCA1 promotes cell proliferation, migration and invasion of human leukemia cells via sponging miR-126. Eur Rev Med Pharmacol Sci. 2018;22:2233–2245. doi: 10.26355/eurrev_201804_14809. [DOI] [PubMed] [Google Scholar]
- 63.Sun S, Wu Y, Guo W, Yu F, Kong L, Ren Y, Wang Y, Yao X, Jing C, Zhang C, et al. STAT3/HOTAIR signaling axis regulates HNSCC growth in an EZH2-dependent manner. Clin Cancer Res. 2018;24:2665–2677. doi: 10.1158/1078-0432.CCR-16-2248. [DOI] [PubMed] [Google Scholar]
- 64.Yang R, Wang M, Zhang G, Bao Y, Wu Y, Li X, Yang W, Cui H. E2F7-EZH2 axis regulates PTEN/AKT/mTOR signalling and glioblastoma progression. Br J Cancer. 2020;123:1445–1455. doi: 10.1038/s41416-020-01032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han H, Wang S, Meng J, Lyu G, Ding G, Hu Y, Wang L, Wu L, Yang W, Lv Y, et al. Long noncoding RNA PART1 restrains aggressive gastric cancer through the epigenetic silencing of PDGFB via the PLZF-mediated recruitment of EZH2. Oncogene. 2020;39:6513–6528. doi: 10.1038/s41388-020-01442-5. [DOI] [PubMed] [Google Scholar]
- 66.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 67.Chen P, Wan D, Zheng D, Zheng Q, Wu F, Zhi Q. Long non-coding RNA UCA1 promotes the tumorigenesis in pancreatic cancer. Biomed Pharmacother. 2016;83:1220–1226. doi: 10.1016/j.biopha.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 68.Yang C, Li X, Wang Y, Zhao L, Chen W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene. 2012;496:8–16. doi: 10.1016/j.gene.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Gong Y, Jin B, Wu C, Yang J, Wang L, Zhang Z, Mao Z. Long non-coding RNA urothelial carcinoma associated 1 induces cell replication by inhibiting BRG1 in 5637 cells. Oncol Rep. 2014;32:1281–1290. doi: 10.3892/or.2014.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan J, Li X, Wu W, Xue M, Hou H, Zhai W, Chen W. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016;382:64–76. doi: 10.1016/j.canlet.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Li T, Xiao Y, Huang T. HIF1-α-ainduced upregulation of lncRNA UCA1 promotes cell growth in osteosarcoma by inactivating the PTEN/AKT signaling pathway. Oncol Rep. 2018;39:1072–1080. doi: 10.3892/or.2018.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma H, Su R, Feng H, Guo Y, Su G. Long noncoding RNA UCA1 promotes osteosarcoma metastasis through CREB1-mediated epithelial-mesenchymal transition and activating PI3K/AKT/mTOR pathway. J Bone Oncol. 2019;16:100228. doi: 10.1016/j.jbo.2019.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu ZR, Yan L, Liu YT, Cao L, Guo YH, Zhang Y, Yao H, Cai L, Shang HB, Rui WW, et al. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat Commun. 2018;9:4624. doi: 10.1038/s41467-018-06853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu C, Luo J. Long non-coding RNA (lncRNA) urothelial carcinoma-associated 1 (UCA1) enhances tamoxifen resistance in breast cancer cells via inhibiting mTOR signaling pathway. Med Sci Monit. 2016;22:3860–3867. doi: 10.12659/MSM.900689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Popova NV, Jücker M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int J Mol Sci. 2021;22:1743. doi: 10.3390/ijms22041743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Li H, Cui Z, Zhou Z, Chen S, Ma J, Hou L, Pan X, Li Q. Long non-coding RNA UCA1 relieves cardiomyocytes H9c2 injury aroused by oxygen-glucose deprivation via declining miR-122. Artif Cells Nanomed Biotechnol. 2019;47:3492–3499. doi: 10.1080/21691401.2019.1652630. [DOI] [PubMed] [Google Scholar]
- 77.Wang QS, Zhou J, Li X. LncRNA UCA1 protects cardiomyocytes against hypoxia/reoxygenation induced apoptosis through inhibiting miR-143/MDM2/p53 axis. Genomics. 2020;112:574–580. doi: 10.1016/j.ygeno.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Zhang C. Silence of long non-coding RNA UCA1 inhibits hemangioma cells growth, migration and invasion by up-regulation of miR-200c. Life Sci. 2019;226:33–46. doi: 10.1016/j.lfs.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Li X, Wu S, Xue M, Chen W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci. 2014;105:951–955. doi: 10.1111/cas.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Andel H, Kocemba KA, Spaargaren M, Pals ST. Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options. Leukemia. 2019;33:1063–1075. doi: 10.1038/s41375-019-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang B, Fang S, Cheng Y, Zhou C, Deng F. The long non-coding RNA, urothelial carcinoma associated 1, promotes cell growth, invasion, migration, and chemo-resistance in glioma through Wnt/β-catenin signaling pathway. Aging (Albany NY) 2019;11:8239–8253. doi: 10.18632/aging.102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu HW, Liu XD. UCA1 promotes papillary thyroid carcinoma development by stimulating cell proliferation via Wnt pathway. Eur Rev Med Pharmacol Sci. 2018;22:5576–5582. doi: 10.26355/eurrev_201809_15821. [DOI] [PubMed] [Google Scholar]
- 83.Liu H, Wang G, Yang L, Qu J, Yang Z, Zhou X. Knockdown of long non-coding RNA UCA1 increases the tamoxifen sensitivity of breast cancer cells through inhibition of Wnt/β-Catenin Pathway. PLoS One. 2016;11:e0168406. doi: 10.1371/journal.pone.0168406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, Gao J, Yu Y, Zhao Z, Pan Y. Long non-coding RNA UCA1 targets miR-185-5p and regulates cell mobility by affecting epithelial-mesenchymal transition in melanoma via Wnt/β-catenin signaling pathway. Gene. 2018;676:298–305. doi: 10.1016/j.gene.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 85.Zhu G, Liu X, Su Y, Kong F, Hong X, Lin Z. Knockdown of urothelial carcinoma-associated 1 suppressed cell growth and migration through regulating miR-301a and CXCR4 in osteosarcoma MHCC97 Cells. Oncol Res. 2018;27:55–64. doi: 10.3727/096504018X15201143705855. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Chen T, Chen Z, Lian X, Wu W, Chu L, Zhang S, Wang L. MUC 15 promotes osteosarcoma cell proliferation, migration and invasion through livin, MMP-2/MMP-9 and Wnt/β-catenin signal pathway. J Cancer. 2021;12:467–473. doi: 10.7150/jca.49641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gui X, Li H, Li T, Pu H, Lu D. Long Noncoding RNA CUDR regulates HULC and β-catenin to govern human liver stem cell malignant differentiation. Mol Ther. 2015;23:1843–1853. doi: 10.1038/mt.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lombard DB, Cierpicki T, Grembecka J. Combined MAPK Pathway and HDAC Inhibition Breaks Melanoma. Cancer Discov. 2019;9:469–471. doi: 10.1158/2159-8290.CD-19-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kong L, Wu Q, Zhao L, Ye J, Li N, Yang H. Upregulated lncRNA-UCA1 contributes to metastasis of bile duct carcinoma through regulation of miR-122/CLIC1 and activation of the ERK/MAPK signaling pathway. Cell Cycle. 2019;18:1212–1228. doi: 10.1080/15384101.2019.1593647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li D, Hao S, Zhang J. Long non-coding RNA UCA1 exerts growth modulation by miR-15a in human thyroid cancer TPC-1 cells. Artif Cells Nanomed Biotechnol. 2019;47:1815–1822. doi: 10.1080/21691401.2019.1606007. [DOI] [PubMed] [Google Scholar]
- 91.Nixon CC, Mavigner M, Sampey GC, Brooks AD, Spagnuolo RA, Irlbeck DM, Mattingly C, Ho PT, Schoof N, Cammon CG, et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature. 2020;578:160–165. doi: 10.1038/s41586-020-1951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng Q, Lin Z, Li X, Xin X, Wu M, An J, Gui X, Li T, Pu H, Li H, Lu D. Inflammatory cytokine IL6 cooperates with CUDR to aggravate hepatocyte-like stem cells malignant transformation through NF-κB signaling. Sci Rep. 2016;6:36843. doi: 10.1038/srep36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Q, Zhao MW, Yang P. LncRNA UCA1 suppresses the inflammation via modulating miR-203-mediated regulation of MEF2C/NF-κB signaling pathway in epilepsy. Neurochem Res. 2020;45:783–795. doi: 10.1007/s11064-019-02952-9. [DOI] [PubMed] [Google Scholar]
- 94.Qin L, Jia Z, Xie D, Liu Z. Knockdown of long noncoding RNA urothelial carcinoma-associated 1 inhibits cell viability, migration, and invasion by regulating microRNA-182 in gastric carcinoma. J Cell Biochem. 2018;119:10075–10086. doi: 10.1002/jcb.27344. [DOI] [PubMed] [Google Scholar]
- 95.de Bock CE, Demeyer S, Degryse S, Verbeke D, Sweron B, Gielen O, Vandepoel R, Vicente C, Vanden Bempt M, Dagklis A, et al. HOXA9 cooperates with activated JAK/STAT signaling to drive leukemia development. Cancer Discov. 2018;8:616–631. doi: 10.1158/2159-8290.CD-17-0583. [DOI] [PubMed] [Google Scholar]
- 96.Jiang H, Zhu M, Wang H, Liu H. Suppression of lncRNA MALAT1 reduces pro-inflammatory cytokines production by regulating miR-150-5p/ZBTB4 axis through JAK/STAT signal pathway in systemic juvenile idiopathic arthritis. Cytokine. 2021;138:155397. doi: 10.1016/j.cyto.2020.155397. [DOI] [PubMed] [Google Scholar]
- 97.Yang W, Qian Y, Gao K, Zheng W, Wu G, He Q, Chen Q, Song Y, Wang L, Wang Y, et al. LncRNA BRCAT54 inhibits the tumorigenesis of non-small cell lung cancer by binding to RPS9 to transcriptionally regulate JAK-STAT and calcium pathway genes. Carcinogenesis. 2021;42:80–92. doi: 10.1093/carcin/bgaa051. [DOI] [PubMed] [Google Scholar]
- 98.Li JL, Liu XL, Guo SF, Yang Y, Zhu YL, Li JZ. Long noncoding RNA UCA1 regulates proliferation and apoptosis in multiple myeloma by targeting miR-331-3p/IL6R axis for the activation of JAK2/STAT3 pathway. Eur Rev Med Pharmacol Sci. 2019;23:9238–9250. doi: 10.26355/eurrev_201911_19416. [DOI] [PubMed] [Google Scholar]
- 99.Liu J, Luo C, Zhang C, Cai Q, Lin J, Zhu T, Huang X. Upregulated lncRNA UCA1 inhibits trophoblast cell invasion and proliferation by downregulating JAK2. J Cell Physiol. 2020;235:7410–7419. doi: 10.1002/jcp.29643. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, Yao G, Li L, Ma Z, Chen J, Chen W. LncRNA-UCA1 inhibits the astrocyte activation in the temporal lobe epilepsy via regulating the JAK/STAT signaling pathway. J Cell Biochem. 2020;121:4261–4270. doi: 10.1002/jcb.29634. [DOI] [PubMed] [Google Scholar]
- 101.Xu J, Shao T, Song M, Xie Y, Zhou J, Yin J, Ding N, Zou H, Li Y, Zhang J. MIR22HG acts as a tumor suppressor via TGFβ/SMAD signaling and facilitates immunotherapy in colorectal cancer. Mol Cancer. 2020;19:51. doi: 10.1186/s12943-020-01174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang ZS, Wang J, Zhu BQ, Ge L. Long noncoding RNA UCA1 promotes multiple myeloma cell growth by targeting TGF-beta. Eur Rev Med Pharmacol Sci. 2018;22:1374–1379. doi: 10.26355/eurrev_201803_14481. [DOI] [PubMed] [Google Scholar]
- 103.Shu T, He L, Wang X, Pang M, Yang B, Feng F, Wu Z, Liu C, Zhang S, Liu B, et al. Long noncoding RNA UCA1 promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells via miRNA-145-5p/SMAD5 and miRNA-124-3p/SMAD4 axis. Biochem Biophys Res Commun. 2019;514:316–322. doi: 10.1016/j.bbrc.2019.04.140. [DOI] [PubMed] [Google Scholar]
- 104.Li Z, Liu H, Zhong Q, Wu J, Tang Z. LncRNA UCA1 is necessary for TGF-β-induced epithelial-mesenchymal transition and stemness via acting as a ceRNA for Slug in glioma cells. FEBS Open Bio. 2018;8:1855–1865. doi: 10.1002/2211-5463.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang M, Zhao Y, Zhang Y, Wang D, Gu S, Feng W, Peng W, Gong A, Xu M. LncRNA UCA1 promotes migration and invasion in pancreatic cancer cells via the Hippo pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1770–1782. doi: 10.1016/j.bbadis.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Tian Y, Zhang X, Hao Y, Fang Z, He Y. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res. 2014;24:335–341. doi: 10.1097/CMR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 107.Liu Q, Li Y, Lv W, Zhang G, Tian X, Li X, Cheng H, Zhu C. UCA1 promotes cell proliferation and invasion and inhibits apoptosis through regulation of the miR129-SOX4 pathway in renal cell carcinoma. Onco Targets Ther. 2018;11:2475–2487. doi: 10.2147/OTT.S160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duan Q, Xu M, Wu M, Zhang X, Gan M, Jiang H. Long noncoding RNA UCA1 promotes cell growth, migration, and invasion by targeting miR-143-3p in oral squamous cell carcinoma. Cancer Med. 2020;9:3115–3129. doi: 10.1002/cam4.2808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Li ZG, Xiang WC, Shui SF, Han XW, Guo D, Yan L. 11 Long noncoding RNA UCA1 functions as miR-135a sponge to promote the epithelial to mesenchymal transition in glioma. J Cell Biochem. 2020;121:2447–2457. doi: 10.1002/jcb.29467. [DOI] [PubMed] [Google Scholar]
- 110.Sun S, Gong C, Yuan K. LncRNA UCA1 promotes cell proliferation, invasion and migration of laryngeal squamous cell carcinoma cells by activating Wnt/β-catenin signaling pathway. Exp Ther Med. 2019;17:1182–1189. doi: 10.3892/etm.2018.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen X, Wang Z, Tong F, Dong X, Wu G, Zhang R. lncRNA UCA1 Promotes Gefitinib Resistance as a ceRNA to Target FOSL2 by Sponging miR-143 in Non-small Cell Lung Cancer. Mol Ther Nucleic Acids. 2020;19:643–653. doi: 10.1016/j.omtn.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.He C, Lu X, Yang F, Qin L, Guo Z, Sun Y, Wu J. LncRNA UCA1 acts as a sponge of miR-204 to up-regulate CXCR4 expression and promote prostate cancer progression. Biosci Rep. 2019;39:BSR20181465. doi: 10.1042/BSR20181465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han R, Chen S, Wang J, Zhao Y, Li G. LncRNA UCA1 affects epithelial-mesenchymal transition, invasion, migration and apoptosis of nasopharyngeal carcinoma cells. Cell Cycle. 2019;18:3044–3053. doi: 10.1080/15384101.2019.1667707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Yu Y, Gao F, He Q, Li G, Ding G. lncRNA UCA1 functions as a ceRNA to promote prostate cancer progression via sponging miR143. Mol Ther Nucleic Acids. 2020;19:751–758. doi: 10.1016/j.omtn.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gharib E, Anaraki F, Baghdar K, Ghavidel P, Sadeghi H, Nasrabadi PN, Peyravian N, Aghdaei HA, Zali MR, Mojarad EN. Investigating the diagnostic performance of HOTTIP, PVT1, and UCA1 long noncoding RNAs as a predictive panel for the screening of colorectal cancer patients with lymph node metastasis. J Cell Biochem. 2019;120:14780–14790. doi: 10.1002/jcb.28739. [DOI] [PubMed] [Google Scholar]
- 116.Esfandi F, Taheri M, Kholghi Oskooei V, Ghafouri-Fard S. Long noncoding RNAs expression in gastric cancer. J Cell Biochem. 2019;120:13802–13809. doi: 10.1002/jcb.28653. [DOI] [PubMed] [Google Scholar]
- 117.Pan J, Xie X, Li H, Li Z, Ren C, Ming L. Detection of serum long non-coding RNA UCA1 and circular RNAs for the diagnosis of bladder cancer and prediction of recurrence. Int J Clin Exp Pathol. 2019;12:2951–2958. [PMC free article] [PubMed] [Google Scholar]
- 118.Hu ML, Wang XY, Chen WM. TGF-β1 upregulates the expression of lncRNA UCA1 and its downstream HXK2 to promote the growth of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22:4846–4854. doi: 10.26355/eurrev_201808_15620. [DOI] [PubMed] [Google Scholar]
- 119.Zheng ZK, Pang C, Yang Y, Duan Q, Zhang J, Liu WC. Serum long noncoding RNA urothelial carcinoma-associated 1: A novel biomarker for diagnosis and prognosis of hepatocellular carcinoma. J Int Med Res. 2018;46:348–356. doi: 10.1177/0300060517726441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mirzajani S, Ghafouri-Fard S, Habibabadi JM, Arsang-Jang S, Omrani MD, Fesharaki SSH, Sayad A, Taheri M. Expression analysis of lncRNAs in refractory and non-refractory epileptic patients. J Mol Neurosci. 2020;70:689–698. doi: 10.1007/s12031-019-01477-8. [DOI] [PubMed] [Google Scholar]
- 121.Yu X, Wang R, Han C, Wang Z, Jin X. A panel of urinary long non-coding RNAs differentiate bladder cancer from urocystitis. J Cancer. 2020;11:781–787. doi: 10.7150/jca.37006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local cancer recurrence: The realities, challenges, and opportunities for new therapies. CA Cancer J Clin. 2018;68:488–505. doi: 10.3322/caac.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yao F, Wang Q, Wu Q. The prognostic value and mechanisms of lncRNA UCA1 in human cancer. Cancer Manag Res. 2019;11:7685–7696. doi: 10.2147/CMAR.S200436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo N, Sun Q, Fu D, Zhang Y. Long non-coding RNA UCA1 promoted the growth of adrenocortical cancer cells via modulating the miR-298-CDK6 axis. Gene. 2019;703:26–34. doi: 10.1016/j.gene.2019.03.066. [DOI] [PubMed] [Google Scholar]
- 125.Kang K, Huang YH, Li HP, Guo SM. Expression of UCA1 and MALAT1 long-chain non-coding RNAs in esophageal squamous cell carcinoma tissues is predictive of patient prognosis. Arch Med Sci. 2018;14:752–759. doi: 10.5114/aoms.2018.73713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang X, Yu L, Bao J, Jiang P, Yan F. Function of long noncoding RNA UCA1 on gastric cancer cells and its clinicopathological significance in plasma. Clin Lab. 2019 Nov 1; doi: 10.7754/Clin.Lab.2019.181233. (Epub ahead of print). doi: 10.7754/Clin.Lab.2019.181233. [DOI] [PubMed] [Google Scholar]
- 127.Yang L, Zhang L, Lu L, Wang Y. lncRNA UCA1 increases proliferation and multidrug resistance of retinoblastoma cells through downregulating miR-513a-5p. DNA Cell Biol. 2020;39:69–77. doi: 10.1089/dna.2019.5063. [DOI] [PubMed] [Google Scholar]
- 128.Wang J, Ye C, Liu J, Hu Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501:1034–1040. doi: 10.1016/j.bbrc.2018.05.104. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y, Liu Y, Xu X. Knockdown of LncRNA-UCA1 suppresses chemoresistance of pediatric AML by inhibiting glycolysis through the microRNA-125a/hexokinase 2 pathway. J Cell Biochem. 2018;119:6296–6308. doi: 10.1002/jcb.26899. [DOI] [PubMed] [Google Scholar]
- 130.Yang YN, Zhang R, Du JW, Yuan HH, Li YJ, Wei XL, Du XX, Jiang SL, Han Y. Predictive role of UCA1-containing exosomes in cetuximab-resistant colorectal cancer. Cancer Cell Int. 2018;18:164. doi: 10.1186/s12935-018-0660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu HY, Bai WD, Ye XM, Yang AG, Jia LT. Long non-coding RNA UCA1 desensitizes breast cancer cells to trastuzumab by impeding miR-18a repression of Yes-associated protein 1. Biochem Biophys Res Commun. 2018;496:1308–1313. doi: 10.1016/j.bbrc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 132.Xiao Y, Jiao C, Lin Y, Chen M, Zhang J, Wang J, Zhang Z. lncRNA UCA1 contributes to imatinib resistance by acting as a ceRNA against miR-16 in chronic myeloid leukemia cells. DNA Cell Biol. 2017;36:18–25. doi: 10.1089/dna.2016.3533. [DOI] [PubMed] [Google Scholar]
- 133.Wang X, Yang B, Ma B. The UCA1/miR-204/Sirt1 axis modulates docetaxel sensitivity of prostate cancer cells. Cancer Chemother Pharmacol. 2016;78:1025–1031. doi: 10.1007/s00280-016-3158-8. [DOI] [PubMed] [Google Scholar]
- 134.Zhen S, Lu J, Chen W, Zhao L, Li X. Synergistic antitumor effect on bladder cancer by rational combination of programmed cell death 1 blockade and CRISPR-Cas9-mediated long non-coding RNA urothelial carcinoma associated 1 knockout. Hum Gene Ther. 2018;29:1352–1363. doi: 10.1089/hum.2018.048. [DOI] [PubMed] [Google Scholar]
- 135.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liang F, Ren C, Wang J, Wang S, Yang L, Han X, Chen Y, Tong G, Yang G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis. 2019;8:59. doi: 10.1038/s41389-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.