Abstract
Preeclampsia is a progressive hypertensive disorder of pregnancy affecting 2%–8% of pregnancies globally. Preexisting chronic hypertension is a major risk factor associated with developing preeclampsia, and growing evidence suggests a role for the gut microbiome in the development of preeclampsia. However, neither alterations in the gut microbiome associated with preeclampsia nor the mechanisms involved are fully understood. In this study, we tested the hypothesis that normal gestational maternal gut microbiome remodeling is impaired in the Dahl salt-sensitive (Dahl S) rat model of superimposed preeclampsia. Gut microbiome profiles of pregnant Dahl S, normal pregnant Sprague–Dawley (SD), and matched virgin controls were assessed by 16S rRNA gene sequencing at baseline; during early, middle, and late pregnancy; and 1-wk postpartum. Dahl S rats had significantly higher abundance in Proteobacteria, and multiple genera were significantly different from SD rats at baseline. The pregnant SD displayed a significant increase in Proteobacteria and genera such as Helicobacter, but these were not different between pregnant and virgin Dahl S rats. By late pregnancy, Dahl S rats had significantly lower α-diversity and Firmicutes compared with their virgin Dahl S controls. β-diversity was significantly different among groups (P < 0.001). KEGG metabolic pathways including those associated with short-chain fatty acids were different in Dahl S pregnancy but not in SD pregnancy. These results reveal an association between chronic hypertension and gut microbiome dysbiosis which may hinder pregnancy-specific remodeling in the gut microbial composition during superimposed preeclampsia.
Keywords: chronic hypertension, dysbiosis, microbiome, preeclampsia, pregnancy
INTRODUCTION
Preeclampsia is a progressive hypertensive disorder of pregnancy affecting 2%–8% of pregnancies globally (1). The classic diagnostic characteristics of preeclampsia are new-onset hypertension and proteinuria after the 20th week of pregnancy, and it may negatively affect other organs such as the liver and the brain (2). Furthermore, preeclampsia is a leading cause of maternal and fetal deaths, and previous reports suggest that preeclampsia predisposes both the mother and offspring to developing cardiovascular diseases later in life (3, 4). Our understanding of the mechanisms driving the pathophysiology of the disease is incomplete. However, available evidence suggests that preeclampsia is a multisystem syndrome of complex etiologies ranging from uteroplacental ischemia to immune system imbalance (5–7) and dysregulated metabolic processes like dyslipidemia (8, 9). Unfortunately, early delivery of the fetus is currently the only definitive cure. Preexisting chronic hypertension is a leading risk factor for developing superimposed preeclampsia (10, 11).
The human microbiome collectively refers to genomes of microorganisms on and in the human body with the majority residing in the gut (12). The gut microbiome is known to undergo both transient and long-term changes in its bacterial composition in response to the host’s metabolic state changes. Although alteration in the microbiome is believed to be essential in regulating normal body functions like energy homeostasis and immunity, disruption in the microbial population homeostasis, or dysbiosis has been widely associated with disease (13–17).
Emerging evidence has associated disease promoting dysbiosis to cardiometabolic disorders such as hypertension. Recent studies in humans have suggested that pregnancy is associated with alterations in gut microbiome homeostasis (18, 19), and dysbiosis of the gut has been suggested to contribute to adverse pregnancy outcomes such as preterm birth and preeclampsia (20–23). Furthermore, these studies demonstrated that improving the gut microbiome through intrapartum probiotic supplementation ameliorated the incidence of adverse pregnancy outcomes (24–26). There is not enough evidence distinguishing adaptive dysbiosis from pathogenic dysbiosis in pregnancy. More studies are needed to establish the specific gut microbiome changes occurring over the course of a normal pregnancy to better understand dysbiosis in pregnancy disorders.
We proposed that examining the gut microbiome changes in a spontaneous animal model of preeclampsia would provide preclinical evidence for the role of dysbiosis in the pathophysiology of preeclampsia and potentially uncover therapeutic targets for the disease. The research presented here sought to understand and establish a timeline of pregnancy-specific changes in the gut microbiome over the course of a healthy pregnancy and evaluate whether spontaneous superimposed preeclampsia is associated with gut dysbiosis. We have previously demonstrated that the Dahl salt-sensitive (Dahl S) rat spontaneously exhibits a preeclamptic phenotype during pregnancy (27). We hypothesized that preeclampsia superimposed on chronic hypertension and kidney disease would be associated with impaired maternal gut microbiome remodeling. To test this hypothesis, we characterized fecal microbial populations of the female Dahl S rat and the Sprague–Dawley (SD) rat, a well-established rodent model of normal pregnancy. We expected the Dahl S rat to be a good preclinical model to study longitudinal pregnancy gut microbiome changes as it develops the disease phenotype spontaneously with no surgical, dietary, or pharmacologic interventions needed. We compared the fecal microbiome before pregnancy, at early, mid, and late gestation, and 1 wk postpartum.
METHODS
Animals
Dahl salt-sensitive rats (SS/jr) were obtained from the colonies maintained by Dr. Michael Garrett at the University of Mississippi Medical Center. Sprague–Dawley (SD) rats were purchased from Harlan Laboratories (Indianapolis, IN). Both strains were housed in the same room in conventional cages (2 or 3 rats/cage) for 5 mo before starting the study, and studies began when rats were approximately 7 mo old. This study used female rats, and the use of males was exclusively limited to breeding purposes. All rats were maintained on low salt rodent chow (TD7034, 0.3% NaCl, Harlan Teklad, Madison, WI) and water ad libitum on a 12-h light-dark cycle. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were monitored by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Rats were randomly divided into four groups: SD virgin; SD pregnant; Dahl S virgin; and Dahl S pregnant (n = 6 or 10/group). When rats were ∼7mo-old, timed breeding was performed for each strain, and presence of sperm in vaginal smears was marked as gestational day one (GD1). Rats were housed in conventional caging and grouped based on strain and pregnancy status. In the pregnant groups, pups were removed within 24 h of birth to minimize confounding effects due to lactation and offspring feces on the maternal gut microbiome. Each rat was placed in in a clean empty cage and freshly excreted samples (as generated) were immediately transferred to Eppendorf tubes and stored at −80°C. Baseline fecal samples were collected, and samples were collected again on the following days GD6, GD13, and GD20 for pregnant groups and their age-matched virgin controls. Samples were also collected 1 wk postpartum. These are denoted as D28 for simplicity; however, there was variation in the exact day from conception based on natural variation in pregnancy length. Two baseline samples were collected on different days to account for a potential influence of the estrous cycle or any normal day-to-day variation on the microbiome. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
16S rRNA Microbiome DNA Extraction and Sequencing
Fecal samples were provided to the UMMC Molecular and Genomics Core Facility for the isolation of DNA and 16S sequencing. DNA was isolated using MoBio PowerMag Soil Kit (recommended for isolation of DNA from stool samples) on the KingFisher Flex automated DNA/RNA isolation system (96 samples throughput). Subsequently, samples underwent an initial quality control step to determine DNA concentration (Nanodrop One and Qubit Fluorimeter) and integrity (Qiagen QIAxcel advanced system). Samples that passed quality parameters (minimum concentration and quality DNA) were used to amplify the 16S V3 and V4 region bacterial ribosomal RNA using 16S Amplicon PCR Forward Primer = 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 16S Amplicon PCR Reverse Primer = 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC using a limited cycle PCR per Illumina protocol. Illumina sequencing adapters and dual‐index barcodes were added to the amplicon target using Illumina NexteraXT indices (Illumina, San Diego, CA) which allows up to 384 samples to be indexed and run in a single library preparation (Illumina, San Diego, CA). For the current experiment, n = 191 samples were pooled into a single library for sequencing. The library was sequenced using MiSeq Reagent Kitv2 (600 cycles) on Illumina MiSeq platform. The run generated 13.5 Gb at QC30 = 74% with 21 million reads passing filter.
Microbiome Data Processing and Analyses
The sequencing reads were automatically uploaded and evaluated for quality using the Illumina BaseSpace Cloud. The BaseSpace QIIME analysis pipeline (http://qiime.org/) was used for demultiplexing and quality filtering, OTU picking, taxonomic assignment, and phylogenetic reconstruction, and diversity analyses. Sequencing reads were classified against the Greengenes database (http://greengenes.lbl.gov/cgi-bin/nph-index.cgi), which allows up to species-level sensitivity. The *.biom file generated from the QIIME output was analyzed using online MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/) for statistical analysis and data visualization following default parameters (low count filter: minimum count of 4; prevalence in samples by at least 20%; and low variance filter of 10% based on the interquartile range) (28, 29).
α-Diversity and β-Diversity
Differential abundance analysis of α-diversity was assessed by the Shannon diversity measure (t test/ANOVA) among groups. Principal coordinates analysis (PCoA) was applied to assess between-subject diversity (β-diversity) in microbial community composition. PCoA was used to generate orthogonal summary measures of bacterial composition based on a distance matrix of microbial abundance (Bray-Curtis). Linear discriminate analysis effect size (LEfSe) (30) analysis was performed to identify the taxa characterizing the differences among groups, the taxa with a high LDA score (greater than 3 orders of magnitude).
Functional Annotation and Profiling
The 16S rRNA sequencing data was used to predict the metabolic potentials of microbial species based on their phylogenetic distances or sequence similarity to species with sequenced and annotated whole genomes. PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states) (31) was used to predict functional potentials by generating relative Kyoto Encyclopedia of Genes and Genomes (KEGG) orthologs (KOs) abundance levels. The resulting tables containing relative KO abundance levels were further explored through the Shotgun Data Profiling (SDP) module for overall functional profiling. Diversity overview and association analysis for fecal microbiota functional capabilities were examined.
Statistics
α- and β-diversity indices were calculated using MicrobiomeAnalyst by ANOVA and PERMANOVA, respectively. The MicrobiomeAnalyst generated taxa abundances were further analyzed for timed differences using GraphPad Prism software (GraphPad Prism 7.01). Statistical analyses were performed by Student’s t test to compare two groups and ANOVA followed by Dunnett test for multiple groups’ comparisons. Data are presented as means ± SE. A probability value <0.05 was considered for statistical significance.
RESULTS
Dahl S and SD Rats have Inherently Distinct Microbial Profiles
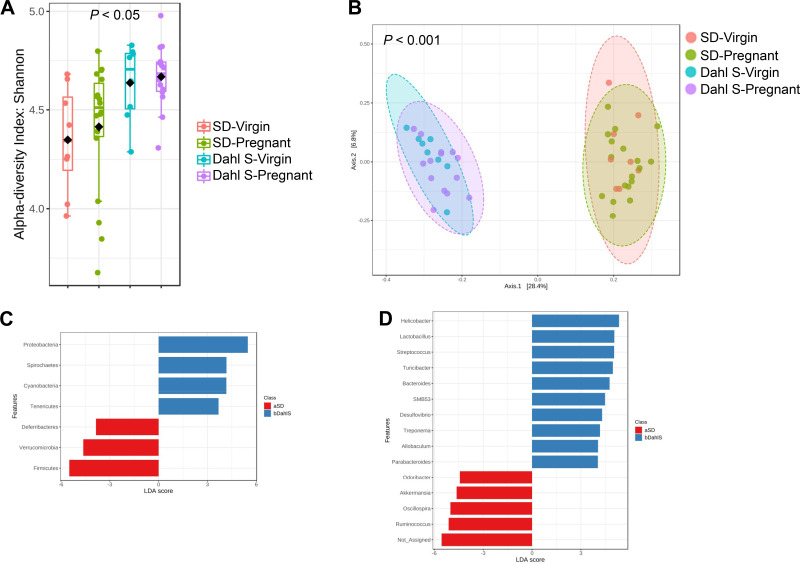
Before characterizing the changes in the gut microbiome during pregnancy, baseline similarities and differences in the gut microbial population between female Dahl S and SD rats were assessed by comparing fecal microbiome composition by 16S rRNA-Amplicon sequencing. Two baseline samples were collected and analyzed to account for potential gut microbiome variations due to animals’ estrous cycles and handling. Dahl S rats demonstrated a higher Shannon index of α-diversity than SD rats (Fig. 1A). Principal coordinates analysis (PCoA) revealed that animals were separately clustered by strain (Fig. 1B). Linear discriminate analysis (LEfSe) at the phylum and genus taxonomic levels was used to identify differentially abundant taxa associated with each strain. To that end, 7 phyla were significantly different between strains. Four phyla (Proteobacteria, Spirochaetes, Cyanobacteria, and Tenericutes) were significantly upregulated in the Dahl S whereas Deferribacteres, Verrucomicrobia, and Firmicutes were significantly downregulated compared with the SD rats (Fig. 1C). Furthermore, numerous other genera were observed to be differentially abundant. Bacterial strains from genera such as Helicobacter, Streptococcus, and Treponema were predominant in Dahl S compared with SD. In contrast, Dahl S rats had significantly downregulated taxa from the genera Akkermansia, Ruminococcus, and Roseburia compared with the SD rats (Fig. 1D).
Figure 1.
Female Dahl S and SD rats have distinct taxonomic composition and phylogenetic diversity at baseline. A: α diversity presented as Shannon index. Each dot represents an individual rat. Pink bars represent virgin SD; green bars represent SD rats that were later mated; blue bars represent virgin Dahl S; and purple bars represent Dahl S rats that were later mated. B: PCoA plot representing β-diversity, each dot represents an individual rat. The distance between the two clusters indicates that the microbial population is significantly distinct between strains. The pink ellipse represents virgin SD; the green ellipse represents SD rats that were later mated; the blue ellipse represents virgin Dahl S; and the purple ellipse represents Dahl S rats that were later mated. C: LEfSe bar plot of differentially abundant phyla. Blue bars represent phyla that are more abundant in Dahl S and red bars represent phyla that are abundant in SD. D: LEfSe bar plot of differentially abundant genera. Blue bars represent genera that are more abundant in Dahl S and red bars represent genera that are more abundant in SD. n = 6 or 10 female rats. Dahl S, Dahl salt-sensitive; LEfSe, linear discriminate analysis effect size; PCoA, principle coordinates analysis; SD, Sprague Dawley.
Markers of Dysbiosis
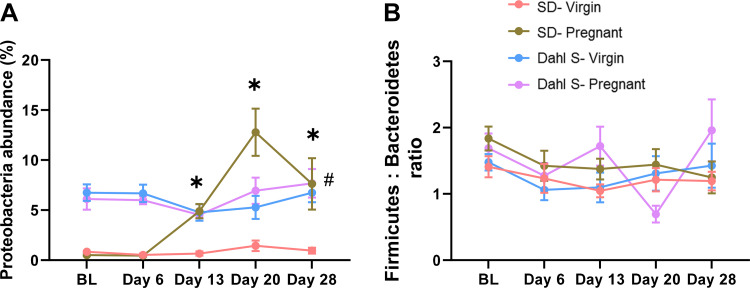
Next, changes in gut microbiome composition during preeclamptic pregnancy were assessed by evaluating changes in the fecal microbial population of the Dahl S rat at early, mid, and late gestation and 1 wk postpartum. In parallel, fecal microbial population of the SD rats at these pregnancy stages was also examined to uncover the gut microbial changes associated with a reference normotensive pregnancy. Commonly used markers of gut dysbiosis were evaluated, namely, the abundance of Proteobacteria phylum and Firmicutes to Bacteroidetes ratio (F:B). Overall, the abundance of the Proteobacteria remained low in the virgin SD group, whereas the pregnant SD group showed gestational stage-dependent elevations in the Proteobacteria population which peaked by late pregnancy. The Dahl S rat exhibited higher Proteobacteria abundance at baseline compared with the SD rat. However, no differences were observed in the phylum between virgin and pregnant Dahl S as they maintained similar levels regardless of their pregnancy status (Fig. 2A). Both SD (virgin & pregnant) and virgin Dahl S rats maintained their baseline F:B ratio at the studied time points. However, pregnant Dahl S rats displayed fluctuations in the F:B ratio displaying the highest ratio mid-pregnancy before subsiding to the lowest ratio by late pregnancy (Fig. 2B). Collectively, these results suggest that normal pregnancy is associated with transient dysbiosis predominantly characterized by upregulation of Proteobacteria, a pattern not displayed in the Dahl S pregnancy.
Figure 2.
Dahl S rats display dysbiosis. A: percent Proteobacteria phylum abundance. B: Firmicutes to Bacteroidetes ratio. The pink line represents virgin SD; the green line represents the pregnant SD; the blue line represents virgin Dahl S; and the purple line represents the pregnant Dahl S. BL: baseline; day 6: gestation day 6 (early); day 13: gestation day 13 (middle); day 20: gestation day 20 (late); and day 28: day 28 (1 wk postpartum). Data are expressed as means ± SE. *P < 0.05 vs. matched SD virgin. #P < 0.05 Dahl S (virgin and pregnant groups) vs. SD virgin. n = 6 or 10 female rats. Dahl S, Dahl salt-sensitive; SD, Sprague Dawley.
Gestational Gut Microbiome Profiling
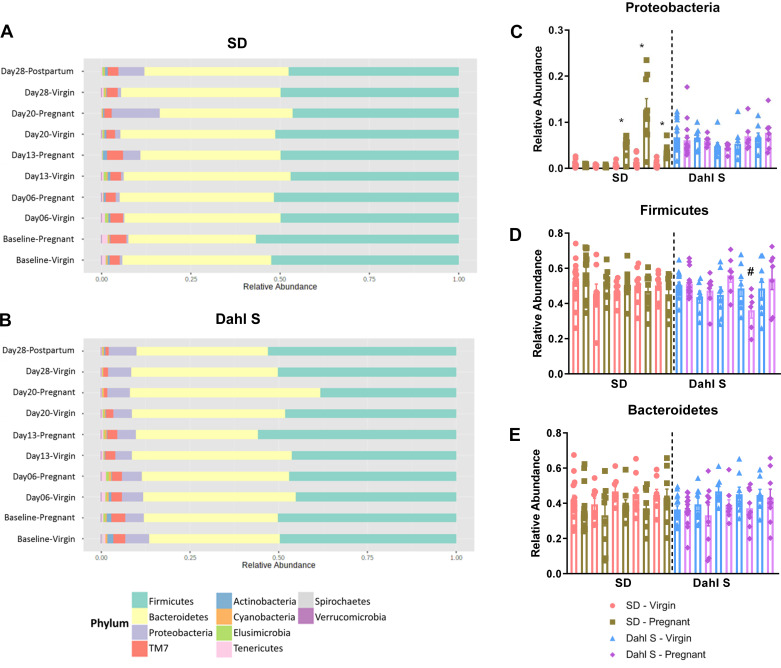
To assess whether preexisting gut dysbiosis impairs gut microbiome remodeling during pregnancy, gut microbiome taxonomic changes during pregnancy were assessed. No changes were observed in the relative abundance of Firmicutes or Bacteroidetes at any gestation stage. SD rats showed a significant increase in the Proteobacteria as previously stated (Fig. 3A). The Dahl S rat exhibited no change in Bacteroidetes relative abundance during pregnancy but had a significant decrease in the Firmicutes around late pregnancy (Fig. 3B) which resolved after birth. By further comparing changes in the top 3 phyla between virgin and pregnant animals, we observed that the SD rats showed a pregnancy specific increase in Proteobacteria whereas the Dahl S had no changes in this phylum during pregnancy (Fig. 3C). The Dahl S rat underwent changes in Firmicutes at late pregnancy but this was not mirrored in the SD rat (Fig. 3D). Both strains had no changes in Bacteroidetes across pregnancy (Fig. 3E).
Figure 3.
Dahl S and SD have different microbial population adaptations to pregnancy. A: stacked bar plot depicting time-course changes in phyla relative abundance of SD before, during and after pregnancy. B: stacked bar plot depicting time-course changes in phyla relative abundance of Dahl S before, during, and after pregnancy. C: bar graph representing relative abundance of the Proteobacteria phylum. D: bar graph representing relative abundance of the Firmicutes phylum. E: bar graph representing relative abundance of the Bacteroidetes phylum. Pink bars represent virgin SD; green bars represent pregnant SD; blue represents virgin Dahl S; and purple bars represent pregnant Dahl S. *P < 0.05 vs. matched SD virgin. #P < 0.05 vs. matched Dahl S virgin, n = 6 or 10 female rats. Dahl S, Dahl salt-sensitive; SD, Sprague Dawley.
Diversity of the Pregnancy Microbial Composition
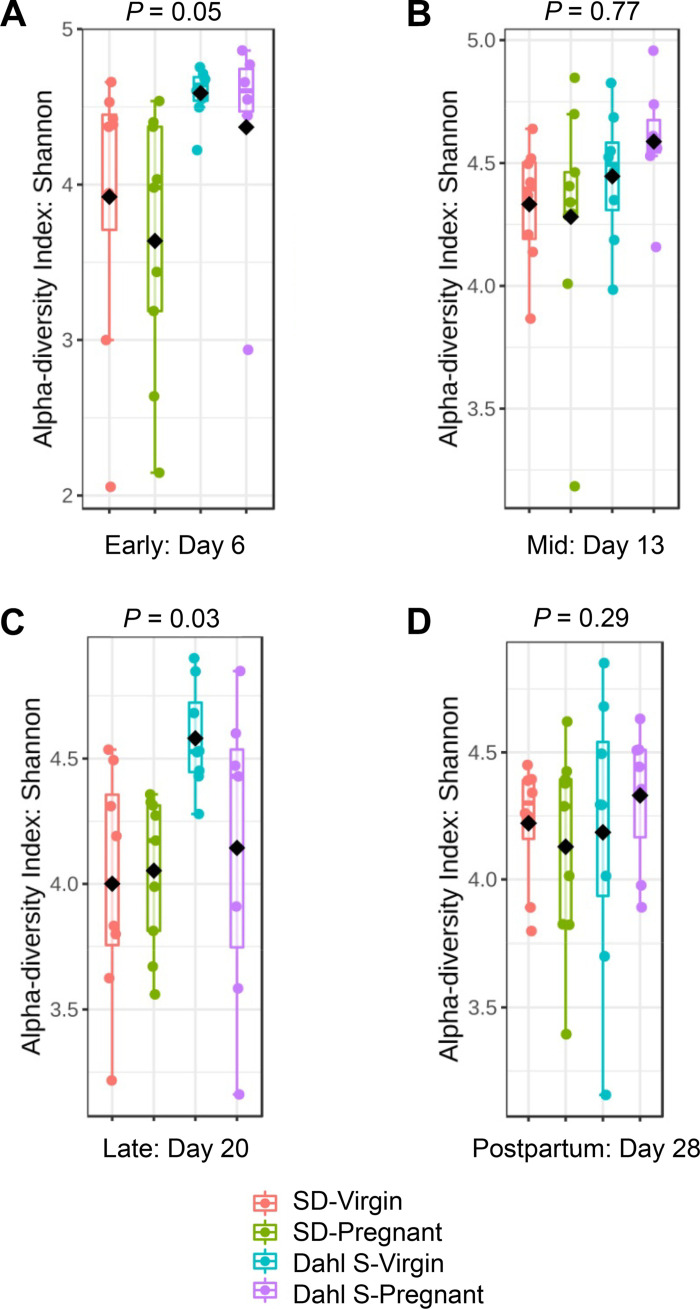
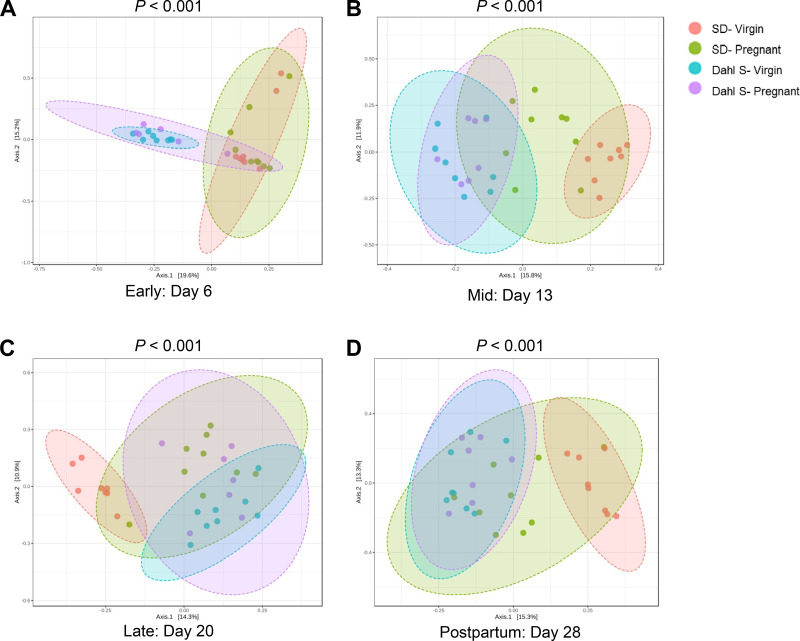
Diversity analysis was done at the OTU feature-level for total diversity representation. Although there were no differences in α-diversity between pregnant and virgin groups during early and mid-pregnancy, pregnant Dahl S group had significantly lower α-diversity than the Dahl S virgins during late pregnancy (Fig. 4). β-Diversity was significantly different between strains with Dahl S and SD demonstrating distinct clustering regardless of time point studied (P < 0.001). However, both strains demonstrated a similar divergence by their pregnancy status (Fig. 5). Interestingly, at the 1 wk postpartum time point, the pregnant SD group clustered separately from their virgin counterparts whereas the Dahl S groups resumed the initial pattern of overlapping clusters.
Figure 4.
The microbial α-diversity (Shannon index) significantly changed between mid-to-late pregnancy in Dahl S rats. A: changes during early pregnancy represented as gestation day 6 data. B: changes around midpregnancy represented as day 13 data. C: changes around late pregnancy represented as day 20 data. D: changes 1 wk postpartum denoted as day 28 data. Each dot represents an individual rat. Pink bars represent virgin SD; green bars represent pregnant SD; blue bars represent virgin Dahl S; and purple bars represent pregnant Dahl S. n = 6 or 10 female rats. Dahl S, Dahl salt-sensitive; SD, Sprague Dawley.
Figure 5.
Changes in the beta-diversity during the time course of pregnancy in Dahl S and SD rats. PCoA plots representing beta-diversity during (A) early pregnancy, day 6; (B) midpregnancy, day 13; (C) late pregnancy, day 20; and (D) 1 wk postpartum, day 28. Each dot represents an individual rat. The pink ellipse represents virgin SD; the green ellipse represents pregnant SD; the blue ellipse represents virgin Dahl S; and the purple ellipse represents pregnant Dahl S. n = 6–10 female rats. Dahl S, Dahl salt-sensitive; PCoA, principle coordinates analysis; SD, Sprague Dawley.
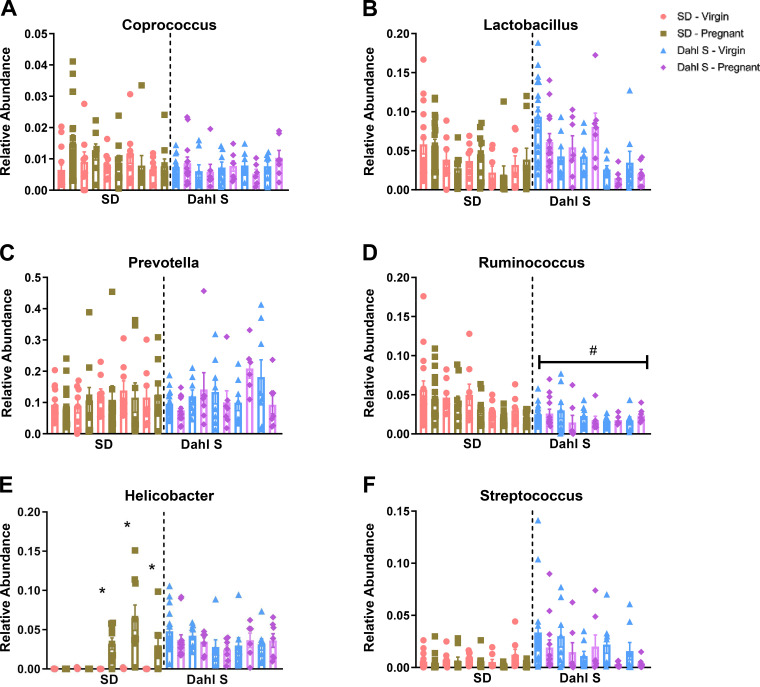
Differentially Changed Genera during Preeclampsia
The use of Proteobacteria abundance and the Firmicutes to Bacteroidetes ratio as markers of disease promoting dysbiosis is an evolving concept in the field. Thus, we assessed bacterial changes at the genus level to further identify temporal alterations in the Dahl S and SD rat pregnancies. Similar levels of Coprococcus, Prevotella, and Lactobacillus at the genus level were observed (Fig. 6, A–C). However, compared with the SD rats, the Dahl S rats had significantly lower relative abundance of the Ruminococcus genus (Fig. 6D). Through the LEfSe algorithm, these genera and additional ones such as Paraprevotella, Clostridium, Dehalobacterium, Deftia, Bilophila, and Mucispirillum were observed to be more abundant in the SD than Dahl S rats. In contrast, SD pregnant groups were associated with a decrease in abundance in some of these genera such as Dehalobacterium, Paraprevotella, Clostridium, and Ruminococcus (Supplemental Figs. S2, S3, S4, and S5, all Supplemental material is available at https://doi.org/10.6084/m9.figshare.12999740).
Figure 6.
Genera enrichment changes across pregnancy stages between strains. Relative abundance in Coprococcus (A), relative abundance in Lactobacillus (B), relative abundance in Prevotella (C), relative abundance in Ruminococcus (D), relative abundance in Helicobacter genus (E), and relative abundance in Streptococcus genus (F). Pink bars represent virgin SD; green bars represent pregnant SD; blue bars represent virgin Dahl S; and purple bars represent pregnant Dahl S. *P < 0.05 vs. gestation stage matched virgin controls. #P < 0.05 vs. baseline virgin SD. n = 6 or 10 female rats. Dahl S, Dahl salt-sensitive; SD, Sprague Dawley.
The SD pregnant group exhibited significant increase in Helicobacter from middle through late pregnancy; the abundance of the genera was observed to decrease 1 wk postpartum. The abundance of Helicobacter was observed to be more abundant in Dahl S rats irrespective of their pregnancy status (Fig. 6E). Dynamic changes in the abundance of Streptococcus within groups that were not specific to pregnancy occurred in both strains (Fig. 6F). LEfSe results confirmed elevated abundance Helicobacter in the pregnant SD rat compared with the matched SD virgins. Bacteria of the Desulfovibrio genus were higher during early to midpregnancy (GD6-13) which was followed by increased abundance of Bacteroides and Treponema (Supplemental Figs. S2 and S3) in the late pregnancy stage of SD pregnant compared with the SD virgin. Although the genera of Helicobacter; Bacteroides; Streptococcus; Desulfovibrio; Treponema; and Parabacteroides are more abundant in Dahl S than SD rats, there were no differences in these genera’s abundance between Dahl S pregnant and virgin groups (Supplemental Figs. S2, S3, and S4). Lastly, some increased abundance in Helicobacter and Treponema genera did not normalize 1 wk postpartum in SD rats whereas they remained equally elevated in virgin and pregnant Dahl S groups.
KEGG Functional Diversity
PICRUSt analysis was used to predict the functional capacity of microbial species in normal and preeclamptic pregnancies to better understand the differences in their metabolic potentials.
Changes in metabolic pathways that occur in pregnancy were assessed by comparing pregnant rats to their counterpart virgins within each strain. Functional diversity profile indicated that several pathways including lipid and amino acid metabolic pathways were downregulated during late pregnancy in the Dahl S compared with virgin Dahl S rats but unchanged in the SD rats. (Supplemental Fig. S6). Association analysis showed that among the significantly changed microbial pathways in pregnant Dahl S compared with virgin Dahl S rats were SCFAs related pathways, butanoate, and propanoate metabolism. These pathways were not different comparing pregnant SD to virgin SD rats (Table 1). The full KEGG analysis is available in Supplemental Tables S1, S2, S3, and S4.
Table 1.
Metabolic pathways associated with SCFAs
| Pathway Name | Butanoate Metabolism | Propanoate Metabolism |
|---|---|---|
| SD | 32 (0.43) | 29 (0.57) |
| Dahl S | 33 (0.01)* | 31 (0.01)* |
Number of gene hits (P value), *P < 0.05 vs. SD.
Dahl S, Dahl salt-sensitive; SD, Sprague Dawley.
DISCUSSION
The overall objective of this study was to characterize and establish the timeline of the gut microbial changes during pregnancy and evaluate whether spontaneous superimposed preeclampsia is associated with gut dysbiosis. The key findings are that 1) healthy pregnancy is characterized by transient dysbiosis occurring predominantly mid to late pregnancy and 2) the female Dahl S rat exhibits gut dysbiosis outside of pregnancy and does not exhibit the pregnancy-specific gut microbiome remodeling observed in normotensive pregnancy. We report that the virgin female Dahl S rat fed a low salt rodent chow (0.3% salt) exhibits gut microbiome dysbiosis characterized by increased α-diversity and differentially abundant phyla and genera. Our results are in line with a previously reported association between hypertension and gut microbiota in this model of hypertension (32) although potential microbiome differences may exist due to environmental factors and sex differences. In this study, diversity was evaluated at the OTUs feature-level instead of specific taxa to represent the total diversity and account for OTUs whose taxa are not assigned.
Studies in other animal models of hypertension (33, 34) and in humans (16, 35–37) have also suggested the involvement of gut microbiome dysregulation in hypertension. Emerging reports have explored the potential role of the microbiome in preeclampsia. Some suggest that placental microbiome dysbiosis may contribute to the pathophysiology of the disease (38) whereas others propose that the human placenta does not have a microbiome (39). Oral and vaginal microbiomes have also been suggested to contribute to adverse pregnancy outcomes (40–42). Alterations in the gut microbiome have been consistently suggested to affect pregnancy outcomes (18, 26, 43). We also show that over the course of a normal pregnancy, the gut microbiome displayed pregnancy-specific bacteria shifts which, like many other pregnancy-related metabolic changes, is believed to be beneficial for maternal adaptation to pregnancy and to allow adequate fetal growth (44). Our findings indicate that (33) the Dahl S model of preeclampsia displays markers of disease promoting gut dysbiosis before pregnancy and (38) the normal changes in the gut microbiome observed in healthy pregnancy do not occur in the Dahl S rats, suggesting that gut dysbiosis before pregnancy could hinder the remodeling necessary for a healthy pregnancy.
Normal pregnancy is suggested to be associated with a decrease in α-diversity, an increase in β-diversity and increased abundance in the Proteobacteria phylum (44). Similarly, our results also show an increase in Proteobacteria in the SD rat pregnancy. Although the Dahl S demonstrated significantly more Proteobacteria at baseline, no difference was noted between the virgin and the pregnant group at any time point, suggesting that their development of the pregnancy-specific dysbiosis may be compromised. We also noted that this transient pregnancy-specific dysbiosis may be accompanied by differential changes in specific genera like Helicobacter. The finding is particularly relevant because studies in rodents have shown that changes in a specific bacteria strain can induce phenotypes with characteristics like those seen in preeclampsia. Campylobacter rectus of the Proteobacteria phylum causes abnormal placental architecture, fetal growth restriction and increased pup mortality in mice (45). Porphyromonas gingivalis increases proinflammatory cytokines and fetal growth restriction in mice (46).
Gut-derived short-chain fatty acids (SCFAs) resulting from the fermentation of dietary fiber by gut bacteria are key mediators between dysbiosis and disease. These include anti-inflammatory SCFAs like butyrate and acetate and proinflammatory metabolites such as lactate. Butyrate and acetate have been shown to lower blood pressure (47–49), whereas a study in rats demonstrated that increased lactate producers is associated with hypertension (50). Human studies have shown a correlation between blood pressure and SCFAs production (51, 52). Propionate attenuated hypertension and systemic inflammation in mice (53). Although we did not directly measure levels of these SCFAs in this study, our findings indicate that bacteria genera known to be butyrate producers such as Odoribacter and Roseburia were higher in virgin SD, an established normotensive strain, compared with their pregnant counterparts or the Dahl S rats. In addition, lactate producers such as Streptococcus and Turibacter were significantly higher in Dahl S rats.
Recent gut microbiome-targeted therapies have widely explored probiotics to modulate the dysbiosis and restore gut homeostasis. Although probiotics were shown to be safe in pregnancy, two clinical trials reported that they did not lower the risk of developing gestational diabetes (54, 55). Our understanding of the composition of a healthy pregnancy microbiome is incomplete. The lack of baseline microbiome data, large variation in composition of bacterial floras, variation in the timing of the initiation of interventions, and differences in probiotics composition pose a challenge in assessing their efficacy in pregnancy disorders like preeclampsia. Ongoing clinical trials assessing the effects of probiotics will add to the current growing interest of their therapeutic potential (56, 57). Robles-Vera et al. (58) showed that probiotics prevented dysbiosis and development of endothelial dysfunction and lowered blood pressure in spontaneously hypertensive rats. These findings are particularly interesting because hypertension and endothelial dysfunction play a big role in the pathophysiology of preeclampsia (4, 59).
Defining the specific pathobionts behind the gut microbiome dysbiosis in preeclampsia is vital to elucidating the mechanisms involved. A major advantage of our study was the ability to evaluate microbiome changes in the same group of animals at key stages of pregnancy without surgical/pharmacological interventions typically utilized for other models. The Dahl S rat model reliably exhibits a preeclampsia-like phenotype including high blood pressure, proteinuria, increased uterine artery resistance, and intrauterine growth restriction during pregnancy (27, 60). Other models such as the reduced uterine perfusion pressure rat that relies on surgical intervention at day 14 of pregnancy or models that require injections or dietary changes during mid/late pregnancy do not allow for the assessment of microbiome changes during early pregnancy or the changes occurring solely due to pregnancy itself. A limitation of this study is that we did not measure blood pressure in this cohort of rats. Because we have shown that the development of the maternal syndrome of preeclampsia is very reproducible in this model, we designed the study in the absence of implanted telemetry devices to avoid the potential confounding effects of the surgical procedure. The use of this spontaneous model allowed us to identify pathobionts that are specific to the disease through a controlled study with prepregnancy baseline microbiome and comparisons with microbiome changes in the normal pregnant SD rat. These findings could potentially lead to the identification of novel noninvasive biomarkers and disease specific therapeutic design.
For the current study, another limitation is the lack of direct assessment of circulating SCFAs or other metabolites. Much like our previous findings using this model, data from this study indicate that in this preclinical rodent model of preeclampsia, most changes occur between mid- and late pregnancy stages. Among these include a significant reduction in the Firmicutes phylum, a decrease in α-diversity along with a significant reduction in the metabolic pathways such as lipid, energy, and amino acid metabolism. We have previously reported differential expression in genes involved in the metabolism of lipids during pregnancy in this model compared with the SD pregnancy (61).The decrease in bacterial diversity in the late pregnancy phase has been reported in patients who developed gestational diabetes (62). Wang et al. (63) also recently demonstrated a significant decrease in Firmicutes in preeclamptic but not normal pregnant women between the second and third trimester. Although we did not directly measure circulating gut-derived metabolites, we have shown that producers of butyrate and lactate are different, and that butanoate and propanoate metabolic pathways are changed in superimposed preeclampsia. Future studies will measure these levels and assess the therapeutic effects of microbiome-targeted therapies on disease outcome.
Currently, little is known regarding the role of the gut microbiota in preeclampsia. This can partly be attributed to the fact that there are multiple microbiota environments suggested to contribute to preeclampsia (oral, gut, placental, and vaginal microbiome (18, 38, 41) but not enough evidence on specific mechanisms in the gut. The microbiota can both influence and be affected by preeclampsia characteristics like blood pressure, inflammation, and kidney injury making their interpretation and biological relevance more challenging (37, 43, 51, 64). The data available from human studies evaluated the association between gut microbiota changes and adverse pregnancy outcomes in the second half of pregnancy (42) and do not provide sufficient details on other factors that influence the microbiota like a diet. Studies investigating gut microbiota changes in various phases of pregnancy preeclampsia are needed to separate adaptive changes from disease-promoting microbiota. Available animal studies on gut microbiota and preeclampsia are limited by their inability to distinguish between preeclampsia specific changes due to inherited maternal changes and the influence of environmental factors such as bacteria administration (40).
Currently, the gut microbiota changes specific to preeclampsia and their timeline is not understood. Using spontaneous models of the disease such as the Dahl S rat in controlled experimental settings will greatly contribute to this gap. To our knowledge, this is the first longitudinal study assessing gut microbiota changes in preeclampsia. We identified specifically changed taxa and metabolic pathways which will guide our future work exploring the association between gut microbiota, signaling metabolites, and their related mechanisms in preeclampsia. We acknowledge that without measurement of characteristics of preeclampsia, our data do not show a direct association with these factors. In order to avoid any confounding effect of surgical interventions or stress on the gut microbiota, we did not implant telemetry devices or use tail cuff plethysmography to measure blood pressure directly in these cohorts of hypertensive Dahl S rats and normotensive SD rats. Likewise, we did not obtain urine or blood samples to assess biomarkers of preeclampsia in these rats. Although this is certainly a limitation of the current study, work by our laboratory and others has shown that these two rat strains reproducibly model hypertensive pregnancy and healthy pregnancy, respectively. The Dahl S rats used in this study were obtained from an inbred colony (27, 60) supporting that our findings are likely due to their inherited gut microbiota signature. Although we controlled for environmental influence in the outbred normotensive SD rats by housing them in the same room as our Dahl S rats for 5 mo, there is still a possibility that their microbiota was not normalized. Accordingly, the future experimental design will utilize SD offspring born at the institution to better limit influences associated with external sources.
Perspectives
Current clinical management and treatment of preeclampsia are insufficient, and the current definitive “cure” for the disease is early delivery of the fetus and placenta. The lack of treatment may be largely attributed to the fact that we do not fully understand the pathophysiology. However, a large body of scientific evidence supports the hypothesis that an imbalance in the immune system that is shifted towards a sustained excessive proinflammatory state. The gut microbiome has been reported to contribute to inflammation by secreting inflammatory metabolites like lactate and trimethylamine-N-oxide. These metabolites can be released into circulation if the gut-barrier is compromised and are able to exert systemic effects. Additionally, receptors such as Toll-like receptors are known to be activated by bacterial products such as lipopolysaccharide and have also been implicated to play a role in the development of preeclampsia. Microbiome-targeted therapies may offer new treatment avenues for preeclampsia.
GRANTS
Research reported in this publication was supported by the National Institutes of Health under Award numbers R01HL134711 (to J. M. Sasser) and R01HL137673 (to M. R. Garrett) and by the American Heart Association under Award number 20PRE35120561 (to J. A. Ishimwe). The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the NIGMS, including Mississippi INBRE (P20GM103476), Obesity, Cardiorenal and Metabolic Diseases- COBRE (P20GM104357), and the Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20GM121334).
DISCLAIMERS
The content is solely the responsibility of us and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.I., A.A., M.R.G., and J.M.S. conceived and designed research; J.A.I., A.A., and A.C.J. performed experiments; J.A.I., A.A., A.C.J., M.R.G., and J.M.S. analyzed data; J.A.I., A.A., A.C.J., M.R.G., and J.M.S. interpreted results of experiments; J.A.I. and M.R.G. prepared figures; J.A.I. drafted manuscript; J.A.I., A.A., A.C.J., M.R.G., and J.M.S. edited and revised manuscript; J.A.I., A.A., A.C.J., M.R.G., and J.M.S. approved final version of manuscript.
REFERENCES
- 1.Wagner SJ, Barac S, Garovic VD. Hypertensive pregnancy disorders: current concepts. J Clin Hypertens (Greenwich) 9: 560–566, 2007. doi: 10.1111/j.1524-6175.2007.06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists; Task Force. Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1222–1231, 2013. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 3.Bokslag A, van Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev 102: 47–50, 2016. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc 7: 763–771, 2018. doi: 10.1161/JAHA.118.009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faas MM, De Vos P. Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 69: 125–133, 2018. doi: 10.1016/j.placenta.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Geldenhuys J, Rossouw TM, Lombaard HA, Ehlers MM, Kock MM. Disruption in the regulation of immune responses in the placental subtype of preeclampsia. Front Immunol 9: 1–15, 2018. doi: 10.3389/fimmu.2018.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girardi G. Complement activation, a threat to pregnancy. Semin Immunopathol 40: 103–111, 2018. doi: 10.1007/s00281-017-0645-x. [DOI] [PubMed] [Google Scholar]
- 8.Delhaes F, Giza SA, Koreman T, Eastabrook G, McKenzie CA, Bedell S, Regnault TRH, de Vrijer B. Altered maternal and placental lipid metabolism and fetal fat development in obesity: current knowledge and advances in non-invasive assessment. Placenta 69: 118–124, 2018. doi: 10.1016/j.placenta.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Jose de Lima VI, Roberta de Andrade CI, Enrico Ruschi G III, Sass N, Professor I, Professor A. Serum lipid levels in pregnancies complicated by preeclampsia. Sao Paulo Med J 129: 73–76, 2011. doi: 10.1590/s1516-31802011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappell LC, Enye S, Seed P, Briley AL, Poston L, Shennan AH. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension 51: 1002–1009, 2008. doi: 10.1161/HYPERTENSIONAHA.107.107565. [DOI] [PubMed] [Google Scholar]
- 11.Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, August P, Commentary SE. Angiogenic factors in superimposed preeclampsia during pregnancy. Hypertension 59: 740–746, 2012. doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C, Giglio MG, McDonald D, Franzosa EA, Knight R, White O, Huttenhower C. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 550: 61–66, 2017. [Erratum in Nature 2017]. doi: 10.1038/nature23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Heal Dis 26: 1–9, 2015. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheema MU, Pluznick JL. Gut microbiota plays a central role to modulate the plasma and fecal metabolomes in response to Angiotensin II. Hypertension 74: 184–193, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zárate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158: 705–721, 2014. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR, Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA, Meyer KA. Gut microbiota composition and blood pressure. Hypertension 73: 998–1006, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, , et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589, 2017. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, SPRING Trial Group. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68: 974–981, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 19.Lv LJ, Li SH, Li SC, Zhong ZC, Duan HL, Tian C, Li H, He W, Chen MC, He TW, Wang YN, Zhou X, Yao L, Yin AH. Early-onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front Cell Infect Microbiol 9: 1–15, 2019. doi: 10.3389/fcimb.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Chen Y, Zhou Q, Wang C, Chen L, Di W, Zhang Y. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin Sci (Lond) 134: 289–302, 2020. doi: 10.1042/CS20191253. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Li P, Liu M, Zheng H, He Y, Chen MX, Tang W, Yue X, Huang Y, Zhuang L, Wang Z, Zhong M, Ke G, Hu H, Feng Y, Chen Y, Yu Y, Zhou H, Huang L. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 69: 513–522, 2020. doi: 10.1136/gutjnl-2019-319101. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Nitert MD. Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci Rep 7: 1–10, 2017. doi: 10.1038/s41598-017-03066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince AL, Chu DM, Seferovic MD, Antony KM, Ma J, Aagaard KM. The perinatal microbiome and pregnancy: Moving beyond the vaginal microbiome. Cold Spring Harb Perspect Med 5: a023051–a023051, 2015. doi: 10.1101/cshperspect.a023051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldassarre ME, Di Mauro A, Capozza M, Rizzo V, Schettini F, Panza R, Laforgia N. Dysbiosis and prematurity: is there a role for probiotics? Nutrients 11: 1273, 2019. doi: 10.3390/nu11061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirihara N, Kamitomo M, Tabira T, Hashimoto T, Taniguchi H, Maeda T. Effect of probiotics on perinatal outcome in patients at high risk of preterm birth. J Obstet Gynaecol Res 44: 241–247, 2018. doi: 10.1111/jog.13497. [DOI] [PubMed] [Google Scholar]
- 26.Nordqvist M, Jacobsson B, Brantsæter AL, Myhre R, Nilsson S, Sengpiel V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open 8: e018021, 2018. doi: 10.1136/bmjopen-2017-018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R62–R70, 2015. doi: 10.1152/ajpregu.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong J, Liu P, Zhou G, Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc 15: 799–821, 2020. doi: 10.1038/s41596-019-0264-1. [DOI] [PubMed] [Google Scholar]
- 29.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45: W180–W188, 2017. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60, 2011. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31: 814–821, 2013. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez-Guzmán M, Toral M, Romero M, Jiménez R, Galindo P, Sánchez M, Zarzuelo José M, Olivares M, Gálvez J, Duarte J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59: 2326–2336, 2015. doi: 10.1002/mnfr.201500290. [DOI] [PubMed] [Google Scholar]
- 35.Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, Shuchun L. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci 16: 872–881, 2019. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 1–19, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 7: 1–9, 2017. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VHW. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J Obstet Gynaecol Res 41: 662–669, 2015. doi: 10.1111/jog.12619. [DOI] [PubMed] [Google Scholar]
- 39.de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, Smith GCS. Human placenta has no microbiome but can contain potential pathogens. Nature 572: 329–334, 2019. doi: 10.1038/s41586-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han YW, Redline RW, Li M, Yin L, Hill GB, Mccormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 72: 2272–2279, 2004. doi: 10.1128/iai.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macintyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown R, Teoh TG, Holmes E, Nicoholson JK, Marchesi JR, Bennett PR. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 5: 8988, 2015. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2: 18–15, 2014. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Yang H, Yin Z, Jiang X, Zhong H, Qiu D, Zhu F, Li R. Remodeling of the gut microbiota and structural shifts in preeclampsia patients in South China. Eur J Clin Microbiol Infect Dis 36: 713–719, 2017. doi: 10.1007/s10096-016-2853-z. [DOI] [PubMed] [Google Scholar]
- 44.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Kling Bäckhed H, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150: 470–480, 2012. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Offenbacher S, Riche EL, Barros SP, Bobetsis YA, Lin D, Beck JD. Effects of maternal Campylobacter rectus infection on murine placenta. J Periodontol 76: 2133–2143, 2005. doi: 10.1902/jop.2005.76.11-S.2133. [DOI] [PubMed] [Google Scholar]
- 46.Lin D, Smith MA, Elter J, Champagne C, Downey CL, Beck J, Offenbacher S, Al L, Mmun INI. Porphyromonas gingivalis Infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun 71: 5163–5168, 2003. doi: 10.1128/iai.71.9.5163-5168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM, Durgan DJ. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72: 1141–1150, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Ko E, Samborowska E, Ufnal M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch 471: 1441–1453, 2019. doi: 10.1007/s00424-019-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin^angiotensin system. J Hypertens 35: 1899–1908, 2017. doi: 10.1097/HJH.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM Jr . Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension 176: 139–148, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu C-N, Lu P-C, Hou C-Y, Tain Y-L. Blood pressure abnormalities associated with gut microbiota-derived short chain fatty acids in children with congenital anomalies of the kidney and urinary tract. J Clin Med 8: 1090, 2019. doi: 10.3390/jcm8081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G, Krzesinski J, Melin P, Tullio PD, Jouret F. Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension 74: 1005–1013, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12588. [DOI] [PubMed] [Google Scholar]
- 53.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt K-U, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139: 1407–1421, 2019. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callaway LK, Mcintyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, Tobin JM, Wilkinson S, Kothari A, Morrison M, Rourke PO, Pelecanos A. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 42: 364–371, 2019. doi: 10.2337/dc18-2248. [DOI] [PubMed] [Google Scholar]
- 55.Pellonperä O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, Rönnemaa T, Laitinen K. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a double-blind clinical trial. Diabetes Care 42: 1009–1017, 2019. doi: 10.2337/dc18-2591/-/DC1. [DOI] [PubMed] [Google Scholar]
- 56.Browne PD, Bolte A, Claassen E, Weerth CD. Probiotics in pregnancy: protocol of a double-blind randomized controlled pilot trial for pregnant women with depression and anxiety (PIP pilot trial). Trials 20: 440, 2019. doi: 10.1186/s13063-019-3389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halkjaer SI, Nilas L, Carlsen EM, Cortes D, Halldórsson TI, Olsen SF, Pedersen AE, Krogfelt KA, Petersen AM. Effects of probiotics (Vivomixx ®) in obese pregnant women and their newborn: study protocol for a randomized controlled trial. Trials 17: 491, 2016. doi: 10.1186/s13063-016-1617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robles-Vera I, Toral M, Visitación ND, Sánchez M, Gómez-Guzmán M, Romero M, Yang T, Izquierdo-Garcia JL, Jiménez R, Ruiz-Cabello J, Guerra-Hernández E, Raizada MK, Pérez-Vizcaíno F, Duarte J. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Mol Nutr Food Res 64: 1900616, 2020. doi: 10.1002/mnfr.201900616. [DOI] [PubMed] [Google Scholar]
- 59.Das UN. Cytokines, angiogenic, and antiangiogenic factors and bioactive lipids in preeclampsia. Nutrition 31: 1083–1095, 2015. doi: 10.1016/j.nut.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 60.Terstappen F, Clarke SM, Joles JA, Ross CA, Garrett MR, Minnion M, Feelisch M, van Goor H, Sasser JM, Lely AT. Sodium thiosulfate in the pregnant dahl salt- sensitive rat, a model of preeclampsia. Biomolecules 10: 302–314, 2020. doi: 10.3390/biom10020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeda KJ, Showmaker KC, Johnson AC, Garrett MR, Sasser XJM. Spontaneous superimposed preeclampsia: chronology and expression unveiled by temporal transcriptomic analysis. Physiol Genomics 51: 342–355, 2019. doi: 10.1152/physiolgenomics.00020.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crusell MKW, Brink LR, Nielsen T, Allin KH, Hansen T, Damm P, Lauenborg J, Hansen TH, Pedersen O. Gestational diabetes and the human salivary microbiota: a longitudinal study during pregnancy and postpartum. BMC Pregnancy Childbirth 20: 69, 2020. doi: 10.1186/s12884-020-2764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Shi Z, Yang J, Wei Y, Wang X, Zhao Y. Gut microbiota dysbiosis in preeclampsia patients in the second and third trimesters. Chin Med J (Engl) 133: 1057–1065, 2020. doi: 10.1097/CM9.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Offenbacher S, Riché EL, Barros SP, Bobetsis YA, Lin D, Beck JD. Effects of maternal campylobacter rectus infection on murine placenta, fetal and neonatal survival, and brain development. J Periodontol 76: 2133–2143, 2005. doi: 10.1902/jop.2005.76.11-S.2133. [DOI] [PubMed] [Google Scholar]