Keywords: hearing, lizard, middle ear, population coding, sound localization
Abstract
The configuration of lizard ears, where sound can reach both surfaces of the eardrums, produces a strongly directional ear, but the subsequent processing of sound direction by the auditory pathway is unknown. We report here on directional responses from the first stage, the auditory nerve. We used laser vibrometry to measure eardrum responses in Tokay geckos and in the same animals recorded 117 auditory nerve single fiber responses to free-field sound from radially distributed speakers. Responses from all fibers showed strongly lateralized activity at all frequencies, with an ovoidal directivity that resembled the eardrum directivity. Geckos are vocal and showed pronounced nerve fiber directionality to components of the call. To estimate the accuracy with which a gecko could discriminate between sound sources, we computed the Fisher information (FI) for each neuron. FI was highest just contralateral to the midline, front and back. Thus, the auditory nerve could provide a population code for sound source direction, and geckos should have a high capacity to differentiate between midline sound sources. In brain, binaural comparisons, for example, by IE (ipsilateral excitatory, contralateral inhibitory) neurons, should sharpen the lateralized responses and extend the dynamic range of directionality.
NEW & NOTEWORTHY In mammals, the two ears are unconnected pressure receivers, and sound direction is computed from binaural interactions in the brain, but in lizards, the eardrums interact acoustically, producing a strongly directional response. We show strongly lateralized responses from gecko auditory nerve fibers to directional sound stimulation and high Fisher information on either side of the midline. Thus, already the auditory nerve provides a population code for sound source direction in the gecko.
INTRODUCTION
Location of sound sources is a fundamental task of the auditory system. In mammals, the two ears are unconnected pressure receivers, and sound direction is computed from monaural cues created by sound diffraction by the pinna and from binaural interactions in brain involving parallel processing of interaural time and level differences (ITD and ILD). ITD and ILD cues are generated by sound arrival time differences at the ear and by sound diffraction around the head (1). Both cues are small in animals with small head sizes (2, 3). In early mammals, it has been theorized that the evolution of an extended high-frequency hearing range was driven by selection pressures to extend the range of hearing to frequencies where sound diffraction cues yield reliable directional cues (generally frequencies where the wavelength of sound is smaller than the head dimensions) (4–7).
In other land vertebrates, physical cues generated at the eardrums are very different. Lizards and frogs, and to some extent birds, typically have acoustically coupled ears, where the two ears can interact acoustically through connected middle ear cavities, leading to strongly directional eardrum vibration, even at relatively low frequencies [reviews in Christensen-Dalsgaard (8), van Hemmen et al. (9)]. In these animals, therefore, the first stage of binaural processing is acoustical and occurs at the eardrum.
The most extreme acoustical coupling is found in the lizards, where the middle ears are open and connected through the pharynx. This connection results in very directional ears. In the anole Anolis sagrei, the house gecko Hemidactylus frenatus, and the Tokay gecko Gekko gecko, interaural level differences of up to 30 dB (normalized for sound diffraction around the head) have been measured at the eardrum using laser vibrometry (10, 11), solely generated by acoustical coupling. Furthermore, the coupling enables lizard ears to generate directional cues at frequencies where the wavelengths are up to 10 times larger than the head width of the lizard. For example, the house gecko has a head width of 1 cm and a strongly directional response (maximal ipsi-contralateral difference of 36 dB) at 3 kHz, where the diffraction cues are insignificant (11).
Acoustical coupling also changes interaural time differences by imposing a delay on the transmission through the internal pathways, leading to interaural time differences of up to three times larger than the arrival-time differences (11). The interaural time differences in the Tokay gecko (head width 25 mm) thus approach 300 µs, where the arrival-time differences would be ∼75 µs (12). Analytical and finite-element models of lizard ears have confirmed that both the amplitude and timing effects are effects of the strong coupling of lizard ears (13–15).
To understand the effect of coupled ears upon sound source localization, we first recorded from auditory nerve fibers in the Tokay gecko using dichotic stimulation. These experiments revealed ITD sensitivity in the auditory nerve similar to the ITD tuning found in the mammalian medial superior olive and avian nucleus laminaris (12). The gecko ITD sensitivity emerges from acoustical interactions between the two ears, rather than being computed in brain (12). Dichotic stimulation also showed dependence upon ILDs. Most notably, the interaural transmission gain [the relative amplitude of sound reaching the internal face of the eardrum (11)] and the interaural transmission delay can be calculated from the auditory nerve data. These dichotic experiments allowed for independent delivery of sound stimuli to each ear but could not reveal the auditory nerve responses in natural conditions.
To understand directional processing, it is necessary to study auditory nerve responses in free field. There are two reasons for this. First, and as shown in frogs, additional, nontympanic pathways could exist for sound to stimulate the inner ear [review in Christensen-Dalsgaard (8)]. These pathways could further modify the directional information from the eardrum and contribute to improved directionality in the auditory nerve fibers. Second, there is a fundamental difference between the performance of coupled and uncoupled ears in free field. With coupled ears, ITD and ILD cues are always combined and binaural processing begins at the eardrum. How this processing changes the time and level cues presented to the central nervous system (CNS) neurons is crucial to the subsequent processing by the CNS.
In the present paper, therefore, we investigated the responses of gecko auditory nerve fibers in a free sound field with unobstructed middle ear transmission. We tested whether the auditory nerve responses accurately reflected the vibrations at the eardrum, or showed improved directionality, and used Fisher information (FI) analyses to describe the directionality of the neural responses. Furthermore, since geckos are highly vocal animals, we investigated responses to gecko calls. We show that the auditory nerve fibers show pronounced directional responses that closely follow eardrum directionality. At directions around to the midline, small changes in location yield large changes in rate (16), and high Fisher information values (17). These could be very useful in steering the head of the gecko in the direction of the sound source. A conference proceedings paper with a brief report on some of these data has been published earlier (18).
METHODS
This study was based on data from 13 adult Tokay geckos (G. gecko) of both sexes. All animal care and anesthesia procedures followed the procedures approved by the Danish Animal Experimentation Board (Dyreforsøgstilsynet), J.No. 2009/561–1645.
Laser Vibrometry
Six geckos, weight 45–71 g, were lightly anesthetized using ketamine (100 mg/kg, Ketalar, Warner‐Lambert/Parke‐Davis, Denmark) and placed in the center of an anechoic room. Stimulation and data recording were controlled by Tucker-Davis (TDT) system 2 hardware and customized software (DragonQuest). Stimuli were frequency sweeps [175 ms, 200–7,500 Hz, 16 sweeps, levels of up to 83 dB sound pressure level (SPL)] emitted in an anechoic room from 12 JBL 1 G loudspeakers placed at 30° intervals around the lizard, each at 1-m distance. In this study, we did not see departures from linearity in the eardrum responses, suggesting that the geckos’ acoustic reflex, comparable with the stapedius reflex (10, 19), was not elicited. The anechoic room was custom-made with 30 cm rockwool wedges and had been tested to be anechoic to below 200 Hz. However, some reflections, especially from the laser setup, are probably unavoidable and may explain the spectral ripple in some of the measurements. The signal sent to the loudspeakers was deconvolved with the individual loudspeaker characteristics (measured with a B&K ½ in. microphone at the center of the setup after positioning the animal) by dividing the spectrum of the sweep by the transfer function of the speaker. Eardrum vibrations were measured with a Polytec vibrometer (OFV-505 sensor and OFV-5000 vibrometer; Polytec, Waldbronn, Germany), and we obtained strong reflections directly (no added reflector) from the tip of the extracolumellar attachment close to the center of the eardrum. Sound and laser recordings were averaged over 16 presentations. Sound at the eardrum was measured by a B&K 4182 probe microphone (Naerum, Denmark) with the tip ∼5 mm from the eardrum surface.
Neurophysiology
Animals and recording.
In vivo recordings from 13 geckos, including the six measured using laser vibrometry, were used to investigate the physiology of the auditory nerve. Anesthesia was induced by intramuscular injection of ketamine (∼100 mg/kg). The animals were kept sedated (immobile, with normal breathing) by supplementary injections of ketamine (50 mg/kg) every 2 h. The skin covering the caudal end of the skull was removed using scalpel and fine rongeurs (without drilling) and the brain stem was exposed. Finally, the dura was retracted, allowing access to the auditory nerve and part of the brain stem on the right side of the animal. The animal was placed on a custom-made thermostatically controlled heating platform, allowing the cloacal temperature to be kept at 27°C. The platform was placed in the center of an anechoic room. Single fiber responses were recorded in the auditory nerve using tungsten electrodes (10–20 MΩ, FHC Inc., Bowdoin, ME). The electrode signal was amplified (WPI Cyto 705), AD-converted (TDT AD2, sample rate 22.05 kHz), and spikes were discriminated using customized software (FrogMaster FF). The detection time of each spike was stored (using TDT spike discriminator SD1 and event timer ET1).
Sound stimulation.
Sound stimuli were emitted from 12 JBL 1 G loudspeakers placed equidistant from the animal at 1 m distance [positive angles: ipsilateral (IL), negative angles: contralateral (CL); the animal was facing the 0° loudspeaker]. Calibration and stimulus generation was controlled by a PC and TDT sys2 hardware, using the software FrogMasterFF. The digital signals were sampled at 22.05 kHz. The stimuli were calibrated and centered digitally by measuring the transfer function of each loudspeaker by a B&K ½ in. microphone in the center of the setup. Subsequently, the signal sent to each loudspeaker was divided by this transfer function. Stimuli consisted of 100 ms tone bursts (gated with a cos2 function, 10 ms rise-fall time), click trains, and gecko call elements. These call elements were taken from one of the authors’ field recording of Tokay calls from Doi Chiang Dao, Chiang Mai province, Thailand (JCD, May 1998). The gecko call consists of three distinct elements (Fig. 1), an initial rattle, followed by elements sounding like “ge” and “ko.” Accordingly, we converted the recording to 22.05 kHz sample rate and selected three 370 ms call chunks that contained each of these elements. The call amplitudes were normalized (the nominal amplitude is the peak equivalent dB SPL of the call) and stored in a call library as described previously (20), so identical call sequences were used for all fibers.
Figure 1.

Gecko call elements. Gecko call elements from Tokay gecko calls recorded by one of the authors (J.C.D.) in Doi Chiang Dao National Park, Thailand. The figure shows the three distinct call elements, the initial rattle, usually repeated twice, and the “ge” and “ko” elements, which are usually repeated 5–6 times. Spectrograms were constructed in MATLAB using 256 point Hamming windows, 98% overlap, and 512 point fast Fourier transform (FFT).
Four different types of measurements were performed on each fiber, time permitting. First, response areas were measured by presenting stimuli in a pseudorandom sequence of frequencies (50 ms tone bursts from 200–4,000 Hz) and stimulus levels. Second, we used an automatic frequency tuning curve procedure that compared stimulus-driven responses to 50 ms tone bursts to spontaneous activity (criterion: 10% increase above spontaneous activity with at least one additional spike in the stimulus time window) and adjusted levels using an adaptive method. Third, rate-intensity functions were measured at increasing stimulus levels of either 100 ms tone bursts at a fixed frequency [usually the characteristic frequency (CF) measured from the tuning curve] or call chunks to generate rate-intensity functions. These three measures were all taken with sound stimulation from the 90° direction (IL). Fourth, directional responses were measured using 100 ms tone bursts at constant level and frequency (usually CF measured from the tuning curve) or call chunks emitted from the 12 speaker directions to measure directional sensitivity. If the fiber could be held for sufficient time, we usually measured directional responses at three sound levels at CF. Negative numbers indicate contralateral (−) and positive ipsilateral (+) dominance.
Data Analysis
Depending on the measurement type, recorded spike times were displayed as rate-level curves, response areas, poststimulus time histograms (PSTHs) or polar plots based on spike rates. The directionality of the responses was analyzed in different ways. First, we constructed polar plots from spike rates. All plots were centered on 90°, so were evaluated from −90° to 240°. We quantified a lateralization index (LI) for each fiber from each measurement of its directional response, based on the difference between ipsilateral (IL) and contralateral (CL) spikes, where LI = (ΣIL spikes − ΣCL spikes)/(total number of spikes). This index results in an index between −1 and 1, where negative numbers indicate contralateral and positive ipsilateral dominance. Also, we converted the spike rates measured at each direction to equivalent dB values (21). Here, we interpolated the rate-level curve measured from the 90° direction and read off the dB levels corresponding to the spike rate measured from the different directions. The resulting equivalent dB value is a measure of the effective sound stimulus reaching the inner ear and allows for comparison with laser measurements of eardrum directionality in polar plots constructed at the stimulus frequency.
For tone burst stimulation, response latency was measured as the median first spike following stimulation onset. To evaluate the dependence of latency on sound level, we computed the level-intensity function of each fiber by a power function using linear regression on a log-log plot of level versus latency. The latency as function of direction could then be estimated using the equivalent dBs as input (see example in Fig. 8A). For click and call chunk stimulation, we also evaluated directional latency differences by cross-correlations of the PSTH envelope with the stimulus envelope. Statistical analyses were done in Sigmaplot 12.5 (Systat Software, Inc., San José).
Figure 8.

Equivalent dBs, comparison with eardrum vibrations. The polar plots show equivalent dB values for three auditory fibers (red curves). Equivalent dBs are calculated from the directional responses and the rate level curve of the fiber at the CF (see methods for details). For comparison, eardrum vibration at the CF measured by laser vibrometry in the same animal is also shown (green curve). Laser measurements are normalized to the equivalent dB at 90°. CF, characteristic frequency.
To model binaural processing of neural directional cues from the recorded auditory nerve responses and investigate the codependence on time and rate cues, we implemented a simplified IE model of processing of the recorded spike trains (Fig. 2). In the model, spike trains from each direction are compared with spike trains from corresponding contralateral directions (reflected across the midline, assuming that the auditory pathway is symmetrical across the midline) in the same fiber. We chose the IE configuration to facilitate direct comparison with auditory nerve directionality, although binaural neurons may also be EI (ipsilateral inhibitory, contralateral excitatory). EI and IE models generally produced very similar results, except that their resulting directionalities are reflections across the midline. Inhibition by contralateral spikes was modeled, so that a leading contralateral spike would suppress firing for 5 ms and an ipsilateral spike would produce a spike in the IE neuron (Fig. 2). The model IE neuron received input from one ipsilateral and one contralateral auditory nerve fiber with identical CFs and directional characteristics and to be excited by ipsilateral and inhibited by contralateral spikes. This means that, for example, for 30° sound direction, the IE neuron would compare inputs from −30° contralateral (I) and 30° ipsilateral (E). Conversely, for a −30° sound direction, inputs from 30° would be inhibitory and inputs from −30° excitatory. The procedure makes it possible to calculate a model IE response based on the recorded spike trains from each auditory fiber.
Figure 2.
IE model. The model IE (ipsilateral excitatory, contralateral inhibitory) response is calculated using the spike trains from each sweep of the directional sequence experiments (10 sweeps, i.e., spike trains/direction). Assuming symmetry of the auditory system each spike train is compared with a spike train from the contralateral symmetrical direction. The figure shows an example of an auditory nerve (AN) spike train (right, red color) compared with a contralateral spike train (blue, left) stimulated by a tone burst (black bar). The resulting IE response (middle) is calculated combining the two spike trains such that each contralateral spike suppresses spikes in the following 5 ms interval. In the example, only one spike in the contralateral IE neuron is suppressed (arrow). The bottom row shows the output of the IE neuron. In this particular example, one input spike is suppressed in the left IE neuron. See methods for additional details. CL, contralateral; IL, ipsilateral.
Auditory nerve recordings were fed to the IE model by comparing each ipsilateral spike train with each contralateral spike train. For the 10 sweeps used in the directional sequence experiments, this yielded 100 comparisons for each direction (for the front and back directions, we compared each spike train with one of the nine other spike trains, yielding only 90 comparisons). From the model spike trains, we generated PSTHs and calculated spike rates.
Fisher Information Analysis
Fisher information (FI) (17, 22) is given by the following equation:
| (1) |
where conditional spike count probabilities P are calculated from the spike count rate r in response to the stimulus from direction q.
The spike count probabilities P(r|q) were estimated using two methods. Method 1 was similar to the one used by Fischer and Konishi (23, 24). The spike count histograms were first calculated from the data for each stimulus direction and then smoothed across stimuli and within stimuli as follows: the distribution was linearly interpolated (spike count resolution: 0.1 spikes, and 10 intermediate steps between stimuli, typical resolution: 3°) and convolved with a 2-D-Gaussian kernel (SDs: 0.5° and 0.5 spikes). After convolution, the values of the resulting histograms for the original stimulus directions were used. Within each stimulus direction, the histogram was normalized to become a probability, and we calculated the FI for each neuron from this Gaussian-smoothed probability matrix (see Eq. 1). FI analyses were grouped by frequency, with low-frequency responses < 1,000 Hz, midfrequency 1,000 Hz–1,800 Hz, and high frequency above 1,800 Hz.
In method 2, because the sample size was small (10 spike counts per neuron and direction), we also created a set of FI model neurons [similar to Gordon et al. (17)]—one for each recorded neuron—to calculate FI. For each FI model neuron, the FI was calculated directly from the Gaussian-distributed response probability P(r|q) of the spike count rate r for a given stimulus q (see Eq. 1). Each FI model neuron’s response for each stimulus had the same mean, variance, and maximum rate as the corresponding recorded neuron’s response. The numerical differences were resampled to the size of the original matrix by linear two-point interpolation. Model neurons were divided into either low, medium, or high best frequency groups (as aforementioned, also see results).
RESULTS
Laser Measurements of Eardrum Vibration Showed Strongly Directional Responses
In geckos, laser measurements revealed directional eardrum vibrations, with up to 30 dB amplitude differences between ipsi- and contralateral directions and up to 2 radians directional phase changes. An example for one individual is shown in Fig. 3A. A part of the directionality is caused by sound diffraction across the head and body of the animal. If the diffraction effect is removed by measuring the transfer function amplitude (i.e., the eardrum vibration amplitude spectrum divided by the sound spectrum measured at the eardrum), a robust directionality is seen at frequencies below 3–4 kHz (Fig. 3B) caused by interaural coupling. Thus, the combined effects of interaural coupling and diffraction produce robust ipsi-contralateral differences up to 7 kHz. However, there was considerable variation in the directional responses of the eardrum between individuals (Fig. 3C). The contra-ipsilateral difference is generally larger than 10 dB at frequencies below 2 kHz in all cases, but the frequency location and size of the maximal difference varied between individuals. Thus, the following comparisons between biophysical and neural directionality were based on data collected in the same individual.
Figure 3.
Free-field responses of the eardrum in the Tokay gecko measured by laser vibrometry. A: eardrum vibration transfer function in one individual, showing transfer function, i.e., eardrum vibrations divided by sound measured at the eardrum [color, dB (re 1 mm/s/Pa)] as a function of direction (x-axis, positive angles ipsilateral) and frequency (y-axis, Hz). B: eardrum vibration amplitudes (color scale, dB re 1 mm/s) measured in one individual as function of direction (x-axis, degrees) and frequency (y-axis, Hz) (i.e, including the contribution of diffraction that dominates directionality above about 3,000 Hz). Positive angles are ipsilateral, negative contralateral. C: directional difference (−60°/60°) for the six geckos, showing large individual differences. The curves (one for each individual) show the dB-difference between transfer function with stimulation from −60° to 60° sound directions. CL, contralateral; IL, ipsilateral.
Free-Field Sensitivity to Frequency and Level
We recorded free-field responses from 117 auditory fibers in the Tokay gecko. The fibers have V-shaped tuning curves with a well-defined characteristic frequency (CF). Above threshold, there was a marked response especially at frequencies below the CF, as shown by the response areas (Fig. 4, C–F). Auditory nerve fibers had characteristic frequencies ranging from 200 Hz to 3,200 Hz, with thresholds at CF from 14 dB SPL to 58 dB SPL (median 36 dB SPL) (Fig. 4A). The distribution of CFs was bimodal (Fig. 4B), and high sensitivity was found at both low (∼400 Hz) and high frequencies (∼2,000 Hz, Fig. 4A).
Figure 4.

Free-field frequency tuning and sensitivity of auditory nerve responses. A: scatterplot showing characteristic frequencies (CFs) and threshold at CF for the 117 fibers. The black line connects the most sensitive fibers at each frequency. B: distribution of CFs. C–F: response areas of four fibers showing spike rates (color scale, average of three presentations) in response to 50 ms tone bursts emitted from the 90° direction at different levels and frequencies (200–4,000 Hz in 1/6th octave steps). G: rate-level functions of seven auditory nerve fibers with different CFs, stimulated from the 90° direction. The smooth curves are three-parameter sigmoidal approximations (SigmaPlot 12.5); actual data points plotted for three of the fibers (symbols). The sigmoidal functions were used to convert measured spike rates to equivalent dBs. H: distribution of maximal slopes of all rate-level (R-L) curves, in all cases stimulated from the 90° direction. I: latency-level functions in four fibers stimulated from the 90° direction. The figure shows the measured data points (symbols) as well as power function fits calculated from the measurements (see methods for details).
All rate-level curves showed monotonic increases of spike rate with stimulus level (Fig. 4G). Most curves saturated at high stimulus levels. The maximal spike rates measured were 350 spikes/s, but the saturation spike rate varied, and some fibers saturated at rates below 100 spikes/s. The maximal slope of the rate level functions also varied, with a median maximal slope of 7.5 spikes/(s × dB; based on 181 rate-level curves) (Fig. 3H). All auditory nerve fibers showed a systematic decrease of first-spike latency with tone burst level that were approximated by a power function(latency = a·dBSPLb), where the parameters a and b were estimated from the slope and intercept, respectively, in a linear regression of a log-log plot of latency versus sound level for each fiber (Fig. 4I). The minimal latency measured with tone burst stimulation was 5.1 ms (Fig. 4I).
Directional Responses in Free Field
All auditory nerve fiber responses were directional, with ovoidal directional sensitivity and higher spike rates from ipsilateral stimulus directions (Fig. 5, A–C). Polar plots show the speaker orientation at 30° intervals around the gecko and include the associated PSTHs to directional tone burst stimuli from each speaker. The strongly directional auditory nerve responses (red line, Fig. 5, A–C) typified free-field responses in geckos and were quantified using Fisher information and described using a lateralization index (see A Lateralization Index for Direction below).
Figure 5.

Directional responses of auditory nerve fibers. A–C: directional responses of three auditory nerve fibers recorded from the right auditory nerve in the same individual in response to 100 ms tone bursts at CF, from speakers arrayed around the gecko. Polar plots show responses to speaker orientations at 30° intervals. For each fiber, red lines show the evoked rate (Hz) in response to tone bursts approximately 10–15 dB above threshold to each speaker. The associated PSTHs show responses patterns and spike rate for each speaker location. D: combination line and histogram plot of the distribution of spatial receptive field sizes, defined as the region within which a given stimulus elicited a response greater than 75% of the fiber’s maximum response, for all fibers. All fibers were recorded from the right auditory nerve (red, rhs). These data were reflected to simulate left side responses (blue). E and F: spike rates and mean (black line) for all low, medium, and high best frequency auditory nerve fibers for each of 12 speaker locations, where 0° corresponds to the front of the gecko, and 90° to its right (ipsilateral). Plots are centered on 90°. Note the increases in firing rate with respect to ipsilateral speaker locations. CF, characteristic frequency; rhs, right-hand side.
From the 117 fibers recorded in this study, we obtained 68 recordings with a full directional data set. Within this full directional data set, we were sometimes able to hold the fiber for a sufficient time to make multiple recordings of directional sensitivity over a range of sound pressure levels. We used these repeated measures for construction of a set of model neurons for Fisher information (method 2, see Model neuron measures of Fisher information below). Because the central projections of the different tonotopic locations of gecko ear end in distinct areas of the brain (25) and because of the different peripheral structures for low- and high-frequency sound transduction (26, 27), we divided the fibers according to their best frequencies. Fibers with best frequencies below <1,000 Hz were placed in the low-frequency group (n = 33), fibers with best frequencies 1,000–1,800 Hz in the midfrequency group (n = 18), and fibers with best frequencies above 1,800 Hz in the high-frequency group (n = 17). Despite tonotopic variation, we found no frequency-dependent differences in responses (Table 1).
Table 1.
Peak FI and best ITD values
| Frequency Range | n | FI Peak, Back Hemifield | n | Model FI Peak, Back Hemifield | n | FI Peak, Front Hemifield | n | Model FI Peak, Front Hemifield | n | Best ITD |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2–1 kHz | 27 | 200.4 ± 41.7 | 70 | 206.0 ± 43 | 29 | −30.9 ± 54.8 | 70 | −31.9 ± 48.8 | 29 | 90 ± 29.4 |
| 1–1.8 kHz | 18 | 196.7 ± 56.6 | 43 | 185.7 ± 50.9 | 19 | −23.3 ± 69.4 | 43 | −14.6 ± 56.8 | 19 | 88.3 ± 31.7 |
| 1.8–3 kHz | 17 | 192.3 ± 46.3 | 39 | 207.7 ± 56.4 | 20 | −33.7 ± 57.8 | 39 | −27.2 ± 44.8 | 20 | 84.7 ± 30.7 |
| 0.2–3 kHz | 62 | 197 ± 40 | 68 | −27.8 ± 56 | 71 | 88.6 ± 29.9 |
FI, Fisher information; ITD, interaural time difference.
Individual auditory nerve responses to tone burst stimulation varied (Fig. 5, E–G), but all fibers showed highest probability responses to sound sources in the ipsilateral hemifield or to speaker locations at 60°–120° for most right-hand side (ipsilateral) auditory nerve fibers (Fig. 5D). Although the population response was strongest to stimuli around the 90° speaker, individual auditory nerve fibers could contribute information about other stimulus locations because the distribution of auditory nerve receptive fields was broad. To capture this variability, we adapted a method from Middlebrooks and Knudsen’s (28) work on spatial tuning in the cat inferior colliculus. This defined a fiber’s receptive field as the region within which a given stimulus elicited a response “greater than 75% of the fiber’s maximum response” to that stimulus (Fig. 5D). The 75% criterion was chosen because it fell near the steepest part of the spatial response profile of most fibers. Thus, the histogram bars in Fig. 5D show all locations with responses above 75%, for example, more than 90% of fibers responded above 75% for 60°, 90°, and 120°. Note Fig. 5A shows a fiber with firing rates above 75% for 90°–150°, whereas the fiber in Fig. 5B responded most strongly from 60°–150° and the fiber in Fig. 5C responded above 75% at speaker locations of 30° and from 90°–150°. Spatial receptive fields also varied in width from 30° to omnidirectional responses (Fig. 5, E–G); the width of the receptive field was weakly correlated (R2 = 0.34) with spontaneous rate, in that the highest rate neurons tended to have the widest receptive fields.
Response profiles for individual auditory nerve recordings could be asymmetric, with greater changes in spike rate in either front or back speaker locations (Fig. 5, E–G). To quantify directional responses over the full 360°, we separately analyzed responses in the front and back hemifields (see Fisher Information Was Highest around the Midline below). The front hemifield was defined as a sector extending from −90°, contralateral to the recorded auditory nerve, through 0° to 90°, directly in front of the recorded auditory nerve (Fig. 5, E–G, dashed line). The back hemifield extended from 120° to 240°. Figure 5, E–G shows that receptive fields were not totally symmetric ∼90°; often the back hemifield exhibited diminished responses around 150° speaker location (Fig. 5F).
Fisher Information Was Highest around the Midline
Fisher information is a measure of the amount of information provided by neural spike counts about a particular stimulus value, such as a single stimulus location, and thus quantifies the accuracy with which a stimulus is encoded. To compute FI for the gecko auditory nerve responses [method 1 (23, 24)], we measured the firing rate distributions for each fiber, consisting of 10 recordings for each of 12 directions, for the low, medium, or high best frequency groups described above (Free Field Sensitivity to Frequency and Level). Fisher information was typically maximal at the steepest parts of the tuning curve, which was usually highest contralateral to the midline (Fig. 6). Means for the entire population showed FI peaks in the front hemifield for right auditory nerve fibers around −30° (mean = −27.8° ± 56.7°, n = 68) and behind the animal, between 180°–210° (Fig. 6, D–F, mean 197° ± 40°, n = 62). All groups showed similar increases in FI with changes in firing rate, with no differences between groups (Fig. 6, Table 1).
Figure 6.

Fisher information (FI). A–C: spike rates (blue line, standard deviation gray dashed line) and Fisher information (green line) for three exemplar auditory nerve fibers of low (0.3 kHz), medium (1.4 kHz), and high (3 kHz) best frequency. Rates are shown for each of 12 speaker locations, where 0 corresponds to the front of the gecko and 90° to its right (ipsilateral). Plots are centered on 90°. D–F: combined line and column plots of mean spike rate (black line) for low, medium, and high best frequency groups. Columns show the speaker location associated with the highest FI for each fiber. The front hemifield fibers are shown as green bars and back hemifield as blue bars. Arrows point to FI means (see Table 1). G–I: population model neurons for low, medium, and high best frequency auditory nerve fibers. Model spike rates (blue lines) and FI (dashed green line).
Best ITD and results for the two methods used to compute FI. The Gaussian-smoothed probability matrix and model neuron approach yielded similar measures of peak FI. There were no statistically significant differences between group means as determined by one-way ANOVA for frontal FI peaks [F(2,67) = 3.1337, P = 0.31] or between back FI group means [F(2,60) = 3.15, P = 0.75]. Numbers of fibers (n) for front and back were not equal because some fibers had a single FI peak, or two peaks in front or back space. The bottom row shows the data from all single FI computations, not from the model neuron. Note the front hemifield extends from −90° to +90° and the back hemifield from 120° to 240°. Auditory nerve fibers throughout the tonotopic range provided information about sound source location. A one-way between-subjects ANOVA was conducted to compare the effect of best frequency on FI values in low, medium, and high best frequency responses in the frontal hemifield. There was a small effect of best frequency on FI for the three conditions [F(2,63) = 3.21, P = 0.047] and no statistically significant differences between group means as determined by one-way ANOVA [F(2,67) = 2.54, P = 0.086] for the back hemifield. Both response peaks and FI measures for individual auditory nerve recordings could be asymmetric, however, with higher FI levels from either front or back speaker locations (Fig. 6, A–C). Therefore, and although the population FI means were contralateral to the midline (Fig. 6, Table 1), individual auditory nerve fibers could contribute information about other stimulus locations, because the distribution of auditory nerve receptive fields was broad (Fig. 5D) and fibers from the population could show high FI values for all azimuths (Fig. 6, D–F).
Model neuron measures of Fisher information.
We also used spike rate data to create a population of model neurons for each frequency group to estimate FI at the auditory nerve (Fig. 6, G–I, Table 1). To estimate the average population FI, we used all recordings from the full directional data set, which included multiple recordings of directional sensitivity over a range of sound pressure levels. This data set included a total of 70 low-frequency, 43 midfrequency, and 39 high-frequency responses [method 2 (17)]. To create each FI model neuron, we computed the mean firing rate from each auditory nerve fiber and calculated the average number of spikes for each direction to provide a tuning curve for each neuron. Average spike data were calculated for all neurons and responses were normalized. FI model neurons were divided into either low, medium, or high best frequency groups, as before. For each of the three populations, the FIs of a given stimulus direction were then summed across the neurons to create a measure of FI for each population (Fig. 6, G–I). FI peaks for the model neurons were similarly distributed to the FI peaks computed with the method 1 (Table 1).
A Lateralization Index for Direction
We calculated the lateralization index (LI) of the directional response (see methods for details) to provide a measure of potential binaural performance via, for example, a Braitenberg vehicle (29, 30). LI is a measure of the relative contributions of spikes on each side, as a summed measure of the difference between the response to ipsi- and contralateral directions (Fig. 7A). The highest LI was 0.82 (82% more spikes evoked by ipsilateral than contralateral side stimulation); the median maximal LI was 0.34 (34% of spikes lateralized). LI depended on sound level, as shown by the examples in the LI trajectory plots (Fig. 7B). For most fibers, LI was maximal ∼10–20 dB above threshold and declined at higher levels. We computed LI for an exemplar fiber with 15 dB average directional differences IL-CL, 10 dB average directional difference IL-midline, a threshold at 30 dB SPL, 30 dB dynamic range, no spontaneous activity, and a linear rate level slope of 7.5 spikes/s × dB from threshold (Fig 7B, green curve). LI was affected by the spontaneous rate; generally, fibers with high spontaneous rates had smaller LI values, especially at low sound levels (Fig. 7A).
Figure 7.
Lateralization index (LI) for auditory nerve fibers. A: scatterplot of the LI (see methods for details) as a function of frequency. There is one dot for each nerve fiber at its maximal LI. The two different colors are low-spontaneous (<10 spikes/s open symbols) and high-spontaneous fibers (>10 spikes/s, filled symbols). B: trajectories of LI dependence on level in nine fibers (values in same fiber connected by lines). Note general decline with level. The green curve shows a predicted response of a fiber with 15 dB average directional differences IL-CL, 10 dB average directional difference IL-midline, a threshold at 30 dB SPL, 30 dB dynamic range, no spontaneous activity, and a linear RL slope of 7.5 spikes/s × dB from threshold.
Additional Contributions to Ear Directionality
In frogs, marked extratympanic sensitivity contributes to low-frequency directional responses recorded from the auditory nerve (31–33). To determine if geckos experienced any additional contributions to the directionality, apart from that generated by the acoustical coupling, we calculated equivalent dB values from the directional spike rate and rate-level curves (see methods for details). The equivalent dB value represents the input to the auditory nerve fibers and therefore can be compared with the eardrum directivity measured by laser vibrometry (Fig. 8). Equivalent dB values are shown in red and laser vibrometry values in green, with the two plots normalized so that they coincide on the 90° data point. The shape of the equivalent dB plots was generally very similar to the eardrum directivity, with only small deviations between eardrum directivity and equivalent dB plots; on average 1–4 dB per point (absolute value). The difference between the laser vibrometry values and equivalent dB values was not significant (one-sample t test, two-tailed, P = 0.11, df 14), supporting the hypothesis that geckos do not receive prominent extratympanic cues in the frequency range studied here.
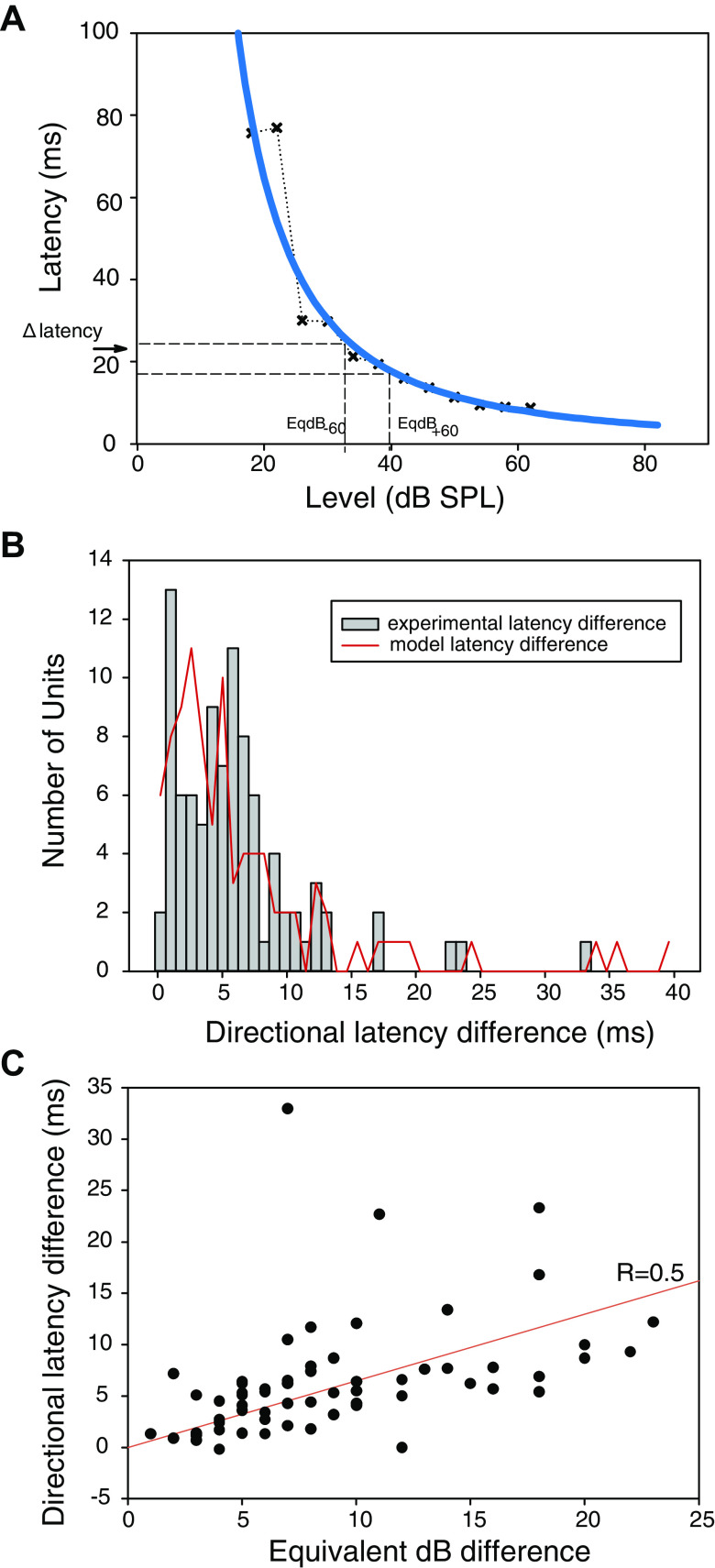
Auditory nerve fibers showed large changes in response latency with direction and these latency differences could provide additional cues for sound localization. The distribution of differences in first-spike latency between 60° and −60° stimulation is shown in Fig. 9B (bars) and yielded a median 5.4 ms difference (n = 93, first quartile 2.7). Figure 8B also shows the latency shift calculated using the latency power function with the equivalent dB values as input (Fig. 9B, red curve, example of calculation Fig. 9A). For this model response the median was 5.1 ms. The latency difference is correlated with equivalent dB directional difference (Fig. 9C). Thus, differences in response latency are largely generated by intensity-latency trading in the fibers, further augmented by the 10 ms rise-fall time of the tone burst stimuli. Cross-correlation of responses to brief impulses showed a directional latency difference of 320 µs, consistent with delay across the head (12).
Figure 9.
Directional latency shift. A: calculated directional latency shift (Δ latency) for an exemplar fiber, based on the equivalent dBs for stimulation from 60° and −60° directions at characteristic frequency (CF). The crosses show first spike latencies measured in one fiber as well as power function calculated from the measurements (see methods for details). B: the distribution of directional latency difference, measured experimentally as the median first spike latency difference with stimulation at CF (bars) and computed (red curve) using the equivalent dB values as shown in the example in Fig. 7A. C: directional latency difference measured as the median first spike latency difference with 60° and −60° stimulation at CF as a function of equivalent dB level. The red line is the regression, R = 0.5.
All Fibers Respond to the Three Components of the Gecko Call
Fibers respond to the call irrespective of CF (Fig. 10A). In all fibers, threshold responses to the three call components, Ge, ko, and rattle, were similar, with median thresholds for call responses of 44 dB SPL calculated for 16 fibers. Examples of directional responses to the three call components in three fibers are shown in Fig. 10B. Call responses were strongly lateralized [compare right (ipsilateral, red) and left PSTHs (contralateral, blue) responses] with a clear representation of the call envelope in the PSTH (Fig. 10C). To facilitate comparison, all responses were taken from the same animal at the same sound level (54 dB SPL). Lateralized responses to calls were found throughout the entire hearing range.
Figure 10.

Directional responses to the three call components. A: rate level responses to the three call components in 14 fibers spanning a range of different characteristic frequencies (CFs). B: polar plots of spike rates (red curve) and poststimulus time histograms (PSTHs) of directional call responses in a nerve fiber (CF 1,800 Hz, level 54 dB SPL). C: PSTH responses to the three call components, Ge, ko, and rattle in six different fibers with different CFs. To facilitate comparisons, these responses were taken from the same animal at the same sound level (54 dB SPL). The PSTHs show strongly lateralized responses [compare right (ipsilateral, red) and left PSTHs (contralateral, blue) responses] with a clear representation of the call envelope in the PSTH.
Generally, lateralization as measured by the lateralization index was slightly smaller than for tone burst stimuli (median ∼0.25 for all three call components, maximal LI ∼0.5). We measured the latency of the call responses by cross-correlating response PSTHs with the call envelope. This was possible at high-stimulus levels, where there were robust responses from all directions. We compared the cross-correlation peak at 60° and −60° stimulus directions and found a latency difference of 2.4 ms with the “Ge” component and 1.6 ms with the rattle component (n = 32). The latency difference of the rattle component is, however, not significantly less than the latency difference of the “Ge” component (two-sided t test, n = 32, P = 0.052, df 25).
Simulated Binaural Comparisons
IE processing in the central auditory system could sharpen the directional response (Fig. 11A, see Fig. 2 and methods for details). In IE model simulations, the IE spike rate was largely unchanged at ipsilateral directions but reduced at frontal and contralateral angles. The effect was quantified by comparing the lateralization index before and after IE processing (Fig. 11B). In all fibers the lateralization index difference increases with level above threshold and the maximal values were found 20 dB or more above threshold, where IE processing generates a lateralization index increase of up to 0.30, corresponding to 30% more lateralized spikes (median: 0.14). The improvement of lateralization by IE processing was slightly larger with call stimulation, especially for the rattle call (median increase of 0.17 in the lateralization index).
Figure 11.
Simulated IE processing. A: polar plot showing the spike rate response of an auditory nerve fiber before (red) and after (green) applying simulated IE processing. B: the effect of IE processing at different intensities. The plot show trajectories of lateralization index (LI) differences in individual fibers (i.e., LI after IE processing − LI before IE processing). The red line is the linear regression of all data, with a correlation coefficient R2 = 0.36.
DISCUSSION
In these first free-field recordings from geckos using naturalistic sound, we show that auditory nerve responses form two broadly tuned directional channels, largely due to the eardrum directionality. We had previously used closed-field stimulation to reveal ITD sensitive responses in the auditory nerve that reflected the acoustic coupling of the eardrums (12). We now report directional responses of the gecko ear and auditory nerve under naturalistic stimulus conditions and show that acoustic coupling leads to strong directionality in the auditory nerve response (Fig. 5).
Earlier studies of gecko auditory nerve fibers also used free-field stimulation, but with a ventral approach to the auditory nerve and consequent opening of the mouth cavity (34, 35). Other studies combined a ventral approach with closed-field stimulation (36) or a dorsal approach with closed-field dichotic stimulation (12). The data reported here in regard to sensitivity and frequency range of CFs (Fig. 4, B and C) are generally most similar to the comprehensive data set reported in Eatock and Manley (34) and Eatock et al. (35), which showed similar sensitivity at low and high frequencies and lowest thresholds around 500 and 2 kHz and comparable tuning curves and rate-level functions. The basic response properties also resemble data recorded using closed-field dichotic stimulation (12).
The presence of strong directionality in the periphery begs the question as to whether sound source estimation increases in accuracy and robustness at higher levels of the gecko central auditory system. This seems likely, as increased accuracy has been observed in other well-studied systems. Toadfish shows sharpening of directional responses from periphery to midbrain (37). In free-tailed bats, ambiguity in interaural level differences sensitivity is reduced between the lateral superior olive and the central nucleus of the inferior colliculus. Furthermore, many more collicular responses were stable across overall sound intensity, indicating that hierarchical transformations increase robustness (38). In barn owls, variability in ITD coding decreases between the first stage of binaural processing in the nucleus laminaris and the second stage in the lemniscal nuclei (24), whereas in rabbits, the encoding of ITD can become sharper as the information ascends through the auditory system (39). Pecka et al. (40) note that improved robustness of ITD information at higher processing stages may be at least as relevant than accuracy.
Lateralization of Sound Sources
Sound source location can be decoded from the relative firing rates of two broadly and inversely tuned channels. Our measures of Fisher information and firing rate both show a main information change across the midline (17, 41), whereas our EI models suggest that binaural comparisons could yield more lateralized responses in the central auditory system. The use of a population code for sound source location would be consistent with other studies, which report the neural resolution of sound source locations to be highest over the slopes, rather than the peaks, of spike rate functions (16, 41, 42).
In the absence of psychophysical observations, we cannot determine how accurately geckos localize sound. Our free field recordings suggest that the gecko central auditory system may emphasize lateralization rather than directionality. Lateralized responses can generate directional responses from simple rule of moving toward the most excited ear, as shown in robotic implementations [review in Braitenberg (29), Shaikh et al. (30), Christensen-Dalsgaard and Manley (43)]. For these reasons, measures of the lateralization index may be more relevant than the standard circular statistics often employed in directional hearing studies [e.g., Mardia (44)]. Our lateralization index (Fig. 7A) suggests that geckos should have a well-developed ability to lateralize sound. Index values were between −1 and 1, where negative numbers indicate contralateral and positive ipsilateral dominance. Values ranged from 0.2 to 0.8, that is, with 20% to 80% of the action potentials being lateralized. The lateralization index is intensity-dependent and generally declines at high intensities (Fig. 7B). It is maximal around 10–20 dB above threshold (depending on directionality and dynamic rate, where the ipsilateral response will be nearly maximal and the contralateral response close to threshold). For example, if the directional difference between eardrum vibration with ipsi- and contralateral stimulation is 20 dB, stimulation 20 dB above threshold will produce a very low contralateral and a strong ipsilateral response, but at higher levels the contralateral response will increase whereas the ipsilateral response will saturate, as shown by the model in Fig. 8B. The decline of lateralization index with level suggests a role for EI processing, as suggested below. The strong lateralization also applies to communication calls, which may be behaviorally important, as geckos are highly vocal animals. The gecko call is multiharmonic, with the fundamental around 400 Hz, but all fibers irrespective of best frequency respond to the call and show a strongly lateralized and robust response to the gecko call components with similar thresholds across CF (Fig. 9), providing a strong population representation of call structure and direction in the auditory nerve.
Spike Timing Appears Unlikely to Contribute to Direction Sensitivity
Both spike rate and spike timing vary with direction, as coupled ears generate large directional envelope timing differences with most natural sounds. These cues are stimulus and level dependent. Factors that contribute to timing differences between ipsi- and contralateral auditory nerve fiber responses include time of arrival differences, the interaural transmission delay produced by interaural coupling, and intensity-latency differences caused by weaker stimulation at contralateral directions [time-intensity trading (45)]. We evaluate timing cues in the following paragraphs and conclude that differences in rate may be more salient than temporal cues.
The minimal ipsi-contralateral delay differences found in our experiments were 300–500 µs, close to the 300 µs transmission delay generated by interaural coupling and measured previously in the gecko, both by laser vibrometry and neurophysiology (12). These delay differences are more than three times larger than the time-of-arrival differences predicted from the head size (∼70 µs in the gecko). However, directional differences in sensitivity produced much larger latency differences (Fig. 9, B and C). Most of these differences were caused by intensity-latency differences due to the steepness of onset of the stimulus envelope. They were relatively large with tone burst stimulation in the present experiments, due to the 10 ms rise-fall time. With a 20 dB difference in sensitivity to ipsi- and contralateral stimulation, the rise-fall time of the cos2 burst shape alone would generate a latency shift of ∼6 ms when the contralateral directions are just above threshold. For the call envelope, however, time differences measured from cross-correlations of the PSTHs were ∼2 ms and most likely also due to time-intensity trading caused by the sloping call envelope. The strong level dependence of latency makes onset timing differences less robust as a directional cue.
In contrast, fine-structure cues may be relatively independent of stimulus level, as has been shown for frog auditory nerve fibers (32, 46). Gecko nerve fibers show phase locking at least up to 1,300–1.500 Hz (12). We have not measured the directional variation in temporal fine structure, but directional phase differences should reflect the delay generated by interaural coupling (i.e., up to ∼300 µs). CNS processing of these phase differences, for example, by coincidence detectors, would be complicated except for directions close to the midline, as the contralateral input would be very reduced. It is, therefore, unlikely that directional hearing in geckos depends strongly on analysis of interaural time differences or onset disparities.
Acoustical Coupling of the Two Tympana is Sufficient to Explain Recorded Directionality
In frogs, extratympanic sources contribute to directional responses recorded from low best frequency auditory nerve fibers (33, 47). The extratympanic sensitivity is probably generated by mechanisms similar to bone conduction in humans (i.e., sound-induced vibrations of the head and body of the animal), and similar mechanisms may be present in lizards. In geckos, however, comparisons of laser vibrometry measures of tympanic directivity (Fig. 8) with neural directionality show that acoustical coupling of the two tympana is “sufficient” to explain the recorded directionality at the frequencies investigated here. We see no evidence for extratympanic directionality in the gecko.
At frequencies above 500 Hz, the ovoidal directionality pattern found in both gecko eardrum and nerve data (Figs. 3 and 5) is similar to auditory nerve directionality in frogs (21, 31), although the equivalent dB difference is up to 10 dB higher than in frogs, probably reflecting the more directional periphery in lizards. At lower frequencies (below 300–400 Hz), however, equivalent dB measurements are markedly different in frogs, showing a figure-eight directionality that does not match the eardrum directionality (21, 31). In frogs, therefore, extratympanic sensitivity (33, 48) imparts increased low-frequency sensitivity and directionality that are markedly different from the tympanic input (31, 32). Even the “earless” frogs (with a nonfunctional middle ear) have comparable auditory sensitivity to eared species at frequencies below ∼900 Hz (47). As the mechanisms of bone conduction appear very general (49, 50), it is puzzling that similar effects are not found in the geckos at low frequencies.
One reason for the difference between lizards and frogs may be that amphibians have a second movable element in the oval window, the operculum, which has been proposed as a conduit for low-frequency sound stimulation (8). Another reason may be that the lizard tympanum is thinner and more sensitive, and thus a more efficient sound receptor at all frequencies studied here (10). The present laser vibrometry measures showed smaller vibrations and higher neural thresholds at low frequencies (200–300 Hz) (Fig. 3A), than at frequencies from 1 to 2 kHz (Fig. 3A), but the eardrum vibrations are probably still considerably larger than the sound-induced vibrations of the head.
Implications for Central Processing
The auditory pathways in geckos are similar to those in other diapsids, including birds and other lizards (25, 51–53). The auditory nerve projects to first-order nuclei, magnocellularis, and angularis (54). The nucleus magnocellularis projects bilaterally to the second-order nucleus laminaris (25). The gecko nucleus laminaris is anatomically similar to laminaris in birds and crocodilians (25, 53, 55–57). Despite these anatomical similarities, our data suggest that it is unlikely that geckos use coincidence detection of temporal fine structure. Instead, and as outlined above, IE/EI processing of strongly directional input from the auditory nerve generates a robust rate code for hemispheric representation of sound source location in the central auditory system. Processing the neural input using an IE model (Fig. 2) and assuming contralateral inhibition within a 5-ms time window, sharpens the lateralized response (Fig. 11, A and B). The resulting sharpening using the actual, recorded spike trains is not as strong as expected, for examples based on even simpler models of binaural comparison such as the IVAD (interaural vibration amplitude difference) model based on eardrum vibration measurements, in Christensen-Dalsgaard and Manley (10), that show a very strong reduction of activity for all CL directions. It is possible that the IE models are ineffectual because inputs from the contralateral ear are typically weaker and delayed with respect to ipsilateral inputs (Fig. 5), which limit the scope for neural binaural interactions.
IE processing would serve to increase the dynamic range of directionality. At high-stimulus levels, where firing is near saturation from favored directions and increased from nonfavored directions, IE processing could produce a significant directional sharpening, as shown by up to 30% increases in the lateralization index (Fig. 11B). Binaural interactions may also be strongly stimulus dependent at high stimulus levels because of latency-intensity trading (see Spike Timing Appears Unlikely to Contribute to Direction Sensitivity above), so the delay of the contralateral neural input would be very dependent on stimulus envelope. The call elements (Fig. 1), especially the rattle call, with a more transient onset also will synchronize the ipsi- and contralateral inputs more effectively and therefore produce a stronger improvement of lateralization by IE interaction (median increase in LI using call element stimulation is 0.17). It should be noted that the model is strongly simplified compared with the actual IE processing, of which we know very little in lizards. A further objection may be that the model IE neuron only has two inputs (one IL and one CL auditory fiber, both with identical characteristics, Fig. 2). This structure enabled simple calculations on each auditory fiber but is likely unrealistic. An EE, or coincidence detector model, produces a similarly weak response, due to the weak and delayed input from contralateral angles (18). Noncoincidence EE responses, however, may be useful to evaluate absolute sound levels, and responses from few brainstem neurons that reflect EE-type processing have been reported (18).
The central auditory processing of sound source location in geckos, as shown by the few free-field units reported from the torus (58), may resemble hemispheric representations in avian and mammalian systems (59–62). A more in-depth analysis of decoding strategies available to the gecko could be the subject of future study, with the current observation that the broad representation of ITD in gecko auditory nerve is consistent with ITD being coded by the overall activity of the entire population (63) and also consistent with high resolution for near-midline sources.
As the auditory nerve is broadly responsive to sounds originating in the ipsilateral hemisphere, with sharp changes in information at front and back, its output may be most appropriate for directing orientation toward frontal sound sources. Geckos may need little additional directional processing other than the aforementioned requirement for dynamic range. In lizards, robotic models produce an efficient steering of the head to face the sound (30), implying a close connection between directional processing and motor control that may also be applicable to other animals (64, 65). Population rate model outputs may be suitable for generating motor commands that orient the gecko toward a sound source (64, 66). Opponent coding channels in brain could inhibit motion toward the ipsilateral side, consistent with this function. Thus, the study of central auditory processing, especially of sound direction, in the gecko should provide further understanding of directional processing.
GRANTS
This work was supported by the University of Southern Denmark (to J. Christensen-Dalsgaard); Velux Foundation Grant SDU2020 (to C. E. Carr); Carlsberg Foundation Grant 2009-01-0684 (to J. Christensen-Dalsgaard); and National Institute on Deafness and Other Communication Disorders (NIDCD) Grant DCD000436 (to C. E. Carr).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C-D. and C.E.C. conceived and designed research; J.C-D. and C.E.C. performed experiments; J.C-D., P.K., J.E.M., and C.E.C. analyzed data; J.C-D., P.K., J.E.M., and C.E.C. interpreted results of experiments; J.C-D. and C.E.C. prepared figures; J.C-D. and C.E.C. drafted manuscript; J.C-D., P.K., and C.E.C. edited and revised manuscript; J.C-D., P.K., and C.E.C. approved final version of manuscript.
REFERENCES
- 1.Blauert J. Spatial Hearing. The Psychophysics of Sound Localization. Cambridge, MA: MIT Press, 1997. [Google Scholar]
- 2.Carlile S, Pettigrew AG. Directional properties of the auditory periphery in the guinea pig. Hear Res 31: 111–122, 1987. doi: 10.1016/0378-5955(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 3.Yin TCT, Smith PH, Joris PX. Neural mechanisms of binaural processing in the auditory brainstem. Compr Physiol 9: 1503–1575, 2019. doi: 10.1002/cphy.c180036. [DOI] [PubMed] [Google Scholar]
- 4.Grothe B, Pecka M. The natural history of sound localization in mammals–a story of neuronal inhibition. Front Neural Circuits 8: 1–19, 2014. doi: 10.3389/fncir.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 90: 983–1012, 2010. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- 6.Heffner RS, Heffner HE. Evolution of sound localization in mammals. In: The Evolutionary Biology of Hearing, edited by Webster DB, Popper AN, Fay RR.. New York: Springer, 1992, p. 691–715. [Google Scholar]
- 7.Masterton B, Heffner H, Ravizza R. The evolution of human hearing. J Acoust Soc Am 45: 966–985, 1969. doi: 10.1121/1.1911574. [DOI] [PubMed] [Google Scholar]
- 8.Christensen-Dalsgaard J. Directional hearing in non-mammalian tetrapods. In: Sound Source Localization, edited by Popper AN, Fay RR.. New York: Springer, 2005, 67–123. [Google Scholar]
- 9.van Hemmen JL, Christensen-Dalsgaard J, Carr CE, Narins PM. Animals and ICE: meaning, origin, and diversity. Biol Cybern 110: 237–246, 2016. doi: 10.1007/s00422-016-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen-Dalsgaard J, Manley GA. Directionality of the lizard ear. J Exp Biol 208: 1209–1217, 2005. doi: 10.1242/jeb.01511. [DOI] [PubMed] [Google Scholar]
- 11.Christensen-Dalsgaard J, Manley GA. Acoustical coupling of lizard eardrums. J Assoc Res Otolaryngol 9: 407–416, 2008. doi: 10.1007/s10162-008-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen-Dalsgaard J, Tang Y, Carr CE. Binaural processing by the gecko auditory periphery. J Neurophysiol 105: 1992–2004, 2011. doi: 10.1152/jn.00004.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livens P, Muyshondt PGG, Dirckx JJJ. Sound localization in the lizard using internally coupled ears: a finite-element approach. Hear Res 378: 23–32, 2019. doi: 10.1016/j.heares.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Vedurmudi APG, Christensen-Dalsgaard J, Young BA, Williams R, van Hemmen JL. How internally coupled ears generate temporal and amplitude cues for sound localization. Phys Rev Lett 116: 028101, 2016. doi: 10.1103/PhysRevLett.116.028101. [DOI] [PubMed] [Google Scholar]
- 15.Vossen C, Christensen-Dalsgaard J, van Hemmen JL. Analytical model of internally coupled ears. J Acoust Soc Am 128: 909–918, 2010. doi: 10.1121/1.3455853. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi TT, Bala ADS, Spitzer MW, Euston DR, Spezio ML, Keller CH. The synthesis and use of the owl's auditory space map. Biol Cybern 89: 378–387, 2003. doi: 10.1007/s00422-003-0443-5. [DOI] [PubMed] [Google Scholar]
- 17.Gordon N, Shackleton TM, Palmer AR, Nelken I. Responses of neurons in the inferior colliculus to binaural disparities: insights from the use of Fisher information and mutual information. J Neurosci Methods 169: 391–404, 2008. doi: 10.1016/j.jneumeth.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Christensen-Dalsgaard J, Carr CE. Processing of directional information in the gecko auditory nerve. Acta Acustica United With Acustica 104: 848–851, 2018. doi: 10.3813/AAA.919242. [DOI] [Google Scholar]
- 19.Wever EG. The Reptile Ear. Princeton, NJ: Princeton University Press, 1978. [Google Scholar]
- 20.Elliott TM, Christensen-Dalsgaard J, Kelley DB. Temporally selective processing of communication signals by auditory midbrain neurons. J Neurophysiol 105: 1620–1632, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng AS. Directional characteristics of the acoustic receiver of the leopard frog (Rana pipiens): a study of eighth nerve auditory responses. J Acoust Soc Am 68: 1107–1114, 1980. doi: 10.1121/1.384981. [DOI] [PubMed] [Google Scholar]
- 22.Lehman EL. Theory of Point Estimation. New York: Wiley & Sons, 1983.doi: 10.1007/978-1-4757-2769-2. [DOI] [Google Scholar]
- 23.Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci 8: 1684–1689, 2005. doi: 10.1038/nn1541. [DOI] [PubMed] [Google Scholar]
- 24.Fischer BJ, Konishi M. Variability reduction in interaural time difference tuning in the barn owl. J Neurophysiol 100: 708–715, 2008. doi: 10.1152/jn.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Y, Christensen-Dalsgaard J, Carr CE. Organization of the auditory brainstem in a lizard, Gekko gecko. I. Auditory nerve, cochlear nuclei, and superior olivary nuclei. J Comp Neurol 520: 1784–1799, 2012. doi: 10.1002/cne.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiappe ME, Kozlov AS, Hudspeth AJ. The structural and functional differentiation of hair cells in a lizard's basilar papilla suggests an operational principle of amniote cochleas. J Neurosci 27: 11978–11985, 2007. doi: 10.1523/JNEUROSCI.3679-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manley GA, Köppl C.Sienknecht UJ. The remarkable ears of geckos and pygopods. In: Insights from Comparative Hearing Research, (Springer Handbook of Auditory Research, vol. 49) edited by Manley GA, Köppl C, Popper AN, Fay RR.. New York: Springer, 2013, p. 111–131. doi: 10.1007/2506_2013_27. [DOI] [Google Scholar]
- 28.Middlebrooks JC, Knudsen EI. A neural code for auditory space in the cat's superior colliculus. J Neurosci 4: 2621–2634, 1984. doi: 10.1523/JNEUROSCI.04-10-02621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braitenberg V. Vehicles: Explorations in Synthetic Psychology. Cambridge, MA: MIT Press, 1984. [Google Scholar]
- 30.Shaikh D, Hallam J, Christensen-Dalsgaard J. From “ear” to there: a review of biorobotic models of auditory processing in lizards. Biol Cybern 110: 303–317, 2016. doi: 10.1007/s00422-016-0701-y. [DOI] [PubMed] [Google Scholar]
- 31.Jørgensen MB, Christensen-Dalsgaard J. Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. I. Spike rate responses. J Comp Physiol A 180: 493–502, 1997. doi: 10.1007/s003590050066. [DOI] [PubMed] [Google Scholar]
- 32.Jørgensen MB, Christensen-Dalsgaard J. Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. II. Spike timing. J Comp Physiol A 180: 503–511, 1997. doi: 10.1007/s003590050067. [DOI] [PubMed] [Google Scholar]
- 33.Wilczynski W, Resler C, Capranica RR. Tympanic and extratympanic sound-transmission in the leopard frog. J Comp Physiol A 161: 659–669, 1987. doi: 10.1007/BF00605007. [DOI] [PubMed] [Google Scholar]
- 34.Eatock RA, Manley GA. Auditory nerve fibre activity in the Tokay gecko. J Comp Physiol 142: 219–226, 1981. doi: 10.1007/BF00605740. [DOI] [Google Scholar]
- 35.Eatock RA, Manley GA, Pawson L. Auditory nerve fibre activity in the tokay gecko. J Comp Physiol 142: 203–218, 1981. doi: 10.1007/BF00605739. [DOI] [Google Scholar]
- 36.Sams-Dodd F, Capranica RR. Representation of acoustic-signals in the 8th nerve of the Tokay gecko. I. Pure-tones. Hear Res 76: 16–30, 1994. doi: 10.1016/0378-5955(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 37.Edds-Walton P, Fay RR. Sharpening of directional responses along the auditory pathway of the oyster toadfish, Opsanus tau. J Comp Physiol A 191: 1079–1086, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Park TJ, Klug A, Holinstat M, Grothe B. Interaural level difference processing in the lateral superior olive and the inferior colliculus. J Neurophysiol 92: 289–301, 2004. doi: 10.1152/jn.00961.2003. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick DC, Batra R, Stanford TR, Kuwada S. A neuronal population code for sound localization. Nature 388: 871–874, 1997. doi: 10.1038/42246. [DOI] [PubMed] [Google Scholar]
- 40.Pecka M, Siveke I, Grothe B, Lesica NA. Enhancement of ITD coding within the initial stages of the auditory pathway. J Neurophysiol 103: 38–46, 2010. doi: 10.1152/jn.00628.2009. [DOI] [PubMed] [Google Scholar]
- 41.Harper NS, McAlpine D. Optimal neural population coding of an auditory spatial cue. Nature 430: 682–686, 2004. doi: 10.1038/nature02768. [DOI] [PubMed] [Google Scholar]
- 42.Butts DA, Goldman MS. Tuning curves, neuronal variability, and sensory coding. PLoS Biol 4: e92, 2006. doi: 10.1371/journal.pbio.0040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen-Dalsgaard J, Manley GA. Sound localization by the internally coupled ears of lizards: from biophysics to biorobotics. J Acoust Soc Am 146: 4718, 2019. doi: 10.1121/1.5138929. [DOI] [PubMed] [Google Scholar]
- 44.Mardia KV. Statistics of Directional Data. New York: Academic Press, 1972. [Google Scholar]
- 45.Rheinlaender J, Mörchen A. Time-intensity trading in locust auditory interneurones. Nature 281: 672–674, 1979. doi: 10.1038/281672a0. [DOI] [Google Scholar]
- 46.Schmitz B, White TD, Narins PM. Directionality of phase locking in auditory nerve fibers of the leopard frog Rana pipiens pipiens. J Comp Physiol A 170: 589–604, 1992. doi: 10.1007/BF00199335. [DOI] [PubMed] [Google Scholar]
- 47.Womack MC, Christensen-Dalsgaard J, Coloma LA, Chaparro JC, Hoke KL. Earless toads sense low frequencies but miss the high notes. Proc R Soc B 284: 20171670, 2017. doi: 10.1098/rspb.2017.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombard RE, Straughan IR. Functional aspects of anuran middle ear structures. J Exp Biol 61: 71–93, 1974. [DOI] [PubMed] [Google Scholar]
- 49.Christensen CB, Lauridsen H, Christensen-Dalsgaard J, Pedersen M, Madsen PT. Better than fish on land? Hearing across metamorphosis in salamanders. Proc R Soc B 282: 20141943, 2015. doi: 10.1098/rspb.2014.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenfelt S. Bone conduction and the middle ear. In: The Middle Ear: Science, Otosurgery and Technology, edited by Puria S, Fay RF, Popper AN.. New York: Springer Science & Business Media, 2013, p. 135–169. doi: 10.1007/978-1-4614-6591-1_6. [DOI] [Google Scholar]
- 51.Foster RE, Hall WC. The organization of central auditory pathways in a reptile, Iguana iguana. J Comp Neurol 178: 783–832, 1978. doi: 10.1002/cne.901780412. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi TT, Konishi M. Projections of the cochlear nuclei and nucleus laminaris to the inferior colliculus of the barn owl. J Comp Neurol 274: 190–211, 1998. doi: 10.1002/cne.902740206. [DOI] [PubMed] [Google Scholar]
- 53.Yan K, Tang Y, Carr CE. Calcium-binding protein immunoreactivity characterizes the auditory system of Gekko gecko. J Comp Neurol 518: 3409–3426, 2010. doi: 10.1002/cne.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szpir MR, Sento S, Ryugo DK. Central projections of cochlear nerve fibers in the alligator lizard. J Comp Neurol 295: 530–547, 1990. doi: 10.1002/cne.902950403. [DOI] [PubMed] [Google Scholar]
- 55.Carr CE, Christensen-Dalsgaard J. Sound localization strategies in three predators. Brain Behav Evol 86: 17–27, 2015. doi: 10.1159/000435946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carr CE, Soares D, Smolders JWT, Simon JZ. Detection of interaural time differences in the alligator. J Neurosci 29: 7978–7990, 2009. doi: 10.1523/JNEUROSCI.6154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kettler L, Carr CE. Neural maps of interaural time difference in the American alligator: a stable feature in modern archosaurs. J Neurosci 39: 3882–3896, 2019. doi: 10.1523/JNEUROSCI.2989-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manley GA. A review of the auditory physiology of reptiles. Prog Sens Physiol 2: 49–134, 1981. doi: 10.1007/978-3-642-68169-1_2. [DOI] [Google Scholar]
- 59.Cazettes F, Fischer BJ, Beckert MV, Peña JL. Emergence of an adaptive command for orienting behavior in premotor brainstem neurons of barn owls. J Neurosci 38: 7270–7279, 2018. doi: 10.1523/JNEUROSCI.0947-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee CC, Middlebrooks JC. Specialization for sound localization in fields A1, DZ, and PAF of cat auditory cortex. J Assoc Res Otolaryngol 14: 61–82, 2012. doi: 10.1007/s10162-012-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lingner A, Pecka M, Leibold C, Grothe B. A novel concept for dynamic adjustment of auditory space. Sci Rep 8: 1–13, 2018. doi: 10.1038/s41598-018-26690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stecker GC, Harrington IA, Middlebrooks JC. Location coding by opponent neural populations in the. PLoS Biol 3: e78, 2005. doi: 10.1371/journal.pbio.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lesica NA, Lingner A, Grothe B. Population coding of interaural time differences in gerbils and barn owls. J Neurosci 30: 11696–11702, 2010. doi: 10.1523/JNEUROSCI.0846-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hancock KE. A physiologically-based population rate code for interaural time differences (ITDs) predicts bandwidth-dependent lateralization. In: Hearing — From Sensory Processing to Perception. edited by Kollmeier B, Klump G, Hohmann V, Langemann U, Mauermann M, Uppenkamp S, Verhey, J. Berlin: Springer-Verlag, 2007, p. 389–397. [Google Scholar]
- 65.Peña JL, Cazettes F, Beckert MV, Fischer BJ. Synthesis of hemispheric ITD tuning from the readout of a neural map: commonalities of proposed coding schemes in birds and mammals. J Neurosci 39: 9053–9061, 2019. doi: 10.1523/JNEUROSCI.0873-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCue MP, Guinan JJ. . Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol 18: 355–360, 1997. [PubMed] [Google Scholar]