Abstract
Comprehensive functional characterization of cardiac tissue includes investigation of length and load dependence. Such measurements have been slow to develop in engineered heart tissues (EHTs), whose mechanical characterizations have been limited primarily to isometric and near-isometric behaviors. A more realistic assessment of myocardial function would include force-velocity curves to characterize power output and force-length loops mimicking the cardiac cycle to characterize work output. We developed a system that produces force-velocity curves and work loops in human EHTs using an adaptive iterative control scheme. We used human EHTs in this system to perform a detailed characterization of the cardiac β-myosin specific inhibitor, mavacamten. Consistent with the clinically proposed application of this drug to treat hypertrophic cardiomyopathy, our data support the premise that mavacamten improves diastolic function through reduction of diastolic stiffness and isometric relaxation time. Meanwhile, the effects of mavacamten on length- and load-dependent muscle performance were mixed. The drug attenuated the length-dependent response at small stretch values but showed normal length dependency at longer lengths. Peak power output of mavacamten-treated EHTs showed reduced power output as expected but also shifted peak power output to a lower load. Here, we demonstrate a robust method for the generation of isotonic contraction series and work loops in engineered heart tissues using an adaptive-iterative method. This approach reveals new features of mavacamten pharmacology, including previously unappreciated effects on intrinsic myosin dynamics and preservation of Frank-Starling behavior at longer muscle lengths.
NEW & NOTEWORTHY We applied innovative methods to comprehensively characterize the length and load-dependent behaviors of engineered human cardiac muscle when treated with the cardiac β-myosin specific inhibitor mavacamten, a drug on the verge of clinical implementation for hypertrophic cardiomyopathy. We find mechanistic support for the role of mavacamten in improving diastolic function of cardiac tissue and note novel effects on work and power.
Keywords: engineered heart tissues, hypertrophic cardiomyopathy, mavacamten, power
INTRODUCTION
Ever since the first derivation of cardiomyocytes from induced pluripotent stem cells approximately a decade ago, enthusiasm has continued to mount for the basic and translational applications of this technology in fields ranging from cardiovascular disease modeling, human cardiac development, high-fidelity drug and toxicity testing, and advanced cellular and tissue regenerative medicine (1). Contractile assays are a key aspect of iPSC-CM characterization for all of the above applications, as efficient cardiac contraction is the fundamental evolutionary purpose of cardiac tissue in which all aspects of myocardial biology, including energetics, electrophysiology, and mechanics, converge. Despite the fact that the physiological function of the heart is to produce work and power under load (2), most assays using iPSC-CMs have examined mechanical behavior under decidedly unphysiological loading conditions (3).
Cardiac contraction can be fundamentally characterized as the production of work and power output at defined afterloads and preloads (4–7). Elegant single cardiomyocyte studies show that the commonly studied adrenergic agonist isoproterenol increases isometric force by 50% but increases work by 400%, demonstrating that work measurements integrate changes in both systolic and diastolic function simultaneously (8). Furthermore, cardiac power output is highly tuned by factors such as myofilament isoform and posttranslational modifications to perform optimally at the physiological load, with important shifts occurring during development (9, 10). Beyond providing a more accurate reflection of contractility, work output was shown by Starling and Visscher (11) in their seminal 1927 paper to directly correlate with oxygen uptake and thereby energy consumption. As far as we are aware, no existing technique is capable of performing these important measurements in iPSC-CM-based systems.
In the work presented here, we demonstrate a robust method for generating a series of isotonic contractions and work loops in engineered heart tissues using an adaptive-iterative method. This method enables a comprehensive characterization of cardiac mechanics that includes measuring performance under a variety of loading conditions that relate meaningfully to in vivo physiology. Having demonstrated the feasibility of the approach, we use it to perform an in-depth characterization of the acute effects of mavacamten, a cardiac myosin-specific inhibitor.
METHODS
iPSC Culture and Differentiation
The human iPSC line used in this study was acquired from the Coriell Institute (GM23338), which has been previously characterized and used in cardiac differentiation (12). iPSC colonies were maintained on growth factor-reduced Matrigel (Corning)-coated tissue culture plates in mTeSR1 medium (Stem Cell Technologies). iPSCs were differentiated into iPSC-derived cardiomyocytes (iPSC-CMs) following a sequential protocol of manipulating the WNT pathway (13), using 12.5 µM CHIR99021 (Stem Cell Technologies) for 24 h and 5 µM IWP4 (Tocris) for 48 h on day 3 after the start of differentiation. Subsequently, the differentiating cultures were maintained in RPMI + B27 minus insulin until day 11, when spontaneous beating was observed. Medium was then changed to RPMI + B27+insulin for 2 days, followed by 4 days of metabolic selection using DMEM with no glucose and 4 mM lactate (14). iPSC-CMs were kept in RPMI+B27+ insulin for 2 more days and then used to make engineered heart tissues (EHTs). iPSC-CMs used in this study were generated from four batches of differentiations using iPSCs between passage 35 and 45.
Engineered Heart Tissue Fabrication
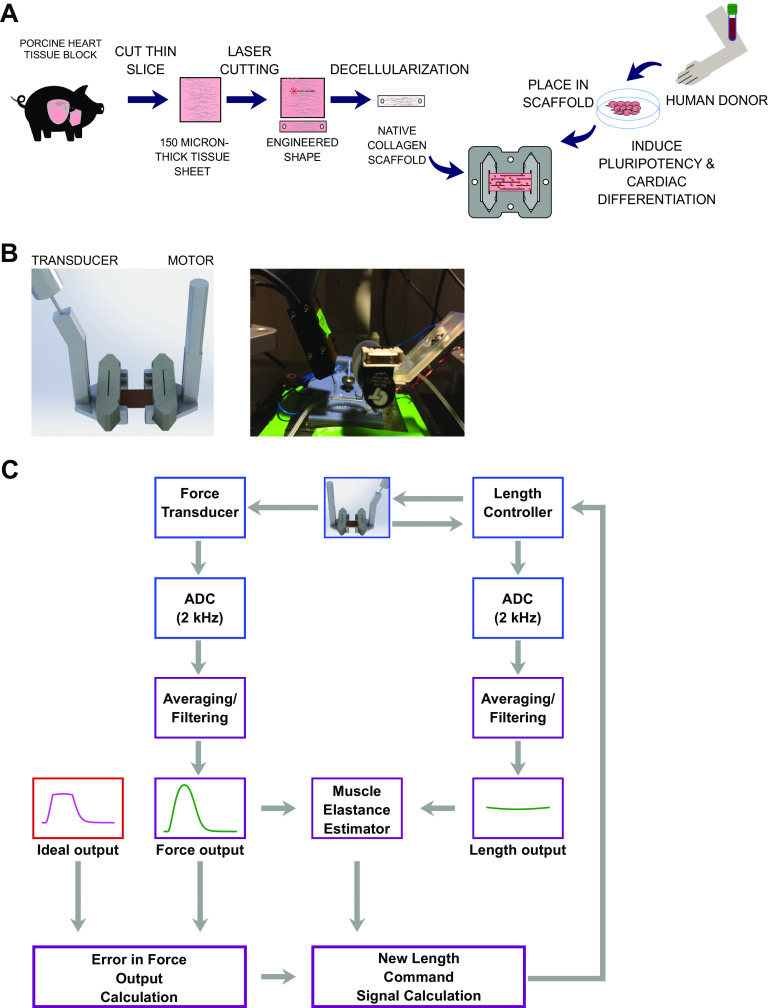
Engineered heart tissues were created from decellularized porcine myocardium based on an optimization of previously published protocols (Fig. 1A) (15, 16). Briefly, we used a cryostat to cut 150-μm sections from porcine left ventricular free wall midmyocardium blocks frozen in crushed dry ice. We then thawed these sections and cut 16 × 3 mm rectangular scaffolds from these sections using a laser cutter oriented such that the long axis of the rectangle aligned with the fiber direction of the tissue. Individual rectangular tissues pieces were fixed into crevices 6 mm apart cut into our custom polytetrafluoroethylene (PTFE) tissue culture cassette (15) using a 0.5-mm thick, 4.2 × 2.2 mm rectangular PFE slab. These native tissues were then decellularized for 2 h in a cellular lysis buffer (10 mM Tris, 0.1% wt/vol EDTA, pH 7.4), followed by 45 min in a detergent solution (0.5% SDS in DPBS) agitated at 50 rpm on a nutator at room temperature. Scaffolds were then washed for 1 h in DPBS on the nutator and then incubated overnight in DMEM with 10% FBS and 2% penicillin-streptomycin before being seeded within the next 2 days. iPSC-CMs were dissociated from culture using TrypLE Select for 15–20 min, followed by washing with PBS and mechanical pipetting. EHTs were subsequently grown in standard media conditions using RPMI with 2% B27 with insulin for 28 days before being utilized for mechanical measurements.
Figure 1.
Engineered heart tissue methods and mechanical testing setup. A: methods for fabrication engineered heart tissue. B: mechanical testing setup. C: workflow for iterative control method. ADC, analog-to-digital converter.
Mechanical Testing Setup
For mechanical testing, EHTs were ejected from their culture frame into a custom-designed, three-dimensional (3D)-printed organotypic testing bath and immersed in Tyrode’s solution at 36°C with constant perfusion, as previously described (15). The two ends of EHTs affixed to the PTFE pieces were mechanically mounted between a World Precision Instruments (WPI KG7) force transducer and an Aurora high-speed length controller (322C) and brought to an optically calibrated distance between the two ends reflecting the length of the EHT during culture (6 mm) (Fig. 1B). EHTs were stimulated using 1-Hz constant current (100 mA) field stimulus during all experiments unless otherwise noted. Before any subsequent mechanical measurements were recorded, EHTs were preconditioned with three cycles of stretch and slack by moving the EHTs between 5.7 mm and 6.6 mm.
Drug Infusion Protocol
Mavacamten (MYK-461, Cayman Chemicals) stock was prepared in DMSO at 1 mM dilution. For drug infusion experiments, mavacamten (mava) was diluted to either 0.33 or 0.5 µM in fresh Tyrode’s solution. Drug solution was infused rapidly into the testing bath over the course of 5 min, allowing for full exchange of bath content. Thereafter, drug solution was continuously perfused during the rest of the experiment. For drug time course experiments, nonlinear regression using a three-parameter mono-exponential decay model was used, where the measured parameter p was fit as a function of time t using the equation p(t) = (p0 − p∞)e−βp + p∞, where the free constants of the fit included the time constant β and the scaling constants p0 and p∞.
Isometric Measurements
Isometric measurements of EHTs were conducted at 6.6-mm EHT length (10% above culture length) at 1-Hz field stimulus. Metrics calculated from the active isometric twitch contractions include peak force (PF), time from stimulus to peak contraction (TTP), time from peak to 50% relaxation (RT50), and normalized tension-time integral (nTTI). Normalized tension-time integral (nTTI) was calculated as the area under the curve of the twitch normalized to its own maximum peak force using numerical integration in MATLAB.
Length-Dependent Measurements
Length-dependent measurements of active and diastolic force were conducted by stretching EHTs at a continuous linear stretch at a rate of 0.015 mm/s from 5.7 mm to 6.6 mm in length. The diastolic force and active force during this continuous stretch was extracted by a custom MATAB script, as previously described (16). Stretch ratio λ was defined as the ratio of current length l to culture length L0 such that λ = l/L0. To demonstrate the Frank-Starling relation (FSR), we plotted relative active force as the active force at any given length divided by the active force at the shortest stretch ratio (λ = 0.95). Frank startling relation slope (FSRS) was defined as the numerical derivative of the FSR with respect to the stretch ratio such that .
Adaptive Control Technique for Isotonic Clamps
Adaptive control of intact muscle force transient was inspired by the approach first implemented by Peterson et al. 1989 (17). In our version of this approach, a force transient fmeas i(t) during a particular iteration i is compared with an ideal force transient fideal(t) and used to generate a control length signal ci(t) that is applied to the EHT in the subsequent cycle. The force measured in response to applying ci(t) is then used to inform the next iteration (Fig. 1C). Ultimately, this iterative process is continued until the desired force waveform is achieved.
The specific steps of the isotonic adaptive algorithm are as follows. 1) An initial (i = 0) isometric measurement is recorded, consisting of 20 s under 1-Hz field stimulus. Isometric twitches are averaged together to reduce noise and filtered using a first-order Savitsky-Golay filter with a frame width of 41 to create an initial isometric twitch of the EHT, f0(t). 2) For any given isotonic clamp, a threshold is set dictating the force developed during the plateau of the isotonic twitch, e.g., fthreshold. The idealized force transient fideal(t) is generated using f0(t) and fthreshold according to a piecewise function within each time period τ (τ = 1 s for 1 Hz stimulus):
The time constants t1 and t2 are defined as the first time point and last time point, respectively, when fmeas0(t) is greater than fthreshold. In this manner, the isotonic twitch signal that we ideally require from our control loop fideal(t) is generated.
3) The length control signal ci(t) is generated after each iteration i by taking in to account the force transient measured during that iteration fi(t) and the length transient measured during that iteration li(t) and using these to compute a time varying elastance scaling factor ki(t), a cumulative error term ei(t), and the ideal desired force transient fideal(t). These are computed as follows:
4) The length control signal is subject to practical constraints in order to ensure physical realization including that ci(t) < lmax for all t, where lmax is the maximum motor excursion, and that , where c0 is the baseline isometric muscle length.
5) During each iteration of the algorithm (i > 0), the length control signal ci(t) is calculated and used to drive the high-speed length controller for 20 repeats of the given time period τ until a satisfactory isotonic clamp is achieved with a converged length control signal cf(t). Once achieved, a 40-s final record is collected, containing 20 pairs of isometric twitch fiso(t), followed by the converged isotonic twitch ff(t) along with measurements of the actual length measurement of the EHT during this time lf(t).
Analysis of Load Velocity and Load Power Characteristics from Isotonic Twitches
The measurements collected from each successfully clamped EHT at any given isotone include the concurrent isometric twitch force fiso(t), the isotonic twitch force ff(t), and the length measurement of the EHT during the isotonic clamp lf(t). The velocity of shortening during the isotonic twitch v(t) was defined as the numerical derivative of the length, such that . For each set of measurements, the afterload Fa during that clamp is defined as the average force during that shortening phase of the plateau of the isotonic twitch divided by the maximum isometric force during that measurement. For each isotonic twitch, the maximum shortening velocity va is further defined as the maximum speed of shortening during the shortening phase of the isotonic twitch. Each isotonic twitch of each EHT in each condition generated a pair of measurements (Fa,va). Power Pa was calculated as the product of afterload and velocity for each pair of measurements such that Pa = Fava, yielding a set of three values for each isotonic clamp for each EHT in a given condition. For each EHT in a given condition, a series of these sets of triple measurements was generated corresponding to the various imposed isotonic afterloads.
The power and velocity were fit as a function of the afterload according to the classic Hill force-velocity equation (18). In particular, the velocity-load relation was fit using the fminsearch optimization algorithm according to the following equation:
where p0, a, and b are free parameters of the model, identifiable with the constants of Hill’s dynamic muscle function theory.
From the above fit and subsequent simulation over the range of data to verify goodness of fit, three metrics were closely examined for each EHT in a given condition, specifically the maximum theoretical shortening velocity vmax, the maximum power output Pmax, and the optimal afterload at maximum power output Fopt. The maximum theoretical shortening velocity vmax was calculated as the predicted unloaded shortening velocity of a muscle with the constants determined from fitting the data of a particular EHT. The maximum power output Pmax was determined as the predicted peak power output of a muscle, with the constants determined from fitting the data of a particular EHT. The optimal afterload at maximum power output Fopt was the afterload Fa, at which Pmax was found for a given EHT.
Work Loop Measurements and Analysis
In order to perform the work loops, the length signal from a converged successful isotonic clamp cf(t) was modified to create the work loop length signal cw(t) at that particular afterload according to the following piecewise function:
where is the time constant that corresponds to the time at which the maximum shortening is reached in the length signal cf(t):
The relengthening function r(t) was a custom cosine-type function that brought the EHT back to its initial length by the end of the time period τ according to the following
To measure the work loop force profile fw(t), a 40-s record is collected, containing 20 pairs of isometric twitch fiso(t), followed by the work loop twitch fw(t), along with measurements of the actual length of the EHT during this time lw(t).
Although a variety of metrics could be quantified from the work loops, we focused on analysis of two primary metrics from the work loops in EHTs, namely the amount of work performed (W) and the end systolic force-length relationships (ESFLR) The amount of work (W) done by the muscle was computed as the integral of the work loop via numerical integration, calculated as:
The end-systolic force length relationship (ESFLR) was determined to be the slope of the linear regression of the end-systolic force and end-systolic length for a specific EHT in a given condition over its individual range of measured afterloads and preloads.
Statistical Analysis
Drug studies performed in this work were all paired measurements (with and without drug) in the same EHT. Hence, all statistical analyses were done in paired fashion. For testing statistical significance of a single metric before and after treatment, paired Student’s t test was used. For testing statistical significance of metrics collected in repeated measurements, such as those dependent on afterload and stretch before and after treatment, two-way ANOVA with repeated measures by both the factors of EHT identity and the afterload/stretch was performed. Any multiple comparisons conducted were corrected with the Sidak correction method. All statistical analyses were performed in GraphPad Prism 7.01. Statistical significance cutoff was P < 0.05. Symbolic representations of P values were set as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
RESULTS
Implementation of an Adaptive Control Method for Engineered Heart Tissue
Our approach yielded accurate control of engineered heart tissue for acute implementation of isotonic twitches and work loops. The algorithm typically converged to the desired conditions within three to five iterations, achieving the desired load within 100 s. (Fig. 2A). Furthermore, continuous sequences of alternating isometric and isotonic twitches showed that the properties of the muscle are not significantly altered by the muscle length control and movement (Fig. 2B). By performing a series of isotonic twitches at different lengths, force velocity and force power characteristics as well as work loops could be readily measured without changing muscle properties within ∼30 min total per isotonic series (Fig. 2C).
Figure 2.
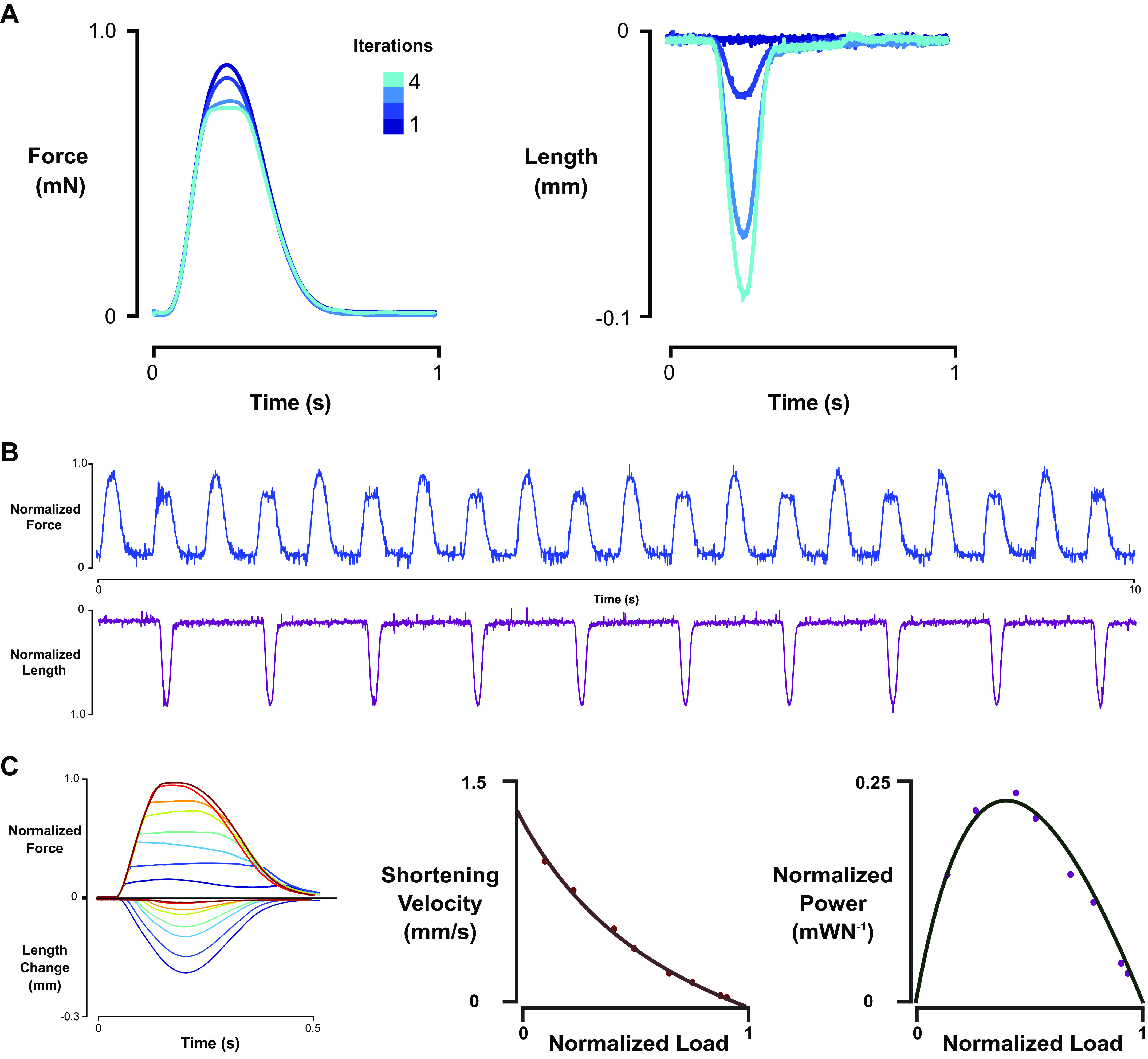
Iterative control method workflow and characteristics, example force-velocity/power curves, and work-preload-afterload curves. A: iterative algorithm typically converged within 4 iterations to desired isotone. B: sequence for final measurement collecting showing beat-to-beat isometric and isotonic twitch, depending on length control command once algorithm converged. C: representative isotonic twitches and derived load-velocity and load-power curves.
Mavacamten Alters Isometric and Preload-Dependent Properties of Cardiac Muscle
In order to demonstrate the system’s ability to study fundamental muscle properties, it was used to characterize responses of human EHTs to the cardiac myosin inhibitor mavacamten (19). Mavacamten is a small molecule on the verge of clinical application for the treatment of hypertrophic cardiomyopathy and possibly heart failure with preserved ejection (20). Extensive characterization of mavacamten’s effect on isometric and length-dependent contractile properties, in addition to work and power measurements, has yet to be performed on intact human-based tissues. Accordingly, we performed a set of comprehensive paired measurements of mavacamten in our human engineered heart tissue system.
We first examined the effect of mavacamten on active contraction (Fig. 3A) by performing paired isometric measurements at 1 Hz and a stretch ratio of 1.1 before and after drug treatment at clinically relevant concentrations of 0.33 and 0.5 µM. After the start of mavacamten infusion, the mechanical properties of muscle reached steady state within 45 min (Fig. 3B). Notably, the time course of twitch kinetics and peak force attenuation had identical time constants of exponential decay (0.0016 ms−1), indicating that the properties of force and kinetics of contraction are inextricably linked through mavacamten’s effects on duty cycle and kinetics of myosin.
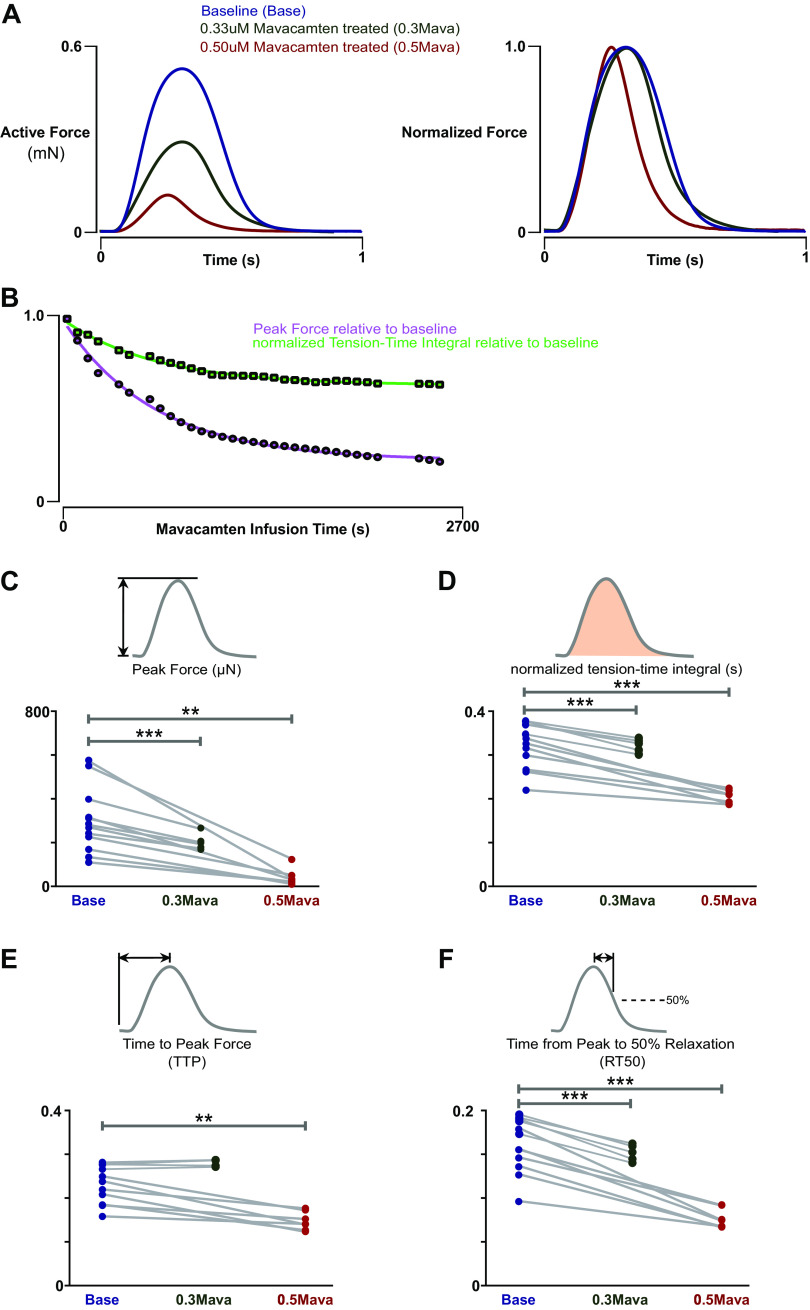
Figure 3.
Isometric characteristics of baseline and mavacamten-treated engineered heart tissues (EHTs). A: representative isometric twitch of an EHT at 10% stretch under 1-Hz stimulus under baseline conditions, with 0.33 µM mavacamten treatment, and with 0.5 µM mavacamten treatment. B: time course of peak force (PF) and normalized tension-time integral (nTTI) as a function of mavacamten infusion time, demonstrating that steady state in isometric twitch is reached within 30 min. The exponential decay constant of PF and nTTI were identical (0.0016 ms−1). C: PF was reduced on average by 40% with 0.33 µM mavacamten treatment (n = 5, paired t test, P = 0.0009) and by 85% with 0.50 µM mavacamten treatment (n = 7, paired t test, P = 0.0075). D: nTTI was reduced on average by 14% with 0.33 µM mavacamten (n = 5, paired t test, P = 0.0008) and by 30% with 0.5 µM mavacamten treatment (n = 7, paired t test, 0.0009). E: TTP (time to peak force) was reduced on average by 43 ms by 0.5 µM mavacamten treatment (n = 7, paired t test, P = 0.0021) and not affected by 0.33 µM mavacamten treatment (n = 5, paired t test). F: RT50 (time from peak to 50% relaxation) was reduced on average by 24% with 0.33 µM mavacamten treatment (n = 5, paired t test, P = 0.0002) and by 45% with 0.5 µM mavacamten treatment (n = 7, paired t test, P = 0.0003). **P < 0.01, ***P < 0.001.
We found that 0.33 µM mavacamten resulted in an average peak force decrease of 40% (P = 0.0009), whereas 0.5 µM mavacamten further decreased the peak force to an average of 85% of baseline (P = 0.0075) (Fig. 3C). We further examined closely the effects of mavacamten on the kinetic properties of active muscle contraction, including time to peak (TTP), time from peak to 50% relaxation (RT50), and normalized tension-time integral (nTTI) (Fig. 3C). nTTI was reduced on average by ∼14% by 0.33 µM mavacamten treatment (P = 0.0008) and was further reduced on average by ∼30% by 0.5 µM mavacamten treatment (P = 0.0009) (Fig. 3D). TTP was not significantly affected by 0.33 µM mavacamten treatment but was reduced on average by ∼20% by 0.5 µM mavacamten treatment (P = 0.0021) (Fig. 3E). RT50 was reduced on average by ∼24% by 0.33 µM mavacamten treatment (P = 0.0002) and was further reduced on average by ∼45% by 0.5 µM mavacamten treatment (P = 0.0003) (Fig. 3F). Overall, mavacamten decreased contractility of human engineered heart tissue in a concentration-dependent fashion primarily through decreasing inotropy and increased lusitropy.
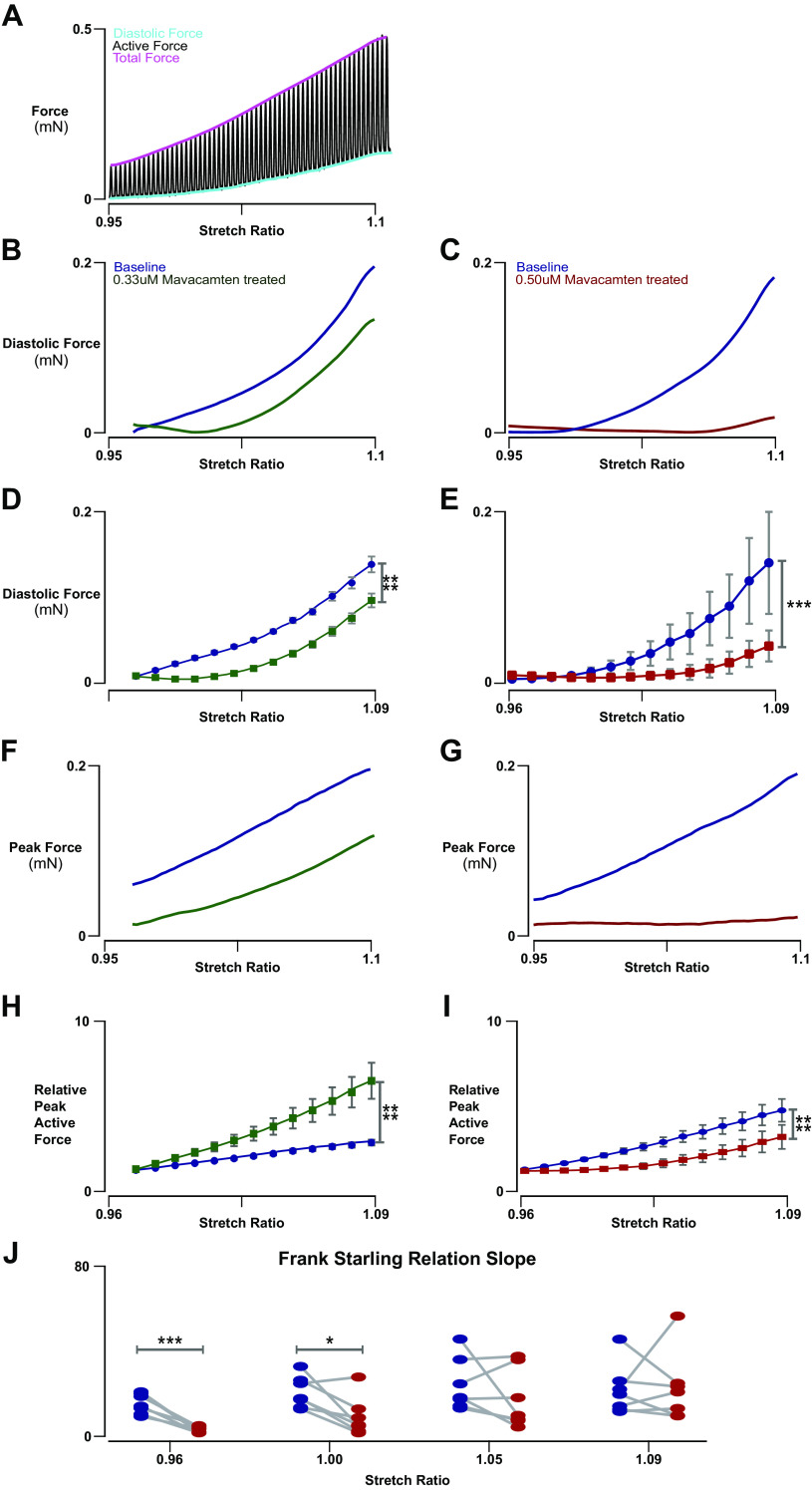
We next examined the effect of length on the active and diastolic force properties of EHTs and how these properties change when treated with mavacamten at both 0.33 and 0.50 µM dosages. EHTs were continuously stretched during simultaneous 1-Hz field stimulus, and active and diastolic force properties were extracted as a function of tissue stretch (Fig. 4A). Mavacamten had striking effects on the diastolic force properties of EHTs (Fig. 4, B and C). In particular, the drug significantly altered the effect of stretch on diastolic force (Fig. 4, D and E), decreasing the diastolic force elicited by lengthening of EHT at both the 0.33 μM concentration of mavacamten (2-way ANOVA, interaction P < 0.0001) by ∼20% at the highest stretch and at the 0.50 μM concentration of mavacamten (2-way ANOVA, interaction P = 0.0010) by ∼60% at the highest stretch. Such findings suggest the presence of a significant crossbridge-based force that contributes to the diastolic stiffness properties of cardiac tissues.
Figure 4.
Force-length characteristics of baseline and mavacamten-treated engineered heart tissues (EHTs). A: representative trace of full-force recording of an EHT undergoing constant slow linear stretch from 0.95 to 1.10 stretch while being stimulated at 1 Hz. B: representative diastolic force-length trace of an EHT from 0.96 to 1.10 stretch ratio at 1-Hz stimulus with and without 0.33 µM mavacamten acute treatment. C: representative diastolic force-length trace of an EHT from 0.95 to 1.10 stretch ratio at 1-Hz stimulus with and without 0.5 µM mavacamten acute treatment. D: 0.33 µM mavacamten significantly altered the effect of stretch on diastolic force (n = 5, 2-way ANOVA, repeated measures by stretch and by drug treatment, interaction P < 0.0001). E: 0.50 µM mavacamten significantly altered the effect of stretch on diastolic force (n = 7, 2-way ANOVA, repeated measures by stretch and by drug treatment, interaction P = 0.0010). F: representative active force-length trace of an EHT from 0.96 to 1.10 stretch ratio at 1-Hz stimulus with and without 0.33 µM mavacamten acute treatment. G: representative active force-length trace of an EHT from 0.95 to 1.10 stretch ratio at 1-Hz stimulus with and without 0.50 µM mavacamten acute treatment. H: 0.33 µM mavacamten significantly altered the effect of stretch on the Frank-Starling active force relationship (n = 5, 2-way ANOVA, repeated measures by stretch and by drug treatment, interaction P < 0.0001). I: 0.50 µM mavacamten significantly altered the effect of stretch on the Frank-Starling active force relationship (n = 7, 2-way ANOVA, repeated measures by stretch and by drug treatment, interaction P < 0.0001). J: mavacamten at 0.50 µM reduced the slope of the Frank-Starling active force relation at lower stretch (n = 7, paired t test, P < 0.0001 at 0.96 stretch ratio). *P < 0.05, ***P < 0.001, and ****P < 0.0001.
Mavacamten also exhibited surprising effects on the length dependence of active force (Fig. 4, F and G). When active force was normalized by active force at initial stretch to quantify the Frank-Starling peak active force-length relationship (Fig. 4H), 0.33 μM mavacamten treatment was found to increase the gain of force by stretch despite resulting in an overall decrease in contractility (2-way ANOVA, interaction P < 0.0001), enhancing Frank-Starling response on average ∼2.5-fold at the highest stretch. However, at the highest concentration of mavacamten of 0.50 μM, the stretch dependence of peak force is altered in a more complex fashion (Fig. 4I) (2-way ANOVA, interaction P < 0.0001). The relationship was found to be biphasic in nature. At low stretch, mavacamten seemed to prevent any additional muscle activation. At higher stretch, the slope of the Frank-Starling relationship was similar to that exhibited under predrug conditions (paired t test; (Fig. 4J). As far as we are aware, this is the first evidence suggesting that mavacamten modulates myosin activity in a stretch-dependent way. This finding also gives indirect support to recent work suggesting that that force feedback, in addition to strain of crossbridges and myofilament constituents, contributes to cardiac length-dependent activation (21, 22).
Mavacamten Alters Preload and Afterload Dependence of Cardiac Power and Work Output
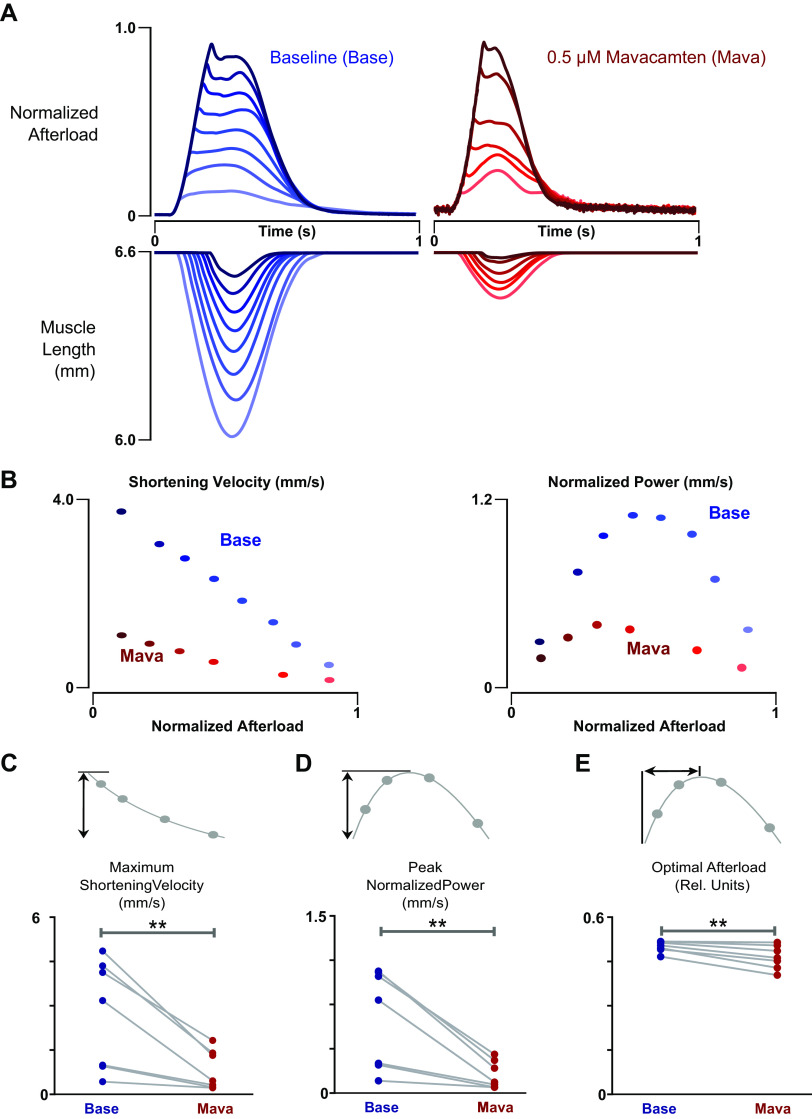
Using adaptive control, we examined for the first time the effect of mavacamten on power production using isotonic load clamp (Fig. 5A). These experiments were conducted at the 0.50 μM concentration of mavacamten in order to ensure that most of the myosins have a drug bound to them, demonstrating the intrinsic effect of the drug without competing indirect effects such as possible cooperative activation. As expected, mavacamten significantly depressed the maximum shortening velocity and peak power output of EHTs (Fig. 5B). However, on closer examination of the power curve parameters (Fig. 5C-E), mavacamten can be seen to reduce optimal afterload by ∼4% in EHTs, shifting the power output curve to the left.
Figure 5.
Load-velocity and power characteristics of baseline and mavacamten treated engineered heart tissues (EHTs). A: representative isotonic twitches of an EHT at 1 Hz and 10% stretch with and without 0.5 µM mavacamten acute treatment. Note that the force records have been normalized to the peak isometric force under the respective conditions. Shading of each force response matches its corresponding length transient shown on the axis immediately below. B: representative shortening velocity and power versus afterload measurements of an EHT at 1 Hz and 10% stretch with and without 0.5 µM mavacamten acute treatment. C: mavacamten reduced maximum shortening velocity (Vmax) by 1.9 mm/s in EHTs (n = 7, paired t test, P = 0.0094). D: mavacamten reduced peak normalized power output (Pmax) of EHTs by 0.47 mm/s (n = 7, paired t test, P = 0.0077). E: mavacamten reduced optimal afterload (Fopt) by ∼4% in EHTs (n = 7, paired t test, P = 0.0066). **P < 0.01.
We also investigated the effect of mavacamten on work production in EHTs (Fig. 6A). Surprisingly, 0.5 µM mavacamten not only depressed the amount of work done but also modified the dependence of work on afterload in EHTs (Fig. 6B) (2-way ANOVA, interaction P = 0.0032). Although unexpected, this could perhaps be an effect of decreased force feedback as an effect of mavacamten on myosin itself. Of great clinical relevance is assessment of the end systolic force-length relation (ESFLR), which is known to be a scalar assessment of the inotropic state of the heart integrative of many factors. We conducted work loops under varying preloads (Fig. 5C) in order to study ESFLR and fit a linear regression to the ESFLR to determine its slope (Fig. 5D). Not unexpectedly, mavacamten decreased the slope of the ESFLR in EHTs (P = 0.0082; Fig. 5E). Overall, this demonstrates that mavacamten reduces the inotropic state of the heart, irrespective of preload and afterload.
Figure 6.

Work loop characteristics of baseline and mavacamten-treated engineered heart tissues (EHTs). A: representative work loops at a preload of 6.6 mm in an EHT before and after treatment with 0.5 µM acute mavacamten. B: mavacamten modifies the dependence of work on afterload in EHTs (n = 7, 2-way ANOVA, repeated measures by stretch and by drug treatment, interaction P = 0.0032). C: representative work loops of an EHT at different preloads before and after 0.5 µM acute mavacamten treatment. D: representative linear fits and measurements of end-systolic force and corresponding end-systolic length of an EHT before and after mavacamten treatment. E: mavacamten decreases the slope of the end-systolic force-length relation in EHTs (n = 6, paired t test, P = 0.0082). **P < 0.01.
DISCUSSION
We present here the first system of which we are aware that is capable of characterizing human engineered heart tissue behavior under a comprehensive set of isometric, isotonic, and working conditions. These assays augment the utility of iPSC-CMs for several applications, including disease modeling, developmental biology, regenerative medicine, and drug testing (3). In this case, the system was used to undertake thorough characterization of the effects of the drug mavacamten, revealing new features of its effects on cardiac contractility.
Power and work output measurements in EHTs in the presence of mavacamten reveal that this drug may address at least two purported pathomechanisms of HCM. First, it counteracts diastolic dysfunction by modulating both diastolic myocardial stiffness and by improving active lusitropic function of cardiac muscles, as determined by isometric and length-dependent measurements of active and diastolic force. Second, mavacamten reduces work and maximum power output with clear potential to ease the energy deficit that is hypothesized to exist in hypertrophic myocardium (23, 24).
Careful testing of the length dependence of twitch force yielded perhaps the most interesting finding of this study. The active force-length relation, i.e., the Frank-Starling curve, becomes biphasic when treated with the higher regime of mavacamten used here. Specifically, under 0.50 μM mavacamten treatment, the slope of the Frank-Starling curve is close to zero near its resting length. However, after surpassing a 1.05 stretch ratio (∼5% stretch), the muscle regains its length sensitivity, the slope of the length-force curve thereafter mirroring the untreated value. Such an unexpected biphasic response to stretch with mavacamten may result from modulation of the super-relaxed (SRX) state of myosin.
The role of the SRX state in cardiac muscle physiology is a relatively new concept (25, 26), its importance gradually coming into focus thanks to structural and computational studies (21, 22, 27, 28). These studies show that the thick filament structural changes involved in the Frank-Starling relationship depend on dynamic recruitment from SRX state through a mechanism that involves sensing of force and not simply length. Mavacamten seemingly can enhance persistence of the SRX state, which in our hands results in biphasic Frank-Starling relationships as well as blunting the afterload and preload dependence of work and power. However, it appears that once the force rises to a sufficient level, SRX crossbridges can be recruited, which has been suggested in ex vivo demembranated cardiac preparation as well (29) but not yet observed in intact human muscle until this present work.
The importance of assessing power and work to give a comprehensive picture of mechanical properties of cardiac tissue are clear even in the seminal works establishing much of the in vitro and ex vivo assessment of cardiac function by Sonnenblick (7) some 50 years ago. Indeed, historic findings (7) show that the load-velocity and load-power relationships set the maximum and minimum inotropic states of the heart under various conditions. However, for any state of inotropy, the actual work of the heart depends on the afterload of the muscle (30, 31). Our techniques developed here allow us to investigate this in engineered heart tissues for the first time and may prove useful in future applications.
At the same time, there are limitations to these methods that should be noted. Foremost is that EHTs still cannot claim complete developmental maturity such as an adult metabolic profile and full adrenergic responsiveness (32), which may somewhat limit full applications of these techniques to unraveling drug responses targeting those pathways. Nevertheless, there have been significant advances in engineered heart tissue technology over the years (33), including the achievement of adult human-like myosin isoform profiles when proper loading conditions are given (34), which are the same conditions employed in the present work. As a consequence, the twitch kinetic parameters compare favorably with those reported in native human trabeculae (35). That said, there are practical limits to the density of cardiomyocytes that can be achieved by reseeding decellularized myocardium, and as such, the ratio of active to passive stress in these EHTs is substantially lower than what would be observed in native tissue. Furthermore, the peak active stress in these EHTs, roughly 1.5 kPa, is only about one-twentieth that of native human myocardium. Although further work is needed to determine the full translatability of our findings to adult human heart muscle, we feel nevertheless that approaches presented here show a robust basis for future work and generation of clinically relevant insights.
Therefore, mavacamten may permit some cardiac reserve for patients taking the drug. In fact, as shown at the lower concentration of mavacamten, the enhancement of length-dependent augmentation in contractility indeed could further increase cardiac output in response to increased filling (36). This is certainly a desirable if unexpected property for a contraction inhibitor. It is interesting to consider the potential implications of these findings on in vivo hemodynamics. As mavacamten eases crossbridges contributing to diastolic stiffness, the increasingly compliant ventricle permits greater filling. With the Frank-Starling behavior still intact at longer fiber lengths, the enhanced filling still elicits an appropriate augmentation in cardiac output. Such findings suggest an explanation for the clinical scenarios encountered in PIONEER-HCM, in which a relatively acute mild to moderate drop in ejection fraction does not precipitate clinical symptoms or biological markers of heart failure, as cardiac output may be preserved or even increased (20).
GRANTS
This work was supported by following sources of funding: National Institute of Health Grant R01-HL-136590 (to S.G.C); a P.D. Soros Fellowship for New Americans, National Institute of Health/National Institute of General Medical Sciences Medical Scientist Training Program Grant (T32GM007205), and an American Heart Association Predoctoral Fellowship (to L.R.S).
DISCLOSURES
S.G.C. has equity ownership in Propria LLC, which has licensed EHT technology used in the research reported in this publication. This arrangement has been reviewed and approved by the Yale University Conflict of Interest Office. L.R.S. and S.S. report no conflict of interest.
AUTHOR CONTRIBUTIONS
L.R.S., S.S., and S.G.C. conceived and designed research; L.R.S. and S.S. performed experiments; L.R.S. analyzed data; L.R.S., S.S., and S.G.C. interpreted results of experiments; L.R.S. prepared figures; L.R.S. drafted manuscript; L.R.S., S.S., and S.G.C. edited and revised manuscript; L.R.S., S.S., and S.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Thomas J. Ryan for assistance with setting up the 322C motor. We thank J. Alex Clark and Aydin Akyol for helping with design and fabrication of the motor mount.
REFERENCES
- 1.Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res 117: 80–88, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter G, Williams SG, Vered Z, Tan LB. Role of cardiac power in heart failure. Curr Opin Cardiol 18: 215–222, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Sewanan LR, Campbell SG. Modelling sarcomeric cardiomyopathies with human cardiomyocytes derived from induced pluripotent stem cells. J. Physiol 598: 2909–2922, 2019. doi: 10.1113/JP276753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iribe G, Helmes M, Kohl P. Force-length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load. AJP Heart Circ Physiol 292: H1487–H1497, 2007. doi: 10.1152/ajpheart.00909.2006. [DOI] [PubMed] [Google Scholar]
- 5.Katz AM. Influence of altered inotropy and lusitropy on ventricular pressure-volume loops. J. Am. Coll. Cardiol, 1988. doi: 10.1016/0735-1097(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 6.Sela G, Landesberg A. The external work-pressure time integral relationships and the afterload dependence of Frank-Starling mechanism. J Mol Cell Cardiol 47: 544–551,2009. doi: 10.1016/j.yjmcc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenblick EH. Force-velocity relations in mammalian heart muscle. Am J Physiol 202: 931–939, 1962. doi: 10.1152/ajplegacy.1962.202.5.931. [DOI] [PubMed] [Google Scholar]
- 8.Helmes M, Najafi A, Palmer BM, Breel E, Rijnveld N, Iannuzzi D, van der Velden J. Mimicking the cardiac cycle in intact cardiomyocytes using diastolic and systolic force clamps; measuring power output. Cardiovasc Res 111: 66–73, 2016. doi: 10.1093/cvr/cvw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung E, Diffee GM. Effect of aging on power output properties in rat skinned cardiac myocytes. J Gerontol A Biol Sci Med Sci 66A: 1267–1273, 2011. doi: 10.1093/gerona/glr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herron TJ, Korte FS, Mcdonald KS, Mc KS, Mc-Donald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression [Online]. Am J Physiol Hear Circ Physiol 281: 1217–1222, 2001. www.ajpheart.org [8 Jul. 2018]. [DOI] [PubMed] [Google Scholar]
- 11.Starling EH, Visscher MB. The regulation of the energy output of the heart. [Online]. J Physiol 62: 243–261, 1927. doi: 10.1113/jphysiol.1927.sp002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng R, Manring H, Papoutsidakis N, Albertelli T, Tsai N, See CJ, Li X, Park J, Stevens TL, Bobbili PJ, Riaz M, Ren Y, Stoddard CE, Janssen PML, Jared Bunch T, Hall SP, Lo YC, Jacoby DL, Qyang Y, Wright N, Ackermann MA, Campbell SG. Patient mutations linked to arrhythmogenic cardiomyopathy enhance calpain-mediated desmoplakin degradation. JCI Insight 4: e128643, 2019. doi: 10.1172/jci.insight.128643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc 8: 162–175, 2013. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, Fukuda K. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12: 127–137, 2013. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Schwan J, Kwaczala AT, Ryan TJ, Bartulos O, Ren Y, Sewanan LR, Morris AH, Jacoby DL, Qyang Y, Campbell SG. Anisotropic engineered heart tissue made from laser-cut decellularized myocardium. Sci Rep 6: 32068, 2016. doi: 10.1038/srep32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sewanan LR, Schwan J, Kluger J, Park J, Jacoby DL, Qyang Y, Campbell SG. Extracellular matrix from hypertrophic myocardium provokes impaired twitch dynamics in healthy cardiomyocytes. JACC Basic Transl Sci 4: 495–505, 2019. doi: 10.1016/j.jacbts.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson JN, Hunter WC, Berman MR. Control of segment length or force in isolated papillary muscle: an adaptive approach. Am J Physiol 256: H1726–H1734, 1989. doi: 10.1152/ajpheart.1989.256.6.H1726. [DOI] [PubMed] [Google Scholar]
- 18.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc London Ser B - Biol Sci 126: 136–195, 1938. [Google Scholar]
- 19.Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, Song Y, Wan W, Leinwand LA, Spudich JA, McDowell RS, Seidman JG, Seidman CE. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351: 617–621, 2016. doi: 10.1126/science.aad3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten treatment for obstructive hypertrophic cardiomyopathy a clinical trial. Ann Intern Med 170: 741–748, 2019. doi: 10.7326/M18-3016. [DOI] [PubMed] [Google Scholar]
- 21.Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, De Tombe PP. Titin strain contributes to the frank-starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci U S A 113: 2306–2311, 2016. doi: 10.1073/pnas.1516732113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kampourakis T, Sun YB, Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci U S A 113: E3039–E3047, 2016. doi: 10.1073/pnas.1602776113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Velden J, Witjas-Paalberends ER, Stienen GJM, Dos Remedios C, Ten Cate FJ, Ho CY, Michels M, Poggesi C. Increased energy utilization for force generation in human familial hypertrophic cardiomyopathy caused by sarcomere gene mutations. Eur. Hear. J 34: P4192–P4192, 2013. doi: 10.1093/eurheartj/eht309.P4192. [DOI] [Google Scholar]
- 24.Witjas-Paalberends ER, Güclü A, Germans T, Knaapen P, Harms HJ, Vermeer AMC, Christiaans I, Wilde AAM, DosRemedios C, Lammertsma AA, Van Rossum AC, Stienen GJM, Van Slegtenhorst M, Schinkel AF, Michels M, Ho CY, Poggesi C, Van Der Velden J. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc Res 103: 248–257, 2014. doi: 10.1093/cvr/cvu127. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, Seidman JG, Ruppel KM, Irving TC, Cooke R, Green EM, Spudich JA. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A 115: E8143–E8152, 2018. doi: 10.1073/pnas.1809540115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawas RF, Anderson RL, Bartholomew Ingle SR, Song Y, Sran AS, Rodriguez HM. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem 292: 16571–16577, 2017. doi: 10.1074/jbc.M117.776815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell KS, Janssen PML, Campbell SG. Force-dependent recruitment from the myosin off state contributes to length-dependent activation. Biophys J 115: 543–553, 2018. doi: 10.1016/j.bpj.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: A potential cardioprotective mechanism in the heart. Biophys J 100: 1969–1976, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awinda PO, Bishaw Y, Watanabe M, Guglin MA, Campbell KS, Tanner BCW. Effects of mavacamten on Ca2+ sensitivity of contraction as sarcomere length varied in human myocardium. Br. J. Pharmacol 177: 5609–55621, 2020. doi: 10.1111/bph.15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez ML, Werner TR, Becker B, Eschenhagen T, Hirt MN. Magnetics-based approach for fine-tuning afterload in engineered heart tissues. ACS Biomater Sci Eng 5: 3663–3675, 2019. doi: 10.1021/acsbiomaterials.8b01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnenblick EH, Downing SE. Afterload as a primary determinat of ventricular performance. Am J Physiol 204: 604–610, 1963. doi: 10.1152/ajplegacy.1963.204.4.604. [DOI] [PubMed] [Google Scholar]
- 32.Chung J-H, Martin BL, Canan BD, Elnakish MT, Milani-Nejad N, Saad NS, Repas SJ, Schultz JEJ, Murray JD, Slabaugh JL, Gearinger RL, Conkle J, Karaze T, Rastogi N, Chen M-P, Crecelius W, Peczkowski KK, Ziolo MT, Fedorov VV, Kilic A, Whitson BA, Higgins RSD, Smith SA, Mohler PJ, Binkley PF, Janssen PML. Etiology-dependent impairment of relaxation kinetics in right ventricular end-stage failing human myocardium. J Mol Cell Cardiol 121: 81–93, 2018. doi: 10.1016/j.yjmcc.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556: 239–243, 2018. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng R, Sewanan L, Stankey P, Li X, Qyang Y, Campbell S. Shortening velocity causes myosin isoform shift in human engineered heart tissues. Circ Res 128: 281–283, 2020. doi: 10.1161/CIRCRESAHA.120.316950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung J-H, Milani-Nejad N, Davis JP, Weisleder N, Whitson BA, Mohler PJ, Janssen PML. Impact of heart rate on cross-bridge cycling kinetics in failing and nonfailing human myocardium. Am J Physiol Heart Circ Physiol 317: H640–H647, 2019. doi: 10.1152/ajpheart.00163.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson LW, Tillisch JH. Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation 74: 1303–1308, 1986. doi: 10.1161/01.cir.74.6.1303. [DOI] [PubMed] [Google Scholar]