Abstract
Recent work identified Gpr160 as a candidate receptor for cocaine- and amphetamine-regulated transcript peptide (CARTp) and described its role in pain modulation. The aims of the present study were to determine if Gpr160 is required for the CARTp’s ability to reduce food intake and water intake and to initially identify the distribution of Gpr160-like immunoreactivity (Gpr160ir) in the rat brain. A passive immunoneutralization approach targeting Gpr160 was used to block the behavioral effects of a pharmacological dose of CARTp in the fourth cerebroventricle (4V) of rats and to determine the importance of endogenously produced CARTp in the control of ingestive behaviors. Passive immunoneutralization of Gpr160 in the 4V blocked the actions of CARTp to inhibit food intake and water intake. Blockade of Gpr160 in the 4V, independent of pharmacological CART treatment, caused an increase in both overnight food intake and water intake. The decrease in food intake, but not water intake, caused by central injection of CARTp was demonstrated to be interrupted by prior administration of a glucagon-like peptide 1 (GLP-1) receptor antagonist. Gpr160ir was observed in several, distinct sites throughout the rat brain, where CARTp staining has been described. Importantly, Gpr160ir was observed to be present in both neuronal and nonneuronal cell types. These data support the hypothesis that Gpr160 is required for the anorexigenic actions of central CARTp injection and extend these findings to water drinking. Gpr160ir was observed in both neuronal and nonneuronal cell types in regions known to be important in the multiple pharmacological effects of CARTp, identifying those areas as targets for future compromise of function studies.
Keywords: cocaine- and amphetamine-regulated transcript, food intake, glial cells, GLP-1, Gpr160, water intake
INTRODUCTION
In 1981, Vale’s group identified a novel, somatostatin-like peptide in ovine hypothalamus (1). Over a decade later, their discovery was recognized to be the protein encoded by a novel mRNA sequence upregulated in rat brain following intraperitoneal injection of psychostimulants (2, 3). Cocaine- and amphetamine-regulated transcript peptide (CARTp), the peptide encoded by that transcript, was found by immunohistochemistry to be present in multiple brain areas relevant for energy balance (4–8). When administered into the cerebroventricular system of rodents, CARTp significantly decreased food intake (9–11), apparently via downstream engagement of glucagon-like peptide 1 (GLP-I) system (12), although it was possible that CRF-expressing neurons were targets as well (13). Importantly, passive immunoneutralization of endogenous CARTp via ventricular antibody administration (14) or intranuclear injection into the nucleus tractus solitarius (NTS) (15) resulted in increased food intake, strongly suggesting a physiologically relevant role for the peptide in feeding behavior.
Multiple sites of action of CARTp have been suggested (12, 16–20), and although the majority of the literature favors an anorexigenic action of CARTp, orexigenic responses to pharmacological administration have been reported (17–30). Genetic models, however, have supported the anorexigenic role of CARTp (31–33). Furthermore, polymorphisms in the human CART gene have been linked to obesity, as well as anxiety and depression (34–40). This has led to the hypothesis that CARTp or analogs might have clinical potential, but the development of suitable CARTp-based therapeutics has languished due to the absence of an identified receptor for the peptide.
Multiple studies (10, 12, 14, 41–47) have suggested that the CARTp receptor(s) is (are) a G protein-coupled receptor (GPCR). To date, over 140 GPCRs remain “orphan receptors,” that is, receptors with an unknown ligand (48, 49). GPCRs are targets for at least one-third of all FDA-approved drugs (48, 49). To achieve the suggested potential for CARTp to be the endogenous ligand from which effective analogs might be designed for the treatment of eating disorders, the cognate receptor had to be identified. We have developed a novel approach to match orphan receptors to their cognate ligands (50). Using our deductive reasoning strategy, we have identified receptors for neuronostatin, proinsulin-connecting peptide, adropin, and phoenixin (50–53). We recently used the strategy to identify the previously orphaned GPCR Gpr160 to be a cognate receptor for CARTp and demonstrated its importance in the regulation of pain (54). Here, we present preliminary evidence for the wide distribution of Gpr160ir expression in brain and demonstrate its importance for the inhibitory effect of CARTp on food and water intakes.
METHODS
Animals and Surgery
All procedures and protocols have been approved by the Saint Louis University Animal Care and Use Committee Protocol No. 2564. Adult male rats (Harlan/Envigo Sprague-Dawley; 225–250 g upon receipt) were housed in groups of three for minimally 4 days of quarantine and then individually housed following surgery with free access to food and water, unless otherwise indicated, under controlled conditions (23°C–25°C, lights on 0600–1800 h).
Animals were anesthetized with a mixture of ketamine (70 mg/kg; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (9 mg/kg; TranquiVed, Vedco, St. Joseph, MO). Buprenorphine LAB SR (1.0 mg/kg; Reckitt & Coleman, Richmond, VA) was administered subcutaneously before surgery. A stainless-steel cannula (23 gauge, 17 mm) was implanted into the fourth cerebroventricle (4V) with the aid of a stereotaxic device. Animals were allowed to recover to presurgery body weight before use (minimum 5 days). Patency and placement of the cannula were determined by fourth ventricle injection of 2-deoxyglucose as previously described (55). Blood glucose was measured by tail snip before and 1 h after injection. Animals displaying a 1.5- to 2-fold increase in blood glucose were used.
Ingestive Behavior Studies
Four protocols were used to determine the role of Gpr160 in CARTp-induced reduction of food intake. Rats (240–300 g following initial quarantine and recovery from surgery) were first habituated to metabolic cages (n = 8, Nalgene; Harvard Apparatus, Holliston, MA) for 3 days with free access to laboratory chow (Cat. No. 1811156, Test Diet, Richmond, IN) and tap water. Food and water intakes and body weights were recorded daily.
Gpr160 Antibody
The antibody (Pa5-33650, Thermo Fisher Scientific, Waltham, MA) was previously validated by our group using a combination of in vivo and in vitro approaches (54).
Protocol 1 (pharmacology).
Passive immunoneutralization was employed using Gpr160 antiserum injected into the 4V of rats. This antibody targets the second extracellular loop to prevent CARTp from accessing potential binding sites. Detailed validation of antibody specificity is presented in our recent manuscript on the role of CARTp in neuropathic pain perception (54). Food and water were removed just before injection of 2 µL of saline containing 2 µg of Gpr160 antiserum or IgG control into the 4V at 1700 h, followed by injection of CARTp (CART 55-102, Phoenix Pharmaceuticals, Burlingame, CA) or vehicle at 1730 h. Food and water were zeroed and replaced at 1800 h. Food and water intakes were recorded every 30 min between 1800 h and 2000 h and again at 0800 h the following day.
Protocol 2 (physiology).
The passive immunoneutralization approach again was performed using Gpr160 antiserum independent of subsequent CARTp treatment to examine the effect of passive immunoneutralization on “lights out” feeding and drinking. Food and water were removed at 1600 h. A single injection of 2 µL of saline containing 2 µg of Gpr160 antiserum or control IgG was administered at 1700 h. Food and water were zeroed and replaced at 1730 h. Food and water intakes were recorded every 30 min between 1730 h and2000 h and again at 0800 h the following day.
Protocol 3 (physiology).
The third protocol used two antiserum injections independent of subsequent pharmacological CARTp treatment, again to examine the effect of passive immunoneutralization on “lights out” feeding and drinking. Food and water were removed at 1600 h. Injection of the first 2 µg of antiserum or control IgG was administered at 1715 h. Food and water were zeroed and replaced at 1730 h followed by a second 2-µg fourth ventricle antiserum or control IgG injection at 1745 h. Food and water intakes were recorded every 30 min from 1730 h to 2000 h and again at 0800 h the following day.
Protocol 4 (pharmacology).
Food and water were removed just before subjecting rats to 4V injection of 20 µg (in 2 µL) of the GLP-1 antagonist, exendin-3 (9-39) (Cat. No. 070–93, Phoenix Pharmaceuticals) or control, followed 30 min later by injections of 1 µg of CARTp or control. Food and water were replaced at 1730 h, and intakes were monitored for 2 h.
Astrocyte/Microglia Purification
One T75 flask/five rats was coated with poly-l-lysine, overnight at 37°C. Poly-l-lysine was removed, and flasks were washed one time with sterile water. Two 100-mm plates were prepared, one filled with 25 mL of cold PBS and one with 5 mL of cold complete media [high-glucose Dulbecco’s modified Eagle’s medium (DMEM), 10% heat-inactivated fetal bovine serum, 10% heat-inactivated horse serum, 1% penicillin-streptomycin (5,000 U/mL), all Thermo Fisher Scientific], and kept on ice. Rats were euthanized by rapid decapitation, and whole brains were removed and placed in the plate containing PBS on ice. The brainstem dorsal vagal complex was isolated in a dish containing cold media, finely chopped, and transferred into a 15-mL conical tube. The resulting tissue minces were vigorously triturated using a series of three pipettes, starting from large to small diameter until homogenous. Remaining pieces were allowed to settle for ∼3 min. The suspension was transferred to a second conical tube, and the previous steps were repeated for a total of three times to remove large particles. After the third separation was completed, warm medium was added to fill the conical tube. The cell suspension was transferred to poly-l-lysine-coated T75 flask. Medium was changed every 2–3 days until cells neared confluence, then the flask was shaken at 250 rpm overnight in a 37°C tissue culture incubator. The following day, the speed was increased to 500 rpm for 4 h, after which the supernatant was spun at 150 g for 5 min (room temperature). This shaking step was able to detach the less adherent microglia. The supernatant containing microglia was then plated in a separate poly-l-lysine-coated T75 flask. The more tightly adherent astrocytes remained attached to the flask. Fresh medium (high-glucose DMEM, 10% FBS, 10% horse serum, 1% penicillin/streptomycin) was added to the flask containing the attached cells.
Astrocyte/Microglia Staining
Cells were plated on poly-l-lysine-coated cover slips in a six-well plate. Cells were fixed with 4% PFA for 10 min at room temperature and then washed three times with PBS before being stored in PBS at 4°C until processed. Blocking solution (PBS, 0.3% Triton X-100, 10% normal donkey serum) was added for 2 h at room temperature. Cells were then incubated with primary antibody in PBS 0.3% Triton X-100 for 24 h at room temperature at the proper dilution [glial fibrillary acidic protein (GFAP) Abcam ab53554 1:5,000; Iba1 Abcam ab5076 1:1,000; NeuN Millipore MAB377 1:1,000; vimentin Abcam ab137321 1:1,000; Gpr160 Invitrogen PA5-33650 1:500). After three PBS washes, cells were incubated with secondary antibodies at a 1:300 dilution for 2 h at room temperature. They then were washed three times with PBS, and cover slips were mounted on microscope slides using ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA).
Immunohistochemistry
Sprague-Dawley rats weighing 225–275 g were injected with pentobarbital (390 mg/mL, 0.1 mL/100g body wt barbiturate overdose). Rats then were transcardially perfused with 300 mL of PBS, followed by 500 mL of freshly prepared, filtered, 4% PFA. Brains were harvested and incubated in 4% PFA for 1 h before being transferred to a 30% sucrose solution until processing. The brains were sectioned into free-floating 30-µm sections using a CM1950 Cryostat (Leica, Wetzlar, Germany) and stored at −20° in a cryoprotectant solution containing 15% sucrose and 30% ethylene glycol in PBS until further processing. Sections were removed from cryoprotectant and washed three times for 5 min each in PBS. Sections then were blocked for 2 h in PBS containing 0.3% Triton X-100 and 10% normal donkey serum, followed by incubation with anti-Gpr160 (Pa5-33650, Thermo Fisher Scientific, Waltham, MA) overnight at a dilution of 1:500 in PBS containing 0.3% Triton X-100 at room temperature. The following day, sections were washed three times for 5 min in PBS, followed by 2-h incubation with secondary donkey anti-rabbit Alexa594 (Jackson Immunoresearch, West Grove, PA) diluted 1:300 in PBS containing 0.3% Triton X-100 while protected from light. After incubation with the secondary antibody, sections were washed three times for 5 min in PBS and mounted on microscope slides using ProLong Gold antifade reagent with DAPI (Thermo Fisher Scientific, Waltham, MA). Slides were imaged on a TCS SP8 confocal microscope (Leica, Wetzlar, Germany).
Statistical Analyses
For in vivo protocols, when comparing results from several groups, data were analyzed by ANOVA with Tukey’s multiple comparisons. When data from only two experimental groups were compared, an independent Student’s t test was used. All data are presented as means ± SE and considered statistically significant if P was ≤0.05.
RESULTS
Passive Immunoneutralization of Gpr160 Blocks the Anorexigenic and Antidipsogenic Effects of CART Peptide in Brainstem
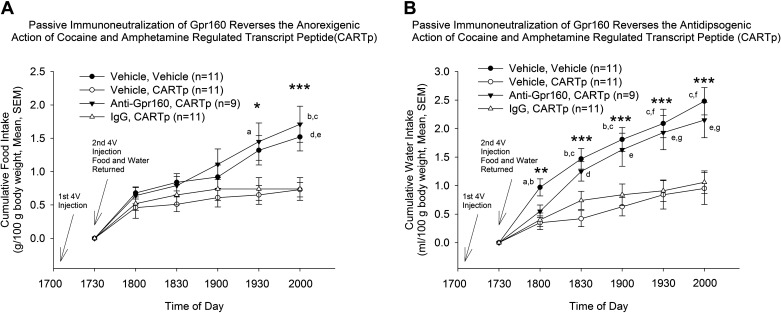
Recent work demonstrated a Gpr160-dependent role of CARTp (54). We, therefore, hypothesized that Gpr160 signaling is required for CARTp-induced anorexia and used an in vivo passive immunoneutralization strategy to test this hypothesis. Fourth ventricle injection of CART peptide resulted in a significant decrease in both food intake (Fig. 1A) and water intake (Fig. 1B). Passive immunoneutralization of Gpr160 in the 4V prevented the anorexigenic and antidipsogenic effects of a subsequent 4V of CARTp (Fig. 1, A and B). There were no statistically significant differences in body weights (g) among the four test groups (Veh/Veh: 265 ± 8, Veh/CARTp: 258 ± 4, anti-Gpr160/CARTp: 261 ± 7, IgG/CARTp: 253 ± 5; F3,38 = 0.723, P = 0.55).
Figure 1.
Passive immunoneutralization of Gpr160 blocks cocaine- and amphetamine-regulated transcript peptide (CARTp)-induced reduction in ingestive behaviors. A: 4 V administration of 2 μg anti-Gpr160 (PA5-33650) prevents the effect of an anorexigenic dose of CARTp injected into the 4 V (ANOVA: *P < 0.05, F3,38 = 3.89; ***P < 0.001, F3,38 = 7.07). Multiple comparisons, Tukey’s: a, P < 0.05 anti-Gpr160/CARTp vs. vehicle/CARTp; b, P < 0.01 anti-Gpr160/CARTp vs. vehicle/CARTp; c, P < 0.001 anti-Gpr160/CARTp vs. IgG/CARTp; d, P < 0.05, vehicle/vehicle vs. vehicle/CARTp; e, P < 0.05, vehicle/vehicle vs. IgG/CARTp. B: 4 V administration of 2 μg anti-Gpr160 (PA5-33650) prevents the effect of an antidipsogenic dose of CARTp injected into the 4 V (ANOVA: **P < 0.01, F3,38 = 4.83; ***P < 0.001, 1830 h F3,38 = 8.81, 1900 h F3,38 = 7.59, 1930 h F3,38 = 7.15, 2000 h F3,38 = 9.06). Multiple comparisons, Tukey’s: a, P < 0.01, vehicle/vehicle vs. vehicle/CARTp; b, P < 0.01 vehicle/vehicle vs. IgG/CARTp; c, P < 0.001 vehicle/vehicle vs. vehicle/CARTp; d, P < 0.01 anti-Gpr160/CARTp vs. vehicle/CARTp; e, P < 0.05, anti-Gpr160/CARTp vs. vehicle/CARTp; f, P < 0.01, vehicle/vehicle vs. IgG/CARTp; g, P < 0.05, anti-Gpr160/CARTp vs. IgG/CARTp.
When the feeding data were analyzed for time versus treatment interactions (two-way ANOVA), a highly significant interactive effect was revealed (F15,228 = 2.86, P < 0.001). The same outcome was revealed when the data were analyzed for time (F5,228 = 161.8, P < 0.0001) and for treatment (F3,228 = 18.76, P < 0.0001). Similarly, when the water drinking data were analyzed for time versus treatment interactions (two-way ANOVA), a highly significant interactive effect was revealed (F15,228 = 2.21, P < 0.01). The same outcome was revealed when the data were analyzed for time (F5,228 = 120.6, P < 0.0001) and for treatment (F3,228 = 22.09, P < 0.0001).
On the following morning, animals exposed to CARTp alone displayed reduced food intake but not water intake across the 18-h sampling period. However, as at the earlier time points, anti-Gpr160-pretreated animals did not respond to CARTp injection with reduced food intake (Table 1).
Table 1.
Cumulative, overnight food and water consumption following fourth ventricle treatments
| Treatment Group | Food (g/100 g Body Wt)F3,38 = 6.85, P < 0.001 | Water (mL/100 g Body Wt)F3,38 = 4.87, P < 0.01 |
|---|---|---|
| Vehicle/vehicle (n = 11) | 5.38 ± 0.32P = 0.01 vs. vehicle/CART | 8.98 ± 0.77P < 0.01 vs. vehicle/CART |
| Vehicle/CART (n = 11) | 3.33 ± 0.41 | 4.83 ± 1.18 |
| Anti-Gpr160/CART (n = 9) | 5.66 ± 0.11P < 0.05 vs. IgG/CART | 7.12 ± 0.61P = 0.50 vs. IgG/CART |
| IgG/CART (n = 11) | 3.71 ± 0.48 | 5.31 ± 0.74 |
Values are means ± SE. ANOVA, Tukey’s multiple comparisons, no significant differences between vehicle/vehicle and anti-Gpr160/CART. CART, cocaine- and amphetamine-regulated transcript.
Effect of Passive Immunoneutralization of Gpr160 in Brainstem on Overnight Food and Water Intake
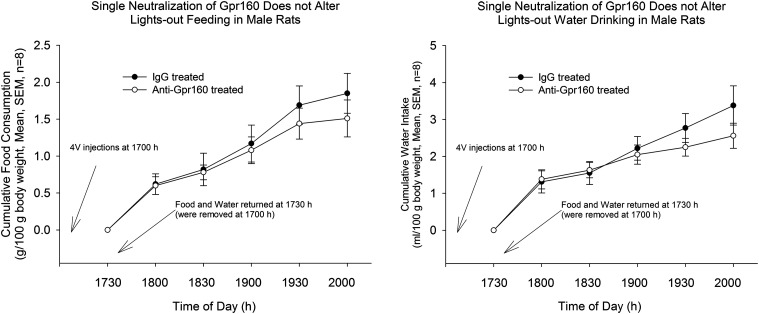
Next, to determine the physiological role of brainstem Gpr160, the passive immunoneutralization approach again was used independently of CARTp injection. Because the pharmacological effect of CARTp administration was reversed by passive immunoneutralization, we hypothesized that Gpr160 antiserum administration before “lights out” would result in increased food intake and water intake compared with controls. A single injection of Gpr160 antiserum (protocol 2) did not lead to a change of ad libitum (lights out) food intake or water intake in rats (Fig. 2).
Figure 2.
Single passive immunoneutralization of Gpr160 fails to alter lights out ingestive behaviors. IgG or anti-Gpr160 (as in Fig. 1) was injected into the 4 V at 1700 h following removal of food and water from the cages. Food and water were returned to the cages at 1730 h and intakes measured at 1800 h and then every 30 min until 2000 h and again the following morning. Results analyzed by unpaired t test, n = 8.
The failure of the same, single dose of anti-Gpr160 that blocked the anorexigenic and antidipsogenic actions of pharmacologically altered ingestive behaviors to significantly alter physiologically driven feeding and drinking suggested that the timing of the neutralization was important. Therefore, we conducted a second experiment in which anti-Gpr160 was administered before food and water return and then 15 min later once feeding and drinking had begun. We reasoned that sufficient antibody would need to be present once ingestion stimulated the endogenous release of CARTp from vagal afferents to significantly dampen physiologically driven food and water consumption. Indeed, that was the case.
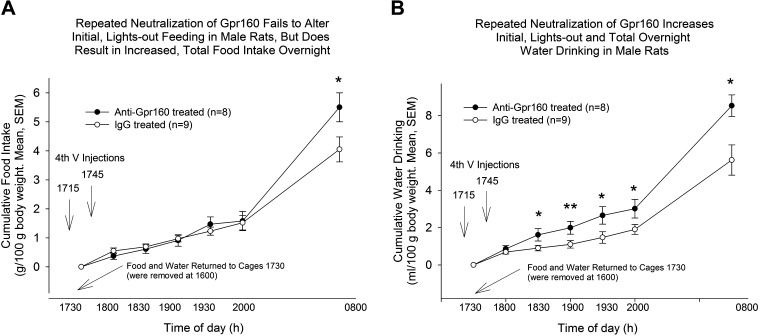
When neutralizing antibody was administered both before (at 1715 h) and shortly after (at 1745 h) food was made available (1730 h), significant effects on ad libitum food intake (P = 0.044) and water consumption (P = 0.012) were observed at the end of the overnight feeding period. During the early observation periods of the lights out phase, no significant effect of anti-Gpr160 on food intake was observed (Fig. 3A); however, water drinking was increased at all time points, with significance attained at all but the first sampling period. *P < 0.05, **P < 0.01 (Fig. 3B).
Figure 3.
Sequential injection passive immunoneutralization of Gpr160. A: sequential fourth ventricle injection passive immunoneutralization with 2 µg of anti-Gpr160 (Pa5-33650) does not affect food intake in male rats during the first 2 h. B: sequential fourth ventricle injection passive immunoneutralization with 2 µg of anti-Gpr160 increases water intake in male rats during the first 2 h. A and B: passive immunoneutralization with 2 µg of anti-Gpr160 increases both overnight food intake and water intake. *P < 0.05, **P < 0.01 vs. IgG pretreatement, unpaired t test, group sizes indicated in the bars.
CARTp-Induced Decrease in Food but Not Water Intake May Be Dependent of Recruitment of Downstream GLP-1 Receptors in Brainstem
Previous research suggests that the anorexigenic effects of lateral cerebroventricle-injected CARTp are dependent on downstream GLP-1 signaling (12). We hypothesized that the anorexigenic effects of fourth ventricle CARTp administration similarly require downstream GLP-1 signaling. To test this hypothesis, a GLP-1 receptor antagonist exendin-3 (9-39) was used to block any potential downstream GLP-1 signaling generated by CARTp. When administered alone into the 4V, 20 µg of exendin-3 (9-39) did not result in any significant change in food intake or water intake (Fig. 4). However, 4V injection of 20 µg of exendin-3 (9-39) reversed the reduction in feeding normally caused by injection of CARTp (Fig. 4A). 4V administration of exendin-3 (9-39) by itself did not significantly alter water drinking compared with controls and did not reverse the antidipsogenic action of CART (Fig. 4B). When intakes were examined the following morning, there were significant differences in food consumption among treatment groups [ANOVA, F3,40 = 5.00, P < 0.01; multiple comparisons, vehicle/vehicle vs. vehicle/CARTp, P < 0.01; vehicle/vehicle vs. exendin-3 (9-39)/CARTp, P < 0.05; vehicle/vehicle vs. exendin-3 (9-39) P < 0.05]. At this time point, there were no significant differences among treatment groups in amounts of water consumed (ANOVA, F3,40 = 1.83, P = 0.158).
Figure 4.
The GLP-1 antagonist exendin-3 (9-39) administered into the 4 V of rats partially reverses the anorexigenic effect of cocaine and amphetamine-regulated transcript peptide (CARTp) (A) but not the antidipsogenic action of CARTp (B). A: ANOVA 1900 h F3,40 = 2.93, *P < 0.05; 1930 h F3,40 = 4.97, **P < 0.01; 2000 h F3,40 = 4.74, **P < 0.01). Multiple comparisons (Tukey’s): a, vehicle/vehicle vs. vehicle/CARTp, P < 0.01; b, vehicle/CARTp vs. exendin-3 (9-39), P < 0.05. t test comparisons of vehicle/CARTp vs. exendin-3 (9-39): c, P < 0.05, d, P < 0.01. B: ANOVA 1930 h F3,40 = 2.82, P = 0.051, 2000 h F3,40 = 3.501, P < 0.05. Multiple comparisons (Tukey’s): a, vehicle/vehicle vs. vehicle/CARTp, P < 0.05.
Gpr160ir Is Expressed Widely Throughout the Rat Brain in Areas Associated with Ingestive Behaviors and Reward
The same antibody used for passive immunoneutralization studies (Abcam Pa5-33650) was used for immunohistochemistry. The specificity of this antibody was validated by our group in a previous study (54). In addition to brainstem nuclei, we examined the localization of Gpr160 immunoreactivity throughout the rat brain (Fig. 5, A–L).
Figure 5.
Rat Gpr160 brain tissue staining. A–L: 30-µm free-floating sections were stained with rabbit anti-Gpr160 (Pa5-33650). Nuclear staining DAPI (blue), Gpr160 staining (red); scale bar = 75 µm, same for all subparts.
Gpr160 immunoreactivity was observed throughout the rostrocaudal extent of the NTS (Fig. 5A). At the level of the AP, dense staining was present lateral to the AP, whereas relatively less staining was present in the commissural NTS. In addition, Gpr160ir was detected diffusely within the AP. Other nuclei known to be important in the regulation of ingestive behaviors where Gpr160ir was observed included the parabrachial nucleus (5C), hypoglossal nucleus (5E), ARC (5B), and PVN (5 D), with broad localization in each of these structures. Gpr160ir was abundantly present in the nucleus accumbens shell (5H), substantia nigra (5I), and amygdala (5 K) and readily detectable in specific cell layers of the hippocampus projecting from the midline and following the hippocampal CA1, CA2, and CA3 areas all the way to the dentate gyrus (Fig. 5F), and the retrochiasmatic area (RCA, Fig. 5L). Gpr160ir was not observed in the nucleus accumbens core (5 G) or the ventral tegmental area (5 J).
Gpr160ir Is Expressed in Glial Cells in Addition to Neurons
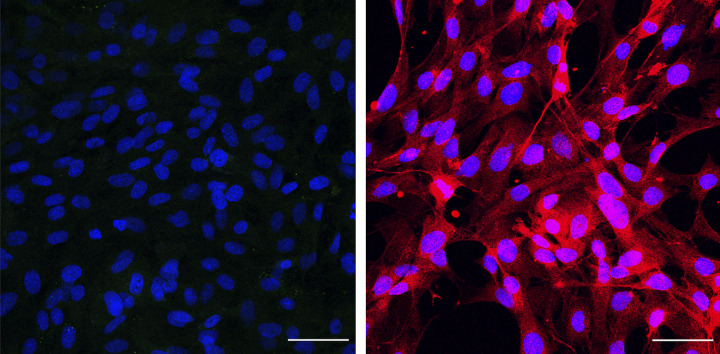
To determine what cell types might express Gpr160ir, coimmunostaining of GPR160 with glial fibrillary acidic protein (GFAP), a marker for astrocytes (Figs. 6 and 7), and ionized calcium-binding adaptor molecule 1 (Iba1), a marker for microglia (Fig. 8, A and B), was performed in cultured cells harvested from rat brainstem and in brainstem tissue slices. In the cultured astrocytes, Gpr160ir colocalization with GFAP was detectable (Fig. 6). However, GFAP and GPR160ir colocalization in brainstem tissue slices was less apparent (Fig. 7).
Figure 6.
Glial fibrillary acidic protein (GFAP) and Gpr160-like immunoreactivity (Gpr160ir) coimmunostaining in cultured astrocytes. Representative image of cultured brainstem astrocytes. Nuclear staining DAPI (blue), Gpr160ir staining (red), GFAP (green), colocalization (yellow). scale bar = 25 µm.
Figure 7.

Glial fibrillary acidic protein (GFAP) and Gpr160-like immunoreactivity (Gpr160ir) coimmunostaining in nucleus tractus solitarius (NTS) tissue section. Representative image of NTS tissue section, nuclear staining DAPI (blue), Gpr160ir staining (red), GFAP astrocyte marker (green).
Figure 8.
Ionized calcium-binding adaptor molecule 1 (Iba1) and Gpr160-like immunoreactivity (Gpr160ir) coimmunostaining in microglia. A: representative image of cultured microglia purified from rat brainstem tissue. B: representative image of rat nucleus tractus solitarius (NTS)/AP brain slice staining. Nuclear staining DAPI (blue), Gpr160ir staining (red), Iba1 microglia marker (green). Inset: scale bar = 5 µm
In cultured microglia, Gpr160ir was detected in almost all cells (Fig. 8A). Colocalization between Gpr160ir and Iba1 was similarly observed in brainstem tissue slices containing the NTS and AP (Fig. 8B). However, in tissue slices, not all microglia exhibited Gpr160ir and not all Gpr160ir was localized to microglia (Fig. 8B).
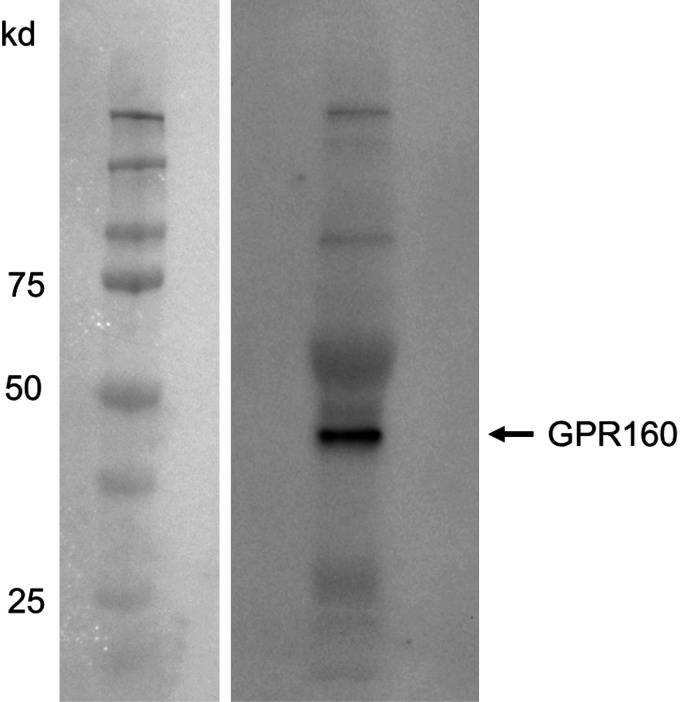
Specific detection of Gpr160ir in cultured microglia was confirmed by Western blot (Abcam ab117074, Fig. 9).
Figure 9.
Western blot of Gpr160 in purified microglia. Five micrograms of protein was subjected to Western blot analyses on 10% SDS-PAGE. Gpr160 antibody (ab117074) was diluted at 1:1,000 and incubated overnight at 4°C. Detected on Chemi Doc Imaging System.
As a control, astrocyte cultures were also stained with NeuN and vimentin to identify other cell types that were copurified with the cultured astrocytes. The primary cultured astrocytes appeared to be copurifying with fibroblasts and not with neurons (Fig. 10, A and B).
Figure 10.
Determining lineage of cells present in astrocyte culture. Left: representative image of background cells identified in astrocyte culture stained for NeuN. Right: representative image of background cells identified in astrocyte culture stained for vimentin. DAPI (blue), NeuN (green), vimentin (red). Scale bar = 50 μm.
To determine if neurons exhibit Gpr160ir in vivo, coimmunostaining with antibodies for GPR160 and NeuN in brain tissue slices was performed (Fig. 11). Colocalization between Gpr160ir and NeuN was detectable in NTS (Fig. 11A). However, the majority of neurons in NTS were not positive for Gpr160ir, as most of the Gpr160ir was localized to nonneuronal cells (Fig. 11A). In the hypoglossal nucleus, a greater percentage of neurons displayed positive Gpr160 immunoreactivity (Fig. 11B). Taken together, these results indicate that Gpr160 is expressed in diverse brain nuclei in multiple cell types.
Figure 11.
NeuN and Gpr160 coimmunostaining in tissue sections. A: representative image of nucleus tractus solitarius (NTS) tissue section. B: representative image of hypoglossal nucleus tissue section. Nuclear staining DAPI (blue), Gpr160-like immunoreactivity (GPR160ir) staining (red), neuronal marker NeuN (green). Scale bar = 75 µm; Inset: scale bar = 3 µm.
DISCUSSION
The previously orphaned GPCR, Gpr160, recently was identified as a receptor for CARTp, and the ligand/receptor pair’s role in the neurotransmission of chronic pain was established (54). We sought to determine if Gpr160 also is required for the anorexigenic actions of CARTp. It is well established that injection of the active form of the CARTp into the rat cerebroventricular system results in a significant reduction in food intake (14, 16, 56). There is still some debate on whether the effect of centrally administered or endogenously produced CARTp is caused by specific satiety signaling or rather a nonspecific aversive state (10, 56). However, CARTp’s expression in the nodose ganglia and release into the NTS, along with its interaction with leptin, ghrelin, and CCK, is suggestive of CARTp’s role as an endogenous satiety factor (28, 30). Another line of evidence in favor of CARTp as an endogenous satiety factor is the fact that in addition to a reduction in food intake, CARTp also reduces gastric emptying, an effect shared by many satiety factors (13, 57). Moran et al. (13) demonstrated that the action of CART administered into the 4V to increase c-Fos expression in the dorsal vagal complex was blocked by administration of a CRF antagonist, suggesting that Gpr160 expression in AP and NTS links CARTp to downstream CRF neurons.
Consistent with the existing literature (56), 4V injection of CARTp resulted in a decrease in food intake in rats (Fig. 1A). Moreover, CARTp administration resulted in a significant decrease in water intake (Fig. 1B), a phenomenon largely unreported in previous lines of research (10, 14, 58). To our knowledge, only one study to date has demonstrated that 4V CARTp injection resulted in a reduction of water intake in rats (56). Another study demonstrated that central CARTp does not result in a reduction of water intake; however, a fragment CART 82–102 was used rather than the well-established bioactive forms (59). Importantly, passive immunoneutralization of Gpr160 prevented a decrease in food intake and water intake caused by CARTp injection (Fig. 1A, 1B), suggesting that CARTp requires Gpr160 to induce its inhibitory effects on food intake and water intake, when CARTp is administered in a pharmacological setting.
To determine the physiological effects of Gpr160 blockade, passive immunoneutralization was used independent of pharmacological CARTp treatment. Previous studies have focused on elucidation of the physiological effects of CARTp using a similar immunoneutralization approach, targeting CART peptide rather than a receptor (14, 15, 58). The first attempt at passive immunoneutralization of Gpr160, using a single injection of antibody, failed to significantly alter food intake or water intake. This may be due to the effects of antibody dilution in the cerebrospinal fluid or due to injection timing relative to the start of the dark-phase feeding period. The second attempt at passive immunoneutralization was modified with one injection before and one injection after returning food and water to the rats, successfully overcoming these limitations (Fig. 3). Thus, we feel that the success of the second passive immunoneutralization protocol (Fig. 3, A and B) was due to the administration of antiserum both before and after the release of endogenous CARTp from vagal afferents.
During the first 2 h after passive immunoneutralization of Gpr160, water intake was increased, whereas food intake was not changed (Fig. 3, A and B). This result was unexpected; however, it is consistent with the one existing study indicating that CART reduced water intake when injected into the fourth ventricle (56). In addition, several previous studies used a liquid diet, making differences in food and fluid intake indistinguishable (10, 12, 13). Although food intake was unchanged during the first 2 h of observation, following the overnight feeding period, Gpr160-immunoneutralized animals displayed significant increases of both food intake and water intake (Fig. 3, A and B).
Interestingly, the kinetics of the change in food intake after passive neutralization of Gpr160 are similar to the kinetics after immunoneutralization of CART peptide in the cerebroventricular system. Lateral ventricle passive immunoneutralization of CART peptide did not result in any changes in food intake during the first 2 h of observation (14). However, during a 12-h (overnight) observation period, passive immunoneutralization of CART peptide resulted in a significant increase in food intake in rats (14), similar to passive immunoneutralization of Gpr160 shown in the present study (Fig. 3A). The authors suggested that CART peptide reduced food intake during the later stages of the feeding period, supporting a role for the peptide as an endogenous satiety factor (14). However, water intake was not reported in this study. It is possible that water intake was increased during the first 2 h after CART peptide neutralization, resulting in a compensatory increase in food intake during the later stages of the feeding period.
Previous research demonstrated that the effects of central CART peptide injection on food intake were dependent on downstream GLP-1 receptor activation (12). Those experiments demonstrated that lateral ventricle injection of a GLP-1 receptor antagonist prevented CART peptide-induced decreases in food intake (12). Our data are consistent with the hypothesis that downstream GLP-1 recruitment may be at least partially responsible for CARTp-mediated reduction of food intake (Fig. 4A). GLP-1 neurons in the brainstem have been established as an important component of acute satiety signaling. AAV-mediated compromise of the GLP-1 receptor has been shown to increase ad libitum food intake by a specific increase in meal size (60). Recent work also has indicated that central or peripheral injection of GLP-1 receptor agonists reduced water intake, both in the presence and absence of food (61). In addition, lateral ventricle injection of a GLP-1 receptor antagonist increased water intake in both ad libitum and water-deprived rats (62). Downstream GLP-1 receptors also were required for c-Fos upregulation in PVN, supraoptic nucleus (SON), NTS and LPBN, indicating that both hypothalamic and brainstem nuclei are activated after lateral ventricle CART peptide injection (12). Our data demonstrate that a GLP-1 receptor antagonist injected into the 4V was sufficient to prevent the anorexigenic actions of a subsequent 4V injection of CART peptide (Fig. 4A), as previously shown after lateral ventricle injection (12). This would suggest that brainstem nuclei are at least partially responsible for the anorexigenic actions of CARTp induced GLP-1 recruitment. That is not to say that hypothalamic nuclei are not involved in the anorectic effect of CARTp in hindbrain feeding centers, as neurons in the brainstem project to the hypothalamus, and it has yet to be determined if this relay is required. We extend these findings to address the CARTp-induced reduction in water intake, revealing that prior administration of exendin-3 (9-39) did not significantly reverse CARTp’s antidipsogenic action (Fig. 4B). It is possible that blockade of CARTp’s antidipsogenic actions would require GLP-1 receptor disruption in the hypothalamus, a region not targeted in our current study, as previous studies demonstrating increased drinking behavior deployed GLP-1 receptor antagonists into the lateral ventricle (62). Alternatively, the antidipsogenic and anorexigenic actions of CARTp, when administered into the 4V, may involve separate as well as shared signaling events.
Gpr160-like immunoreactivity was detected in discrete nuclei throughout the rat brain. Several regions where Gpr160ir was detected overlap with brain regions known to contain CART peptides (4, 5). Importantly, the same Gpr160 antibody used for in vivo passive neutralization was used for staining, and therefore, tissue staining should represent the same binding sites that were disrupted during behavioral experiments. It should be noted that our study was not designed to be a comprehensive survey of Gpr160ir distribution in the whole brain. Instead, we sought to identify sites where Gpr160ir can be visualized that overlap with areas of the brain where actions of CARTp have been reported. These observations will guide future site-specific compromise of Gpr160 in discrete brain areas to determine the physiological function of this receptor.
Areas of interest for our future studies where CARTp immunoreactivity and Gpr160ir were both identified include both forebrain and hindbrain sites. In particular, the NTS, an area that receives gustatory information from the oral cavity (63) and contains GLP-1 (PPG) neurons, and the AP, where CART actions have been described related to gastric emptying (64). In addition, the presence of Gpr160ir in the hypoglossal nucleus where CARTp-positive fibers have been reported suggests a role for the receptor in oromotor function in the rat (65). In the forebrain, this overlap of immunoreactivity is highly present in the ARC, where distinct actions of CART have been described (66, 67), and the PVN, where a potential action of CARTp on thyrotropin-releasing hormone (TRH)-producing neurons has been reported (68, 69). Additional sites where colocalization suggests a requirement of Gpr160 expression for the actions of CARTp include the LPBN, an area known to be important in meal termination and the perception of taste (70–72).
The highest level of Gpr160ir was detected in amygdala, where CARTp has been demonstrated to produce either conditioned place preference or conditioned place aversion depending on the dose (73) and where expression of CARTp has been reported to be altered by stress (74). Thus, behavioral actions of CARTp may be communicated via interaction with Gpr160. These include the hippocampus, where depolarizing actions have been demonstrated (47). In general, the reward systems also deserve future attention because of Gpr160ir localization in the NAcc shell, where CARTp actions related to energy balance have been described (24). Surprisingly, Gpr160ir was not observed in the NAcc core, the site of origin of CART-producing neurons that project to substantia nigra (75) and the VTA, a site where intranuclear CARTp administration blunted the increase locomotor activity caused by systemic cocaine treatment (76).
To determine what cell types expressed Gpr160, we performed coimmunostaining with molecular phenotype markers on purified astrocyte and microglial cultures from rat brainstem tissue (Figs. 6–8, 10, 11). Initial coimmunostaining or GFAP and Gpr160ir identified areas of colocalization in cultured astrocytes, but the results in brainstem tissue slices were inconclusive for at least two potential reasons (Fig. 7). Astrocytes are surrounded by nonastrocytic cells, and it is, therefore, difficult to definitively identify from which cell the Gpr160 staining is derived (Fig. 7). In addition, GFAP was predominantly localized in astrocytic end-feet, whereas Gpr160 staining was mainly detectable in the cell body, and therefore, a high degree of direct colocalization might not be expected. However, given that Gpr160 was detectable in cultured astrocytes, it is likely that GPR160 is also expressed in astrocytes in vivo. We noted that in the purified astrocyte cultures, there was an additional population of GFAP-negative cells (Fig. 7). To determine what other cell types were present, we stained for NeuN and vimentin, a fibroblast marker (Fig. 10, A and B). Fibroblast immunoreactivity was detected in the astrocyte cultures (Fig. 10B) potentially explaining the predominant cell type copurifying with astrocytes. However, one must be careful to recognize that the isolation and culture conditions may have increased artificially the immunoreactive content of the receptor and potentially the number of fibroblasts growing in the cultures.
Colocalization between Gpr160ir and Iba1 was detected in both cultured rat brainstem microglia and brainstem tissue slices (Fig. 8, A and B). In cultured microglia, Gpr160ir was detected in the vast majority of cells, whereas Gpr160ir was only observed in a small subset of microglia in brainstem tissue slices (Fig. 8, A and B). In nonnative conditions, cultured microglia may be more activated by physical manipulation, excess serum required for the culture conditions, or isolation from other cell types. The identification of Gpr160ir on glial cells opens a new possibility that some of the pharmacological actions described for CART peptides are mediated via an interaction with nonneuronal cells in the brain. In support of this possibility, we recently reported the identification of Gpr160ir on astrocytes and microglia in laminae 1–3 of the spinal cord dorsal horn (54).
Neuronal Gpr160ir also was detected in the NTS and hypoglossal nuclei (Fig. 11, A and B). In the commissural NTS, Gpr160 was observed predominantly in nonneuronal cells (Fig. 11A). In contrast to the smaller number of neurons expressing Gpr160 in the NTS, a larger percentage of neurons in the hypoglossal nucleus express the receptor (Fig. 11B). This observation raises the possibility that CART could be acting directly on Gpr160-expressing hypoglossal motor neurons to slow ingestive behavior to an appropriate level throughout a discrete meal, assisting in timely meal termination. The fact that Gpr160 is expressed on multiple cell types will increase the difficulty of identifying the relative influence of each cell type on the combined physiological output, and thus, the development of cell-specific knockdown approaches is required.
Unanswered Questions and Cautions
In our previous manuscript (54), we validated the Gpr160 antibody by a combination of approaches, including the demonstration that the actions of CARTp to increase cFos in KATO III cells and ERK phosphorylation in differentiated PC-12 cells were significantly compromised by prior siRNA treatment targeting Gpr160, that Gpr160 immunoreactivity coimmunoprecipitated with FAM-labelled CARTp, that CARTp colocalized with Gpr160 on cells using the proximity ligation assay, that using RNAscope Gpr160 was localized to neurons (as well as microglia and astrocytes), and that Gpr160 targeting siRNA pretreatment significantly decreased Gpr10 protein levels in KATO III cells as detected by Western blotting and immunofluorescence. However, we cannot rule out the possibility that other feeding-related peptides may interact with the same receptor. Future studies using proximity ligation assays and other methods for assessing physical association between proteins may answer these questions (54). It also is possible that the Gpr160 antibody we used may interact with epitopes shared with several other GPCRs. Significant homologies do exist among members of this class of receptors. Only when each potential candidate is expressed in cells null for the other receptors might this be established by detection of ligand (CARTp) binding and by demonstrating that signaling through that other receptor is not interrupted by the Gpr160 antibody.
We acknowledge that these studies were performed only in male rats and that future studies must address similar issues in female animals. We are well aware of the abundant literature describing potential sex differences in ingestive behaviors, including many important, early references on food intake (77) and water drinking (78); therefore, future studies must examine the role of Gpr160 expression in select brain sites in males and females across all 4 days of the estrous cycle using transgenic animals designed for that purpose.
Perspectives and Significance
Overall, this work demonstrated evidence of an interaction between CART peptide and Gpr160 resulting in effects on food intake and water intake. As there are several biologically active forms of CART peptide, the possibility of additional receptors cannot yet be ruled out (6, 8, 79–81). It will be critical to determine if the activity of CART 62-102 is also dependent on Gpr160 and to identify any differences in intracellular signaling or physiological function between biologically active splice isoforms. Understanding peripheral expression and function of Gpr160 also will be an important next step. The initial anatomical distribution of central Gpr160ir shown here provides a framework for future studies to determine circuit-specific functions of Gpr160. Moreover, the demonstration that not only neurons but also glia express Gpr160 will be important to take into consideration in future studies. Determining the relative impact of each cell type on the phenotypical output will likely require site-specific, cell-type-targeted compromise using modern genetic approaches.
Future work also will be required to fully understand the effects of CART and Gpr160 on both food intake and water intake. CART is currently thought to be an anorexigenic hormone whose receptor has the potential to be targeted as an effective weight-loss therapeutic (9, 79). CARTp is certainly an important regulator of body weight, as deletions or mutations in the CARTp gene result in weight gain in both animal models and humans (31–40). However, data shown here demonstrate a role for CARTp in both water intake and possibly glial function.
The work described here provides important information that will enable a more detailed examination of the anatomic localization of Gpr160ir and the cellular effects of CARTp. Clearly, now our task and that of others will be to identify the phenotypes of cells expressing Gpr160, as well as to develop genetically modified animals in which select populations of Gpr160-expressing cells can be targeted and the resultant behaviors studied.
GRANTS
This work was funded by the National Institutes of Health (DK118340) and departmental funds.
AUTHORS CONTRIBUTIONS
G.L.Y., C.J.H, and W.K.S. conceived and designed research; C.J.H., G.A., L.M.S., M.R.H., G.R.K., W.K.S., and G.L.Y. analyzed data and conducted experiments; C.J.H., G.A., L.M.S., M.R.H., G.R.K., W.K.S., and G.L.Y. interpreted results of experiments; G.L.Y. drafted manuscript; G.R.K., W.K.S., and G.L.Y. edited and revised manuscript; C.J.H., G.A., L.M.S., M.R.H., G.R.K., W.K.S., and G.L.Y. approved final version of manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Spiess J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry 20: 1982–1988, 1981. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- 2.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci 15: 2471–2481, 1995. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglass J, Daoud S. Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene 169: 241–245, 1996. doi: 10.1016/0378-1119(96)88651-3. [DOI] [PubMed] [Google Scholar]
- 4.Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol 9: 823–833, 1997. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- 5.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol 391: 115–132, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Thim L, Kristensen P, Nielsen PF, Wulff BS, Clausen JT. Tissue-specific processing of cocaine- and amphetamine-regulated transcript peptides in the rat. Proc Natl Acad Sci USA 96: 2722–2727, 1999. doi: 10.1073/pnas.96.6.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhar MJ, Adams S, Dominguez G, Jaworski J, Balkan B. CART peptides. Neuropeptides 36: 1–8, 2002. doi: 10.1054/npep.2002.0887. [DOI] [PubMed] [Google Scholar]
- 8.Dey A, Xhu X, Carroll R, Turck CW, Stein J, Steiner DF. Biological processing of the cocaine and amphetamine-regulated transcript precursors by prohormone convertases, PC2 and PC1/3. J Biol Chem 278: 15007–15014, 2003. doi: 10.1074/jbc.M212128200. [DOI] [PubMed] [Google Scholar]
- 9.Thim L, Kristensen P, Larsen PJ, Wulff BS. CART, a new anorectic peptide. Int J Biochem Cell Biol 30: 1281–1284, 1998. doi: 10.1016/S1357-2725(98)00110-1. [DOI] [PubMed] [Google Scholar]
- 10.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol 281: R1862–R1867, 2001. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, Patterson C, Berthoud HR. Fourth ventricular injection of CART peptide inhibits short-term sucrose intake in rats. Brain Res 896: 153–156, 2001. doi: 10.1016/S0006-8993(00)03256-X. [DOI] [PubMed] [Google Scholar]
- 12.Aja S, Ewing C, Lin J, Hyun J, Moran TH. Blockade of central GLP-1 receptors prevents CART-induced hypophagia and brain c-Fos expression. Peptides 27: 157–164, 2006. doi: 10.1016/j.peptides.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Smedh U, Moran TH. Peptides that regulate food intake: separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am J Physiol Regul Integr Comp Physiol 284: R1418–R1426, 2003. doi: 10.1152/ajpregu.00665.2002. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, , et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393: 72–76, 1998. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Krieger JP, Vergara M, Quinn D, McDougle M, de Araujo A, , et al. Blunted vagal cocaine- and amphetamine-regulated transcript promotes hyperphagia and weight gain. Cell Rep 30: 2028–2039.e2024, 2020. doi: 10.1016/j.celrep.2020.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res 818: 499–509, 1999. doi: 10.1016/S0006-8993(98)01349-3. [DOI] [PubMed] [Google Scholar]
- 17.Abbott CR, Rossi M, Wren AM, Murphy KG, Kennedy AR, Stanley SA, , et al. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology 142: 3457–3463, 2001. doi: 10.1210/endo.142.8.8304. [DOI] [PubMed] [Google Scholar]
- 18.Kong W, Stanley S, Gardiner J, Abbott C, Murphy K, Seth A, , et al. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J 17: 1688–1690, 2003. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- 19.Smith KL, Gardiner JV, Ward HL, Kong WM, Murphy KG, Martin NM, , et al. Overexpression of CART in the PVN increases food intake and weight gain in rats. Obesity (Silver Spring) 16: 2239–2244, 2008. doi: 10.1038/oby.2008.366. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Zheng DZ, Zhou JY, Zhou SW. Orexigenic effect of cocaine- and amphetamine-regulated transcript (CART) after injection into hypothalamic nuclei in streptozotocin-diabetic rats. Clin Exp Pharmacol Physiol 37: 989–995, 2010. doi: 10.1111/j.1440-1681.2010.05423.x. [DOI] [PubMed] [Google Scholar]
- 21.Lau J, Farzi A, Qi Y, Heilbronn R, Mietzsch M, Shi YC, , et al. CART neurons in the arcuate nucleus and lateral hypothalamic area exert differential controls on energy homeostasis. Mol Metab 7: 102–118, 2018. doi: 10.1016/j.molmet.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res 957: 298–310, 2002. doi: 10.1016/S0006-8993(02)03640-5. [DOI] [PubMed] [Google Scholar]
- 23.Skibicka KP, Alhadeff AL, Grill HJ. Hindbrain cocaine- and amphetamine-regulated transcript induces hypothermia mediated by GLP-1 receptors. J Neurosci 29: 6973–6981, 2009. doi: 10.1523/JNEUROSCI.6144-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Job MO, Licata J, Hubert GW, Kuhar MJ. Intra-accumbal administration of shRNAs against CART peptides cause increases in body weight and cocaine-induced locomotor activity in rats. Brain Res 1482: 47–54, 2012. doi: 10.1016/j.brainres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol 594: 5791–5815, 2016. doi: 10.1113/JP271538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166: 209–221, 2016. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 151: 3589–3599, 2010. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lartigue G. Putative roles of neuropeptides in vagal afferent signaling. Physiol Behav 136: 155–169, 2014. doi: 10.1016/j.physbeh.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldsinger A, Lu Y, Zhou SY, Wu X, Grabauskas G, Song I, , et al. Cocaine- and amphetamine-regulated transcript is the neurotransmitter regulating the action of cholecystokinin and leptin on short-term satiety in rats. Am J Physiol Gastrointest Liver Physiol 303: G1042–G1051, 2012. doi: 10.1152/ajpgi.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, , et al. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 142: 4394–4400, 2001. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- 32.Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahren B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept 129: 203–211, 2005. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Moffett M, Stanek L, Harley J, Rogge G, Asnicar M, Hsiung H, , et al. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides 27: 2037–2045, 2006. doi: 10.1016/j.peptides.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 34.Guerardel A, Barat-Houari M, Vasseur F, Dina C, Vatin V, Clement K, , et al. Analysis of sequence variability in the CART gene in relation to obesity in a Caucasian population. BMC Genet 6: 19, 2005. doi: 10.1186/1471-2156-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miraglia del Giudice E, Santoro N, Fiumani P, Dominguez G, Kuhar MJ, Perrone L. Adolescents carrying a missense mutation in the CART gene exhibit increased anxiety and depression. Depress Anxiety 23: 90–92, 2006. doi: 10.1002/da.20156. [DOI] [PubMed] [Google Scholar]
- 36.Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology 147: 39–43, 2006. doi: 10.1210/en.2005-0812. [DOI] [PubMed] [Google Scholar]
- 37.Rigoli L, Munafo C, Di Bella C, Salpietro A, Procopio V, Salpietro C. Molecular analysis of the CART gene in overweight and obese Italian children using family-based association methods. Acta Paediatr 99: 722–726, 2010. doi: 10.1111/j.1651-2227.2010.01709.x. [DOI] [PubMed] [Google Scholar]
- 38.del Giudice EM, Santoro N, Cirillo G, D'Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes 50: 2157–2160, 2001. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez G, del Giudice EM, Kuhar MJ. CART peptide levels are altered by a mutation associated with obesity at codon 34. Mol Psychiatry 9: 1065–1066, 2004. doi: 10.1038/sj.mp.4001578. [DOI] [PubMed] [Google Scholar]
- 40.Yamada K, Yuan X, Otabe S, Koyanagi A, Koyama W, Makita Z. Sequencing of the putative promoter region of the cocaine- and amphetamine-regulated-transcript gene and identification of polymorphic sites associated with obesity. Int J Obes 26: 132–136, 2002. doi: 10.1038/sj.ijo.0801848. [DOI] [PubMed] [Google Scholar]
- 41.Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett 384: 198–202, 2005. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 42.Lin Y, Hall RA, Kuhar MJ. CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6-38 as a CART receptor antagonist. Neuropeptides 45: 351–358, 2011. doi: 10.1016/j.npep.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicentic A. CART peptide diurnal variations in blood and brain. Peptides 27: 1942–1948, 2006. doi: 10.1016/j.peptides.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Sloncova E, Elbert T, , et al. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol 559: 109–114, 2007. doi: 10.1016/j.ejphar.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Nagelova V, Pirnik Z, Zelezna B, Maletinska L. CART (cocaine- and amphetamine-regulated transcript) peptide specific binding sites in PC12 cells have characteristics of CART peptide receptors. Brain Res 1547: 16–24, 2014. doi: 10.1016/j.brainres.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 46.Keller PA, Compan V, Bockaert J, Giacobino JP, Charnay Y, Bouras C, , et al. Characterization and localization of cocaine- and amphetamine-regulated transcript (CART) binding sites. Peptides 27: 1328–1334, 2006. doi: 10.1016/j.peptides.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci 21: 7474–7480, 2001. doi: 10.1523/JNEUROSCI.21-19-07474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang XL, Wang Y, Li DL, Luo J, Liu MY. Orphan G protein-coupled receptors (GPCRs): biological functions and potential drug targets. Acta Pharmacol Sin 33: 363–371, 2012. doi: 10.1038/aps.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang Y, Kenakin T, Liu C. Editorial: orphan GPCRs as emerging drug targets. Front Pharmacol 6: 295, 2015. doi: 10.3389/fphar.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol 303: R941–R949, 2012. doi: 10.1152/ajpregu.00336.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yosten GL, Lyu RM, Hsueh AJ, Avsian-Kretchmer O, Chang JK, Tullock CW, , et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol 25: 206–215, 2013. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein LM, Yosten GL, Samson WK. Adropin acts in brain to inhibit water drinking: potential interaction with the orphan G protein-coupled receptor, GPR19. Am J Physiol Regul Integr Comp Physiol 310: R476–R480, 2016. doi: 10.1152/ajpregu.00511.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, , et al. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol 311: R489–R496, 2016. doi: 10.1152/ajpregu.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yosten GL, Harada CM, Haddock CJ, Giancotti LA, Kolar GR, Patel R, Guo C, Chen Z, Zhang J, Doyle TM, Dickenson AH, Samson WK, Salvemini D. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J Clin Invest 130: 2587–2592, 2020. doi: 10.1172/JCI133270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HK. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010. [Erratum in Cell Metab 23: 744, 2016]. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aja S, Robinson BM, Mills KJ, Ladenheim EE, Moran TH. Fourth ventricular CART reduces food and water intake and produces a conditioned taste aversion in rats. Behav Neurosci 116: 918–921, 2002. doi: 10.1037/0735-7044.116.5.918. [DOI] [PubMed] [Google Scholar]
- 57.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse 29: 293–298, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 59.Kask A, Schioth HB, Mutulis F, Wikberg JE, Rago L. Anorexigenic cocaine- and amphetamine-regulated transcript peptide intensifies fear reactions in rats. Brain Res 857: 283–285, 2000. doi: 10.1016/S0006-8993(99)02383-5. [DOI] [PubMed] [Google Scholar]
- 60.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, , et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacol 42: 1471–1479, 2017. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKay NJ, Kanoski SE, Hayes MR, Daniels D. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol 301: R1755–R1764, 2011. doi: 10.1152/ajpregu.00472.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKay NJ, Galante DL, Daniels D. Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J Neurosci 34: 16417–16423, 2014. doi: 10.1523/JNEUROSCI.3267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 89: 531–535, 2006. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smedh U, Scott KA, Moran TH. Fourth ventricular CART peptide induces c-fos in the area postrema and nucleus of the solitary tract via a CRF-receptor dependent mechanism. Neurosci Lett 609: 124–128, 2015. doi: 10.1016/j.neulet.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 65.Wiesenfeld Z, Halpern BP, Tapper DN. Licking behavior: evidence of hypoglossal oscillator. Science 196: 1122–1124, 1977. doi: 10.1126/science.558653. [DOI] [PubMed] [Google Scholar]
- 66.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, , et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998. doi: 10.1016/S0896-6273(00)80656-X. [DOI] [PubMed] [Google Scholar]
- 67.Farzi A, Lau J, Ip CK, Qi Y, Shi YC, Zhang L, , et al. Arcuate nucleus and lateral hypothalamic CART neurons in the mouse brain exert opposing effects on energy expenditure. Elife 7: e36494, 2018. doi: 10.7554/eLife.36494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res 848: 101–113, 1999. doi: 10.1016/S0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- 69.Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, , et al. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci 20: 9224–9234, 2000. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fekete C, Wittmann G, Liposits Z, Lechan RM. Origin of cocaine- and amphetamine-regulated transcript (CART)-immunoreactive innervation of the hypothalamic paraventricular nucleus. J Comp Neurol 469: 340–350, 2004. doi: 10.1002/cne.10999. [DOI] [PubMed] [Google Scholar]
- 71.Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP neurons control meal termination. Cell Metab 23: 811–820, 2016. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baez-Santiago MA, Reid EE, Moran A, Maier JX, Marrero-Garcia Y, Katz DB. Dynamic taste responses of parabrachial pontine neurons in awake rats. J Neurophysiol 115: 1314–1323, 2016. doi: 10.1152/jn.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rademacher DJ, Sullivan EM, Figge DA. The effects of infusions of CART 55-102 into the basolateral amygdala on amphetamine-induced conditioned place preference in rats. Psychopharmacology 208: 499–509, 2010. doi: 10.1007/s00213-009-1748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunter RG, Bellani R, Bloss E, Costa A, Romeo RD, McEwen BS. Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res 1152: 234–240, 2007. doi: 10.1016/j.brainres.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dallvechia-Adams S, Smith Y, Kuhar MJ. CART peptide-immunoreactive projection from the nucleus accumbens targets substantia nigra pars reticulata neurons in the rat. J Comp Neurol 434: 29–39, 2001. doi: 10.1002/cne.1162. [DOI] [PubMed] [Google Scholar]
- 76.Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55-102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides 41: 65–72, 2007. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang GH. Age and sex differences in the amount of food-intake in albino rats. Am J Physiol 71: 729–735, 1925. doi: 10.1152/ajplegacy.1925.71.3.729. [DOI] [Google Scholar]
- 78.Vijande M, Costales M, Schiaffini O, Marin B. Angiotensin II-induced drinking: sexual differences. Pharmacol Biochem Behav 8: 753–755, 1978. doi: 10.1016/0091-3057(78)90278-2. [DOI] [PubMed] [Google Scholar]
- 79.Adams LD, Gong W, Vechia SD, Hunter RG, Kuhar MJ. CART: from gene to function. Brain Res 848: 137–140, 1999. doi: 10.1016/S0006-8993(99)01907-1. [DOI] [PubMed] [Google Scholar]
- 80.Stein J, Steiner DF, Dey A. Processing of cocaine- and amphetamine-regulated transcript (CART) precursor proteins by prohormone convertases (PCs) and its implications. Peptides 27: 1919–1925, 2006. doi: 10.1016/j.peptides.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 81.Hunter RG, Philpot K, Vicentic A, Dominguez G, Hubert GW, Kuhar MJ. CART in feeding and obesity. Trends Endocrinol Metab 15: 454–459, 2004. doi: 10.1016/j.tem.2004.09.010. [DOI] [PubMed] [Google Scholar]