Abstract
Recent evidence indicates a crucial role for G protein-coupled estrogen receptor 1 (GPER1) in the maintenance of cardiovascular and kidney health in females. The current study tested whether GPER1 activation ameliorates hypertension and kidney damage in female Dahl salt-sensitive (SS) rats fed a high-salt (HS) diet. Adult female rats were implanted with telemetry transmitters for monitoring blood pressure and osmotic minipumps releasing G1 (selective GPER1 agonist, 400 μg/kg/day ip) or vehicle. Two weeks after pump implantation, rats were shifted from a normal-salt (NS) diet (0.4% NaCl) to a matched HS diet (4.0% NaCl) for 2 wk. Twenty-four hour urine samples were collected during both diet periods and urinary markers of kidney injury were assessed. Histological assessment of kidney injury was conducted after the 2-wk HS diet period. Compared with values during the NS diet, 24-h mean arterial pressure markedly increased in response to HS, reaching similar values in vehicle-treated and G1-treated rats. HS also significantly increased urinary excretion of protein, albumin, nephrin (podocyte damage marker), and KIM-1 (proximal tubule injury marker) in vehicle-treated rats. Importantly, G1 treatment prevented the HS-induced proteinuria, albuminuria, and increase in KIM-1 excretion but not nephrinuria. Histological analysis revealed that HS-induced glomerular damage did not differ between groups. However, G1 treatment preserved proximal tubule brush-border integrity in HS-fed rats. Collectively, our data suggest that GPER1 activation protects against HS-induced proteinuria and albuminuria in female Dahl SS rats by preserving proximal tubule brush-border integrity in a blood pressure-independent manner.
Keywords: blood pressure, brush border, dietary sodium, female, kidney
INTRODUCTION
It is well established that estradiol elicits protective actions in the cardiovascular and kidney systems in females (1–3). Estradiol is a pleiotropic molecule with many diverse actions, thus resolving the complexity of estrogenic signaling may provide new therapeutic targets for cardiovascular and kidney diseases. An improved understanding of the actions mediated by different estrogen receptors is of fundamental importance from a clinical perspective.
Estradiol actions are mediated via activation of the classical estrogen receptors α and β or the novel G protein-coupled estrogen receptor 1 (GPER1). GPER1 is a seven-transmembrane-spanning receptor that mediates rapid estrogenic signaling (4–7) and is ubiquitously expressed in multiple organ systems, including the cardiovascular and kidney systems (5). Recent evidence from human and animal studies indicates a critical role for GPER1 in the maintenance of cardiovascular health in females (8–12). GPER1 activation ameliorates kidney damage in female mRen2.Lewis rats, a transgenic rat model expressing the murine Ren2 gene, fed a high-salt (HS) diet (13) and reduces blood pressure in ovariectomized mRen2.Lewis (14) and Sprague-Dawley rats (15) fed a normal-salt (NS) diet. Conversely, aged GPER1-knockout mice display an increased blood pressure phenotype (16). Moreover, we recently showed that activation of GPER1 in the renal medulla evokes natriuresis in NS-fed female Sprague-Dawley rats (15). However, the contribution of GPER1 to salt sensitivity and salt-induced kidney injury is not completely understood. Refining our understanding of the protective actions mediated by GPER1 and the underlying mechanisms within the cardiovascular and kidney systems in males and females may improve personalized medicine care to men and premenopausal and postmenopausal women. This is particularly relevant to GPER1 because of the clinical availability of FDA-approved selective estrogen receptor modulators, including tamoxifen and raloxifene, that elicit agonistic activity on GPER1 (17, 18).
Salt-induced hypertension and associated kidney damage affect a large percentage of the population worldwide and constitute a major cause of chronic kidney disease. The Dahl salt-sensitive (SS) rat is a well-established model of SS hypertensive nephropathy that has notable similarities to hypertension in humans (19, 20). Indeed, Dahl SS rats are commonly used to study the relationship between salt-induced hypertension and kidney damage, and early studies revealed that salt sensitivity in this rat model is regulated by sex hormones (21). Ovariectomy augments salt sensitivity in female Dahl SS rats (21, 22), suggesting that the female sex hormones protect against the development of hypertension and end-organ kidney damage. Overall, although numerous recent studies have suggested that estrogen confers cardiovascular protection by facilitating GPER1 signaling (23–25), whether GPER1 activation protects female Dahl SS rats against hypertension and kidney damage is not clear. Therefore, this study was designed to test whether systemic administration of the selective GPER1 agonist, G1, ameliorates hypertension and kidney damage in HS-fed female Dahl SS rats.
MATERIALS AND METHODS
Animal Studies
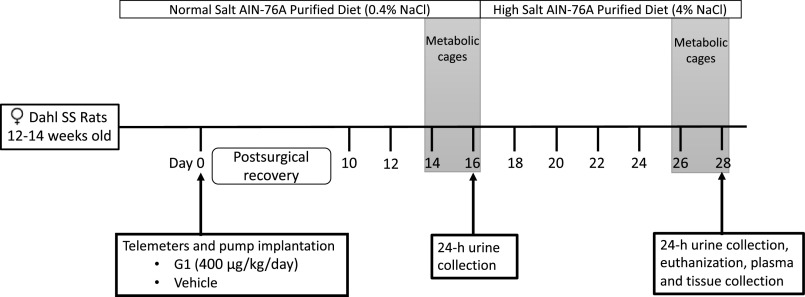
All animal protocols were in accordance with the Guide for the Care and Use of Laboratory Animals and were approved in advance by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. These studies used female Dahl SS rats obtained from our in-house colony. At 12–14 wk of age, rats were anesthetized with Fluriso (2% isoflurane, 502017, VetOne, Boise, ID) and implanted with HD-S10 transmitters (Data Sciences International, Duluth, MN) as previously described (26) to allow continuous monitoring of blood pressure by telemetry. Simultaneously, Alzet osmotic pumps (model 2ML4; Cupertino, CA) were intraperitoneally implanted in the rats to allow the constant administration of G1 (400 µg/kg/day; 1004003, Sandia Biotec Inc., Albuquerque, NM) or vehicle (75% DMSO in saline) for 28 days. Rats were randomly assigned to treatment with vehicle or G1. Rats were allowed 9 days of postsurgical recovery before recording blood pressure, heart rate, locomotor activity, and temperature. Blood pressure was measured for 10 s at 10-min intervals throughout the entire study. Figure 1 illustrates the experimental timeline.
Figure 1.
Experimental timeline. SS, salt-sensitive.
Animals were maintained on an NS diet (AIN-76A Purified Rodent Diet, 0.4% NaCl, DYET No. 100000, Dyets, Inc., Bethlehem, PA), provided ad libitum, in 12-h:12-h light/dark cycle and temperature (18°C –23°C)-controlled rooms. Two weeks after surgery, animals were placed into metabolic cages to acclimate for 2 days, then 24-h urine samples were collected while food and water intake were monitored (Fig. 1). Immediately thereafter, all animals were switched from the NS diet to an HS diet (AIN-76A Purified Rodent Diet, 4.0% NaCl, DYET No. 113756, Dyets, Inc.), on which they remained for 2 wk (Fig. 1). On the last day of the study, 24-h urine samples were again collected after 2 days of acclimation to metabolic cages (Fig. 1). Then, animals were euthanized, plasma was collected, and kidneys were harvested, fixed, and preserved in paraffin for analysis as detailed previously (27). Data were generated from three independent experimental batches of animals conducted at three different times.
Electrolyte and Osmolality Measurement
Urine electrolyte concentrations were determined using an EasyLyte analyzer (Medica, Bedford, MA). Urine osmolality was determined using a vapor pressure osmometer (Osmomat 030, Gonotec, Berlin, Germany).
Creatinine Measurement
Plasma and urine creatinine levels were determined by underivatized, stable isotope dilution LC-MS/MS as previously described (28). An Agilent Infinity 1260 LC and an Agilent Infinity 1290 Autosampler with a 6460 Triple Quad Mass Spectrometer (Agilent Technologies, Wilmington, DE) were used.
Urea Measurement
Plasma urea levels were determined using the Bioassay Systems Urea Assay Kit (50–107-8333 from Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions.
Determination of Urinary Levels of Kidney Injury Markers
Urinary protein excretion was measured by a Bradford assay (Bio‐Rad, Hercules, CA). Urinary levels of albumin, the podocyte damage marker nephrin, and the proximal tubular injury marker kidney injury molecule-1 (KIM-1) were determined using commercially available ELISA kits (GWB-1B2B4B, GenWay Biotech, San Diego, CA; MBS018640, MyBioSource, San Diego, CA; and RKM100, R&D Systems, Minneapolis, MN, respectively).
Histological Assessment of Kidney Damage
Kidneys were fixed overnight in 4% buffered formalin solution at room temperature, transferred to 70% ethanol for 24 h, then embedded in paraffin. Tissues were cut longitudinally into 4-μm thick sections and mounted on Superfrost slides. Kidney structures were stained with Gomori’s Blue Trichrome and evaluated using brightfield microscopy (Olympus BX40; Olympus America, Melville, NY). Twenty photographs (×200 magnification) per cortex were obtained with a digital camera (Olympus DP12; Olympus America) and examined in a blinded manner. In all, 43–58 glomeruli were assessed per slide and assigned a glomerulosclerosis score of 0 (0% of the glomerulus was sclerotic), 1 (≤25% sclerosis), 2 (>25% and ≤50% sclerosis), 3 (>50% and ≤75% sclerosis), or 4 (>75% and ≤100% sclerosis). The degree of proximal tubule dilation was also quantified in blue trichrome-stained slides. A total of 40 microscopy fields per kidney were examined in a blinded manner and assigned a tubule dilation score from 0 (no dilation) to 2 (prominent dilation). The integrity of the proximal tubule brush border was examined using periodic acid-Schiff (PAS) stain. Forty cortical fields per kidney were examined and assigned a score for integrity of 0 (no brush border), 1 (poor integrity), 2 (moderate integrity), or 3 (excellent integrity). All the scores were averaged per animal and experimental group and are reported as means ± SE.
The presence of protein casts in the kidney was evaluated in whole kidney scans (×40 magnification) obtained with a bright-field microscope fitted with a motorized stage and digital camera (Olympus BX40 and DP12 camera; Olympus America). Each blue trichrome-stained kidney scan was color thresholded for the presence of protein casts and quantified using MetaMorph software (Molecular Devices LLC., San Jose, CA; https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/metamorph-microscopy). Data are reported as the average percent area covered by protein casts in each experimental group. Similarly, quantification of kidney interstitial fibrosis was performed in 20 cortical images per kidney using a color thresholding method and quantified using MetaMorph software (27, 29). Data are reported as the average percent area covered by fibrosis in each experimental group.
Immunohistochemical Analysis
Tissue sections were stained with primary antibodies specific for CD3 (1:400; ab16669, Abcam, Cambridge, MA) and ED-1 (1:100; MCA341R, Bio-Rad) and detected with polymer conjugated secondary antibody (RMR622H, Biocare Medical, Concord, CA). Kidney T-cells (CD3+) and macrophages (ED-1+) were quantified by blindly counting 10 microscopic fields (400 × 400 μm, ×200 magnification) in each renal cortex and medulla. Cell numbers are reported as the average of the counts in the 10 fields per renal cortex.
Statistical Analysis
Values are presented as the means ± SE. Mean arterial pressure (MAP) data reported for the first week of HS diet represent averaged data for the sixth–eighth day after increasing the dietary salt intake, which corresponds to the 22nd–24th day after starting G1 or vehicle treatment. Statistical tests used for each data set are specified in the associated figure/table legend. This includes assessment using repeated-measures two-way ANOVA with Sidak’s post hoc test for multiple comparisons. Unpaired t test was also used for data analysis. P values for each data set are specified within the associated figure or table. P values < 0.05 were deemed statistically significant. Telemetry data were analyzed by cosinor analysis using GraphPad Prism v. 8 (GraphPad Software, Inc., La Jolla, CA) to calculate the midline estimating statistic of rhythm (MESOR), acrophase (time of peak), and amplitude (peak minus trough). Statistical analysis was performed using GraphPad Prism v. 8 (GraphPad Software, Inc.).
RESULTS
Data supplements can be found at https://doi.org/10.6084/m9.figshare.13525391.v1.
Blood Pressure
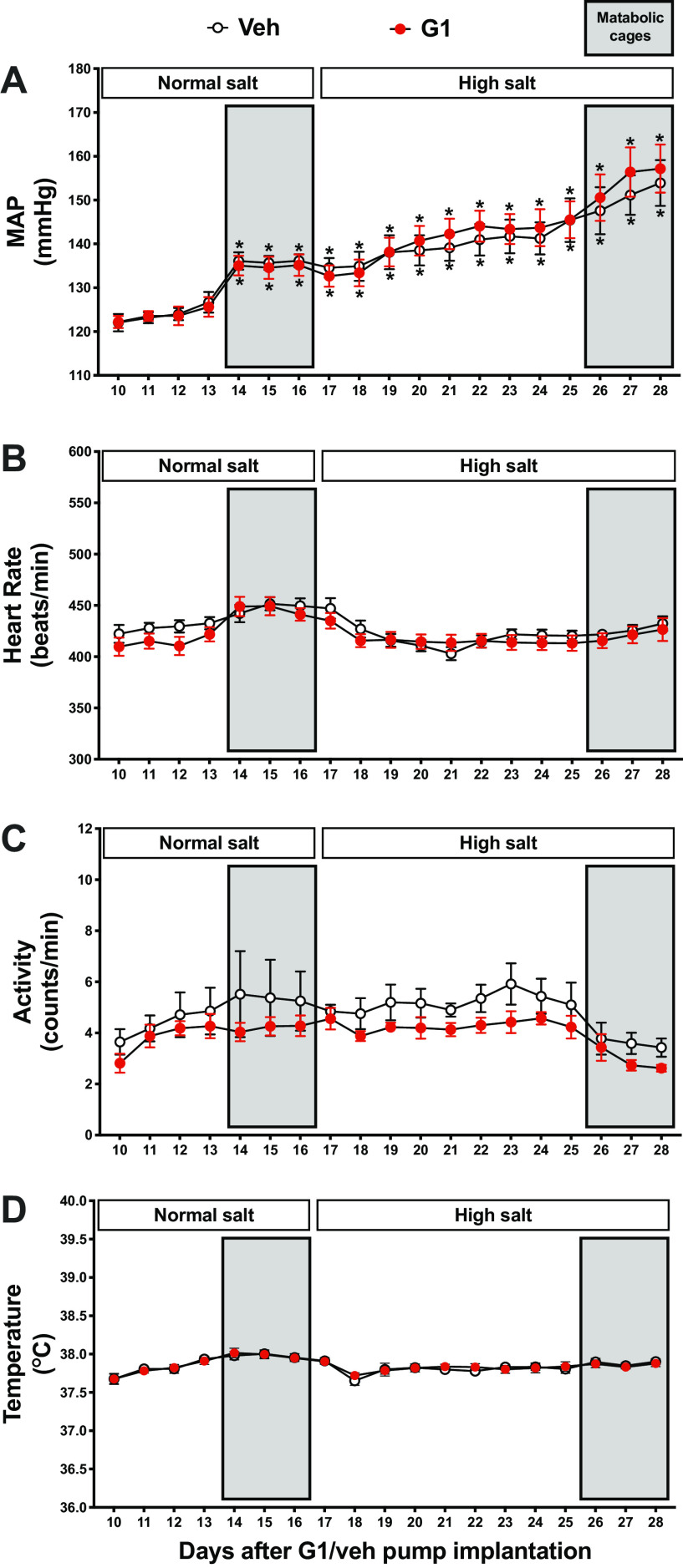
Systemic GPER1 activation by G1 did not attenuate MAP in NS-fed female Dahl SS rats (vehicle vs. G1: 123 ± 2 vs. 124 ± 2 mmHg after 2 wk of G1 or vehicle infusion, n = 5 and 6, respectively, Fig. 2A). Of note, rats experienced slight, yet significant, increases in MAP when placed into metabolic cages to acclimate (Figs. 1 and 2A). Consistent with previous data (21, 22), vehicle-treated rats developed hypertension upon increased dietary salt intake, reaching a MAP of 141 ± 3 mmHg after 1 wk on the HS diet (n = 5, Fig. 2A). This salt-induced increase in MAP was not attenuated in G1-treated animals (144 ± 2 mmHg after 1 wk of HS, n = 6, Fig. 2A). Systolic and diastolic blood pressures followed the same pattern as MAP (Supplemental Fig. S1, A and B). No diet- or G1-induced differences in heart rate, body temperature, or locomotor activity were observed (Fig. 2, B–D).
Figure 2.
G protein-coupled estrogen receptor 1 (GPER1) activation does not affect blood pressure in female Dahl salt-sensitive (SS) rats. Twenty-four-hour MAP (A), heart rate (B), locomotor activity (C), and temperature (D) were recorded during the entire study in conscious female Dahl SS rats. Rats were systemically treated with GPER1 agonist (G1; 400 μg/kg/day) or vehicle throughout the normal salt and subsequent high-salt diet periods. Shaded areas represent the days when the animals were placed in metabolic cages. Data are presented as means ± SE (n = 6 and 5 rats in the G1- and vehicle-treated group, respectively). Statistical comparisons were performed by repeated-measures two-way ANOVA with Sidak’s post hoc test for multiple comparisons. *P < 0.05 vs. corresponding baseline values at the 10th day after starting G1 or vehicle treatment. Two-way ANOVA results: MAP: Pinteraction = 0.8, Ptime < 0.0001, PG1 = 0.8; heart rate: Pinteraction = 0.4, Ptime = 0.0002, PG1 = 0.5; locomotor activity: Pinteraction = 0.9, Ptime = 0.02, PG1 = 0.2; temperature: Pinteraction = 0.9, Ptime < 0.0001, PG1 = 0.9. Veh, vehicle.
Circadian Blood Pressure Rhythm
To examine whether G1 treatment or HS intake affects circadian blood pressure rhythm in female Dahl SS rats, blood pressure was analyzed by cosinor analysis. Statistics, including MESOR, amplitude, and acrophase, calculated by cosinor analysis, are presented in Supplemental Table S1. Tracings for hourly averages of MAP, heart rate, locomotor activity, and temperature are presented in Supplemental Fig. S2. The HS diet significantly increased the blood pressure rhythm in G1- and vehicle-treated rats (Supplemental Table S1, Supplemental Fig. S2A). G1 treatment had no significant effect on MESOR, amplitude, or acrophase in the NS- or HS-diet period (Supplemental Table S1).
Urinary Excretion of Water and Electrolytes
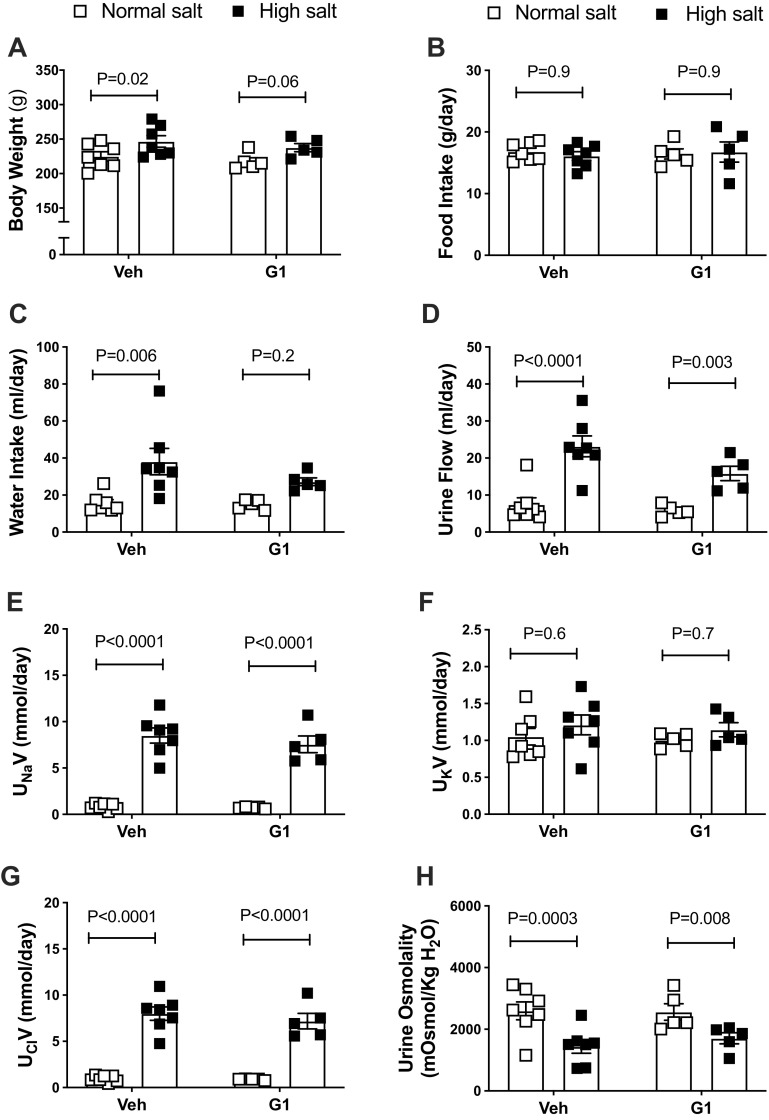
G1 treatment did not alter body weight or food intake during the NS- or HS-diet period (Fig. 3, A and B). The HS diet appeared to increase water intake in vehicle- and G1-treated rats, but this increase did not reach statistical significance for G1-treated animals (Fig. 3C). Notably, previous studies showed that GPER1 activation decreases water intake in female rats (30). The HS diet also significantly increased urine flow, urinary Na+, and Cl− excretion in vehicle- and G1-treated rats (Fig. 3, D–G). Urinary K+ did not differ among the groups, regardless of treatment or diet (Fig. 3F). The HS diet evoked similar declines in urine osmolality in both vehicle- and G1-treated rats (Fig. 3H).
Figure 3.
Body weight and metabolic cage parameters in female Dahl salt-sensitive (SS) rats. Body weight (A), food intake (B), water intake (C), urine flow (D), urinary excretion of Na+ (UNaV) (E), K+ (UKV) (F) and Cl− (UClV) (G), and urine osmolality (H) were measured in female Dahl SS rats systemically treated with GPER1 agonist (G1; 400 μg/kg/day) or vehicle throughout the normal salt (NS) and subsequent high-salt diet (HS) periods. Data are presented as means ± SE (n = 5 and 7 rats in the G1- and vehicle-treated group, respectively. Statistical comparisons were performed by repeated-measures two-way ANOVA with Sidak’s post hoc test for multiple comparisons. Two-way ANOVA results: body weight: Pinteraction = 0.9, PG1 = 0.4, Pdiet = 0.003; food intake: Pinteraction = 0.7, PG1 = 0.8, Pdiet = 0.9; water intake: Pinteraction = 0.3, PG1 = 0.2, Pdiet = 0.003; urine flow: Pinteraction = 0.09, PG1 = 0.1, Pdiet < 0.0001; UNaV: Pinteraction = 0.5, PG1 = 0.4, Pdiet < 0.0001; UKV: Pinteraction = 0.9, PG1 =0.6, Pdiet = 0.3; UClV: Pinteraction = 0.5, PG1 = 0.4, Pdiet <0.0001; urine osmolality: Pinteraction = 0.3, PG1 = 0.7, Pdiet < 0.0001. Veh, vehicle. P values computed by the multiple pairwise comparisons test between NS and HS are displayed on the figures. P values computed by the multiple-comparisons test between G1 and vehicle-treated rats are > 0.05.
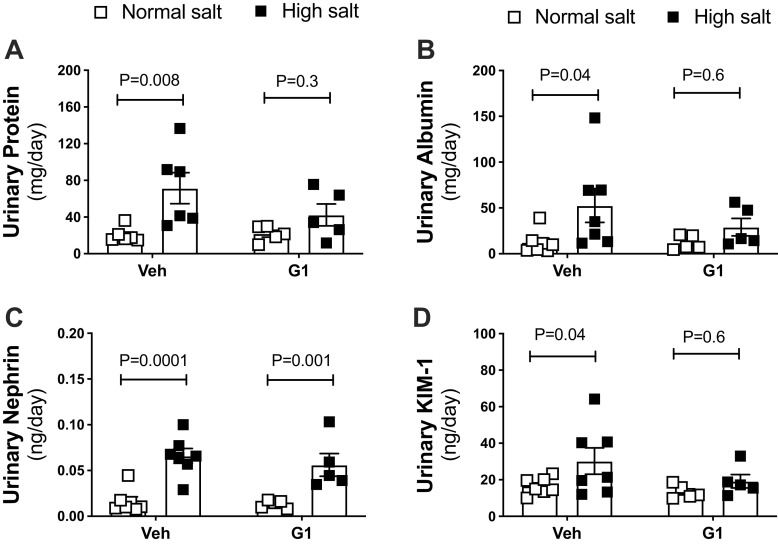
Proteinuria and Albuminuria
G1 treatment did not affect urinary excretion of protein and albumin in the NS-diet period (Fig. 4, A and B). However, G1 treatment did attenuate the increase in urinary excretion of protein and albumin induced by the HS diet (Fig. 4, A and B). G1 treatment did not affect plasma creatinine levels, plasma urea levels, or creatinine clearance in HS-fed rats (Supplemental Table S2).
Figure 4.
G protein-coupled estrogen receptor 1 (GPER1) activation attenuates salt-induced proteinuria, albuminuria, and KIM-1 excretion in female Dahl salt-sensitive (SS) rats. Urinary excretion of protein (A), albumin (B), nephrin (C), and kidney injury molecule-1 (KIM-1, D) were measured in female Dahl SS rats systemically treated with GPER1 agonist (G1; 400 μg/kg/day) or vehicle throughout the normal salt (NS) and subsequent high-salt (HS) diet periods. Data are presented as means ± SE (n = 5 and 7 rats in the G1- and vehicle-treated group, respectively). Statistical comparisons were performed by repeated-measures two-way ANOVA with Sidak’s post hoc test for multiple comparisons. Two-way ANOVA results: protein: Pinteraction = 0.2, PG1 = 0.3, Pdiet = 0.005; albumin: Pinteraction = 0.3, PG1 = 0.4, Pdiet = 0.03; nephrin: Pinteraction = 0.6, PG1 = 0.5, Pdiet < 0.0001; KIM-1: Pinteraction = 0.3, PG1 = 0.2, Pdiet = 0.03. Veh, vehicle. P values computed by the multiple-pairwise comparisons test between NS and HS are displayed on the figures. P values computed by the multiple comparisons test between G1 and vehicle-treated rats are > 0.05.
Urinary Nephrin and KIM-1 Excretion
To identify the nature of the G1-induced protection against proteinuria and albuminuria, urine levels of nephrin, a marker for podocyte damage, and kidney injury molecule-1 (KIM-1), a marker for tubular injury, were assessed. G1 treatment did not alter the urinary excretion of these markers in the NS-diet period (Fig. 4, C and D). Although G1 treatment did not affect the increase in urinary excretion of nephrin induced by the HS diet (Fig. 4C), it did attenuate the HS diet-induced increase in KIM-1 excretion (Fig. 4D).
Glomerular and Proximal Tubular Injury
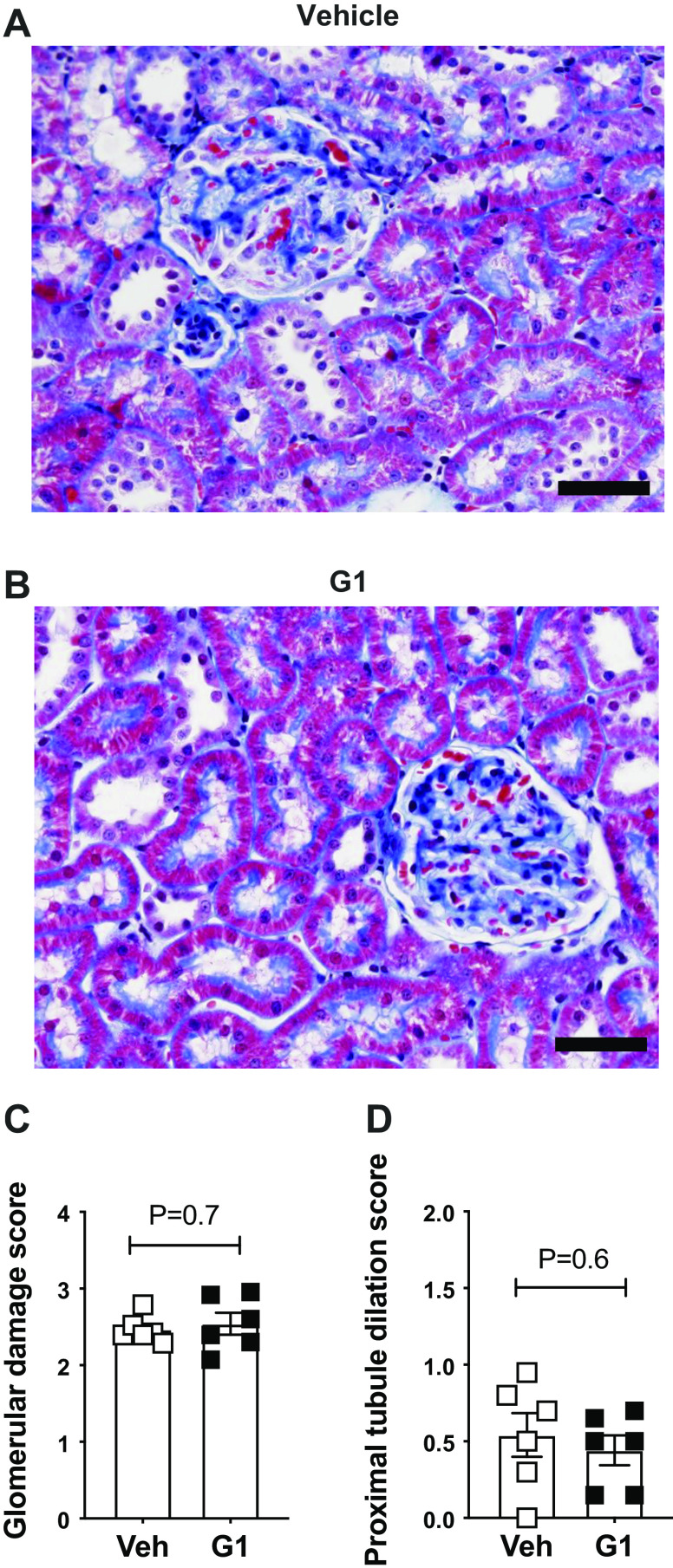
After 2 wk on the HS diet, the degree of glomerulosclerosis was similar in vehicle- and G1-treated rats (Fig. 5, A–C). Likewise, the presence of protein casts in the kidney and the extent of interstitial fibrosis were similar between the two groups, suggesting that systemic GPER1 activation does not prevent this kind of kidney damage (% area covered by protein casts, vehicle vs. G1: 1.7 ± 0.4 vs. 1.8 ± 0.1, respectively, P = 0.7, n = 6/group, Supplemental Fig. S3; % area covered by fibrosis, vehicle vs. G1: 23.7 ± 1.9 vs. 25.6 ± 2.4, P = 0.5, n = 6/group).
Figure 5.
G protein-coupled estrogen receptor 1 (GPER1) activation does not prevent glomerulosclerosis or proximal tubule dilation in high salt-fed female Dahl salt-sensitive (SS) rats. Representative images (A and B) and quantification of glomerular injury (C) and proximal tubule dilation (D) in female Dahl SS rats systemically treated with G1 (400 μg/kg/day) or vehicle (Veh) and fed a high-salt diet for 2 wk. Data are presented as means ± SE (n = 6 rats in each group). Statistical comparisons were performed by unpaired t test. Scale bars = 50 μm.
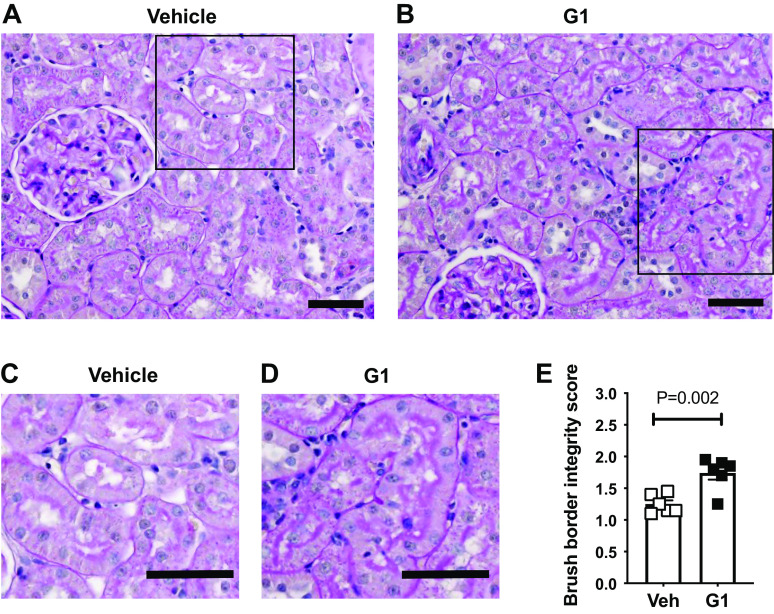
Proximal tubule dilation was also similar in vehicle- and G1-treated rats after 2 wk on the HS diet (Fig. 5D). In contrast, the severe deterioration of the proximal tubule brush border observed in vehicle-treated rats was remarkably preserved in the G1-treated rats (Fig. 6, A–E).
Figure 6.
G protein-coupled estrogen receptor 1 (GPER1) activation improves proximal tubule brush-border integrity in high salt-fed female Dahl salt-sensitive (SS) rats. Representative images of proximal tubule brush-border integrity in female Dahl SS rats systemically treated with vehicle (Veh) (A) or G1 (400 μg/kg/day; B) and fed a high-salt diet for 2 wk. The areas indicated by squares within A and B are shown at higher magnification in C and D. Quantification of the brush-border integrity is graphed in E. Data are presented as means ± SE (n = 6 rats in each group). Statistical comparisons were performed by unpaired t test. Scale bars = 50 μm.
T-Cell and Macrophage Accumulation
Because kidney inflammation has been implicated in the development of salt-sensitive hypertension, we also evaluated the degree of T-cell and macrophage accumulation in the kidneys from vehicle- or G1-treated Dahl SS rats fed a HS diet. After 2 wk on the HS diet, the numbers of T-cells in the kidney cortex and medulla were similar in the two groups (vehicle vs. G1, cortex: 15.7 ± 1. vs. 19.3 ± 2.4 cells/field, P = 0.2; medulla: 37.4 ± 3.9 vs. 36.2 ± 2.3 cells/field, P = 0.5, respectively, n = 6/group, Supplemental Fig. S4), as were the numbers of macrophages (vehicle vs. G1, cortex: 5.3 ± 1.0 vs. 5.7 ± 1.4 cells/field, P = 0.8; medulla: 8.5 ± 0.7 vs. 10.7 ± 1.3 cells/field, respectively, P = 0.9, n = 6/group, Supplemental Fig. S4). Thus, treatment with G1 does not decrease the salt-sensitive immune cell accumulation in the kidneys of female Dahl SS rats.
DISCUSSION
Recent evidence suggests that the novel estrogen receptor GPER1 has a pivotal role in the maintenance of cardiovascular and kidney health in females. The major finding of this study is that systemic activation of GPER1 in female Dahl SS rats abrogates HS-induced proteinuria and albuminuria via preserving the integrity of the proximal tubule brush border, without affecting HS-induced hypertension.
In the current study, challenging female Dahl SS rats with a HS diet for 2 wk increased proteinuria, albuminuria, and blood pressure, similar to previous observations documenting salt sensitivity and kidney injury in female Dahl SS rats (21, 31). Nevertheless, systemic activation of GPER1 did not reduce the salt-induced increase in blood pressure. These findings are consistent with those from studies by Lindsey et al. (13) conducted in female mRen2.Lewis rats, suggesting that G1 abolishes kidney damage in a blood pressure-independent manner. In contrast, it has been demonstrated that GPER1 activation by G1 lowers blood pressure acutely in male Sprague-Dawley rats (16) and chronically in ovariectomized mRen2.Lewis rats (13) and ovariectomized Sprague-Dawley rats (15). These previous studies suggest that the blood pressure-lowering actions of GPER1 are mainly evident under conditions of ovarian hormonal deficiency. Thus, we speculate that the lack of a blood pressure-lowering action in the current study may be related to the use of ovary-intact female rats, in which the endogenous ligand for GPER1, estradiol, was available. Although it is clear that ovariectomy potentiates HS-induced hypertension in female Dahl SS rats (21, 22), it remains to be determined whether G1 supplementation in ovariectomized Dahl SS rats can reduce this effect. Similarly, whether G1 supplementation induces an antihypertensive effect in male Dahl SS rats remains to be tested.
Hypertension that develops in salt-loaded Dahl SS rats is associated with impairment of kidney pressure natriuresis resulting in sodium retention (32) and impairment of Na+-K+-ATPase contributing to the HS-induced hypertension (33, 34). We recently provided new evidence that GPER1 activation decreases renal tubular reabsorption of Na+, at least partially, by decreasing the activity of Na+-K+-ATPase in the outer medullary region of the kidney (15). Specifically, we demonstrated that in female Sprague-Dawley rats fed an NS diet, acute renal medullary interstitial infusion of G1 evokes an increase in urinary Na+ excretion via an endothelin-1-dependent pathway (15, 35). Our current results did not show differences in urinary Na+ excretion in response to systemic GPER activation in female Dahl SS rats. It has not been determined whether increased dietary salt-loading regulates the pronatriuretic response to renal medullary GPER1 activation. Notably, salt loading increases renal cortical GPER1 mRNA and protein expression in mRen2.Lewis rats (13). Additional studies are required to determine whether the pronatriuretic actions induced by GPER1 are impaired under hypertensive conditions.
Evidence indicates that both the glomerulus and the proximal tubule are responsible for the increases in albumin excretion observed in salt-loaded male Dahl SS rats (36). In the current study, we show that systemic activation of GPER1 abrogates proximal tubule injury, but not glomerular injury, as indicated by histological and urinary markers. In contrast, Hutchens et al. (37) showed that estradiol reduces postischemia glomerular hyperpermeability via a GPER1-dependent mechanism, suggesting that GPER1 activation improves glomerular permeability in this model of kidney injury. Salt loading in the Dahl SS rat model elicits marked tubular damage, which is characterized by expansion and necrosis of proximal tubule epithelial cells (36). Herein, we demonstrate that G1 preserves proximal tubule brush-border integrity and prevents the salt-induced increase in KIM-1 excretion. Similarly, G1 reduced proteinuria and renal hypertrophy in female mRen2.Lewis rats fed a HS diet (13). These observations suggest that systemic GPER1 activation improves tubular protein reabsorption and G1 administration may present an effective treatment for salt-induced kidney injury when combined with agents that restore glomerular integrity.
The expression of KIM-1, a transmembrane protein, in the proximal tubule and its shedding into the urine are exaggerated after an insult to the kidney such as ischemia reperfusion injury (38) or HS diet (27). Our findings demonstrate that treatment with G1 successfully decreases the urinary excretion of KIM-1, suggesting that G1 specifically protects the proximal tubule against HS-induced damage. Kwekel et al. (39) demonstrated that baseline expression of KIM-1 in the kidney is markedly distinct in male and female F344 rats at different ages, with a higher expression of KIM-1 in females versus males at 8 wk of age that sharply falls and reverses at 15 wk of age (exaggerated KIM-1 expression by males from 15 wk to 104 wk of age). Whether this pattern of kidney KIM-1 expression is similarly affected by sex and age in the Dahl SS rat remains unknown.
The ability of G1 to ameliorate kidney damage in salt-loaded animals further supports the notion that estrogenic actions protect the kidney against salt-induced complications. Further studies are required to identify the mechanism(s) underlying the protective effects of G1 on proximal tubule brush-border integrity and determine whether tubular protein reabsorption, via megalin and/or cubilin, also improves in response to GPER1 activation.
Evidence from clinical trials indicates that the proximal tubule is the major contributor in the progression of chronic kidney disease (40–42). During chronic kidney disease, the increase in tubular flow workload that occurs to compensate for changes in glomerular filtration can cause increased reactive species production and, consequently, a vicious cycle of injury and nephron loss (42, 43). Importantly, GPER1-mediated protection against salt-induced kidney injury has been attributed to attenuation of tubular oxidative stress (13). Similarly, several studies have reported that GPER1 has an antioxidant role in cardiovascular disease (44–46). Of note, increased oxidative stress has been reported in the Dahl SS rat model (47–49). Whether the protection against proximal tubular function deterioration mediated by GPER1 in salt-loaded female Dahl SS rats involves reducing oxidative stress remains unknown, however.
GPER1 is ubiquitously expressed throughout multiple organ systems in the body, including the kidney (5). Specifically, GPER1 expression has been reported in the renal tubular and epithelial cells (13, 50). Supporting our present findings, Lindsey et al. (13) showed that GPER1 is highly expressed in the brush border of the proximal tubule. It is noteworthy that renal GPER1 expression and cellular localization are influenced by the estrus cycle (50). Whether the dynamic changes in the expression and localization of GPER1 in the kidney regulate the protective actions of GPER1 in the kidney is unknown.
Despite extensive evidence from preclinical research pointing to protective effects of estrogen against cardiovascular disease, earlier clinical trials, including the Women Health Initiative, showed that hormonal replacement to postmenopausal women is associated with increased cardiovascular risk (51, 52). Recent clinical trials and additional analysis of the Women Health Initiative data demonstrate that estrogen replacement therapy initiated in recently menopausal women was associated with favorable outcomes on cardiovascular disease and mortality, pointing to a critical role for the timing of initiating estrogen therapy in menopausal women on influencing the impact on health outcomes (53–55). With recent advancement of our understanding of estrogen genomic and nongenomic actions in light of the discovery of the new GPER1, clinical research in this area is necessary to provide new targets for the development of drugs for cardiovascular and renal protection.
Preclinical drug development studies are instrumental for the initiation of human clinical trials as they help delineate the drug-dosing regimen, pharmacokinetic profile, general safety, and toxicity profile. In consistency with previous studies (14, 15, 45, 56), the present study identifies a systemic pharmacological dose of G1 of 400 μg/kg/day. Notably, contradictory results exist regarding GPER1 function in cancer biology, being apoptotic or proliferative (57–61). In addition, the results of studies focusing on the impact of GPER1 on bone health are also contrasting (62–66). Improving our understanding of the role of GPER1 in carcinogenesis and skeletal metabolism in males and females and the potential interactions with other estrogen receptor subtypes is warranted for the development of therapeutic agents that target GPER1.
Perspectives and Significance
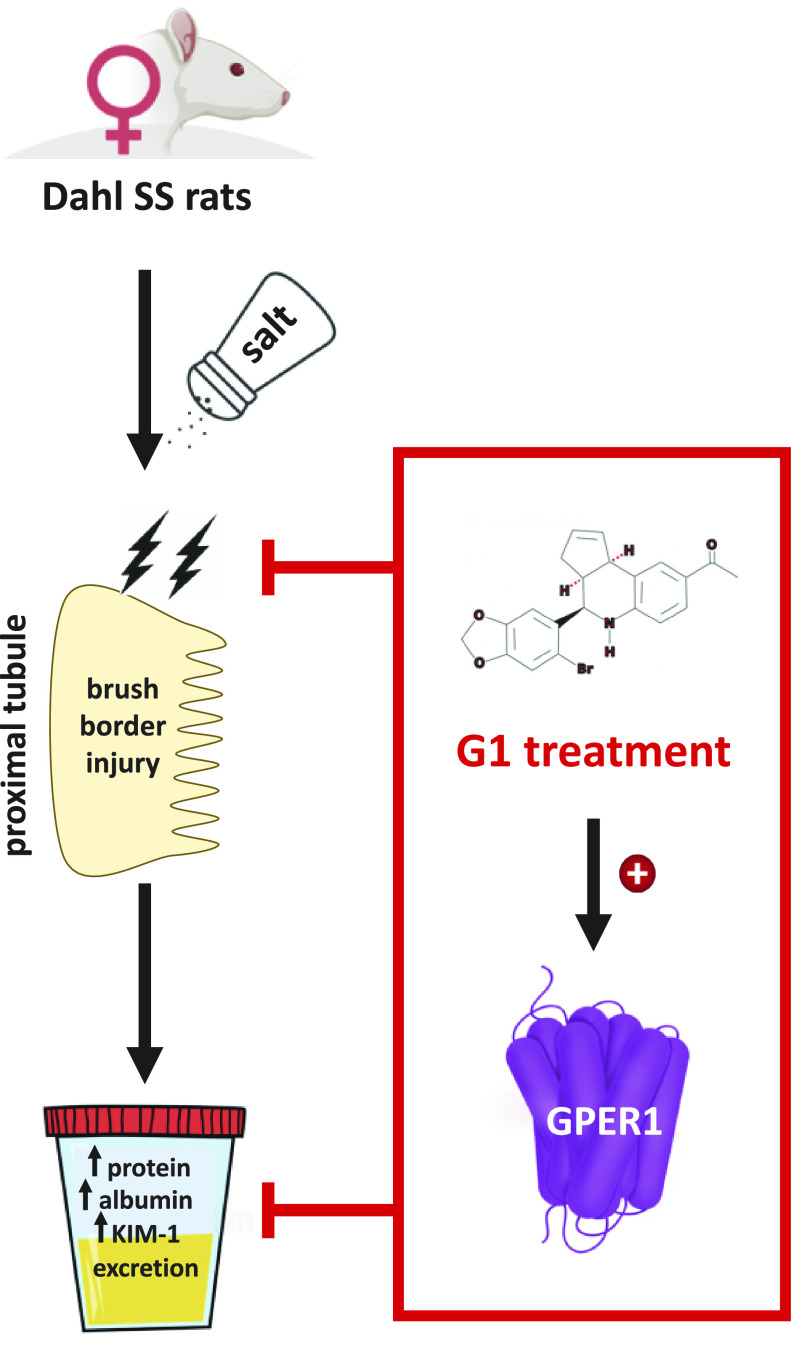
Herein, we provide new evidence that the selective GPER1 agonist G1 attenuates salt-induced proteinuria, albuminuria, and KIM-1 excretion and improves renal proximal tubular brush-border integrity in female Dahl SS rats (Fig. 7), in a blood pressure-independent manner—findings that are in agreement with previous reports (13). These findings support the emerging recognition of GPER1 as a potential therapeutic target for the management of salt-induced kidney damage in females. Our results also underscore the importance of assessing molecular mechanisms driving physiological and pathophysiological processes using both male and female animal models. Targeting GPER1 may be of especial importance in women exhibiting resistant hypertension, as our findings suggest that pharmacological activation of GPER1 with G1 could be protective against hypertensive kidney damage, without ameliorating the increase in blood pressure.
Figure 7.
Hypothetical schematic of the proposed renoprotective actions of G protein-coupled estrogen receptor 1 (GPER1) activation in female Dahl salt-sensitive (SS) rats.
GRANTS
This project was supported by National Institutes of Health (NIH) Grant K99DK119413 and American Heart Association (AHA) Grant 18CDA34110010 (to E. Y. Gohar); NIH Grant P30 DK 079337 to the O’Brien Kidney Research Center; NIH Grant P01 HL136267 and AHA SRG grant (to J.S. Pollock and D. M. Pollock); and NIH Grant K01HL145324 (to C. De Miguel).
DISCLOSURES
E. Y. Gohar is affiliated with the Department of Pharmacology and Toxicology, Faculty of Pharmacy, Alexandria University, Egypt. The other authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.Y.G., D.M.P., and C.D.M conceived and designed research; E.Y.G., R.N.A., E.M.D., M.K.B., and C.J.M. performed experiments; E.Y.G., M.K.B., and C.D.M analyzed data; E.Y.G., J.S.P., D.M.P., and C.D.M. interpreted results of experiments; E.Y.G. and C.D.M prepared figures; E.Y.G. drafted manuscript; E.Y.G., R.N.A., E.M.D., M.K.B., C.J., J.S.P., D.M.P., and C.D.M edited and revised manuscript; E.Y.G., R.N.A., E.M.D., M.K.B., C.J., J.S.P., D.M.P., and C.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate Dr. Xiaofen Liu’s outstanding assistance with immunohistochemistry studies, Dr. Binli Tao’s excellent help with KIM-1 and nephrin measurements, and Jackson Colson’s excellent technical assistance with husbandry of the rats.
REFERENCES
- 1.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 37: 746–756, 2017. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki H, Kondo K. Chronic kidney disease in postmenopausal women. Hypertens Res 35: 142–147, 2012. doi: 10.1038/hr.2011.155. [DOI] [PubMed] [Google Scholar]
- 3.Valdivielso JM, Jacobs-Cacha C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens 28: 1–9, 2019. doi: 10.1097/MNH.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 4.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153: 2953–2962, 2012. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ 10: 4, 2019. doi: 10.1186/s13293-019-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7: 715–726, 2011. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632, 2005. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 8.Arefin S, Simoncini T, Wieland R, Hammarqvist F, Spina S, Goglia L, Kublickiene K. Vasodilatory effects of the selective GPER agonist G-1 is maximal in arteries of postmenopausal women. Maturitas 78: 123–130, 2014. doi: 10.1016/j.maturitas.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297: H1806–1813, 2009. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman RD, Gros R, Ding Q, Hussain Y, Ban MR, McIntyre AD, Hegele RA. A common hypofunctional genetic variant of GPER is associated with increased blood pressure in women. Br J Clin Pharmacol 78: 1441–1452, 2014. doi: 10.1111/bcp.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gohar EY. G protein-coupled estrogen receptor 1 as a novel regulator of blood pressure. Am J Physiol Renal Physiol 319: F612–F617, 2020. doi: 10.1152/ajprenal.00045.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res 94: 96–104, 2012. doi: 10.1093/cvr/cvs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58: 665–671, 2011. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2. Lewis rats. Endocrinology 150: 3753–3758, 2009. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gohar EY, Daugherty EM, Aceves JO, Sedaka R, Obi IE, Allan JM, Soliman RH, Jin C, De Miguel C, Lindsey SH, Pollock JS, Pollock DM. Evidence for G-protein-coupled estrogen receptor as a pronatriuretic factor. J Am Heart Assoc 9: e01511, 2020. [32390531] doi: 10.1161/JAHA.119.015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat 89: 89–97, 2009. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 19.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension 23: 531–550, 1994. doi: 10.1161/01.HYP.23.4.531. [DOI] [PubMed] [Google Scholar]
- 20.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Twin studies in Barbados. Hypertension 15: 803–809, 1990. doi: 10.1161/01.HYP.15.6.803. [DOI] [PubMed] [Google Scholar]
- 21.Dahl LK, Knudsen KD, Ohanian EV, Muirhead M, Tuthill R. Role of the gonads in hypertension-prone rats. J Exp Med 142: 748–759, 1975. doi: 10.1084/jem.142.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartori-Valinotti JC, Venegas-Pont MR, Lamarca BB, Romero DG, Yanes LL, Racusen LC, Jones AV, Ryan MJ, Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt-sensitive rats. Steroids 75: 794–799, 2010. doi: 10.1016/j.steroids.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Madungwe NB, da Cruz Junho CV, Bopassa JC. Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol 174: 4329–4344, 2017. doi: 10.1111/bph.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalves GK, Scalzo S, Alves AP, Agero U, Guatimosim S, Reis AM. Neonatal cardiomyocyte hypertrophy induced by endothelin-1 is blocked by estradiol acting on GPER. Am J Physiol Cell Physiol 314: C310–C322, 2018. doi: 10.1152/ajpcell.00060.2017. [DOI] [PubMed] [Google Scholar]
- 25.Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav 6: e00449, 2016. doi: 10.1002/brb3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin C, Jeon Y, Kleven DT, Pollock JS, White JJ, Pollock DM. Combined endothelin a blockade and chlorthalidone treatment in a rat model of metabolic syndrome. J Pharmacol Exp Ther 351: 467–473, 2014. doi: 10.1124/jpet.114.215566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Miguel C, Sedaka R, Kasztan M, Lever JM, Sonnenberger M, Abad A, Jin C, Carmines PK, Pollock DM, Pollock JS. Tauroursodeoxycholic acid (TUDCA) abolishes chronic high salt-induced renal injury and inflammation. Acta Physiol (Oxf) 226: e13227, 2019. doi: 10.1111/apha.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young S, Struys E, Wood T. Quantification of creatine and guanidinoacetate using GC-MS and LC-MS/MS for the detection of cerebral creatine deficiency syndromes. Curr Protoc Hum Genet 17: 13, 2007. [18428409] doi: 10.1002/0471142905.hg1703s54. [DOI] [PubMed] [Google Scholar]
- 29.De Miguel C, Hamrick WC, Hobbs JL, Pollock DM, Carmines PK, Pollock JS. Endothelin receptor-specific control of endoplasmic reticulum stress and apoptosis in the kidney. Sci Rep 7: 43152, 2017. doi: 10.1038/srep43152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santollo J, Daniels D. Activation of G protein-coupled estrogen receptor 1 (GPER-1) decreases fluid intake in female rats. Horm Behav 73: 39–46, 2015. doi: 10.1016/j.yhbeh.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 35: 484–489, 2000. doi: 10.1161/01.HYP.35.1.484. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Galicia M, Frohlich B, Roman RJ. Induction of P4504α activity improves pressure-natriuresis in Dahl S rats. Hypertension 31: 232–236, 1998. doi: 10.1161/01.HYP.31.1.232. [DOI] [PubMed] [Google Scholar]
- 33.Fedorova OV, Kolodkin NI, Agalakova NI, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension 37: 462–466, 2001. doi: 10.1161/01.HYP.37.2.462. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Yan Y, Liu L, Xie Z, Malhotra D, Joe B, Shapiro JI. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. J Biol Chem 286: 22806–22813, 2011. doi: 10.1074/jbc.M111.246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gohar E, Pollock DM. Functional Interaction of Endothelin receptors in mediating natriuresis evoked by G protein-coupled estrogen receptor 1. J Pharmacol Exp Ther 376: 98–105, 2021. doi: 10.1124/jpet.120.000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endres BT, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Kamocka MM, McDermott-Roe C, Staruschenko A, Molitoris BA, Geurts AM, Palygin O. Intravital imaging of the kidney in a rat model of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F163–F173, 2017. doi: 10.1152/ajprenal.00466.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–385, 2012. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwekel JC, Desai VG, Moland CL, Vijay V, Fuscoe JC. Sex differences in kidney gene expression during the life cycle of F344 rats. Biol Sex Differ 4: 14, 2013. doi: 10.1186/2042-6410-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perazella MA. Clinical approach to diagnosing acute and chronic tubulointerstitial disease. Adv Chronic Kidney Dis 24: 57–63, 2017. doi: 10.1053/j.ackd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Schnaper HW. The tubulointerstitial pathophysiology of progressive kidney disease. Adv Chronic Kidney Dis 24: 107–116, 2017. doi: 10.1053/j.ackd.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shalamanova L, McArdle F, Amara AB, Jackson MJ, Rustom R. Albumin overload induces adaptive responses in human proximal tubular cells through oxidative stress but not via angiotensin II type 1 receptor. Am J Physiol Renal Physiol 292: F1846–F1857, 2007. doi: 10.1152/ajprenal.00265.2006. [DOI] [PubMed] [Google Scholar]
- 44.De Francesco EM, Rocca C, Scavello F, Amelio D, Pasqua T, Rigiracciolo DC, Scarpelli A, Avino S, Cirillo F, Amodio N, Cerra MC, Maggiolini M, Angelone T. Protective role of GPER agonist G-1 on cardiotoxicity induced by doxorubicin. J Cell Physiol 232: 1640–1649, 2017. doi: 10.1002/jcp.25585. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Kashyap S, Murphy B, Hutson DD, Budish RA, Trimmer EH, Zimmerman MA, Trask AJ, Miller KS, Chappell MC, Lindsey SH. GPER activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 310: H953–H961, 2016. doi: 10.1152/ajpheart.00631.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Sun X, Lin MS, Ferrario CM, Van Remmen H, Groban L. G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Transl Res 199: 39–51, 2018. doi: 10.1016/j.trsl.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manning RD Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25: 311–317, 2005. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 48.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD Jr.. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 41: 1346–1352, 2003. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 49.Meng S, Roberts LJ 2nd, Cason GW, Curry TS, Manning RD Jr.. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol 283: R732–R738, 2002. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 50.Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, Thomas P, Filardo EJ. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol 382: 950–959, 2014. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, , et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291: 1701–1712, 2004. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 52.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML, WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 299: 1036–1045, 2008. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 53.Manson JE, Bassuk SS, Kaunitz AM, Pinkerton JV. The Women's Health Initiative trials of menopausal hormone therapy: lessons learned. Menopause 27: 918–928, 2020. [DOI] [PubMed] [Google Scholar]
- 54.Naftolin F, Friedenthal J, Nachtigall R, Nachtigall L. Cardiovascular health and the menopausal woman: the role of estrogen and when to begin and end hormone treatment. F1000Res 8: 1576, 2019. doi: 10.12688/f1000research.15548.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thaung Zaw JJ, Howe PRC, Wong RHX. Postmenopausal health interventions: time to move on from the Women's Health Initiative? Ageing Res Rev 48: 79–86, 2018. doi: 10.1016/j.arr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Davis GK, Newsome AD, Cole AB, Ojeda NB, Alexander BT. Chronic estrogen supplementation prevents the increase in blood pressure in female intrauterine growth-restricted offspring at 12 months of age. Hypertension 73: 1128–1136, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broselid S, Cheng B, Sjöström M, Lövgren K, Klug-De Santiago HL, Belting M, Jirström K, Malmström P, Olde B, Bendahl PO, Hartman L, Fernö M, Leeb-Lundberg LM. G protein-coupled estrogen receptor is apoptotic and correlates with increased distant disease-free survival of estrogen receptor-positive breast cancer patients. Clin Cancer Res 19: 1681–1692, 2013. doi: 10.1158/1078-0432.CCR-12-2376. [DOI] [PubMed] [Google Scholar]
- 58.De Francesco EM, Pellegrino M, Santolla MF, Lappano R, Ricchio E, Abonante S, Maggiolini M. GPER mediates activation of HIF1alpha/VEGF signaling by estrogens. Cancer Res 74: 4053–4064, 2014. doi: 10.1158/0008-5472.CAN-13-3590. [DOI] [PubMed] [Google Scholar]
- 59.Hsu LH, Chu NM, Lin YF, Kao SH. G-protein coupled estrogen receptor in breast cancer. Int J Mol Sci 20: 306, 2019. doi: 10.3390/ijms20020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ignatov A, Ignatov T, Weissenborn C, Eggemann H, Bischoff J, Semczuk A, Roessner A, Costa SD, Kalinski T. G-protein-coupled estrogen receptor GPR30 and tamoxifen resistance in breast cancer. Breast Cancer Res Treat 128: 457–466, 2011. doi: 10.1007/s10549-011-1584-1. [DOI] [PubMed] [Google Scholar]
- 61.Poola I, Abraham J, Liu A, Marshalleck JJ, Dewitty RL. The cell surface estrogen receptor, G protein- coupled receptor 30 (GPR30), is markedly down regulated during breast tumorigenesis. Breast Cancer (Auckl) 1: 65–78, 2008. doi: 10.4137/bcbcr.s557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res 26: 298–307, 2011. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang WB, Cong Y, Ru JY, Ying SQ, Zhu T, Wang DS, Liu XW, Liu G, Zhao JN. Osteoprotective effect of combination therapy of low-dose oestradiol with G15, a specific antagonist of GPR30/GPER in ovariectomy-induced osteoporotic rats. Biosci Rep 35: e00239, 2015. doi: 10.1042/BSR20150146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin X, Li L, Wu S, Tian J, Zheng W. Activation of GPR30 promotes osteogenic differentiation of MC3T3-E1 cells: an implication in osteoporosis. IUBMB Life 71: 1751–1759, 2019. doi: 10.1002/iub.2118. [DOI] [PubMed] [Google Scholar]
- 65.Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150: 687–698, 2009. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 66.Ribeiro M, Sousa C, Rufino AT, Judas F, Mendes AF. Expression and function of the nonclassical estrogen receptor, GPR30, in human cartilage and chondrocytes. J Cell Physiol 235: 8486–8494, 2020. doi: 10.1002/jcp.29691. [DOI] [PubMed] [Google Scholar]