Keywords: estrogen, fat taste, fatty acids, mice, sex differences, taste
Abstract
Sex as a biological variable has been the focus of increasing interest. Relatively few studies have focused, however, on differences in peripheral taste function between males and females. Nonetheless, there are reports of sex-dependent differences in chemosensitivity in the gustatory system. The involvement of endogenous changes in ovarian hormones has been suggested to account for taste discrepancies. Additionally, whether sex differences exist in taste receptor expression, activation, and subsequent signaling pathways that may contribute to different taste responsiveness is not well understood. In this study, we show the presence of both the nuclear and plasma membrane forms of estrogen receptor (ER) mRNA and protein in mouse taste cells. Furthermore, we provide evidence that estrogen increases taste cell activation during the application of fatty acids, the chemical cue for fat taste, in taste receptor cells. We found that genes important for the transduction pathway of fatty acids vary between males and females and that these differences also exist across the various taste papillae. In vivo support for the effect of estrogens in taste cells was provided by comparing the fatty acid responsiveness in male, intact female, and ovariectomized (OVX) female mice with and without hormone replacement. In general, females detected fatty acids at lower concentrations, and the presence of circulating estrogens increased this apparent fat taste sensitivity. Taken together, these data indicate that increased circulating estrogens in the taste system may play a significant role in physiology and chemosensory cellular activation and, in turn, may alter taste-driven behavior.
NEW & NOTEWORTHY Using molecular, cellular, and behavioral analyses, this study shows that sex differences occur in fat taste in a mouse model. Female mice are more responsive to fatty acids, leading to an overall decrease in intake and fatty acid preference. These differences are linked to sex hormones, as estradiol enhances taste cell responsiveness to fatty acids during periods of low circulating estrogen following ovariectomy and in males. Estradiol is ineffective in altering fatty acid signaling during a high-estrogen period and in ovariectomized mice on hormone replacement. Thus, taste receptor cells are a direct target for actions of estrogen, and there are multiple receptors with differing patterns of expression in taste cells.
INTRODUCTION
The taste system plays an important role in nutrient recognition and therefore in shaping the diet of all organisms. In addition to recognizing essential nutrients, the taste system also provides animals the ability to avoid toxins and spoiled foods. Beyond the five basic taste modalities, there are an increasing number of compounds that have been shown to activate the peripheral taste system, including fatty acids, astringents, metal ions, and CO2 (1–6), among others. Of these, the taste of fat and its underlying mechanisms has been the most intensely studied nonconventional taste. Significant research has been dedicated to understanding how the body responds to dietary fat (fatty acids), due in no small part to the rise of obesity and related diseases. In addition to the texture-based oral sensations, fat has been shown to elicit gustatory cues through various taste-specific analyses (2, 7–10). With this information and that of many other bodies of work using various techniques and model systems, fat taste has been suggested to reflect a sixth basic or primary tastant (10–13).
To satisfy diverse physiological needs, males and females require different food intake and storage (14). In both sexes, energy homeostasis is under the control of a variety of hormones secreted from the gut, pancreas, adipose tissue, and gonads. However, gonadal hormones are the primary sources of sex differences in ingestive behavior (15). The taste system constitutes the initial sensory cue for food intake and contributes to preferences and perceptions. Studies involving sex differences in the taste system are sparse; however, existing data support that males and females may differ in their food preferences, sensitivities, and overall intake (16–18). Additionally, some of these differences have been attributed to gonadal hormones, although not mechanistically (19, 20). Estrogen, a primarily female hormone, reduces food intake and body weight and exerts a profound effect on meal size (21). Estrogen decreases energy intake, acting in conjunction with other circulating factors such as leptin and ghrelin to exert tonic inhibition (22, 23). Although largely unknown, taste cells have the machinery for these circulating factors, which may modify the sensitivity of these cells in accordance with the nutritional need of the organism (24, 25).
Although much is known about the role of estrogen and its receptors in the control of energy balance, less is known about sex differences in the taste system, and almost nothing about whether and how circulating estradiol influences taste cells and/or responses to specific tastants. Estrogen signaling is mediated via two classical nuclear receptors, ERα and ERβ, as well as the nonclassical membrane form G protein-coupled estrogen receptor 1 (GPER1). 17β-Estradiol is a potent agonist for all three ERs. There are, however, conflicting data on the role of each receptor in energy regulation.
In the present study, we have attempted to characterize sex differences in the taste system by focusing on the effects of polyunsaturated fatty acids in the peripheral taste systems of male, female, and ovariectomized mice. In addition to utilizing cell-based assays and behavioral assays, we have characterized expression of the three major estrogen receptors. The results of these experiments are consistent with a sex difference in fatty acid signaling in mice, and one of the contributors to this difference lies in the effects of circulating estrogen.
METHODS
Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Utah State University and the University of Central Florida and were performed in accordance with American Veterinary Medical Association guidelines. All experiments were performed on adult C57BL/6J (wild-type) mice, transgenic mice expressing enhanced green fluorescent protein (EGFP) under control of the glutamic acid decarboxylase 67 (GAD67); GFP-GAD67) promoter on a C57BL/6 background from The Jackson Laboratory (Bar Harbor, ME), and the phospholipase Cβ2 (PLCβ2)-EGFP transgenic strain on C57BL/6 background (26). PLCβ2-EGFP mice were generously provided by Dr. Nirupa Chaudhari (University of Miami School of Medicine). Mice were maintained on a 12-h:12-h light-dark schedule and given ad libitum access to chow and water, unless otherwise specified.
Ovariectomy and Hormone Assessment
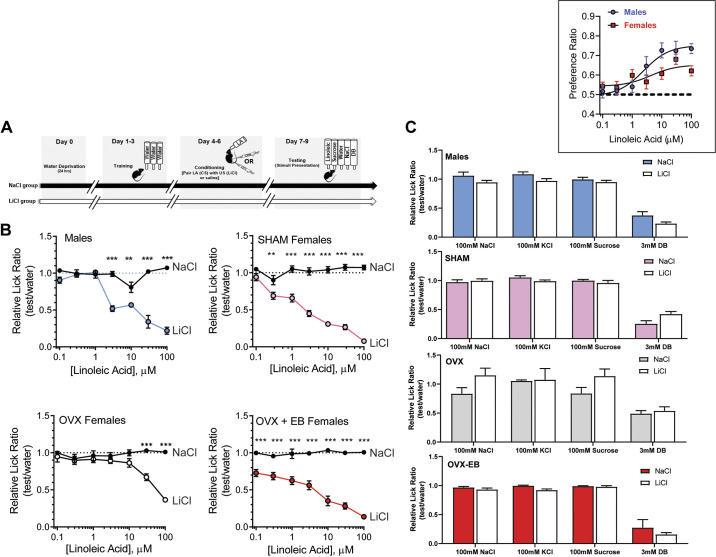
Animals were anesthetized using 2% isoflurane and bilaterally ovariectomized (OVX). Mice received carprofen (20 mg/kg) before surgery and 12 h after surgery. SHAM-OVX females received the analgesic and anesthesia, dorsal skin incision, and suturing similarly to that of the OVX animals without removal of the ovaries. Mice recovered for 1 wk before being used for further experimentation. For SHAM-OVX females, regularity of estrous cycle phases was tracked for 7 consecutive days before conditioned taste aversion (CTA) testing. Given that environmental conditions and stimuli can influence estrous cycle synchrony, cycle phases were not further determined in the SHAM females during CTA testing. To study the effect of estradiol (E2), 8-wk-old female mice were treated with a hormonal regimen to mimic the physiological range of estradiol levels for a young adult female mouse. OVX mice received intrascapular subcutaneous injections of either 2 µg of 17β-estradiol benzoate (EB; Sigma, St. Louis, MO) dissolved in 0.1 mL of sesame oil or the vehicle (sesame oil) alone. This hormone treatment was repeated every 4 days and lasted the duration of the CTA assay. Because estradiol may influence the acquisition of conditioned taste aversions (27), CTA tests were performed starting on day 2 of injections to avoid the estradiol-enhanced LiCl-induced effects (see Fig. 6).
Figure 6.
Ovariectomized (OVX) females showed reduced aversion to linoleic acid (LA) in a conditioned taste aversion assay (CTA). A: schematic representation of the behavioral experimental procedure. B: lick ratios for various concentrations of LA (0.1-100 µM), expressed as means ± SE in LiCl and NaCl groups of male, intact female, OVX female, and OVX female with hormone replacement mice on day 2 after CTA. Data between LiCl and NaCl groups and LA detection concentration were compared using two-way ANOVA followed by Tukey–Kramer method for post hoc multiple comparison. Results are expressed as the mean ± SE of each group, male (n = 8), SHAM-OVX (n = 9), OVX-E2 (n = 7), and OVX (n = 10). *Significant differences between NaCl-injected (closed circle and LiCl-injected (open circles) groups: **P < 0.001, ***P < 0.0001). C: aversion to LA did not generalize to other tastants. Inset: preference ratio for LA (LA intake/[LA intake + water intake]) in male (n= 7) and female (n= 7) mice during two-bottle 48-h LA vs. water tests.
Uterine weights have been previously used as a marker for estrogenicity, as there is a positive correlation between uterine weights and increased estradiol levels (28–30). To evaluate successful ovariectomy, wet uterine weights were collected at the end of experiments. The uterus was cut just above its junction with the cervix and gently stripped of fat and connective tissue and weighed. Absolute mass was calculated, and uterine weight was found to increase with estradiol replacement in the OVX mice.
To determine the stage in the estrous cycle, a standard vaginal lavage and swabs were applied (31). Briefly, vaginal secretions (cells) were collected via plastic Pasteur pipettes or cotton-tipped swabs after flushing of the vaginal canal with 0.9% NaCl. For swabs, a cotton-tipped swab was gently turned and rolled against the vaginal wall and then removed. Cells were transferred to a glass slide by rolling the swab across the slide. The slide was then air dried and fixed in methanol followed by staining using a Differential Quick Stain Kit (Polysciences). Alternatively, a wet smear was collected in 0.9% NaCl with a fine-tip pipette and observed under light microscopy. Both wet smear and swabbing provided ample samples to distinguish readily the different cycles. To minimize the incidence of missing cycles, sample collection was performed in the early afternoon. Cyclical changes in food intake and preference have been reported to coincide with the estrous cycle, food intake decreases beginning at diestrus 2 and continuing into proestrus in female rodents (21, 32–34). Accordingly, as is commonly done, to delineate the high- versus low-estrogen phases, diestrus 2 and proestrus were designated as “early phases,” when estradiol levels are beginning to rise and have peaked, and estrus and metestrus/diestrus 1 as “late phases,” as estradiol circulation decreases and/or plateaus, respectively.
Solutions
Standard saline solution (Tyrode's) contained (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 10 Na pyruvate, pH 7.40 adjusted with NaOH; 305–315 mOsm. 17β-Estradiol (10 nM E2) was purchased from Sigma (St. Louis, MO) and made in 100% ethanol, and working concentrations were made from stock on the day of experiment and dissolved in standard Tyrode’s solution. Linoleic acid (LA) is a polyunsaturated fatty acid (PUFA) that has been considered the prototypical stimulus for fatty acid taste and representative of other PUFAs (2). LA was purchased from Sigma (St. Louis, MO) and was prepared as described before (35). Briefly, LA was prepared as stock solutions (25 mg/mL) in 100% ethanol and stored under nitrogen at −20°C, and working concentrations (0.1–100 µM) were made immediately before the experiment. LA was prepared in deionized water for behavioral experiments and dissolved in standard Tyrode’s in all other experiments. Enzyme cocktail components collagenase A (1 mg/mL), dispase II (2.5 mg/mL), and trypsin inhibitor (1 mg/mL) were purchased from Sigma (St. Louis, MO). G-1 (100 nM), an agonist of GPER1, G-15 (1 μM), an antagonist of GPER1, were purchased from Tocris Bioscience (Minneapolis, MN).
Taste Cell Isolation
Individual taste cells were isolated from the tongues by use of isolation procedures that have been described before (7). Briefly, adult mice were euthanized by exposure to CO2, followed by cervical dislocation. Males and female groups (intact and OVX) were used in these experiments. The tongue was removed and placed in Tyrode’s solution. The dorsal side of the tongue was then injected with an enzyme cocktail in Tyrode’s solution. The tongue was incubated in Tyrode’s solution and bubbled with O2 for 30–40 min at room temperature. Following the incubation, the lingual epithelium was removed from the underlying muscle layer with forceps and pinned out in a Sylgard-lined petri dish. The lingual epithelium was incubated in divalent cation-free (Ca2+- and Mg2+-free) Tyrode’s solution for 10 min at room temperature. Individual taste cells in taste buds were removed by gentle suction with a ∼100- to 150-µm fire-polished pipette using a low-magnification dissection microscope. Taste cells were plated onto 15-mm glass coverslips coated with Corning Cell-Tak Cell and Tissue Adhesive (Corning, PN 354240) for calcium imaging. For RNA isolation and RT-PCR assays, the same general protocol was followed, taste cells and taste buds were expelled into a 1.5 microcentrifuge tube containing RNALater, and different tubes were used for each taste papilla. Additionally, control nontaste tissue epithelium from the intermolar eminence was excised for RNA isolation.
Intracellular Calcium Imaging
Individual taste cells were measured by ratiometric calcium imaging using Fura 2-AM as described previously (7). Briefly, taste cells were loaded with intracellular calcium ([Ca2+]i) indicator Fura 2-AM (Invitrogen), and InCyt Im2 software (Intracellular Imaging, Inc.) was used to control the experiment and collect the data. To record changes in [Ca2+]i, cells were excited at 340 and 380 nm, and emission was recorded at 510 nm. To identify the subset of EGFP-expressing cells in the GFP-GAD67 or GFP-PLCβ2 transgenic lines, cells were excited at 490 nm, and emission was recorded at 510 nm and marked as GFP-expressing cells within the software before recording the Fura 2 signal. The F340/F380 ratio of each cell was converted to [Ca2+]i based on a calcium calibration kit (Invitrogen). A series of LA concentrations in random order were perfused over taste cells at a flow rate of 4 mL/min, followed by 1 min of 0.1% fatty acid-free BSA solution, and then Tyrode’s solution was applied until the calcium signal returned to baseline. To observe the effect of estradiol on taste cells, a physiological concentration (10 nM) of 17β-estradiol was used. This level of estradiol has been shown previously to similarly match the low-basal circulating levels found in cycling animals and have been used to treat various types of cells to study the effects of estrogen on cellular activity (36–38). Calcium responses from LA-17β were calculated using mean percent change from LA-alone calcium responses.
Behavioral Assays
Two-bottle preference tests were used to assess the preference for linoleic acid-containing solutions compared with water during a 48-h period. Naïve mice were given a series of two-bottle preference tests for various concentrations of linoleic acid solution. The solutions were presented in order of increasing concentration; each concentration was given for 2 consecutive test days. The side of the cage containing the stimulus bottle was alternated daily. Water was available ad libitum for 48 h following the 2 consecutive test days, as described previously (39). Briefly, animals had access to the solutions 23 h/day, and the remaining hour was used to maintain and record the data. Additionally, in females, the hour was used to determine their estrous cycle stage. The same females were used to examine the preference for linoleic acid in the early and late estrous cycle phases. Only females with a stable 4- to 5-day estrous cycle were used for further data analysis. Daily measurements of body weight and fluid intake were made throughout the experiment. The ratio of stimulus intake compared with the total intake of stimulus and water was considered a measure of the preference ratio, (LA intake)/(total intake).
Procedures for the conditioned taste aversion assay (CTA) have been described in detail previously (7, 40). Briefly, a CTA was performed to assess whether manipulation of estrogen secretion alters the ability to detect polyunsaturated long-chain fatty acids such as linoleic acid. Mice were divided into four groups (SHAM-OVX females, OVX females, estrogen-treated OVX females, and males). Each group was further assigned to two categories to receive either LiCl (experimental group) or saline (control group) injections. Animals had access ad libitum to water until 24 h before conditioning and then were placed on a 23.5-h water restriction schedule. Mice were trained on the MS-160 Davis Rig apparatus (DiLog Instruments) for 3 days before conditioning, using water as stimuli. On the 3 conditioning days, mice received 1 mL of intraoral application of 100 µM LA (conditioned stimulus, CS), and then immediately paired with intraperitoneal (ip) injections of either 150 mM LiCl to cause gastric malaise (unconditioned stimulus, US) or 150 mM NaCl (saline) as a control solution. The ip injections were dose dependent based on the individual mouse’s body weight (20 mL/kg body wt dosage). Following conditioning days, mice were tested for avoidance of the CS in 3 consecutive days in the Davis Rig chamber. In addition to water, mice were tested with seven concentrations of the CS (0.1–100 µM LA), 100 mM sucrose, 100 mM NaCl, 100 mM KCl, and 3 mM denatonium benzoate (see Fig. 6). Each daily test consisted of two blocks of 13 randomly ordered trials with stimulus duration of 5 s and maximum wait times of 150 s until the first lick; each stimulus trial was preceded by a water rinse trial with a duration of 2 s. Trials with zero licks were not included in subsequent analyses. After each conditioning and testing days, animals were given 30-min access to water. This level of water deprivation caused maximal lick rates (∼5 licks/min) to water and other preferred (nonaversive) taste stimuli on testing days.
Immunofluorescence Staining
Female mice were deeply anesthetized with isoflurane and transcardially perfused with 4% paraformaldehyde (PFA, wt/vol) in 0.1 M phosphate buffer (pH 7.4). The tongues were removed, postfixed in PFA for 1–2 h, and incubated in 30% sucrose (wt/vol) in 0.1 M PBS (pH 7.4) overnight at 4°C. Blocks of tissue containing circumvallate and fungiform papillae were embedded and frozen in OCT compound (Tissue-Tek). Frozen coronal sections (20 μm) were cut using a cryostat and mounted on Superfrost glass slides. For fungiform papillae sections, the slides were microwaved in citric buffer, pH 6.0, to facilitate retrieval of the target antigens. For the circumvallate sections, the staining steps were performed without using the antigen retrieval method. Sections were washed with 0.1 M PBS, and nonspecific binding was blocked for 1 h in blocking solution (5% serum, 0.3% Triton X-100, and in PBS). Primary antibodies [1:100 anti-GPR30, Santa Cruz, (sc134576) and 1:100 anti-ERα, Millipore (06935)]; were diluted in blocking solution and applied to sections for incubation overnight at room temperature. For GPER1, the amount of Triton X-100 was decreased to 0.1% in the antibody diluent. For ERα, the amount of Triton X-100 remained at 0.3% to ensure antibody permeation into cells. After a rinse in PBS, the sections were incubated with 1:500 diluted goat-anti rabbit Alexa Fluor 594 (Invitrogen) for 2 h. After a washing with PBS, nuclei were stained with 0.5 mg/mL Hoechst 33258 in 0.1% PBS-Tween 20 (PBST) for 10 min at room temperature. The sections were rinsed three times in PBS, and coverslips were mounted with Fluoromount G (Southern Biotech). Negative controls were obtained by replacing the primary antibodies with the antibody diluent in PBS to assess any background signal. Sections were imaged with a laser scanning confocal microscope (Zeiss, LSM710) equipped with 405, 488, 561, and 633 laser lines. Images were processed and analyzed with ImageJ (41).
Total RNA Isolation and Real-Time Quantitative PCR
Taste buds and nontaste epithelium were isolated as described above, and total RNA was isolated using an RNeasy Micro Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. DNase digestion (2 h) was performed after RNA isolation by using RNase-free DNase set (Qiagen, Germantown, MD). The concentration and purity of the RNA were measured using a NanoDrop spectrophotometer (Thermo Scientific), and the mRNA integrity was determined using an Experion Bioanalyzer, Experion Highsens Chips, and Experion software (Bio-Rad Laboratories, Inc.) following the manufacturer’s recommended protocol. First-strand cDNA was transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), using 1 µg of RNA. Control samples without reverse transcriptase were run alongside to test for genomic DNA (gDNA) contamination. To check for gDNA, glyceraldehyde 3-phosphate dehydrogenase (Gapdh) primers were used and amplicons identified by electrophoresis on 2% agarose gel. Only samples without gDNA were used for further analysis. Real-time PCR analyses were performed on the SmartCycler from Cepheid (Sunnyvale, CA) to measure relative abundance of the following genes in cDNA: estrogen receptor alpha (ERα; Esr1; Mm00433149 m1), estrogen receptor beta (ERβ; Esr2; Mm00599821 m1), G protein-coupled estrogen receptor 1 (Gper1; Mm02620446 s1), cluster of differentiation 36 (CD36; Cd36; Mm00432403 m1), G protein-coupled receptor 120 (GPR120; Ffar4; Mm00725193 m1), transient receptor potential channel type M5 (Trpm5; Mm01129032_m1; Thermo Fisher scientific), and GAPDH (sense/antisense/probe: TGCACCACCAACTGCTTAGGGATGCAGGGATGATGTTC/ATCACGCCACAGCTTTCCAGAGGG; Integrated DNA Technologies, Coralville, IA). Following RT-PCR, quantification of gene expression was determined using the cycle threshold (CT). Three biological replicates were measured for each gene, and within each sample triplicate reactions were conducted. Relative gene expression was evaluated using the comparative CT (ΔΔCT) method. Target genes were compared with the endogenous control, GAPDH, and relative gene expressions were normalized to the lowest ΔCT value, the most highly expressed gene in the tissues. For gene expression of estrogen receptors, the highest-expressing gene was Gper1 in females during the early phases of the estrous cycle and was thus used as the calibrator. The highest-expressing gene involving fat taste transduction was Trpm5 in females during the early phases of the estrous cycle and was therefore used as the calibrator. Mean relative gene abundance in each sample ± SE was determined with the 2−ΔΔCT method as has been done previously (42).
Experimental Design and Statistical Analyses
Calcium imaging data analyses were based on the area under the curve (AUC) and analyzed in Origin 8-9.6 (OriginLab, Northampton, MA), and GraphPad Prism version 8.0 (GraphPad Software, Inc., San Diego, CA) was used to perform statistical analyses. For gene expression, two-way ANOVA with Tukey–Kramer method for post hoc multiple comparisons were performed in specific genes and between males and female groups. For Ca2+ imaging experiments, n = 1 was defined as one cell, and cells were obtained from multiple animals across multiple independent experiments on different days. To determine dose-response profiles of fatty acids in taste receptor cells (TRCs), the half- maximal effective concentration (EC50) values were estimated by fitting a four-parameter logistic dose-response curve to estimated values resulting from the model in addition to a one-way ANOVA with Tukey–Kramer method for post hoc multiple comparison. To determine E2 responsiveness in TRCs, one-way ANOVA, followed by Tukey’s multiple comparison post hoc tests were used to evaluate differences in calcium responses (AUC) between groups. To evaluate E2 responsiveness in GFP-PLCβ2 and GFP-GAD67 TRCs, an unpaired, two-tailed t test was used. In the two-bottle preference test, differences between males and females were analyzed for statistical significance using a one-way ANOVA, followed by Tukey’s multiple comparison post hoc tests. In the conditioned taste aversion (CTA) assays, two-way ANOVA with Tukey–Kramer method for post hoc multiple comparisons were performed to test differences between LiCl- and saline-injected mice within each group. Fatty acid detection concentrations were determined as the lowest concentration that a statistically significant difference was seen between the LiCl- and saline-injected mice within each group.
RESULTS
Sex Differences and Estrous Cycle-Dependent Expression Patterns of Estrogen Receptors in Taste Cells
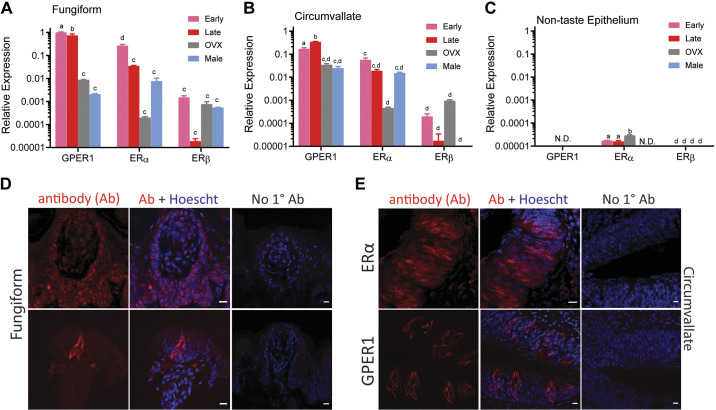
To assess sex-specific differences in the expression of ERs, RNA from circumvallate papillae, fungiform papillae, and nontaste tissue were collected from both males and females at the four phases of the estrous cycle and OVX females. In taste cells, Esr1 (ERα), Esr2 (ERβ) and the G-protein coupled estrogen receptor 1 (Gper1) transcripts were observed (Fig. 1, A–C). The highest expression of ERs was found in the fungiform papillae (Fig. 1A) compared with the circumvallate papillae (Fig. 1B) and nontaste tissue (Fig. 1C). Gper1 was highly expressed during the early phase of the cycle in the fungiform taste cells and high in the late phase in the vallate taste cells. GPER1 expression was downregulated in OVX females in both taste papillae. Moreover, ERα mRNA expression displayed peak levels during the early phases as opposed to the late phases of the cycle in both types of papillae. ERβ did not show expression differences between the papillae in females regardless of hormone status, and between male and female taste cells. There was a sex difference in ER expression: males showed generally lower mRNA levels across the ER transcripts. Although very low, ERα and ERβ receptor mRNA was also detectable in nontaste epithelium tissue (Fig. 1C).
Figure 1.
Estrogen receptor (ER) expression in mouse taste cells is altered during estrous. Quantitative real time PCR was used to determine relative expression of ERs in taste buds from the fungiform papillae (A), circumvallate papillae (B), and nontaste epithelium (C). Bars depict mean ± SE gene expression relative to the internal calibrator [early-phase G protein-coupled estrogen receptor 1 (GPER1) in fungiform papillae] of 3 independent biological replicates using GAPDH as the housekeeping gene. N.D. denotes gene was not detected in the qPCR assay. Letters (a,b,c,d) above bars indicate statistical grouping variables determined using 2-way ANOVA corrected for multiple comparisons using Tukey–Kramer method. D and E: confocal images illustrating ER localization in taste cells from fungiform and circumvallate papillae, respectively. ERα is found primarily as a nuclear receptor in taste cells and the nonclassical ER; GPER1 protein is localized in taste cells as membrane and cytosolic receptor. Scale bars, 10 µm.
Estrogen Receptor Proteins Are Expressed in Taste Cells
We next performed immunofluorescence staining of coronal sections of fungiform and vallate papillae of mouse tissue with specific anti-ER antibodies. There are reports that show the nonspecificity of the ERβ antibodies that is commonly used to detect ER proteins (43). We also found exceedingly low gene expression of ERβ in taste tissue compared with the other estrogen receptors. For these reasons, we focused on examining only the protein expression of ERα and GPER1 in taste cells. GPER1 proteins were present as membrane form extranuclear receptors and were not present in nontaste tissue. ERα was abundantly expressed in taste cells, and the mRNA could be found in nontaste tissue (Fig. 1, D and E), protein staining was also evident outside of the taste buds (Fig. 1, D and E).
GPER1 Is Expressed in Receptor (Type II) Cells but Not in Presynaptic (Type III) Cells
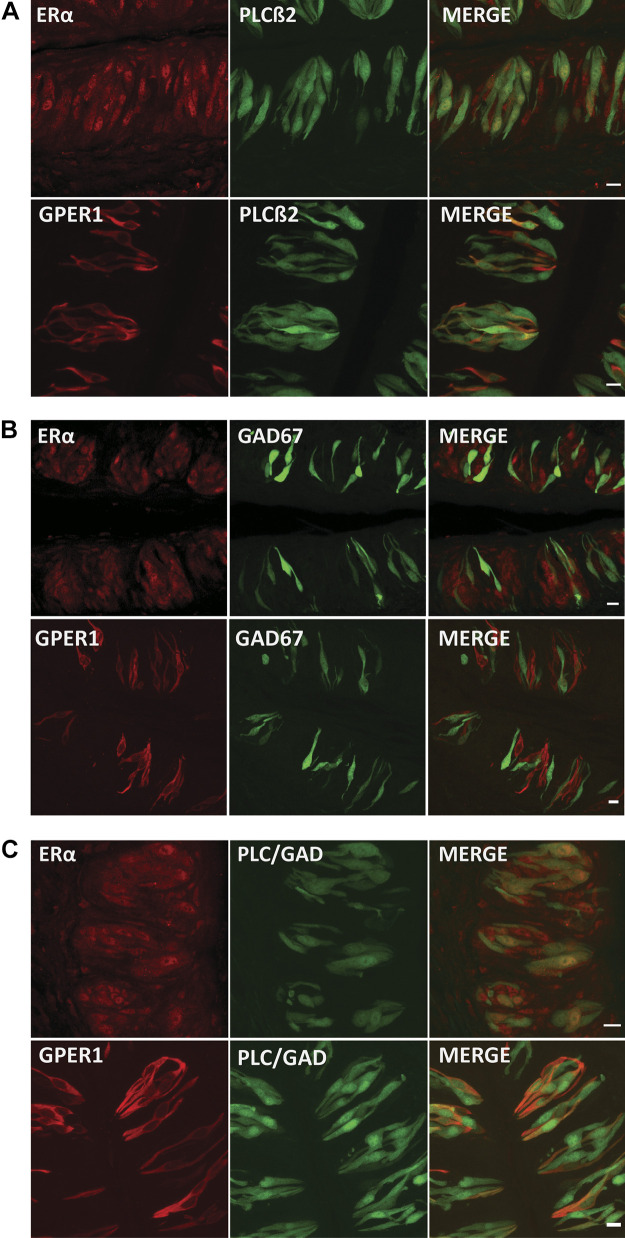
Next, the specificity of ERs in taste tissue suggested that estrogen might have a function in taste. To address this issue, we used GFP transgenic mice expressing markers for presynaptic (type III) cells and receptor (type II) cells and costained taste tissue from these mice for ERs. We found that estrogen receptors (ERα and GPER1) in taste cells colocalized with a subset of cells expressing PLCβ, a marker for type II taste cells, suggesting that estrogen plays a role in those cells that express the signaling pathway for bitter, sweet, umami, and fatty acids (Fig. 2A). Moreover, we found that GPER1 was almost completely absent in GAD67-expressing cells, a marker for type III taste cells (Fig. 2B). ERα colocalized with small number of GAD67-expressing cells as a nuclear receptor protein. It has been shown that estrogens alter the function of glial cells in the central nervous system (44, 45). Additionally, Curtis (19) found that estrogen enhances salt taste sensitivity and leads to decreased salt consumption. Although unclear, some type I taste cells are thought to transduce salt taste (46). Accordingly, we investigated the presence of ERs in cells that were not GAD67- and not PLCβ-expressing cells. We bred transgenic mice expressing GFP in both type II and type III cells and looked for expression in cell types that did not express GFP. Although this approach does not ensure that non-GFP cells only consist of type I cells, nuclear-only ERα was found in some GFP-expressing cells and in many non-GFP cells. On the other hand, GPER1 primarily costained with GFP-only expressing cells (Fig. 2C).
Figure 2.
Estrogen receptors (ERs) are present in type II cells. A: representative immunofluorescent images for ERα and G protein-coupled estrogen receptor 1 (GPER1; red) in vallate taste buds from mice expressing GFP in phospholipase Cβ2 (PLCβ2)-positive (type II) cells, and the corresponding merged image. Yellow color indicates both ERs and PLCβ2 within the same taste cells. Note that some ER-positive cells are not colocalized with PLCβ2. Scale bars, 10 µm. B: immunofluorescence for ERs and a marker [GFP-glutamic acid decarboxylase 67 (GAD67)] for type III cells in mouse vallate taste buds. Scale bars, 10 µm. C: immunofluorescence assays for ERs in double-GFP transgenic mice show that there was little GPER1 receptor expression in the population of non-type II and non-type III vallate taste cells. ERα colocalize with GFP-positive cells and are apparent in some non-GAD67- or PLCβ2-expressing cells (green). Scale bars, 10 µm.
Sex Differences in the Molecular Elements of Fat Taste Detection
Given that estrogen receptor expression was present predominantly in type II taste cells, we examined potential sex differences in genes that underlie fatty acid transduction, a process that is largely limited to this cell type. In the fungiform papillae, Trpm5 transcripts significantly varied at phases of the estrous cycle and between males and females. Trpm5 transcripts were more abundant in males and females in early phases of the estrous cycle compared with females in the late phases of the estrous cycle. The levels of Cd36, Ffar4 (GPR120), transient receptor potential channel type M4 (Trpm4) TrpM4, and potassium voltage-gated channel subfamily A member 5 (Kv1.5; Kcna5) were not significantly different between males and females and at phases of the estrous cycle. In the vallate papillae, the Cd36, Gpr120, Trpm5, and Trpm4 mRNA levels were comparable between males and females in early phases of the estrous cycle but showed lower mRNA levels in the late phases of the estrous cycle. Kcna5 transcript levels of males and females were roughly comparable to those of females in late phases of the estrous cycle in other genes.
The transcript levels of all genes in OVX females showed a steadily decreasing trend (Fig. 3A).
Figure 3.
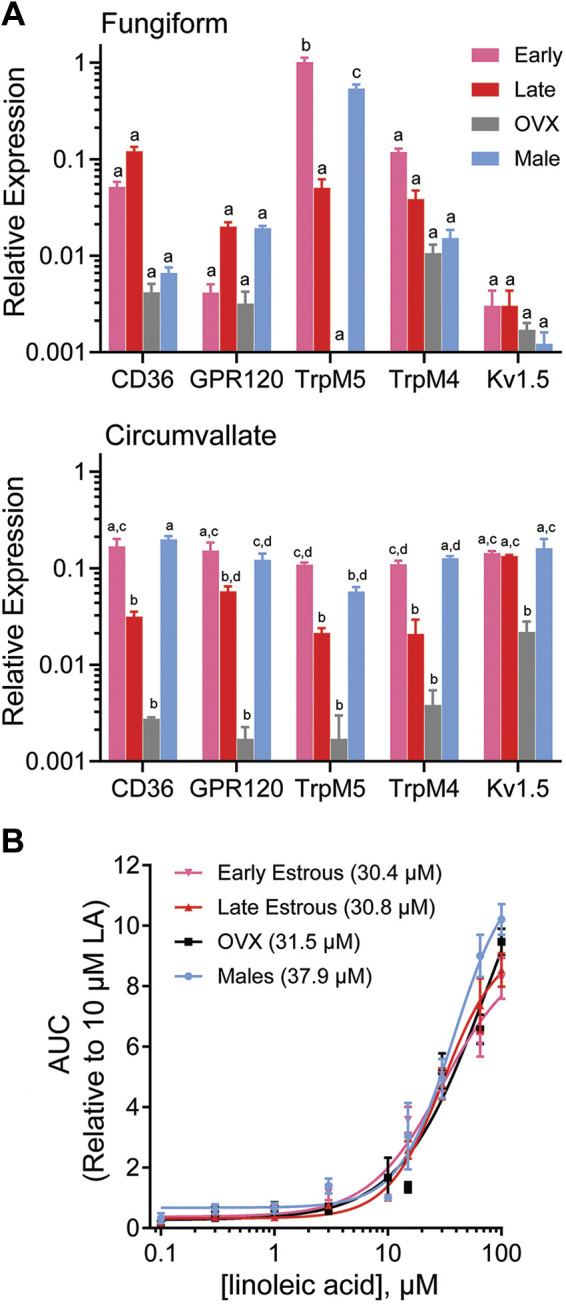
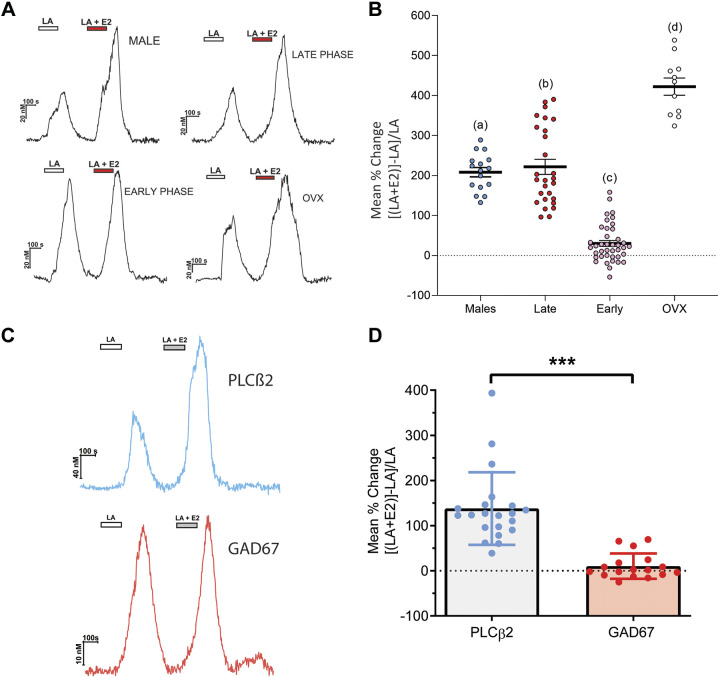
Expression and function of fatty acid signaling elements in male and female mice. A: bars depict mean ± SE gene expression relative to the internal calibrator TrpM5 during early phases of the estrous cycle in fungiform papillae of 3 independent biological replicates, using GAPDH as the housekeeping gene in male and female groups of fungiform and vallate taste cells. Letters (a,b,c,d) above bars indicate statistical grouping variables determined using two-way ANOVA corrected for multiple comparisons using Tukey–Kramer method. B: dose-response profiles for linoleic acid (LA) in males, females in early and late stages of the estrous cycle, and ovariectomized (OVX) females. Changes in intracellular calcium ([Ca2+]i) in response to perfusion with LA (0.1 μM to100 μM) were measured by ratiometric Fura 2-based imaging, and area under the curve (AUC) measurements were calculated during stimulus response. One-way ANOVA with Tukey–Kramer method for post hoc multiple comparisons was used to compare the groups. Sigmoidal curves were fitted using normalized response data points in GraphPad Prism. All data are presented as means ± SE. The EC50 for male, females in early estrous phases, females in late estrous phases, and OVX females were 37.9, 30.4, 30.8, and 31.5 µM, respectively.
Increased Cellular Response to LA is Estrogen Mediated
On the basis of the previous data showing that genes involved in fat taste transduction vary between males and females and at the phases of the estrous cycle, we sought to determine whether this translated to response changes in taste cells. We have also previously shown that fatty acids, particularly LA, can activate taste cells in a dose-dependent manner (7). We conducted concentration-response experiments in taste cells of LA at a range of concentrations from 0.1 µM to 100 µM. We used isolated taste cells from cycling females during early and late stages of the estrous cycle, OVX females, and males. A one-way ANOVA showed no significant difference in LA responses between groups (F(24,47) = 0.06450, P > 0.05). We also found a similar dose-response relationship for LA in taste cells (Fig. 3B). In addition, we characterized fatty acid responsiveness as the LA concentration producing a half-maximal response (EC50), i.e., the concentration of LA eliciting a half maximal intracellular calcium change (Fmax/2), by fitting concentration-response data with a logistic relation. We observed a higher EC50 in males than in females, indicating that females regardless of hormone status appear to be slightly more responsive to LA than their male counterparts. We found no difference between fungiform and circumvallate papillae in the concentration-response function, so the combined data were analyzed.
Using calcium imaging, we examined fatty acid responses in taste cells in the presence and absence of exogenous estradiol (E2, 10 nM). In all experiments, E2 alone did not produce a measurable change in intracellular calcium. The effect of E2 was evident only in combination with fatty acid stimulation and was seen independently of stimulus order. A one-way ANOVA showed the effect of exogenous E2 on taste cells from female groups (early phase, late phase, and OVX) and males was significant (F(47) = 113.7, P < 0.001). This effect of estradiol on LA responses was dependent on the stage of the estrous cycle; exogenous estradiol had little to no effect on female taste cells during the earlier phases of the cycle, a period when high endogenous estradiol was present at cell collection. However, cells collected during conditions of low circulating estrogen (late phase) showed that addition of estradiol greatly enhanced responsiveness to fatty acids (Fig. 4, A and B). This enhancement was also seen in taste cells collected from male mice. Furthermore, taste cells from OVX females also showed an increase in fatty acid responses during estrogen application. There were also greater responses in cells from OVX compared with cells from male mice when exposed to exogenous E2 (Fig. 4B). Collectively, our data are consistent with a role for estrogen in the peripheral taste system and specifically in the modulation of cellular responses to fatty acids (fat taste).
Figure 4.
Acute exposure of estrogen increases taste cell responses to linoleic acid. A: Representative traces of the intracellular calcium ([Ca2+]i) change induced by 10 µM linoleic acid (LA) and LA with 17β-estradiol (E2, 10 nM) in taste cells of wild-type (WT) males, females during early phases, late phases, and ovariectomized (OVX) females. B: summary data showing mean percent change of responding taste cells comparing LA (10 µM) alone to the combination of LA and estradiol (10 nM). Individual data points represent one taste cell (WT males, n =16; females during estrous phase, n = 28; females during proestrus phase, n = 40; and ovariectomized females, n = 11). Different letters represent different statistical groups determined by one-way ANOVA with Tukey–Kramer method for post hoc multiple comparisons. C: enhancement of fatty acid responses by estrogen is found only in type II cells. Taste cell responses were recorded from isolated taste cells (n = 37) of female mice on estrous stage. Cells were sequentially stimulated with LA (10 µM) and LA + E2 (10 nM) in random order. Traces show responses from phospholipase Cβ2 (PLCβ2)-GFP cells (blue) and glutamic acid decarboxylase 67 (GAD67)-GFP cells (red). D: increased responsiveness to the combination of LA and E2 was observed primarily in cells expressing PLCβ2-GFP but not cells expressing GAD67-GFP. ***P < 0.001 as indicated by Student’s t test.
Furthermore, we tested whether the ability of estradiol to modulate fatty acid responses was specific in type II or type III taste cells. Because the acute actions of estradiol were evident primarily during the late phases of the estrous cycle, late-phase female mice expressing GFP under control of the PLCβ2 (type II) or GAD67 (type III) promoters were used for calcium imaging. Using a similar paradigm to that in Fig. 4A, we found that only PLCβ2 cells (type II cells) showed an estradiol-mediated enhancement of LA responses (t(48) =6.238, P < 0.0001). Comparatively, there was no obvious enhancement of LA responses by estradiol observed in GAD67-expressing (type III) cells (Fig. 4, C and D).
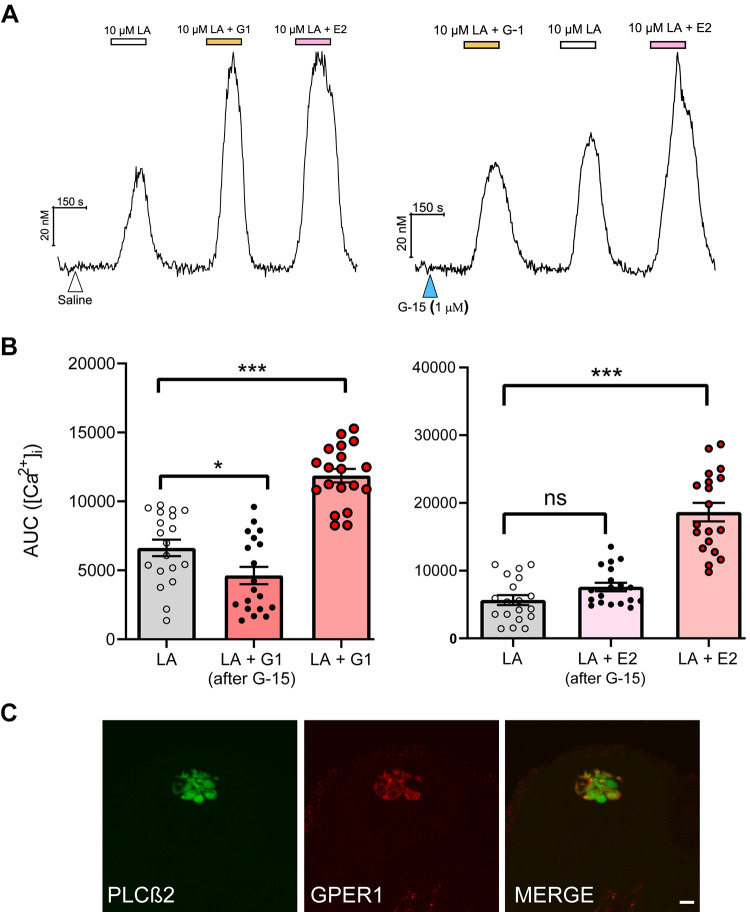
Because of the timing of our responses, we initially hypothesized that our rapid increase in cytosolic calcium upon fatty acid activation in taste cells was likely mediated by stimulation of a membrane form of ER. Whereas it has been reported that predominately nuclear ERα and ERβ can mediate membrane estrogen signaling, typically rapid effects are most likely mediated by membrane-associated estrogen receptors such as GPER1 (47). Previous studies have indicated that estrogen is involved in regulation of [Ca2+]i release via membrane-associated ERs (mERs) including GPER1 (48, 49). Moreover, we found GPER1 to be the only ER expressed exclusively in taste cells. We used a pharmacological approach to test for the involvement of GPER1 in mammalian taste cells. The GPER1 agonist, G-1, with linoleic acid, showed an increase in intracellular calcium, indicating that GPER1 has a specific role in taste cells [Fig. 5, A and B; F(2,54) = 42.01, P < 0.0001]. On the other hand, administration of the GPER1 antagonist G-15 60 seconds before the combination of fatty acid and E2 exposure significantly attenuated estrogen’s ability to enhance LA responses [Fig. 5, A and B; F(2,54) = 52.80, P < 0.0001]. This acute response was found in fungiform taste papillae cells. To determine whether this acute response might involve PLCβ2, fungiform slices that expressed GFP in PLCβ2 promoter were stained with GPER1 antibody. GPER1 proteins were highly colocalized with PLCβ2-expressing cells (Fig. 5C).
Figure 5.
Estrogen enhancement of fatty acid responses is dependent upon G protein-coupled estrogen receptor (GPER1). A: representative traces of [Ca2+]i were recorded in taste cells during bath stimulation of linoleic acid (LA; 10 µM) in the absence or presence of estradiol (E2; 10 nM) and the GPER1 agonist, G-1 (100 nM) and after brief pretreatment with saline or the GPER1 antagonist, G-15 (1 µM). B: fungiform taste cells were stimulated with LA in absence or presence of E2 and G-1. Before stimulation, taste cells were pretreated with G-15 (1 µM) for 60 s. Area under the curve (AUC) measurements were calculated during stimulus response. All data are presented as means ± SE. C: immunostaining of fungiform taste papillae taste buds showing specific labeling of phospholipase Cβ2 (PLCβ2; green) and GPER1 (red); GPER1 proteins are present in PLCβ2-positive cells similar to that found in the circumvallate papillae (Fig. 2A). *P < 0.05, ***,P < 0.001, ns, Not significant.
Estrogen Increases LA Detection Levels and Alters Preference in Taste-Guided Behaviors
We have previously reported that female rats detected fatty acids at lower concentrations and showed an increased avoidance to linoleic acid in a conditioned taste aversion test compared with their male counterparts (40). To directly examine sex differences in LA responses, we compared the lowest avoidance concentration of linoleic acid in males and females following formation of a CTA against 100 µM LA. A two-way ANOVA showed that LiCl-injected mice in all the groups avoided licking LA at low concentrations, whereas the saline-injected control group licked LA relatively at the same rate as water; males F(11) = 31.18, P < 0.0001, SHAM-OVX, F(11) = 30.90, P < 0.000, OVX + EB, F(11) = 17.14, P < 0.0001, and OVX F(11) = 12.46, P < 0.001 (Fig. 6B). In line with previous data, intact female mice detected lower concentrations of LA than males: 0.3 µM and 3 µM LA, respectively. To examine the effect of circulating hormones in females on taste responsiveness, female mice underwent ovariectomy and were exposed to the same behavioral paradigm. OVX females showed significantly decreased LA responsiveness. Estradiol has been shown to alter taste responsiveness and modify tastant-specific behaviors; we assessed the effect of chronic estradiol in linoleic acid responsiveness (17, 18, 50, 51). OVX mice treated with estradiol displayed greater behavioral response to linoleic acid than non-treated OVX mice, suggesting that estradiol may influence fatty acid detection in females. We found LiCl-mediated aversion to linoleic acid did not generalize to other control tastants (Fig. 6C).
To validate the effectiveness of the ovariectomies and the estrogen replacement treatment, adult C57BL/6J mice were euthanized after the completion of the CTA experiments, and uteri were excised and weighed. Changes in uterine wet weight (milligrams) in each group across experimental conditions (LiCl and NaCl) were analyzed. Uterine weights (means ± SE) for the LiCl and NaCl groups were as follows: Sham (non-OVX): 97.0 ± 2.1 mg (LiCl), 105.1 ± 6.8 mg (NaCl); OVX: 23.0 ± 1.8 mg (LiCl), 28.2 ± 1.9 mg (NaCl); OVX + EB: 109.8 ± 4.3 mg (LiCl), 116.1 ± 4.5 mg (NaCl). One-way ANOVA followed by Tukey–Kramer method for post hoc multiple comparisons showed the OVX group had the lowest uterine weight compared with SHAM females and OVX females with E2 replacement. The OVX groups were significantly different from the corresponding SHAM and OVX + EB groups [SHAM, n = 7, OVX + EB, n = 5, OVX + Oil, n = 7; NaCl: F(2,16) = 94.49, P < 0.0001; LiCl: F(2,16) = 329.5, P < 0.0001].
In the two-bottle preference assay, a dose-response curve was used to characterize LA preference for males and females. Preference LA concentrations ranging from 0.1 µM to 100 µM were plotted for males and females. There was no significant difference in preference for LA in females at the four phases of the estrous cycle, so the average preference ratio was plotted for females. Males showed trends toward a greater preference for fatty acids over water at the higher concentrations of LA (≥10 µM; Fig. 6, inset). However, there was no significant difference in the concentration of LA that produced the half-maximal preference for linoleic acid (EC50) between males (2.4 µM) and females (4.3 µM).
DISCUSSION
The present study supports a role for estrogen in peripheral taste detection in mice. Several results provide evidence in support of this interpretation. 1) Two of the three types of estrogen receptors, ERα and GPER1, are significantly expressed in mouse taste cells to varying degrees, consistent with taste cells being susceptible to both genomic and nongenomic (rapid) effects of this hormone. 2) Estrogen (estradiol) enhances fatty acid-induced calcium responses in isolated taste cells, an effect that is largely restricted to type II cells. 3) Estrogen influences the responsiveness to free fatty acids in in vivo assays of taste function.
Our initial focus was to determine whether estrogen receptors are expressed in mouse taste cells (TRCs). Using both qPCR and immunohistochemistry, we showed that ERα and GPER1 were present in mouse taste cells. Additionally, in female mice, the relative expression of ERα and GPER1 was phase dependent during the estrous cycle. The expression of GPER1 and ERα in the fungiform taste papillae showed significant increases in the early stage (high estradiol) compared with the late stage of the estrous cycle. Our immunohistochemical experiments further illustrated the localization of ERs in taste cells. GPER1 appeared as extranuclear receptors while ERα appeared as a nuclear receptor in taste tissue as well as in surrounding nontaste tissue. Our results implicate both rapid actions of estrogens via GPER1, while ERα might be involved in modulating long-term transcriptional events involving cellular signaling pathways in the taste system.
Taste cells are classified into four discrete cell types, fulfilling roles as basal cells, sustentacular cells (type I), receptor cells (type II), and presynaptic cells (type III), in simplest terms. Within the three cell types in the taste buds exist different pathways containing distinct mechanisms for the initial recognition events for salt, sour, sweet, bitter, umami, and fatty acids (52–55). Although comparatively understudied, sex differences have been implicated in taste of sweet (20), salt (19, 56), and fatty acids (40, 51, 57). However, the mechanisms underlying these sex differences remain unclear, although a logical link lies in the effects of sex hormones in the taste system (58). We found that GPER1 colocalized with PLCβ2, a marker for type II cells, but are apparently absent in GAD67-expressing (type III) cells, whereas ERα appears to be present in most taste cell types. In recent next-generation RNA sequencing (RNA-seq) data examining the specific connection of sweet and bitter TRCs, Lee et al. demonstrated that ERα is present in both bitter- and sweet-modulating taste cells whereas ERβ transcript remained only in sweet-responsive taste cells (59). A second study using RNA-seq (60) by examining specific genetic profiles of taste cells did not report the presence of ERs in type II and type III taste cells. The discrepancy reported in those studies could be due to methodological differences including but not limited to RNA-seq library preparation approaches, reporter markers for taste cell types, and analytical methods. Nevertheless, we showed that there is a discernable presence of ER protein especially in type II taste cells.
To further support the evidence that estradiol influences fat taste signaling, we examined the transcript levels of known genes that regulate fat chemosensation and transduction. More notably, we found that Trpm5, which is a critical component of the fat taste signaling cascade (7), is highly variable between males and females and changes in females according the phases of the estrous cycle. Additionally, the levels of Trpm5 are more abundant in fungiform taste cells than vallate taste cells; this differential transcript abundance may contribute to the sex differences in fat chemosensitivity. Additionally, females during the early phases of estrous cycle and males show remarkably similar patterns of gene expression compared with females in late phases of the estrous cycle in vallate papillae. Ovariectomy in females resulted in a decrease in gene expression, alluding to the possibility that the fluctuations of gonadal hormones in females contribute to the adjustments of gene expression involved in taste function. Of note, ERα is primarily localized in the nuclear region of taste cells. Similar to other physiological processes, it is possible that it binds to estrogen-responsive elements or other transcriptional factors and regulates the gene transcription involving taste sensitivity (61). Our data are suggestive of the important role of Trpm5 and other genes as important mediators in studying sex differences in taste compounds such as sweet, bitter, umami, and fat. Further research is needed to reveal the underlying mechanisms of 17β-estradiol and the consequences of molecular alterations in the taste system.
To date, there is no consensus mouse model that expresses unequivocally a universally accepted marker for identification of all type I cells, which constitute the most cells in each taste bud. The expression of nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) and the glial glutamate-aspartate transporter (GLAST) have been previously used to identify type I cells (62). However, these protein markers have had problems identifying type I cells exclusively (63). To gauge evidence of ER expression in type I cells, we used tissue expressing both GFP in type II and type III cells. Although other cells such as basal cells are present in taste buds and we cannot be certain that low GFP expression might mask the contribution of some type II or III cells, we found the presence of ERα in a significant fraction of cells that did not express PLCβ2 or GAD67. The predominant cells in the taste bud are these glial-like cells; accordingly, we reason that ERα is present in at least a subpopulation of type I cells. Analogous to the glial cells in the rest of the nervous system, type I cells may elicit estrogen-mediated protection to the rest of the taste bud (64). However, ERs in type I cells are not involved in the cell-based responses to fatty acids seen in the present study. The function of ERs in type I taste cells remains unknown and will require additional targeted research.
Unlike other cells (65), estrogen alone did not alter [Ca2+]i in taste cells. We propose a mechanism involving estrogen in taste cells where it enhances the rise in cytosolic calcium upon activation with linoleic acid. Moreover, this enhanced activation is estrous-phase specific; higher estrogen phases (proestrus and diestrus II) are not impacted significantly by exogenous application of estradiol. It is feasible that high circulating estrogen at the time of cell isolation leads to a decrease in subsequent cell responsiveness through an unknown mechanism involving changes in receptor expression or responsiveness. Cells were more prone to estrogenic activation during the latter phases of the estrous cycle, when endogenous estrogen is limited. Compared with our gene expression data, we observed results in apparent conflict with our functional cellular data. Females in early phases of the estrous cycle showed upregulation of genes in a similar pattern to males, whereas in the functional Ca2+ imaging data, late-phase females and males showed similar responses to fatty acid and E2 application. We hypothesize that it is feasible that the higher levels of estradiol in early phase females may be modulating genes involved in fat detection and that the acute exposure of exogenous E2 is more apparent in late-phase females. Although estrogen certainly is playing a role in modulating fatty acid responsiveness at the molecular, cellular, and behavioral levels based upon the data in the present work, it does not preclude involvement of additional steroid and nonsteroid hormones contributing to these changes as well. Further studies aimed at looking at the involvement of other endocrine pathways in the OVX ± E2 model will be needed to generate a more complete picture of the hormonal influence on (fatty acid) taste.
The enhancement of the LA-induced calcium rise by estrogen was rapid and was seen within a few minutes of application. Therefore, we initially focused on the membrane ER, GPER1, to determine whether it was responsible for the changes in cell-induced responses seen. Using a GPER1 agonist (G-1) we saw an increase in intracellular Ca2+ similar to the effect induced by E2 in fat-responsive cells, and the GPER1 antagonis G-15, attenuated this increased response. In other cell types, GPER1-coupled Ca2+ responses were mediated by signaling cascades involving inositol trisphosphate receptor and leading to calcium release from the intracellular stores (66). While both a release from the intracellular Ca2+ pool and an extracellular influx are possible, we found that PLCβ2 proteins are highly colocalized with GPER1 proteins. These data, along with the idea that E2 is acting in concert with fatty acids, prompted us to conclude that the source of the fast-acting calcium rise is via the PLC pathway. Our results are consistent that GPER1 is involved in rapid actions of estrogen in taste cells, particularly fatty acid-activated taste cells. A recent study has implicated a role for GPER1 in the etiology of diabetes and obesity, providing an interesting parallel to the current findings (67). Studies in the taste system that contributed to the development of the well-regarded signaling pathways have not taken the contribution of cycling hormones into consideration. Thus, the observation that taste cells can elicit variable responses based on the sex of the animal and current hormonal status is largely understudied. We provide a possible mechanism that explains the reported behavioral and cellular differences in males and females regarding fatty acid signaling in the peripheral taste system.
Estrogen is a critical regulator of nutrient metabolism. Estradiol has been shown to play a significant role in food intake, and loss-of-function promotes metabolic disturbance including overeating and weight gain (68, 69). Although these studies provide data on the role of estradiol in food intake, little is known about the effects of estradiol in nutrient detection. There is evidence suggesting that estrogen may influence taste sensitivity and threshold for some sapid molecules. Compared with males, females showed enhanced sensitivity to salt and umami tastants and decreased detection of sweet tastants (20, 57). It was also previously reported that female rats were more responsive to fatty acids and did not discriminate between different types of fatty acids as well as their male counterparts (40). The CTA results (Fig. 6) showed that intact females could detect (avoid) LA at lower concentrations than males. Calcium imaging data (Fig. 3B) also revealed this difference in responsiveness (via EC50 values) and, although significant, may not be large enough to argue that sensitivity or affinity is a behaviorally relevant difference between the sexes. However, removal of ovaries diminished LA responsiveness, and this effect was reversed by exogenous administration of estradiol. In our experiments, we compared males and females, but more detailed experiments are needed to illustrate that short-term and transient changes of the estrous cycle contribute to the detection of fatty acids at the behavioral level.
The findings from these experiments have illustrated that females are significantly more responsive to fatty acid taste than males and that this responsiveness is partly due to the presence of estrogen. We postulate that the changes in responsiveness of the taste system to fatty acid stimuli are intimately related to estrogenic perturbation in taste cells. The specific roles of estrogen receptors in food intake have been reported. Due to the dynamic locations of ERs in cells, it is possible that each ER receptor subtype contributes uniquely to overall food intake but might vary across species and the nutritional state of the organism. Overall, our results validate that steroid hormones influence taste-guided behaviors in males and females. Particularly, estrogen can enhance fatty acid taste behaviors in females and is an important component of the underlying process influencing sex differences in fat taste responses.
Conclusions
Sex differences in the taste system are not well understood yet may be important for recognizing dietary nutrients and directing subsequent intake for males and females. We show that sex differences of mice in cellular and behavioral responses to fatty acids, which contribute to the taste of fat, may be due to estrogenic effects on fat chemosensory pathways. Identification of the estrogen receptors in specific types of taste cells may help explain the cellular mechanisms of estrogen influence on taste processing, given the cyclical secretion of estradiol in the female mice and its effects on fat perception. By providing connections between nutrient sensing and sex hormones, new therapeutic practices for processes related to the control of food intake may be available.
Importantly, our study demonstrates that taste receptor cells are a direct target for actions of estrogen and that there are multiple receptors with differing patterns of expression in taste cells. Estrogens exert their action by increasing cellular responses to fatty acids in taste cells and can significantly increase fatty acid taste responsiveness in taste-guided behaviors. These results lay the foundation for the role of estrogen in peripheral nutrient detection and its regulation.
GRANTS
This research was supported by National Institute on Deafness and Other Communication Disorders Award R01DC013318.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S.D. and T.A.G. conceived and designed research; N.S.D., A.N.C., B.J.M., and Y.L. performed experiments; N.S.D., A.N.C., and T.A.G. analyzed data; N.S.D., Y.L., and T.A.G. interpreted results of experiments; N.S.D., A.N.C., and T.A.G. prepared figures; N.S.D. drafted manuscript; A.N.C., Y.L., and T.A.G. edited and revised manuscript; N.S.D., A.N.C., B.J.M., Y.L., and T.A.G. approved final version of manuscript.
REFERENCES
- 1.Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science 326: 443–445, 2009. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbertson TA. Gustatory mechanisms for the detection of fat. Curr Opin Neurobiol 8: 447–452, 1998. doi: 10.1016/S0959-4388(98)80030-5. [DOI] [PubMed] [Google Scholar]
- 3.Iiyama S, Toko K, Matsuno T, Yamafuji K. Responses of lipid membranes of taste sensor to astringent and pungent substances. Chem Senses 19: 87–96, 1994. doi: 10.1093/chemse/19.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Montell C. Gustatory receptors: not just for good taste. Curr Biol 23: R929–932, 2013. doi: 10.1016/j.cub.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riera CE, Vogel H, Simon SA, Damak S, Le Coutre J. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J Neurosci 29: 2654–2662, 2009. doi: 10.1523/JNEUROSCI.4694-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zajac AL, Lei W, Christensen CM, Margolskee RF, Bouysset C, Golebiowski J, Zhao H, Fiorucci S, Jiang P. Metal ions activate the human taste receptor TAS2R7. Chem Senses 44: 339–347, 2019. doi: 10.1093/chemse/bjz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci 31: 8634–8642, 2011. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattes RD. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol Behav 104: 624–631, 2011. doi: 10.1016/j.physbeh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizushige T, Inoue K, Fushiki T. Why is fat so tasty? Chemical reception of fatty acid on the tongue. J Nutr Sci Vitaminol (Tokyo) 53: 1–4, 2007. doi: 10.3177/jnsv.53.1. [DOI] [PubMed] [Google Scholar]
- 10.Running CA, Craig BA, Mattes RD. Oleogustus: the unique taste of fat. Chem Senses 40: 507–516, 2015. doi: 10.1093/chemse/bjv036. [DOI] [PubMed] [Google Scholar]
- 11.Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: a sixth taste modality? Physiol Rev 96: 151–176, 2016. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 53: 82–92, 2014. doi: 10.1016/j.plipres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Pittman DW. Role of the gustatory system in fatty acid detection in rats. In: Fat Detection: Taste, Texture, and Post Ingestive Effects, edited by Montmayeur JP and le Coutre J.. Boca Raton (FL): CRC Press/Taylor & Francis, 2010. [Google Scholar]
- 14.Wu BN, O'Sullivan AJ. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J Nutr Metab 2011: 391809, 2011. doi: 10.1155/2011/391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104: 517–524, 2011. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke SNDA, Ossenkopp KP. Taste reactivity responses in rats: influence of sex and the estrous cycle. Am J Physiol Regul Integr Comp Physiol 274: R718–R724, 1998. doi: 10.1152/ajpregu.1998.274.3.R718. [DOI] [PubMed] [Google Scholar]
- 17.Curtis KS, Contreras RJ. Sex differences in electrophysiological and behavioral responses to NaCl taste. Behav Neurosci 120: 917–924, 2006. doi: 10.1037/0735-7044.120.4.917. [DOI] [PubMed] [Google Scholar]
- 18.Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav 80: 657–664, 2004. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Curtis KS. Estradiol and osmolality: behavioral responses and central pathways. Physiol Behav 152: 422–430, 2015. doi: 10.1016/j.physbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis KS, Stratford JM, Contreras RJ. Estrogen increases the taste threshold for sucrose in rats. Physiol Behav 86: 281–286, 2005. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Butera PC. Estradiol and the control of food intake. Physiol Behav 99: 175–180, 2010. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56: 1051–1058, 2007. [Erratum in Diabetes 56: 2649, 2017]. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 23.Mela V, Vargas A, Meza C, Kachani M, Wagner EJ. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J Steroid Biochem Mol Biol 160: 15–26, 2016. doi: 10.1016/j.jsbmb.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A 97: 11044–11049, 2000. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin YK, Egan JM. Roles of hormones in taste signaling. Results Probl Cell Differ 52: 115–137, 2010. doi: 10.1007/978-3-642-14426-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCbeta2 promoter in a functional class of taste receptor cells. Chem Senses 31: 213–219, 2006. doi: 10.1093/chemse/bjj021. [DOI] [PubMed] [Google Scholar]
- 27.de Beun R, Jansen E, Smeets MA, Niesing J, Slangen JL, van de Poll NE. Estradiol-induced conditioned taste aversion and place aversion in rats: sex- and dose-dependent effects. Physiol Behav 50: 995–1000, 1991. doi: 10.1016/0031-9384(91)90427-P. [DOI] [PubMed] [Google Scholar]
- 28.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 26: 1103–1109, 2002. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto YK, Kasai M, Tomihara K. The enhancement effect of estradiol on contextual fear conditioning in female mice. PLoS One 13: e0197441, 2018. e0197441. doi: 10.1371/journal.pone.0197441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modder UI, Riggs BL, Spelsberg TC, Fraser DG, Atkinson EJ, Arnold R, Khosla S. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur J Endocrinol 151: 503–510, 2004. doi: 10.1530/eje.0.1510503. [DOI] [PubMed] [Google Scholar]
- 31.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 32.Choleris E, Clipperton-Allen AE, Gray DG, Diaz-Gonzalez S, Welsman RG. Differential effects of dopamine receptor D1-type and D2-type antagonists and phase of the estrous cycle on social learning of food preferences, feeding, and social interactions in mice. Neuropsychopharmacology 36: 1689–1702, 2011. doi: 10.1038/npp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ter Haar MB. Circadian and estrual rhythms in food intake in the rat. Horm Behav 3: 213–219, 1972. doi: 10.1016/0018-506X(72)90034-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Z, Liu X, Senthil Kumar SP, Zhang J, Shi H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm Behav 63: 533–542, 2013. doi: 10.1016/j.yhbeh.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav 86: 681–690, 2005. doi: 10.1016/j.physbeh.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee J, Cardarelli RA, Cantaut-Belarif Y, Deeb TZ, Srivastava DP, Tyagarajan SK, Pangalos MN, Triller A, Maguire J, Brandon NJ, Moss SJ. Estradiol modulates the efficacy of synaptic inhibition by decreasing the dwell time of GABAA receptors at inhibitory synapses. Proc Natl Acad Sci U S A 114: 11763–11768, 2017. doi: 10.1073/pnas.1705075114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17Beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res 76: 688–696, 2004. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci 24: 5315–5321, 2004. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers KP, Sclafani A. Development of learned flavor preferences. Dev Psychobiol 48: 380–388, 2006. doi: 10.1002/dev.20147. [DOI] [PubMed] [Google Scholar]
- 40.Pittman DW, Smith KR, Crawley ME, Corbin CH, Hansen DR, Watson KJ, Gilbertson TA. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Senses 33: 449–460, 2008. doi: 10.1093/chemse/bjn012. [DOI] [PubMed] [Google Scholar]
- 41.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol 289: C868–C880, 2005. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- 43.Andersson S, Sundberg M, Pristovsek N, Ibrahim A, Jonsson P, Katona B, Clausson CM, Zieba A, Ramström M, Söderberg O, Williams C, Asplund A. Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun 8: 15840, 2017. 15840. doi: 10.1038/ncomms15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim Biophys Acta 1800: 1106–1112, 2010. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89: 567–578, 1999. doi: 10.1016/S0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 46.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9: 1, 2008. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, Black MA, Donato AL, Arterburn JB, Oprea TI, Prossnitz ER, Dun NJ, Jordan VC. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res 70: 1184–1194, 2010. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, Macpherson LJ, Parada CA, Zuker CS, Ryba NJP. Rewiring the taste system. Nature 548: 330–333, 2017. doi: 10.1038/nature23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler MJ, Hildebrandt RP, Eckel LA. Selective activation of estrogen receptors, ERalpha and GPER-1, rapidly decreases food intake in female rats. Horm Behav 103: 54–61, 2018. doi: 10.1016/j.yhbeh.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding X, Gao T, Gao P, Meng Y, Zheng Y, Dong L, Luo P, Zhang G, Shi X, Rong W. Activation of the G protein-coupled estrogen receptor elicits store calcium release and phosphorylation of the mu-opioid receptors in the human neuroblastoma SH-SY5Y cells. Front Neurosci 13: 351, 2019. doi: 10.3389/fnins.2019.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci 30: 12950–12957, 2010. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke SN, Bernstein IL. NaCl preference increases during pregnancy and lactation: assessment using brief access tests. Pharmacol Biochem Behav 68: 555–563, 2001. doi: 10.1016/S0091-3057(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 53.Stratford JM, Curtis KS, Contreras RJ. Chorda tympani nerve transection alters linoleic acid taste discrimination by male and female rats. Physiol Behav 89: 311–319, 2006. doi: 10.1016/j.physbeh.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol 10: 519–527, 2000. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 55.Kinnamon SC. Neurosensory transmission without a synapse: new perspectives on taste signaling. BMC Biol 11: 42, 2013. doi: 10.1186/1741-7007-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D, Archer N, Duesing K, Hannan G, Keast R. Mechanism of fat taste perception: Association with diet and obesity. Prog Lipid Res 63: 41–49, 2016. doi: 10.1016/j.plipres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 18: 485–497, 2017. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frye CA, Demolar GL. Menstrual cycle and sex differences influence salt preference. Physiol Behav 55: 193–197, 1994. doi: 10.1016/0031-9384(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 59.Stratford JM, Curtis KS, Contreras RJ. Linoleic acid increases chorda tympani nerve responses to and behavioral preferences for monosodium glutamate by male and female rats. Am J Physiol Regul Integr Comp Physiol 295: R764–R772, 2008. doi: 10.1152/ajpregu.00916.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin LJ, Sollars SI. Contributory role of sex differences in the variations of gustatory function. J Neurosci Res 95: 594–603, 2017. doi: 10.1002/jnr.23819. [DOI] [PubMed] [Google Scholar]
- 61.Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, Margolskee RF. Whole transcriptome profiling of taste bud cells. Sci Rep 7: 7595, 2017. doi: 10.1038/s41598-017-07746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorski J, Hansen JC. The “one and only” step model of estrogen action. Steroids 49: 461–475, 1987. doi: 10.1016/0039-128x(87)90088-2. [DOI] [PubMed] [Google Scholar]
- 63.Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol 497: 1–12, 2006. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chem Senses 39: 3–16, 2014. doi: 10.1093/chemse/bjt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhandapani KM, Brann DW. Estrogen-astrocyte interactions: implications for neuroprotection. BMC Neurosci 3: 6, 2002. doi: 10.1186/1471-2202-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology 145: 3788–3795, 2004. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 67.Sharma G, Hu C, Staquicini DI, Brigman JL, Liu M, Mauvais-Jarvis F, Pasqualini R, Arap W, Arterburn JB, Hathaway HJ, Prossnitz ER. Preclinical efficacy of the GPER-selective agonist G-1 in mouse models of obesity and diabetes. Sci Transl Med 12, 2020. doi: 10.1126/scitranslmed.aau5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lizcano F, Guzmán G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int 2014: 757461, 2014. doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 32: 949–958, 2008. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]