Keywords: batokines, brown adipose tissue, thermogenesis
Abstract
Brown adipose tissue (BAT) has been encouraged as a potential treatment for obesity and comorbidities due to its thermogenic activity capacity and contribution to energy expenditure. Some interventions such as cold and β-adrenergic drugs are able to activate BAT thermogenesis as well as promote differentiation of white adipocytes into brown-like cells (browning), enhancing the thermogenic activity of these cells. In this mini-review, we discuss new mechanisms related to BAT and energy expenditure. In this regard, we will also discuss recent studies that have revealed the existence of important secretory molecules from BAT “batokines” that act in autocrine, paracrine, and endocrine mechanisms, which in turn may explain some of the beneficial roles of BAT on whole body glucose and fat metabolism. Finally, we will discuss new insights related to BAT thermogenesis with an additional focus on the distinct features of BAT metabolism between rodents and humans.
INTRODUCTION
Obesity is characterized by excessive fat accumulation in the body and is the main factor for insulin resistance, type 2 diabetes, and cardiovascular disease. This adiposity is composed of white (WAT) and brown adipose tissue (BAT), characterized by very dynamic plasticity with varied metabolic actions and contributions. WAT is involved with energy storage and brown/beige adipocytes play an important role in thermogenesis and energy expenditure. The balance between energy intake and utilization favors healthy or unhealthy metabolism (1). Therefore, understanding the metabolic participation of adipose tissue subtypes in animals and humans will contribute to future treatment strategies to fight obesity. BAT contains small lipid droplets and mitochondria with a higher capacity to metabolize glucose and fatty acids, increasing heat production (thermogenesis). Thus, BAT activation may be a therapeutic strategy in the treatment of obesity and related diseases (2). With the advances in technology in the last decade, many discoveries and mechanisms have been made in the field of adipose tissue and its contribution to metabolic health; however, the majority of these findings were in rodents. This mini-review will focus on the advances in BAT biology in the last three years, and whether these mechanisms are translatable to humans.
The Metabolic Role of White and Brown Adipose Tissue
White and brown adipocytes are present in rodents and humans, participating significantly in whole body metabolism. The distribution of adipose tissue is anatomically distinct, with WAT present in visceral and subcutaneous depots, and BAT found in small depots in cervical, paravertebral, axillary, and clavicular regions in adult humans (3). The differences between visceral and subcutaneous fat are very well known, where subjects with increased central obesity (visceral fat) are more prone to cardiovascular risk (1). On the other hand, there is a negative correlation with subcutaneous fat mass (flanks, hips) and metabolic syndrome in humans, demonstrating the distinct metabolic aspects of these fat depots (1). Although WAT has the primary role in energy storage and immune-endocrine functions, subcutaneous WAT (scWAT) has low lipolysis rates and non-esterified fatty acid (NEFA) release and visceral WAT has higher lipolysis and NEFA release (4). These distinct metabolic contributions of differently located adipocytes may be due to a distinct genetic signature. A growing body of evidence suggests new adipocyte subtypes, even in a single fat depot (5, 6). Emerging studies using single-cell transcriptomics are contributing to deeper understanding of the identity and development of adipocytes (7–9). The study by Lee et al. (10) showed at least three subtypes of WAT from different precursor cells and characterized by distinct molecular markers, metabolic contribution, and response to external stimuli. In addition, the distribution of these WAT subtypes composes the fat pads in different amounts, and differences in this distribution may explain the heterogeneous metabolic phenotype found in humans.
WAT and BAT adipocytes have different morphologies, where WAT contains a single large lipid droplet and a few mitochondria (11, 12), whereas BAT contains small multilocular lipid droplets, elevated mitochondria content, and high sympathetic nervous system (SNS) innervation (11, 12). In response to cold, diet, or β3-adrenergic stimuli, BAT is activated and dissipates energy through the uncoupling of oxidative phosphorylation in the inner mitochondrial membrane, resulting in heat production (thermogenesis) and contributing to energy expenditure (11, 12). The mitochondria respiration uncoupling is mediated by uncoupling protein 1 (UCP1, one of the main markers of BAT) in a process called nonshivering thermogenesis. BAT activity is related to NEFA and glucose metabolization, but also to releasing factors from BAT (“batokines”) that may play a role in other metabolic organs, such as fibroblast growth factors 21 (FGF21), microRNAs, Irisin/FNDC5 (fibronectin type III domain-containing protein 5), interleukin-6 (IL-6), and 12,13-dihydroxy-9Z-octadecenoic acid (12, 13-diHOME) (13). Besides WAT and BAT adipocytes, beige adipocytes are inducible fat cells that emerge within WAT depots. Following external stimuli such as cold and sympathetic activation, white adipocytes in the subcutaneous pads become brown-like adipocytes, a process called “browning,” with similar characteristics to BAT cells, showing multilocular lipid droplets, mitochondria content, and thermogenic capacity (2). In humans, 10 days of cold exposure induced UCP1 and mitochondrial uncoupled respiration in the abdominal subcutaneous WAT, suggesting the beiging of WAT (14). The phenotype shifting of white to beige cells is very relevant to whole body metabolism, since beige adipocytes are able to contribute to energy expenditure and release other adipokines that improve metabolic health, including transforming growth factor-beta 2 (TGFβ2), branched fatty acid esters of hydroxy fatty acids (FAHFAs), adiponectin, and leptin (15–18). Recent evidence showed the presence of UCP1-independent thermogenesis in beige adipocytes (19). Ikeda et al. demonstrated an ATP-dependent Ca2+ cycling through sarco/endoplasmic reticulum Ca2+-ATPase 2b (SERCA2b) and ryanodine receptor 2 (RyR2). The authors proposed that under cold exposure, norepinephrine is able to bind to α1 and β3-adrenergic receptors (β3-AR) increasing the Ca2+ flux through SERCA2b and RyR2. This Ca2+ flux goes to mitochondria through the voltage-dependent anion channel (VDAC) and mitochondrial calcium uniporter (MCU), activating pyruvate dehydrogenase (PDH) and ATP synthesis (19). This mechanism was confirmed in UCP1 knockout mice and pig and human adipocytes lacking UCP1.
BAT-related thermogenesis has been very well detailed in the last decade; however, additional and new mechanisms that could potentiate future strategies to treat metabolic diseases in humans are necessary. To better understand this, the next topic will elucidate important differences in BAT biology between rodent models and humans.
BAT DIFFERENT ASPECTS IN RODENTS AND HUMANS
Using rodents in experimental studies can provide deep understanding of the BAT contribution to whole body metabolism; however, some points should be addressed when we translate these data to humans. Although the majority of fat depots are similar in rodents and humans, mice BAT is localized mainly in the interscapular region (iBAT) and some small depots in the cervical spine, around the heart and kidneys (11). BAT is highly activated in newborns contributing to the nonshivering thermogenesis, and also present in infants and adolescents. In adulthood, the location of BAT is similar to adolescence; however, BAT mass and activity decline with aging, compromising its activity (11). It is important to highlight the limitations of BAT mass quantification and the lack of a standard method. The study by Leitner et al. (20) applied positron emission tomography-computed tomography (PET-CT) with a 3D-axial region of interest (ROI) to calculate the total BAT volume in humans, independently of the fluorodeoxyglucose (18FDG) uptake. The authors highlight that active BAT is found in humans; however, less than one-half of these fat depots are stimulated by cold exposure. Another important aspect when rodents are compared with humans is the temperature where the rodents are housed. A lot of studies maintain rodents at ∼22°C, a temperature different from their thermoneutrality (29–30°C), which may influence metabolic behavior in response to the experimental design (21). Alternatively, humans can protect themselves against a cold challenge, leading to lower BAT activity during life. In addition, the differences in the activity of β-AR subtypes between rodents and humans may explain the challenge to safely activate BAT thermogenesis in humans. Recently, Blondin et al. (22) suggested that human brown adipocyte thermogenesis is mainly activated by β2-AR. Finally, the differences in oxidative and thermogenic capacity between humans and other mammals should be addressed.
Due to these differences between humans and rodents, translating new mechanisms of BAT activation to individuals with metabolic disease is a challenging process. BAT activity in humans is classically 18FDG uptake by PET-CT. Several protocols of cold exposure (∼18°C) with different times of exposure led to increased glucose uptake in BAT (up to 10-fold) (23–25). In addition, BAT activity is higher during winter than summer in humans (26). Another well-utilized strategy to promote health benefits is physical exercise. Several studies in rodents and humans using physical exercise protocols showed conflicting results regarding glucose uptake and BAT activation (27, 28). Although some rodent studies indicate improved insulin sensitivity and glucose uptake in BAT in response to physical exercise, others did not find alterations (28, 29). In humans, there is evidence that physical activity and athletes have lower BAT glucose uptake (30–32). This fact could be due to the higher skeletal muscle glucose uptake requirement during high-activity periods. A pharmacological strategy to induce BAT activity is the use of β3-AR agonists; however, the dose and specificity of the drug should be addressed to avoid cardiovascular outcomes in the patients (33).
NEW MECHANISMS REGARDING BAT METABOLISM
Diet-Induced Thermogenesis: Role of Leptin
Food intake and thermogenesis controls are complex and highly relevant processes for understanding the increase in body adiposity. Food-induced thermogenesis is a contributing factor for regulating body weight, especially considering that this response appears to be attenuated in obese individuals (34, 35), although not in all studies (36, 37). Understanding this metabolic disorder has been the study object of several researchers, including the analysis and interaction between the central nervous system (CNS) and tissues involved in the thermal effect of food and the body’s energy expenditure (38, 39). Aspects such as meal size, the proportion of carbohydrates and proteins, and circulating insulin levels influence the post-meal thermogenic response (40, 41). Furthermore, it has recently been demonstrated that the leptin-adrenal medulla-adipose tissue axis is necessary and responsible for the meal-induced increases in body temperature in rodents (39). Perry et al. (39) found that meal-induced increase in plasma leptin concentrations in 48-h fasted lean rats led to a CNS-induced increase in adrenal medullary secretion of catecholamines, resulting in a postprandial increase in body temperature. Importantly, there was a critical plasma leptin threshold for this effect of ∼2 ng/mL which when exceeded in high-fat fed obese rats eliminated this thermogenic response to feeding (39). Whether these findings translate to humans remains to be determined and will be challenging given that many of these effects are only observed with plasma leptin concentrations less than 1 ng/mL in rodents.
The key contribution of BAT to postprandial thermogenesis and increase in body temperature is evidenced by the findings that interscapular BATectomy reduced the thermogenic effect of food and leptin by ∼60% (39). Thus, BAT appears to be a crucial mediator of leptin-induced thermogenesis. Also, catecholamine’s lipolytic effect on WAT and the consequent availability of substrates is essential in the thermogenic response of BAT after meals (39). Corroborating this point, treatment with a small molecule ATGL inhibitor, atglistatin, abolished leptin’s thermogenic effect which was abrogated with an infusion of Liposyn/heparin, to raise plasma fatty acid concentrations (39). In addition, a recent study has demonstrated that branched-chain amino acids (BCAAs) are a crucial substrate for BAT thermogenesis and the defects in BCAA catabolism in the BAT accelerates diet-induced obesity in mice (42). These findings suggest that BCAAs in addition to exogenous fatty acids also appear to play an important role in the BAT-mediated postprandial thermogenesis.
Metabolic Sink for BCAA
As well as understanding macronutrients in postprandial thermogenesis, recent studies increased the knowledge about the oxidation of BCAAs in BAT (42, 43). Supplementation with BCAAs has some beneficial outcomes in healthy individuals; however, increased concentrations of BCAAs in the circulation are observed in obese and diabetic subjects. Neinast et al. (43) showed balanced BCAA oxidation among the organs, but genetic and environmental alterations led to decreased BCAA oxidation in the liver and adipose tissues, resulting in higher BCAA levels in the circulation and overflow to skeletal muscle, causing lipotoxicity and insulin resistance. In the same way, Yoneshiro et al. (42) observed increased BCAA utilization in the mitochondria of BAT after cold exposure in mice and humans, promoting systemic BCAA clearance. This process requires SLC25A44 as a BCAA transporter in mitochondria, and defects in BAT BCAA oxidation can lead to impaired thermogenesis and may contribute to diet-induced obesity and glucose intolerance.
Sirt5 and NAD+ Metabolism
Recently, two works highlighted the importance of sirtuin 5 (SIRT5) in BAT function. The study of Shuai et al. (44) demonstrated the role of SIRT5 in brown adipocyte differentiation through an indirect effect on histone modifications, resulting in impaired energy expenditure and glucose tolerance in SIRT5 knockout mice. Complementing these data, Wang et al. (45) showed the role of SIRT5 in the mitochondrial protein succinylation and malonylation in BAT. In this study, BAT-specific SIRT5 KO mice presented metabolic inflexibility and impaired mitochondrial homeostasis. Furthermore, the increasing succinylation of UCP1 led to its stability and function (45). Because sirtuins are nicotinamide adenine dinucleotide (NAD+)-dependent acetylases, some studies have shown that NAD+ biosynthesis may be essential to the adaptive thermogenesis of BAT and whole body metabolism (46, 47). NAD+ biosynthesis is mediated by the rate-limiting enzyme nicotinamide phosphoribosyltransferase (Nampt), and its deletion in WAT and BAT led to impaired thermogenic genes and mitochondrial function in BAT, accompanied by a blunted thermogenic response to acute cold exposure, prolonged fasting, and β3-adrenergic stimuli. However, treatment with nicotinamide mononucleotide rescued the abnormalities found in Nampt knockout mice (47). These studies emphasize the importance of SIRT5 and NAD+ biosynthesis to BAT thermogenic capacity and homeostasis.
Sympathetic Innervation: CLSTN3β and IL-17F
BAT thermogenesis is mediated by the SNS that drives neurotransmitter noradrenaline to activate β3-AR. Furthermore, the specific characteristics of each adipocyte are associated with its sympathetic innervation. Recently, the study by Zeng et al. (48) showed the role of calsyntenin 3β (CLSTN3β), a mammal-specific membrane protein, to promote sympathetic innervation in brown and beige adipocytes. Through loss and gain of function experiments, the authors demonstrated that CLSTN3β is localized in the endoplasmic reticulum and induces the secretion of calcium-binding protein B (S100b) from brown adipocytes. S100b, in turn, induces sympathetic axon outgrowth. For example, the ablation of CLSTN3β led to defective energy expenditure and adaptive thermogenesis, and greater susceptibility to the deleterious effects of a high-fat diet; however, the adipose-specific CLSTN3β-transgenic mice showed opposite responses and enhanced thermogenesis (48). Thus, this study supports understanding of one pathway involved in the communication of adipocyte-sympathetic innervation through a neurotrophic factor. Moreover, CLSTN3 was identified as a top-marker gene for multilocular perirenal BAT in humans, supporting its role in BAT activity (49).
Another study showed the communication between γδ T cells in the promotion of sympathetic innervation (50). Mice lacking γδ T cells are cold tolerant, and the reason for this is the interleukin-17F (IL-17F) cytokines produced by these cells. As a receptor for IL-17, IL-17RC is more abundantly expressed in thermogenic adipocytes and its deletion in adipocytes leads to defective thermogenesis and susceptibility to diet-induced obesity and glucose tolerance (50). Using proteomics and RNA sequencing, the authors revealed the participation of TGFβ in this process, more specifically TGFβ1. Finally, the model proposed is that TGFβ1 stimulates sympathetic innervation in BAT in response to IL-17F/IL-17RC signaling, describing the importance of immune-regulatory mechanisms that may drive BAT activity and thermogenesis.
Batokines
Although BAT thermogenesis has been deeply studied in recent years, brown adipocytes are also able to secrete endocrine factors, called “batokines,” with important participation in whole body metabolic health. These “batokines” can exert autocrine, paracrine, and endocrine actions. Several “batokines” have been reported previously, such as FGF21, bone morphogenetic protein 8b (BMP8b), IL-6, vascular endothelial growth factor A (VEGFA), insulin like growth factor 1 (IGF1), neuregulin 4 (NRG4), prostaglandins, endocannabinoids, and microRNAs (13). More recently, a new translational paracrine mechanism was described, in which it was observed that exercise and cold increase the FGF6 and FGF9 expression by FGFR3 receptors and prostaglandins, and activate UCP1 expression in BAT and scWAT. Interestingly, the authors observed that FGF9 and FGFR3 were more highly expressed in BAT deep neck biopsies than the superficial neck WAT, and FGFR3 expression in human abdominal scWAT was negatively correlated with the body mass index (BMI) and fasting plasma insulin (51). Another new mechanism involving the FGF family was described by Ruan et al. (52), who observed that the adenosine A2A receptor (A2AR) induces FGF21 expression and this batokine acts in hypertensive cardiac remodeling. In addition, CXCL14 (C-X-C motif chemokine ligand-14) was identified as a novel batokine in the control of thermogenesis by M2 macrophages. CXCL14 improved glucose homeostasis in obese mice and promoted browning effects in WAT (53). In this context, as scientific technology progresses, through high-sensitivity mass-spectrometry-based proteomics, 101 proteins were identified expressed exclusively in brown adipocytes derived from the supraclavicular brown adipose of adult humans (54). Among these possible novel “batokines,” ependymin-related protein 1 (EPDR1) demonstrated important participation in energy homeostasis in mice and a role in adipocyte thermogenic differentiation. EPDR1 was also detected in human plasma, suggesting important participation in human metabolism (54).
Physical Exercise
In this endocrine context, Kong et al. (55) verified that the reduction in the transcription factor interferon regulatory factor 4 (IRF4) in BAT induces myostatin secretion to circulation, and reduces mammalian target of rapamycin (mTOR) signaling in type IIB muscle fibers, mitochondrial function in muscle, and exercise capacity. Furthermore, physical exercise stimulates the lipid 12,13-diHOME in BAT, and increases fatty acid oxidation and fatty acid uptake in skeletal muscle. Increased exercise-induced circulating levels of 12,13-diHOME were detected in humans and mice (56). In addition, 12,13-diHOME was increased after cold exposure in humans and mice. 12,13-diHOME stimulates fatty acid uptake into BAT and its lipids levels are negatively correlated with BMI and insulin resistance in humans (57). Finally, as mentioned earlier, the role of physical exercise to stimulate BAT glucose uptake is controversial and requires further investigation.
FUTURE PERSPECTIVES RELATED TO BAT IN THE TREATMENT OF METABOLIC SYNDROME
Advances in the field of molecular biology and understanding of adipose tissue, in general, have provided new insights regarding the role of adipocytes in whole body metabolism in the context of several diseases. Characterizing different subtypes of adipocytes may be a key step to understanding the development of obesity, insulin resistance, and type 2 diabetes. Moreover, emerging studies looking at long-noncoding RNAs (lncRNA) demonstrated a role in the adipocyte thermogenic program (i.e., Blnc1, lncBATE1, lncBATE10, UC.417, AK079912, H19, GM13133) (58). The lncRNAs have metabolic implications due to their capacity to interact with DNA, with RNAs, and with proteins. A recent study described LINC00473 as a primate-specific lncRNA, with a role in the development of human thermogenic adipocytes (59). LINC00473 was found in the cytoplasm interacting with lipid droplets and mitochondria forming a multimeric complex which includes Perilipin 1 (PLIN1), and resulting in lipolysis and mitochondrial respiration activation (59). Knowledge about the role of lncRNAs in human metabolism is very recent, and further studies could provide new insights in the field.
Although some studies failed in the past due to drug selectivity, oral viability, and adverse effects, recent works showed a positive role of mirabegron in the activation of BAT. The approval of β3-AR agonist mirabegron for the treatment of overactive bladder enabled additional clinical investigations with BAT in humans. With respect to these recent studies, the first investigated acute doses of 50 mg and 200 mg of mirabegron in 12 healthy men demonstrated some dose-dependent effects in WAT lipolysis, BAT activity, and resting energy expenditure (REE) (60). Following this first study, another work from the same group treated 14 healthy women with 100 mg mirabegron for 4 wk, and found increased REE, insulin sensitivity, BAT activity, circulating adiponectin, HDL, and bile acids, compared with day 0 (61). Interestingly, some effects were observed on day 1, such as higher REE, BAT activity, adiponectin, WAT lipolysis, and liver fatty oxidation, showing an acute effect of mirabegron compared with day 0. Moreover, skeletal muscle and scWAT activity were unchanged, suggesting a major role of BAT in response to mirabegron. The data of this study also suggest that chronic treatment with mirabegron is effective in increasing BAT activity even in subjects with low BAT mass (61). The positive effects of this study were accompanied by an acute increase in the heart rate and systolic blood pressure on day 1 of mirabegron treatment (100 mg), and higher baseline values on day 28. Corroborating with these findings, the study by Finlin et al. (62) treated 13 obese, insulin-resistant subjects with 50 mg mirabegron for 12 wk and found improved glucose homeostasis, reduced skeletal muscle triglycerides, and higher scWAT lipolysis after the treatment. In addition, the same group showed a positive role of treatment with 50 mg mirabegron for 10 wk to induce scWAT beiging in obese subjects (14). The inclusion of mixed ethnicities and small sample sizes in these studies should be addressed. In both studies, there were no differences in the blood pressure and heart rate with mirabegron treatment (50 mg) (14, 62). Future randomized clinical trials in larger populations will contribute to information in this field. As well as the positive effects of mirabegron, a recent study showed that the activation of BAT lipolysis mediated by mirabegron exacerbates atherosclerosis in mice and may contribute to cardiovascular outcomes and cerebrovascular disease in patients with atherosclerosis (63). Taken together, these recent studies advanced the understanding of BAT activation in humans and may confer better understanding about the role of BAT thermogenesis in the treatment of metabolic syndrome. Figure 1 summarizes the recent findings discussed here. Moreover, new studies using drugs that induce BAT activity in humans should look not only at BAT glucose uptake, but also at some already described “batokines.” Understanding the secretome of BAT in humans will also contribute to the development of new interventions to treat metabolic disease in the future, implicating in the promotion of metabolic health worldwide.
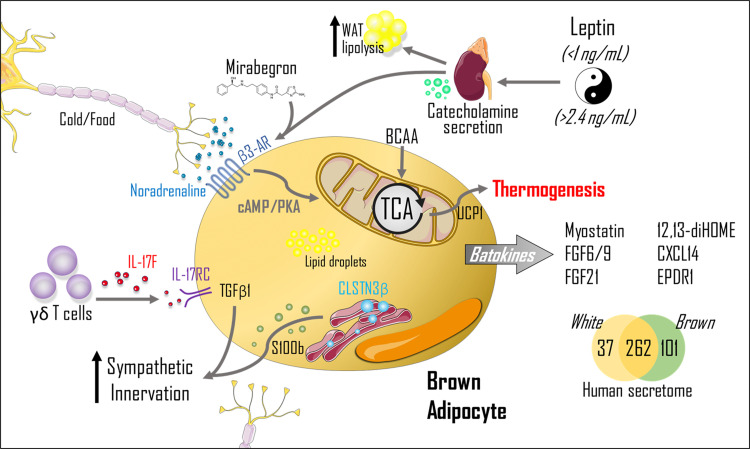
Figure 1.
Schematic figure summarizing the recent studies involving brown adipose tissue thermogenesis and secretome. The classical pathway involved in the thermogenesis process is initiated with a central stimulus (cold/food) where noradrenaline is released and activates the β3-adrenergic receptor (β3-AR), leading to cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathway and heat production/thermogenesis through mitochondrial uncoupling by uncoupling protein 1 (UCP1). Variations in plasma leptin concentrations are associated with changes in catecholamine concentrations, contributing to diet-induced thermogenesis. The branched-chain amino acids (BCAA) oxidation in mitochondria makes an important contribution to brown adipose tissue (BAT) thermogenesis. Calsyntenin 3β (CLSTN3β) are proteins located in the endoplasmic reticulum responsible for inducing the secretion of calcium-binding protein B (S100b), which will stimulate sympathetic innervation growth. In addition, γδ T cells contribute to the increase in sympathetic innervation through the interleukin-17F (IL-17F)/ interleukin-17 receptor C (IL-17RC)/transforming growth factor-beta 1 (TGFβ1) axis, highlighting the importance of immune cells in the control of adipocyte metabolism. The drug mirabegron has been described in the activation of β3-AR in humans, contributing to BAT glucose uptake and whole body glucose homeostasis and energy expenditure. Leptin stimulates adrenal medulla activation and contributes to the regulation of postprandial thermogenesis by the actions of catecholamines. Emerging “batokines” also drive signals to other metabolic tissues, contributing to body homeostasis. Recently described “batokines” are myostatin, fibroblast growth factors 6/9 (FGF6/9), fibroblast growth factors 21 (FGF21), 12,13-dihydroxy-9Z-octadecenoic acid (12,13/diHOME), C-X-C motif chemokine ligand-14 (CXCL14), and ependymin-related protein 1 (EPDR1); however, a recent human secretome identified 101 batokines, and it is probable the role of these “batokines” will be described in the near future. TCA, tricarboxylic acid cycle. We would like to thank SMART Servier for the images used in the figure of this manuscript (https://smart.servier.com).
GRANTS
The work was supported by the National Council for Scientific and Technological Development (CNPq; Process No. 306535/2017-3), Coordination for the Improvement of Higher Education Personnel (CAPES; finance code 001), São Paulo Research Foundation (FAPESP; Process Nos. 2019/00227-1; 2018/20872-6; 2019/11338-9; 2018/07568-6), and National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) (R01 DK113984, R01 DK116774, R01 DK119968, R01 DK114793, RC2 DK120534, and P30 DK045735).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.C.G. prepared figures; R.C.G., J.R.P., G.I.S., and V.R.M. drafted manuscript; R.C.G., J.R.P., G.I.S., and V.R.M. edited and revised manuscript; R.C.G., J.R.P., G.I.S., and V.R.M. approved final version of manuscript.
REFERENCES
- 1.Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest 129: 3990–4000, 2019. doi: 10.1172/jci129187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab 1: 189–200, 2019. doi: 10.1038/s42255-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend KL, Tseng Y-H. Of mice and men: novel insights regarding constitutive and recruitable brown adipocytes. Int J Obes Suppl 5, Suppl 1, S15–S20, 2015. doi: 10.1038/ijosup.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61: 1691–1699, 2012. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez AK, Dankel SN, Rastegarpanah B, Cai W, Xue R, Crovella M, Tseng Y-H, Kahn CR, Kasif S. Single-cell transcriptional networks in differentiating preadipocytes suggest drivers associated with tissue heterogeneity. Nat Commun 11: 2117, 2020. doi: 10.1038/s41467-020-16019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song A, Dai W, Jang MJ, Medrano L, Li Z, Zhao H, Shao M, Tan J, Li A, Ning T, Miller MM, Armstrong B, Huss JM, Zhu Y, Liu Y, Gradinaru V, Wu X, Jiang L, Scherer PE, Wang QA. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J Clin Invest 130: 247–257, 2020. doi: 10.1172/JCI129167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Percec I, Seale P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364: eaav2501, 2019. doi: 10.1126/science.aav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min SY, Desai A, Yang Z, Sharma A, DeSouza T, Genga RMJ, Kucukural A, Lifshitz LM, Nielsen S, Scheele C, Maehr R, Garber M, Corvera S. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc Natl Acad Sci USA 116: 17970–17979, 2019. doi: 10.1073/pnas.1906512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff K-U, Soldati G, Wolfrum C, Deplancke B. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559: 103–108, 2018. doi: 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Luong Q, Sharma R, Dreyfuss JM, Ussar S, Kahn CR. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J 38: e99291, 2019. doi: 10.15252/embj.201899291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda K, Maretich P, Kajimura S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab 29: 191–200, 2018. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Gurmaches J, Hung CM, Guertin DA. Emerging complexities in adipocyte origins and identity. Trends Cell Biol 26: 313–326, 2016. doi: 10.1016/j.tcb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13: 26–35, 2017. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 14.Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, Harfmann B, Jones KA, Johnson ZR, Westgate PM, Alexander CM, Sullivan PG, Dupont-Versteegden EE, Kern PA. Human adipose beiging in response to cold and mirabegron. JCI Insight 3: e121510, 2018. doi: 10.1172/jci.insight.121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooqi IS, O’Rahilly S. 20 Years of leptin: human disorders of leptin action. J Endocrinol 223: T63–T70, 2014. doi: 10.1530/joe-14-0480. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Nigro P, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng YH, Goodyear LJ. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1: 291–303, 2019. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 55: 2319–2326, 2012. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 18.Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, Dhaneshwar AS, Hammarstedt A, Smith U, McGraw TE, Saghatelian A, Kahn BB. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159: 318–332, 2014. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23: 1454–1465, 2017. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, Tal I, Dieckmann W, Gupta G, Kolodny GM, Pacak K, Herscovitch P, Cypess AM, Chen KY. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA 114: 8649–8654, 2017. doi: 10.1073/pnas.1705287114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong JMA, Sun W, Pires ND, Frontini A, Balaz M, Jespersen NZ, Feizi A, Petrovic K, Fischer AW, Bokhari MH, Niemi T, Nuutila P, Cinti S, Nielsen S, Scheele C, Virtanen K, Cannon B, Nedergaard J, Wolfrum C, Petrovic N. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat Metab 1: 830–843, 2019. doi: 10.1038/s42255-019-0101-4. [DOI] [PubMed] [Google Scholar]
- 22.Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, Jespersen NZ, Kooijman S, Boon MR, Fortin M, Phoenix S, Frisch F, Guérin B, Turcotte ÉE, Haman F, Richard D, Picard F, Rensen PCN, Scheele C, Carpentier AC. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab 32: 287–300.e7, 2020. doi: 10.1016/j.cmet.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanssen MJW, Hoeks J, Brans B, Van Der Lans AAJJ, Schaart G, Van Den Driessche JJ, Jörgensen JA, Boekschoten MV, Hesselink MKC, Havekes B, Kersten S, Mottaghy FM, Van Marken Lichtenbelt WD, Schrauwen P. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21: 863–865, 2015. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes 38: 812–817, 2014. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 26.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaspar RC, Muñoz VR, Kuga GK, Nakandakari SCBR, Minuzzi LG, Botezelli JD, da Silva ASR, Cintra DE, de Moura LP, Ropelle ER, Pauli JR. Acute physical exercise increases leptin-induced hypothalamic extracellular signal-regulated kinase1/2 phosphorylation and thermogenesis of obese mice. J Cell Biochem 120: 697–704, 2019. doi: 10.1002/jcb.27426. [DOI] [PubMed] [Google Scholar]
- 28.Peres Valgas da Silva C, Hernández-Saavedra D, White JD, Stanford KI. Cold and exercise: therapeutic tools to activate brown adipose tissue and combat obesity. Biology (Basel) 8: 9, 2019. doi: 10.3390/biology8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, Sindeldecker DA, May FJ, Lauritzen HPMM, Goodyear LJ, Stanford KI. Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience 11: 425–439, 2019. doi: 10.1016/j.isci.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motiani P, Virtanen KA, Motiani KK, Eskelinen JJ, Middelbeek RJ, Goodyear LJ, Savolainen AM, Kemppainen J, Jensen J, Din MU, Saunavaara V, Parkkola R, Löyttyniemi E, Knuuti J, Nuutila P, Kalliokoski KK, Hannukainen JC. Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men. Diabetes Obes Metab 19: 1379–1388, 2017. doi: 10.1111/dom.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singhal V, Maffazioli GD, Ackerman KE, Lee H, Elia EF, Woolley R, Kolodny G, Cypess AM, Misra M. Effect of chronic athletic activity on brown fat in young women. PLoS One 11: e0160129, 2016. doi: 10.1371/journal.pone.0160129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EBM, Van Der Lans AAJJ, Broeders EPM, Mottaghy FM, Schrauwen P, Van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes 39: 1696–1702, 2015. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- 33.Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21: 33–38, 2015. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaak EE, Hul G, Verdich C, Stich V, Martinez JA, Petersen M, Feskens EFM, Patel K, Oppert JM, Barbe P, Toubro S, Polak J, Anderson I, Astrup A, Macdonald I, Langin D, Sørensen T, Saris WH; NUGENOB Consortium. Impaired fat-induced thermogenesis in obese subjects: the NUGENOB study. Obesity (Silver Spring) 15: 653–663, 2007. doi: 10.1038/oby.2007.606. [DOI] [PubMed] [Google Scholar]
- 35.de Jonge L, Bray GA. The thermic effect of food and obesity: a critical review. Obes Res 5: 622–631, 1997. doi: 10.1002/j.1550-8528.1997.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 36.Marques-Lopes I, Forga L, Martínez JA. Thermogenesis induced by a high-carbohydrate meal in fasted lean and overweight young men: insulin, body fat, and sympathetic nervous system involvement. Nutrition 19: 25–29, 2003. doi: 10.1016/s0899-9007(02)00950-4.] [DOI] [PubMed] [Google Scholar]
- 37.Tentolouris N, Pavlatos S, Kokkinos A, Perrea D, Pagoni S, Katsilambros N. Diet-induced thermogenesis and substrate oxidation are not different between lean and obese women after two different isocaloric meals, one rich in protein and one rich in fat. Metabolism 57: 313–320, 2008. doi: 10.1016/j.metabol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Henningsen JB, Scheele C. Brown adipose tissue: a metabolic regulator in a hypothalamic cross talk? Annu Rev Physiol. In press. doi: 10.1146/annurev-physiol-032420-042950. [DOI] [PubMed] [Google Scholar]
- 39.Perry RJ, Lyu K, Rabin-Court A, Dong J, Li X, Yang Y, Qing H, Wang A, Yang X, Shulman GI. Leptin mediates postprandial increases in body temperature through hypothalamus-adrenal medulla-adipose tissue crosstalk. J Clin Invest 30: 2001–2016, 2020. doi: 10.1172/JCI134699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kassis A, Godin JP, Moille SE, Nielsen-Moennoz C, Groulx K, Oguey-Araymon S, Praplan F, Beaumont M, Sauser J, Monnard I, Kapp AF, Ammon-Zufferey C, Frei N, Guignard L, Delodder F, Mace K. Effects of protein quantity and type on diet induced thermogenesis in overweight adults: a randomized controlled trial. Clin Nutr 38: 1570–1580, 2019. doi: 10.1016/j.clnu.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Robinson SM, Jaccard C, Persaud C, Jackson AA, Jequier E, Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr 52: 72–80, 1990. doi: 10.1093/ajcn/52.1.72. [DOI] [PubMed] [Google Scholar]
- 42.Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, , et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572: 614–619, 2019. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, Li X, Zhan L, White E, Anthony TG, Rabinowitz JD, Arany Z. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab 29: 417–429.e4, 2019. doi: 10.1016/j.cmet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuai L, Zhang LN, Li BH, Tang CL, Wu LY, Li J, Li JY. SIRT5 regulates brown adipocyte differentiation and browning of subcutaneous white adipose tissue. Diabetes 68: 1449–1461, 2019. doi: 10.2337/db18-1103. [DOI] [PubMed] [Google Scholar]
- 45.Wang GX, Meyer JG, Cai W, Softic S, Li ME, Verdin E, Newgard C, Schilling B, Kahn CR. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol Cell 74: 844–857.e7, 2019. doi: 10.1016/j.molcel.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crisol BM, Veiga CB, Lenhare L, Braga RR, Silva VRR, da Silva ASR, Cintra DE, Moura LP, Pauli JR, Ropelle ER. Nicotinamide riboside induces a thermogenic response in lean mice. Life Sci 211: 1–7, 2018. doi: 10.1016/j.lfs.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi S, Franczyk MP, Chondronikola M, Qi N, Gunawardana SC, Stromsdorfer KL, Porter LC, Wozniak DF, Sasaki Y, Rensing N, Wong M, Piston DW, Klein S, Yoshino J. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc Natl Acad Sci USA 116: 23822–23828, 2019. doi: 10.1073/pnas.1909917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, Spiegelman BM. Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis. Nature 570: E32, 2019. doi: 10.1038/s41586-019-1267-3. [DOI] [PubMed] [Google Scholar]
- 49.Jespersen NZ, Feizi A, Andersen ES, Heywood S, Hattel HB, Daugaard S, Peijs L, Bagi P, Feldt-Rasmussen B, Schultz HS, Hansen NS, Krogh-Madsen R, Pedersen BK, Petrovic N, Nielsen S, Scheele C. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol Metab 24: 30–43, 2019. doi: 10.1016/j.molmet.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, Desai BN, Banks AS, Lowell BB, Mathis D, Spiegelman BM. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578: 610–614, 2020. doi: 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shamsi F, Xue R, Huang TL, Lundh M, Liu Y, Leiria LO, Lynes MD, Kempf E, Wang CH, Sugimoto S, Nigro P, Landgraf K, Schulz T, Li Y, Emanuelli B, Kothakota S, Williams LT, Jessen N, Pedersen SB, Böttcher Y, Blüher M, Körner A, Goodyear LJ, Mohammadi M, Kahn CR, Tseng YH. FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat Commun 11: 1421, 2020. doi: 10.1038/s41467-020-15055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, Gao PJ. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab 28: 476–489.e5, 2018. doi: 10.1016/j.cmet.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Cereijo R, Gavaldà-Navarro A, Cairó M, Quesada-López T, Villarroya J, Morón-Ros S, Sánchez-Infantes D, Peyrou M, Iglesias R, Mampel T, Turatsinze JV, Eizirik DL, Giralt M, Villarroya F. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab 28: 750–763.e6, 2018. doi: 10.1016/j.cmet.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, Brunner AD, Larsen TJ, Bayarri-Olmos R, Prabhakar BS, Helgstrand C, Severinsen MCK, Holst B, Kjaer A, Tang-Christensen M, Sanfridson A, Garred P, Privé GG, Pedersen BK, Gerhart-Hines Z, Nielsen S, Drucker DJ, Mann M, Scheele C. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab 30: 963–975.e7, 2019. doi: 10.1016/j.cmet.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, Dawes BA, Tsai L, Kahn BB, Spiegelman BM, Liu T, Rosen ED. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 28: 631–643.e3, 2018. doi: 10.1016/j.cmet.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, Chen EY, Gao F, Narain NR, Distefano G, Shettigar VK, Hirshman MF, Ziolo MT, Kiebish MA, Tseng YH, Coen PM, Goodyear LJ. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27: 1111–1120.e3, 2018. doi: 10.1016/j.cmet.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, Baer LA, May FJ, Gao F, Narain NR, Chen EY, Kiebish MA, Cypess AM, Blüher M, Goodyear LJ, Hotamisligil GS, Stanford KI, Tseng Y. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med 23: 631–637, 2017. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun L, Lin JD. Function and mechanism of long noncoding RNAs in adipocyte biology. Diabetes 68: 887–896, 2019. doi: 10.2337/dbi18-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran K-V, Brown EL, DeSouza T, Jespersen NZ, Nandrup-Bus C, Yang Q, Yang Z, Desai A, Min SY, Rojas-Rodriguez R, Lundh M, Feizi A, Willenbrock H, Larsen TJ, Severinsen MCK, Malka K, Mozzicato AM, Deshmukh AS, Emanuelli B, Pedersen BK, Fitzgibbons T, Scheele C, Corvera S, Nielsen S. Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473. Nat Metab 2: 397–412, 2020. doi: 10.1038/s42255-020-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baskin AS, Linderman JD, Brychta RJ, McGehee S, Anflick-Chames E, Cero C, Johnson JW, O’Mara AE, Fletcher LA, Leitner BP, Duckworth CJ, Huang S, Cai H, Martin Garraffo H, Millo CM, Dieckmann W, Tolstikov V, Chen EY, Gao F, Narain NR, Kiebish MA, Walter PJ, Herscovitch P, Chen KY, Cypess AM. Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by A b3-adrenergic receptor agonist. Diabetes 67: 2113–2125, 2018. doi: 10.2337/db18-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, Fink YA, Kapuria D, Cassimatis TM, Kelsey N, Cero C, Sater ZA, Piccinini F, Baskin AS, Leitner BP, Cai H, Millo CM, Dieckmann W, Walter M, Javitt NB, Rotman Y, Walter PJ, Ader M, Bergman RN, Herscovitch P, Chen KY, Cypess AM. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest 130: 2209–2219, 2020. doi: 10.1172/jci131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finlin BS, Memetimin H, Zhu B, Confides AL, Vekaria HJE, Khouli RH, Johnson ZR, Westgate PM, Chen J, Morris AJ, Sullivan PG, Dupont-Versteegden EE, Kern PA. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest 130: 2319–2331, 2020. doi: 10.1172/jci134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sui W, Li H, Yang Y, Jing X, Xue F, Cheng J, Dong M, Zhang M, Pan H, Chen Y, Zhang Y, Zhou Q, Shi W, Wang X, Zhang H, Zhang C, Zhang Y, Cao Y. Bladder drug mirabegron exacerbates atherosclerosis through activation of brown fat-mediated lipolysis. Proc Natl Acad Sci USA 116: 10937–10942, 2019. doi: 10.1073/pnas.1901655116. [DOI] [PMC free article] [PubMed] [Google Scholar]