Abstract
Physical inactivity influences the development of muscle insulin resistance yet is far less understood than diet-induced muscle insulin resistance. Progress in understanding the mechanisms of physical inactivity-induced insulin resistance is limited by a lack of an appropriate preclinical model of muscle insulin resistance. Here, we discuss differences between diet and physical inactivity-induced insulin resistance, the advantages and disadvantages of the available rodent inactivity models to study insulin resistance, and our current understanding of the mechanisms of muscle insulin resistance derived from such preclinical inactivity designs. The burgeoning rise of health complications emanating from metabolic disease presents an alarming issue with mounting costs for health care and a reduced quality of life. There exists a pressing need for more complete understanding of mechanisms behind the development and progression of metabolic dysfunction. Since lifestyle modifications such as poor diet and lack of physical activity are primary catalysts of metabolic dysfunction, rodent models have been formed to explore mechanisms behind these issues. Particularly, the use of a high-fat diet has been pervasive and has been an instrumental model to gain insight into mechanisms underlying diet-induced insulin resistance (IR). However, physical inactivity (and to some extent muscle disuse) is an often overlooked and much less frequently studied lifestyle modification, which some have contended is the primary contributor in the initial development of muscle IR. In this mini-review we highlight some of the key differences between diet- and physical inactivity-induced development of muscle IR and propose reasons for the sparse volume of academic research into physical inactivity-induced IR including infrequent use of clearly translatable rodent physical inactivity models.
Keywords: insulin sensitivity, metabolism, mice, muscle disuse, skeletal muscle
INTRODUCTION
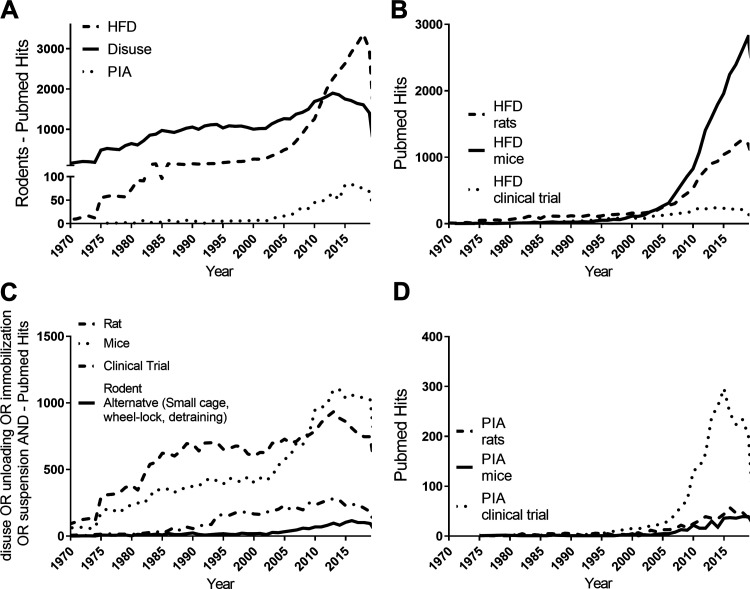
The main causes of obesity and metabolic diseases are attributable to lifestyle, namely the balance of physical activity and energy intake (1). Physical activity is a major player behind the prevention and treatment for obesity (2) and its metabolic comorbidities (3–6). Indeed, the negative consequences of physical inactivity are many (3), and health impairments can occur independent of obesity or adiposity. The United States Department of Health and Human Services estimates that ∼80% of Americans do not meet physical activity requirements and that this level of physical inactivity amounts to ∼$117 billion in healthcare costs (7). Furthermore, these costs increase with worsening levels of physical inactivity (8). Using PubMed searches for key terms (Fig. 1), one can see that physical inactivity is an often overlooked and much less frequently studied lifestyle modification in comparison to a high-fat diet (HFD), particularly in rodents (Fig. 1, A and B) (9). Unfortunately, the mechanisms of physical inactivity-induced insulin resistance (IR) have been minimally studied in rodents due to several less recognized barriers to its investigation (discussed below). As a result, PubMed searches again reveal there is an abundance of very extreme rodent models of muscle disuse (Fig. 1C), but fewer experimental models that mimic human conditions of physical inactivity and that of insulin resistance (IR) (distinctions between disuse and physical inactivity are presented in the next section). A majority of the rodent models that broadly represent situations that induce physical inactivity were purposely designed to study structural and functional changes of muscle with disuse like muscle atrophy or weakness, but not metabolic changes (i.e., insulin resistance). In fact, a 2005 review by Morey-Holton et al. (10) (summarizing the rodent hindlimb unloading literature at that time), reported that only 23 out of 1113 publications covered the topic of energy metabolism. This pattern has not changed much 15 years later. Comparatively, very few models of physical inactivity and/or muscle disuse in rodents have focused on IR as a primary outcome, either in the whole animal or specifically in the muscle. Thus, it is no surprise that PubMed searches also reveal a greater amount of research on physical inactivity-induced IR that has been conducted in clinical compared to preclinical settings (Fig. 1D). Although clinical studies are extremely valuable, they are extremely expensive to conduct and potentially invasive with muscle biopsies and clamp studies. Even so, it is rare or ethically impossible to focus beyond blood and muscle in many clinical trials. Rodent studies do provide the option of examining the physiology of other tissues (e.g. liver, visceral fat, heart, kidneys, and brain). Additionally, it is more challenging to obtain mechanistic information of causation with clinical studies. Improved preclinical representations of physical inactivity-induced IR in rodents would be less expensive and beneficial to unravel molecular mechanisms such as with genetic manipulation.
Figure 1.
PubMed searches for number of hits per year for “high-fat diet rodents” compared to “physical inactivity rodents” and “disuse OR unloading OR immobilization OR suspension and rodents” (A); “high-fat diet rats” vs. “high-fat diet mice” vs. “high-fat diet clinical trial” (B); “disuse OR unloading OR immobilization OR suspension and Rat” vs. “disuse OR unloading OR immobilization OR suspension AND mice” vs. “disuse OR unloading OR immobilization OR suspension – clinical Trial” vs. “small cage OR detraining OR wheel-lock OR physical inactivity AND rodent” (C); “physical inactivity rats” vs. “physical inactivity mice” vs. “physical inactivity clinical trial” (D). HFD = high-fat diet, PIA = physical inactivity.
EXTREME YET LESS CLINICALLY RELEVANT RODENT INACTIVITY MODELS
Most of what is known regarding physical inactivity in mice has been garnered from studies of muscle disuse using the hindlimb unloading (HU), cast or staple immobilization (HI), and denervation (DEN) models. These models are considered extreme versions of physical inactivity in which there is no load bearing and typically focused on hindlimb muscle-isolated effects. The benefit of these models, at least in the case of HI and DEN, would be the ability to utilize a unilateral design to examine localized responses with the other limb serving as an internal control. Nonetheless, these models often fail to recapitulate the full spectrum of whole-body effects of physical inactivity observed in humans. Moreover, these models typically force the rodent to use their forelimbs for locomotion, thereby impacting overall physical activity. Although we broadly classify HU, HI, and DEN in a “muscle disuse” subset of physical inactivity, there are some important distinctions to make between these three muscle disuse models. Hindlimb immobilization induces isometric contractions during the first few days (11), whereas DEN is characterized with more complete cessation of muscle contraction. Hindlimb unloading results in reductions of mechanical loading as well, however, unrestricted range of motion is one of the distinguishing factors of the HU model resulting in periodic “free-wheeling” of the hindlimbs over the time course of disuse. Moreover, hindlimb unloading results in loss of lean fat mass in both mice and rats (12–14), which is not consistent in humans where fat gain is more representative during physical inactivity (15). These (rapid hindlimb movement and fat loss) might be some possible reasons why HU sometimes results in increased muscle insulin sensitivity, whereas HI and DEN result in muscle IR (Table 1).
Table 1.
Comparison of rodent physical inactivity and disuse models on muscle atrophy and insulin sensitivity at the muscle and whole body level
| Rodent Inactivity Model | Description | Muscle Atrophy | Insulin Sensitivity |
|
|---|---|---|---|---|
| Muscle Specific | Whole Body (Insulin or glucose tolerance test) | |||
| Spaceflight | Exposure of rodents to microgravity. Rare, expensive, confounders of stress, radiation, etc. | ↓ | ↑ SL, ↓FT (16) | ? |
| Hindlimb unloading | Suspending a rodent by their tail for a period of time to unload the hindlimbs. Designed as a spaceflight analog | ↓ | ↑1 day to 5 wk (16–24);↓6 h to 1 day (25, 26) | ↓(27); ↑(13, 28, 29) |
| Hindlimb cast or staple immobilization | Local immobilization of a hindlimb, typically with a cast or a staple | ↓↓ | ↓(30, 31) | ? |
| Denervation | Block of muscle contraction by interrupting the nerve signal to the muscle by physical or chemical means | ↓↓ | ↓(19, 25, 32) | ? |
| Small cage | Restricting physical activity by reducing the living space by varying degrees | ↓(33) ↕ST only; ↕ST, ↓FT (34); ↓both (35) | ↓14 days (33); ↓8 days (36) | ↓7 days (35); ↔14 dys (33, 35); ↓8 days (36); ↔19 wk (37) |
| Wheel lock | Making the standard sedentary laboratory rodent physically active with voluntary wheel running and then locking the wheel to induce physical inactivity | ↔(9) | ↓R(38), M? | Insulin ↑(39); 7 days small cage, ↔(40) |
| Detraining | Making the standard sedentary laboratory rodent physically active with forced treadmill training and then stopping the training | ? | ↓∼2 days (41, 42) | ? |
| Fasting | The reduced amount of food will have secondary cause of making a mouse less active | ↓ | ↑(43, 44) | ↑(43, 44) |
M, mouse; R, rat; ST, slow-twitch muscle; FT, fast-twitch muscle.
LESS EXTREME YET MORE TRANSLATABLE RODENT INACTIVITY MODELS
Other models of physical inactivity that are more representative of the whole body phenotypic effects of human physical inactivity have been used, albeit much less frequently and with reduced attention on muscle IR. These encompass rodent models of detraining where mice or rats are forced to train using treadmill or swimming exercises or voluntarily trained with running wheels for a period of time before the training stimulus is removed. With the running wheels, the rodent is placed in a new standard cage without a wheel or kept in the cage with a running wheel, but the wheel is locked (wheel lock). Other methods to modify the size of the rodent cage have been used. Increasing the size of the cage can induce greater physical activity, whereas decreasing the size of the cage may reduce physical activity. Although this small cage approach is not a new method for use with rats (33–35, 40, 45–48), its use has been re-established in a few recent studies yet now adapted to mice and in the context of muscle adaptation and IR (36, 37). Additional studies using the small mouse cage model over short- and long-term periods and improved assessments of whole body and muscle IR (hyperinsulinemic-euglycemic clamps, muscle 2-deoxy-D-glucose (2DG) uptake) are needed. A very recent study has even demonstrated that prohibiting climbing is another effective method to induce physical inactivity phenotypes (37). Combining detraining with some of the above methods (HU, small cage) has also been considered to further accelerate physical inactivity-induced effects (36, 40, 49).
DIFFERENCES BETWEEN DIET- AND INACTIVITY-INDUCED IR
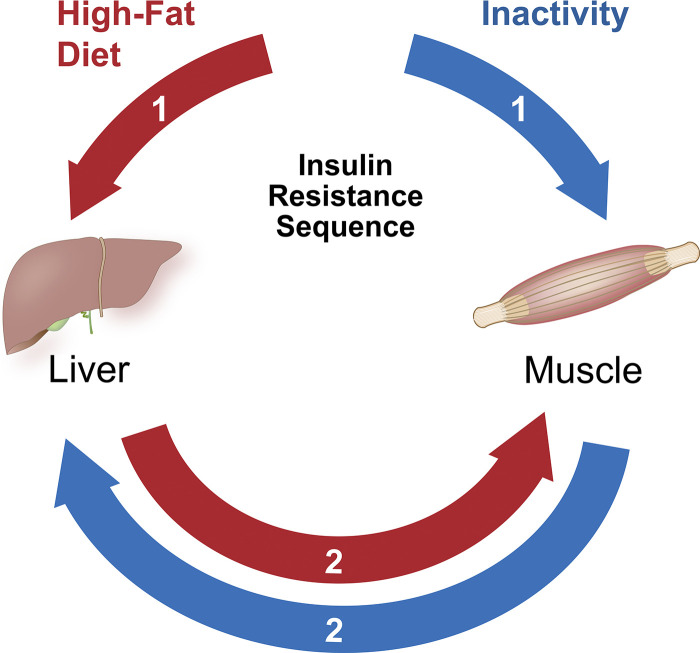
High-fat diet studies in rodents have been thoroughly used to inform the mechanisms of inactivity-induced IR. Although there may be some overlap at certain points of disease progression between the lifestyle factors of diet and physical activity, there are drastic differences in the early development of IR between the scenarios. Skeletal muscle is the initial and primary site of IR following physical inactivity and disuse (50), unlike HFD where the liver or another tissue (i.e., fat) may be the main organ initially influenced (50). First, at the onset of the exposure, inactivity-induced skeletal muscle IR is more potent (i.e., greater magnitude of change) than diet-induced IR in skeletal muscle (51). Second, disuse and physical inactivity-induced muscle IR occurs before or even in the absence of hepatic IR (52, 53), whereas under HFD conditions, hepatic impairments almost always occur before muscle IR (54) (Fig. 2). Third, the development of inactivity-induced IR (as determined by insulin-stimulated glucose uptake) occurs more rapidly than IR induced by HFD. Some studies have shown that skeletal muscle IR is prominent within at least 3 h of denervation (rat) (32), 4 h of hindlimb unloading (rat) (25), 6 h of hindlimb immobilization (mice) (30), 1 day of hindlimb unloading (rat) (26) or immobilization (mice) (31), 40–42 h of detraining (rat) (41, 42), and 2 days of running wheel-lock (rat) (5) in rodents. Similarly, with human studies, inactivity-induced IR is rapidly seen as soon as 1 day of sitting (53), 3 days of bed rest (55, 56), and 1 week of reduced ambulation (57). In contrast, IR with HFD treatment becomes more evident in muscle after ∼ 3 weeks of HFD exposure (54). This general pattern of a delay in HFD-induced muscle IR (versus physical inactivity- induced IR) is apparent across many species (58). Even though excess lipids in circulation will induce muscle IR when rapidly infused to supraphysiological levels (59, 60), the physiological irrelevance of that observation does provide strong evidence of an early developmental role of muscle IR with HFD. Finally, a fourth distinction is that during the initial stages of IR development, HFD-induced IR may be mediated by circulating factors, whereas physical inactivity- and disuse-induced IR is not (61).
Figure 2.
High-fat diet results in insulin resistance in liver prior to skeletal muscle. In contrast, physical inactivity or disuse results in insulin resistance to skeletal muscle and later may develop into liver insulin resistance. Numbers denote order of tissue insulin resistance. Graphical illustration was created by Diana Lim.
MECHANISMS RELATED TO THE EARLY EVENTS OF PHYSICAL INACTIVITY- AND/OR DISUSE-INDUCED MUSCLE IR IN RODENTS
Most of what is known regarding the mechanisms underlying disuse-induced IR arises from work in the 1980-1990s using rodent models of DEN and some limited studies using HI or HU. Importantly, these mechanisms have not been examined in more translationally relevant physical inactivity rodent models. Following disuse, the rapid and nearly immediate (within hours) decrease in insulin sensitivity occurs concurrently with inactivation of glycogen synthase (62) but is unrelated to GLUT4 expression (63), glucocorticoids, adenosine, prostaglandins, circulating factors, insulin binding to its receptor (64, 65), changes in hexokinase activity (66), distal glycogenolysis (66, 67), or elevated glycogen content (61, 66). Of note, prolonged disuse leads to a decrease in GLUT4 expression, possibly worsening the severity of disuse-induced IR (33, 68–70). Nonetheless, the rapid induction of IR following disuse is likely due to a defect in intracellular signaling (71), albeit one that is downstream of the receptor tyrosine kinase (72). Since the effect of DEN is rapid, some have suggested that proteolysis or an early post-receptor defect (64, 65) may be linked to the initial IR following DEN (61). The literature has majorly focused on the early development (within hours to a few days) of disuse-induced IR and points to inactivity-induced dysregulation of insulin signaling downstream of the insulin receptor. However, it is unknown if these same potential mechanisms are observed with longer term disuse (1–2 days versus 1–2 weeks) as IR worsens weeks later when adiposity develops and other tissues develop IR (15).
Although IR induced by physical inactivity, disuse, and HFD may have the same end point, the path to that end point is divergent. Overfeeding (HFD) and increased contractile activity (i.e., electrical stimulation) independently increase long chain fatty acids in muscle, yet have an opposite effect on insulin sensitivity (73). Whereas lipid transport is decreased during muscle disuse (74), yet both disuse and HFD typically induce IR. At face value this is paradoxical during the condition of contractile activity (physical activity), since lipid influx and specifically that of saturated free fatty acids like palmitate are known triggers of muscle IR. In fact, palmitate transport is increased with increased contractile activity, but decreased during denervation, a phenomenon that correlates with the abundance of membrane-bound fatty acid transport proteins (74). Thus, HFD and lack of contractile activity results in increased IR, but likely through different mechanisms. These mechanisms warrant further investigation with more translatable models of physical inactivity.
CURRENT BARRIERS WITH RODENT MODELS OF PHYSICAL INACTIVITY
Methodological Limitations
To compare the rodent models of inactivity-induced IR to the phenotype observed in human studies of inactivity, it is important to use similar methods to assess glucose metabolism and IR (58). In humans (and rodents), the gold standard method to assess IR is the hyperinsulinemic-euglycemic clamp, which has the ability to not only assess the whole-body insulin sensitivity but also contributions from the glucose disposal and appearance rates from central and peripheral tissues (58). Tissue-specific glucose uptake in humans may also be directly assessed by the use of stable isotope and tissue biopsies, particular in skeletal muscle. Many rodent inactivity studies discussed above utilize the ex vivo glucose uptake assay as their primary outcome for skeletal muscle insulin sensitivity. Glucose tolerance tests, insulin tolerance tests, and plasma glucose and insulin assessments were most commonly used. No rodent studies have yet used the hyperinsulinemic-euglycemic clamp to report inactivity-induced IR. Although hyperinsulinemic-euglycemic clamps would be optimal to study IR as a result of physical inactivity, there are likely reasons why this method has not been used to date. One of which is that this method requires a stressful catheter implantation surgery before the clamp procedure that is often followed by a bout of forced inactivity during recovery from the surgery. Thus, any result due to the intervention is likely to be confounded due to the insult of the surgery/recovery period. Although the 2DG method is an excellent way to assess changes in insulin sensitivity that occur in rodent skeletal muscle, these data are not directly numerically comparable to data collected with the clamp method. Thus, the most relevant comparison is with the rodent ex vivo muscle glucose uptake data to the stable-isotope/muscle biopsy glucose uptake data with the clamp procedure in humans. An oral glucose tolerance test (or in the case of rodents through an i.p. mediated glucose tolerance test), although useful to assess whole-body glucose handling and more readily performed, does not provide information on organ-specific responses. Overall, our ability to compare rodent and human inactivity studies are inundated by differing methods of assessment (58).
IR in Nonmuscle Tissue Depots
It is also not possible to make a conclusive statement in rodent disuse models whether IR occurs primarily in the skeletal muscle, or whether it also occurs in other tissues. Unlike in human studies of physical inactivity, IR appears to be primarily driven by skeletal muscle (50). Several reports suggest that heart, muscle, liver, bone, and visceral fat tissues reduce in size with HU (12–14). This is likely due to reduced rates of protein and adipose tissue triglyceride turnover typifying a negative net energy balance (12), instead of the positive energy balance observed in human models of physical inactivity. One might infer that reduced tissue mass in rodent inactivity models would promote an overall reduced ability to clear circulating glucose because the reduced tissue masses would have a reduced sum of peripheral tissue glucose uptake. Part of this problem is because few studies have examined non-muscle tissue (liver, heart, and adipose) insulin resistance responses following disuse (12, 27, 75, 76). One report suggests greater gluconeogenesis in the liver with HU (76). Another study demonstrates reduced mitochondrial content and fat oxidation capacity in the liver with locking voluntary running wheels (27). More studies are needed to examine non-muscle tissue insulin resistance with rodent models of physical inactivity. Therefore, it remains to be seen whether rodent inactivity models promote IR across various tissue depots and their contributions.
Obvious but Often Neglected Differences between Rodent and Human Metabolism and Physical Activity Status
When examining localized effects of disuse between rodents and humans, an important observation is the magnitude in IR responsiveness between rodents and humans. For example, limb immobilization in humans results in muscle IR, but to a smaller magnitude compared to rodent hindlimb immobilization (77). There are several factors that may explain the divergent IR responses of humans and rodents using models of physical inactivity (mostly disuse). First, rodents (especially mice) have a higher insulin responsiveness (i.e., the capacity to respond to insulin) than humans (58). Second, liver metabolism accounts for half of the metabolic rate in a mouse, which is a much higher contribution compared to humans (78). Third, rodents are not inactive in the wild (79) as they are in the laboratory where there is no survival advantage for them to be active (4). So often, it is more difficult to induce a change in insulin resistance unless it is isolated to a limb and extreme (denervation and immobilization). In fact, some have postulated that control rodents in the laboratory are “metabolically morbid,” because they quickly become insulin-resistant, overweight, hypertensive, and are at greater risk of premature death (80). This is likely due to their imposed sedentary state and lack of adequate environmental stimuli (9, 80). Because of the high glucose metabolism and insulin responsiveness in rodents and their reduced physical activity status (in case of laboratory rodents), there is high potential to mask subtle effects of inactivity.
FUTURE DIRECTIONS
We find ourselves with the untenable task of forcing inactivity on a sedentary rodent. Data from step reduction studies in humans offers some insight as to why this has been a failed endeavor. Krogh-Madsen et al. (81) showed that change in steps was not correlated with the change in insulin sensitivity (hyperinsulinemic-euglycemic clamp) following reduced activity in young men. We show similar findings (r = 0.151, P = 0.64) in older adults (82). Together, this suggests that the magnitude of the decrease in physical activity levels after a certain point is not proportional to a continued change in insulin sensitivity. Thus, it is interesting to speculate that the inactivity “threshold” may have already been attained in the sedentary laboratory rodent. Yet, even with these limitations, general patterns with rodent research have been initially impactful to provide general advancement to the field. For example, work by Kump and Booth (38) inspired the human step reduction study in Pederson’s (81) laboratory highlighted above. Both the rodent and human studies just mentioned agreed in showing that insulin signaling was impaired upon the onset of inactivity. We highlight that there are areas where the rodent and human model do agree, in general, but if we are to advance the field further we need more translatable rodent models. Recent developments by various laboratories hold promising models to examine the mechanisms of inactivity-induced muscle IR. Methods to normalize the rodent physical activity levels back to their intrinsically active state with wheel running (5), wheel running combined with wheel-lock (55), small cage housing (33, 45), or HU (49) may open up promising opportunities to future research regarding the mechanisms of inactivity-induced muscle IR. However, these models have not yet been validated with the hyperinsulemic-euglycemic clamp, and it would appear there is a limited window (in the first 1–2 weeks) to capture the effect of physical inactivity before the rapid metabolism of the rodent compensates in a new homeostasis and effects of inactivity are no longer apparent (83). Alternatively, genetic modifications of behavior (84) to alter physical activity may also provide a novel way to manipulate rodent physical activity and to more mechanistically address the development of inactivity-induced IR. These models are promising, but still much work and validation must be conducted to find an appropriate model with translational applicability.
GRANTS
This work was supported by National Institutes of Health Grants R01AG050781, R01DK107397, and F32AR072481.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.T.R. and M.J.D. conceived and designed research; P.T.R. and M.J.D. performed experiments; P.T.R., C.E.P., and M.J.D. analyzed data; P.T.R., J.M.M., C.E.P., K.F., and M.J.D. interpreted results of experiments; P.T.R. and M.J.D. prepared figures; P.T.R., J.M.M., K.F., and M.J.D. drafted manuscript; P.T.R., J.M.M., C.E.P., K.F., and M.J.D. edited and revised manuscript; P.T.R., J.M.M., C.E.P., K.F., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Diana Lim for the graphical illustration.
REFERENCES
- 1.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 56: 441–447, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy RL, Chokkalingham K, Srinivasan R. Obesity in the elderly: who should we be treating, and why, and how? Curr Opin Clin Nutr Metab Care 7: 3–9, 2004. doi: 10.1097/00075197-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thyfault JP, Booth FW. Lack of regular physical exercise or too much inactivity. Curr Opin Clin Nutr Metab Care 14: 374–378, 2011. doi: 10.1097/MCO.0b013e3283468e69. [DOI] [PubMed] [Google Scholar]
- 6.Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol (1985) 111: 1218–1224, 2011. doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. Strategic goal 2: Protect the health of Americans where they live, learn, work, and play assistant secretary for planning and evaluation https://www.hhs.gov/about/strategic-plan/strategic-goal-2/index.html.
- 8.Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis 57: 315–323, 2015. doi: 10.1016/j.pcad.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts MD, Company JM, Brown JD, Toedebusch RG, Padilla J, Jenkins NT, Laughlin MH, Booth FW. Potential clinical translation of juvenile rodent inactivity models to study the onset of childhood obesity. Am J Physiol Regul Integr Comp Physiol 303: R247–R258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 10: 7–40, 2005. doi: 10.1016/s1569-2574(05)10002-1. [DOI] [PubMed] [Google Scholar]
- 11.Fournier M, Roy RR, Perham H, Simard CP, Edgerton VR. Is limb immobilization a model of muscle disuse? Exp Neurol 80: 147–156, 1983. doi: 10.1016/0014-4886(83)90011-0. [DOI] [PubMed] [Google Scholar]
- 12.Bederman IR, Lai N, Shuster J, Henderson L, Ewart S, Cabrera ME. Chronic hindlimb suspension unloading markedly decreases turnover rates of skeletal and cardiac muscle proteins and adipose tissue triglycerides. J Appl Physiol (1985) 119: 16–26, 2015. doi: 10.1152/japplphysiol.00004.2014. [DOI] [PubMed] [Google Scholar]
- 13.Keune JA, Wong CP, Branscum AJ, Iwaniec UT, Turner RT. Bone marrow adipose tissue deficiency increases disuse-induced bone loss in male mice. Sci Rep 7: 46325, 2017. doi: 10.1038/srep46325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wade CE, Baer LA, Wu X, Silliman DT, Walters TJ, Wolf SE. Severe burn and disuse in the rat independently adversely impact body composition and adipokines. Crit Care 17: R225, 2013. doi: 10.1186/cc13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol (1985) 111: 1201–1210, 2011. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 16.Tischler ME, Henriksen EJ, Munoz KA, Stump CS, Woodman CR, Kirby CR. Spaceflight on STS-48 and earth-based unweighting produce similar effects on skeletal muscle of young rats. J Appl Physiol (1985) 74: 2161–2165, 1993. doi: 10.1152/jappl.1993.74.5.2161. [DOI] [PubMed] [Google Scholar]
- 17.Bonen A, Elder GC, Tan MH. Hindlimb suspension increases insulin binding and glucose metabolism. J Appl Physiol (1985) 65: 1833–1839, 1988. doi: 10.1152/jappl.1988.65.4.1833. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen EJ, Ritter LS. Effect of soleus unweighting on stimulation of insulin-independent glucose transport activity. J Appl Physiol (1985) 74: 1653–1657, 1993. doi: 10.1152/jappl.1993.74.4.1653. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen EJ, Rodnick KJ, Mondon CE, James DE, Holloszy JO. Effect of denervation or unweighting on GLUT-4 protein in rat soleus muscle. J Appl Physiol (1985) 70: 2322–2327, 1991. doi: 10.1152/jappl.1991.70.5.2322. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen EJ, Tischler ME. Time course of the response of carbohydrate metabolism to unloading of the soleus. Metabolism 37: 201–208, 1988. doi: 10.1016/0026-0495(88)90096-0. [DOI] [PubMed] [Google Scholar]
- 21.Henriksen EJ, Tischler ME, Johnson DG. Increased response to insulin of glucose metabolism in the 6-day unloaded rat soleus muscle. J Biol Chem 261: 10707–10712, 1986. [PubMed] [Google Scholar]
- 22.Koebel DA, Fell RD, Steffen JM. Increased glucose and 2-deoxy-D-glucose uptake in skeletal muscle of suspended rats. Aviat Space Environ Med 64: 1016–1022, 1993. [PubMed] [Google Scholar]
- 23.Qi Z, Zhang Y, Guo W, Ji L, Ding S. Increased insulin sensitivity and distorted mitochondrial adaptations during muscle unloading. Int J Mol Sci 13: 16971–16985, 2012. doi: 10.3390/ijms131216971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stump CS, Balon TW, Tipton CM. Effects of insulin and exercise on rat hindlimb muscles after simulated microgravity. J Appl Physiol (1985) 73: 2044–2053, 1992. doi: 10.1152/jappl.1992.73.5.2044. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen EJ, Tischler ME. Glucose uptake in rat soleus: effect of acute unloading and subsequent reloading. J Appl Physiol (1985) 64: 1428–1432, 1988. doi: 10.1152/jappl.1988.64.4.1428. [DOI] [PubMed] [Google Scholar]
- 26.O’Keefe MP, Perez FR, Kinnick TR, Tischler ME, Henriksen EJ. Development of whole-body and skeletal muscle insulin resistance after one day of hindlimb suspension. Metabolism 53: 1215–1222, 2004. doi: 10.1016/j.metabol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Linden MA, Meers GM, Ruebel ML, Jenkins NT, Booth FW, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Hepatic steatosis development with four weeks of physical inactivity in previously active, hyperphagic OLETF rats. Am J Physiol Regul Integ Comp Physiol 304: R763–R771, 2013. doi: 10.1152/ajpregu.00537.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon OS, Nelson DS, Barrows KM, O'Connell RM, Drummond MJ. Intramyocellular ceramides and skeletal muscle mitochondrial respiration are partially regulated by Toll-like receptor 4 during hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 311: R879–R887, 2016. doi: 10.1152/ajpregu.00253.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, Bikman BT, McClain DA, O'Connell RM, Drummond MJ. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am J Physiol Endocrinol Metab 309: E11–E21, 2015. doi: 10.1152/ajpendo.00124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson WF, Watson PA, Booth FW. Glucose uptake and glycogen synthesis in muscles from immobilized limbs. J Appl Physiol 56: 431–435, 1984. doi: 10.1152/jappl.1984.56.2.431. [DOI] [PubMed] [Google Scholar]
- 31.Seider MJ, Nicholson WF, Booth FW. Insulin resistance for glucose metabolism in disused soleus muscle of mice. Am J Physiol Endocrinol Metab 242: E12–E18, 1982. doi: 10.1152/ajpendo.1982.242.1.E12. [DOI] [PubMed] [Google Scholar]
- 32.Turinsky J, Bayly BP, O'Sullivan DM. 1,2-Diacylglycerol and ceramide levels in rat liver and skeletal muscle in vivo. Am J Physiol Endocrinol Metab 261: E620–E627, 1991. doi: 10.1152/ajpendo.1991.261.5.E620. [DOI] [PubMed] [Google Scholar]
- 33.Fushiki T, Kano T, Inoue K, Sugimoto E. Decrease in muscle glucose transporter number in chronic physical inactivity in rats. Am J Physiol Endocrinol Metab 260: E403–E410, 1991. doi: 10.1152/ajpendo.1991.260.3.E403. [DOI] [PubMed] [Google Scholar]
- 34.Takemura A, Roy RR, Edgerton VR, Ishihara A. Biochemical adaptations in a slow and a fast plantarflexor muscle of rats housed in small cages. Aerosp Med Hum Perform 87: 443–448, 2016. doi: 10.3357/AMHP.4436.2016. [DOI] [PubMed] [Google Scholar]
- 35.Barański S, Kwarecki K, Szmigielski S, Rozyński J. Histochemistry of skeletal muscle fibres in rats undergoing long-term experimental hypokinesia. Folia Histochem Cytochem (Krakow) 9: 381–386, 1971. [PubMed] [Google Scholar]
- 36.Mahmassani ZS, Reidy PT, McKenzie AI, Petrocelli JJ, Matthews OC, de Hart NM, Ferrara PJ, O’Connell RM, Funai K, Drummond MJ. Absence of MyD88 from skeletal muscle protects female mice from inactivity-induced adiposity and insulin resistance. Obesity (Silver Spring) 28: 772–782, 2020. doi: 10.1002/oby.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roemers P, Hulst Y, van Heijningen S, van Dijk G, van Heuvelen MJG, De Deyn PP, van der Zee EA. Inducing physical inactivity in mice: preventing climbing and reducing cage size negatively affect physical fitness and body composition. Front Behav Neurosci 13: 221, 2019. doi: 10.3389/fnbeh.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005. doi: 10.1113/jphysiol.2004.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kump DS, Laye MJ, Booth FW. Increased mitochondrial glycerol-3-phosphate acyltransferase protein and enzyme activity in rat epididymal fat upon cessation of wheel running. Am J Physiol Endocrinol Metab 290: E480–E489, 2006. doi: 10.1152/ajpendo.00321.2005. [DOI] [PubMed] [Google Scholar]
- 40.Mondon CE, Dolkas CB, Reaven GM. Effect of confinement in small space flight size cages on insulin sensitivity of exercise-trained rats. Aviat Space Environ Med 54: 919–922, 1983. [PubMed] [Google Scholar]
- 41.Host HH, Hansen PA, Nolte LA, Chen MM, Holloszy JO. Rapid reversal of adaptive increases in muscle GLUT-4 and glucose transport capacity after training cessation. J Appl Physiol (1985) 84: 798–802, 1998. doi: 10.1152/jappl.1998.84.3.798. [DOI] [PubMed] [Google Scholar]
- 42.Kawanaka K, Tabata I, Katsuta S, Higuchi M. Changes in insulin-stimulated glucose transport and GLUT-4 protein in rat skeletal muscle after training. J Appl Physiol (1985) 83: 2043–2047, 1997. doi: 10.1152/jappl.1997.83.6.2043. [DOI] [PubMed] [Google Scholar]
- 43.Le Marchand Y, Loten EG, Assimacopoulos-Jeannet F, Forgue ME, Freychet P, Jeanrenaud B. Effect of fasting and streptozotocin in the obese-hyperglycemic (ob/ob) mouse: apparent lack of a direct relationship between insulin binding and insulin effects. Diabetes 26: 582–590, 1977. doi: 10.2337/diab.26.6.582. [DOI] [PubMed] [Google Scholar]
- 44.Le Marchand-Brustel Y, Freychet P. Effect of fasting and streptozotocin diabetes on insulin binding and action in the isolated mouse soleus muscle. J Clin Invest 64: 1505–1515, 1979. doi: 10.1172/JCI109609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marmonti E, Busquets S, Toledo M, Ricci M, Beltrà M, Gudiño V, Oliva F, López-Pedrosa JM, Manzano M, Rueda R, López-Soriano FJ, Argilés JM. A rat immobilization model based on cage volume reduction: a physiological model for bed rest? Front Physiol 8: 184–184, 2017. doi: 10.3389/fphys.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuart DG. Effects of muscle atrophy on motor control. In: NASA Technical Reports Server. Tucson, AZ, United States: Arizona Water Resources Research Center, 1985. [Google Scholar]
- 47.Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol 44: 1320–1327, 2004. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol (1985) 100: 249–257, 2006. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]
- 49.McKenzie AI, Reidy PT, Nelson DS, Mulvey JL, Yonemura NM, Petrocelli JJ, Mahmassani ZS, Tippetts TS, Summers SA, Funai K, Drummond MJ. Pharmacological inhibition of TLR4 ameliorates muscle and liver ceramide content after disuse in previously physically active mice. Am J Physiol Regul Integr Comp Physiol 318: R503–R511, 2020. doi: 10.1152/ajpregu.00330.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 37: 802–806, 1988. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 51.Han XX, Fernando PK, Bonen A. Denervation provokes greater reductions in insulin-stimulated glucose transport in muscle than severe diabetes. Mol Cell Biochem 210: 81–89, 2000. doi: 10.1023/A:1007108025929. [DOI] [PubMed] [Google Scholar]
- 52.Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Alsted TJ, Kiens B, Stallknecht B, Dela F, Vaag A. Increased rate of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. Am J Physiol Endocrinol Metab 298: E555–E564, 2010. doi: 10.1152/ajpendo.00223.2009. [DOI] [PubMed] [Google Scholar]
- 53.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 60: 941–949, 2011. doi: 10.1016/j.metabol.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 55.Lipman RL, Raskin P, Love T, Triebwasser J, Lecocq FR, Schnure JJ. Glucose intolerance during decreased physical activity in man. Diabetes 21: 101–107, 1972. doi: 10.2337/diab.21.2.101. [DOI] [PubMed] [Google Scholar]
- 56.Smorawiński J, Kaciuba-Uściłko H, Nazar K, Kubala P, Kamińska E, Ziemba AW, Adrian J, Greenleaf JE. Effects of three-day bed rest on metabolic, hormonal and circulatory responses to an oral glucose load in endurance or strength trained athletes and untrained subjects. J Physiol Pharmacol 51: 279–289, 2000. [PubMed] [Google Scholar]
- 57.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299: 1261–1263, 2008. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 58.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab 307: E859–E871, 2014. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 59.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011, 2002. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 60.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48: 1836–1841, 1999. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 61.Sowell MO, Dutton SL, Buse MG. Selective in vitro reversal of the insulin resistance of glucose transport in denervated rat skeletal muscle. Am J Physiol Endocrinol Metab 257: E418–E425, 1989. doi: 10.1152/ajpendo.1989.257.3.E418. [DOI] [PubMed] [Google Scholar]
- 62.Sowell MO, Robinson KA, Buse MG. Phenylarsine oxide and denervation effects on hormone-stimulated glucose transport. Am J Physiol Endocrinol Metab 255: E159–E165, 1988. doi: 10.1152/ajpendo.1988.255.2.E159. [DOI] [PubMed] [Google Scholar]
- 63.Didyk RB, Anton EE, Robinson KA, Menick DR, Buse MG. Effect of immobilization on glucose transporter expression in rat hindlimb muscles. Metabolism 43: 1389–1394, 1994. doi: 10.1016/0026-0495(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 64.Forsayeth JR, Gould MK. Inhibition of insulin-stimulated xylose uptake in denervated rat soleus muscle: a post-receptor effect. Diabetologia 23: 511–516, 1982. doi: 10.1007/BF00254301. [DOI] [PubMed] [Google Scholar]
- 65.Smith RL, Lawrence JC. Jr.. Insulin action in denervated skeletal muscle: evidence that the reduced stimulation of glycogen synthesis does not involve decreased insulin binding. J Biol Chem 260: 273–278, 1985. doi: 10.1016/S0021-9258(18)89727-7. [DOI] [PubMed] [Google Scholar]
- 66.Burant CF, Lemmon SK, Treutelaar MK, Buse MG. Insulin resistance of denervated rat muscle: a model for impaired receptor-function coupling. Am J Physiol Endocrinol Metab 247: E657–E666, 1984. doi: 10.1152/ajpendo.1984.247.5.E657. [DOI] [PubMed] [Google Scholar]
- 67.Turinsky J. Dynamics of insulin resistance in denervated slow and fast muscles in vivo. Am J Physiol Regul Integr Comp Physiol 252: R531–R537, 1987. doi: 10.1152/ajpregu.1987.252.3.R531. [DOI] [PubMed] [Google Scholar]
- 68.Block NE, Menick DR, Robinson KA, Buse MG. Effect of denervation on the expression of two glucose transporter isoforms in rat hindlimb muscle. J Clin Invest 88: 1546–1552, 1991. doi: 10.1172/JCI115465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coderre L, Monfar MM, Chen KS, Heydrick SJ, Kurowski TG, Ruderman NB, Pilch PF. Alteration in the expression of GLUT-1 and GLUT-4 protein and messenger RNA levels in denervated rat muscles. Endocrinology 131: 1821–1825, 1992. doi: 10.1210/endo.131.4.1396328. [DOI] [PubMed] [Google Scholar]
- 70.Elmendorf JS, Damrau-Abney A, Smith TR, David TS, Turinsky J. Phosphatidylinositol 3-kinase and dynamics of insulin resistance in denervated slow and fast muscles in vivo. Am J Physiol Endocrinol Metab 272: E661–E670, 1997. doi: 10.1152/ajpendo.1997.272.4.E661. [DOI] [PubMed] [Google Scholar]
- 71.Lauritzen HPMM, Ploug T, Ai H, Donsmark M, Prats C, Galbo H. Denervation and high-fat diet reduce insulin signaling in T-tubules in skeletal muscle of living mice. Diabetes 57: 13–23, 2008. doi: 10.2337/db07-0516. [DOI] [PubMed] [Google Scholar]
- 72.Burant CF, Treutelaar MK, Buse MG. In vitro and in vivo activation of the insulin receptor kinase in control and denervated skeletal muscle. J Biol Chem 261: 8985–8993, 1986. [PubMed] [Google Scholar]
- 73.Holloway GP, Han XX, Jain SS, Bonen A, Chabowski A. Chronic muscle stimulation improves insulin sensitivity while increasing subcellular lipid droplets and reducing selected diacylglycerol and ceramide species in obese Zucker rats. Diabetologia 57: 832–840, 2014. doi: 10.1007/s00125-014-3169-0. [DOI] [PubMed] [Google Scholar]
- 74.Koonen DP, Benton CR, Arumugam Y, Tandon NN, Calles-Escandon J, Glatz JF, Luiken JJ, Bonen A. Different mechanisms can alter fatty acid transport when muscle contractile activity is chronically altered. Am J Physiol Endocrinol Metab 286: E1042–E1049, 2004. doi: 10.1152/ajpendo.00531.2003. [DOI] [PubMed] [Google Scholar]
- 75.Du F, Ding Y, Zou J, Li Z, Tian J, She R, Wang D, Wang H, Lv D, Chang L. Morphology and molecular mechanisms of hepatic injury in rats under simulated weightlessness and the protective effects of resistance training. PloS One 10: e0127047, 2015. doi: 10.1371/journal.pone.0127047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stein TP, Schluter MD, Galante AT, Soteropoulos P, Ramirez M, Bigbee A, Grindeland RE, Wade CE. Effect of hind limb muscle unloading on liver metabolism of rats. J Nutr Biochem 16: 9–16, 2005. doi: 10.1016/j.jnutbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Richter EA, Kiens B, Mizuno M, Strange S. Insulin action in human thighs after one-legged immobilization. J Appl Physiol (1985) 67: 19–23, 1989. doi: 10.1152/jappl.1989.67.1.19. [DOI] [PubMed] [Google Scholar]
- 78.Kummitha CM, Kalhan SC, Saidel GM, Lai N. Relating tissue/organ energy expenditure to metabolic fluxes in mouse and human: experimental data integrated with mathematical modeling. Physiol Rep 2: e12159, 2014. doi: 10.14814/phy2.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meijer JH, Robbers Y. Wheel running in the wild. Proc R Soc B 281: 20140210, 2014. doi: 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin B, Ji S, Maudsley S, Mattson MP. Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA 107: 6127–6133, 2010. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985) 108: 1034–1040, 2010. [Erratum in J Appl Physiol (1985) 108:1034, 2010]. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 82.Reidy PT, McKenzie AI, Mahmassani Z, Morrow VR, Yonemura N, Hopkins PN, Marcus RL, Rondina MT, Lin YK, Drummond MJ. Skeletal muscle ceramides and relationship to insulin sensitivity after 2 weeks of simulated sedentary behavior and recovery in healthy older adults. J Physiol 596: 5217–5236, 2018. doi: 10.1113/JP276798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Callahan ZJ, Oxendine M, Wheatley JL, Menke C, Cassell EA, Bartos A, Geiger PC, Schaeffer PJ. Compensatory responses of the insulin signaling pathway restore muscle glucose uptake following long-term denervation. Physiol Rep 3: e12359, 2015. doi: 10.14814/phy2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberts MD, Ruegsegger GN, Brown JD, Booth FW. Mechanisms associated with physical activity behavior: insights from rodent experiments. Exerc Sport Sci Rev 45: 217–222, 2017. doi: 10.1249/JES.0000000000000124. [DOI] [PubMed] [Google Scholar]