Abstract
Acute aerobic exercise produces post-exercise hypotension (PEH). Chinese populations have lower prevalence of cardiovascular disease compared to Caucasians. PEH may be associated cardiovascular disease through its influence on hypertension. The purpose of this study was to compare PEH between Caucasian and Chinese subjects following acute aerobic exercise. 62 (30 Caucasian and 32 Chinese, 50% male) subjects underwent measurement of peripheral and central hemodynamics as well as arterial and cardiac evaluations, 30 min and 60 min after 45 min of treadmill exercise. Caucasians exhibited significantly higher baseline BP than the Chinese. While the reduction in brachial artery systolic BP was greater in Caucasian than in the Chinese, there was no difference in changes in carotid systolic BP between the groups. The increase in cardiac output and heart rate was greater in the Chinese than Caucasians, but total peripheral resistance and leg pulse wave velocity decreased by a similar magnitude in the Chinese and Caucasian subjects. We conclude that acute aerobic exercise produces a greater magnitude of PEH in peripheral systolic BP in Caucasian compared to Chinese subjects. The different magnitude in PEH was caused by the greater increase in cardiac output mediated by heart rate, with no change in stroke volume. It is possible that initial BP differences between races influenced the findings.
Keywords: post exercise hypotension, cardiac output, total peripheral resistance, racial differences
Introduction
Several studies have reported that an acute bout of aerobic exercise may transiently decrease resting blood pressure (BP), a response that may last several hours [23]. This phenomenon is referred to as “post exercise hypotension” (PEH) and has been described in both hypertensive and normotensive individuals [11, 23]. PEH can be considered an important strategy to help control resting BP [11]. Thus, the reduction in BP after acute exercise is of clinical relevance in BP regulation [14, 15] especially in hypertensive individuals [24]. It is generally accepted that, in most subjects following aerobic exercise, PEH is due to a persistent drop in systemic vascular resistance that is not completely offset by increases in cardiac output (CO) [14, 16], although endurance-trained men achieve this reduced mean arterial pressure via reductions in CO [42]. Furthermore, autonomic nervous modulation [36], vasodilatory substances [20] and cardiac load [23] may contribute to PEH. However, the precise mechanisms of PEH are unknown.
The magnitude and duration of PEH varies, depending on factors such as exercise mode [44], intensity [23], duration [13], initial BP [10, 30] and posture during recovery [4]. The general consensus is that moderate-intensity aerobic exercise promotes a greater and longer reduction in BP than resistance exercise [11] and central BP may be different than peripheral BP [5]. To date, most research in PEH has focused on brachial artery [32, 41] and central aortic [19, 25] BP. Carotid artery BP, another important indicator of central BP, is an important contributor to carotid compliance and brain blood flow [35]. A previous study [5] reported that carotid BP changed differently from brachial BP after resistance exercise. To date no studies have investigated the effects of moderate-intensity aerobic exercise on carotid artery BP, and there is a lack of information comparing the BP response following exercise in the brachial, aortic and carotid artery. This may be important as the site of measurement may provide differential information.
A previous studies [29] reported that Chinese (Ch) populations (living in China) have a lower prevalence of cardiovascular disease compared with their Caucasian (Ca) counterparts (living outside of China). In addition, Ch exhibited better endothelial function compared to Ca as they age, suggesting that BP control may differ between Ch and Ca. This can be explored through investigations of PEH. However, it remains unknown whether there are different changes in central and peripheral BP following moderate-intensity aerobic exercise between Ca and Ch (who live in the same country). Therefore, the present investigation was conducted to study the effect of an acute bout of aerobic exercise on BP from brachial artery, carotid artery and aortic artery before and 30-min and 60-min post exercise in Ca and Ch. In addition, this study intended to identify some potential variables that contribute to the changes of BP in Ca and Ch.
Methods
Participants
62 (15 Ca men, 15 Ca women, 16 Ch men and 16 Ch women) healthy, nonsmoking, normotensive (systolic BP < 140 mmHg and diastolic BP < 90 mmHg) non-obese (body mass index < 30 kg/m2) participants between the ages of 18 and 40 years volunteered to participate in this study. Participants were classified as sedentary based on their exercise habits for the past 6 months (no structured exercise activity of any kind lasting longer than 30 min more than once per week). All participants were free of known cardiovascular or metabolic disease as determined from a medical history questionnaire and were not taking medication known to affect BP or heart rate (including anti-inflammatories). Participants were self-defined as Ca or Ch (28 participants born in China) and Ch decent (4 participants born in USA), and self-reporting that both parents were of Ca or Ch descent. All participants signed informed consent, and the study was approved by the University of Illinois at Urbana-Champaign institutional review board. This study meets the International Journal of Sports Medicine’s ethical standards [17].
Study design
All participants reported to the laboratory on 2 separate occasions and the time interval between each visit was 48 h to 2 weeks. Participants were instructed to abstain from caffeine, alcohol and vigorous exercise for 24 h and were at least 3 h post-prandial prior to testing. Following completion of the physical activity and health history questionnaire, measurements of height, weight and brachial artery BP, aerobic capacity (VO2peak) and maximal heart rate (HRmax) were performed during the first visit. During the second visit, participants were required to rest in the supine position for a period of 10 min in a temperature-controlled room before testing. While still in supine position, they then underwent brachial artery, carotid and central aortic BP, carotid and central aortic pulse wave analysis (PWA) as well as measurement of carotid-femoral (c-f), carotid-radial (c-a) and femoral-ankle (f-a) pulse wave velocity (PWV), carotid artery maximum diameter (Dmax), minimum diameter (Dmin) and cardiac function parameters. Following resting data acquisition, participants underwent a supervised aerobic treadmill exercise bout (45 min, 70% of heart rate reserve (HRR)). After the completion of the exercise protocol, participants resumed the supine position for recovery data acquisition. All measurements were obtained again 30 min and 60 min after the exercise bout per previous PEH protocols [39].
Since exercise induced hypotension is most consistently observed between 15:00 and 20:00 [16, 21], the second visits was at the same time (15:00–20:00) each day for each subject to avoid diurnal variations [21, 22].
Anthropometries
Height and weight were measured as previously described [18]. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
VO2peak test
Aerobic capacity was assessed using the Bruce treadmill protocol [2]. HR was measured using a Polar Heart Rate Monitor (Polar Electro, Woodbury, NY, USA) and expired air was analyzed with a Quark b2 breath-by-breath metabolic system (Cosmed, Rome, Italy). Rating of perceived exertion (RPE) was assessed once every exercise stage and at peak exercise using the Borg scale [33]. The test was terminated when participants met 2 of the following 4 criteria: (1) a plateau in HR despite an increase in workload, (2) a plateau in oxygen uptake with an increase in work load, (3) the inability to maintain adequate pace to keep up with treadmill speed, and (4) a respiratory exchange ratio > 1.1 [28]. Maximal heart rate (HRmax) was obtained to calculate the heat rate used during the exercise session as: HRtarget = (HRmax – HRrest) × 70% + HRrest, where HRtarget and HRrest are the heart rate during exercise session and heart rate at rest.
Brachial artery blood pressure assessment
Brachial artery systolic BP (SBP) and diastolic BP (DBP) were measured at the right brachial artery with the participants in supine position using an automated oscillometric cuff (HEM-907 XL; Omron, Shimane, Japan). All resting brachial artery BP measurements were made in duplicate with a 1-min rest between measurements. If the 2 values were not within 5 mmHg, a third measurement was taken until 2 values within 5 mmHg of each other were obtained. BP Values within 5 mmHg of each other were averaged and used for subsequent analysis. Mean arterial pressure (MAP) was calculated as: MAP = [(2 × DBP) + SBP]/3.
Wave reflection and aortic BP
Radial and carotid artery pressure waveforms were obtained with the participants in the supine position, from a 10-s epoch using applanation tonometry (Millar Instruments, Houston, TX, USA) and calibrated using brachial artery MAP and DBP [7]. Using a generalized validated transfer function [43] a central aortic pressure waveform was reconstructed from the radial artery pressure waveform (SphygmoCor; AtCor Medical, Sydney, Australia) to obtain central aortic BP, first systolic peak (P1) and end systolic pressure (ESP).
Pulse wave velocity assessment
All measurements were conducted using previously described techniques [45]. Distances from the carotid artery to the suprasternal notch, from the suprasternal notch to the femoral artery, from the suprasternal notch to the radial artery and from femoral artery to ankle artery were measured as straight lines with a tape measure and recorded to the nearest mm. The distance from the carotid artery to the suprasternal notch was then subtracted from both the suprasternal notch to femoral artery segment length (c-f PWV) and from the suprasternal notch to radial artery segment length (c-r PWV), and the distance from the femoral artery to ankle artery (f-a PWV) to account for differences in the direction of pulse wave propagation. The same high-fidelity strain gauge transducer (Millar Instruments, Houston, TX, USA) was used to obtain consecutive pressure waveforms in the supine position from the right common carotid artery and the right femoral artery for c-f PWV, and from the right common carotid artery and the right radial artery for c-r PWV, and from the right femoral artery and right ankle artery for f-a PWV. PWV was calculated from the distances between measurement points and the measured time delay between 10 proximal and distal waveforms (SphygmoCor; AtCor Medical). The peak of an in-phase R wave recorded from the ECG was used as a timing marker and further used to obtain heart rate [46].
Carotid stiffness assessment
Carotid artery diameter (Dmax and Dmin) was measured by ultrasonography (SSD-α10, Aloka, Tokyo, Japan). The cephalic portion of carotid artery was imaged in a longitudinal section, 1–2 cm proximal to the bifurcation, using a 7.5-MHz linear-array probe. The intima-media thickness (IMT) measurement was made at end diastole using B-mode ultrasonography.
Total peripheral resistance (TPR) index assessment
The TPR index was calculated by dividing aortic mean arterial pressure by the cardiac output (CO) indexed to body size.
Cardiac function assessment
Stroke volume (SV), CO, end diastolic volume (EDV), and end systolic volume (ESV) were assessed by two-dimensional echocardiography using ultrasonography (SSD-α10, Aloka, Tokyo, Japan). With subjects in the left lateral position, measurements were obtained using the 2-chamber apical view. The interior of the left ventricle was traced manually during both end systole and end diastole. Volumes were measured using Simpson’s rule. SV was calculated by subtracting EDV from ESV. CO was calculated as HR multiplied by SV. 3 beats were measured and the average of the measurement was reported. Ejection fraction (EF) was calculated from the ventricular volumes and expressed as percent. Two-dimensional guided M-mode echocardiography of left ventricular size was performed using standard procedures as suggested by the American Society of Echocardiography (ASE) [26]. Left ventricular mass (LVM) was calculated according to the ASE recommended formula, which estimates LVM from linear dimensions based on modeling the left ventricle as a prolate ellipse of revolution [26]. This formula has been validated against necropsy findings [6]. Fractional shortening (FS) was calculated from the ventricular diameters and expressed as a percentage.
Acute exercise session
An acute bout of 45-min supervised treadmill exercise was performed at 70 % of HRR in a temperature-controlled room. HR was measured using a Polar Heart Rate Monitor (Polar Electro, Woodbury, NY, USA). HRmax was attained from previous VO2peak test.
Statistical analysis
All data are presented as mean ± SE. A one-way multivariate ANOVA was performed to determine racial differences in descriptive characteristics between Ca and Ch. A 2 × 3 (2 race × 3 time-points) ANOVA with repeated measures was conducted on all dependent variables to compare the differences between races and time. We also conducted a repeated measures analysis on the change from baseline (2 race × 2 time). When a significant race-by-time interaction was detected, post hoc t-tests were performed to determine where the difference occurred. Age was used as covariate. Significance was declared if p < 0.05. Statistical Package for the Social Sciences (SPSS, Chicago, IL) version 17.0 was used.
Results
Subject characteristics are presented in Table 1. No significant differences were found between groups for BMI, VO2peak, HRrest, HRmax, IMT and LVM, but age (24 ± 4 years in Ca vs. 28 ± 4 years in Ch), body mass (68 ± 9 kg in Ca vs. 62 ± 10 kg in Ch), and height (172 ± 10 cm in Ca vs. 165 ± 8 cm in Ch) were significantly different between groups (P < 0.05).
Table 1.
Physical and functional characteristic of the participants.
| Variables | Chinese | Caucasian |
|---|---|---|
| male/female | 16/16 | 15/15 |
| age (years) | 28 ± 4 | 24 ± 4 * |
| body mass (kg) | 62 ± 10 | 68 ± 9 * |
| height (cm) | 165 ± 8 | 172 ± 10 * |
| BMI (kg.m−2) | 22.5 ± 2.6 | 23.1 ± 2.5 |
| VO2peak (ml.kg−1.min−1) | 43.3 ± 6.3 | 47.0 ± 9.6 |
| HRrest (b.min−1) | 68 ± 11 | 64 ± 13 |
| HRmax (b.min−1) | 195 ± 8 | 193 ± 10 |
| IMT (mm) | 0.40 ± 0.05 | 0.40 ± 0.46 |
| LVM (g) | 161 ± 50 | 170 ± 45 |
BMI, body mass index; VO2peak, peak oxygen update; HRrest, heart rate at rest; HRmax, maximal heart rate; IMT, carotid artery intima-media thickness; LVM, left ventricle mass
Significantly different compared to Chinese (P < 0.05)
Table 2 shows the data comparing the hemodynamic variables following exercise between Ch and Ca. There was significantly higher baseline brachial, carotid and central aortic SBP in Ca than Ch (P < 0.05, respectively). Brachial artery SBP decreased significantly from baseline following a bout of exercise (P < 0.05 for a time effect) and with a greater decrease in Ca than in Ch (p < 0.05) at 30-min and 60-min post exercise (Fig. 1). There were significant decreases in carotid and aortic SBP following exercise, and significant race-by-time interactions were found for brachial and carotid SBP (P < 0.05), but there was no race-by-time interaction for aortic SBP (P = 0.065).
Table 2.
Race interval hemodynamics.
| Variables | Race | Pre-exercise | Post-30 | Post-60 |
|---|---|---|---|---|
| brachial systolic blood pressure (mmHg) #‡ | Ch | 110 ± 11 | 108 ± 11 | 106 ± 9 |
| Ca | 121 ± 9 * | 117 ± 12 | 115 ± 11 | |
| brachial diastolic blood pressure (mmHg) ‡ | Ch | 60 ± 5 | 61 ± 5 | 62 ± 6 |
| Ca | 66 ± 6 * | 65 ± 5 | 64 ± 5 | |
| brachial mean arterial pressure (mmHg) #‡ | Ch | 76 ± 6 | 77 ± 6 | 77 ± 5 |
| Ca | 82 ± 6 * | 83 ± 6 | 81 ± 6 | |
| carotid systolic blood pressure (mmHg) ‡ | Ch | 101 ± 14 | 103 ± 15 | 101 ± 14 |
| Ca | 112 ± 12 * | 114 ± 16 | 108 ± 16 | |
| carotid diastolic blood pressure (mmHg) | Ch | 60 ± 6 | 61 ± 5 | 60 ± 6 |
| Ca | 66 ± 6 * | 65 ± 6 | 64 ± 5 | |
| carotid mean arterial pressure (mmHg) | Ch | 77 ± 6 | 77 ± 6 | 76 ± 7 |
| Ca | 84 ± 5 * | 84 ± 7 | 82 ± 7 | |
| aortic systolic blood pressure (mmHg) | Ch | 92 ± 7 | 92 ± 77 | 90 ± 11 |
| Ca | 100 ± 6 * | 98 ± 8 | 95 ± 7 | |
| aortic diastolic blood pressure (mmHg) | Ch | 62 ± 6 | 64 ± 6 | 62 ± 7 |
| Ca | 67 ± 6 * | 67 ± 6 | 66 ± 5 | |
| aortic mean arterial pressure (mmHg) | Ch | 76 ± 6 | 77 ± 6 | 74 ± 7 |
| Ca | 82 ± 6 * | 81 ± 7 | 79 ± 6 | |
| heart rate (b.min−1) #‡ | Ch | 68 ± 11 | 87 ± 10 | 77 ± 13 |
| Ca | 64 ± 14 | 78 ± 14 | 70 ± 12 | |
| stroke volume (ml) | Ch | 73 ± 23 | 68 ± 18 | 68 ± 20 |
| Ca | 79 ± 20 | 71 ± 18 | 74 ± 17 | |
| cardiac output (l·min−1) #‡ | Ch | 4.5 ± 1.3 | 5.5 ± 1.6 | 5.0 ± 1.4 |
| Ca | 5.0 ± 1.3 | 5.3 ± 1.5 | 5.1 ± 1.3 | |
| total peripheral resistance (mmHg.min.l−1) * | Ch | 0.056 ± 0.01 | 0.052 ± 0.009 | 0.055 ± 0.011 |
| Ca | 0.062 ± 0.028 | 0.058 ± 0.018 | 0.06 ± 0.021 | |
| central pulse wave velocity (m.s−1) | Ch | 5.6 ± 1.1 | 5.7 ± 1.2 | 5.7 ± 1.1 |
| Ca | 5.6 ± 1 | 5.4 ± 0.6 | 5.4 ± 0.7 | |
| brachial pulse wave velocity (m.s−1) | Ch | 7.0 ± 1.0 | 6.9 ± 1.1 | 6.8 ± 1.2 |
| Ca | 7.1 ± 1 | 7.1 ± 1 | 7.2 ± 1 | |
| leg pulse wave velocity (m.s−1) # | Ch | 8.2 ± 1.2 | 7.8 ± 1.2 | 8.1 ± 1.2 |
| Ca | 8.9 ± 1.3 * | 8.4 ± 1.5 | 8.4 ± 0.9 | |
| end systolic volume (ml) ‡ | Ch | 35 ± 12 | 39 ± 14 | 36 ± 11 |
| Ca | 42 ± 15 * | 40 ± 14 | 42 ± 13 | |
| end diastolic volume (ml) ‡ | Ch | 108 ± 32 | 107 ± 29 | 104 ± 29 |
| Ca | 121 ± 31 | 110 ± 27 | 116 ± 27 | |
| carotid maximum diameter (mm) | Ch | 7.3 ± 0.5 | 7.2 ± 0.6 | 7.5 ± 0.5 |
| Ca | 7.3 ± 0.6 | 7.0 ± 0.6 | 7.4 ± 0.6 | |
| carotid minimum diameter (mm) | Ch | 6.7 ± 0.5 | 6.7 ± 0.6 | 7.0 ± 0.5 |
| Ca | 6.6 ± 0.6 | 6.5 ± 0.6 | 6.8 ± 0.6 | |
| ejection fraction (%) | Ch | 67 ± 5 | 64 ± 6 | 65 ± 6 |
| Ca | 66 ± 6 | 65 ± 6 | 64 ± 6 | |
| fractional shortening (%) | Ch | 37 ± 4 | 35 ± 4 | 36 ± 4 |
| Ca | 36 ± 5 | 36 ± 4 | 35 ± 5 | |
| carotid first systolic peak (mmHg) # | Ch | 34 ± 11 | 36 ± 11 | 32 ± 13 |
| Ca | 38 ± 13 | 39 ± 11 | 35 ± 12 | |
| aortic first systolic peak (mmHg) | Ch | 28 ± 6 | 27 ± 6 | 26 ± 6 |
| Ca | 32 ± 6 * | 31 ± 5 | 29 ± 5 | |
| carotid end systolic pressure (mmHg) # | Ch | 84 ± 15 | 77 ± 8 | 78 ± 7 |
| Ca | 91 ± 7 * | 85 ± 8 | 85 ± 7 | |
| aortic end systolic pressure (mmHg) | Ch | 80 ± 8 | 77 ± 7 | 75 ± 8 |
| Ca | 88 ± 7 * | 84 ± 9 | 82 ± 7 |
P < 0.05 vs. Ch at rest.
P < 0.05 time effect.
P < 0.05 race-by-time interaction
Fig. 1.
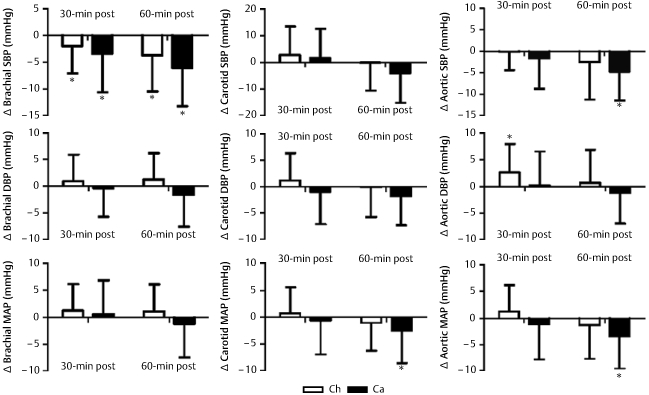
Graphic representation of changes from baseline in brachial, carotid and aortic blood pressure following exercise in Chinese (Ca) and Caucasian (Ca) participants. Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) are shown. * P < 0.05, significantly different from baseline.
Brachial, carotid and aortic DBP at baseline were significantly higher in Ca than Ch (P < 0.05, respectively) (Table 2). Significant race-by-time interactions were found for brachial DBP (P < 0.05), but there was no time effect (P = 0.204). There was no time effect in carotid (P = 0.314) and aortic DBP (P = 0.401), nor were there any race-by-time interactions (P = 0.26 for carotid and P = 0.204 for aortic DBP).
Brachial, carotid and aortic MAP were also significantly higher in Ca than Ch at baseline (P < 0.05, respectively) (Table 2).While there was a significant time effect (P < 0.05) and race-by-time interaction (P < 0.05) for brachial MAP, the time effect was not found in the change value from baseline in brachial MAP (Fig. 1). There was no time effect (P = 0.312 for carotid and P = 0.17 for aortic MAP), nor were there any race-by-time interactions (P = 0.12 for carotid and P = 0.105 for aortic MAP) for carotid and aortic MAP (Table 2).
As shown in Fig. 2 (and Table 2), there was a time effect (P < 0.05) and significant race-by-time interaction (P < 0.05) for CO, with a greater increase in Ch than in Ca (p < 0.05) (Fig. 1). This increase was explained by increased HR (significant interaction P < 0.05) as no significant changes were found in SV (P = 0.42 for time and P = 0.334 for race-by-time). TPR decreased significantly from baseline during recovery (P < 0.05 for a time effect), but there was no significant race-by-time interaction (P = 0.486) (Fig. 2). A significant race-by-time interaction was seen for both EDV and ESV (P < 0.05, respectively), while no time effect was observed (P = 0.657 for EDV and P = 0.875 for ESV). Additionally, there were no time effect for EF (P = 0.722) and FS (P = 0.716), nor were there any time-by-race interactions (P = 0.38 for EF and P = 0.075 for FS).
Fig. 2.
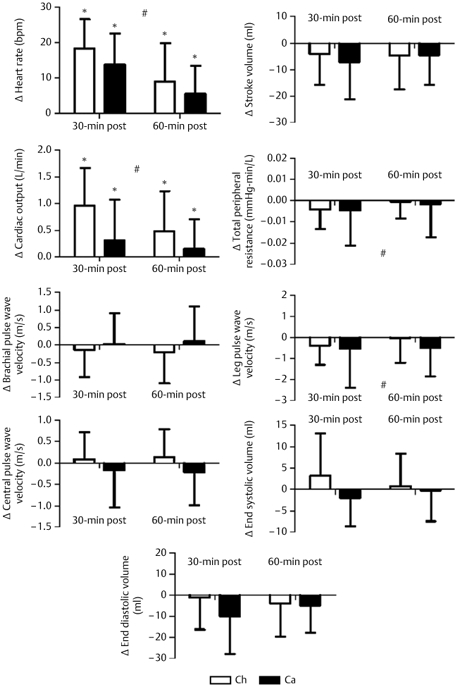
Graphic representation of changes from baseline following exercise in Chinese (Ch) and Caucasian (Ca) participants. The change in heart rate, stroke volume, cardiac output, total peripheral resistance, brachial pulse wave velocity, central pulse wave velocity, leg pulse wave velocity, left ventricle end systolic volume and end diastolic volume are shown. * P < 0.05, significant change from baseline. # P < 0.05, time effect.
As depicted in Table 2 (and Fig. 2), leg PWV decreased significantly from baseline following a bout of exercise (P < 0.05 for a time effect), but there was no time-by-race interaction (P = 0.207). Neither time effect nor time-by-race interactions was found in brachial PWV (P = 0.391 for time and P = 0.38 for race-by-time) and central PWV (P = 0.718 for time and P = 0.075 for race-by-time).
Discussion
This is the first study comparing the PEH response following an acute bout of moderate-intensity aerobic exercise between Ch and Ca. In the present study, we examined the changes in peripheral (brachial) and central (carotid and aortic) artery BP at pre-, 30-min and 60-min post-exercise in Ca and Ch. Our main finding was that there was a significant reduction in brachial SBP in both Ca and Ch after acute exercise, and this reduction was greater in Ca, who also had a higher initial BP, than in Ch. This is in agreement with the literature suggesting that PEH is greater in subjects with a higher initial BP level [10, 30] However, the precise mechanism of the higher initial BP producing the greater PEH is unclear.
Although BP was higher in Ca at baseline, the determinants of BP (cardiac output and peripheral resistance) were not statistically different, probably due to the higher variance in these variables compared to BP. However, the higher peripheral resistance in the Ca group is likely the cause of the higher BP at baseline (although this was not statistically significant). This would be consistent with higher sympathetic activity at baseline (producing increased peripheral resistance through peripheral vasoconstriction) [31]. The difference in the BP response following acute exercise was likely due to a lesser increase in cardiac output in Ca (although not statistically different), since the change in peripheral resistance after exercise was almost identical between groups. Thus, the influence of sympatholysis following acute exercise was likely similar between groups (since peripheral resistance changes were nearly identical) [9]. Nevertheless, it is possible that sympathetic activity may still exhibit a small influences on peripheral resistance, coupled with a larger influence on cardiac output, resulting in a larger drop in BP (due to reduced changes in cardiac output) in the Ca group. This raises interesting questions of potential differences in autonomic control of blood flow between Ch and Ca individuals, which needs to be explored in future studies.
Consistent with previous findings [3, 23, 31] an acute bout of aerobic exercise significantly reduced brachial artery SBP, and our data suggest that acute aerobic exercise produces more beneficial changes in BP for Ca than for Ch. The results of previous studies, which investigated the changes of MAP and DBP following acute exercise, are conflicting. A previous study [3] reported that both MAP and DBP decreased following acute aerobic exercise, while other studies indicated that there was no change in MAP or DBP following exercise [37]. In this study, neither brachial artery MAP nor DBP was found to change significantly following exercise.
An interesting finding in our study was that while both central (aortic and carotid) and peripheral (brachial) BP decreased following exercise, only brachial BP decreased significantly more in Ca. There was also, however, a significant interaction for carotid SBP (but no significant differences between races in the change values). Others have also found differential responses between peripheral and central BP changes following exercise. Yan et al. [48] showed greater changes in central but not peripheral BP in Ca compared to African-Americans following maximal exercise. Although our current findings support differential changes in peripheral and central BP following exercise, the present findings showed differential responses between groups in peripheral, but not central BP. This may be a function of the difference in populations (comparing Ca with Ch vs. African-Americans), the type of exercise used (submaximal vs. maximal) or other unknown factors. Considering that central BP is a stronger predictor of clinical outcome than brachial BP [38], the differential effect of exercise on central compared to peripheral blood pressure may be important and needs to be explored in future research.
Compared with pre-exercise values, HR is consistently elevated following exercise, and this is probably the product of higher temperatures [15] and changes in autonomic function [45]. Our findings are in agreement with previous studies [15, 34] showing that an acute exercise produces a significant increase in recovery HR. It is unclear why HR increased more in Ch than Ca following exercise. It may be due to a different regulatory capacity of autonomic balance or body temperature. However, the mechanisms involved in this response were not investigated in this study. Similar to Halliwill et al. [16], who have shown that SV is maintained during exercise recovery, our present data showed no effect on SV following acute exercise. Thus, our data suggest that the different magnitudes in increases in HR, with no change in SV, contribute to the different magnitude of increase in CO between Ch and Ca.
It is suggested that both active and inactive tissue vasodilate, thereby causing the reduction in TPR [40]. In this current study, a significant reduction in TPR was observed following exercise, with no different between races. Although we did not have measures of active and inactive skeletal muscle vasodilatation, an increase in vasodilatation would be associated with a decrease in artery stiffness [12]. A high degree of arterial stiffness may contribute to a high BP [8], and it is widely accepted that PWV is an important parameter for evaluating arterial stiffness [18]. We found that, compared with baseline values, there was a decrease in lower limb PWV following a bout of acute exercise, with no difference between races. Interestingly, there was no significant decrease in upper limb (brachial) and central PWV. Our data are thus in partial agreement with the aforementioned model for the reduction in TPR, suggesting that sustained post-exercise vasodilatation is present in active lower limb skeletal muscle, not upper limbs and trunk, which may play a primary role in reducing TPR. It is unclear why vasodilatation increased only in lower limbs, without any change in both upper limbs and trunk. It is possible that skeletal muscle contraction in the legs increases local blood flow to meet the changing metabolic demands [47], resulting in the change for local blood flow shear stress, and to stimulate endothelial cells to produce more nitric oxide and thereby produce vasodilatation in lower limbs [1]. Unfortunately, we did not measure the change of nitric oxide in this study.
The rhythmically contracting skeletal muscles in the legs produce the post-exercise vasodilatation. Consequently, there is an increase in venous pooling, generating a drop in central venous pressure and left ventricular preload [14, 42]. Halliwill et al. [14] suggested that SV is maintained through an increase in contractility and a decrease in afterload. As HR is increased, with no change in SV, CO is slightly elevated. Because, however, this increase does not completely counteract the reduction in TPR, BP is reduced, thereby resulting in PEH. We found no evidence for a reduction in left ventricular preload or an increase in contractility in either race. We did not have measures of central venous pressure or direct measures of preload, but a decrease in preload would be expected to produce a reduction in EDV [40]. EDV did not change significantly in either race in this present study. Thus, it is unlikely that left ventricular preload decreased in our study. Similarly, as ESV did not change significantly in either race, no significant change was likewise found in either EF or FS. There was thus no evidence for an increase in left ventricular contractility. Consequently, our data do not support the aforementioned model for the maintenance of SV in the current of PEH. Our study showed that, despite a slight difference in ESV pre-exercise, SV did not change significantly or differently between Ch and Ca.
The clinical implications of our findings are not clear at this time. Although PEH has been predictive of the BP response to exercise training [27], both groups in our study reduced BP following acute exercise. It is unknown if the differential magnitude of the PEH is also predictive of differential BP responses to exercise training between Ca and Ch individuals. It is possible that the differential responses between central and peripheral BP may be clinically important, which needs to be explored in future studies. In conclusion, the results of this study indicated that moderate-intensity aerobic exercise produces a greater magnitude for PEH in peripheral SBP in Ca than Ch. The reason for the PEH in Ch appears to differ from Ca, with greater increase in CO caused by higher HR, with no change in SV, this increase is not substantial enough to counteract the similar decrease in TPR. The difference in initial BP may contribute to the difference changes for PEH between Ca and Ch, suggesting that the higher initial BP may be responsible for the greater magnitude in PEH.
Acknowledgements
This study was funded by the National Institute of Health, 1R01HL093249-01A1, awarded to Bo Fernhall.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1.Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, Joannides R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension 2010; 55: 674–680 [DOI] [PubMed] [Google Scholar]
- 2.Bruce RA. Methods of exercise testing. Step test, bicycle, treadmill, isometrics. Am J Cardiol 1974; 33: 715–720 [DOI] [PubMed] [Google Scholar]
- 3.Chan HH, Burns SF. Oxygen consumption, substrate oxidation, and blood pressure following sprint interval exercise. Appl Physiol Nutr Metab 2013; 38: 182–187 [DOI] [PubMed] [Google Scholar]
- 4.de Tarso VFP, Nakamura FY, Polito MD. Influence of recovery posture on blood pressure and heart rate after resistance exercises in normotensive subjects. J Strength Cond Res 2009; 23: 2487–2492 [DOI] [PubMed] [Google Scholar]
- 5.DeVan AE, Anton MM, Cook JN, Neidre DB, Cortez-Cooper MY, Tanaka H. Acute effects of resistance exercise on arterial compliance. J Appl Physiol 2005; 98: 2287–2291 [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458 [DOI] [PubMed] [Google Scholar]
- 7.Fahs CA, Yan H, Ranadive S, Rossow LM, Agiovlasitis S, Wilund KR, Fernhall B. The effect of acute fish-oil supplementation on endothelial function and arterial stiffness following a high-fat meal. Appl Physiol Nutr Metab 2010; 35: 294–302 [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo VN, Yugar-Toledo JC, Martins LC, Martins LB, de Faria AP, de Haro MC, Sierra C, Coca A, Moreno H. Vascular stiffness and endothelial dysfunction: Correlations at different levels of blood pressure. Blood Press 2012; 21: 31–38 [DOI] [PubMed] [Google Scholar]
- 9.Forjaz CL, Ramires PR, Tinucci T, Ortega KC, Salomao HE, Ignes EC, Wajchenberg BL, Negrao CE Jr, Mion D. Postexercise responses of muscle sympathetic nerve activity and blood flow to hyperinsulinemia in humans. J Appl Physiol 1999; 87: 824–829 [DOI] [PubMed] [Google Scholar]
- 10.Forjaz CL, Tinucci T, Ortega KC, Santaella DF Jr, Mion D, Negrao CE. Factors affecting post-exercise hypotension in normotensive and hypertensive humans. Blood Press Monit 2000; 5: 255–262 [DOI] [PubMed] [Google Scholar]
- 11.Gomes AP, Doederlein PM. A review on post-exercise hypotension in hypertensive individuals. Arq Bras Cardiol 2011; 96: e100–e109 [PubMed] [Google Scholar]
- 12.Grignola JC, Domingo E, Aguilar R, Vazquez M, Lopez-Messeguer M, Bravo C, Roman A. Acute absolute vasodilatation is associated with a lower vascular wall stiffness in pulmonary arterial hypertension. Int J Cardiol 2013; 164: 227–231 [DOI] [PubMed] [Google Scholar]
- 13.Guidry MA, Blanchard BE, Thompson PD, Maresh CM, Seip RL, Taylor AL, Pescatello LS. The influence of short and long duration on the blood pressure response to an acute bout of dynamic exercise. Am Heart J 2006; 151: 1322–1325 [DOI] [PubMed] [Google Scholar]
- 14.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev 2001; 29: 65–70 [DOI] [PubMed] [Google Scholar]
- 15.Halliwill JR, Buck TM, Lacewell AN, Romero SA. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol 2013; 98: 7–18 [DOI] [PubMed] [Google Scholar]
- 16.Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol 2000; 89: 1830–1836 [DOI] [PubMed] [Google Scholar]
- 17.Harriss DJ, Atkinson G. Ethical standards in sport and exercise science research: 2014 update. Int J Sports Med 2013; 34: 1025–1028 [DOI] [PubMed] [Google Scholar]
- 18.Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med 2007; 28: 197–203 [DOI] [PubMed] [Google Scholar]
- 19.Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA, Fernhall B. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis 2009; 207: 220–226 [DOI] [PubMed] [Google Scholar]
- 20.Ito D, Ito O, Cao P, Mori N, Suda C, Muroya Y, Takashima K, Ito S, Kohzuki M. Effects of exercise training on nitric oxide synthase in the kidney of spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2013; 40: 74–82 [DOI] [PubMed] [Google Scholar]
- 21.Jones H, George K, Edwards B, Atkinson G. Effects of time of day on post-exercise blood pressure: circadian or sleep-related influences? Chronobiol Int 2008; 25: 987–998 [DOI] [PubMed] [Google Scholar]
- 22.Jones H, Taylor CE, Lewis NC, George K, Atkinson G. Post-exercise blood pressure reduction is greater following intermittent than continuous exercise and is influenced less by diurnal variation. Chronobiol Int 2009; 26: 293–306 [DOI] [PubMed] [Google Scholar]
- 23.Keese F, Farinatti P, Pescatello L, Cunha FA, Monteiro WD. Aerobic exercise intensity influences hypotension following concurrent exercise sessions. Int J Sports Med 2012; 33: 148–153 [DOI] [PubMed] [Google Scholar]
- 24.Kenney MJ, Seals DR. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 1993; 22: 653–664 [DOI] [PubMed] [Google Scholar]
- 25.Kingsley JD, McMillan V, Figueroa A. Resistance exercise training does not affect postexercise hypotension and wave reflection in women with fibromyalgia. Appl Physiol Nutr Metab 2011; 36: 254–263 [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463 [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Goodman J, Nolan R, Lacombe S, Thomas S. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med Sci Sports Exerc 2012; 44: 1644–1652 [DOI] [PubMed] [Google Scholar]
- 28.McConnell TR. Practical considerations in the testing of VO2max in runners. Sports Med 1988; 5: 57–68 [DOI] [PubMed] [Google Scholar]
- 29.McCrohon JA, Woo KS, Celermajer DS. A comparison of endothelial function in Caucasian and Chinese women before and after the meno-pause. Maturitas 2000; 35: 31–37 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald JR, MacDougall JD, Hogben CD. The effects of exercise duration on post-exercise hypotension. J Hum Hypertens 2000; 14: 125–129 [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34: 724–728 [DOI] [PubMed] [Google Scholar]
- 32.Moraes MR, Bacurau RF, Simoes HG, Campbell CS, Pudo MA, Wasitiski F, Pesquero JB, Wurtele M, Araujo RC. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J Hum Hypertens 2012; 26: 533–539 [DOI] [PubMed] [Google Scholar]
- 33.Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 1983; 15: 523–528 [PubMed] [Google Scholar]
- 34.Ohuchi H, Suzuki H, Yasuda K, Arakaki Y, Echigo S, Kamiya T. Heart rate recovery after exercise and cardiac autonomic nervous activity in children. Pediatr Res 2000; 47: 329–335 [DOI] [PubMed] [Google Scholar]
- 35.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension 2012; 59: 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rezk CC, Marrache RC, Tinucci T Jr, Mion D, Forjaz CL. Post-resistance exercise hypotension, hemodynamics, and heart rate variability: influence of exercise intensity. Eur J Appl Physiol 2006; 98: 105–112 [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez D, Silva V, Prestes J, Rica RL, Serra AJ, Bocalini DS Jr, Pontes FL. Hypotensive response after water-walking and land-walking exercise sessions in healthy trained and untrained women. Int J Gen Med 2011; 4: 549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50: 197–203 [DOI] [PubMed] [Google Scholar]
- 39.Rossow L, Fahs CA, Guerra M, Jae SY, Heffernan KS, Fernhall B. Acute effects of supramaximal exercise on carotid artery compliance and pulse pressure in young men and women. Eur J Appl Physiol 2010; 110: 729–737 [DOI] [PubMed] [Google Scholar]
- 40.Rossow L, Yan H, Fahs CA, Ranadive SM, Agiovlasitis S, Wilund KR, Baynard T, Fernhall B. Postexercise hypotension in an endurance-trained population of men and women following high-intensity interval and steady-state cycling. Am J Hypertens 2010; 23: 358–367 [DOI] [PubMed] [Google Scholar]
- 41.Santana HA, Moreira SR, Neto WB, Silva CB, Sales MM, Oliveira VN, Asano RY, Espindola FS, Nobrega OT, Campbell CS, Simoes HG. The higher exercise intensity and the presence of allele I of ACE gene elicit a higher post-exercise blood pressure reduction and nitric oxide release in elderly women: an experimental study. BMC Cardiovasc Disord 2011; 11: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senitko AN, Charkoudian N, Halliwill JR. Influence of endurance exercise training status and gender on postexercise hypotension. J Appl Physiol 2002; 92: 2368–2374 [DOI] [PubMed] [Google Scholar]
- 43.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006; 47: 1203–1208 [DOI] [PubMed] [Google Scholar]
- 44.Terblanche E, Millen AM. The magnitude and duration of post-exercise hypotension after land and water exercises. Eur J Appl Physiol 2012; 112: 4111–4118 [DOI] [PubMed] [Google Scholar]
- 45.Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 2002; 15: 445–452 [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Aretid BJ, Shu YE, MacKay LS, Webb DJ, Cockcroft JR. Pulse-wave analysis: clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vase Biol 2002; 22:147–152 [DOI] [PubMed] [Google Scholar]
- 47.Williams JT, Pricher MP, Halliwill JR. Is postexercise hypotension related to excess postexercise oxygen consumption through changes in leg blood flow? J Appl Physiol 2005; 98: 1463–1468 [DOI] [PubMed] [Google Scholar]
- 48.Yan H, Ranadive SM, Heffernan KS, Lane AD, Kappus RM, Cook MD, Wu PT, Sun P, Harvey IS, Woods JA, Wilund KR, Fernhall B. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol 2014; 306: H60–H68 [DOI] [PMC free article] [PubMed] [Google Scholar]


