Abstract
Reviews on the cost/outcome of donor human milk (DHM) for infants requiring care in the neonatal intensive care unit (NICU) setting have been undertaken. However, the cost‐effectiveness evidence is unclear. Therefore, we conducted a systematic review of published full economic evaluations of DHM versus standard feeding in infants in neonatal care with the aim of undertaking a narrative synthesis of the cost‐effectiveness evidence and critical appraisal of the methods used. MEDLINE, EMBASE, Web of Science, Cochrane Library, Centre for Reviews and Dissemination (CRD) and PROSPERO databases were searched. Studies were included if they were full economic evaluations (model‐based or trial‐based), the participants were infants in neonatal units requiring nutritional support, the intervention was DHM and the comparator was any standard feeding option. There were no restrictions on outcome measures. Two authors independently assessed eligibility, extracted data, assessed quality and cross‐checked results, with disagreements resolved by consensus. Information extracted focused on study context, and economic evaluation methods and results. Of 2861 studies, seven were included. Six (86%) studies originated from high‐income countries. Four (57%) of the studies were model‐based. Although we could not directly compare the different studies, due to the heterogenous nature of health and economic parameters used in the studies, all DHM interventions indicated cost‐effective or cost saving results. This review suggests that economic evaluation of DHM interventions is an expanding area of research. Although these interventions show promise, future economic evaluations of DHM interventions need to explicitly provide more details on long‐term costs and consequences.
Keywords: breast milk, cost‐effectiveness, donor human milk, economic evaluation, health state, infant formula, milk bank
Key messages.
Feeding infants with DHM reduces the risk of necrotising enterocolitis by approximately two‐thirds compared with formula milk.
In comparison with formula milk, the use of DHM in the clinical setting is uncommon. Barriers to the use of DHM may be the high cost and/or lack of availability.
This study is novel as it is the first study to review the methods used for full economic evaluations in this field.
This review suggests that economic evaluations of DHM interventions is an expanding area of research. Although all studies indicated that DHM interventions are cost‐effective or cost saving, the review found that there was heterogeneity with respect to methods used, and further research and transparency is needed.
1. INTRODUCTION
Maternal breast milk is a natural prophylactic and is associated with improved infant outcomes in lowering the incidence of severe complications of preterm birth, such as necrotising enterocolitis (NEC), neonatal infections and retinopathy of prematurity (ROP) (Fengler, Heckmann, Lange, Kramer, & Flessa, 2020). This supports mother's own milk (MOM) as the optimal choice for infants (National Institute for Health and Care Excellence [NICE], 2008; World Health Organization [WHO], 2011). However, mothers with premature or low birthweight (LBW)/very low birthweight (VLBW) infants may not be able to (1) produce enough milk and (2) breastfeed due to being HIV positive or having breast cancer or they may have died during childbirth (Murguia‐Peniche & Kirsten, 2014). In these circumstances, the World Health Organization (WHO) and others (e.g., the European Society for Paediatrics Gastroenterology and Nutrition) recommend using DHM that provides a number of benefits over formula milk, such as a lower risk of NEC in the infant due to the presence of active enzymes and anti‐infective properties in the breastmilk (Arslanoglu et al., 2013; WHO, 2011). A recent Cochrane review reported that feeding infants DHM reduced the risk of NEC by approximately two‐thirds compared with formula milk (Quigley, Embleton, & McGuire, 2018). Another study indicated that feeding DHM may reduce the risk of bronchopulmonary dysplasia (BPD) (Villamor‐Martínez et al., 2018).
The use of DHM is not common and not all preterm infants who are likely to benefit receive DHM, as it is more expensive than formula milk (Fengler et al., 2020; Hagadorn, Brownell, Lussier, Parker, & Herson, 2016; Place, 2010; Zipitis, Ward, & Bajaj, 2015). Availability of DHM is also a limiting factor. For example, some European countries have only one or two milk banks (European Milk Bank Association, 2020). A recent systematic review indicated that DHM is likely to provide short‐term cost savings by reducing the incidence of NEC (Buckle & Taylor, 2017). However, the cost‐effectiveness of DHM is still unclear.
This paper reports on a systematic review of published full economic evaluations of DHM versus standard feeding in infants in neonatal care with the aim of undertaking a narrative synthesis of the cost‐effectiveness evidence, appraising the methods used and assessing the quality of the economic evaluations using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) (Husereau et al., 2013) and Phillips (Philips, Bojke, Sculpher, Claxton, & Golder, 2006) checklists. To our knowledge, this is the first systematic review of published full economic evaluations of DHM.
2. METHODS
The systematic review follows the reporting guidelines of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) (Liberati et al., 2009). The protocol is registered with the international prospective register of systematic reviews (PROSPERO) database (reference number CRD42019139251).
2.1. Search strategy
The following electronic databases were searched: MEDLINE (Ovid); MEDLINE in‐process and non‐index citations; EMBASE (Ovid); Web of Science; all Cochrane Libraries; Centre for Reviews and Dissemination (CRD): (Database of Abstracts of Reviews of Effects [DARE], the National Health Service Economic Evaluation Database [NHS EED], Health Technology Assessment [HTA]); and PROSPERO. The original searches were conducted on 19 September 2019, and auto alerts were set up until 30 June 2020 to identify any additional studies since the searches were conducted. There was no limitation regarding the year of publication, and all published records were searched. Search strategies included Medical Subject Headings (MeSH) terms and text words of key papers that were identified beforehand. Search strategies that were developed for MEDLINE were adapted as appropriate for the other bibliographic databases with additional supplementary searches carried out as necessary. Searches of grey literature and screening of reference lists in relevant identified articles were also performed to identify potential additional studies. The MEDLINE search strategy is presented in Data S1.
2.1.1. Eligibility criteria
Studies were included or excluded based on the following criteria:
Types of study/article: full economic evaluations were included (studies in which both the costs and the outcomes of the alternatives are examined and in which a comparison of two or more interventions or case alternatives are undertaken) including trial‐based and model‐based evaluations. All five types of economic evaluations (cost‐benefit analysis [CBA], cost‐consequence analysis [CCA], cost‐effectiveness analysis [CEA], cost‐minimisation analysis [CMA], and cost‐utility analysis [CUA]) were included. Partial economic evaluations, systematic reviews, meta‐analyses, qualitative studies, conference abstracts, editorials, short commentary and study protocols were excluded.
Participants/population: infants in neonatal units requiring nutritional support—new‐born, premature and those of LBW and VLBW—and neonatal units offering nutritional support to infants were included.
Intervention(s) and exposure(s): DHM for infant feeding where a mother is unable to provide breast milk or where there is insufficient supply of breast milk to sustain the infants' nutritional requirements.
Comparator(s)/control: all alternative infant feeding options including infant formula—of any variety (fortified and unfortified); MOM with formula milk were included.
Outcome(s): no restrictions on outcomes measures. Potentially relevant costs, effectiveness and cost‐effectiveness outcomes were as follows: costs of the DHM provision, direct medical care costs, direct non‐medical costs, indirect costs, societal costs, incidence of feeding intolerance, weight gain, incidence and severity of NEC and any other infections, duration of hospital stays, incremental cost‐effectiveness ratios (ICER) (e.g., cost per disability‐adjusted life year [DALY] averted, cost per quality‐adjusted life year [QALY] gained, additional DHM cost per case of averted NEC).
Other criteria: there were no restrictions based on evaluation perspective taken, time horizon for evaluation and country where the studies were conducted. No setting was formally defined within the search for this intervention, but premature babies and those with LBW/VLBW are routinely managed in neonatal units (Hagadorn et al., 2016).
2.2. Study selection procedure
The review followed a two‐stage method. First, two reviewers independently screened the titles and abstracts of all publication records identified by the searches against the selection criteria. Disagreements were resolved by retrieval of the full publication and consensus agreement or discussion with a third reviewer. Second, full copies of all studies deemed potentially relevant were obtained, and two reviewers independently assessed these with respect to the inclusion/exclusion criteria. Records rejected at full text stage, and reasons for exclusion were documented. The literature search results were exported into EndNote X9 Reference package (Thomson Reuters, Philadelphia, PA, USA).
2.3. Data extraction
The study characteristics and findings were extracted by one reviewer, using an electronic, pre‐existing cost‐effectiveness form, which was adapted for extracting data for this systematic review. The whole process was independently checked for completeness and accuracy by a second reviewer. Extracted information included the following:
Details of study context (authors, publication year, country, setting, data source, study population, intervention and comparators).
A detailed account of the economic evaluation methods and results (type of economic evaluation, outcome measures, model type, study perspective, time horizon, currency and price year, discount rate, resource use/costs, analytical methods, results, sensitivity analyses, generalisability, conclusion, source of funding and conflicts of interest).
Missing data were requested from study authors.
2.4. Quality assessment of included studies
To allow a comparison of the economic evaluation methods used in the studies, the reporting quality of both the trial‐based and model‐based economic evaluations were assessed using the CHEERS checklist (Husereau et al., 2013), which is a commonly used generic quality assessment tool of reporting standards. The quality of each model‐based economic evaluation was further assessed using the Philips checklist (Philips et al., 2006) to provide more specific data for a specific audience (e.g., health economists' modellers). The quality assessment checklists provide a systematic and critical descriptive overview of key methodological elements. Quality assessment was undertaken by one reviewer and was independently checked for completeness and accuracy by a second reviewer.
3. RESULTS
Figure 1 illustrates the flow of papers identified, screened and included in the review. Of the 5559 studies identified in the initial literature search, 2861 were screened. From the screened papers, 2818 were excluded based on titles and abstracts. Forty‐three articles were considered potentially relevant and remained for subsequent detailed assessment. Of these, seven were in line with the eligibility criteria. Therefore, these articles were included in the analysis and synthesis. The most common reasons for exclusion were non‐eligible study/article type or non‐eligible intervention. Full details of the excluded studies are presented in Data S2.
FIGURE 1.
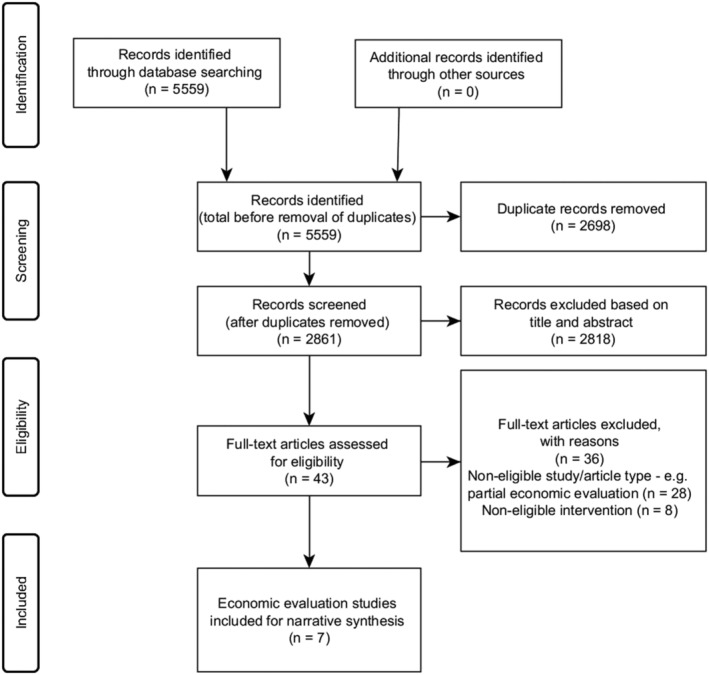
PRISMA flow diagram for the selection of studies
3.1. Details of study context
Full details of study context are presented in Table 1. A large proportion of the studies (n = 6) were published between 2012 and 2019 (Assad, Elliott, & Abraham, 2016; Dritsakou et al., 2016; Ganapathy, Hay, & Kim, 2012; Hampson, Roberts, Lucas, & Parkin, 2019; Taylor, Joolay, Buckle, & Lilford, 2018; Trang et al., 2018), apart from one published in 2002 (Arnold, 2002). The vast majority of the studies (n = 6) originated from high‐income countries, mainly the United States (n = 4) (Arnold, 2002; Assad et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019), with one each from Canada (Trang et al., 2018) and Greece (Dritsakou et al., 2016). The Organisation for Economic Co‐operation and Development (OECD) defines high‐income countries as those with a gross national income (GNI) per capita of US$12,375 or more, with upper middle‐income countries as those with a GNI per capita between US$3996 and US$12,374 (OECD, 2019). Only one study was from an upper middle‐income country—South Africa (Taylor et al., 2018). In terms of study setting, all took place in NICU settings. More than half of the economic evaluations (n = 4) were model‐based (Arnold, 2002; Ganapathy et al., 2012; Hampson et al., 2019; Taylor et al., 2018) compared with trial‐based evaluations (Assad et al., 2016; Dritsakou et al., 2016; Trang et al., 2018).
TABLE 1.
Summary of general characteristics of the studies
| Authors, year | Country | Setting | Data source | Study population | Subgroups | Intervention | Comparator |
|---|---|---|---|---|---|---|---|
| Arnold, 2002 | USA | NICU | Data from published studies and hospital | Model 1: 21 VLBW infants <1500 g, model 2: 200 premature and VLBW infants, model 3: 189 premature infants | Medical NEC and surgical NEC | Banked donor milk: DHM and fortifier | MOM |
| Assad et al., 2016 | USA | Level III community NICU | Single centre retrospective chart review | 293 preterm infants between gestational ages 23 to 34 weeks and birth weights between 490 and 1700 g | None | EHM using either MOM or DHM and DHM‐derived fortifier | Bovine‐based fortifier and maternal milk; mixed combination of maternal milk, bovine‐based fortifier and formula; and formula |
| Dritsakou et al., 2016 | Greece | Tertiary perinatal centre, NICU and DHM bank | Prospective matching | 100 LBW infants (group I), 100 LBW infants (group II) | None | Mother's breast milk supplemented with donor milk | Donor milk followed by PTF |
| Ganapathy et al., 2012 | USA | NICU | RCT and data from hospital discharges | 207 VLBW infants (RCT), 2560 EP infants in the final analytic sample derived from data | No NEC, medical NEC, and surgical NEC | Human milk‐based diet composed of mother's milk fortified with a donor human milk‐based HMF | Mother's milk fortified with a bovine milk‐based HMF |
| Hampson et al., 2019 | USA | NICU | Data from published studies (RCTs and cohort) | A hypothetical population of 1000 VLBW babies, all of whom are assumed to be admitted to a NICU | Medical NEC and surgical NEC | Babies receive an EHMD. They are fed with mother's expressed breast milk supplemented with a human milk based fortifier | Usual practice of care: babies are fed with mother's expressed breast milk supplemented with a cow's milk based fortifier |
| Taylor et al., 2018 | South Africa | Neonatal units | Clinical data and published evidence | 10,000 VLBW infants, four groups based on birthweight: (500–750 g, 751–1000 g, 1001–1250 g, 1251–1500 g) | Four birthweight groups | DHM | Formula milk |
| Trang et al., 2018 | Canada | Tertiary NICU | Double‐blinded RCT | 363 VLBW infants <1500 g | None | DHM | Bovine‐based PTF |
Abbreviations: DHM, donor human milk; EHM, entirely human milk; EHMD, exclusive human milk diet; EP, extremely premature; HMF, human milk fortifier; LBW, low birthweight; MOM, mother's own milk; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PTF, preterm formula; RCT, randomised controlled trial; VLBW, very low birthweight.
The number of participants in each study differed considerably. For trial‐based studies, this ranged from 200 (Dritsakou et al., 2016) to 363 (Trang et al., 2018); and for model‐based studies, ranged from 410 (Arnold, 2002) to 10,000 (Taylor et al., 2018). Apart from two (29%) studies which included LBW infants (Assad et al., 2016; Dritsakou et al., 2016), the remainder only included VLBW or extremely premature (EP) infants (Arnold, 2002; Ganapathy et al., 2012; Hampson et al., 2019; Taylor et al., 2018; Trang et al., 2018). As per our inclusion criteria, all interventions were DHM. A range of comparators were identified, such as MOM.
3.2. Review of economic evaluation methods and results
A detailed account of the economic evaluation methods and results is presented in Tables 2, 3 and 4.
TABLE 2.
Detailed account of the economic evaluation methods—Part 1
| Authors, year | Type of economic evaluation/outcomes | Model type | Study perspective | Time horizon | Price year/currency | Discount rate | Resource use and costs | Detail resource use and costs (DHM/other diet provision) |
|---|---|---|---|---|---|---|---|---|
| Arnold, 2002 | CMA/savings to a health care system or individual family for NEC/sepsis prevention | Three models of cost‐effectiveness analysis | Health care/payer | 2 months | Model 1: not stated, Model 2: 1998, Model 3: 1994/US$ | N/A | Direct cost, cost reduction from shorter hospital stays as a result of NEC/sepsis prevention, costs to an individual state | Fortifier: $1.00/packet |
| Assad et al., 2016 | CCA/hospital stays, NEC/intolerance incidence, weight gain, time to full feed, hospitalisation costs | N/A | Health care | Enrolled 2009–2014 | Not stated/US$ | Not stated | Hospitalisation costs: length of stay for VLBW infants, physician charges | EHM group: donor milk and donor milk‐derived fortifier costs ($125–$250/100 ml bottle) |
| Dritsakou et al., 2016 | CCA/hospital stays, viral infections, duration of enteral gavage feeding, NICU/hospitalisation costs | N/A | Health care | 8 months | Not stated/€ | N/A | NICU/hospitalisation costs: doctors/prescriptions, enteral feeding (nasogastric tubes and syringes), parenteral feeding (intravenous bags and syringes) | Formula feeding (bottles, quantities of formula), breast feeding (storage bags), human milk fortifiers, milk transport, pasteurisation donor milk costs |
| Ganapathy et al., 2012 | CCA/hospital stays, NICU/NEC/hospitalisation costs | Assumed a decision model | Health care/payer | Not stated | 2011/US$ | Not stated | NICU/NEC (medical and surgical)/hospitalisation costs, net savings in hospital costs | Prolact/H2MF: $6.25/ml, DHM: $3.00/ounce ($0.10/ml); bovine milk‐based HMF: $1.30/packet, PTF: $1.00/ounce ($0.03/ml) |
| Hampson et al., 2019 | CCA/deaths (initial hospital stay), cases of NEC (medical and surgical), cases of late onset sepsis and other infections, NEC/hospitalisation/sepsis costs | Decision tree | Health care, sensitivity analysis: societal | Not stated | 2016/US$ | 3% for both costs and benefits | Hospitalisation costs: initial stay for VLBW infants/NEC (medical and surgical)/sepsis costs, sensitivity analysis: societal costs | 30 ml Prolact+ 6 product: $187.50, DHM: $183; total EHMD cost: $7731; cow's milk: $226 |
| Taylor et al., 2018 | CUA/incidence, severity of NEC, cost/DALY averted | Cohort Markov decision | Health care | 14 weeks | 2015/US$, converted at PPP | N/A | Neonatal care up to point of death/initial discharge: length of stay, NEC surgery | DHM: $0.1371/ml, 75.7 for 100 ml; formula milk: $0.0529/ml, 68.9 for 236 ml |
| Trang et al., 2018 | CEA/incidence of NEC, hospitalisation/post discharge costs, extra DHM cost/case of averted NEC | N/A | Societal | 18 months | 2015/Canadian $ | Not stated | Hospitalisation/readmissions costs: physician fees; enteral feeds, indirect, informal/non‐medical costs, societal costs | DHM unit cost: 4.95 (3–7.6) Canadian $/ounce; bovine‐based PTF: 0.13 Canadian $/ounce; fortifier: 0.14 Canadian $/ounce |
Abbreviations: CCA, cost‐consequence analysis; CEA, cost‐effectiveness analysis; CMA, cost‐minimisation analysis; CUS, cost‐utility analysis; DALY, disability‐adjusted life year; DHM, donor human milk; EHM, entirely human milk; EHMD, exclusive human milk diet; HMF, human milk fortifier; N/A, not applicable; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PTF, preterm formula; PPP, purchasing power parity; VLBW, very low birthweight.
TABLE 3.
Detailed account of the economic evaluation methods and results—Part 2
| Authors, year | Analytical methods | Results (incremental costs and outcomes) | |
|---|---|---|---|
| Arnold, 2002 | Three models of cost analysis, statistical analysis methods: Not stated | Model 1: $8800 could be saved per infant, every $1 spent on DHM leads to a save of $11–$37 in NICU costs. Model 2: save of $48,150 in additional hospital stay days, assuming that each infant is discharged 15 days earlier. Model 3: a case of confirmed NEC not requiring surgery cost: additional $138,000 per infant and a case of NEC requiring surgery cost: Additional $238,000 per infant | Not stated |
| Assad et al., 2016 | STATA statistical software version 13: Fisher's exact test and linear regression analyses | Feeding intolerance, number of days to full feeds and incidence of NEC were lower, and total hospitalisation costs were lower by up to $106,968 per infant in those fed an EHM diet compared with other groups. Average weight gain per day was similar among the four groups (18.5 to 20.6 g per day). Mixed group had the highest number of days to full feeds and total hospitalisation costs | Not stated |
| Dritsakou et al., 2016 | SPSS version 19: independent Student's t test, Mann–Whitney test, Chi‐squared test, Fisher's exact test, and logistic regression analyses | Infants fed with their mother's milk had significantly shorter hospital stays and lower hospitalisation costs. In group I infants, the duration of enteral feeding was shorter, resulting in significantly lower costs. Up to 8 months of age, group I infants experienced fewer episodes of viral infections, and cost of each doctor visit and drug prescription was lower for these infants | Not stated |
| Ganapathy et al., 2012 | Excel 2003: cost calculator for the model and a separate analysis of hospital discharges | Incremental costs of medical/surgical NEC over/above average costs incurred for EP infants without NEC: $74,004 and $198,040 per infant, respectively. EP infants fed with 100% human milk‐based: lower NICU length of stay and total costs of hospitalisation: Savings of 3.9 NICU days and $8167/EP infant | One‐way/two‐way percentage changes in parameters. Cost savings from donor HMF strategy were sensitive to price/quantity of donor HMF, percentage reduction in risk of overall and surgical NEC achieved and incremental costs of surgical NEC |
| Hampson et al., 2019 | Microsoft excel: decision tree model: main analysis, or ‘base case’, sensitivity analyses | EHMD substantially reduces mortality/improves other health outcomes, as well as generating substantial cost savings of $16,309 per infant by reducing adverse clinical events. Cost savings increase to $117,239 per infant when wider societal costs are included. Holding other factors constant, EHMD would still reduce costs if baseline incidence of NEC in usual care group was as low as 7% | (1) Various threshold analyses to explore incidence rates of late onset sepsis/NEC: EHMD to be cost saving. (2) Lower/higher cost scenarios. (3) Some examples of wider societal costs. (4) Case where mortality for usual care group was estimated from retrospective cohort study, with treatment effect of EHMD on mortality taken from trial |
| Taylor et al., 2018 | Microsoft excel: cohort Markov decision: various scenario analyses | Prioritising infants in lowest birthweight groups: Save the most lives, whereas prioritising infants in highest birthweight groups: the highest cost savings. All allocation scenarios would be considered very cost‐effective in South Africa compared with use of formula; ‘worst case’ ICER was $619/DALY averted | Probabilistic SA. Dirichlet distribution: proportion of infants; beta distribution: risk of NEC with formula milk; log normal distribution: relative risk of any NEC with donor milk or relative risk of surgical NEC with donor milk |
| Trang et al., 2018 | SAS version 9.4: nonparametric regression analyses for costs, Cochran–mantel–Haenszel statistics for outcomes, linear regression statistics or Wilcoxon rank tests for continuous outcomes | Incidence of NEC differed between groups (all stages 3.9% DHM, 11.0% PTF; P = 0.01). Costs to 18 months did not differ with a mean of 217,624 and 217,245 in DHM and PTF groups. Incremental cost: 379. Post discharge costs were lower in DHM 46,440 than PTF group 55,102 (P = 0.04). DHM cost an additional $5328/case of averted NEC (ICER: $5328 per case of averted NEC) | Deterministic SA. Costs excluding infants: received exclusively mother's milk during intervention and infants: had incomplete family questionnaires. ICER: DHM costs, formal medical costs, physician fees from birth to 18 months, caregiver wages to reflect Ontario minimum wage and national Canadian wage, and NEC stage ≥II instead of NEC stage ≥I as health outcome. Scatter plots/CEACs |
Abbreviations: CEACs, cost‐effectiveness acceptability curve; DALY, disability‐adjusted life year; DHM, donor human milk; EHM, entirely human milk; EHMD, exclusive human milk diet; EP, extremely premature; HMF, human milk fortifier; ICER, incremental cost‐effectiveness ratio; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; SA, sensitivity analysis.
TABLE 4.
Detailed account of the economic evaluation methods and results—Part 3
| Authors, year | Generalisability | Conclusion | Source of funding | Declared conflicts of interest |
|---|---|---|---|---|
| Arnold, 2002 | Costs saved could be applied to other quality health care programmes/services that would reduce disparity of care, including improving breastfeeding education and support among minority populations of women who initiate breastfeeding at much lower rates | From three models calculated, it would be cost‐effective for a payer to prevent a substantial percentage of NEC cases at a fraction of the cost. Cost of using banked DHM to feed premature infants is inconsequential when compared with the savings from NEC prevention | Not stated | Not stated |
| Assad et al., 2016 | Not stated | Implementing EHM diet in VLBW infants has led to a significant decrease in incidence of NEC. Other benefits of this diet include decreased feeding intolerance, shorter time to full feeds, shorter length of stay, and lower hospital/physician charges for EP and VLBW infants | Not stated | None |
| Dritsakou et al., 2016 | Not stated | Feeding LBW infants predominantly with their mother's milk reduces hospital and health service usage costs | Not stated | Not stated |
| Ganapathy et al., 2012 | Not stated | Compared with feeding EP infants with mother's milk fortified with bovine milk‐based supplements, a 100% human milk‐based diet that includes mother's milk fortified with donor human milk‐based HMF may result in potential net savings on medical care resources by preventing NEC | Prolacta Bioscience | None |
| Hampson et al., 2019 | They cannot draw any strong conclusions on generalisability of these results to other settings, as clinical and resource use data are all specific to the United States. The extent of the cost savings shown by their analysis suggests that it is worth investigating the likelihood that EHMD is cost‐effective in other settings | EHMD is dominant in cost‐effectiveness terms that it is both cost saving and clinically beneficial, for VLBW babies in a US‐based setting. These findings indicate that the use of EHMD rather than usual care in a US setting would reduce costs for health care payer and lead to improved health outcomes for VLBW babies | Prolacta Bioscience | Two authors declare competing interests |
| Taylor et al., 2018 | Data were relied on clinical data provided by one hospital, and there may be variation across hospitals even within one country. Although published data for some parameters do exist for high income countries (most notably the United States), for example, rate of surgical NEC, these data are not applicable to many middle‐income settings due to lack of specialist neonatal equipment such as ventilators | There is an argument to increase supply of DHM in middle‐income countries. The analysis could be extended by taking a longer term perspective, using data from more than one country and exploring use of donor milk as an adjunct to mother's own milk, rather than a pure substitute for it | National Institute for Health Research | None |
| Trang et al., 2018 | The extent to which results are generalizable to other settings in which mother's milk feeding or costs might differ is uncertain | In a high mother's milk use setting, total costs from a societal perspective to 18 months of providing supplemental DHM versus PTF to VLBW infants did not differ, although post discharge costs were lower in DHM group. Although supplemental DHM was not cost saving, it reduced NEC supporting its use over PTF | Canadian Institutes of Health Research and Ontario Ministry of Health | None |
Abbreviations: DHM, donor human milk; EHM, entirely human milk; EHMD, exclusive human milk diet; EP, extremely premature; HMF, human milk fortifier; LBW, low birthweight; NEC, necrotizing enterocolitis; PTF, preterm formula; VLBW, very low birthweight.
3.2.1. Type of economic evaluation and outcome measures
Focusing on the methods of economic evaluation, more than half of the studies (n = 4) performed a CCA (Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019) and used a broad range of outcome measures including: duration of hospital stays (Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019); incidence and severity of NEC (Assad et al., 2016) and other infections such as sepsis (Hampson et al., 2019), viral infection (Dritsakou et al., 2016); incidence of feeding intolerance (Assad et al., 2016); duration of enteral gavage feeding (Dritsakou et al., 2016); time to full feed (Assad et al., 2016); weight gain (Assad et al., 2016); and NICU (Dritsakou et al., 2016; Ganapathy et al., 2012), NEC (medical and surgical) (Ganapathy et al., 2012; Hampson et al., 2019), sepsis (Hampson et al., 2019) and hospitalisation costs (Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019). Of the remaining three (43%) studies, one conducted a CMA (Arnold, 2002) using savings to a health care system or individual family for NEC/sepsis prevention. Another conducted a CEA (Trang et al., 2018) using incidence of NEC, hospitalisation and post discharge costs and additional DHM cost per case of averted NEC. The final study conducted a CUA (Taylor et al., 2018) using the incidence and severity of NEC, and cost per DALY averted.
CMA focuses solely on costs' differences therefore questions such as ‘Is the extra effectiveness worth the extra cost?’ cannot be answered (Hoch & Dewa, 2005). One particular study conducted a CMA, effectiveness data were taken from a single, non‐randomised study comparing the use of fortified MOM with formula (Schanler, Shulman, & Lau, 1999). They assumed the effectiveness outcomes to be equal for DHM and MOM because there was a lack of available effectiveness data for DHM. This is problematic as DHM is unlikely to be as effective as MOM (Renfrew et al., 2009). Measurement and valuation of DALY were not reported in the CUA study (Taylor et al., 2018).
3.2.2. Type of modelling approach taken
With respect to modelling, one study used three models for the cost‐effectiveness analysis (Arnold, 2002); another a single cost‐effectiveness model (assumed to be a decision model) (Ganapathy et al., 2012); a third used a decision tree model (Hampson et al., 2019); and the fourth a probabilistic cohort Markov decision model (Taylor et al., 2018). The studies by Taylor et al. (2018) and Hampson et al. (2019) justified their model choice. The remaining two model‐based studies did not provide any justification for their model choice (Arnold, 2002; Ganapathy et al., 2012).
3.2.3. Evaluation perspective taken, time horizon considered and price year/currency
All studies clearly reported their study perspective. The majority (n = 5) were from a healthcare perspective (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Taylor et al., 2018); two (29%) were from a societal perspective (Hampson et al., 2019; Trang et al., 2018). The time horizons of the studies differed, ranging from 2 months for the model‐based study by Arnold (2002) to 18 months for the trial‐based study by Trang et al. (2018). None of these studies justified their choice of time horizon. One (14%) study only stated the enrolment time frame for the infants (Assad et al., 2016). Two (29%) studies did not explicitly state their time horizon (Ganapathy et al., 2012; Hampson et al., 2019). Four (57%) studies specified their price year, and three (43%) studies did not (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016). All studies reported their currency. For the study by Taylor et al. (2018) costs were converted to 2015 US Dollars at Purchasing Power Parity (PPP) using the World Bank exchange rates.
3.2.4. Choice of discount rate
For less than half of the studies (n = 3) (Arnold, 2002; Dritsakou et al., 2016; Taylor et al., 2018), discounting was not appropriate as the time horizons were less than 1 year (Drummond, Sculpher, Claxton, Stoddart, & Torrance, 2015). Three (43%) studies did not state a discount rate (Assad et al., 2016; Ganapathy et al., 2012; Trang et al., 2018), whereas one (Hampson et al., 2019) reported using an annual discount rate of 3%, for both costs and benefits as recommended by the US Second Panel on Cost‐Effectiveness in Health and Medicine (Sanders et al., 2016).
3.2.5. Resource use and costs
The choice of inclusion of a particular type of resource use and cost varied according to the study purpose, perspective, time horizon and the nature of the intervention/comparator being evaluated. Costs tended to be categorised into direct medical care costs (e.g., NICU, NEC treatment [medical and surgical], sepsis and hospitalisation costs) (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019; Taylor et al., 2018; Trang et al., 2018); informal and non‐medical care costs (e.g., caregiver transportation and labour market earnings lost) (Trang et al., 2018); indirect costs incurred by institutions (e.g., administration, human resources and plant operations) (Trang et al., 2018); societal costs (Hampson et al., 2019; Trang et al., 2018); enteral feeding costs (Dritsakou et al., 2016; Trang et al., 2018); parenteral feeding costs (Dritsakou et al., 2016); and resource use and costs of the DHM/other diet provision such as formula milk (detailed information is presented in Table 2) (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019; Taylor et al., 2018; Trang et al., 2018).
Only two (29%) studies stated the costs which were excluded from their costing. These were discharges with an average daily cost of <$100 and infants who died within the first 3 days of life for the study by Ganapathy et al. (2012) and costs to parents and society and long‐term health service costs by Taylor et al. (2018).
3.2.6. Sensitivity analysis undertaken
Fewer than half of the studies (n = 3) did not perform any type of sensitivity analysis to assess the robustness of their results (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016). One of the trial‐based studies conducted a deterministic sensitivity analysis (Trang et al., 2018), whereas three of the model‐based studies conducted at least one type of sensitivity analysis in line with recommendations. These were one‐way and two‐way percentage changes in the parameters used to build the expected costs calculator (Ganapathy et al., 2012); four sensitivity analyses to explore the impact on the results if alternative input values were used in the model (Hampson et al., 2019) and a probabilistic sensitivity analysis (Taylor et al., 2018).
3.2.7. Narrative synthesis of cost‐effectiveness evidence
We cannot compare results of economic evaluations that assess health care interventions, which have been conducted in different regions/settings and times. This is due to notable differences in the funding of health care systems, the treatments and care pathways, and baseline population and demographic characteristics around the world. Despite the heterogenous methods of economic evaluations used prohibiting direct comparison between studies, all DHM interventions indicated cost‐effective or cost saving results. The costs saved in each study, using DHM, differed considerably. This ranged from $8167 (Ganapathy et al., 2012) to $238,000 (Arnold, 2002) per infant.
Arnold (2002) stated that three models of cost analysis were presented to indicate the savings that could accrue to a health care system or individual family if banked donor milk were provided as first feedings when MOM was not available. Model 1 noted that $8800 could be saved per infant (every $1 spent on DHM could lead to a saving of $11–$37 in NICU costs). Model 2 indicated that there would be a saving of $48,150 in additional hospital stay days, assuming that each infant was discharged 15 days earlier. Model 3 showed that a case of confirmed NEC not requiring surgery would cost an additional $138,000 per infant, and a case of NEC requiring surgery would cost an additional $238,000 per infant. Therefore, they concluded that the cost of using banked donor milk to feed premature infants is relatively inconsequential when compared with the savings from NEC prevention.
Assad et al. (2016) noted that implementing an entirely human milk (EHM) diet in VLBW infants resulted in a significant decrease in the incidence of NEC (1.10% in the EHM group vs. 10% in the bovine and mixed groups, P < 0.011). Other benefits of this diet included reductions in feeding intolerance, time to full feeds, length of hospital stay, and hospital and physician charges (by up to $106,968 per infant) for EP and VLBW infants.
Dritsakou et al. (2016) concluded that LBW infants fed predominantly with their mother's milk supplemented with donor milk had significantly lower hospital stays (mean length of stay 21 vs. 26 days, P < 0.001) and NICU/hospitalisation costs. In these infants, the duration of enteral gavage feeding was shorter, and they experienced fewer episodes of viral infections.
The results by Ganapathy et al. (2012) indicated that EP infants fed a 100% human milk‐based diet had lower expected NICU length of stay and hospitalisation costs, resulting in net direct savings of almost four NICU days and $8167 per EP infant.
The analysis by Hampson et al. (2019) determined that an exclusive human milk diet (EHMD) reduces mortality and improves other health outcomes, as well as generating substantial cost savings of $16,309 per infant by reducing adverse clinical events. Cost savings increase to $117,239 per infant when wider societal costs are included. Holding other factors constant, an EHMD would still reduce costs if the baseline incidence of NEC in the usual care group was as low as 7%.
Taylor et al. (2018) concluded that prioritising infants in the lowest birthweight groups would save the most lives, whereas prioritising infants in the highest birthweight groups would lead to the highest cost savings. All allocation scenarios would be considered very cost‐effective in South Africa compared with the use of formula milk. The ‘worst case’ ICER was $619 per DALY averted. However, the analysis could be extended by taking a longer time horizon, using data from more than one country and exploring the use of DHM as an adjunct to MOM.
The findings by Trang et al. (2018) indicated that incidence of NEC was significantly lower in the DHM (3.9%) compared with the preterm formula (PTF) group (11.0%). Post discharge costs were significantly lower in the DHM $46,440 compared with the PTF group $55,102. DHM cost an additional $5328 per case of averted NEC (ICER: $5328 per case of averted NEC). However, total costs from a societal perspective to 18 months of providing supplemental DHM versus PTF to VLBW infants did not vary.
3.2.8. Generalisability
Three (43%) of the studies did not report any information regarding the generalisability of their results (Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012). There were various points of view regarding the generalisability of findings by the remaining studies. It was noted by Arnold (2002) that costs saved could be applied to other quality health care services which would decrease disparity of care, including improving breastfeeding education and support among women from minority populations who initiate breastfeeding at much lower rates. Hampson et al. (2019) pointed out that as the clinical and resource use data are all specific to the US, no strong conclusions on the generalisability of their findings to other settings can been made. However, cost savings of their analysis suggests that it is worth investigating the likelihood that an EHMD is cost‐effective in other settings. Data from the study by Taylor et al. (2018) was based on clinical data provided by one hospital, and there might be differences between hospitals even within a single country. The authors mentioned that although published data for some parameters exist for high income countries, mostly the US, these data cannot be applied to many middle‐income settings because of the lack of specialist neonatal equipment. Trang et al. (2018) noted that the extent to which the findings are generalizable to other settings is uncertain.
3.3. Quality assessment of the included studies
The quality assessment results are presented in Table 5. The quality of both the trial‐based and model‐based economic evaluations was assessed using the CHEERS checklist (Husereau et al., 2013)—a 26‐item instrument with a total of six domains. None of the included studies fulfilled all of the quality criteria although none were ranked as ‘worthless’. More than half of the studies fulfilled a large number of the quality criteria. The criteria which were least well addressed were the assumptions, the characterising uncertainty and heterogeneity, the generalisability and the source of funding. The quality of any model‐based economic evaluations was further assessed using the Philips checklist (Philips et al., 2006). Only two of the studies fulfilled a large number of the quality criteria according to the Phillips checklist. The criteria that were least well addressed were the justifications, the assumptions, the uncertainties and the heterogeneity.
TABLE 5.
Critical appraisal of the economic evaluation studies using the CHEERS and Philips checklists
| Authors, year | CHEERS checklist: All seven studies | Philips checklist: Only four model‐based studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Partially completed | Not applicable | Total score | Yes | No | Partially completed | Not applicable | Unclear | Total score | |
| Arnold, 2002 | 14 | 5 | 5 | 2 | 14/26 | 16 | 10 | 17 | 10 | 3 | 16/56 |
| Assad et al., 2016 | 15 | 7 | 2 | 2 | 15/26 | ||||||
| Dritsakou et al., 2016 | 14 | 7 | 2 | 3 | 14/26 | ||||||
| Ganapathy et al., 2012 | 19 | 4 | 2 | 1 | 19/26 | 14 | 12 | 18 | 7 | 5 | 14/56 |
| Hampson et al., 2019 | 22 | 1 | 2 | 1 | 22/26 | 31 | 6 | 11 | 7 | 1 | 31/56 |
| Taylor et al., 2018 | 21 | 1 | 3 | 1 | 21/26 | 36 | 6 | 8 | 2 | 4 | 36/56 |
| Trang et al., 2018 | 21 | 3 | 0 | 2 | 21/26 | ||||||
4. DISCUSSION
To the best of our knowledge, this is the first study to conduct a systematic review of the methods used for published full economic evaluations of DHM versus standard feeding in infants in neonatal care. The scaling up of breastfeeding can help to prevent an estimated 823,000 child deaths and 20,000 breast cancer deaths every year (Victora et al., 2016).
The review identified some emerging patterns. We found that among the economic evaluations, all DHM interventions indicated cost‐effective or cost saving results. The majority of the studies reported clinical outcome measures (e.g., incidence of NEC). Only one of the seven studies (Taylor et al., 2018) reported health‐related outcome measures (e.g., DALY) commonly used within economic evaluations. None of the included studies explicitly reported that they were CBA. However, four of them applied a CCA approach with costs saved as an outcome, which is a form of CBA. Consideration of a broader range of outcomes going beyond the health sector allows for inclusion of benefits and costs from multiple sectors. Efforts are being made to adapt and develop methodologies to promote the use of this type of economic evaluation (Frew, 2017).
Model‐based evaluations offer the opportunity to improve the generalisability of findings and assess the longer term costs and benefits of DHM versus standard feeding. They are important as they are widely used as policy‐making tools that can inform resource allocation decisions. However, none of the model‐based studies offered data based on a longer time horizon, and furthermore, the results from one of these studies was based on a small sample size (Arnold, 2002). Furthermore, two of the studies did not make explicit mention of procedures for checking their models (Arnold, 2002; Ganapathy et al., 2012), and one study did not assess the sensitivity of their findings to the model choice (Arnold, 2002). More importantly, the clinical and resource use data are all specific to one country. Therefore, the generalisability of findings to other contexts, particularly from developed to developing country settings, would be questionable (Musgrove & Fox‐Rushby, 2006).
More than half of the evaluations in this review either did not apply a discount rate, because it was not applicable, or applied one which was a recommended discount rate according to the relevant country guidelines. Three remaining studies did not apply a discount rate. Of those, one had a stated time horizon (Trang et al., 2018). The other two did not state a time horizon, making it difficult to judge if they (Assad et al., 2016; Ganapathy et al., 2012) neglected to follow discounting guidelines.
Methods for collecting resource use and the type of costs included were found to differ across the studies. The majority of the studies did not report what cost components were excluded from their estimates. Future studies should clearly specify which costs are included and excluded. Informal and non‐medical care costs, indirect costs and societal costs were only collected by two studies (Hampson et al., 2019; Trang et al., 2018). It is considered good practice to report findings both with and without informal/indirect costs. Including these types of costs (e.g., costs incurred by families) may alter the management recommendations. To be able to determine the macroeconomic benefits of DHM by reducing the incidence of NEC, an analysis of the lifetime costs would be useful. However, a lifetime model for the economic impact of DHM versus formula feed would be subject to extreme assumptions, which would introduce excessive uncertainties. Establishing causality in this area is extremely challenging and relies on huge amounts of data, which may not even exist. Longitudinal analysis is an option; however, it is costly and slow.
It is inevitable that an economic evaluation contains some degree of uncertainty in its assessment. In order to assess the level of uncertainty, one can apply various sensitivity analyses, which are a set of techniques that seek to analyse how sensitive the results are to uncertain parameters. The choice of sensitivity analysis may depend on the methodology applied, type of economic evaluation (trial‐based or model‐based) or the setting in which the intervention was conducted. However, three of the studies did not perform any type of sensitivity analysis to assess the robustness of their results, and no justification for the exclusion was given (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016).
Two of the model‐based studies (Arnold, 2002; Ganapathy et al., 2012) did not have sufficient methodological quality to provide convincing evidence of the cost‐effectiveness of DHM. Also, although all DHM interventions in this review appear cost‐effective or even cost saving using standard rules of cost‐effectiveness, there is variation by intervention design. However, the narrative synthesis of the cost‐effectiveness evidence, appraising the methods applied and assessing the quality of the included studies are useful for informing health economists/modellers and future research direction in this area.
4.1. Comparison with previous systematic reviews
The main intervention being evaluated in Renfrew et al. economic evaluations' review (Renfrew et al., 2009) was the provision of a lactation consultant to help mothers breastfeed their own infants. The use of DHM, as an adjunct to MOM, was considered in a secondary analysis. However, this comparison was not between exclusive use of DHM and exclusive use of formula. The study by Buckle and Taylor (2017) reviewed cost and cost‐effectiveness of DHM to prevent NEC. Therefore, they mainly focused on one particular outcome. They also only included one full economic evaluation based on their research question and the date they conducted their review. They concluded that it is likely that the use of DHM is cost‐effective; however, they suggested that to strengthen the evidence base, there is a need for conducting comprehensive full economic evaluations of the use of DHM versus standard feeding in infants.
In general, the two previous reviews mainly focused on cost analysis and partial economic evaluations, and summarised and compared the costs and outcomes of a range of interventions and comparators (Buckle & Taylor, 2017; Renfrew et al., 2009). Neither of them reviewed the full economic evaluation methods in the way they have been outlined in this paper. By imposing no restrictions on outcome measures, findings of this study showed that all DHM interventions indicated cost‐effective or cost saving results. Additionally, appraising the methods of full economic evaluations adds to the literature as it provides researchers with detailed information of the methods applied in each study which could help them to produce high quality and useful research.
4.2. Strengths and limitations of this review
One of the most important strengths of this systematic review is the comprehensive search strategy used which contained a broad range of electronic databases of published studies. Furthermore, there were no country restrictions. There was one publication from South Africa as a middle‐income country. Also, this review considered a wide range of evaluations: the use of DHM with different durations versus other standard feeding options. All the major short‐ and long‐term health and associated cost consequences related to the use of DHM compared with standard feeding options were considered. In addition, more than half of the studies fulfilled a large number of the quality criteria.
Our review also had some limitations. We only included full economic evaluations. Therefore, some important data contained within partial evaluations might have been missed. The shortcomings of the included studies and underlying evidence base were further limitations. Synthesising the evidence base was challenging due to the heterogeneous nature of the methods applied. Potential publication bias may also be a problem. It is possible that any study that did not find positive evidence of the use of DHM did not conduct an economic evaluation or, if they did, failed to get it published.
4.3. Challenges associated with conducting economic evaluations in this space
Researchers in this space usually will not calculate DALYs or QALYs gained. This is due to the short time frames and because they cannot survey the infants with preference‐based measures that are needed in order to derive utilities. The utility measures most appropriate for these analyses would be the DALYs or QALYs gained/lost by the parents of the infants. Also, capturing particularly long‐term health and non‐health costs and consequences of DHM interventions is challenging. There are additional other challenges including: lack of funding, scarcity of robust local clinical data, sourcing unit costs and managing lack of equivalent threshold values for outcome gains.
5. CONCLUSIONS
This systematic review suggests that economic evaluation of DHM interventions is an expanding area of research, and current economic evaluations are mainly set in developed countries. All DHM interventions indicated cost‐effective or cost saving results. However, the review found heterogeneity with respect to the economic evaluation methods used. So, to strengthen the evidence base further and increase comparability across interventions, we recommend a comprehensive approach to evaluate cost‐effectiveness to capture, particularly, long‐term health and non‐health costs and consequences of DHM. Furthermore, a careful description should be provided of how the costs of DHM have been generated and its implications on healthcare.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
HM conceptualised and designed the study. MJ ran the search strategies. HM, MJ and MZ screened titles and abstracts. HM and MZ assessed full‐text articles for eligibility. HM and MZ developed the data extraction instrument. MZ extracted the data and assessed the quality of the included studies. HM independently checked data extraction and quality assessments for completeness and accuracy. MZ analysed the data and interpreted the results. MZ drafted the initial manuscript. HM critically reviewed the manuscript for important intellectual content and revised the manuscript. MJ and MZ also reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supporting information
Data S1. Medline (Ovid) Search Strategy
Data S2. List of Studies Excluded at Full Text Review
Zanganeh M, Jordan M, Mistry H. A systematic review of economic evaluations for donor human milk versus standard feeding in infants. Matern Child Nutr. 2021;17:e13151. 10.1111/mcn.13151
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the article text, tables, figure and references in the tables (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019; Taylor et al., 2018; Trang et al., 2018) and supporting information.
REFERENCES
- Arnold, L. D. W. (2002). The cost‐effectiveness of using banked donor milk in the neonatal intensive care unit: Prevention of necrotizing enterocolitis. Journal of Human Lactation: Official Journal of International Lactation Consultant Association, 18(2), 172–177.Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=12033080 [DOI] [PubMed] [Google Scholar]
- Arslanoglu, S. , Corpeleijn, W. , Moro, G. , Braegger, C. , Campoy, C. , Colomb, V. , … Hojsak, I. (2013). Donor human milk for preterm infants: Current evidence and research directions. Journal of Pediatric Gastroenterology and Nutrition, 57(4), 535–542. 10.1097/MPG.0b013e3182a3af0a [DOI] [PubMed] [Google Scholar]
- Assad, M. , Elliott, M. J. , & Abraham, J. H. (2016). Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. Journal of Perinatology: Official Journal of the California Perinatal Association, 36(3), 216–220. 10.1038/jp.2015.168 [DOI] [PubMed] [Google Scholar]
- Buckle, A. , & Taylor, C. (2017). Cost and cost‐effectiveness of donor human milk to prevent necrotizing enterocolitis: Systematic review. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine, 12(9), 528–536. 10.1089/bfm.2017.0057 [DOI] [PubMed] [Google Scholar]
- Dritsakou, K. , Liosis, G. , Valsami, G. , Polychronopoulos, E. , Souliotis, K. , & Skouroliakou, M. (2016). Mother's breast milk supplemented with donor milk reduces hospital and health service usage costs in low‐birthweight infants. Midwifery, 40, 109–113. 10.1016/j.midw.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Drummond, M. F. , Sculpher, M. J. , Claxton, K. , Stoddart, G. L. , & Torrance, G. W. (2015). Methods for the economic evaluation of health care programmes. Oxford, UK: Oxford University Press. [Google Scholar]
- European Milk Bank Association . (2020). European Milk Bank Association (2020, October 10). Retrieved from https://europeanmilkbanking.com/
- Fengler, J. , Heckmann, M. , Lange, A. , Kramer, A. , & Flessa, S. (2020). Cost analysis showed that feeding preterm infants with donor human milk was significantly more expensive than mother's milk or formula. Acta Paediatrica, 109(5), 959–966. 10.1111/apa.15087 [DOI] [PubMed] [Google Scholar]
- Frew, E. (2017). Aligning health economics methods to fit with the changing world of public health. Applied Health Economics and Health Policy, 15(3), 287–289. 10.1007/s40258-017-0319-9 [DOI] [PubMed] [Google Scholar]
- Ganapathy, V. , Hay, J. W. , & Kim, J. H. (2012). Costs of necrotizing enterocolitis and cost‐effectiveness of exclusively human milk‐based products in feeding extremely premature infants. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine, 7(1), 29–37. 10.1089/bfm.2011.0002 [DOI] [PubMed] [Google Scholar]
- Hagadorn, J. I. , Brownell, E. A. , Lussier, M. M. , Parker, M. G. , & Herson, V. C. (2016). Variability of criteria for pasteurized donor human milk use: A survey of US neonatal intensive care unit medical directors. Journal of Parenteral and Enteral Nutrition, 40(3), 326–333. 10.1177/0148607114550832 [DOI] [PubMed] [Google Scholar]
- Hampson, G. , Roberts, S. L. E. , Lucas, A. , & Parkin, D. (2019). An economic analysis of human milk supplementation for very low birth weight babies in the USA. BMC Pediatrics, 19(1), 337. 10.1186/s12887-019-1691-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch, J. S. , & Dewa, C. S. (2005). An introduction to economic evaluation: What's in a name? The Canadian Journal of Psychiatry, 50(3), 159–166. 10.1177/070674370505000305 [DOI] [PubMed] [Google Scholar]
- Husereau, D. , Drummond, M. , Petrou, S. , Carswell, C. , Moher, D. , Greenberg, D. , … Loder, E. (2013). Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Effectiveness and Resource Allocation, 11(1), 6–11. 10.1186/1478-7547-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. , … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6(7), e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murguia‐Peniche, T. , & Kirsten, G. F. (2014). Meeting the challenge of providing neonatal nutritional care to very or extremely low birth weight infants in low‐resource settings. In Nutritional care of preterm infants (Vol. 110, pp. 278–296). Basel: Karger Publishers. [DOI] [PubMed] [Google Scholar]
- Musgrove, P. , & Fox‐Rushby, J. (2006). Cost‐effectiveness analysis for priority setting (2nd ed.). Washington (DC): World Bank. [PubMed] [Google Scholar]
- NICE . (2008). Maternal and child nutrition: National Institute for Health and Care Excellence. London: National Institute for Health and Care Excellence. [Google Scholar]
- OECD . (2019). Organisation for Economic Co‐operation and Development (OECD): Better policies for better lives (2020, November 30). Retrieved from http://www.oecd.org/countries/
- Philips, Z. , Bojke, L. , Sculpher, M. , Claxton, K. , & Golder, S. (2006). Good practice guidelines for decision‐analytic modelling in health technology assessment. PharmacoEconomics, 24(4), 355–371. 10.2165/00019053-200624040-00006 [DOI] [PubMed] [Google Scholar]
- Place, M. (2010). Donor breast milk banks: The operation of donor milk bank services. [PubMed]
- Quigley, M. , Embleton, N. D. , & McGuire, W. (2018). Formula versus donor breast milk for feeding preterm or low birth weight infants. The Cochrane Database of Systematic Reviews, 6, CD002971. 10.1002/14651858.CD002971.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrew, M. J. , Craig, D. , Dyson, L. , McCormick, F. , Rice, S. , King, S. E. , … Williams, A. F. (2009). Breastfeeding promotion for infants in neonatal units: A systematic review and economic analysis. Health Technology Assessment (Winchester), 13(40), 1–iv. 10.3310/hta13400 [DOI] [PubMed] [Google Scholar]
- Sanders, G. D. , Neumann, P. J. , Basu, A. , Brock, D. W. , Feeny, D. , Krahn, M. , … Prosser, L. A. (2016). Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: Second panel on cost‐effectiveness in health and medicine. JAMA, 316(10), 1093–1103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- Schanler, R. J. , Shulman, R. J. , & Lau, C. (1999). Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics, 103(6 Pt 1), 1150–1157. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi? T=JS&PAGE=reference&D=medc2&NEWS=N&AN=10353922 [DOI] [PubMed] [Google Scholar]
- Taylor, C. , Joolay, Y. , Buckle, A. , & Lilford, R. (2018). Prioritising allocation of donor human breast milk amongst very low birthweight infants in middle‐income countries. Maternal & Child Nutrition, 14(Suppl 6), e12595. 10.1111/mcn.12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang, S. , Zupancic, J. A. F. , Unger, S. , Kiss, A. , Bando, N. , Wong, S. , … O'Connor, D. L. (2018). Cost‐effectiveness of supplemental donor milk versus formula for very low birth weight infants. Pediatrics, 141(3), e20170737. 10.1542/peds.2017-0737 [DOI] [PubMed] [Google Scholar]
- Victora, C. G. , Bahl, R. , Barros, A. J. , França, G. V. , Horton, S. , Krasevec, J. , … Rollins, N. C. (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- Villamor‐Martínez, E. , Pierro, M. , Cavallaro, G. , Mosca, F. , Kramer, B. W. , & Villamor, E. (2018). Donor human milk protects against bronchopulmonary dysplasia: A systematic review and meta‐analysis. Nutrients, 10(2), 238. 10.3390/nu10020238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2011). Guidelines on optimal feeding of low birth‐weight infants in low‐and middle‐income countries. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- Zipitis, C. S. , Ward, J. , & Bajaj, R. (2015). Use of donor breast milk in neonatal units in the UK. Archives of Disease in Childhood. Fetal and Neonatal Edition, 100(3), F279–F281. 10.1136/archdischild-2014-307606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Medline (Ovid) Search Strategy
Data S2. List of Studies Excluded at Full Text Review
Data Availability Statement
The data that support the findings of this study are available in the article text, tables, figure and references in the tables (Arnold, 2002; Assad et al., 2016; Dritsakou et al., 2016; Ganapathy et al., 2012; Hampson et al., 2019; Taylor et al., 2018; Trang et al., 2018) and supporting information.


