Abstract
Women of reproductive age (WRA) need adequate nutrient intakes to sustain a healthy pregnancy, support fetal growth, and breastfeed after childbirth. However, data on women's dietary intake in low‐ and middle‐income countries (LMICs) are limited, and assessment of differences between dietary intakes of pregnant or lactating women compared with that of nonpregnant, nonlactating (NPNL) women is untested. Using single, multiple‐pass 24‐h dietary recall data from a sample of WRA residing in rural Bangladesh, we examined women's dietary intakes for energy, protein, calcium, iron, vitamin A, and dietary diversity for three groups: NPNL (n = 2,903), pregnant (n = 197), and lactating women (n = 944). We used equivalence testing to examine similarity in adjusted intakes for pregnant versus NPNL women and lactating versus NPNL women with a predetermined equivalence threshold based on recommendations specific for each reproductive stage. On average, both pregnant and lactating women had insufficient intakes for all dietary measures. Although statistically significant differences were observed between pregnant and NPNL women for energy intake and dietary diversity and between lactating and NPNL women for energy and protein intake, the magnitudes of these differences were too small to reject equivalence. Statistical similarity was also evident in all micronutrients and dietary diversity for both two‐group comparisons. Understanding statistical differences and similarities between dietary measures of women in distinct reproductive stages has important implications for the relevance, appropriateness, and evaluation of maternal diet‐enhancing interventions in LMICs, especially during pregnancy and lactation, when demand for macronutrients and micronutrients is elevated.
Keywords: assessment of women's diets, Bangladesh, equivalence test, maternal nutrition, pregnant and lactating women
Key Messages.
This study demonstrates the important use of equivalence testing—an appropriate statistical test to hypotheses that aim to look for statistical similarity rather than disparity when performing large‐scale dietary assessments.
Application of equivalence testing to assessment of women's diets suggest that macronutrient and micronutrient intakes of pregnant or lactating women are not only suboptimal but also statistically equivalent to those of nonpregnant, nonlactating women in rural Bangladesh.
When assessing intakes in large samples, statistically significant differences can co‐occur with statistically significant similarities, which may lead to divergent conclusions on the need for diet‐enhancing interventions in regions where maternal nutritional problems are endemic.
Testing for both differences and equivalencies in dietary assessments can improve decision‐making on the need, relevance, and appropriateness of diet‐enhancing interventions aimed at improving maternal nutrition.
1. INTRODUCTION
Women of reproductive age (WRA) need increased nutrient intakes to sustain a healthy pregnancy, support fetal growth, and breastfeed after childbirth. In low‐ and middle‐income countries (LMICs), poor dietary intake among WRA significantly contributes to maternal malnutrition (Alam, Van Raaij, Hautvast, Yunus, & Fuchs, 2003; Kramer, 1987; Roseboom, de Rooij, & Painter, 2006), which increases the risk for adverse maternal and fetal complications during pregnancy, delivery, and postpartum (Rasmussen, 2007; Rasmussen & Kjolhede, 2004; Robinson et al., 2013; Van Lieshout, Taylor, & Boyle, 2011).
Recent World Health Organization (WHO) guidelines recommend food as the main vehicle to fulfill the higher nutritional demands of pregnancy and lactation, with supplementation only when needed (WHO, 2016a). In LMICs, the latter half of this guideline has been addressed through evidence‐based maternal micronutrient supplementation (Bangladesh National Nutrition Policy 2015, 2015; Nguyen et al., 2017; WHO, 2012; WHO, 2013; WHO 2016b). However, assessing the feasibility of achieving the first half of the recommendation remains difficult for policy action in the absence of a population‐level dietary assessment (Blumfield, Hure, Macdonald‐Wicks, Smith, & Collins, 2012; Coates, Colaiezzi, Bell, Charrondiere, & Leclercq, 2017; Lander et al., 2017; Picciano, 2003; Zeisel, 2009). Little is known about what women eat in different stages of the reproductive cycle in LMICs, particularly in South Asia, where maternal undernutrition is widespread (Akseer et al., 2017) and maternal overweight is rising (Balarajan & Villamor, 2009; Chowdhury, Adnan, & Hassan, 2018). This knowledge gap limits our understanding of the relevance, timing, and potential consequences of maternal nutrition interventions in LMICs.
Researchers who have examined women's diets in LMICs attribute the lack of dietary diversity and inadequate nutrient intakes among WRA to the monotonous nature of their diets, their poor socioeconomic status, and a lack of maternal nutrition support programs (Darnton‐Hill & Mkparu, 2015; Kavle & Landry, 2018; Lee, Talegawkar, Merialdi, & Caulfield, 2013; Nguyen et al., 2018). Contextual factors other than gender‐inequitable intrahousehold food allocation also constrain food intake among pregnant and lactating women, such as “eating down” during pregnancy for the fear of delivering large babies, or cultural taboos that impose postpartum food restrictions (Christian et al., 2006; Harding et al., 2017; Kavle & Landry, 2018; Shannon, Mahmud, Asfia, & Ali, 2008).
This literature suggests that WRA in LMICs are unlikely to increase their energy, protein, and micronutrient intakes during pregnancy and lactation to the amounts recommended by the Institute of Medicine or other national bodies with the result that, at a population level, diets of pregnant and lactating women are similar to that of nonpregnant, nonlactating (NPNL) women. However, this hypothesis has not previously been tested for two reasons. First, data on nutrient intakes of WRA are limited in their geographical representation (Arsenault et al., 2013; Harris‐Fry et al., 2015), with sample sizes ranging from 10–;600 women from one or two reproductive stages (Leroy, Ruel, Sununtnasuk, & Ahmed, 2018; Nguyen et al., 2018), or represent women from over a decade ago when overnutrition among childbearing women in LMICs was rare (Alam et al., 2003; Huybregts, Roberfroid, Kolsteren, & Van Camp, 2009; Rao et al., 2003). Second, the hypothesized similarity in the diets of women in different reproductive stages cannot be tested using traditional t‐tests because they are designed to evaluate statistical differences. Equivalence tests, which evaluate similarity rather than difference, are more appropriate for establishing statistical similarity (Barker, Luman, McCauley, & Chu, 2002; Limentani, Ringo, Ye, Bergquist, & MCSorley, E. O., 2005; Walker & Nowacki, 2011). These tests have not been used to assess disparities in dietary intakes of WRA based on their reproductive state.
We analyzed diets of WRA by using dietary data from a large survey representative of rural Bangladesh to (i) compare nutrient intakes of pregnant, lactating, and NPNL women to recommendations that are specific for each stage of reproduction, (ii) demonstrate the use of equivalence testing to evaluate whether the hypothesized similarity in diets of women in different reproductive stages is statistically significant at a population level, and (iii) compare results from equivalence testing and the traditional t‐test to determine whether the nutrient intakes of pregnant and lactating women, adjusted for covariates, are statistically similar to or different from those of NPNL women.
2. METHODS
2.1. Data and sampling
We used data from the Bangladesh Integrated Household Survey (BIHS‐1), a representative socioeconomic household survey of rural Bangladesh with data on individual‐level food consumption (Ahmed et al., 2013). The survey was conducted from October 2011 to March 2012—a period that excludes the Ramadan fasting months. Data were collected in 5,503 households in 275 villages using a two‐stage stratified sampling approach. In the first stage, villages were randomly selected from seven strata (administrative divisions) with probability proportional to the number of households in each stratum. In the second stage, 20 households were randomly selected from each village. Household interviews were conducted using a multimodular questionnaire in Bengali by trained male and female interviewers who interviewed an adult male and an adult female, respectively. Details on the survey methodology and the instrument have been published elsewhere (Ahmed et al., 2013).
Apart from income expenditure and assets at the household level, the BIHS also included individual‐level food consumption, demographic characteristics, (age, sex, education level, occupation, etc.), anthropometric measurements, and the reproductive status of women. Individual‐level food consumption data were collected using multiple‐pass, 24‐h recall and food‐weighing methods for meals prepared at home. The primary female food preparer and server was interviewed to record recipes and weight of raw ingredients of food items prepared in the last 24 h and the quantity of food items consumed by each household member for three main meals, that is, breakfast (“morning”), lunch (“noon”), and dinner (“night”). Snacks consumed outside of these mealtimes were recorded separately as “snacks.” The place of meal consumption was also noted (e.g., at home, work, and restaurant) In this analysis, we accounted for all foods that respondents reported to have consumed in the 24‐h period, regardless of the mealtime or place of food consumption.
2.2. Sample selection
We restricted our analyses to women 15–;49 years of age (i.e., WRA) who were primary respondents of the survey, were ever‐married (because in Bangladeshi culture, women bear children after marrying; Islam, Islam, Hasan, & Hossain, 2017; National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International, 2016), and were either the female head of the household or were closely related to the male head of the household (i.e. wife, daughter, or daughter‐in‐law; Figure S1). We excluded WRA for whom data on reproductive status were missing or self‐reported data on dietary intake were unavailable. Lastly, we excluded participants with implausible caloric intakes for at‐home meals in rural Bangladeshi households, defined as intakes beyond 4,205 kcal/day. This value is the upper bound of the 95% confidence interval for the mean per capita caloric consumption for rural Bangladeshi adults that was recently estimated by Sununtnasuk and Fiedler (Sununtnasuk & Fiedler, 2017) and compares with the 99th percentile of caloric intake for our sample of WRA. Our final sample size consists of 4,044 WRA: 197 pregnant, 944 lactating, and 2,903 NPNL women.
2.3. Outcome variables
We assessed actual dietary intakes of our sample on five nutrients: energy (kcal/day), protein (g/day), calcium (mg/day), iron (mg/day), and vitamin A (retinol activity equivalents, measured in mcg/day). We used the BIHS 24‐h food recall data to estimate quantity of foods consumed and food composition tables (FCTs) by the Institute of Nutrition and Food Sciences (INFS), University of Dhaka (Shaheen et al, 2013), to analyze nutrient content of each consumed food. Nutritive values for 228 of the 290 reported food items were obtained from the INFS FCT. Where information on the nutrient content of a food item was missing from the INFS FCT, we first used information found in the FCT developed by Helen Keller International specifically developed for Bangladesh (Hill, Hassan, Karim, & Duthie, 1988) and then information found in the recent FCT developed for Indian foods by the National Institute of Nutrition, India (Longvah, Ananthan, Bhaskarachary, & Venkaiah, 2017). Questions on intake of prenatal iron and folic acid supplements and on receipt of a postnatal dose of vitamin A were limited to mothers of children under 2 years of age. Given that only 768 women who responded to these questions overlapped with our WRA sample of 4,044, we restricted our analyses to dietary intake of micronutrients.
We also identified food groups consumed by each subject to assess diets for a sixth outcome measure—the women's dietary diversity score (WDDS). WDDS is a well‐established proxy for diet quality specifically developed for WRA with revised guidelines published in 2016 by FAO of the United Nations for use at the population level (Martin‐Prével et al., 2015). The WDDS is calculated based on consumption of 10 food groups, and consumption of ≥5 out of the 10 food groups in a day indicates a high probability of micronutrient adequacy of women's diets for 11 micronutrients. The 10 food groups are as follows: (1) grains, white roots and tubers, and plantains, (2) pulses (beans, peas and lentils), (3) nuts and seeds, (4) dairy, (5) meat, poultry and fish, (6) eggs, (7) dark green leafy vegetables, (8) other Vitamin A‐rich fruits and vegetables, (9) other vegetables, and (10) other fruits.
2.4. Exposure variable
Our exposure of interest is the current physiological status of women aged 15–;49 years, who were classified into three groups: pregnant, lactating, and NPNL. This categorical variable was created from two questions administered to female respondents: if they were pregnant, and if they were lactating. If a respondent answered yes to both (26 WRA), we classified them as “pregnant,” as it is biologically possible for lactating women to become pregnant (Bongaarts & Potter, 2013).
2.5. Confounders
To reduce confounding bias, we used a set of covariates that influence diet during pregnancy and lactation. These variables were identified a priori, based on relevant literature (Arsenault et al., 2013; Harris‐Fry, Shrestha, Costello, & Saville, 2017; Lee et al., 2013; Leroy et al., 2018; Rashid, Smith, & Rahman, 2011), plausibility, and observations from qualitative reports (Craig, Jeyanthi, Pelto, Willford, & Stoltzfus, 2018; Kavle & Landry, 2018). We included three broad categories of control variables: (i) individual‐level factors (age, relationship to household head, education level, anthropometric measurements), (ii) household‐level factors (household size, income calculated as total monthly expenditure, ownership of livestock, participation in fisheries for self‐consumption, food security status), and (iii) community‐level factors (exposure to visits by community health worker).
2.6. Statistical analysis
We used survey data analysis in Stata 15 for univariate and bivariate analysis to account for the complex survey design and to generalize findings to a sample representative of rural Bangladeshi WRA. Specifically, we used the “svyset” command to account for the stratified multistage sampling with administrative divisions as strata and clustered sampling at the village and household level. We specified sampling weights at the household level and used the “subpop” option in Stata that is designed specifically to analyze survey data for a subpopulation of interest. With the NPNL group as the comparator, we used adjusted Wald tests to compare groupwise means and t‐tests to compare proportions between two groups. For comparing the actual intake with dietary recommendations for WRA, we referred to recommended dietary allowances from India (National Institute of Nutrition, Hyderabad, India, 2011) for two reasons: (i) dietary recommendations for energy during pregnancy and lactation were unavailable from the national dietary guidelines from Bangladesh (Nahar, Faruque, & Mannan, 2013) and (ii) available dietary requirements for Bangladeshi adults closely resemble those for the Indian population because of their shared ethnic origin.
Multiple linear regression models were fitted to identify significant individual‐, household‐, and community‐level covariates for the association between reproductive status of WRA and energy intake. Each covariate was individually tested for potential confounding at p < 0.05 using bivariate linear regressions with caloric intake as the outcome. Backward stepwise linear regression was used to eliminate variables until all the covariates in the multivariate model were significant at p < 0.05. These included age, age at marriage, height, education level, income earning status, a dummy variable for skipping at least one meal at the individual level, household income, and food insecurity status at the household level. Once we identified this final set of covariates, multivariable models for other dietary measures were fitted using the same set of covariates for consistency. Because intakes of protein, calcium, iron, and vitamin A were skewed (Figures S3–;S6), these outcomes were log‐transformed before fitting any linear models. For each step in the model‐fitting process, residual plots were assessed for normality of residuals.
Finally, we performed equivalence testing to assess for statistically significant similarity and differences in intakes of pregnant versus NPNL women and lactating versus NPNL women. Figure 1 illustrates the difference between traditional difference testing and equivalence testing. An important aspect of using equivalence tests is to specify an a priori equivalence interval or δ that is both practically and statistically important to suggest equivalence or “similarity.” In the absence of published studies defining a δ of practical significance and tests for an appropriate equivalence interval when comparing dietary intakes, we use a prespecified threshold of 60% of the additional nutrient intakes recommended for pregnant and lactating women. As seen in Tables 1a and 1b, δ for calories for pregnant women is 210 kcal (i.e., 60% of the additional 350 kcal/day recommended during pregnancy) and that for lactating women is 312 kcal (60% of the additional 520 kcal/day recommended during lactation). These values correspond to an additional daily serving of cereal and pulse during pregnancy and an additional meal with cereal, pulse, and milk/fruit/vegetables during lactation. For dietary diversity, we chose an equivalence interval of one additional food group, based on recent findings that suggest that women with a WDDS score of minimum six food groups are more likely to achieve adequate micronutrient intakes during pregnancy (Nguyen et al., 2018), compared with the minimum score of five food groups during the NPNL stage.
FIGURE 1.
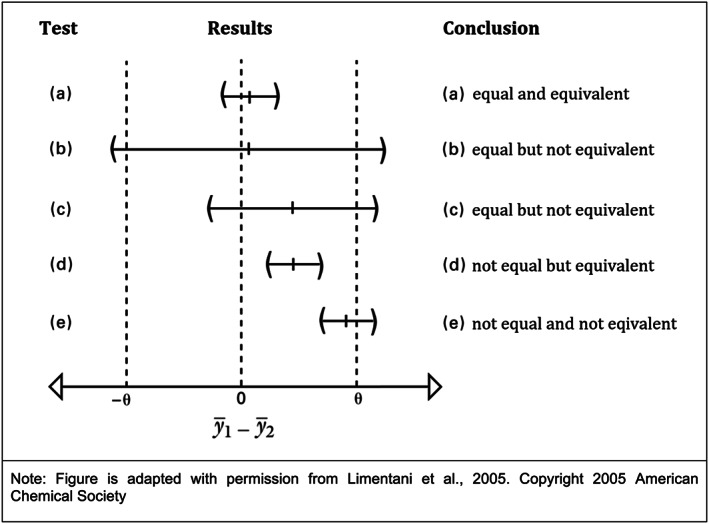
Equivalence testing versus difference testing. For testing equivalence, a reversal of the traditional null and alternative hypotheses is required, such that we can then demonstrate equivalence that is statistically significant, practical, and precise. The “two‐sided equivalence test” (TOST), designed specifically for equivalence testing, begins with a null hypothesis that the two mean values are not equivalent, then attempts to demonstrate that they are equivalent (or “similar”) within an a priori determined “equivalence interval” that provides evidence of statistically significant equivalence or similarity. Unlike the two‐sample t‐test, TOST penalizes poor precision and/or small n values and places the burden on the analyst to prove that the parameters are equivalent. The figure below, adapted with permission from Limentani et al. (2005), illustrates the differences between the conclusions one can draw from the traditional t‐test versus those from an equivalence test, based on confidence intervals for mean difference , and equivalence interval ±θ
TABLE 1a.
Predefined equivalence interval “δ” for testing equivalence in dietary intake between pregnant and NPNL women
| Dietary measure | Recommended intake for NPNL women | Recommended intake for PW | δ PW |
|---|---|---|---|
| Energy (kcal/day) | 2,230 | +350 | ±210 |
| Protein (g/day) | 55 | +23 | ±13.8 |
| Vitamin A—Retinol Adult Equivalent (μg/day) | 600 | +200 | ±120 |
| Calcium (mg/day) | 600 | +600 | ±360 |
| Iron (mg/day) | 21 | +14 | ±8.4 |
| Women's dietary diversity score | 5 | ‐ | ±1 |
Note. Recommended intakes for NPNL women and PW are based on recommended dietary allowances for Indians.
Abbreviations: NPNL, nonpregnant, nonlactating; PW, pregnant women.
TABLE 1b.
Predefined equivalence interval “δ” for testing equivalence in dietary intake between lactating and NPNL women
| Dietary measure | Recommended intake for NPNL women | Recommended intake for LW | δ LW |
|---|---|---|---|
| Energy (kcal/day) | 2,230 | +520 | ±312 |
| Protein (g/day) | 55 | +19 | ±10.8 |
| Vitamin A—Retinol Adult Equivalent (μg/day) | 600 | +350 | ±210 |
| Calcium (mg/day) | 600 | +600 | ±360 |
| Iron (mg/day) | 21 | +0 | n/a |
| Women's dietary diversity score | 5 | ‐ | ±1 |
Note. Recommended intakes for LW are based on recommended dietary allowances for Indians.
Abbreviations: NPNL, nonpregnant, nonlactating; LW, lactating women.
Group‐specific adjusted means obtained from multivariate models were subject to equivalence testing with the prespecified δ for two comparisons: pregnant versus NPNL women and lactating versus NPNL women. Note that the use of group‐specific adjusted means assists in addressing concerns regarding outcomes with skewed distributions. Additionally, we performed post hoc power calculations to assess if we had adequate power to declare statistically significant equivalence or difference for the observed mean difference and variance. These calculations are based on statistical procedures recommended by Chow and Wang for bioequivalence trials (Chow & Wang, 2001). R statistical software, Version 1.1.453, was used to perform two one‐sided tests (TOST). An α of 0.05 yielded 90% confidence intervals for equivalence testing because the formula for confidence interval in TOST is 100(1‐2α)%. Lastly, because recommendations for iron intake are the same during lactation and the NPNL period, δ could not be defined and, therefore, the equivalence test for iron could not be performed for lactating versus NPNL women.
3. RESULTS
3.1. Sample characteristics
The proportions of NPNL, pregnant, and lactating women in our sample were 71.8%, 4.9%, and 23.3%, respectively. The majority of women in each group had completed at least primary school, were currently married to the household head, and belonged to food‐secure, livestock‐owning households with a median income ranging from 23 to 28 US dollars per month (Table 2). NPNL women were significantly older (by 8–;9 years) than women in the other two groups; the average age at marriage was less than 18 years for all groups. Although women from all three groups had similar average height and normal body mass index, lactating women were significantly lighter and thinner than the NPNL group. The proportions of income‐earning women among pregnant and among lactating women were significantly lower than the NPNL group; fewer than half of the pregnant women earned some income. In all groups, 95%–;96% women consumed at least three main meals. A significantly greater proportion of pregnant and lactating women reported being visited by community health worker (˜24%–;27%) compared with NPNL women (14%).
TABLE 2.
Selected characteristics of the study sample
| NPNL women (n = 2,903) | Pregnant women (n = 197) | Lactating women (n = 944) | |
|---|---|---|---|
| Individual characteristics | |||
| Age, years | 34.7 ± 7.5 a | 26.0 ± 6.2 *** | 26.8 ± 5.9 *** |
| Adolescents (15‐19y; %) | 1.4 | 10.5 *** | 4.7 *** |
| Age at marriage, years | 16.6 ± 2.8 | 17.3 ± 3.2 ** | 17.1 ± 2.7 *** |
| Weight, kg | 48.6 ± 8.9 | 49.6 ± 8.5 | 45.1 ± 7.4 *** |
| Height, cm | 150.6 ± 5.7 | 150.8 ± 6.1 | 150.7 ± 6.3 |
| Height below height 145 cm (%) | 15.4 | 14.9 | 13.2 |
| BMI, kg/m2 | 21.4 ± 3.6 | 21.71 ± 3.1 | 19.9 ± 2.9 *** |
| Marital status (%) | |||
| Currently married | 96.0 | 100.0 *** | 99.1 *** |
| Widowed | 3.1 | 0.0 *** | 0.6 *** |
| Divorced/separated | 0.9 | 0.0 *** | 0.3 * |
| Relationship with HH head(%) | |||
| Self (i.e., is female head of the HH) | 16.2 | 9.6 ** | 11.8 ** |
| Wife | 82.1 | 83.9 | 85.8 ** |
| Daughter | 0.4 | 1.7 | 0.5 |
| Daughter‐in‐law | 1.3 | 4.8 * | 2.0 |
| Education (%) | |||
| No schooling | 44.3 | 32.0 ** | 29.6 *** |
| Primary | 28.3 | 26.5 | 31.6 |
| Secondary | 25.7 | 40.5 *** | 36.9 *** |
| High school or higher | 1.7 | 1.0 | 1.9 |
| Has a child <2 years of age (%) | 2.2 | 10.0 ** | 71.4 *** |
| Income‐earning (%) | 64.6 | 46.9 *** | 53.2 *** |
| Consumed at least three main meals (%) | 94.9 | 96.4 | 96.3 |
| HH characteristics | |||
| HH size | 4.2 ± 1.4 | 3.7 ± 1.4 *** | 4.5 ± 1.4 *** |
| Monthly HH expenditure, USD b | 28.3 c (21.1–;38.8) | 30.3 (22.5–;39.4) | 23.1 *** (17.7–;31.3) |
| Food‐secure HH, % | 74.5 | 80.8 * | 74.2 |
| Owns livestock, % | 81.6 | 79.2 | 74.6 *** |
| Engaged in fishing for self‐consumption, % | 32.9 | 33.1 | 32.9 |
| Community level characteristics | |||
| Visited by CHW | 14.4 | 23.8 * | 27.1 *** |
Note. All comparisons are relative to NPNL women as the reference category
Abbreviations: BMI, body mass index; CHW, community health worker; HH, household; NPNL, nonpregnant, nonlactating women.
Mean ± SD (all such values).
USD = US dollars, 1 USD = 82 Bangladeshi Taka (on average since 2012).
Median (Q1–;Q3); all such values.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Comparison of nutrient intakes with reproductive stage‐specific recommendations
Pregnant and lactating women had lower than recommended intakes for all nutrients (Table 3). In both adjusted and unadjusted models, mean intakes of energy and protein among NPNL women were slightly higher than the recommended amounts, whereas pregnant and lactating met 85%–;95% of their energy and protein recommendations on average. For all micronutrients, mean intakes for all groups of women—whether unadjusted or adjusted—fell short of recommendations by 30%–;80%. Deficits were most pronounced for iron and calcium in pregnant women and for vitamin A and calcium in lactating women. Lastly, the expected pattern of incremental intakes recommended for pregnant and lactating women compared with the referent group of NPNL women was virtually absent for all nutrient intakes.
TABLE 3.
Comparison of mean nutrient intakes and WDDS‐10 to reproductive‐stage specific recommendations, and results from difference testing (i.e., traditional t‐test) on mean intakes between NPNL and pregnant women, and NPNL and lactating women
| Dietary outcome | NPNL women (n = 2,709) | Pregnant women (n = 179) | Lactating women (n = 944) |
|---|---|---|---|
| Energy (kcal/d) | |||
| Recommended | 2,230 | 2,580 | 2,750 |
| Unadjusted | 2,359.8 ± 543.4 a | 2,459.5 ± 643.2 * | 2,438.5 ± 577.4 *** |
| Adjusted b | 2,364.6 ± 234.4 | 2,450.1 ± 229.4 *** | 2,440.5 ± 219.5 *** |
| Protein (g/day) | |||
| Recommended | 55 | 78 | 74 |
| Unadjusted | 62.0 ± 20.8 | 66.6 ± 25.4 * | 63.2 ± 21.6 |
| Adjusted | 62.1 ± 23.4 | 62.8 ± 25.0 | 64.5 ± 23.7 ** |
| Vitamin A (mcg/day) | |||
| Recommended | 600 | 800 | 950 |
| Unadjusted | 233.2 ± 484.4 | 283.1 ± 523.5 | 219.5 ± 466.4 |
| Adjusted | 217.5 ± 596.0 | 235.7 ± 430.6 | 215.7 ± 432.7 |
| Dietary calcium (mg/day) | |||
| Recommended | 600 | 1,200 | 1,200 |
| Unadjusted | 413.2 ± 366.5 | 468.6 ± 404.5 * | 421.8 ± 381.9 |
| Adjusted | 405.9 ± 437.9 | 425.1 ± 351.5 | 430.9 ± 415.5 |
| Dietary iron (mg/day) | |||
| Recommended | 21 | 35 | 21 |
| Unadjusted | 11.6 ± 5.4 | 12.8 ± 6.4 * | 11.5 ± 5.2 |
| Adjusted | 11.5 ± 6.3 | 12.1 ± 6.7 | 11.8 ± 5.8 |
| WDDS‐10 | |||
| Recommended | 5 | 5 | 5 |
| Unadjusted | 3.7 ± 1.0 | 3.7 ± 1.0 | 3.7 ± 1.1 |
| Adjusted | 3.73 ± 0.44 | 3.67 ± 0.41 * | 3.71 ± 0.43 |
Abbreviations: NPNL, nonpregnant, nonlactating; WDDS‐10, women's dietary diversity score out of 10 food groups.
Mean ± SD (all such values).
Adjusted for participant's age, age at marriage, height, education, income‐earning status, number of meals skipped, household income, household food security status, and accounting for the complex survey design.
p < 0.05.
p < 0.01.
p < 0.001.
3.3. Results from difference testing (i.e., traditional t‐test) on mean intakes between NPNL and pregnant women, and NPNL and lactating women
Mean nutrient intakes of pregnant and lactating women obtained from bivariate analysis (i.e., unadjusted means) were statistically significantly different for some intakes as compared with the NPNL group (Table 3). We found a significant difference of about 100 kcal/day between the unadjusted mean energy intake of pregnant and NPNL women. Similarly, although the unadjusted mean energy intake of lactating women was significantly higher than that of NPNL women, the mean difference was less than 80 kcal/day. Adjustment for covariates did not change these results. Although PW had significantly higher intakes for protein, dietary calcium, and dietary iron compared with the NPNL group, these differences were no longer significant in adjusted models. In case of lactating versus NPNL women, no statistically significant differences were found for unadjusted intakes of protein or micronutrients; however, there was a statistically significant difference of 2.4 g/day in adjusted protein intake. Lastly, the mean WDDS‐10 was about 3.7 for all groups and was lower than the minimum dietary diversity cut‐off of 5 for all WRA. In the adjusted model, mean WDDS‐10 for pregnant women (3.67 ± 0.41) was significantly (but slightly) lower than that of NPNL women (3.73 ± 0.44).
3.4. Comparison of results from equivalence and difference testing on adjusted means
In testing differences and equivalencies between adjusted mean intakes of nutrients and the WDDS‐10 score for pregnant versus NPNL women and lactating versus NPNL women, we obtained two combinations: one with differing results from the t‐test and equivalent test (“not equal but equivalent”), and another with consistent results from the two tests (“equal and equivalent”). These combinations are described below and are illustrated in Figures 2 and 3. Further details on the confidence intervals around adjusted means are available in Table S1, and individual graphs for the dietary measures are in Figures S8 and S9.
FIGURE 2.
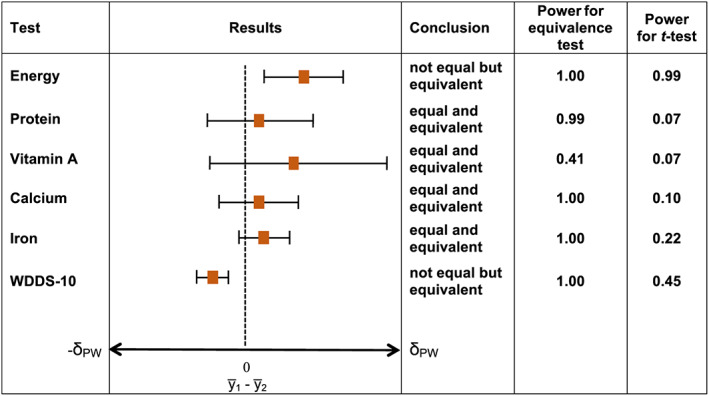
Significant differences and equivalencies in nutrient intakes for pregnant women versus nonpregnant, nonlactating women. Note. Significant difference and equivalence tested at α = 0.05; horizontal T‐shaped bars depict 95% confidence intervals for difference testing. Difference is statistically significant and conclusion is “not equal” if 95% confidence interval does not include zero. Equivalence is statistically significant and conclusion is “equivalent” if 90% confidence interval (obtained from two one‐sided tests with an α of 0.05 and using the formula 100[1–;2α]%) is contained in the interval with endpoints −δ PW and +δ PW defined for each nutrient in Table 1a
FIGURE 3.
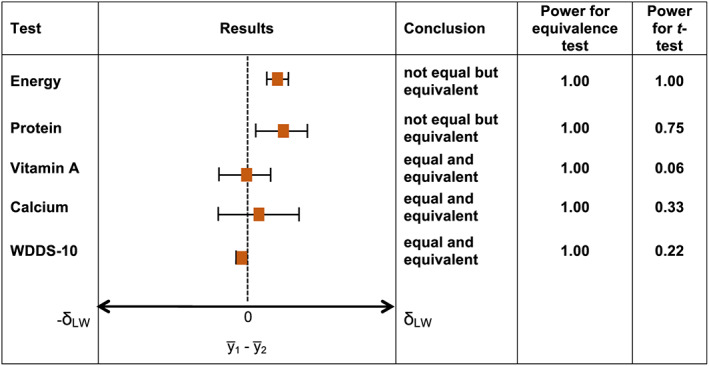
Significant differences and similarities in nutrient intakes for lactating women (LW) versus nonpregnant, nonlactating women. Note. Significant difference and equivalence tested at α = 0.05; horizontal T‐shaped bars depict 95% confidence intervals for difference testing. Difference is statistically significant and conclusion is “not equal” if 95% confidence interval does not include zero. Equivalence is statistically significant and conclusion is “equivalent” if 90% confidence interval (obtained from two one‐sided tests with an α of 0.05 and using the formula 100[1–;2α]%) is contained in the interval with endpoints −δ PW and +δ PW defined for each nutrient in Table 1b
Not equal but equivalent: Energy intakes and WDDS‐10 values for pregnant versus NPNL women and energy and protein intake for lactating versus NPNL women were significantly different for the equality of means. However, we could not reject the null hypothesis of equivalence (or similarity) with the predefined equivalence margins (±60% for energy and nutrient intakes and ±1 food group for WDDS‐10) at α = 0.05. Sensitivity tests with lower equivalence margins of 50% and 40% did not change these results (Figures S10–;S13).
Equal and equivalent: for all comparisons of micronutrient intakes, for protein for pregnant versus NPNL women, and for WDDS‐10 for lactating versus NPNL women, differences between the means were not significantly different and fell within the equivalence bounds. Sensitivity tests with lower equivalence margins of 50% and 40% did not change these results.
4. DISCUSSION
Drawing on data representative of rural Bangladesh, we found that macronutrient and micronutrient intakes of pregnant or lactating women are both suboptimal and statistically equivalent to those of women who were NPNL. Although significant differences were present for four out of the 12 pairwise comparisons after controlling for covariates (i.e., for adjusted energy and dietary diversity in pregnant vs. NPNL women, and for adjusted energy and protein intake in lactating vs. NPNL women), the magnitudes of these differences were small. When we performed equivalence tests at a predetermined equivalence threshold, we found evidence for equivalence for all group comparisons on all dietary measures at p < 0.05. These results did not change with narrower equivalence thresholds, which confirms that nutrient intakes of pregnant and lactating women are essentially equivalent to those of NPNL women.
Although research continues to highlight multiple nutrient deficiencies among Bangladeshi WRA by comparing reported intakes to dietary recommendations (Arsenault et al., 2013; Leroy et al., 2018; Nguyen et al., 2018), our study is the first to use dietary recommendations on a large, representative sample from an LMIC to demonstrate that, at a population level, there is no meaningful difference in the macronutrient or micronutrient intakes of pregnant or lactating women compared to NPNL women, when there should be one. For example, compared with the recommended additional daily energy intake of 350 kcal during pregnancy and 520 kcal during lactation, pregnant and lactating women consumed only 86 and 76 kcal on average above the adjusted mean energy intake of NPNL women. Although negligible, these differences in energy were statistically significant and hence deemed as “not equal.” We obtained similar results when we compared differences in diet diversity between pregnant and NPNL women and protein intake between lactating and NPNL women. Finding a negligible but statistically significant difference (which is highly likely in large samples; Barker et al., 2002) could convey that women who are pregnant or lactating have significantly greater intakes than women who are not. Such understanding may inadvertently understate the need for programmatic action to enhance maternal diets in LMICs. However, the risk for programmatic inaction could be avoided if, along with difference testing, equivalence testing is performed to determine whether differences in subgroups are also statistically equivalent at a predetermined threshold. In this sample, the statistically significant increments are insufficient to support the high nutritional demands placed by fetal growth and tissue expansion during pregnancy and milk production during lactation (United Nations University, World Health Organization, Food and Agriculture Organization of the United Nations, 2004).
When equivalence and difference testing identify intakes of pregnant and lactating as “equal and equivalent” to NPNL, the need for enhancing maternal diets becomes even more pronounced. In our study, we found that this was true for both two‐group comparisons for all micronutrients. It was also true for the comparison on dietary diversity between lactating and NPNL women. Intakes for all micronutrients fell short of the recommendations by 30%–;80% in all WRA subgroups in adjusted as well as unadjusted models, and results are consistent with existing studies (Arimond et al., 2010; Arsenault et al., 2013; Leroy et al., 2018; Nguyen et al., 2018; Ruel, Deitchler, & Arimond, 2010). The mean dietary diversity score in all three groups of women was 3.7 out of 10 food groups, which is lower than the cut‐off of five food groups associated with micronutrient adequacy in WRA. These findings suggest that neither pregnancy nor lactation results in women consuming an adequate or varied diet. Given the critical role of these nutrients to support the unique metabolic states during pregnancy and lactation, programs to improve maternal diets through nutrition‐specific and nutrition‐sensitive interventions are needed to improve maternal and child nutrition outcomes associated with the first 1,000 days of life.
Although multiple nutritional deficits are evident in all our sample subgroups, the relatively high intakes of energy and protein among NPNL found in our sample were also found among Bangladeshi adolescents and WRA by Leroy et al. (2018). Bangladesh is undergoing an economic and nutritional transition, accompanied by a rapid increase in overnutrition and decline in undernutrition among WRA (Bhutta et al., 2013; Chowdhury et al., 2018). Designing food‐based maternal nutrition interventions in this context poses challenges. Future trials could test for differences and similarities in women's diets to assess whether diet‐enhancing interventions generated a statistically significant and meaningful difference, and whether diet‐restricting interventions, if appropriate to address overnutrition, achieved practical equivalence within recommended dietary allowances.
There are two important considerations when performing equivalence tests for topics in public health nutrition. First is the careful selection of the equivalence interval for statistical and practical significance (Barker et al., 2002; Limentani et al., 2005). In the absence of previous research on the application of equivalence testing to dietary intakes in different reproductive stages, we chose a prespecified threshold of 60% of the additional nutrient recommendations for pregnancy and lactation. We restricted our analysis to this threshold for analytical fidelity and because the corresponding equivalence margins of 210 kcal (for pregnant vs. NPNL women) and of 312 kcal (for lactating vs. NPNL women) correspond to the practical guidelines on consuming, at a minimum, an additional daily serving of staples during pregnancy and an additional meal during lactation (Linkages, AED and CORE Nutrition Working Group, USAID, 2004). Although our core findings remained unchanged in post hoc sensitivity analyses with 40% and 50% thresholds, further research is needed to determine the magnitude of tolerable difference, especially to evaluate dietary intakes among WRA as risk factors for maternal and child health outcomes.
Another important consideration is that equivalence testing requires an adequate sample size (Shieh, 2016). The availability of detailed individual‐level dietary intake data from a nationally representative sample of rural Bangladesh enabled us to apply equivalence (and difference) testing to a large sample of WRA subgroups. Nevertheless, using survey data for performing equivalence tests also entails that the subsamples for comparison should be obtained carefully. In our study, the sample size of pregnant women (n = 197) was much smaller relative to the comparator group, that is, NPNL women (n = 2,903). Such relatively small subgroup of pregnant women is expected in data obtained secondarily from surveys, especially those conducted in developing countries where women often do not know or do not report pregnancy in the first trimester. Therefore, we adopted a “maximum sample” approach in our secondary analyses of the BIHS data in order to obtain as many eligible candidates, including pregnant women on whom dietary data is already scant. Although this approach resulted in a smaller sample of pregnant WRA with self‐reported dietary data compared with the overall BIHS sample of WRA who reported their status as “pregnant” (274 out of 6,062 WRA), the proportion of pregnant women in our WRA sample (4.9%) was nearly identical to that observed in the BIHS WRA sample (4.5%). We did not use a priori measures to determine the required sample size for the planned analysis. However, similar to other secondary data analyses, we performed post hoc power analysis that incorporates the observed effect size and variance. As seen in Figures 2 and 3, equivalence was observed for almost all group comparisons with a power of ˜1.00. For vitamin A between pregnant and NPNL women, we obtained a power of 0.41, which suggests that for the a priori‐defined equivalence margin for vitamin A (i.e., 120 μg), there is a 41% chance that such margin is truly statistically equivalent. One could achieve a higher power by increasing the sample size or by declaring a wider equivalence threshold. The former approach is not feasible in survey data analysis if we choose eligible candidates uniformly across WRA subsamples, and the latter would defeat the purpose of testing equivalence at a prespecified threshold.
To our knowledge, no other studies have applied TOST to establish statistical similarity in dietary intakes of women across reproductive stages in an LMIC. Apart from using dietary data from a large survey representative of rural Bangladesh, our study places the equivalence test in a regression analysis framework with individual‐, household‐, and community‐level covariates that enables us to evaluate similarity in adjusted nutrient intakes and adds depth to the test.
Our study has some limitations. We analyzed nutrient intakes from a single 24‐h dietary recall. This is acceptable for population‐level estimation of mean intakes and regression analyses (National Institutes of Health, National Cancer Institute, n.d.). However, 24‐h recall dietary data are prone to inaccurate recall, which results in measurement error (Bell, Saltzman, & Coates, 2016). In view of the lack of gold‐standard measurements on diets in LMICs (Coates et al., 2017), we are unable to assess the extent of this mismeasurement. For our sample of rural Bangladeshi women, misreporting of diet may be relatively limited given their fixed meal pattern (three meals per day), the monotonous nature of their day‐to‐day diet, and rare occurrences of eating outside the home. (For instance, more than 97% of our sample of WRA reported consuming breakfast, lunch, and dinner, whereas only 21% reported consuming snacks, and the proportion of WRA reporting consumption of foods at home ranged from 95% for snacks and 98%–;99% for the main meals.) To minimize bias from misreporting, we selected only those women for whom we have self‐reported dietary intake data for the 24‐h reference period during which dietary intakes were recorded. We were unable to adjust for two important covariates: intake of iron supplements and trimester of pregnancy. Women who take iron supplements may have higher iron intake than we estimate. It is possible that some women, unaware of their pregnancy in the first trimester, may not have the opportunity, knowledge, or desire to adjust their dietary intake. However, because data on prevalence of iron supplementation were missing for a large proportion of our sample and data on gestational phase or antenatal nutrition knowledge and practices were not collected, we were unable to adjust for these covariates.
We demonstrate that intakes of WRA are not responsive to the conditions of pregnancy and lactation and that appropriate statistical tests, such as the equivalence test, should be applied to uncover the hypothesized similarities in diets of women in different reproductive stages. Our results highlight the value of equivalence testing—a relatively new but more appropriate statistical test for hypotheses in nutrition research that aim to identify statistical similarity rather than difference. Beyond contributing to improved methodological rigor in nutrition research, equivalence testing in our study reinforces the need for interventions designed to improve women's diets during nutritionally vulnerable stages of pregnancy and lactation and for evaluation of such interventions by establishing that, if there is a statistical difference, it is a meaningful one.
CONFLICTS OF INTEREST
We declare no conflict of interest.
CONTRIBUTIONS
GWG conceived the research idea, performed the research, analyzed the data, and wrote the first draft. JH advised on research methods, assisted in interpreting the results, and critically revised the manuscript. KD, RK, PM, and KMR advised on research methods and commented on the manuscript. All authors read and approved the final manuscript.
Supporting information
Table S1. Confidence intervals around adjusted means
Figure S1. Final analytical sample.
Figure S2. Boxplot of energy intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S3. Boxplot of protein intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S4. Boxplot of calcium intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S5. Boxplot of iron intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S6. Boxplot of vitamin A intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S7. Boxplot of women's dietary diversity score (WDDS) by reproductive status among 15‐49y old rural Bangladeshi women
Figure S8. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 60% for pregnant vs. NPNL women
Figure S9. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 60% for lactating vs. NPNL women
Figure S10. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 50% for pregnant vs. NPNL women
Figure S11. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 50% for lactating vs. NPNL women
Figure S12. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 40% for pregnant vs. NPNL women
Figure S13. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 40% for lactating vs. NPNL women
ACKNOWLEDGMENTS
We thank Wahid Quabili for assistance in accessing and using the Bangladesh Integrated Household Survey data.
Wable Grandner G, Dickin K, Kanbur R, Menon P, Rasmussen KM, Hoddinott J. Assessing statistical similarity in dietary intakes of women of reproductive age in Bangladesh. Matern Child Nutr. 2021;17:e13086. 10.1111/mcn.13086
REFERENCES
- Ahmed, A. U. , Ahmad, K. , Chou, V. , Hernandez, R. , Menon, P. , Naeem, F. , … Yu, B. (2013). The status of food security in the feed the future zone and other regions of Bangladesh: Results from the 2011–;2012 Bangladesh Integrated Household Survey. Dhaka: Project Report Submitted to the US Agency for International Development. International Food Policy Research Institute. [Google Scholar]
- Akseer, N. , Kamali, M. , Arifeen, S. E. , Malik, A. , Bhatti, Z. , Thacker, N. , … Bhutta, Z. A. (2017). Progress in maternal and child health: How has South Asia fared? British Medical Journal, 357, j1608. 10.1136/bmj.j1608 [DOI] [PubMed] [Google Scholar]
- Alam, D. S. , Van Raaij, J. M. A. , Hautvast, J. G. A. J. , Yunus, M. , & Fuchs, G. J. (2003). Energy stress during pregnancy and lactation: Consequences for maternal nutrition in rural Bangladesh. European Journal of Clinical Nutrition, 57(1), 151–;156. 10.1038/sj.ejcn.1601514 [DOI] [PubMed] [Google Scholar]
- Arimond, M. , Wiesmann, D. , Becquey, E. , Carriquiry, A. , Daniels, M. C. , Deitchler, M. , … Torheim, L. E. (2010). Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource‐poor settings. The Journal of Nutrition, 140(11), 2059S–;2069S. 10.3945/jn.110.123414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault, J. E. , Yakes, E. A. , Islam, M. M. , Hossain, M. B. , Ahmed, T. , Hotz, C. , … Brown, K. H. (2013). Very low adequacy of micronutrient intakes by young children and women in rural Bangladesh is primarily explained by low food intake and limited diversity. The Journal of Nutrition, jn.112.169524. 10.3945/jn.112.169524 [DOI] [PubMed] [Google Scholar]
- Balarajan, Y. , & Villamor, E. (2009). Nationally representative surveys show recent increases in the prevalence of overweight and obesity among women of reproductive age in Bangladesh, Nepal, and India. The Journal of Nutrition, 139(11), 2139–;2144. 10.3945/jn.109.112029 [DOI] [PubMed] [Google Scholar]
- Bangladesh National Nutrition Policy 2015 . (2015). Retrieved October 28, 2017, from http://nsb-bangladesh.org/sites/default/files/BGD_National_Nutrition_Policy_2015.pdf
- Barker, L. E. , Luman, E. T. , McCauley, M. M. , & Chu, S. Y. (2002). Assessing equivalence: An alternative to the use of difference tests for measuring disparities in vaccination coverage. American Journal of Epidemiology, 156(11), 1056–;1061. 10.1093/aje/kwf149 [DOI] [PubMed] [Google Scholar]
- Bell, W. F. , Saltzman, E. , & Coates, J. (2016). Accuracy of self‐reported dietary intake in low‐ and middle‐income countries: A review of the literature. The FASEB Journal, 30(1 Supplement), lb417–;lb417. http://www.fasebj.org/content/30/1_Supplement/lb417 [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … Group, T. L. N. I. R., & others . (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382(9890), 452–;477. http://www.sciencedirect.com/science/article/pii/S0140673613609964 [DOI] [PubMed] [Google Scholar]
- Blumfield, M. L. , Hure, A. J. , Macdonald‐Wicks, L. , Smith, R. , & Collins, C. E. (2012). Systematic review and meta‐analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutrition Reviews, 70(6), 322–;336. 10.1111/j.1753-4887.2012.00481.x [DOI] [PubMed] [Google Scholar]
- Bongaarts, J. , & Potter, R. E. (2013). Fertility, biology, and behavior: An analysis of the proximate determinants (1st ed.). New York: Academic Press. https://www.elsevier.com/books/fertility-biology-and-behavior/bongaarts/978-0-08-091698-9 [Google Scholar]
- Chow, S.‐C. , & Wang, H. (2001). On sample size calculation in bioequivalence trials. Journal of Pharmacokinetics and Pharmacodynamics, 28(2), 155–;169. 10.1023/A:101150303235 [DOI] [PubMed] [Google Scholar]
- Chowdhury, M. A. B. , Adnan, M. M. , & Hassan, M. Z. (2018). Trends, prevalence and risk factors of overweight and obesity among women of reproductive age in Bangladesh: A pooled analysis of five national cross‐sectional surveys. BMJ Open, 8(7), e018468. 10.1136/bmjopen-2017-018468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, P. , Srihari, S. B. , Thorne‐Lyman, A. , Khatry, S. K. , LeClerq, S. C. , & Shrestha, S. R. (2006). Eating down in pregnancy: Exploring food‐related beliefs and practices of pregnancy in rural nepal. Ecology of Food and Nutrition, 45(4), 253–;278. 10.1080/03670240600846336 [DOI] [Google Scholar]
- Coates, J. C. , Colaiezzi, B. A. , Bell, W. , Charrondiere, U. R. , & Leclercq, C. (2017). Overcoming dietary assessment challenges in low‐income countries: Technological solutions proposed by the International Dietary Data Expansion (INDDEX) Project. Nutrients, 9(3), 289. 10.3390/nu9030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, H. C. , Jeyanthi, R. , Pelto, G. , Willford, A. C. , & Stoltzfus, R. J. (2018). Using a cultural‐ecological framework to explore dietary beliefs and practices during pregnancy and lactation among women in Adivasi communities in the Nilgiris Biosphere Reserve, India. Ecology of Food and Nutrition, 57(3), 165–;186. 10.1080/03670244.2018.1445088 [DOI] [PubMed] [Google Scholar]
- Darnton‐Hill, I. , & Mkparu, U. C. (2015). Micronutrients in pregnancy in low‐ and middle‐income countries. Nutrients, 7(3), 1744–;1768. 10.3390/nu7031744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, K. L. , Matias, S. L. , Mridha, M. K. , Vosti, S. A. , Hussain, S. , Dewey, K. G. , & Stewart, C. P. (2017). Eating down or simply eating less? The diet and health implications of these practices during pregnancy and postpartum in rural Bangladesh. Public Health Nutrition, 20(11), 1928–;1940. 10.1017/S1368980017000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris‐Fry, H. , Azad, K. , Kuddus, A. , Shaha, S. , Nahar, B. , Hossen, M. , … Fottrell, E. (2015). Socio‐economic determinants of household food security and women's dietary diversity in rural Bangladesh: A cross‐sectional study. Journal of Health, Population and Nutrition, 33, 2. 10.1186/s41043-015-0022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris‐Fry, H. , Shrestha, N. , Costello, A. , & Saville, N. M. (2017). Determinants of intra‐household food allocation between adults in South Asia—A systematic review. International Journal for Equity in Health, 16(1), 107. 10.1186/s12939-017-0603-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, D. , Hassan, N. , Karim, R. , & Duthie, M. R. (1988). Tables of nutrient composition of Bangladeshi foods. Bangladesh: Helen Keller International (HKI). [Google Scholar]
- Huybregts, L. F. , Roberfroid, D. A. , Kolsteren, P. W. , & Van Camp, J. H. (2009). Dietary behaviour, food and nutrient intake of pregnant women in a rural community in Burkina Faso. Maternal & Child Nutrition, 5(3), 211–;222. 10.1111/j.1740-8709.2008.00180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. M. , Islam, M. K. , Hasan, M. S. , & Hossain, M. B. (2017). Adolescent motherhood in Bangladesh: Trends and determinants. PLoS ONE, 12(11), e0188294. 10.1371/journal.pone.0188294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavle, J. A. , & Landry, M. (2018). Addressing barriers to maternal nutrition in low‐ and middle‐income countries: A review of the evidence and programme implications. Maternal & Child Nutrition, 14(1), e12508. 10.1111/mcn.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M. S. (1987). Determinants of low birth weight: Methodological assessment and meta‐analysis. Bulletin of the World Health Organization, 65(5), 663–;737. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2491072/ [PMC free article] [PubMed] [Google Scholar]
- Lander, R. L. , Hambidge, K. M. , Krebs, N. F. , Westcott, J. E. , Garces, A. , Figueroa, L. , … Stolka, K. B. (2017). Repeat 24‐hour recalls and locally developed food composition databases: A feasible method to estimate dietary adequacy in a multi‐site preconception maternal nutrition RCT. Food & Nutrition Research, 61(1), 1311185. 10.1080/16546628.2017.1311185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. E. , Talegawkar, S. A. , Merialdi, M. , & Caulfield, L. E. (2013). Dietary intakes of women during pregnancy in low‐ and middle‐income countries. Public Health Nutrition, 16(8), 1340–;1353. 10.1017/S1368980012004417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, J. L. , Ruel, M. , Sununtnasuk, C. , & Ahmed, A. (2018). Understanding the determinants of adolescent nutrition in Bangladesh. Annals of the new York Academy of Sciences., 1416, 18–;30. 10.1111/nyas.13530 [DOI] [PubMed] [Google Scholar]
- Limentani, G. B. , Ringo, M. C. , Ye, F. , Bergquist, M. L. , & MCSorley, E. O. (2005). Beyond the t‐test: Statistical equivalence testing. Analytical Chemistry, 77(11), 221 A–;226 A. 10.1021/ac053390m [DOI] [PubMed] [Google Scholar]
- Linkages, AED and CORE Nutrition Working Group, USAID . (2004, August). Maternal Nutrition During Pregnancy and Lactation‐ A dietary guide . https://coregroup.org/wp-content/uploads/2017/09/Maternal-Nutrition-During-Pregnancy-and-Lactation.pdf
- Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. (2017). Indian Food Composition Tables. National Institute of Nutrition, Hyderabad, India. Retrieved from International Network of Foods Data System (INFOODS) website: http://www.fao.org/infoods/infoods/tables-and-databases/asia/en/
- Martin‐Prével, Y. , Allemand, P. , Wiesmann, D. , Arimond, M. , Ballard, T. , Deitchler, M. , … Mousi, M. (2015). Moving forward on choosing a standard operational indicator of women's dietary diversity. Rome: Food and Agriculture Organization of the United Nations. http://www.fao.org/3/a-i4942e.pdf [Google Scholar]
- Nahar, Q. , Faruque, O. , & Mannan, M. A. (2013). Dietary guidelines for Bangladesh. Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders [BIRDEM], Dhaka. [Google Scholar]
- National Institute of Nutrition, Hyderabad, India . (2011). Dietary guidelines for Indians—A Manual (Vol. 2). http://ninindia.org/dietaryguidelinesforninwebsite.pdf
- National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International . (2016). Bangladesh Demographic and Health Survey 2014. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICF International. https://dhsprogram.com/pubs/pdf/FR311/FR311.pdf [Google Scholar]
- National Institutes of Health, National Cancer Institute . (n.d.). Dietary Assessment Primer: Choosing an approach for dietary assessment . https://dietassessmentprimer.cancer.gov/approach/
- Nguyen, P. H. , Sanghvi, T. , Kim, S. S. , Tran, L. M. , Afsana, K. , Mahmud, Z. , … Menon, P. (2017). Factors influencing maternal nutrition practices in a large scale maternal, newborn and child health program in Bangladesh. PLoS ONE, 12(7), e0179873. 10.1371/journal.pone.0179873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, P. H. , Huybregts, L. , Sanghvi, T. G. , Tran, L. M. , Frongillo, E. A. , Menon, P. , & Ruel, M. T. (2018). Dietary diversity predicts the adequacy of micronutrient intake in pregnant adolescent girls and women in Bangladesh, but use of the 5‐group cutoff poorly identifies individuals with inadequate intake. The Journal of Nutrition, 148(5), 790–;797. 10.1093/jn/nxy045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciano, M. F. (2003). Pregnancy and lactation: Physiological adjustments, nutritional requirements and the role of dietary supplements. The Journal of Nutrition, 133(6), 1997S–;2002S. http://jn.nutrition.org/content/133/6/1997S, 10.1093/jn/133.6.1997S [DOI] [PubMed] [Google Scholar]
- Rao, S. , Kanade, A. , Margetts, B. M. , Yajnik, C. S. , Lubree, H. , Rege, S. , … Study, P. M. N. (2003). Maternal activity in relation to birth size in rural India. The Pune Maternal Nutrition Study. European Journal of Clinical Nutrition, 57(4), 531–;542. 10.1038/sj.ejcn.1601582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid, D. A. , Smith, L. C. , & Rahman, T. (2011). Determinants of dietary quality: Evidence from Bangladesh. World Development, 39(12), 2221–;2231. 10.1016/j.worlddev.2011.05.022 [DOI] [Google Scholar]
- Rasmussen, K. M. , & Kjolhede, C. L. (2004). Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics, 113(5), e465–;e471. 10.1542/peds.113.5.e465 [DOI] [PubMed] [Google Scholar]
- Rasmussen, K. M. (2007). Association of maternal obesity before conception with poor lactation performance. Annual Review of Nutrition, 27, 103–;121. 10.1146/annurev.nutr.27.061406.093738 [DOI] [PubMed] [Google Scholar]
- Robinson, M. , Zubrick, S. R. , Pennell, C. E. , Van Lieshout, R. J. , Jacoby, P. , Beilin, L. J. , … Oddy, W. H. (2013). Pre‐pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. Journal of Developmental Origins of Health and Disease, 4(1), 42–;48. 10.1017/S2040174412000578 [DOI] [PubMed] [Google Scholar]
- Roseboom, T. , de Rooij, S. , & Painter, R. (2006). The Dutch famine and its long‐term consequences for adult health. Early Human Development, 82(8), 485–;491. 10.1016/j.earlhumdev.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Ruel, M. T. , Deitchler, M. , & Arimond, M. (2010). Developing simple measures of women's diet quality in developing countries: Overview. The Journal of Nutrition, 140(11), 2048S–;2050S. 10.3945/jn.110.123695 [DOI] [PubMed] [Google Scholar]
- Shaheen, N. , Rahim, A. T. , Mohiduzzaman, M. d. , Banu, C. P. , Bari, M. d. , Tukun, A. B. , … Stadlmayr, B. (2013). Food Composition Table for Bangladesh. Dhaka, Bangladesh: Institute of Nutrition and Food Science Centre for Advanced Research in Sciences University of Dhaka. http://www.fao.org/fileadmin/templates/food_composition/documents/FCT_10_2_14_final_version.pdf [Google Scholar]
- Shannon, K. , Mahmud, Z. , Asfia, A. , & Ali, M. (2008). The social and environmental factors underlying maternal malnutrition in rural Bangladesh: Implications for reproductive health and nutrition programs. Health Care for Women International, 29(8), 826–;840. 10.1080/07399330802269493 [DOI] [PubMed] [Google Scholar]
- Shieh, G. (2016). Exact power and sample size calculations for the two one‐sided tests of equivalence. PLoS ONE, 11(9), e0162093. 10.1371/journal.pone.0162093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sununtnasuk, C. , & Fiedler, J. L. (2017). Can household‐based food consumption surveys be used to make inferences about nutrient intakes and inadequacies? A Bangladesh Case Study. Food Policy., 72, 121–;131. 10.1016/j.foodpol.2017.08.018 [DOI] [Google Scholar]
- United Nations University, World health organization, Food and agriculture organization of the United Nations . (2004). HumanEnergy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Rome, 17‐24 October 2001 (Vol. 1). FAQ 2004. http://www.fao.org/3/y5686e/y5686e00.htm#Contents [Google Scholar]
- Van Lieshout, R. J. , Taylor, V. H. , & Boyle, M. H. (2011). Pre‐pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: A systematic review. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, 12(5), e548–;e559. 10.1111/j.1467-789X.2010.00850.x [DOI] [PubMed] [Google Scholar]
- Walker, E. , & Nowacki, A. S. (2011). Understanding equivalence and noninferiority testing. Journal of General Internal Medicine, 26(2), 192–;196. 10.1007/s11606-010-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2012). WHO Guideline: Daily oral iron supplementation during pregnancy . http://www.ncbi.nlm.nih.gov/books/NBK132263/
- World Health Organization . (2013). Guideline: Calcium supplementation in pregnant women. Geneva: World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/85120/9789241505376_eng.pdf;jsessionid=518FF3C22B92CF4F6792DF07AE2E5093?sequence=1 [PubMed] [Google Scholar]
- World Health Organization . (2016a). WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization. http://www.who.int/nutrition/publications/guidelines/antenatalcare-pregnancy-positive-experience/en/ [PubMed] [Google Scholar]
- World Health Organization. (2016b). Guideline: Daily iron supplementation in adult women and adolescent girls. Geneva: World Health Organization. http://www.ncbi.nlm.nih.gov/books/NBK361888/ [PubMed] [Google Scholar]
- Zeisel, S. H. (2009). Is maternal diet supplementation beneficial? Optimal development of infant depends on mother's diet. The American Journal of Clinical Nutrition, 89(2), 685S–;687S. 10.3945/ajcn.2008.26811F [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Confidence intervals around adjusted means
Figure S1. Final analytical sample.
Figure S2. Boxplot of energy intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S3. Boxplot of protein intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S4. Boxplot of calcium intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S5. Boxplot of iron intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S6. Boxplot of vitamin A intake by reproductive status among 15‐49y old rural Bangladeshi women
Figure S7. Boxplot of women's dietary diversity score (WDDS) by reproductive status among 15‐49y old rural Bangladeshi women
Figure S8. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 60% for pregnant vs. NPNL women
Figure S9. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 60% for lactating vs. NPNL women
Figure S10. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 50% for pregnant vs. NPNL women
Figure S11. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 50% for lactating vs. NPNL women
Figure S12. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 40% for pregnant vs. NPNL women
Figure S13. Results of equivalence and difference test for adjusted mean nutrient intakes and WDDS at delta = 40% for lactating vs. NPNL women


