Abstract
Female‐initiated HIV prevention methods, such as oral pre‐exposure prophylaxis (PrEP) and the vaginal ring, may be important risk reduction strategies for breastfeeding women. Given their novelty, information about the sociocultural context and how it influences perceptions of and support for their use during breastfeeding is lacking. To address this gap, we conducted 23 focus group discussions separately with pregnant and breastfeeding women, male partners and grandmothers (N = 196) and 36 in‐depth interviews with key informants in Malawi, South Africa, Uganda and Zimbabwe. We analysed the data using a framework analysis method. Overall, breastfeeding was the norm, and participants described the transference of health (e.g., nutrition) and disease (e.g., HIV) to children through breast milk. Participants considered the early breastfeeding period as one of high HIV transmission risk for women. They explained that male partners tend to seek outside sexual partners during this period because women need time to recover from delivery, women focus their attention on the child, and some men are disgusted by breast milk. Participants highlighted concerns about the drugs in oral PrEP transferring to the child through breast milk, but fewer worried about the effects of the vaginal ring because the drug is localized. Women, grandmothers and key informants were supportive of women using these HIV prevention methods during breastfeeding, while male partners had mixed opinions. These findings can be used to tailor messages for promoting the use of PrEP or the vaginal ring during breastfeeding in sub‐Saharan Africa.
Keywords: breast milk, breastfeeding, HIV prevention, qualitative, sub‐Saharan Africa
Key messages.
Participants explained that men in sub‐Saharan Africa seek outside sex partners during the early breastfeeding period, which puts women and their breastfeeding children at increased risk of HIV transmission.
Women, grandmothers and key informants were supportive of women using HIV prevention methods during breastfeeding, while male partners had mixed opinions.
Participants worried about the drugs in oral PrEP transferring to the child through breast milk but had fewer worries about the vaginal ring because the drug is localized.
Future trials of female‐initiated HIV prevention methods should address concerns about the methods' effects on breast milk and offer women strategies to safely engage their male partners in conversations about the methods.
1. INTRODUCTION
With the continued high fertility rate of 4.8 live births per woman in sub‐Saharan Africa, many women in the region go through multiple pregnancies and periods of lactation during their adolescence and adulthood (Bongaarts, 2017; The World Bank, 2019). The median breastfeeding duration in sub‐Saharan Africa remains long, ranging from 12 months in South Africa to 23 months in Malawi (Aboud et al., 2009; National Statistical Office [Malawi] & ICF, 2017). HIV prevalence estimates among women of reproductive age vary by country in sub‐Saharan Africa (e.g., 7.5% in Uganda, 10.8% in Malawi, 16.7% in Zimbabwe and 26.0% in South Africa), and estimates for east and southern Africa are the highest globally (Human Sciences Research Council, 2018; NSO [Malawi] & ICF, 2017; Uganda Bureau of Statistics & ICF, 2018; Zimbabwe National Statistics Agency & ICF International, 2016). Breastfeeding women are at risk of acquiring HIV because of behavioural, sociocultural and biological factors, and new HIV infections carry a higher risk of transmission to the baby (Drake, Wagner, Richardson, & John‐Stewart, 2014). The risk of HIV transmission from mother to child from untreated postnatally‐acquired HIV is estimated at 29% (Dunn, Newell, Ades, & Peckham, 1992).
The high prevalence of HIV, particularly among women of reproductive age, and the power imbalances among many heterosexual sub‐Saharan couples make it is difficult for women to negotiate the use of HIV prevention methods, such as male and female condoms or sexual abstinence, when men have more power (Leddy, Chakravarty, Dladla, de Bruyn, & Darbes, 2016). Therefore, female‐initiated HIV prevention methods are essential for preventing transmission among breastfeeding women (Ramjee & Daniels, 2013; Sia et al., 2016; UNAIDS, 2016). To ensure that female‐initiated HIV prevention methods will be used during breastfeeding, it is important to understand beliefs about risks of acquiring and transmitting HIV during breastfeeding and perceptions of the link between breastfeeding, HIV risk and maternal and child health. Previous research on breastfeeding and HIV focused on prevention of mother‐to‐child transmission programmes (Buskens, Jaffe, & Mkhatshwa, 2007; Chinkonde, Hem, & Sundby, 2012; Desclaux & Alfieri, 2009; Maman et al., 2012; Østergaard & Bula, 2010; Tuthill, McGrath, & Young, 2014; Young et al., 2011), particularly under earlier World Health Organization (WHO) recommendations related to HIV and infant feeding (WHO, UNICEF, UNFPA, & UNAIDS, 2003, 2007). WHO previously recommended that to prevent HIV transmission through breast milk, women living with HIV choose either to exclusively formula feed or to exclusively breastfeed and then stop breastfeeding at 6 months, neither of which were culturally normative in most sub‐Saharan African settings (Chinkonde, Sundby, & Martinson, 2009; Desclaux & Alfieri, 2009; Østergaard & Bula, 2010). Several studies showed that it was challenging for women to follow the guidelines because of their own beliefs about the importance of breastfeeding for child health and as a key element of parenting, the stigma of not breastfeeding in a setting where breastfeeding is normative, and the pressure from family members to breastfeed (Buskens et al., 2007; Chinkonde et al., 2012; Leshabari, Blystad, & Moland, 2007; Maman et al., 2012). Those studies point to the importance of understanding cultural norms and health beliefs when proposing guidelines for the use of new methods, such as oral PrEP or the vaginal ring. To our knowledge, there is no research on beliefs related to risk of HIV acquisition and transmission during breastfeeding or use of methods for preventing sexual transmission of HIV during breastfeeding.
This paper fills this gap through qualitative research with pregnant and breastfeeding women, male partners, grandmothers and community key informants in preparation for a phase IIIB trial of two new biomedical HIV prevention methods during breastfeeding—daily Truvada™ (tenofovir diproxyl fumarate and embricitabine) for oral PrEP and the monthly dapivirine vaginal ring—in four sub‐Saharan African countries. Truvada is an oral pill that has proven safe and effective among breastfeeding women who are at risk of HIV (Fonner et al., 2016; Mofenson, Baggaley, & Mameletzis, 2017). The dapivirine vaginal ring is a microbicide embedded silicone ring, which is effective at preventing HIV in nonpregnant women and currently in the approval process (Baeten et al., 2016; Nel et al., 2016). Oral PrEP and the dapivirine ring are important HIV prevention methods because they can be initiated by women, whereas it is difficult for women to initiate or have control over other HIV prevention methods. Our aims with this analysis were to describe the sociocultural context in which female‐initiated HIV prevention methods will be used and to understand how the sociocultural context may influence perceptions of and support for use of female‐initiated HIV prevention methods during breastfeeding.
2. METHODS
2.1. Study design
Microbicide Trials Network (MTN) 041 = MAMMA (Microbicide/PrEP Acceptability among Mothers and Male Partners in Africa) study was a multisite qualitative study (clinicaltrials.gov NCT03648931). A total of 232 participants were enrolled between May and November 2018 in Blantyre, Malawi; Johannesburg, South Africa; Kampala, Uganda; and Chitungwiza, Zimbabwe. The sites were chosen because they are MTN clinical trials sites. All four countries have generalized HIV epidemics and high HIV prevalence, especially in urban and peri‐urban areas, where the sites are located. The South Africa site is in an inner‐city neighbourhood with high population density. The Zimbabwe site is in a high‐density township from which people commute to Harare, the capital, which is 30 km away. The Uganda site is in the capital, and the Malawi site is in the economic capital, which has the lowest population density of the four sites.
Figure 1 shows the number and types of participants by data collection method and country. Twenty‐three single‐sex focus group discussions (FGDs) were conducted across the four sites with participants who were independently recruited into the following groups: (1) HIV‐uninfected women aged 18 to 40 who were currently or recently (in the past 2 years) pregnant or breastfeeding (referred to as women), (2) men aged 18+ with female partners who were currently or recently pregnant or breastfeeding (referred to as male partners) and (3) mothers of women who were currently or recently pregnant or breastfeeding or mothers of men whose female partner was currently or recently pregnant or breastfeeding (referred to as grandmothers). FGD participants were recruited from various community settings (e.g., through street outreach, community advisory boards, word of mouth, construction sites [men only] and religious gatherings [grandmothers only]) and antenatal/postnatal clinics [women only]). Key informants participated in in‐depth interviews (IDIs). They were purposively sampled from professional groups that have direct involvement in pregnant and breastfeeding women's well‐being, including clinicians, traditional care providers, social service/community health workers and community leaders.
FIGURE 1.
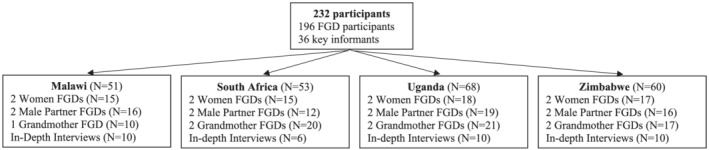
Study sample by country and type of participant
2.2. Data collection
FGDs and IDIs were conducted by trained social scientists who were native speakers of the local languages and fluent in English. They used different semi‐structured question guides for women, male partners, grandmothers and key informants. The guides are available on the MTN website (https://mtnstopshiv.org/research/studies/mtn-041). They were translated into Chichewa in Malawi, Zulu in South Africa, Luganda in Uganda and Shona in Zimbabwe. Topics discussed included HIV risk perception and sexual health practices, health‐related decision‐making and opinions about two HIV prevention methods: daily oral PrEP and a monthly vaginal ring. Questions were asked first relative to pregnancy/pregnant women, followed by probes on how they relate to lactation/breastfeeding women.
A brief educational video about the two HIV prevention methods (https://mtnstopshiv.org/research/studies/mtn-041/mtn-041-videos) was shown just before the section of the FGD or IDI about the products, and participants had an opportunity to handle placebo products at that time. The pills were identical to Truvada (Gilead Sciences, Foster City, CA), and a silicone elastomer placebo ring was identical to the dapivirine vaginal ring (International Partnership for Microbicides, Silver Spring, MD). Demographic information was collected from all participants through interviewer‐administered questionnaires. FGDs and IDIs were audio‐recorded, transcribed and translated into English. Pseudonyms were used to represent different participants in each FGD transcript. Additional details about study procedures can be found in van der Straten et al. (2020).
2.3. Data analysis
The data were analysed using framework analysis, a qualitative method for applied research (Srivastava & Thomson, 2009). A codebook of inductive and deductive codes (Data S1) was iteratively developed with staff from the study sites using a modified socio‐ecological framework as previously described (van der Straten et al., 2020). A team of five qualitative analysts, three of whom were involved throughout the study, coded the transcripts using Dedoose software v7.0.23 and maintaining ~80% intercoder reliability. Analysts met regularly to discuss emergent themes and come to consensus on intercoder discrepancies. For this analysis, data coded with the code BREASTFEEDING (applied to all discussions pertaining only to the breastfeeding or post‐delivery period) were extracted. Breastfeeding‐related data were stratified by participant type and summarized in analytical memos that outlined key themes and included illustrative quotations. Analysts held biweekly meetings during the memoing process to discuss emerging themes and similarities or differences across participant types and study sites. Summary memos were reviewed by and discussed with representatives from each study site for insight on local cultural and contextual findings.
2.4. Ethical considerations
The MTN‐041 protocol was approved by the Western Institutional Review Board, Olympia, Washington, USA, and by local IRBs at each of the study sites. The study was overseen by the regulatory infrastructure of the U.S. National Institutes of Health and the Microbicide Trials Network. All participants provided written informed consent.
3. RESULTS
Participants' socio‐economic characteristics are shown in Table 1. Half of the women were currently pregnant, and three‐quarters of them had ever breastfed. Nearly all of the women were financially dependent on their primary sex partner, although in some sites more than half of the women reported that they also earned their own income. Overall, three‐quarters of male partners earned an income; however, the percentage was much lower in South Africa. Grandmothers were 50 years of age on average, indicating that about half of them were still women of reproductive age. Because of the criteria used for selecting professionals as key informants, they had higher levels of education than other participants. Women reported that male partners (63%) and grandmothers (19%) had the most influence on their decision‐making during breastfeeding (data not shown).
TABLE 1.
Sociodemographic characteristics of participants
| Variable | Malawi | South Africa | Uganda | Zimbabwe | Total |
|---|---|---|---|---|---|
| Women | (N = 15) | (N = 15) | (N = 18) | (N = 17) | (N = 65) |
| Mean age (range) | 26.7 (21–34) | 28.0 (22–40) | 27.2 (19–40) | 26.6 (19–38) | 27.1 (19–40) |
| Earns an income | 9 (60%) | 0 (0%) | 12 (71%) | 6 (35%) | 27 (42%) |
| Secondary education completed | 6 (40%) | 11 (73%) | 4 (22%) | 12 (71%) | 33 (51%) |
| Marital status | |||||
| Single, never married | 0 (0%) | 14 (93%) | 1 (6%) | 0 (0%) | 15 (23%) |
| Married | 14 (93%) | 1 (7%) | 16 (89%) | 16 (94%) | 47 (72%) |
| Separated/divorced | 1 (7%) | 0 (0%) | 1 (6%) | 1 (6%) | 3 (5%) |
| Financial/material support from primary sex partner | 15 (100%) | 14 (93%) | 17 (100%) | 16 (100%) | 62 (98%) |
| Mean pregnancies (range) | 2.5 (1–5) | 3.1 (1–7) | 1.9 (1–3) | 2.3 (1–5) | 2.4 (1–7) |
| Currently pregnant | 8 (53%) | 6 (40%) | 11 (65%) | 7 (41%) | 32 (50%) |
| Ever breastfed | 12 (80%) | 10 (67%) | 15 (83%) | 11 (65%) | 48 (74%) |
| Abstained from sex after most recent delivery | 5 (33%) | 2 (13%) | 10 (56%) | 6 (35%) | 23 (35%) |
| Male partners | (N = 16) | (N = 12) | (N = 19) | (N = 16) | (N = 63) |
| Mean age (range) | 30.2 (19–53) | 33.0 (27–49) | 32.4 (23–54) | 27.0 (19–45) | 30.6 (19–54) |
| Earns an income | 13 (81%) | 4 (33%) | 17 (90%) | 14 (88%) | 48 (76%) |
| Secondary education completed | 6 (38%) | 8 (67%) | 9 (47%) | 12 (75%) | 35 (56%) |
| Grandmothers | (N = 10) | (N = 20) | (N = 21) | (N = 17) | (N = 68) |
| Mean age (range) | 50.6 (39–69) | 54.9 (36–67) | 47.1 (37–63) | 49.3 (36–100) | 50.5 (36–100) |
| Earns an income | 7 (70%) | 3 (15%) | 19 (91%) | 11 (65%) | 40 (59%) |
| Secondary education completed | 1 (10%) | 6 (30%) | 6 (29%) | 6 (35%) | 19 (28%) |
| Key informants | (N = 10) | (N = 6) | (N = 10) | (N = 10) | (N = 36) |
| Mean age (range) | 50.4 (25–73) | 42.7 (31–68) | 49.7 (32–78) | 53.2 (32–79) | 49.7 (25–79) |
| Female | 6 (60%) | 4 (67%) | 7 (70%) | 8 (80%) | 25 (69%) |
| Earns an income | 9 (90%) | 6 (100%) | 10 (100%) | 10 (100%) | 35 (97%) |
| Secondary education completed | 8 (80%) | 6 (100%) | 7 (70%) | 7 (70%) | 28 (78%) |
The main themes that emerged during this analysis were the (1) transference of health and disease through breast milk, (2) risks of sex during breastfeeding to maternal and child health, (3) reasons male partners seek other sex partners during the breastfeeding period, (4) beliefs about the effects of HIV prevention methods on breast milk and (5) support for HIV prevention methods during breastfeeding. Table S1 shows the themes and subthemes with illustrative quotes by type of participant. The themes and subthemes were quite similar across participant groups and countries, but differences are noted where applicable.
3.1. Transference of health and disease through breast milk
Across participant groups and study sites, breastfeeding was the norm and participants stated that generally women are expected to breastfeed their babies. Many participants described breast milk as beneficial for the child and explained that breast milk supply, quality and flow could be influenced by a woman's diet and her level of stress. They described how maternal and child nutrition are linked through breast milk. Ngwanenyana, a woman in South Africa (age 26), said, ‘I think breastfeeding mothers should continue with a healthy diet because whatever they eat goes to the child’. Participants also discussed the importance of good maternal nutrition and consumption of enough fluids for adequate breast milk production and listed a wide variety of foods that could facilitate it. Mbuya Peter, a grandmother in Zimbabwe (age 45), said, ‘You should eat food that makes you produce adequate milk for the baby. That is what we encourage breastfeeding women to do’. Conversely, some participants said that a mother should avoid certain foods and fluids and should not take nonprescribed drugs because they could affect the child through breast milk. For example, a nurse from South Africa (age 53) said,
She should not drink alcohol, she should not smoke tobacco, and she should not eat food that will affect the baby, because she is breastfeeding … They [mothers] must stay away from taking medication that is not prescribed by the doctor because what they take, they give to the baby through breast milk.
In addition, some participants mentioned the importance of women reducing stress and being relaxed during breastfeeding to make sure their milk flows. Pizza, a male partner in Zimbabwe (age 28), explained, ‘Our women should not be stressed. I think it helps in the production of milk because when she is relaxed and not stressed, she produces more milk’.
Participants also understood that HIV could be transmitted from a mother to child through breast milk. Red, a woman in South Africa (age 30), explained,
When you have sex with your partner while breastfeeding, everything that you do goes to the baby, so the baby can be infected like that. Because if you are HIV positive and breastfeeding, your baby will end up being positive also.
3.2. Risks of sex during breastfeeding to maternal and child health
Participants described cultural beliefs and practices related to postpartum abstinence, which traditionally lasted at least 3 months and provided an opportunity for the woman to recover and focus her attention on breastfeeding and taking care of the baby. Guest, a grandmother in South Africa (age 67), explained, ‘I think for three months she has not fully recovered to do penetrative sex. We have to tell the truth. No, the inside has not healed’. Kevin, a male partner in Uganda (age 35), also pointed to the importance of waiting for 3 months, so the woman could regain her strength, but noted that male partners cannot always wait that long. He said, ‘When a woman has just given birth, she is weak, but some men do not give them those three months to get healed’.
Many of the focus group participants considered having sex during the early postpartum period to be dangerous for the woman because her uterus is ‘wet’ and ‘hot’ and her immunity is low, which makes her especially susceptible to sexually transmitted infections, including HIV. Violet, a grandmother in South Africa (age 62) explained, ‘So, he would come to you and you are still wet … Men say a woman who has just delivered a baby is hot and enjoyable sexually and then he leaves you with a sickness’. Cissy, a grandmother in Uganda (age 49) described how a breastfeeding mother's immunity plays a role in HIV acquisition. She said, ‘It is easy for a breastfeeding mother to be infected with HIV because her immunity is still low’.
Many participants said that the postpartum abstinence period has become shorter in recent years as women in settings with high HIV prevalence have felt pressured to resume sex early to keep their male partners from seeking other partners. Women fear that their male partner will acquire HIV during the early breastfeeding period then transmit it to them and they will inadvertently transmit it to their babies through breast milk. Kheliwe, a woman in Malawi (age 25), explained,
After their wives have delivered, a lot of men like going out. Since they take it that the wives have not fully recovered, that they are still not well. So, they end up sleeping with prostitutes. Once they resume their sexual relationship with their wives, they end up infecting them [with HIV], and as a result, these breastfeeding women also infect their babies with the virus via infected breast milk.
To try to avoid a situation where a male partner infects his female partner and his child as a result of his infidelity, participants explained that health personnel advise women to resume sex after 6 weeks of postpartum abstinence or as soon as they feel ready. Lucy, a woman in Malawi (age 29), explained,
The hospital personnel just advise us on the six weeks period [of abstinence], just because of how the men behave nowadays, but we are not supposed to start sex as early as that, because of what one goes through, and the need to be fully recovered and ensure that the discharge has completely ceased prior to resuming sex.
Key informants also described the shortening of the postpartum abstinence period. A nurse in Zimbabwe (age 50) said, ‘We realized that in this HIV era, in those six weeks someone might have already gone outside [to find another sex partner] … So, when the mother feels that she is now fit, they can resume sex’.
3.3. Reasons male partners seek other sex partners during breastfeeding period
All participant groups explained that the early breastfeeding period is a time of risk of HIV transmission for women. Participants outlined three main reasons for increased risk at this time: women are focused on their babies and male partners may see this as uninviting, male partners dislike the smell of breast milk and women's lack of hygiene during breastfeeding, and male partners are simply unwilling to wait to have sex until the woman heals from the delivery. For example, a female religious leader from Zimbabwe (age 51) explained how women attend to their babies and ignore their male partners during the early breastfeeding period, which pushes the male partners to seek outside sex partners. She said,
The woman will not be effective sexually. She will be busy with concentrating on the baby and all the other chores. She will be tired and will not give the man enough attention. So, when he goes out to look for other women, he gets infected with HIV and he goes to infect his wife back home.
Moses, a male partner in Uganda (age 23), had a similar comment. He said,
The HIV virus comes in as a result of committing adultery. In most cases, love ceases to exist [between a man and woman] when a woman gives birth. The woman diverts her love to the baby. The man may find it hard to endure the situation …. So, if he commits adultery and in the process contracts HIV, he will infect the breastfeeding woman.
Participants also explained that male partners may be put off by the breastfeeding process or the milk itself, and some male partners even consider it to be dangerous. Asanda, a male partner from South Africa (age 30), said, ‘When [the breast milk] is full and all over, I cannot stand it. That milk is dangerous’. Women and grandmothers explained that if a woman does not bathe regularly and maintain good hygiene, this can drive the man to seek other sex partners. For example, Jane, a woman from Zimbabwe (age 30), said,
Women when they do not keep themselves smart, like bathing regularly, they smell like milk and most men dislike that, the smell of milk and so forth. So, most of the times most men go out and become unfaithful during that time. So, you can see that you will be at high risk of getting HIV because most men feel disgusted by women when they are breastfeeding, especially if you don't bathe.
Although there was a lot of discussion across groups about male partners' infidelity during breastfeeding, participants agreed that not all male partners went in search of other partners during the breastfeeding period. Some men noted that they should be patient with their female partners and not pressure them to have sex right away because it is not good for the health of the baby or the woman. Pan, a male partner in Uganda (age 30), explained, ‘On that issue, I see some men who are patient. There are some who decide to wait until their partners get well. You wait for those months for her to get well’.
3.4. Beliefs about the effects of HIV prevention methods on breast milk
In accordance with the concept of transference of health and disease through breast milk, many participants' beliefs about female‐initiated HIV prevention methods were related to the potential effect on breast milk taste or production or the possible harm to the child with exposure to the drugs through breast milk. Overall, participants seemed to have more concerns about use of oral PrEP than the vaginal ring during breastfeeding because drugs in oral PrEP were perceived as circulating throughout the body, including in breast milk, whereas the drugs in the vaginal ring were thought to be more localized. Some of them worried about oral PrEP harming the baby, who would be exposed to the PrEP drugs through breast milk, or oral PrEP affecting breast milk production. A pharmacist in Uganda (age 25) explained, ‘No! Breastfeeding women should not take these drugs because some drugs pass through breast milk and they can go to the baby through breast milk’. Peter, a male partner (age 24) in Uganda, explained that drugs can also affect the quantity of breast milk produced. ‘My partner is breastfeeding and … she very well knows that there are some tablets women may use and they reduce breast milk production. Won't my partner have reduced breast milk production because of these products?’
Many participants thought the vaginal ring would not have such an impact on breast milk because drug release is localized, as was explained in the video shown to participants during the FGDs, and hence may not affect the blood or travel to the breast milk. However, a few participants worried that the ring might make the breast milk bitter or could dry up production. Ngwanenyana, a woman in South Africa (age 26), explained, ‘Since it was explained that the ring protects only the vaginal part so I don't think it's going to have an effect on the child while breastfeeding because the medicine doesn't go up to the breasts it only settles on the vagina’. Whereas Mpho, another woman in the same group (age 27), asked, ‘What if it [vaginal ring] … causes the breast milk to be bitter?’
3.5. Support for HIV prevention methods during breastfeeding
Most women liked the idea of HIV prevention methods they could initiate themselves because they would protect them and the baby. Agatha (age 21), a woman in Uganda, summarized this point as follows,
They [women] might be motivated [by] the fact that it helps to protect her and the baby from getting infected which enables her to breastfeed her baby knowing that it is safe. She will know that in case her partner engages into sex with other women she will be able to protect herself and her baby.
Like women, grandmothers and key informants were generally supportive of women using HIV prevention methods during breastfeeding because the methods would protect the woman and her child from acquiring HIV, even if her male partner was not faithful during the postpartum period. Mbuya Shava, a grandmother in Zimbabwe (age 43), said, ‘I think they [other grandmothers in the community] will support it because … no one wants her child to live with HIV’. Key informants thought that if women were well educated about HIV prevention methods, they would use them because the products would protect the baby. A nurse from South Africa (age 68) explained, ‘If they are given the reassurance that this medication [oral PrEP] is safe then they can easily accept it. Most [breastfeeding] mothers do care about protecting their babies. That is their concern, a healthy baby that is not infected’.
Male partners had mixed opinions about their female partner using female‐initiated HIV prevention methods. Some male partners expressed support, but said they wanted to be involved in choosing the method and discussing it openly with their female partner. Other male partners thought conversations with their female partner about product use would be easier when the product is being chosen and used for protecting the baby. For example, Mastermind, a male partner in South Africa (age 29), referring to using oral PrEP during breastfeeding said, ‘Once you have the product knowledge and we know why we want to use it, it becomes easier for both of us that it is for the safety of our child’. Male partners expressed their concerns about discovering that their female partner is using a female‐initiated HIV prevention method during breastfeeding. They said this would be a breach of trust in the relationship, indicating either that she believes he has other partners or she has other partners herself. Huawei, a male partner in South Africa (age 29), said,
Yeah, communication is the key, as my brother is saying. [If] she just comes up with the pills [oral PrEP], the first thing is communication so that I know about these pills. Now that I know [she is using] these pills, I wouldn't feel good as it would mean there is no trust in our relationship.
4. DISCUSSION
The focus of this study was to describe the sociocultural context in four sub‐Saharan African countries where female‐initiated HIV prevention methods will be tested and to learn about perceptions of and support for female‐initiated HIV prevention methods by women and key influencers, including male partners, grandmothers, health providers and community leaders. Key elements of the sociocultural context were related to perceptions of the role of HIV prevention during breastfeeding, the importance of postpartum abstinence for maternal and child health and the competing risks for women of abstinence versus resuming sex during the early breastfeeding period. Although participants acknowledged that oral PrEP and the vaginal ring could protect a woman's well‐being during breastfeeding, much of the narrative was centred around the importance of protecting a woman's health as a way of protecting her child's health. This is consistent with research on the value of children in African cultures (Dyer, 2007). Participants described how postpartum abstinence allows mothers to recover and focus their energy on breastfeeding and taking care of the child. Their discussions indicated that in contexts with high HIV prevalence, women must balance the maintenance of a long period of abstinence against the fear that their male partner will seek other sex partners while waiting and he may then infect her with HIV when they resume sexual relations (Mbekenga, Pembe, Darj, Christensson, & Olsson, 2013). Early resumption of sexual activity was also seen as putting the woman at risk of acquiring HIV because she is not fully recovered from the delivery, her womb/body is more receptive to infections and her immunity is low. Participants' perception of higher HIV risk is consistent with studies in sub‐Saharan Africa showing that HIV risk in breastfeeding women is increased (Drake et al., 2014; Kinuthia et al., 2015) and men report more concurrent sex partners after their female partner delivers (Kinuthia et al., 2017; Mbekenga et al., 2013).
Across participant groups, the main concern with oral PrEP and the vaginal ring as HIV prevention methods was the possibility of drug exposure to the child through breast milk. Participants were generally more worried about the drugs in oral PrEP than the vaginal ring getting into breast milk or changing or drying up breast milk. These fears need to be addressed in patient education when moving to the next stages of testing oral PrEP and the vaginal ring with breastfeeding women. Educational materials could point to pharmacokinetic and safety studies of infant exposure to antiretrovirals in oral PrEP through breast milk (Mugwanya et al., 2016; Nagot et al., 2016; Noguchi et al., 2016, 2019; Waitt, Garner, Bonnett, Khoo, & Else, 2015) and to the extensive testing of the safety of child exposure to antiretrovirals in utero and through breast milk in prevention of mother‐to‐child transmission programmes (Fowler et al., 2016; Jamieson et al., 2012; World Health Organization, 2016).
Women, grandmothers and key informants were generally supportive of breastfeeding women using female‐initiated HIV prevention methods. They were aware of the challenges women face in balancing between having a period of postpartum abstinence to recover from delivery and not wanting to wait too long to resume sex postpartum because of concerns about male partner infidelity. They stated that these HIV prevention methods would provide women with a way to protect themselves and their child from HIV transmission during breastfeeding. Some male partners were also supportive but wanted to be involved in conversations about the use of HIV prevention methods by their female partners and were concerned about female partners deciding to use a method without their knowledge. The power imbalance in many households in sub‐Saharan Africa means that men's greater power in the relationship gives them leeway to seek outside partners, while simultaneously making it difficult for women to bring up issues related to sex and HIV prevention, including negotiating condom use and less risky sexual behaviours (Langen, 2005). Strategies for improved partner communication are needed to ensure that women who choose to use these types of HIV prevention methods feel comfortable disclosing their use and do not face increased risk of intimate partner violence (Cabral et al., 2018; Hartmann et al., 2019; Montgomery et al., 2019; Palanee‐Phillips et al., 2018).
The strengths of this study were the collection of data in multiple countries, the selection of sites with high HIV prevalence and the similarity in responses on most topics across four different types of participants, which indicates the salience of the themes. The main limitation of this study was the small number of FGDs per participant type per site, which did not allow for cross‐country comparisons. However, the study was intended for overall analysis, and we found many similarities in responses across the study sites. Another limitation was that the study had a stronger emphasis on pregnancy, which was asked about first then followed by probes on breastfeeding, so the depth and specificity of responses related to breastfeeding were likely less detailed.
In conclusion, this study described how the sociocultural context in four sub‐Saharan countries may influence the uptake of female‐initiated HIV prevention methods. Participant responses highlighted the struggle breastfeeding women face, especially during the early postpartum period. On the one hand, women can choose to focus their attention on their child and their own recovery from delivery during a period of postpartum abstinence. On the other hand, they may decide to resume sex with their male partner to discourage him from infidelity and possibly infecting the woman and child with HIV. In this context, women, grandmothers and key informants were supportive of female‐initiated HIV prevention methods, while male partners had mixed opinions. Participants flagged some concerns with the drugs in these methods, especially oral PrEP, getting into breast milk and affecting the child. Educational materials used to explain or promote female‐initiated HIV prevention methods in sub‐Saharan Africa should address concerns about the methods' effects on breast milk by describing the safety of antiretroviral drugs, allaying male partners concerns about women's use of the methods by emphasizing the protection they offer the child and providing women strategies to safely engage their male partners in conversations about the methods.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
VLF contributed to the analysis and drafted the paper. IM was a data analyst and contributed to the paper. JR was the project coordinator, a data analyst and reviewed the paper. MC, RN, LS and FT were site coordinators, and FM was a site sub‐investigator and interviewer. They reviewed the analytic memos and the manuscript. AvdS was the protocol chair, led study development and implementation, contributed to the analysis and reviewed the paper.
Supporting information
Data S1 Supporting Information
Table S1. Key themes, sub‐themes and illustrative quotes by type of study participant
ACKNOWLEDGMENTS
Study Team Leadership: Ariane van der Straten, Women's Global Health Imperative (WGHI) RTI International (Protocol Chair); Petina Musara, University of Zimbabwe College of Health Sciences Clinical Trials Research Centre (Protocol Co‐chair); Julia Ryan, Women's Global Health Imperative (WGHI) RTI International (Research Public Health Analyst); Jeanna Piper, U.S. National Institute of Allergy and Infectious Diseases (Medical Officer); Teri Senn, National Institute of Mental Health (NIMH Chief); Nicole Macagna, FHI 360 (Clinical Research Manager)
Study Sites and Site Investigators of Record: Malawi, Blantyre site (Johns Hopkins Project‐College of Medicine, University of Malawi): Frank Taulo; South Africa, Johannesburg site (Wits Reproductive Health and HIV Institute): Krishnaveni Reddy; Uganda, Kampala site (Makerere University—Johns Hopkins University Research Collaboration): Juliane Etima; Zimbabwe, Chitungwiza, Zengeza site (University of Zimbabwe College of Health Sciences Clinical Trials Research Centre): Petina Musara
Data management was provided by the Women's Global Health Imperative Program (RTI International, Berkeley, CA).
The study was designed and implemented by the Microbicide Trials Network (MTN) funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co‐funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Flax VL, Hawley I, Ryan J, et al. After their wives have delivered, a lot of men like going out: Perceptions of HIV transmission risk and support for HIV prevention methods during breastfeeding in sub‐Saharan Africa. Matern Child Nutr. 2021;17:e13120. 10.1111/mcn.13120
REFERENCES
- Aboud, S. , Msamanga, G. , Read, J. S. , Wang, L. , Mfalila, C. , Sharma, U. , … Fawzi, W. W. (2009). Effect of prenatal and perinatal antibiotics on maternal health in Malawi, Tanzania, and Zambia. International Journal of Gynaecology and Obstetrics, 107(3), 202–207. 10.1016/j.ijgo.2009.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten, J. M. , Palanee‐Phillips, T. , Brown, E. R. , Schwartz, K. , Soto‐Torres, L. E. , Govender, V. , … MTN‐020 ASPIRE Study Team . (2016). Use of a vaginal ring containing dapivirine for HIV‐1 prevention in women. New England Journal of Medicine, 375(22), 2121–2132. 10.1056/NEJMoa1506110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaarts, J. (2017). Africa's unique fertility transition. Population and Development Review, 43(S1), 39–58. 10.1111/j.1728-4457.2016.00164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskens, I. , Jaffe, A. , & Mkhatshwa, H. (2007). Infant feeding practices: Realities and mind sets of mothers in South Africa. AIDS Care, 19(9), 1101–1109. 10.1080/09540120701336400 [DOI] [PubMed] [Google Scholar]
- Cabral, A. , Baeten, J. M. , Ngure, K. , Velloza, J. , Odoyo, J. , Haberer, J. E. , … Heffron, R. (2018). Intimate partner violence and self‐reported pre‐exposure prophylaxis interruptions among HIV‐negative partners in HIV serodiscordant couples in Kenya and Uganda. Journal of Acquired Immune Deficiency Syndromes, 77(2), 154–159. 10.1097/QAI.0000000000001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkonde, J. R. , Hem, M. H. , & Sundby, J. (2012). HIV and infant feeding in Malawi: Public health simplicity in complex social and cultural contexts. BMC Public Health, 12(1), 700. 10.1186/1471-2458-12-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkonde, J. R. , Sundby, J. , & Martinson, F. (2009). The prevention of mother‐to‐child HIV transmission programme in Lilongwe, Malawi: Why do so many women drop out. Reproductive Health Matters, 17(33), 143–151. 10.1016/S0968-8080(09)33440-0 [DOI] [PubMed] [Google Scholar]
- Desclaux, A. , & Alfieri, C. (2009). Counseling and choosing between infant‐feeding options: Overall limits and local interpretations by health care providers and women living with HIV in resource‐poor countries (Burkina Faso, Cambodia, Cameroon). Social Science and Medicine, 69(6), 821–829. 10.1016/j.socscimed.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Drake, A. L. , Wagner, A. , Richardson, B. , & John‐Stewart, G. (2014). Incident HIV during pregnancy and postpartum and risk of mother‐to‐child HIV transmission: A systematic review and meta‐analysis. PLoS Medicine, 11(2), e1001608. 10.1371/journal.pmed.1001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, D. T. , Newell, M. L. , Ades, A. E. , & Peckham, C. S. (1992). Risk of human immunodeficiency virus type 1 transmission through breastfeeding. The Lancet, 340(8819), 585–588. [DOI] [PubMed] [Google Scholar]
- Dyer, S. J. (2007). The value of children in African countries: Insights from studies on infertility. Journal of Psychosomatic Obstetrics and Gynaecology, 28(2), 69–77. 10.1080/01674820701409959 [DOI] [PubMed] [Google Scholar]
- Fonner, V. A. , Dalglish, S. L. , Kennedy, C. E. , Baggaley, R. , O'Reilly, K. R. , Koechlin, F. M. , … Grant, R. M. (2016). Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. Aids, 30(12), 1973–1983. 10.1097/QAD.0000000000001145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, M. G. , Qin, M. , Fiscus, S. A. , Currier, J. S. , Flynn, P. M. , Chipato, T. , … Mofenson, L. M. (2016). Benefits and risks of antiretroviral therapy for perinatal HIV prevention. New England Journal of Medicine, 375(18), 1726–1737. 10.1056/NEJMoa1511691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, M. , Palanee‐Phillips, T. , O'Rourke, S. , Adewumi, K. , Tenza, S. , Mathebula, F. , … Montgomery, E. T. (2019). The relationship between vaginal ring use and intimate partner violence and social harms: Formative research outcomes from the CHARISMA study in Johannesburg, South Africa. AIDS Care, 31(6), 660–666. 10.1080/09540121.2018.1533227 [DOI] [PubMed] [Google Scholar]
- Human Sciences Research Council (HSRC) . (2018). The fifth South African national HIV prevalence, incidence, behaviour and communication survey, 2017: HIV impact assessment summary report. Cape Town, South Africa: HSRC Press.
- Jamieson, D. J. , Chasela, C. S. , Hudgens, M. G. , King, C. C. , Kourtis, A. P. , Kayira, D. , … van der Horst, C. (2012). Maternal and infant antiretroviral regimens to prevent postnatal HIV‐1 transmission: 48‐week follow‐up of the BAN randomised controlled trial. Lancet, 379(9835), 2449–2458. 10.1016/S0140-6736(12)60321-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuthia, J. , Drake, A. L. , Matemo, D. , Richardson, B. A. , Zeh, C. , Osborn, L. , … John‐Stewart, G. (2015). HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics: A cohort study. Aids, 29(15), 2025–2033–2033. 10.1097/QAD.0000000000000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuthia, J. , Richardson, B. A. , Drake, A. L. , Matemo, D. , Unger, J. A. , McClelland, R. S. , & John‐Stewart, G. (2017). Sexual behavior and vaginal practices during pregnancy and postpartum: Implications ofr HIV prevention strategies. Journal of Acquired Immune Deficiency Syndromes, 74(2), 142–149. 10.1097/QAI.0000000000001225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen, T. T. (2005). Gender power imbalance on women's capacity to negotiate self‐protection against HIV/AIDS in Botswana and South Africa. African Health Sciences, 5(3), 188–197. 10.5555/afhs.2005.5.3.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy, A. , Chakravarty, D. , Dladla, S. , de Bruyn, G. , & Darbes, L. (2016). Sexual communication self‐efficacy, hegemonic masculine norms and condom use among heterosexual couples in South Africa. AIDS Care, 28(2), 228–233. 10.1080/09540121.2015.1080792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshabari, S. C. , Blystad, A. , & Moland, K. M. (2007). Difficult choices: Infant feeding experiences of HIV‐positive mothers in northern Tanzania. SAHARA‐J: Journal of Social Aspects of HIV/AIDS, 4(1), 544–555. 10.1080/17290376.2007.9724816 [DOI] [PubMed] [Google Scholar]
- Maman, S. , Cathcart, R. , Burkhardt, G. , Omba, S. , Thompson, D. , & Behets, F. (2012). The infant feeding choices and experiences of women living with HIV in Kinshasa, Democratic Republic of Congo. AIDS Care, 24(2), 259–265. 10.1080/09540121.2011.597708 [DOI] [PubMed] [Google Scholar]
- Mbekenga, C. K. , Pembe, A. B. , Darj, E. , Christensson, K. , & Olsson, P. (2013). Prolonged sexual abstinence after childbirth: Gendered norms and perceived family health risks. Focus group discussion in a Tanzanian suburb. BMC International Health and Human Rights, 13, 4. 10.1186/1472-698X-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofenson, L. M. , Baggaley, R. C. , & Mameletzis, I. (2017). Tenofovir disoproxil fumurate safety for women and their infants during pregnancy and breastfeeding. Aids, 31(2), 213–232. 10.1097/QAD.0000000000001313 [DOI] [PubMed] [Google Scholar]
- Montgomery, E. T. , Roberts, S. T. , Nel, A. , Malherbe, M. , Torjesen, K. , Bunge, K. , … Palanee‐Phillips, T. (2019). Social harms in female‐iniatated HIV prevention method research: State of the evidence. Aids, 33(14), 2237–2244. 10.1097/QAD.0000000000002346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugwanya, K. K. , Hendrix, C. W. , Mugo, N. R. , Marzinke, M. , Katabira, E. T. , Ngure, K. , … Baeten, J. M. (2016). Pre‐exposure prophylaxis use by breastfeeding HIV‐uninfected women: A prospective short‐term study of antiretroviral excretion in breast milk and infant absorption. PLoS Medicine, 13(9), e1002132. 10.1371/journal.pmed.1002132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagot, N. , Kankasa, C. , Tumwine, J. K. , Meda, N. , Hofmeyr, G. J. , Vallo, R. , … Rekacewicz, C. (2016). Extended pre‐exposure prophylaxis with lopinavir‐ritonavir versus lamivudine to prevent HIV‐1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): A randomised controlled trial. Lancet, 387(10018), 566–573. 10.1016/S0140-6736(15)00984-8 [DOI] [PubMed] [Google Scholar]
- National Statistical Office [Malawi] , & ICF . (2017). Malawi demographic and health survey 2015–2016 . Zomba, Malawi and Rockville, Maryland, USA: NSO and ICF.
- Nel, A. , van Niekerk, N. , Kapiga, S. , Bekker, L. G. , Gama, C. , Gill, K. , … Ring Study Team . (2016). Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. New England Journal of Medicine, 375(22), 2133–2143. [DOI] [PubMed] [Google Scholar]
- Noguchi, L. M. , Hoesley, C. , Kelly, C. , Scheckter, R. , Bunge, K. , Nel, A. , … Beigi, R. H. (2019). Pharmacokinetics of dapivirine transfer into blood plasma, breast milk, and cervicovaginal fluid of lactating women using the dapivirine ring. Antimicrobial Agents and Chemotherapy, 63(3), e01930–e01918. 10.1128/AAC.01930-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, L. M. , Montgomery, E. T. , Biggio, J. R. , Hendrix, C. W. , Bogen, D. L. , Hillier, S. L. , … Beigi, R. H. (2016). Detectable tenofovir levels in breastfeeding infants of mothers exposed to topical tenofovir. Antimicrobial Agents and Chemotherapy, 60(9), 5616–5619. 10.1128/AAC.00645-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard, L. R. , & Bula, A. (2010). “They call our children ‘Nevirapine babies?’”: A qualitative study about exclusive breastfeeding among HIV positive mothers in Malawi. African Journal of Reproductive Health, 14(3), 213–222. [PubMed] [Google Scholar]
- Palanee‐Phillips, T. , Roberts, S. T. , Reddy, K. , Govender, V. , Naidoo, L. , Siva, S. , … Montgomery, E. T. (2018). Impact of partner‐related social harms on women's adherence to the dapivirine vaginal ring during a Phase III trial. Journal of Acquired Immune Deficiency Syndromes, 79(5), 580–589. 10.1097/QAI.0000000000001866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjee, G. , & Daniels, B. (2013). Women and HIV in sub‐Saharan Africa. AIDS Research and Therapy, 10(1), 30. 10.1186/1742-6405-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia, D. , Onadja, Y. , Hajizadeh, M. , Heymann, S. J. , Brewer, T. F. , & Nandi, A. (2016). What explains gender inequalities in HIV/AIDS prevalence in sub‐Saharan Africa? Evidence from the demographic and health surveys. BMC Public Health, 16(1), 1136. 10.1186/s12889-016-3783-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, A. , & Thomson, S. B. (2009). Framework analysis: A qualitative method for applied policy research. Journal of Administration and Governance, 4(2), 72–79. [Google Scholar]
- van der Straten, A. , Ryan, J. H. , Reddy, K. , Etima, J. , Taulo, F. , Mutero, P. , … MTN‐041/MAMMA Study Team . (2020). Influences on willingness to use vaginal or oral PrEP during pregnancy and breastfeeding in Africa: The Multisite MAMMA study. Journal of the International AIDS Society, 23, e25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank . (2019). Fertility rate, total (births per woman)—Sub‐Saharan Africa. Retrieved from https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=ZG
- Tuthill, E. , McGrath, J. , & Young, S. (2014). Commonalities and differences in infant feeding attitudes and practices in the context of HIV in Sub‐Saharan Africa: A metasynthesis. AIDS Care, 26(2), 214–225. 10.1080/09540121.2013.813625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda Bureau of Statistics (UBOS) , & ICF . (2018). Uganda demographic and health survey 2016. Kampala, Uganda and Maryland, USA: UBOS and ICF.
- UNAIDS . (2016). More investment needed in developing female‐controlled HIV prevention options. Retrieved from https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2016/february/20160223_CROI
- Waitt, C. J. , Garner, P. , Bonnett, L. J. , Khoo, S. H. , & Else, L. J. (2015). Is infant expsoure to antiretroviral drugs during breastfeeding quantitatively important? A systematic review and meta‐analysis of pharmacokinetic studies. Journal of Antimicrobial Chemotherapy, 70(7), 1928–1941. 10.1093/jac/dkv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO , UNICEF , UNFPA , & UNAIDS . (2003). HIV and infant feeding: A guide for health‐care managers and supervisors. Geneva, Switzerland: WHO.
- WHO , UNICEF , UNFPA , & UNAIDS . (2007). HIV and infant feeding: New evidence and programmatic experience: Report of atechnical consultation held on behalf of the Inter‐agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and their Infants, Geneva, Switzerland, 25–27 October 2006. Geneva, Switzerland: WHO.
- World Health Organization . (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Retrieved from http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- Young, S. L. , Mbuya, M. N. N. , Chantry, C. J. , Geubbels, E. P. , Israel‐Ballard, K. A. , Cohan, D. , … Latham, M. C. (2011). Current knowledge and future research on infant feeding in the context of HIV: Basic, clinical, behavioral, and programmatic perspectives. Advances in Nutrition, 2(3), 225–243. 10.3945/an.110.000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimbabwe National Statistics Agency and ICF International . (2016). Zimbabwe demographic and health survey 2015: Final report. Rockville, Maryland, USA: Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information
Table S1. Key themes, sub‐themes and illustrative quotes by type of study participant


